Abstract
Background:
Alcohol is one of the most commonly abused drugs worldwide. Cessation from chronic alcohol consumption can result in the presentation of withdrawal symptoms that commonly promote relapse in individuals with alcohol use disorder (AUD). Thus, preclinical models of voluntary alcohol consumption in which animals manifest spontaneous signs of withdrawal after alcohol cessation can be useful to study AUD and its treatment. The intermittent two-bottle choice paradigm (I2BC) has been extensively used to examine alcohol intake in rodents. However, previous studies have reported conflicting observations regarding its potential to result in the spontaneous manifestation of withdrawal upon alcohol cessation.
Methods:
We employed a battery of behavioral tests to examine the emergence of affective and physical signs of withdrawal in female and male mice exposed to ethanol in the I2BC for 10 weeks. Specifically, mice of both sexes undergoing 24-h withdrawal from the I2BC were tested for physical signs of withdrawal, anxiety-like behavior in the open field arena (OFA) and elevated plus maze (EPM), and anxiety/compulsive-like behavior in the marble burying test (MBT). The main outcomes from these tests were combined into a behavioral severity score to describe the overall behavioral phenotype.
Results:
Both female and male mice undergoing withdrawal from the I2BC displayed elevated physical signs of withdrawal, and anxiety-associated behavior in the EPM and MBT. Analysis of the overall behavioral severity revealed more severe phenotypes in female and male mice undergoing withdrawal from the I2BC compared to controls. Additionally, stratification of the subjects based on severity scores demonstrated differential distribution of severities between the exposure groups.
Conclusions:
Overall, we confirmed that a significant fraction of mice chronically exposed to alcohol in the I2BC display spontaneous withdrawal, and that computing a severity score from a combination of behavioral metrics can be a useful approach for pre-clinical research to model evaluation tools used in patients with AUD.
Keywords: alcohol, intermittent two-bottle choice, spontaneous withdrawal, physical and affective behaviors, behavioral severity score
INTRODUCTION
Alcohol is one of the most commonly used drugs worldwide (WHO, 2018). According to the 2019 National Survey on Drug Use and Health (NSDUH), the vast majority of adults (86%) in the United States (US) has tried alcohol at least once in their lifetime (Substance Abuse and Mental Health Services Administration (SAMHSA), 2019). Moreover, that same year, more than 14 million adults aged 18 or older were diagnosed with alcohol use disorder (AUD) (Substance Abuse and Mental Health Services Administration (SAMHSA), 2019). The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) describes AUD as a chronic cerebral impairment characterized by compulsive alcohol consumption with symptoms that can vary in number and nature among individuals with the disorder (NIAAA, 2013). These symptoms are related to level and frequency of intake, alcohol-associated social or occupational dysfunction, and the emergence of negative affective symptoms when drinking is discontinued. In particular, aversive symptoms of withdrawal pose a challenge to those attempting to become abstinent, as evidenced by positive correlations with relapse risk and negative correlation with treatment outcomes in patients with AUD (see review by Witkiewitz and Villarroel, 2009). It is important to emphasize that not all AUD patients present the same symptomatology, and that typology research has detected distinct AUD subtypes (see review by Leggio et al., 2009). Aligned with this, the current DSM-5 AUD diagnostic tool allows the subclassification of patients based on number of criteria met by the individual [i.e., mild (2-3), moderate (4-5), or severe (6-11)]. Additionally, the Clinical Institute for Withdrawal Assessment for Alcohol-revised (CIWA-Ar) scale is a widely used 10-item diagnostic tool used to determine the severity of the alcohol withdrawal syndrome (AWS) in AUD patients (Sullivan et al., 1989). Clinically, the CIWA-Ar score ranges from 0 to 67 and can be used to determine the optimal treatment for a patient with AWS. For example, inpatient treatment with supportive pharmacotherapy – commonly benzodiazepines, the ‘gold standard’ in the treatment of AWS – is recommended for patients presenting severe withdrawal syndrome (Muncie et al., 2013). The description of sub-types of AUD may be useful in identifying individuals at high risk, formulate prognoses, and determine appropriate specialty treatment.
Pre-clinical models of alcohol-related behaviors and comparable subclassifications can be useful to both pinpoint the neural substrates of AUD and assess the efficacy of novel drugs to modulate the symptoms of the disease. Due to the complexity of human behavior and the influence of social and environmental factors on alcohol use, a subset of AUD symptoms such as loss of control, social/occupational impairments, desire/failure to quit are difficult to study in laboratory animals. However, laboratory animal models exist for the tolerance that follows repeated alcohol exposure (both functional and metabolic) and the negative affect associated with discontinuation of chronic alcohol (see review by Crabbe et al., 2011). Of particular translational value are rodent models of alcohol self-administration capable of establishing dependence, manifested by the development of tolerance and the onset of withdrawal signs upon cessation. An extensively utilized paradigm is the intermittent access to 20% ethanol with a two-bottle choice (I2BC), a model of chronic voluntary oral alcohol consumption in rodents (Hwa et al., 2011; Melendez, 2011; Simms et al., 2008; Wise, 1973). In this paradigm, daily average doses greater than 10 g/kg ethanol are observed in mice, with sex significantly influencing levels of alcohol consumption (see Table 1). Both the free-choice nature of the paradigm and the possibility of establishing alcohol dependence per os are features that make this a good model of human-like drinking with high face validity. It has been shown that rats voluntarily consuming ethanol in the I2BC display sex-dependent alterations in anxiety-, compulsive-, and depressive- like behaviors, anhedonia and lower pain thresholds during acute withdrawal (i.e., 24 h after removal of ethanol bottle) (Gregor et al., 2019; Li et al., 2019). Moreover, some of these affective behaviors seem to remain altered in 28-day abstinent rats (i.e., protracted withdrawal) (Li et al., 2019). However, although the I2BC paradigm was described in mice about a decade ago, the number of studies examining the spontaneous manifestation of physical and/or affective signs of alcohol withdrawal is quite small. Table 1 summarizes findings from studies assessing acute withdrawal following I2BC in mice. To our knowledge, most studies have employed no more than two behavioral tests to evaluate withdrawal-related behaviors. Nonetheless, performing more comprehensive testing and employing methods that are similar to those used in humans may result in increased translational value of pre-clinical studies. As suggested before, stratification of groups based on a global behavioral score could be utilized to investigate genetic influencers on ethanol withdrawal. This approach may also be beneficial when testing the efficacy of a novel compound to reduce signs of ethanol withdrawal in experimental subjects with severe withdrawal phenotypes.
Table 1.
Findings from studies examining alcohol withdrawal-related behaviors in mice chronically exposed to ethanol in the intermittent two-bottle choice.
| Mouse Strain | Sex | Average Ethanol Dose (g/kg/day) | Duration of I2BC | Behavioral Test | Early Withdrawal Phenotype | |
|---|---|---|---|---|---|---|
| Hwa et al., 2011 | C57BL/6J | Females | 32.12 ± 1.45 | 16 w | - | - |
| Males | 20.10 ± 0.48 | 16 w | HIC | Increased 8-h HIC score Increased AUC of HIC score vs time curve |
||
| Hwa et al., 2015 | Swiss-derived, Carworth Farm Webster (CRW) | Males | 14.39 ± 0.35 | 8 w | HIC | Increased 8-h HIC Score |
| In-house Aggression test | Increased aggression against a male
conspecific in home cage Suppressed social contact with intruder male |
|||||
| Bloch et al., 2020 | C57BL/6J | Females | ~22 | 7 w | EZM | No effect* |
| Males | ~12 | 7 w | EZM | Trend of increased open zone entries
(disinhibition-like behavior) * Increased locomotion and average velocity |
||
| Nennig et al., 2020 | C57BL/6J | Males | ~20 | 4 w | SI following Subthreshold SDS | Decreased social interactions |
| Quijano Cardé et al., 2021 | C57BL/6J | Females | 15.90 ± 0.85 | 6 w | SS | Significant main effect of withdrawal on total number of signs, with increased shakes, scratches, grooming and other signs of withdrawal |
| Males | 11.11 ± 0.85 | 6 w | SS |
The following abbreviations are used: EPM = elevated plus maze, OFA = open field arena, MBT = marble burying test, EZM = elevated zero maze, HIC = handling-induced convulsion, SDS = social defeat stress, SI = social interaction, SS = somatic signs, AUC = area under the curve.
