Abstract
Background and Objectives:
The distinct illness trajectory after acute ischemic stroke demands a better understanding of the utilization of palliative care consultations (PCC) for this patient cohort. This study sought to determine the prevalence, predictors, and outcomes associated with PCC for patients hospitalized with severe ischemic stroke.
Methods:
This multicenter cohort study was conducted at four hospitals (2 comprehensive and 2 primary stroke centers) between 01/2016 and 12/2019. We included all patients with a discharge diagnosis of ischemic stroke and an initial National Institutes of Health Stroke Scale (NIHSS) of 10 or greater. We compared patient sociodemographic, clinical and care characteristics as well as hospital outcomes between patients who did and did not receive PCC.
Results:
The study included 1297 patients hospitalized with severe ischemic stroke. PCC occurred for 20% of all patients and this proportion varied across institutions from 11.9% to 43%. Less than half (43%) of patients who died in the hospital. In multivaraible analysis, PCC was less likely in female patients (OR .76, 95% CI .59, .99, p=.04) but more likely in patients with higher NIHSS (OR1.95, 95% CI 1,13, 3.37, p=.02). Patients with PCC had higher rates of moving to a plan focused on comfort measures (CMO) (p <.01) and removal of artificial nutrition as part of a move to CMO (p<.01). In a sub analysis of patients who died in the hospital and received PCC, patients who died on or before hospital day 3 were less likely to receive PCC than patients who died on or after hospital day 4 (24% v. 51%) (p=<.01).
Conclusions:
Most patients with severe stroke do not receive PCC, even among those who experience in-hospital death. The results of this study indicate there are missed opportunities for PCC to help reduce suffering after severe stroke.
Background
Stroke is a leading cause of mortality in the United States resulting in one death every four minutes (1). Among patients who experience a nonfatal stroke, the risk of all-cause mortality at 28 days, 1 year, and 5 years after stroke is 28%, 41%, and 60%, equating to an almost 5-fold increase in risk of death compared with the general population (2). Of the 795,000 strokes that occur each year, nearly 1 in 4 occur in people who have had a previous stroke (1). As the population ages, stroke prevalence, and mortality could potentially double (3). Patients who survive severe strokes have high levels of disability due to impaired cognitive and physical function (3–5). Given the high morbidity and mortality after stroke, the acuteness of stroke onset, and the difficult decisions about goals of care, use of life sustaining treatments, and end of life care, patients with stroke and their families may benefit from palliative care (6–9). However, the distinct illness trajectory of stroke, including the sudden onset, physical and cognitive disabilities and uncertain prognosis, suggest the possible need for a distinct approach to palliative care after stroke; perhaps specifically one that moves away from the traditional, historic approaches that were developed in a cancer setting (7, 10).
Palliative care is medical care for patients living with serious illness which focuses on improving the quality of life for patients and their caregivers, providing care that is consistent with preferences, and addressing pain and symptom relief (11). Additionally, palliative care focuses on social, emotional, and spiritual support. Palliative care may be provided with an increasing emphasis on comfort measures as illness progresses; when curative treatment is no longer a possibility, patients may transition to hospice. Palliative care may be provided by the patient’s primary medical team (primary palliative care) or, in cases where the needs of patients or their families are more complex or difficult, specialty palliative care clinicians may be consulted (12).
In disease courses such as heart failure and cancer, palliative care interventions, including care provided by specialty trained palliative care physicians and nurses, may improve certain aspects of patient care such as quality of life, mood, and healthcare utilization (13–16). Although use of palliative care specialists is established in some life threatening and life ending diseases, little is known about optimal integration of palliative care specialists among the stroke population (4, 17–18). Additionally, few studies have explored the landscape of palliative care use among patients hospitalized for severe stroke (3,6,19–22). The need for further studies regarding palliative care after severe stroke was emphasized in an American Heart Association statement on palliative care and end-of-life care after stroke in 2014 (3). This study sought to fill gaps in the literature by describing the characteristics, predictors and current practice of palliative care consultation as well as outcomes associated with palliative care consultation among patients hospitalized with severe stroke.
Methods
Study Design and Population
We conducted a retrospective multicenter cohort study of patients suffering severe stroke between January 1, 2016 and December 31, 2019 at four hospitals in one city in the Midwestern United States, including a 1262-bed teaching hospital with a comprehensive stroke center certification, a 825-bed comprehensive stroke center, a 462-bed primary stroke center, and a 315-bed safety-net hospital with a primary stroke center certification. Stroke center certification denotes shown ability to deliver appropriate clinical rapid stroke assessment and care. At the time of data collection, three of the four hospitals offered thrombectomy. All hospitals admitted patients to the hospitalist team and utilized neurologists as consultants during stroke care delivery. Additionally, all hospitals had inpatient PCC services and none had a standard or protocol regarding when to consult palliative care. Institutional Review Board approval was obtained from Indiana University.
