Abstract
Bacterial pneumonia is one of the most important causes of mortality in the United States. The bacteria Klebsiella pneumoniae (KP) accounts for a significant proportion of community and hospital-acquired infections. Here, we determine that the Holy basil (Ocimum sanctum) extract improves cell viability and dampens the pro-inflammatory cytokine response in an in vitro model of pneumonia. For this, A549, a human alveolar basal epithelial cell line, was subjected to a lethal KP model following a 24-hour pretreatment with basil extract. Bacteremia, cell viability, apoptosis, MTT assay, phagocytic capacity, cytokines, and Khe gene expression were assessed in these cells following pneumonia. Cell morphology analysis showed that Holy basil protected A549 cells from KP infection-mediated effects by inhibiting cell death due to apoptosis. Additionally, in the presence of basil, A549 cells demonstrated significantly higher bactericidal capacity and phagocytosis. Administration of Holy basil led to reduced expression of HIF-1/2a, NF-κB, and Khe in the KP infected cells while increasing IFN-γ expression. Our results suggest that basil significantly reduced cell death in the setting of KP infection, likely via attenuation of cytokine and IFN-γ mediated signaling pathways. Holy basil is a promising therapeutic agent for managing and treating bacterial pneumonia based on its potency.
Keywords: Holy basil, Phagocytosis, Apoptosis, Pneumonia, Human lung epithelial cells
1. INTRODUCTION
Bacterial pneumonia is one of the leading causes of death in the United States of America(Cilloniz, Martin-Loeches, Garcia-Vidal, San Jose, & Torres, 2016). Hospital-acquired bacterial pneumonia (White, Blainey, Harrison, & Clarke, 1981), particularly ventilator-associated pneumonia (VAP), is a significant reason behind nosocomial infections. The incidences associated with VAP have remained steady despite introducing the VAP bundle in the care of the critically ill (P. R. Miller et al., 2001). Klebsiella pneumoniae is a gram-negative encapsulated bacillus that causes a significant proportion of community and hospital-acquired pneumonia infections (Broug-Holub et al., 1997; V. A. Dolgachev, Yu, Reinke, Raghavendran, & Hemmila, 2012).
Holy basil (Ocimum sanctum Linn.) is considered a sacred plant in India and is attributed to many medicinal properties by Ayurveda, the Indian medicine system (Baliga et al., 2013; Cohen, 2014; Jamshidi & Cohen, 2017; Pattanayak, Behera, Das, & Panda, 2010). The plant Holy Basil belongs to the family Lamiaceae (Pattanayak et al., 2010). Holy Basil is a traditional home medicine used for various conditions such as the common cold, bronchitis, fever, and its anti-inflammatory and analgesic properties (Baliga et al., 2013; Jamshidi & Cohen, 2017; Singh, Rehan, & Majumdar, 2001). It is also known to improve appetite and cure skin diseases, gastric disorders, and hepatic abnormalities (Mondal, Mirdha, & Mahapatra, 2009; Pattanayak et al., 2010). The herbal preparations are considered moderate in efficacy and are less toxic than the most commonly used pharmaceutical drugs (Baliga et al., 2013; Jamshidi & Cohen, 2017).
Historically, medicinal plants have been of notable importance as a source for novel drugs with anti-microbial activity. All traditional medicinal herbs have made considerable contributions to human health. Additionally, plants are considered one of the most important sources of secondary metabolites and essential oils (Pattanayak et al., 2010; Uma Devi, Ganasoundari, Vrinda, Srinivasan, & Unnikrishnan, 2000). Previous studies have suggested that Holy basil has multiple biological properties, including immunomodulatory and anti-microbial effects (Khan et al., 2010; Saharkhiz et al., 2015). This study found that Holy basil protects lung epithelial cells from a fatal pneumonia infection. Here, we show that the application of holy basil extract improves cell viability and dampens the pro-inflammatory cytokine response in an in vitro model of pneumonia.
MATERIALS AND METHODS
2.1. Reagents
Organic Holy Basil powder (Organic Tulsi Holy Basil TBC 0.4 OZ; Pride of India, Walmart, USA) and organic Holy Basil Juice (Basil Juices, Ramdev products, Atlanta, GA) were obtained from commercially available sources. Fresh Thai basil leaves were purchased from local stores (basil leaves, Kroger, Ann Arbor, Michigan). A549, a human lung epithelial cell line (ATCC® CCL-185™), was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Klebsiella pneumonia, strain 43816, serotype 2 was also obtained from American Type Culture Collection (ATCC, Manassas, VA). The blood agar plates were obtained from Fisher Scientific (Thermo-Fisher Scientific, Lenexa, KS). The tryptic soy broth was brought from Sigma (Sigma-Aldrich Co,22092 St. Louis, MO). The MTT assay reagent was obtained from Sigma (Sigma-Aldrich Co, Cat. No. 11 465 007 001, St. Louis, Missouri, USA). The ELISA kit was received from Bio-Rad (Bio-Rad; Bio-Plex Pro™ Human Cytokine Screening Panel, 48-Plex; 12007283). The Trypan blue solution from Sigma (Sigma-Aldrich Co, Cat. No. T8154–20ML, St. Louis, Missouri, USA). Fluorescein isothiocyanate (FITC) was gained from Sigma (Sigma-Aldrich, St. Louis, MO). The Apostat detection kit was obtained from R&D (Catalog number FMK012, R&D Systems, Minneapolis, MN). The M-MLV reverse transcriptase kit was received from Life Technologies (Life Technologies Corporation, Grand Island, NY, USA). TaqMan gene expression reagents or genes are obtained from the Applied BioSystem SYBR Green Master PCR mix (Applied BioSystems, Foster City, CA).