Significant main effect of chronic exposure on open zone entries in the absence of sex-by-exposure interaction. Independent statistical analyses for each sex revealed a non-significant trends of increased open zone entries, distance and velocity in males (p-values ≤ 0.081), but not in females.
Therefore, the goal of this study was to employ a battery of behavioral tests to examine the withdrawal syndrome in mice of both sexes voluntarily consuming alcohol in the I2BC for 10 weeks. All the tests utilized in this study evaluate behaviors that can be presented by drug-naïve mice at baseline but are exacerbated by withdrawal from ethanol (Perez et al., 2015; Perez and De Biasi, 2015). We hypothesized that mice exposed to ethanol in the I2BC would display worse physical and affective behaviors upon spontaneous alcohol withdrawal compared to alcohol-naïve mice, and we set out to determine potential sex-specific effects of chronic alcohol exposure. In addition to the results of our studies, we present the utilization of behavioral severity scores that summarize the overall severity of the physical and affective behaviors, and allow the stratification of experimental subjects into mild, moderate and severe phenotypes (Bock et al., 2013; O’Neal et al., 2020).
MATERIALS AND METHODS
Animals
For this study, we employed a total of 55 adult C57Bl/6J mice of both sexes. The mice were obtained from a C57Bl/6J colony maintained in our laboratory at the University of Pennsylvania. Throughout the study, the mice were group-housed (2-5/cage) in standard ‘shoebox’ cages (7.6 in x 15 in x 5.1 in) with 1 cm of corn cob bedding and a cotton nestlet. All animals had ad libitum access to fluid and food (Labdiet, 5053, PMI, Brentwood, MO). The mice were housed in a temperature- and humidity-controlled room (65-75 °F, 40-60% relative humidity) with a 12-h light/dark cycle. All procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
Reagents
Ethanol solutions were prepared by diluting 190 proof ethanol (Decon Labs, Inc., King of Prussia, PA) in filtered water.
Chronic Ethanol Exposure
We employed the intermittent access to ethanol in a two-bottle choice (I2BC) drinking paradigm for chronic ethanol exposure. This protocol has been extensively used in the field to promote home-cage self-administration of ethanol in mice (Hwa et al., 2011; Melendez, 2011). For the I2BC paradigm, we habituated group-housed adult mice (post-natal day (PND) 60) to the two-bottle setup for one week prior to the initiation of ethanol drinking. The bottles used in our study were 25-mL conical tubes with one-hole rubber stoppers with straight stainless-steel, open-tip sippers. The I2BC consisted of two phases: an initial 1-week escalation period during which mice were provided 24-h access to ethanol in increasing concentrations (3, 6, 10% v/v) every other day, followed by 9 weeks of intermittent access to 20% ethanol (three 24-h sessions/week). All drinking sessions started 1 h after the onset of the dark phase of the light cycle. The position of the ethanol bottle was alternated daily to avoid side preference. Bottles were weighed to the nearest hundredth at the start and end of each drinking session to determine 24-h ethanol and water consumption. Mice were weighed at the beginning of every drinking session to determine ethanol dose (g/kg), which was calculated using reported densities for ethanol solution in water at room temperature (i.e., ρ3%_ethanol = 0.99384 g/mL, ρ6%_ethanol = 0.98973 g/mL, ρ10%_ethanol = 0.98476 g/mL, ρ20%_ethanol = 0.97359 g/mL, and ρ100%_ethanol = 0.789 g/mL) and sum of body weights for each cage. Ethanol preference was calculated as the ratio of ethanol to total fluid intake (mL ethanol solution/mL total fluid).
Behavioral Testing to Assess Spontaneous Ethanol Withdrawal
To examine the effect of chronic ethanol consumption on affective and physical behaviors, we compared age-matched (PND 140-150) ethanol-naïve controls to mice undergoing 24-h withdrawal from the I2BC. For all the behavioral tests, the mice were habituated to the testing room for 30-45 min and were tested during the dark portion of the light cycle. The testing room was equipped with sound attenuating walls and dimmable lights. The luminosity in the behavioral room was measured using a LX-105 Lutron Digital Light Meter. The tests were performed in order of increasing stress: (1) physical signs, (2) MBT, (3) OFA, and (4) EPM. Our group has previously used this battery of behavioral tests to assess spontaneous ethanol withdrawal in mice (Perez and De Biasi, 2015). Three behavioral tools for the assessment of anxiety-associated behaviors were included given that previous work has shown that outcomes from different extensively used anxiety tests in rodents do not always correlate, suggesting that they measure distinct dimensions of anxiety that may be governed by different neuronal networks. Integration of various behavioral tests can provide information about the multidimensional nature of emotional status during withdrawal, suggested by our group and others (Costa Goes et al., 2009; Gangitano et al., 2009; Metten et al., 2018; Ramos et al., 2008, but also see review by Ramos, 2008).
In this study, tests were separated by 3 to 4 days (i.e., one withdrawal cycle) to minimize influences of previous tests on behavioral outcomes. The general study design is summarized in Figure 1E.
Figure 1. Concentration-response curves of ethanol consumption and preference for female and male mice in the intermittent two-bottle choice (I2BC) paradigm.
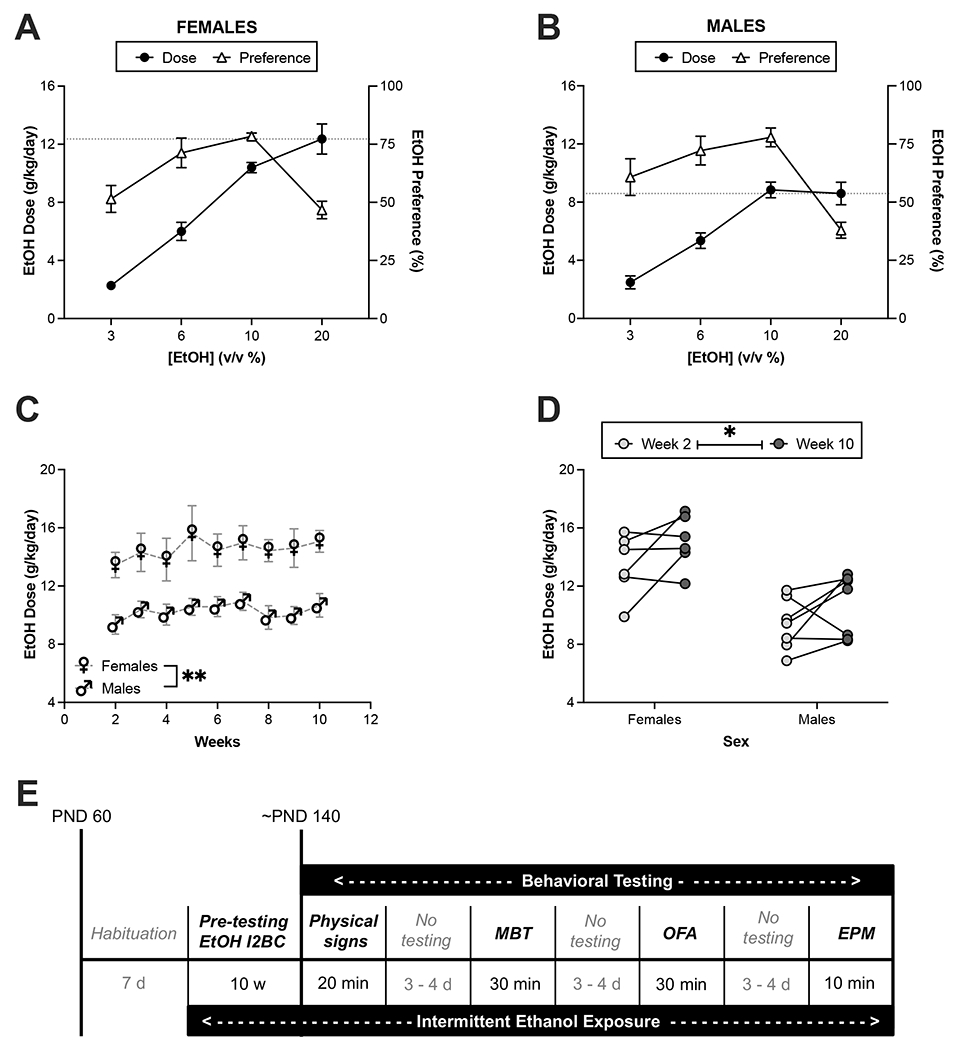
Group-housed female and male C57Bl/6J mice were chronically exposed to ethanol in the I2BC. Concentration-response curves for females (A) and males (B) are shown using data from the first four sessions of the I2BC. Circles (●) represent ethanol intake and triangles (△) represent the preference for the ethanol bottle at the specified concentration. The dotted lines indicate dose at 20% ethanol on the first session of week 2 (session 4). Weekly dose during 20% ethanol access (weeks 2 – 10) for males and females (C). Comparison of ethanol dose during weeks 2 and 10 revealed an increase in both females and males (D). Following chronic ethanol exposure in the I2BC, mice were tested in a battery of behavioral tests (E). Cage numbers are 6 for females and 7 for males. Total animal numbers are 16 for females and 15 for males. **p<0.01 for main effect of sex, *p<0.05 for main effect of week. EtOH = ethanol, MBT = marble burying test, OFA = open field arena, EPM = elevated plus maze.