Eligible patients were identified by the clinical stroke coordinator, a specialized stroke nurse at each hospital. We included all patients with a severe ischemic stroke defined by: (1) a discharge diagnosis of ischemic stroke as identified by International Classification of Diseases, 10th Revision codes; and (2) an initial National Institutes of Stroke Scale Score (NIHSS) of 10 or greater. Patients with a discharge diagnosis of intracranial hemorrhage (ICH) were excluded, as were patients with a discharge diagnosis of transient ischemic attack (TIA) or ischemic stroke with an NIHSS of 9 or lower. Patients with ICH were excluded as ICH is distinct from ischemic stroke and this study focuses on one disease.
Outcome Measures
A standardized chart review tool was designed by the research team to collect patient information from the electronic medical record (EMR) including: 1) demographics (age, gender, race, ethnicity); 2) clinical characteristics (history of prior stroke, NIHSS score on admission, receipt of tissue plasminogen activator (tPA), thrombectomy, intensive care unit (ICU) utilization, mechanical ventilation); 3) palliative care specialist consultation (PCC); and 4) patient outcomes (length of hospital stay, use of a percutaneous gastrostomy feeding tube (PEG Tube) or tracheostomy, a change in code status, transition to comfort measures only, in-hospital mortality, hospice consultation). Patient race and ethnicity were collected because prior studies have shown differential use of palliative care in stroke care between races and ethnicities (21). Sixteen chart reviewers were trained in data abstraction and a kappa statistic was performed on twenty charts. The minimum of 80% agreement was met in order to ensure interrater reliability among chart reviewers. Data was deidentified upon extraction from the EMR and collected and stored in a REDCap database (23).
Statistical Analysis
Descriptive analysis was conducted to determine the prevalence of palliative care consultations following acute severe stroke. Logistic regression was conducted to describe patient and clinical characteristics associated with a palliative care consultation after severe ischemic stroke. Additionally, bivariate analysis was conducted to determine patient and clinical characteristics associated with early PCC (defined as PCC on or before hospital day 3) and late PCC (defined as PCC on or after hospital day 4). Outcomes associated with palliative care consultation after severe stroke, as well as outcomes associated with early and late PCC were analyzed using chi square analysis. Hospital location was adjusted for in this analysis, using hospital as a random effect. This allowed for the effect of hospital to be accounted for in the model, while understanding that the included hospitals are a random sample of possible hospitals. Multivaraible analysis with receipt of PCC as the dependent variable was conducted using all variables from the bivariate analysis with a p-value ≤.20 as well as variables selected a-priori including age, gender, and race. All statistical tests were performed using SAS Version 9.0 (Carry, North Carolina).
Results
Baseline Characteristics
The study included 1297 patients hospitalized with severe ischemic stroke (Table 1). The patients were 54% female, 81% white, and had a median age of 70 years old. The median NIHSS was 17. One in three (501, 39%) patients had a history of prior stroke. One-third of patients received intravenous (tPA) (n=430, 33%) and/ or mechanical thrombectomy (n=405, 32%), and 17% received both tPA and mechanical thrombectomy (n=223).
Table 1.
Patient Demographic and Clinical Characteristics
| All Patients (n=1297) | |
|---|---|
|
| |
| Gender | |
|
| |
| Female | 698 (53.9) |
|
| |
| Male | 597 (46.1) |
|
| |
| Age | 70.1 (58.1, 82.0) |
|
| |
| Race | |
|
| |
| White | 1046 (81.2) |
|
| |
| Black | 192 (14.9) |
|
| |
| Other/ Unknown | 51 (4.0) |
|
| |
| NIHSS (categorical) | |
| 10 – 20 | 848 (65.4) |
| >=21 | 449 (34.6) |
|
| |
| Prior CVA/TIA | |
| First Stroke (no prior) | 789 (61.2) |
| Secondary Stroke (yes prior) | 501 (38.8) |
|
| |
| tPA | |
| Yes | 430 (33.3) |
| No | 863 (66.7) |
|
| |
| Thrombectomy | |
| Yes | 405 (31.6) |
| No | 878 (68.4) |
Characteristics of Patients with Palliative Care Specialist Consultation
Palliative care specialist consultation (PCC) was received by 20% (256) of patients (Table 2). The proportion of PCC varied across the four institutions from 11.9% of patients suffering severe stroke receiving palliative care to 43% of patients. At the time of PCC, 66% (170) of patients were in the ICU, 31% (78) were receiving mechanical ventilation, and 2% (6) were receiving dialysis. Setting goals of care was indicated as the primary reason for the PCC (239, 93%). Median time from hospital admission to PCC was 4 days (IQR: 2–7), and PCCs were most frequently placed early during the hospitalization (39% occurring on or by hospital day 2) with declining frequency on each successive day (Figure 1). Approximately 25% of PCC occurred on or after hospital day 7. Among those who died in-hospital, the median day between PCC and death was 4 days (Table 2).