2.2. Quantification of bacterial colony-forming units
A small aliquot (400 µl, 1% solution) of basil was plated on blood agar plates (Thermo-Fisher Scientific, Lenexa, KS). This bacterium was cultured overnight in trypticase soy broth (Becton Dickinson) with re-inoculation the following morning into a new medium and culture bacteria into a logarithmic growth phase. Optical density (OD) was measured at a wavelength of 600 nm, and serial dilutions were then made to reach a concentration of 500 CFU of bacteria were spread on the plate. The plate was incubated overnight at 37° C in an incubator. The number of bacterial colonies was manually counted and compared in KP alone and KP plus basil treated groups. (V. Dolgachev et al., 2018).
2.3. Cell culture
Human lung epithelial cells (A549) (ATCC, Manassas, VA) were grown as a monolayer in 5% CO2 at 37 °C in DMEM with L-glutamine (Invitrogen Carlsbad, California, USA) medium supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, St. Louis, Missouri, USA), 100 U/mL penicillin G, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, California, USA) in a humidified 95% air/5% CO2 atmosphere at 37°C. The medium was replaced twice a week. When confluent, cells were split using standard trypsinization procedures and used most of the study passage of cells less than 10. Cells were cultured in 96 wells culture plates or 8-well chambered plates for various experiments (Veluthakal, Suresh, & Kowluru, 2009).
2.4. Preparation of aqueous Basil extract
Three different types of basil extracts were tested in our preliminary studies. The first one involved holy basil extract obtained by mechanically grinding dried Holy basil leaves. Dried extract powder (500 mg) was boiled in distilled water (10 mL) for 10 min at 100° C cooled, centrifuged at 500 × g for 15 min, and filtered using Corning® square bottle-top vacuum filter systems (Shetty, Udupa, & Udupa, 2008; Shivananjappa, Mhasavade, & Joshi, 2013). The medium was collected in microcentrifuge tubes and stored at −20° C till further use. We also used the commercially manufactured Holy Basil juice (Vedic Juices, Atlanta, GA). Thai basil leaves were crushed manually using mortar and pestle, and the extract was filter-sterilized (Thai basil extract). Cells were pretreated with basil extracts and incubated for 24 hours before bacterial incubation. We determined the optimal concentration by a dose-response assay as detailed in the manuscript.
2.5. Klebsiella pneumoniae preparation (KP)
Pneumonia, strain 43816, serotype 2 was purchased from ATCC; Manassas, VA, USA). This pneumonia strain was cultured overnight in trypticase soy broth (Becton Dickinson, San Diego, CA, USA) with re-inoculation the following morning into a fresh medium. The pneumonia was centrifuged at 600 g and 4°C for 10 min, washed with saline, centrifuged, and then resuspended in sterile saline. Optical density was read on a spectrophotometer (Milton Roy, Rochester, NY, USA) at a wavelength of 600 nm. Appropriate serial dilutions were subsequently performed to achieve a concentration of 100–500 colony forming units (CFUs) of bacteria per 30 µl of inoculum(Suresh, Dolgachev, et al., 2019).
2.6. KP administration to A549 cells
Human lung epithelial cells were plated at densities of 4 × 105 cells/well in 6-well plates. The next day (24 hours of incubation), the medium was changed to fresh DMEM followed by 24-hour culture. Cells were then treated with and without basil (1%) for 24 hours at 37 °C in 5% CO2. After incubation with basil, the medium was discarded, and cells were added to a new antibiotic-free medium before being treated with KP (100–500 CFU). The cells were then incubated for an additional 24 hours, after which samples (culture medium and cell lysates) were collected and used for further experiments as described below (Suresh et al., 2012).
2.7. Cell viability
Following the bacterial treatment, attached cells were dislodged using trypsin-EDTA and pooled with cells suspended in the media. The cell pellet was obtained after centrifugation and was resuspended in 10 µl PBS and incubated with an equal volume of Trypan blue for 5 min at room temperature. Countess cell counter was used to quantitate cells (Invitrogen, Carlsbad, CA). The experiments were performed in quadruplicate (Chen, Huang, Yang, & Qiu, 2013).