Physical Signs of Withdrawal –
Previous studies in mice have reported alterations in physical behaviors – such as shaking, scratching, grooming, and chewing – during withdrawal from chronic ethanol (Perez et al., 2015; Perez and De Biasi, 2015). To examine these somatic signs, mice were tested in standard housing cages with 1 cm corn cob bedding. The physical behaviors were scored for 20 min, and the experimenter did not get closer than one foot from the cage to avoid disturbing the natural behavior of the mice. The luminosity in the testing room was 3-4 lux for the examination of physical signs of withdrawal.
Open Field Arena (OFA) –
The mice were tested for anxiety-like behavior in the OFA, a 40 cm x 40 cm x 20 cm box made of opaque, white Plexiglass, for 30 min. ANY-maze™ behavioral tracking software was used to quantify the time spent and the distance traveled in the center (20 cm x 20 cm) and outer zones of the OFA. The total distance traveled was used to examine locomotor behavior. The time spent exploring the center zone was used to investigate anxiety-like behavior. Lastly, the number of fecal boli in the maze was recorded at the end of the test. The luminosity in the center of the OFA was set at 7-8 lux for this test.
Elevated Plus Maze (EPM) –
Anxiety-like behavior was also examined for 10 min in the EPM, a cross-shaped maze with two non-adjacent corridors (25 cm x 7 cm) with black walls (height: 15 cm, closed arms) and the other two corridors with no walls (open arms). All corridors were connected by a 7 cm x 7 cm center area, and the maze was elevated 50 cm above the floor. The ANY-maze™ tracking software was used to monitor the behavior of the mice throughout the EPM. Total number of entries was used to assess locomotive behavior. The time spent exploring the open arms and the entry ratio were used to investigate anxiety-like behavior. Similar to the OFA, the number of fecal boli on the maze was counted at the end of the test. The luminosity above the open arms of the maze was 7-8 lux for the EPM test.
Marble Burying Test (MBT) –
The MBT test was used to examine anxiety/compulsive-like behavior. Each mouse was placed in a standard housing cage that contained 5 cm of corn cob bedding and 20 clear marbles (13 mm diameter) evenly spaced throughout the cage for 30 min. The number of buried marbles (75-100% of marble must be covered in bedding) was manually scored at the end of the test. The total amount of marbles buried was used as a measure of compulsive-like behavior. The luminosity on the testing area was 7-8 lux for MBT.
Determination of Behavioral Severity Scores –
In order to investigate whether a combination of outcomes from all behavioral tests utilized in this study could describe the withdrawal state of the mice, we utilized four behavioral metrics: (1) Total occurrence of somatic signs, (2) Number of marbles buried, (3) Total time in the center zone of the OFA, and (4) Open arms entry ratio in the EPM. The raw values of these outcomes were normalized to z-scores and multiplied by the direction of the effect of withdrawal. Then, all z-scores for each mouse were added to obtain a severity score (SS; see Table 2). Lastly, cutoff criteria previously utilized in the addiction field were used to classify the mice into low (SS < −1), moderate (−1 ≤ SS ≤ +1), or high (SS > +1) severity (Bock et al., 2013; O’Neal et al., 2020).
Table 2. Behavioral outcomes and formulas used to determine severity scores.
Severity scores (SS) were calculated using behavioral metrics from tests examining physical, compulsive- and anxiety-like behaviors exacerbated in mice during spontaneous 24-h withdrawal from the intermittent-two bottle choice (I2BC). Each behavioral outcome (i) was normalized to a z-score (z) using its global average () and standard deviation (SD, ). The z-scores were multiplied by the direction (d) of the effect of withdrawal. A SS was computed as the summation of all z-scores for each mouse.
| Behavioral metric (i) | Direction (d) | z-score (z) | Severity Score (SS) |
|---|---|---|---|
| Total number of physical signs (1) | +1 | ||
| Number of marbles buried (2) | +1 | ||
| Time in the center zone of the OFA (3) | −1 | ||
| Open Arms Entry Ratio in the EPM (4) | −1 |
Data and Statistical Analyses
Behaviors in the OFA and EPM were collected using ANY-Maze™ and all data were analyzed using GraphPad Prism (v9.1.2). The Grubbs’ test was used to examine extreme values in the data. Measures from the behavioral tests were compared using two-way analysis of variance (ANOVA). The temporal effect of chronic exposure on the behavior in the OFA and EPM was assessed using two-way ANOVA with repeated measures. Statistically significant interactions between chronic exposure and sex or time were followed by Bonferroni adjusted post-hoc tests. Correlation coefficients between the behavioral outcomes utilized to calculate SS were computed using Pearson’s r correlation test. Lastly, the maximum likelihood ratio Chi-square test was utilized to examine whether chronic exposure influenced the prevalence of each severity category in the groups. Summarized data are presented as average ± standard error of the mean (S.E.M.), with individual data points where appropriate. The significance level was set to 5% in all tests.
RESULTS
Voluntary oral ethanol consumption in the intermittent two-bottle choice (I2BC)
For the chronic exposure, group-housed female and male mice consumed ethanol in the I2BC for 10 weeks. During the first four sessions, the mice had access to different concentrations of ethanol in ascending order (Figure 1A and 1B). Two-way ANOVA with repeated measures of the escalation period revealed significant main effects of ethanol concentration (Fconcentration [1.9,21.4] = 100.9, p<0.0001) and sex (Fsex [1,11] = 6.1, p=0.0316), and a significant sex-by-concentration interaction (Fint [3,33] = 5.2, p=0.0045) on the self-administered dose of ethanol. Bonferroni’s multiple comparisons test only detected an almost significant difference on the first day of 20% ethanol between females and males (p=0.0647). Interestingly, we observed inverted U-curves for preference in both females and males, with significant main effect of concentration (Fconcentration [1.9,20.9] = 19.9, p<0.0001) but not sex-by-concentration interaction (Fint [3,33] = 1.1, p=0.3798). Sex did not significantly affect the effect of ethanol concentration on preference (Fsex[1,11] = 0.002, p=0.9655). Post-hoc analysis of the main effect of ethanol concentration on preference revealed that preference decreases at 20% ethanol compared to 6% (p<0.0001) and 10% (p<0.0001). Furthermore, analysis of the first derivative of the ethanol preference curve showed a local maximum at 10%, with the slope of the curve switching from positive (x = 8, f′(x) = +1.594) to negative (x = 15, f′(x) = −3.614). Analysis across time of 20%-ethanol sessions revealed a close-to-significant effect of time on daily ethanol dose (Fweek[3.7,40.3] = 2.2, p=0.0865), and a significant difference between females and males (F[1, 11] = 13.3, p=0.0039 ; Figure 1C). To further investigate whether mice escalate their consumption after chronic ethanol exposure, ethanol doses between weeks 2 and 10 of the I2BC were compared using a two-way ANOVA with repeated measures. This analysis revealed a significant main effect of week (Fweek[1, 11] = 5.2, p=0.0438; Figure 1D), with an overall percentage increase of 15.3 ± 6.4%. By week 10, the average daily intake was 15.1 ± 0.7 and 10.7 ± 0.8 g/kg for females and males, respectively.
Assessment of physical and affective signs of withdrawal
Following 10 weeks of ethanol exposure in the I2BC, mice were examined using a battery of behavioral tests. The first test assessed the manifestation of physical behaviors that tend to be more prevalent in mice undergoing withdrawal. The Grubbs’ test detected a significant outlier (p<0.05) in the control-male group, which was excluded from data analysis. Analysis of the total occurrence of somatic signs revealed a significant main effect of exposure (F[1,44] = 5.7, p=0.0216; Figure 2A). We did not detect a significant main effect of sex (p=0.1562) nor sex-by-exposure interaction (p=0.9397). Unpaired one-tail t-tests were utilized to determine the effect of chronic exposure on each recorded sign for each sex (Table 3). In this group of mice, we observed that in males, the effect seems to be driven by number of shakes (p=0.0406) and chewing (p=0.0143), whereas in females it seems to be driven by grooming (p=0.0175).