Table 2.
Palliative Care Utilization Following Acute Severe Stroke
| All Hospitals n (%) | Hospital 1 (n=106) | Hospital 2 (n=210) | Hospital 3 (n=563) | Hospital 4 (n=418) | |
|---|---|---|---|---|---|
| Palliative care consultation | 256 (19.7) | 32 (30.2) | 91 (43.3) | 84 (14.9) | 49 (11.7) |
| Median number of days between PCC and death | 4 (2, 11); 125/256 | 9 (2, 53) | 5 (1.5, 15) | 3 (2, 6.5) | 2 (1, 4) |
| Service ordering palliative care consultation | |||||
| Internal medicine | 192 (77.4) | 30 (100) | 80 (87.9) | 69 (86.3) | 13 (27.7) |
| Neurology | 43 (17.3) | 0 (0) | 5 (5.5) | 7 (8.8) | 31 (66.0) |
| Other | 13 (5.2) | 0 (0) | 6 (6.6) | 4 (5.0) | 3 (6.4) |
| Reason for palliative care consultation | |||||
| Communication | 20 (7.8) | 0 (0) | 2 (2.2) | 2 (2.4) | 16 (32.7) |
| Goals of care | 239 (93.4) | 30 (93.8) | 86 (94.5) | 77 (91.7) | 46 (93.9) |
| Pain | 3 (1.2) | 0 (0) | 1 (1.1) | 1 (1.2) | 1 (2.0) |
| Code status | 53 (20.7) | 3 (9.4) | 28 (30.8) | 5 (6.0) | 17 (34.7) |
| Other | 41 (16.0) | 5 (15.6) | 19 (20.9) | 13 (15.5) | 4 (8.2) |
| ICU Utilization at the time of PCC | 170 (66.4) | 28 (87.5) | 48 (52.8) | 66 (78.6) | 28 (57.1) |
| Mechanical Ventilation at the time of PCC | 78 (30.6) | 15 (46.9) | 14 (15.4) | 33 (39.8) | 16 (32.7) |
| Median days on vent | 5 (2.5, 10) | 11 (3, 15) | 3.5 (2, 7) | 4 (2, 8) | 5 (3, 8.5) |
| Dialysis at time of PCC | 6 (2.3) | 0 (0) | 2 (2.2) | 2 (2.4) | 2 (4.1) |
| Median (IQR) number of days until PCC from admission | 4 (2, 7) | 4.5 (2, 7) | 2 (1, 4) | 4 (2, 8) | 6.5 (3, 10.5 |
Figure 1.
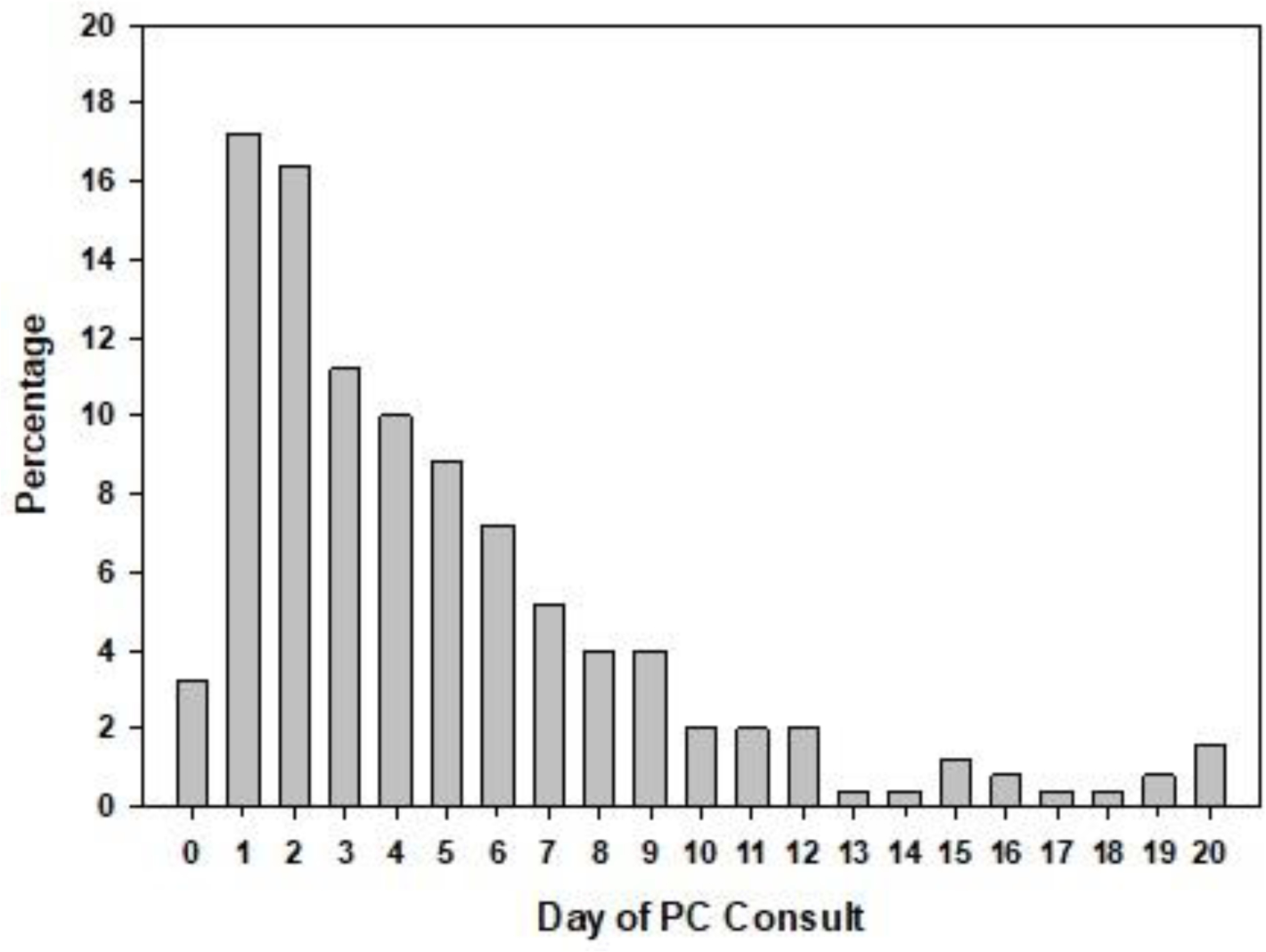
Day of initial palliative care consultation and length of Hospital stay
In bivariate analysis, compared to patients with stroke who did not receive PCC, those who received PCC were older (mean age 81 years vs. 68), had a higher mean NIHSS score (20 vs.17), and had less often received tPA (13% vs. 87%) or thrombectomy (12% vs 88%) (Table 3). After adjusting for potential confounders in multivaraible analysis, only higher NIHSS (OR 2.17) and female gender (OR 0.76) remained significantly associated with PCC. Receipt of tPA or thrombectomy were no longer associated with PCC. We found no significant difference in race between patients receiving PCC and those not (Table 3).
Table 3.
Bivariate and Multivariate Patient Characteristics Associated with Palliative Care Consultation after Severe Stroke
| No Palliative Care Consult (n=1041)* | Palliative Care Consult (n=256)* | p-value | Multivariate analysis Odds Ratio 95% CI p-value | Early PCC (n=125) | Late PCC (n=130) | p-value | Multivariate analysis Odds Ratio 95% CI p-value | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gender | ||||||||
|
| ||||||||
| Female | 548 (78.5) | 150 (21.5) | .09 | 0.76 (0.59,0.99); p=.0419 | 82 (54.7) | 68 (45.3) | .03 | 1.60 (0.91, 2.83); p=.1036 |
|
| ||||||||
| Male | 491 (82.2) | 106 (17.8) | reference | 43 (41.0) | 62 (59.1) | Reference | ||
|
| ||||||||