Figure 2. Mice undergoing spontaneous ethanol withdrawal display increased number of physical signs and compulsive-like behavior compared to ethanol-naïve mice.
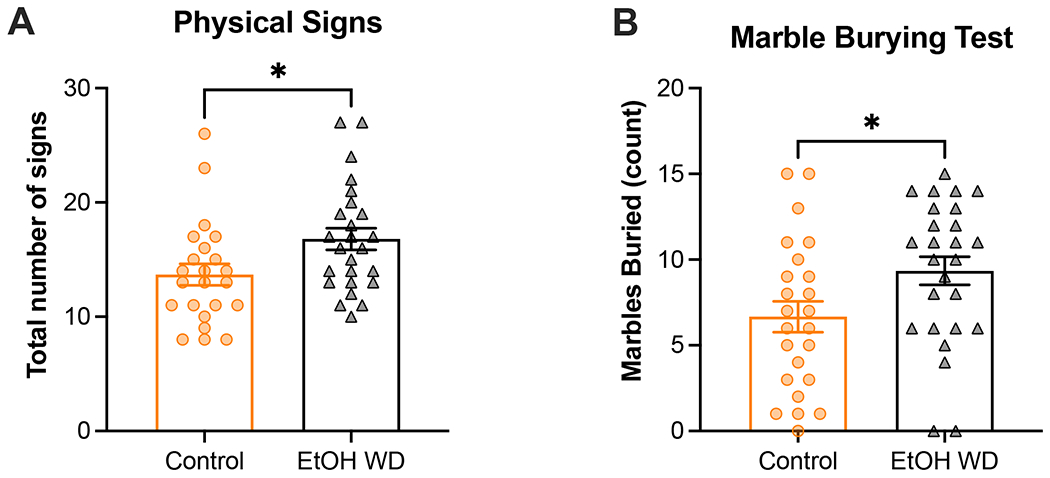
Following 10 weeks of ethanol exposure in the intermittent two-bottle choice (I2BC) paradigm, female and male C57Bl/6J mice were examined for the spontaneous emergence of physical signs of withdrawal (A) and compulsive-like behavior (B) 24h after removal of the ethanol bottle. Age-matched C57Bl/6J mice never exposed to ethanol served as controls. A significant, sex-independent, increase in the occurrence of somatic signs was observed in mice undergoing withdrawal compared to controls (A). Additionally, female and male mice undergoing ethanol withdrawal buried significantly more marbles than control mice (B). Animal numbers are 11-13 males/group and 12 females/group. *p<0.05 for main effect of chronic exposure. EtOH WD = 24-h ethanol withdrawal from I2BC.
Table 3. Effect of ethanol withdrawal on manifestation of each physical sign evaluated in the study.
The occurrence of shaking, scratching, chewing, and grooming was evaluated in ethanol-naïve (Control) and -withdrawal mice of both sexes. Average and standard error of the mean (SEM) are shown for each group. Unpaired one-tail t-tests were used to evaluated whether the occurrence of these signs was elevated in mice undergoing 24-withdrawal from the I2BC compared to same-sex controls. P-values < 0.05 are in bold.
| Sign | Females | Males | ||||
|---|---|---|---|---|---|---|
| Control | Withdrawal | p-value | Control | Withdrawal | p-value | |
| Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | |||
| Shaking | 3.1 (0.51) | 3.4 (0.40) | 0.3067 | 2.5 (0.49) | 3.8 (0.50) | 0.0406 |
| Scratching | 2.3 (0.35) | 3.1 (0.56) | 0.1093 | 2.3 (0.57) | 1.4 (0.11) | 0.0848 |
| Chewing | 3.2 (0.50) | 3.3 (0.57) | 0.4567 | 2.1 (0.64) | 4.7 (0.87) | 0.0143 |
| Grooming | 5.6 (0.71) | 8.0 (0.81) | 0.0176 | 5.3 (0.73) | 6.0 (0.41) | 0.1870 |
The second test that was employed to examine withdrawal was the marble burying test to investigate anxiety and compulsive-like behavior (Figure 2B). Using a two-way ANOVA, we detected a significant effect of exposure on number of marbles buried (F[1,46] = 4.7, p=0.0363) but not a significant main effect of sex (p=0.9795) or sex-by-exposure interaction (p=0.8737).
The last two tests that were included in the battery of behavioral tests have been extensively utilized to assess anxiety-like behavior in rodents (Bolivar et al., 2000; Carobrez and Bertoglio, 2005; Lister, 1987; Valle, 1970). Starting with the OFA, two-way ANOVA of time spent in the center zone did not detect significant effect of exposure (F[1, 45] = 2.1, p=0.1574), sex (F[1, 45] = 0.03, p=0.8537), nor interaction (F[1, 45] = 0.85, p=0.3615). We only observed a sex-specific effect of exposure on time spent exploring the center zone of the maze when we did a temporal examination of exploratory behavior in the OFA. This revealed that ethanol-exposed male mice spent less time in the center of the OFA compared to control male mice (F [1, 23] = 4.6, p=0.0431; Supplemental Figure 1B), which was not observed in females (pexposure=0.7628, pinteraction=0.4237; Supplemental Figure 1A). Although the effect of exposure in males was observed in the absence of a significant time-by-exposure interaction (F[2,46] = 1.9, p=0.1522), the trend of the data suggests that the difference between exposure groups is driven by the behavior displayed during the last third (i.e., 20-30 min) of the OFA test. Also, no effect of chronic exposure was observed on locomotion as analyzed using a two-way ANOVA (p=0.2943; Figure 3B). In addition, we counted the number of fecal boli in the maze after testing each mouse in the OFA (Figure 3C). Previous studies have shown that defecation may also be indicative of anxiety-like behavior in the OFA. However, two-way ANOVA failed to detect a main effect of exposure on number of fecal boli in the OFA (p=0.7574). Lastly, we analyzed behavior in the EPM, where analysis of entry ratio (i.e., entries to open arms divided by total number of entries) revealed a main effects of exposure (F[1,45] = 4.7, p<0.05) and sex (F[1,45] = 9.97, p<0.01), but no sex-by-exposure interaction (F[1,45] = 0.13, p=0.7197) (Figure 4A). Similar to OFA, we did not observe any alterations in locomotion in the EPM due to chronic ethanol exposure (p=0.3489; Figure 4B). Due to the short duration of the EPM, defecation was less prevalent in this test compared to OFA, thus these data were not statistically analyzed. Qualitatively, defecation was more prevalent in female (25%) than male (4%) mice, but the prevalence between exposure groups was similar (13% in control and 16% in ethanol withdrawal; data not shown). Temporal examination of time in the open arms is shown in Supplemental Figure 2. Using two-way ANOVA with repeated measures we detected a close-to-significant main effect of exposure in males (F[1,23] = 3.3, p=0.0817; Supplemental Figure 2B) that was not observed in females (p=0.8039; Supplemental Figure 2A). Here we also observed a significant time-by-exposure interaction in the males (F[4,92] = 3.6, p=0.0091). Interestingly, we observed a decrease in the time spent exploring the open arms after 2 min, regardless of chronic exposure. This perhaps suggests that novelty to the maze profoundly influences the ability to detect an effect of ethanol withdrawal on the EPM, especially in the males (unpaired t-test: +p0-2min=0.0180).
Figure 3. Ethanol withdrawal did not increase anxiety-like behavior in the open field arena (OFA).
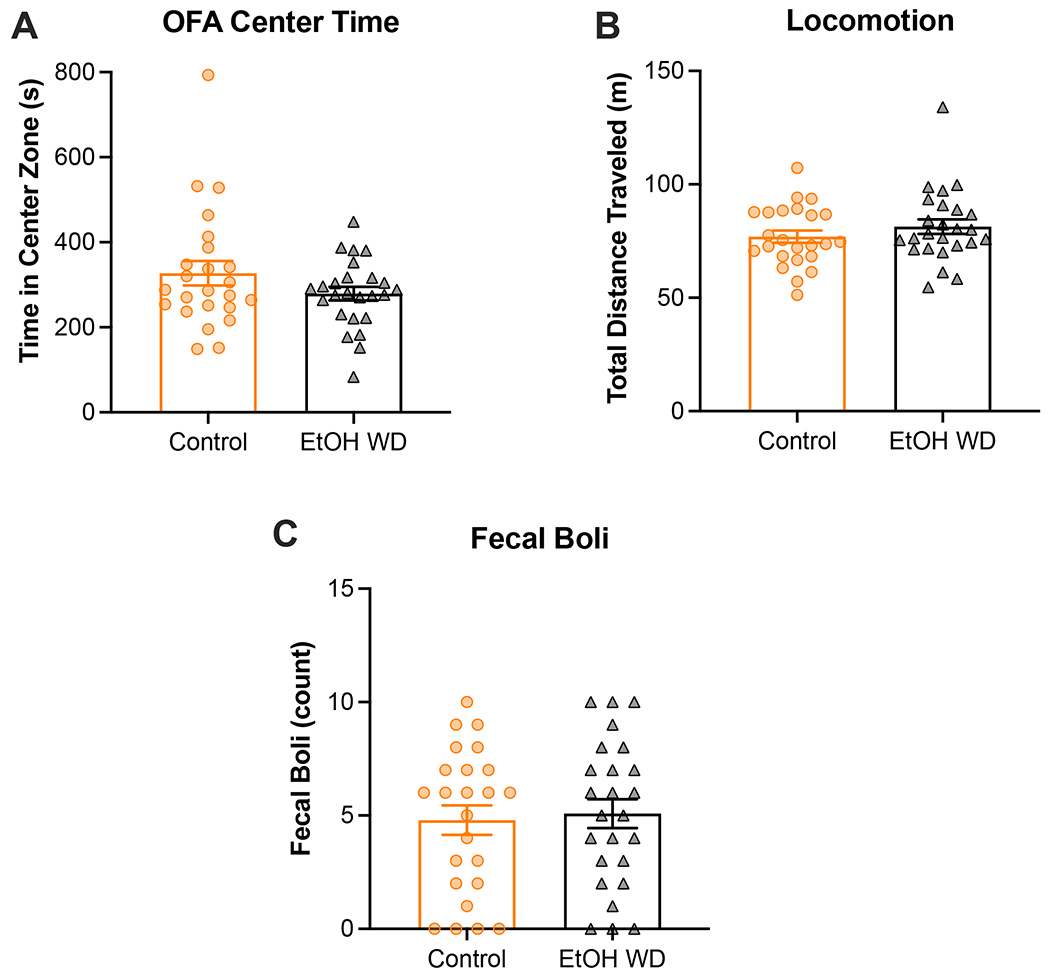
The open field arena (OFA) was used to investigate whereas female and male mice undergoing withdrawal from the I2BC display anxiety-like behavior compared to ethanol-naïve mice. No significant effect of ethanol exposure was observed on anxiety-like behavior as measured by the total time spent in the center zone of the OFA (A). All groups displayed similar levels of locomotive activity in the OFA (B). Chronic exposure did not affect average number of fecal boli in the OFA (C). Animal numbers are 12 control-females, 12 ethanol-females, 12 control-males, 13 ethanol-males. EtOH WD = 24-h ethanol withdrawal from I2BC.
Figure 4. Anxiety-like behavior is elevated in the elevated plus maze during spontaneous ethanol withdrawal.
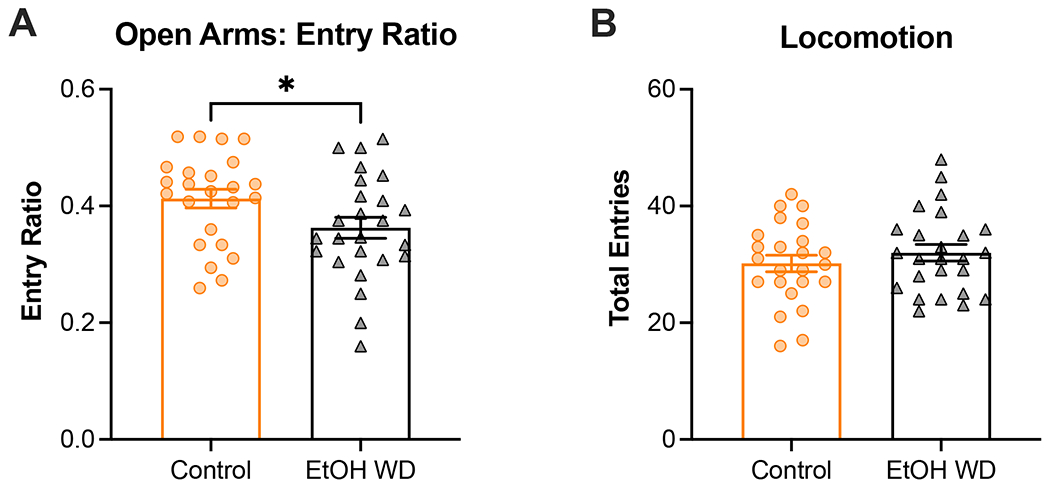
The elevated plus maze (EPM) was used to assess anxiety-like behavior in ethanol-naïve and ethanol-exposed female and male mice. Ethanol-exposed female and male mice displayed increased anxiety-associated behavior in the EPM as measured by open arm entry ratio (A). Similar locomotion in the EPM was observed in all groups (B). Animal numbers are 12 control-females, 12 ethanol-females, 12 control-males, 13 ethanol-males. *p<0.05 for main effect of chronic exposure. EtOH WD = 24-h ethanol withdrawal from I2BC.
Severity of withdrawal syndrome
A combination of behavioral metrics was utilized to compare the overall physical and affective signs in ethanol-exposed versus control mice. Only one mouse was excluded from this analysis because it was an outlier in the test for physical signs of withdrawal (1 control male). Pearson correlation analysis showed an almost significant positive correlation between two measures of anxiety-like behavior: time spent in the center of the OFA and entry ratio in the EPM (r = 0.205, p=0.081; Figure 5A). Also, albeit only marginally significant (r = −0.239, p=0.051), EPM Entry Ratio was negatively correlated with Marbles Buried. Lastly, we observed an unexpected significantly negative correlation between Total Signs and Marbles Buried (r = −0.313, p=0.015). No significant correlation was observed between Total Signs and the measures of anxiety-like behavior. This observation may indicate that ethanol-exposed subjects only manifest some – and not all – signs of withdrawal following chronic alcohol consumption, further highlighting the importance of utilizing a battery of behavioral tests to examine withdrawal.
Figure 5. Correlation of behavioral metrics and distribution of severe phenotypes within groups.
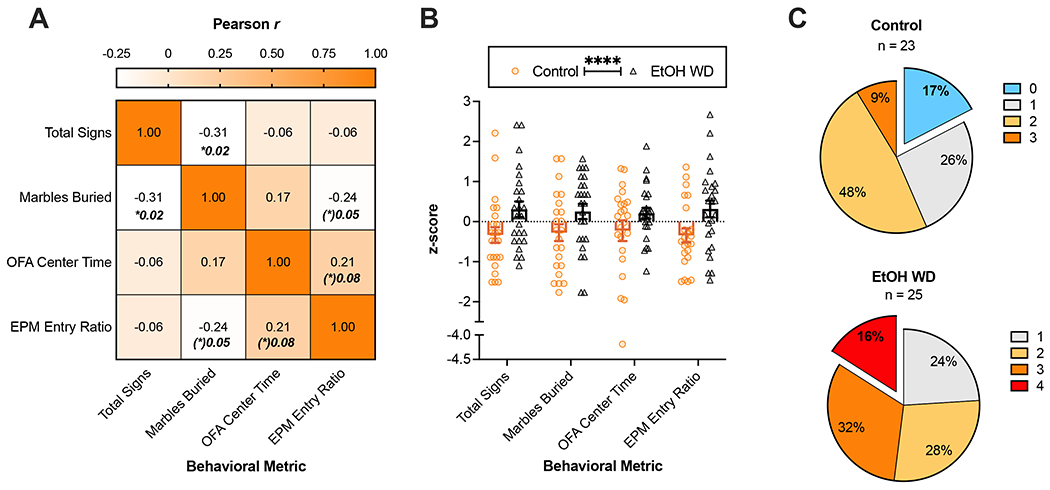
Outcomes from the behavioral tests employed in ethanol naïve and I2BC-exposed mice were examined. (A) Correlation matrix of the main outcomes of the battery of behavioral tests. The color gradient represents the Pearson correlation coefficient, which is also displayed in the center of each cell. Only significant or marginally significant p-values are displayed in bold. (B) Analysis of the z-scores of each behavioral metric shows a significant main effect of withdrawal (****p<0.0001). The pie charts in (C) summarize the occurrence of mice with above-median severity in the number of tests indicated by color. Seventeen percent of the mice showed below-median severity in all the tests (blue) in the control group, a stratum that is absent in the EtOH WD group. Conversely, mice with above-median phenotypes in all the tests (red) were only observed in the EtOH WD group (16%). Overall, the cumulative frequencies were different between control and EtOH WD groups (Two-sample K-S test p<0.05). Animal numbers are 12 control-females, 12 ethanol-females, 11 control-males, 13 ethanol-males. OFA = Open Field Arena, EPM = Elevated Plus Maze, EtOH WD = 24-h ethanol withdrawal from I2BC.