| Age | 67.6 (57.1,78.) | 81.3 (67.1,88.6) | <.01 | 0.99 (0.99,1.00); p=.0773 | 84.8 (72.2, 90.1) | 77.4 (63.8, 86.2) | <.00 | 1.02 (1.00, 1.04); p=.0728 |
|
| ||||||||
| Race | .67 | |||||||
|
| ||||||||
| White | 833 (79.6) | 213 (20.4) | .38 | Reference | 107 (50.2) | 106 (49.8) | Reference | |
|
| ||||||||
| Black | 161 (83.9) | 31 (16.2) | 0.80 (0.55,1.16); p=.4837 | 13 (41.9) | 18 (58.1) | 0.67 (0.28, 1.61); p=.3284 | ||
|
| ||||||||
| Other/ Unknown | 40 (78.4) | 11 (21.6) | 0.90 (0.46,1.78); p=.9772 | 5 (45.5) | 6 (54.6) | 1.30 (0.32, 5.25); p=.5250 | ||
|
| ||||||||
| NIHSS (categorical) | ||||||||
| 10 – 20 | 719 (84.8) | 129 (15.2) | <.01 | Reference | 59 (45.7) | 70 (54.3) | .29 | Reference |
| >=21 | 322 (71.7) | 127 (28.3) | 2.17 (1.60,2.94); p<.0001 | 66 (52.4) | 60 (47.6) | 1.51 (0.88, 2.59); p=.1320 | ||
|
| ||||||||
| Prior CVA/TIA | ||||||||
| First Stroke (no prior) | 639 (81.0) | 150 (19.0) | .50 | Reference | 75 (50.3) | 74 (49.7) | .56 | Reference |
| Secondary Stroke (yes prior) | 398 (79.4) | 103 (20.6) | 0.81 (0.2, 1.08); p=.1484 | 48 (46.6) | 55 (53.4) | 0.99 (0.57, 1.72); p=.9718 | ||
|
| ||||||||
| tPA | ||||||||
| Yes | 376 (87.4) | 54 (12.6) | <.01 | 1.07 (0.81,1.43); p=.6228 | 21 (38.9) | 33 (61.1) | .09 | 1.21 (0.60, 2.44); p=.6016 |
| No | 661 (76.6) | 202 (78.9) | 104 (51.7) | 97 (48.3) | ||||
|
| ||||||||
| Thrombectomy | ||||||||
| Yes | 355 (87.7) | 50 (12.4) | <.01 | 1.00 (0.74,1.34); p=.9828 | 12 (24.5) | 37 (75.5) | <.01 | 3.56 (1.65, 7.66); p=.0012 |
| No | 674 (76.8) | 204 (23.2) | 111 (54.4) | 93 (45.6) | ||||
Characteristics of Patients with Early versus Late Palliative Care Specialist Consultation
In bivariate analysis, compared to patients who received late PCC, those who received early PCC were more often female (54% v. 41% male), were older (mean age 85 years vs. 77) and less often received tpa or thrombectomy (Table 3). After multivaraible analysis, only thrombectomy remained independently associated with the timing of PCC (OR 3.56 for patients receiving late compared to early PCC, p < 0.01) (Table 3).
Palliative Care Specialist Consultation and Outcomes
During the index hospitalization, 16% (212) of patients died and 186 of those (88%) died in the setting of comfort measures only (Table 4). In bivariate analysis, compared to patients who did not receive a PCC, patients with PCC were more likely to receive hospice consultation (p<.01); to be moved to comfort measures only (CMO) (p <.01); and undergo removal of artificial nutrition as part of a move to CMO (p<.01) (Table 4). PCC was also associated with longer length of hospital stay (p<.01), and longer ICU stay (p<.01).
Table 4.
Outcomes associated with Palliative Care Consultation after severe stroke
| All Patients (n=1297) | No Palliative Care Consult (n=1041) | Palliative Care Consult (n=256) | p-value | Early PCC (n=125) | Late PCC (n=130) | p-value | |
|---|---|---|---|---|---|---|---|
| In Hospital Mortality | 212 (16.4) | 121 (57.1) 11.7% | 91 (42.9) 35.8% | <.01 | 43 (47.8) 34.7% | 47 (52.2) 36.4% | .77 |