Analysis of the dimensionally-reduced behavioral metrics (z-scores) also showed a significant effect of withdrawal (F[1,184] = 16.4, p<0.0001; Figure 5B). Three-way ANOVA did not detect significant main effect of sex (F[1,176] = 0.48, p=0.4892), sex-by-exposure interaction (F[3,176] = 0.13, p=0.3369), nor three-way interaction (F[3,176] = 0.19, p=0.9036). The z-scores separated by sex are shown in Supplemental Figure 3. Each behavioral metric was split at the median to determine the number of tests in which each animal had a severe phenotype (Figure 5C). Qualitative evaluation of the resulting sub-populations revealed that extreme strata (i.e., all below-median severity (0) or all above-median severity (4)) were mutually exclusive in our data set. Specifically, whereas in the control group 17% of the mice had below-median severity in all the tests, 16% of the animals undergoing ethanol withdrawal showed above-median severity in all the tests. Intermediate sub-populations (i.e., 1 to 3 above-median behaviors) were present in both exposure groups. The cumulative frequencies were significantly different between control and ethanol withdrawal groups (Two-sample Kolmogorov-Smirnov test: D(48) = 0.3930, p=0.0494).
The z-scores were utilized to compute the severity scores (SS) shown in Figure 6A–B. Figure 6A shows a heatmap of SS with each column representing the exposure group and sex on the y-axis. Two-way ANOVA of those data revealed significantly higher SS in ethanol-exposed mice compared to control mice (F[1, 44] = 20.7, p=0.0003), in a sex-independent manner (psex=0.6020, pinteraction=0.2868). Interestingly, we observed distinct prevalence of severity categories between the exposure groups (Figure 6B), as assessed using the maximum likelihood ratio Chi-square test (χ2(2) = 18.97, p<0.0001). In summary, the majority of the mice in the control group displayed ‘Low’ severity scores (65%), whereas ‘High’ was the most prevalent category in mice undergoing withdrawal (52%). The significant difference in the observed distributions suggests that the overall behavioral phenotype of the mice in the battery of behavioral tests utilized in this study is significantly affected by chronic ethanol exposure in the I2BC. Overall, ethanol intake did not significantly correlate with behavioral severity in mice undergoing ethanol withdrawal (r2h= −0.1387, p2h= 0.5085; r24h= 0.0068, p24h= 0.9742) (Supplemental Figure 4).
Figure 6. Assessment of strata within groups reveals that ethanol withdrawal from I2BC has a profound effect on behavioral severity scores and classification.
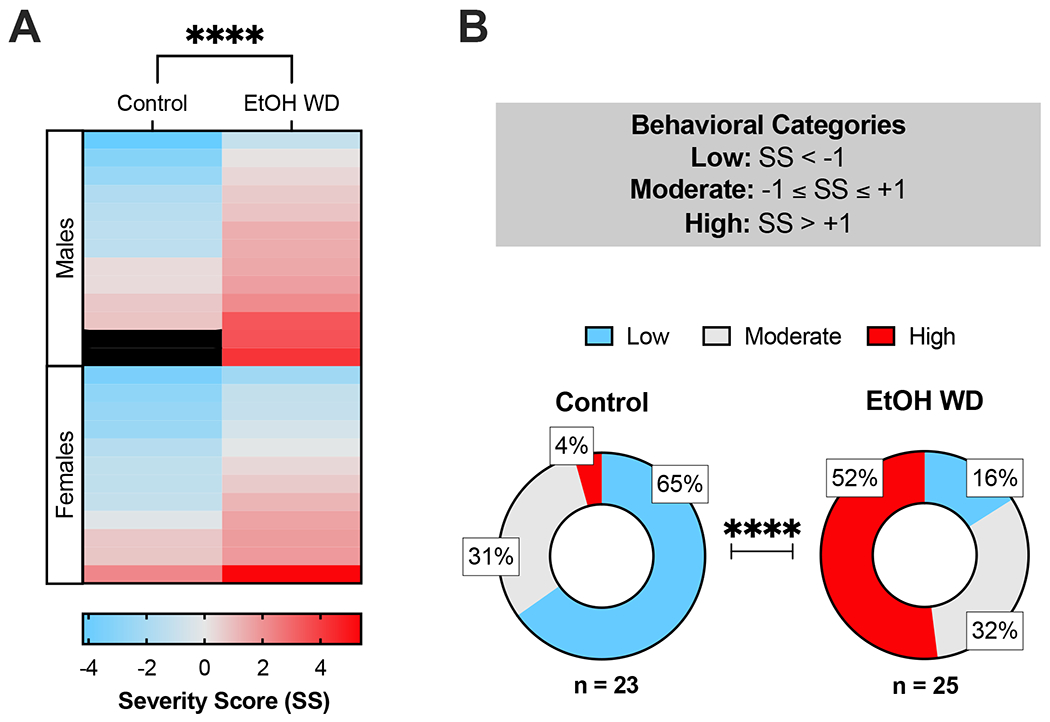
Ethanol naïve and withdrawn mice of both sexes were examined in a battery of tests assessing physical and affective signs of spontaneous ethanol withdrawal from the intermittent two-bottle choice (I2BC) paradigm. To obtain a summarizing behavioral severity score (SS), we normalized and combined four behavioral metrics from all tests: (1) Total occurrence of somatic signs, (2) Number of marbles buried, (3) Total time in the center zone of the OFA, and (4) Open arms entry ratio in the EPM. (A) Heatmap of SS facetted by chronic exposure and sex. The color gradient represents the magnitude and direction of the SS (Low = blue, Moderate = light gray, High = red). Mice of both sexes undergoing 24-h withdrawal from the I2BC displayed more severe behavioral phenotypes compared to control mice (****p<0.0001). (B) Prevalence of behavioral severity categories in each group. Withdrawal from chronic ethanol exposure affected the observed distribution of levels of severity (χ2(2) = 18.97, p<0.0001). Animal numbers are 12 control-females, 12 ethanol-females, 11 control-males, 13 ethanol-males. OFA = Open Field Arena, EPM = Elevated Plus Maze, EtOH WD = 24-h ethanol withdrawal from I2BC, SS = Severity Score.
DISCUSSION
In the present study, we employed a battery of behavioral tests to characterize the withdrawal phenotype in mice chronically exposed to alcohol per os via the I2BC drinking paradigm. Overall, we show that mice undergoing 24-h withdrawal tend to display worse behavioral phenotypes than ethanol-naïve mice.
Ethanol withdrawal impairs physical and affective behaviors independently of sex
An important goal of this study was to investigate sex-dependent effects of ethanol withdrawal. Overall, we only detected a significant difference between females and males in ethanol intake during the I2BC exposure, which has been previously reported by our group and others (Hwa et al., 2011; Quijano Cardé et al., 2021). During behavioral testing, we only identified an increase in withdrawal-related anxiety-like behavior in the OFA that was unique to male mice at the 24-h time point when we performed a temporal examination of time spent in the center zone (Supplemental Figure 2). However, analysis of total time spent in the center zone of the OFA was not significantly affected by 24-h withdrawal from the I2BC in mice of either sex. Our laboratory has previously shown that mice treated with ethanol using a 9-day chronic injection procedure (i.e., 2 g/kg/day ethanol, IP) or via ethanol liquid diet for 6 weeks display increased anxiety-like behavior in the OFA at 24-h withdrawal in the absence of sex differences (Perez and De Biasi, 2015). However, mice in these procedures reach blood ethanol concentrations (BEC) that are much higher than those observed in mice voluntarily consuming ethanol in the I2BC (Hwa et al., 2011; Quijano Cardé et al., 2021). Therefore, because the emergence of withdrawal may depend on level of intake and ethanol clearance, a temporal assessment of the withdrawal phenotype could possibly determine whether anxiety peaks at a different time point in mice undergoing withdrawal from the I2BC. For instance, a previous study showed that handling-induced convulsions (HICs) in mice undergoing withdrawal from a 16-week I2BC exposure peak 8 h after the last ethanol session (Hwa et al., 2011), suggesting that this could be another time point worth examining for behavioral testing. Alternatively, considering that physical symptoms (e.g. tremors, diaphoresis) tend to appear earlier than the affective ones in patients with AWS (Mirijello et al., 2015), it is also plausible that different time points are needed to capture the peaks of somatic and affective signs of withdrawal from the I2BC. Certainly, further research designed to better characterize the time course of ethanol withdrawal in mice and investigate possible sex-specific ethanol-related behaviors is warranted, especially considering that women in the US have shown an 83.7% increase in the prevalence of 12-month DSM-IV AUD in the past decade (Grant et al., 2017).
Lack of correlation between behavioral outcomes underscores distinct underlying causes
We did not observe a significant correlation between outcomes of anxiety-related behaviors. Previous work employing a multidimensional approach to investigate the emergence of alcohol withdrawal following exposure to aerosolized ethanol also reported lack of correlation between anxiety-associated outcomes and results from tests examining different domains of withdrawal (Metten et al., 2018). The lack of correlation between physical and affective manifestations of withdrawal – which have different times of presentation and duration in humans (Mirijello et al., 2015) – provide evidence that these phenomena are governed by distinct physiological and neurobiological processes.
We also examined the correlation between level of ethanol intake and severity of withdrawal, two traits that were shown to positively correlate in a previous study (Phillips et al., 1994). However, in our dataset, we did not see significant correlation between these two ethanol-related behaviors (i.e., higher intake did not lead to more severe withdrawal; Supplemental Figure 4). In humans, daily alcohol intake and duration of alcohol abuse also do not predict the severity of AWS (Goodson et al., 2014). In addition, previous studies investigating the correlation of these two traits across inbred, selected, and mutant mouse lines have presented data that suggest distinct genetic factors influencing susceptibility to display high ethanol consumption or experience severe withdrawal (Blednov et al., 2012; Crabbe and Phillips, 2004; Metten et al., 1998), supporting the hypothesis that intake and withdrawal have potentially different genetic bases. It is challenging to make inferences about the influences of genetics on the relationship between these traits in the present study given that we used inbred C57BL/6J mice. However, we cannot discard possible effects of de novo mutations and genetic variability among mice used in this study as they came from 13 different breeders (Chebib et al., 2021). Moreover, inter-individual phenotypic variation can also be influenced by environmental and epigenetic factors. For instance, a study using a C57 mouse sub-strain (i.e., C57Bl/6NCrl) showed large degree of between-subject variation in ethanol drinking that correlated with the expression of hundreds of transcripts in the nucleus accumbens, prefrontal cortex and ventral midbrain region across several gene ontology groups (Wolstenholme et al., 2011). Interestingly, the same study reported that level of intake correlated with expression of a network of genes involved in the modification and maintenance of chromatin architecture and regulation of transcription. This suggests that epigenetic mechanisms that result from exposure to ethanol and/or other environmental stimuli may also influence the presentation of withdrawal and can affect the relationship between these traits. Future studies with increased statistical power and more detailed characterization of the intake level/pattern are needed to clarify how ethanol-related changes in gene expression influence the spontaneous manifestation of physical and affective signs of withdrawal and alter their correlation.
The presence of sub-populations suggests different sensitivities to ethanol withdrawal
Our analyses highlight the presence of sub-populations in our control and ethanol withdrawal groups, which resembles clinical observations. In humans, the prevalence of DSM-5 AUD (5%) is much lower than that of past-month alcohol use (51%) and binge drinking (24%) in persons aged 12 years or older (Substance Abuse and Mental Health Services Administration (SAMHSA), 2019). This discrepancy indicates that only a subset of individuals with heavy alcohol use transition to pathological drinking. This is partly due to the multifactorial nature of the disorder, as AUD is influenced by complex interactive effects of social, environmental, and genetic factors, resulting in a highly heterogenous disorder with differing symptomatology and severities. In other words, the clinical manifestation of AUD can vary substantially among individuals living with this debilitating condition, where only some experience symptoms of withdrawal. In accordance with this variability in phenotype, the CIWA-Ar diagnostic tool allows the stratification of patients based on global AWS severity. The evaluation of the efficacy of pharmacological or cognitive behavioral therapies within each stratum can be powerful for the identification of a therapeutic effect.
The US Food & Drug Administration (FDA) has proposed the use of enrichment strategies in clinical investigations of novel pharmacotherapeutics, in which any patient characteristic is utilized to select the study population in which the detection of a drug effect is more likely (FDA, 2019). For example, a recent meta-regression analysis of randomized controlled trials (RCTs) for alcohol dependence (AD) revealed that baseline drinking risk levels and pre-treatment abstinence duration profoundly influences alcohol-related responses to placebo (Scherrer et al., 2021), resulting in increased variability in the control group and decreased statistical power to detect an effect of the experimental drug. Other findings supporting the power of stratifying subjects in AUD research come from recent randomized clinical trials in which the efficacies of prazosin (Sinha et al., 2021) and gabapentin (Anton et al., 2020) to treat AUD symptoms were significantly moderated by AWS severity (i.e., low vs high). Thus, in this study we show that a score computed from the combined outcomes of various behavioral tests can be used to define strata and determine the severity of the withdrawal ‘syndrome’ in mice chronically exposed to alcohol. In theory, identifying subjects with moderate-to-high severity phenotypes could allow the utilization of enrichment strategies in pre-clinical studies investigating the efficacy of novel compounds to ameliorate withdrawal. The same approach could also be useful when analyzing mouse lines genetically modified to carry mutations with potential effects of alcohol-related behaviors.
Overall, our results suggest that the I2BC paradigm can be used in pre-clinical studies of ethanol withdrawal, as confirmed by increased behavioral severity and altered prevalence of behavioral severity strata 24 h after the last drinking session in the ethanol-exposed group compared to control. Evaluation of the severity in each test revealed that 52% of the ethanol-exposed mice had a high SS and only 16% of the mice in the ethanol group had above-median severity in all behavioral metrics. This is consistent with human population studies that have found that only a subset of individuals with an AUD exhibit AWS, with anxiety being among the most common symptoms of alcohol withdrawal (Caetano et al., 1998; Hall and Zador, 1997; Sanvisens et al., 2021). Therefore, pre-clinical investigators in the alcohol field are exhorted to take these percentages into consideration when determining sample sizes and designing experiments to examine ethanol withdrawal. Moreover, characterization of withdrawal severity in mice with similar levels of ethanol exposure may increase our understanding of genetic influencers on sensitivity to develop debilitating symptoms of withdrawal upon cessation.
Using this framework to investigate other ethanol-related behaviors
In closure, it is important to emphasize that variability in phenotypic severity and/or pattern is not unique to the manifestation of alcohol withdrawal but is also observed in paradigms assessing other alcohol-associated behaviors in rodents - such as operant and in-cage self-administration. Because previous clinical studies have shown that AWS history can be used as a prognostic indicator of the efficacy of medications under the investigation for AUD, severity scores (such as the one described in this manuscript) may be used to generate sub-populations and investigate levels of responses among them. Alternatively, for drugs proposed to reduce alcohol intake by modulating alcohol reward, results from the conditioned place preference (CPP) paradigm may be used as a proxy for categorization and investigated for its modulation of the efficacy of such compounds. Although speculative, this approach could result in increased translational value of pre-clinical studies investigating the efficacy of novel drugs for AUD and could better inform the design of clinical trials. Altogether, we hope our findings invite researchers to appreciate the heterogeneity among experimental subjects that parallels human observations, to embrace the variability in the data that allows categorization, and to use it to leverage new knowledge of the mechanisms of AUD and its treatment.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. Lorenzo Leggio for his useful comments and discussions on the manuscript, and Tyisha Hundley and Yael A. Day for their technical assistance with the chronic I2BC exposure.