| Hospice Consultation | 155 (12.0) | 61 (39.4) 5.9% | 94 (60.7) 36.7% | <.01 | 54 (57.5) 43.2% | 40 (42.6) 30.8% | .04 |
| Length of Hospital Stay | 7 (4, 11) | 7 (4, 11) | 8 (5, 13) | .01 | 5 (3, 9) | 11.5 (8, 18) | <.01 |
| Length of stay in ICU | 4 (2, 6) | 4 (2, 6) | 5 (3, 8) | <.01 | 3 (2, 5) | 7 (4, 12) | <.01 |
| PEG Tube | 201 (15.6) | 154 (76.6) 14.9% | 47 (23.4) 18.4% | .17 | 13 (27.7) 10.4% | 34 (72.3) 26.2% | <.01 |
| TRACH | 33 (2.6) | 21 (63.6) 2.0% | 12 (36.4) 4.7% | .01 | 0 (0) 0% | 12 (100) 9.2% | <.01 |
| Pt. moved to comfort measures | 288 (22.4) | 141 (49.0) 13.7% | 147 (51.0) 57.7% | <.01 | 73 (50.0) 58.9% | 73 (50.0) 56.2% | .66 |
| Extubation as a part of move to comfort measures | 136 (10.6) | 85 (62.5) 8.3% | 51 (37.5) 20.1% | <.01 | 19 (37.3) 15.3% | 32 (62.8) 24.6% | .06 |
| Withdrawal of artificial nutrition was part of a move to comfort measures | 123 (9.6) | 50 (40.7) 4.9% | 73 (59.4) 28.7% | <.01 | 31 (43.1) 25.2% | 41 (56.9) 31.5% | .26 |
| Patient resuscitated during hospitalization | 21 (1.6) | 18 (85.7) 1.7% | 3 (14.3) 1.2% | .53 | 1 (33.3) 0.8% | 2 (66.7) 1.5% | >.99 |
| Change in Code Status (yes) | 337 (26.1) | 183 (54.3) 17.7% | 154 (45.7) 60.4% | <.01 | 63 (41.2) 50.8% | 90 (58.8) 69.2% | <.01 |
| Code Status change | |||||||
| Full code | 9 (2.7) | 7 (77.8) 3.8% | 2 (22.2) 1.3% | .06 | 0 (0) 0% | 2 (100) 2.2% | .27 |
| Partial code | 22 (6.5) | 16 (72.7) 8.7% | 6 (27.3) 3.9% | 1 (16.7) 1.6% | 5 (83.3) 5.6% | ||
| Do not resuscitate | 306 (90.8) | 160 (52.3) 87.4% | 146 (47.7) 94.8% | 62 (42.8) 98.4% | 83 (57.2) 92.2% | ||
| Discharge location | |||||||
| Home | 183 (16.8) | 171 (93.4) 18.6% | 12 (6.6) 7.0% | <.01 | 9 (75.0) 10.2% | 3 (25.0) 3.6% | .15 |
| Other Health Care Facility | 514 (47.1) | 476 (92.6) 51.7% | 38 (7.4) 22.1% | 16 (42.1) 18.2% | 22 (57.9) 26.2% | ||
| Acute Care Facility | 273 (25.0) | 218 (79.9) 23.7% | 55 (20.2) 32.0% | 24 (43.6) 27.3% | 31 (56.4) 36.9% | ||
| Inpatient Hospice | 55 (5.0) | 20 (36.4) 2.2% | 35 (63.6) 20.4% | 20 (57.1) 22.7% | 15 (42.9) 17.9% | ||
| Home Hospice | 52 (4.8) | 21 (40.4) 2.3% | 31 (59.6) 18.0% | 19 (61.3) 21.6% | 12 (38.7) 14.3% | ||
| D/C to another hospital | 11 (1.0) | 11 (100) 1.2% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | ||
| Other | 4 (0.4) | 3 (75.0) 0.3% | 1 (25.0) 0.6% | 0 (0) 0% | 1 (100) 1.2% |
Values are medians (IQRs) for continuous variables and frequencies (percentages for categorical variables are listed as row percentages in parentheses and column percentages with a %); p-values are from Wilcoxon and Chi-Square tests (verified with Fisher’s Exact where necessary), respectively. Frequencies may not add to column totals due to missing data.
indicates the category “N/A Due to Death” was removed from this one variable.