SOURCES OF SUPPORT
This work was supported by the National Institutes of Health grant U01 AA025931 to MDB.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Anton RF, Latham P, Voronin K, Book S, Hoffman M, Prisciandaro J, Bristol E (2020) Efficacy of Gabapentin for the Treatment of Alcohol Use Disorder in Patients With Alcohol Withdrawal Symptoms A Randomized Clinical Trial. JAMA Intern Med 180:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Mayfield RD, Belknap J, Harris RA (2012) Behavioral actions of alcohol: phenotypic relations from multivariate analysis of mutant mouse data. Genes Brain Behav 11:424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch S, Rinker JA, Marcus MM, Mulholland PJ (2020) Absence of effects of intermittent access to alcohol on negative affective and anxiety-like behaviors in male and female C57BL/6J mice. Alcohol 88:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R, Hoon Shin J, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA (2013) Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci 16:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L (2000) Habituation of Activity in an Open Field: A Survey of Inbred Strains and F 1 Hybrids, Behavior Genetics. Gershenfeld and Paul. [DOI] [PubMed] [Google Scholar]
- Caetano R, Clark C, Greenfield T (1998) Prevalence, trends, and incidence of alcohol withdrawal symptoms: analysis of general population and clinical samples - PubMed. Alcohol Heal Res World 22:73–79. [PMC free article] [PubMed] [Google Scholar]
- Carobrez A, Bertoglio L (2005) Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29:1193–1205. [DOI] [PubMed] [Google Scholar]
- Chebib J, Jackson BC, López-Cortegano E, Tautz D, Keightley PD (2021) Inbred lab mice are not isogenic: genetic variation within inbred strains used to infer the mutation rate per nucleotide site. Heredity (Edinb) 126:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Goes T, Dias Antunes F, Teixeira-Silva F (2009) Trait and state anxiety in animal models: Is there correlation? Neurosci Lett 450:266–269. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Kendler KS, Hitzemann RJ (2011) Modeling the diagnostic criteria for alcohol dependence with genetic animal models. Curr Top Behav Neurosci 13:187–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ (2004) Pharmacogenetic studies of alcohol self-administration and withdrawal. Psychopharmacology (Berl) 174:539–560. [DOI] [PubMed] [Google Scholar]
- Food & Drug Administration (2019) Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products.
- Gangitano D, Salas R, Teng Y, Perez EE, De Biasi M (2009) Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav 8:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson CM, Clark BJ, Douglas IS (2014) Predictors of Severe Alcohol Withdrawal Syndrome: A Systematic Review and Meta-Analysis. Alcohol Clin Exp Res 38:2664–2677. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001-2002 to 2012-2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 74:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor DM, Zuo W, Fu R, Bekker A, Ye J-H (2019) Elevation of Transient Receptor Potential Vanilloid 1 Function in the Lateral Habenula Mediates Aversive Behaviors in Alcohol-withdrawn Rats. Anesthesiology 130:592–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Zador D (1997) The alcohol withdrawal syndrome. Lancet 349:1897–1900. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, Debold JF, Miczek KA (2011) Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 35:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Nathanson AJ, Shimamoto A, Tayeh JK, Wilens AR, Holly EN, Newman EL, Debold JF, Miczek KA (2015) Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology (Berl) 232:2889–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Kenna GA, Fenton M, Bonenfant E, Swift RM (2009) Typologies of alcohol dependence. From Jellinek to genetics and beyond. Neuropsychol Rev. [DOI] [PubMed] [Google Scholar]
- Li J, Chen P, Han X, Zuo W, Mei Q, Bian EY, Umeugo J, Ye J (2019) Differences between male and female rats in alcohol drinking, negative affects and neuronal activity after acute and prolonged abstinence. Int J Physiol Pathophysiol Pharmacol 11:176. [PMC free article] [PubMed] [Google Scholar]
- Lister R (1987) The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 92:180–185. [DOI] [PubMed] [Google Scholar]
- Melendez RI (2011) Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res 35:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK (1998) High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome 9:983–990. [DOI] [PubMed] [Google Scholar]
- Metten P, Schlumbohm JP, Huang LC, Greenberg GD, Hack WR, Spence SE, Crabbe JC (2018) An alcohol withdrawal test battery measuring multiple behavioral symptoms in mice | Elsevier Enhanced Reader. Alcohol 68:19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirijello A, D’angelo C, Ferrulli A, Vassallo G, Antonelli M, Caputo F, Leggio L, Gasbarrini A, Addolorato G (2015) Identification and Management of Alcohol Withdrawal Syndrome. Drugs 75:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncie HL, Yasinian Y, Oge L (2013) Outpatient Management of Alcohol Withdrawal Syndrome. Am Fam Physician 88:589–595. [PubMed] [Google Scholar]
- Nennig SE, Fulenwider HD, Eskew JE, Whiting KE, Cotton MR, McGinty GE, Solomon MG, Schank JR (2020) Intermittent Ethanol Access Increases Sensitivity to Social Defeat Stress. Alcohol Clin Exp Res 44:600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA (2013) Alcohol Use Disorder : A Comparison Between DSM – IV and DSM – 5. NIH Publ 5–6. [Google Scholar]
- O’Neal TJ, Nooney MN, Thien K, Ferguson SM (2020) Chemogenetic modulation of accumbens direct or indirect pathways bidirectionally alters reinstatement of heroin-seeking in high- but not low-risk rats. Neuropsychopharmacology 45:1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez E, Quijano-Cardé N, De Biasi M (2015) Nicotinic Mechanisms Modulate Ethanol Withdrawal and Modify Time Course and Symptoms Severity of Simultaneous Withdrawal from Alcohol and Nicotine. Neuropsychopharmacology 40:2327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez EE, De Biasi M (2015) Assessment of affective and somatic signs of ethanol withdrawal in C57BL/6J mice using a short-term ethanol treatment. Alcohol 49:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK (1994) Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res 18:931–941. [DOI] [PubMed] [Google Scholar]
- Quijano Cardé NA, Perez EE, Feinn R, Kranzler HR, De Biasi M (2021) Antagonism of GluK1-containing kainate receptors reduces ethanol consumption by modulating ethanol reward and withdrawal. Neuropharmacology 199:108783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A (2008) Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci 29:493–498. [DOI] [PubMed] [Google Scholar]
- Ramos A, Pereira E, Martins GC, Wehrmeister TD, Izídio GS (2008) Integrating the open field, elevated plus maze and light/dark box to assess different types of emotional behaviors in one single trial. Behav Brain Res 193:277–288. [DOI] [PubMed] [Google Scholar]
- Sanvisens A, Zuluaga P, Short A, Rubio G, Gual A, Torrens M, Fuster D, Bolao F, Rodríguez de Fonseca F, Muga R (2021) Sex-specific Associations of Alcohol Withdrawal in Patients Admitted for the Treatment of Alcohol Use Disorder. J Addict Med 15:68–73. [DOI] [PubMed] [Google Scholar]
- Scherrer B, Guiraud J, Addolorato G, Aubin H-J, de Bejczy A, Benyamina A, van den Brink W, Caput F, Demattels M, Goudriaan AE, Gual A, Kiefer F, Leggio L, Lesch O-M, Maremmani I, Nutt DJ, Paille F, Perney P, Poulnais R, Raffaillac Q, Rehm J, Rolland B, Simon N, Söderpalm B, Sommer WH, Walter H, Spanagel R (2021) Baseline severity and the prediction of placebo response in clinical trials for alcohol dependence: A meta-regression analysis to develop an enrichment strategy. Alcohol Clin Exp Res 00:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Wemm S, Fogelman N, Milivojevic V, Morgan PM, Angarita GA, Hermes G, Fox HC (2021) Moderation of Prazosin’s Efficacy by Alcohol Withdrawal Symptoms. Am J Psychiatry 178:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) (2019) Results from the 2019 National Survey on Drug Use and Health: Detailed Tables, SAMHSA, CBHSQ. Available at: https://www.samhsa.gov/data/sites/default/files/reports/rpt29394/NSDUHDetailedTabs2019/NSDUHDetTabsSect2pe2019.htm Accessed September 2, 2021.
- Sullivan J, Sykora K, Schneiderman J, Naranjo C, Sellers E (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 84:1353–1357. [DOI] [PubMed] [Google Scholar]
- Valle F (1970) Effects of strain, sex, and illumination on open-field behavior of rats. Am J Psychol 83:103–111. [PubMed] [Google Scholar]
- WHO (2018) World Health Organization Global status report on alcohol and health 2018.
- Wise RA (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29:203–210. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Villarroel NA (2009) Dynamic Association Between Negative Affect and Alcohol Lapses Following Alcohol Treatment. J Consult Clin Psychol 77:633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