PCC was associated with patients not dying in the hospital (p<.01) and with being discharged to hospice (either in-patient, p<.01 or home, p<.01). Compared to patients who received late PCC, patients who received early PCC were more likely to receive hospice consultation (58% v. 43%) (p=.04) (Table 4). In a sub analysis of patients who died in the hospital and received PCC, patients who died on or before hospital 3 were less likely to receive PCC than patients who died on or after hospital day 4 (24% v. 51%) (p=<.01).
Compared to early PCC, late PCC was associated with tracheostomy utilization among patients who received PCC and tracheostomy (0% early v. 100% late) (p<.01) (Table 4). Among patients who received PEG Tube and PCC, late PCC was associated with PEG Tube (72% late PCC v. 28% early PCC) (p<.01) (Table 4). Among patients in the ICU who received PCC, the mean length of stay in the ICU for patients who received early PCC was 3 days, compared to 7 days for patients who received late PCC (p<.01) (Table 4).
Discussion
In this large multi-center retrospective study, palliative care specialists were consulted for one in five patients after severe stroke with a wide variation across hospitals. Palliative care consultation was associated with greater stroke severity, suggesting it is often reserved for the sickest patients. However, there may have been missed opportunities for PCC. Patients who had disability severe enough to require a percutaneous feeding were no more likely to receive a PCC, and among those patients, fewer than one in four received a PCC. PCC was significantly associated with use of tracheostomy for long-term ventilator support, but was still only received in fewer than half of cases. Patients and families with feeding or respiratory difficulties may have high distress and could benefit from PCC. Also, patients with severe stroke may benefit from PCC directed goals of care decisions when considering these interventions. Goals of care conversations include discussions related to the patient’s values, goals, and treatment preferences (25). The variability in PCC consultation among our four hospitals suggests a lack of standardization of who receives PCC after severe stroke.
This study also found that PCC was overwhelmingly consulted regarding setting goals of care. Goals of care conversations should be a core competency for all neurologist who treat stroke patients (26). More research is needed on the quality of these conversations and the role of medical documentation for its assessment. Given the high hospital mortality of this patient group and the difficulty in accurate and consistent prediction of recovery early on, PCC may be appropriate to help patients set goals of care regarding interventions during the hospitalization (26–30). Evidence from the cancer literature suggests that having goals of care conversations early may help patients avoid the unwanted use of aggressive interventions and suffering at end-of-life (31–34). Although early goals of care conversations are optimal, approximately 25% of PCC occurred on or after hospital day 7 in this patient population suggesting the potential need for earlier PCC to set goals of care with patients suffering severe stroke.
After severe stroke, patients with a higher NIHSS who did not receive either tPA and/or thrombectomy had the highest prevalence of PCC. This occurred even when adjusting for decompensation within the first 48 hours of hospital admission. It is likely that the non-receipt of these evidence-based interventions is a marker of worse outcome and possibly more palliative care needs. Conversely, patients who receive tPA and thrombectomy may be less likely to receive PCC because once aggressive care has been initiated, patients, families, and clinicians may wish to give the patient more time to recover.
The majority of PCC occurred early; however, there was a substantial number (25%) of patients who received PCC late in their hospitalization. Among those with PCC, late consultation was associated with receipt of tPA, which may have been because of early hopes for recovery that were not met. Patients who died late during the hospitalization were five times more likely to receive PCC than patients who died early. In essence, the data shows that patients are either admitted with an apparent poor prognosis and receive PCC upon admission, or after some time in the hospital, they decline or do not show signs of recovery and receive PCC.
Limitations
This study has several limitations. First, the chart review was conducted at hospitals in one city and therefore, may not fully represent the practices in other regions or hospital settings. However, these hospitals do represent two different levels of stroke care, and differ in the presence of trainees. Second, due to the retrospective design of this study, the causal relationship between PCC and important outcomes cannot be assessed and should be explored in future prospective studies. One possibility for variation between hospitals is the structure of the palliative care services. Future research with prospective observations in a larger number of participating sites needs to be done to investigate these differences, potentially using mixed methods including interviews with practitioners and patient family members.
Conclusions
The proportion of patients who receive PCC varies widely across hospitals, suggesting there may be missed opportunities to provide palliative care support after severe stroke. Importantly, low rate of PCC, does not in and of itself necessarily mean a low rate of people being managed with appropriate palliative care principles as patients may have been managed using palliative care skills by stroke staff without the requirement for PCC. Interventions may be useful that promote PCC through the use of prompts, such as a checklist or trigger protocol for identifying situations when a PCC consultation may be valuable and promoting consultation earlier in the hospital course (7). There may be more opportunities for through both neurologists and PCC to optimize setting goals of care and to help reduce suffering after severe stroke. More research is needed to identify areas of missed opportunity to better determine the timing and role of PCC after severe stroke.
Contributor Information
Amber R. Comer, Indiana University School of Health and Human Sciences, 1050 Wishard Blvd, RG3034, Indianapolis, IN 46202.
Linda S. Williams, Richard L. Roudebush Veterans Affairs Medical Hospital, Indiana University School of Medicine, Regenstrief Institute, Inc.
Stephanie Bartlett, Indiana University School of Health and Human Sciences.
Lynn D’Cruz, Indiana University School of Health and Human Sciences.
Katlyn Endris, Indiana University School of Health and Human Sciences.
McKenzie Marchand, Indiana University School of Health and Human Sciences.
Isabel Zepeda, Indiana University School of Health and Human Sciences.
Sumeet Toor, Indiana University School of Medicine.
Carly Waite, Indiana University School of Health and Human Sciences.
Areeba Jawed, University Of Michigan.
Robert Holloway, University of Rochester.
Claire J. Creutzfeldt, University of Washington.
James E. Slaven, Indiana University School of Medicine.
Alexia M. Torke, Indiana University School of Medicine; Indiana University Center for Aging Research, Regenstrief Institute; Daniel F. Evans Center for Spiritual and Religious Values in Healthcare.
References
- 1.Centers for Disease Control and Prevention. Stroke Facts. https://www.cdc.gov/stroke/facts.htm. Accessed March 24, 2021.
- 2.Bronnim-Hansen H, Davidsen M, Thorvaldsen P. Long-term survival and causes of death after stroke. Stroke. 2001;32:2131–2136. [DOI] [PubMed] [Google Scholar]
- 3.Holloway RG, Arnold RM, Creutzfeldt CJ, Lewis EF, Lutz BJ, McCann RM, et al. Palliative and End-of-Life Care in Stroke: A Statement for Healthcare Professionals from the American Heart Association/ American Stroke Association. Stroke. 2014;45:00–00. [DOI] [PubMed] [Google Scholar]
- 4.Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag. 2016;12:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Almenkerk S, Depla M, Smalbrugge M, Eefsting JA, Hertog C. Institutionalized stroke patients: Status of functioning of an under researched population. JAMDA. 2021;13:634–639. [DOI] [PubMed] [Google Scholar]
- 6.Burton CR, Payne S, Addington-Hall J, Jones A. The palliative care needs of acute stroke patients: a prospective study of hospital admissions. Age and Ageing. 2010;39(5):554–559. [DOI] [PubMed] [Google Scholar]
- 7.Creutzfeldt CJ, Holloway RG, Curtis JR. Palliative care: A core competency for stroke neurologists. Stroke. 2015;46:2714–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creutzfeldt CJ, Robinson MT, Holloway RG. Neurologists as primary palliative care providers: Communication and practice approaches. Neurology Clinical Practice. 2016;6(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens T, Payne SA, Burton C. et al. Palliative care in stroke: a critical review of the literature. Palliat. Med 2007;21(4):323–331. [DOI] [PubMed] [Google Scholar]
- 10.Creutzfeldt CJ, Longstreth WT, Holloway RG. Predicting decline and survival in severe acute brain injury: the fourth trajectory. BMJ. 2015;351:h3904. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health & Human Services. National Institute on Aging. What are palliative care and hospice care? https://www.nia.nih.gov/health/what-are-palliative-care-and-hospice-care. Accessed March 24, 2021.
- 12.Quill TE, Abernethy AP. Generalists plus Specialist Palliative Care – Creating a More Sustainable Model. N Engl J Med. 2013;368:1173–1175. [DOI] [PubMed] [Google Scholar]
- 13.Quinn KL, Shurrab M, Gitau K, Kavalieratos D, Isenberg SR. et al. Association of receipt of palliative care interventions with health care use, quality of life, and symptom burden among adults with chronic non-cancer illness: a systematic review and meta-analysis. JAMA. 2020;324(14):1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavalieratos D, Corbelli J, Zhand D. Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA. 2016;315(20):2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Palma R, Fortuna D, Hegarty SE, Louis DZ, et al. Effectiveness of palliative care services: A population-based study of end-of-life care for cancer patients. Palliat. Med 2018;32(8):1344–1352. [DOI] [PubMed] [Google Scholar]
- 16.Triplett DP, LeBrett WG, Bryant AK, Bruggeman, et al. Effect of palliative care on aggressiveness of end-of-life care among patients with advanced cancer. JCO Oncology practice. 2017;13(9):e760–e769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steigleder T, Kollmar R, Ostgathe C. Palliative care for stroke patients and their families: barriers for implementation. Front. Neurol 2019;10:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton CR, Payne S. Integrating palliative care within acute stroke services: developing a programme theory of patient and family needs, preferences and staff perspectives. BMC Palliative Care. 2012;11(22):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holloway RG, Ladwig S, Robb J, Kelly A, et al. Palliative care consultations in hospitalized stroke patients. J. Palliat Med 2010;13(4):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eastman P, McCarthy G, Brand CA, Weir L et al. Who, why and when: stroke care unit patients seen by a palliative service within a large metropolitan teaching hospital. BMJ Supportive & Palliative Care. 2013;3:77–83. [DOI] [PubMed] [Google Scholar]
- 21.Williams MT, Zimmerman E, Barry M, Trantum L, et al. A retrospective review of patients with acute stroke with and without palliative care consultations. Am J Hosp Palliat Care. 2019;36(1):60–64. [DOI] [PubMed] [Google Scholar]
- 22.Riesinger R, Altmann K, Lorenzl S. Involvement of specialist palliative care in a stroke unit in Austria – Challenges for families and stroke teams. Front. Neurol 2021;12:1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson T, Walton S, Levine S, Dister E, Baron A, O’Mahony S. Racial and ethnic disparity in palliative care and hospice use. Am J Manag Care. 2020;23(2):e36–e40. [DOI] [PubMed] [Google Scholar]
- 24.Comer AR, Fettig L, Torke AM. Identifying Goals of Care. Med Clin North Am. 2020;104(5):767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creutzfelt CJ, Holloway RG, Curtis JR. Palliative Care: A Core Competency for Stroke Neurologists. Stroke. 2015;46(9):2714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J et al. The REDCap consortium: Building an international community of software partners. J. Biomed Inform 2019;95:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAteer R, Wellbery C. Palliative care: benefits, barriers, and best practices. Am Fam Physician. 2013;88(12):807–813. [PubMed] [Google Scholar]
- 29.Brizzi K, Creutzfeldt CJ, Neuropalliative care: a practical guide for the neurologist. Semin Neurol. 2018;38(5):569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creutzfeldt CJ, Robinson MT. Neuropalliative care of stroke. UptoDate;2021. [Google Scholar]
- 31.Hwang F, Boardingham C, Walther S, Mosenthal AC. et al. Establishing goals of care for patients with stroke and feeding problems: an interdisciplinary trigger-based continuous quality improvement project. JPSM. 2018; 56(4):588–593. [DOI] [PubMed] [Google Scholar]
- 32.Eastman P, Le B. Palliative care and stroke: In: MacLeod R, van den (eds) Textbook of Palliative Care. Springer; 2018. [Google Scholar]
- 33.Rowland K, Schumann SA. Palliative care: Earlier is better. J Fam Pract. 2010;59(12): 695–698. [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmermann C, Swami N, Krzyzanowska M, Hannon B, et al. Early palliative care for patients with advanced caner: a cluster-randomized control trial. The Lancet. 2014;383(9930):1721–1730. [DOI] [PubMed] [Google Scholar]