2.8. Detection of Caspase Activation in A549 cells (ApoStat)
Intracellular caspase activity was measured by Apostat (Catalog # FMK012, R&D Systems, Minneapolis, MN) as per the manufacturer’s instructions. A549 cells were cultured and treated in the presence and absence of basil, followed by KP treatment (30µl, 500 CFU) for 24 hours and then stained with ApoStat for 30 min. Cells were collected, washed, and assayed by flow cytometry (FACS Calibur; Becton, Dickinson), and data is to determine the cells expressing (percentage) active caspases. Data were analyzed using Flow Jo software (Tree Star, Inc., Ashland, OR)(Suresh et al., 2016).
2.9. Tetrazolium dye reduction assay of bacterial killing (MTT)
Bacterial (KP) killing of A549 cells was quantified using a tetrazolium dye reduction assay, as described previously(Chen et al., 2013; Guo & Cheng, 2018). Human lung epithelial cells (A549) were briefly quantified after pneumonia and basil using a tetrazolium dye reduction assay. A549 were collected and seeded into (triplicate) 96-well plates, one experimental (37 °C) plate, and one control (4 °C). After incubation with basil (1% solution), the medium was discarded, and cells were added to a new antibiotic-free medium. Both plates were infected with pneumonia (2.3 × 108 CFU/ml) for 30 min at 37 °C. The experimental plate cells were washed and then incubated at 37 °C for 90 min, whereas removing the medium from the control plate, washed, and then lysed with 0.5% saponin in tryptic soy broth (Sigma, St. Louis, MO) and placed at 4 °C. After 90 min, the experimental plate cells were lysed with 0.5% saponin in tryptic soy broth. Both dishes were then incubated at 37 °C for 2.5 hours. A total of 5 mg/ml MTT (Sigma-Aldrich Co, St. Louis, Missouri, USA) was added to the plates and incubated for 30 min, and the absorbance was read at 595 nm. The results were expressed as a percentage of survival of ingested bacteria normalized to the rate of control plates.
2.10. Preparation of FITC labeling of KP
KP was grown in tryptic soy broth (Sigma-Aldrich, St. Louis, MO), and bacterial absorption was determined by the absorbance at 600 nm and was compared with a predetermined standard curve. For FITC labeling, a KP culture was centrifuged and washed twice by resuspending cell pellets in 1 ml sterile PBS and transferred into a clean tube. KP was heat-killed by autoclaving for 20 min and resuspended at 109 CFU/ml in 0.1 M NaHCO3 (pH 9.2). A total FITC (Sigma-Aldrich, St. Louis, MO) in DMSO was added to heat-killed KP (FITC-KP) and allowed to incubate in the dark (1h) on a rocker at room temperature (RT). Following FITC labeling, heat-killed KP was washed three times and resuspended in 1 ml sterile PBS. FITC labeled KP aliquots were prepared and stored at −80°C until use (Ballinger et al., 2006).
2.11. In vitro phagocytosis assays
Phagocytosis by A549 cells was examined as described previously(Guo & Cheng, 2018; Simamura, Hirai, Shimada, & Koyama, 2001). Briefly, cells were cultured in the presence or absence of basil (400 µl of 1% solution). After 24 hours, serum-free media and 10 µl of FITC-labeled KP (50 µl/well) were added to each well. Incubated 2 hours at 37° C, Trypan blue was added to quench extracellular fluorescence, and phagocytosis was quantified. The normalized fluorescence intensity value was obtained by dividing the sample well’s fluorescence intensity values by the corresponding raw absorbance values.
2.12. TaqMan quantitative PCR
Total RNA was prepared from human lung epithelial cell lysate in the presence and absence of KP and reverse transcribed into cDNA using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT; Thermo Fisher Scientific). cDNA was then amplified by real-time qPCR using an ABI Prism 7700 sequence detection system. TaqMan gene expression reagents, universal Master PCR Mix (Thermo Fisher Scientific) were used to detect gene expression. For the TaqMan experiments, all gene was purchased from the Applied Biosystems. The gene expression was analyzed as the fold change compared to the corresponding control. The fold difference in mRNA expression (IL-1β, IL-6, TNF-α, IFN-γ, MIP-2, IL-10, VEGF-A, NOS-2, and Arginase-1) between treatment groups was determined By Applied Biosystems (Cavalcanti, Brelaz, Neves, Ferraz, & Pereira, 2012; V. A. Dolgachev et al., 2012).
2.13. Multiplex Immunoassay
The levels of cytokines (interleukins, IL-2, IL-4, IL-6, IL-8, IL-10, tumor necrosis factor (TNF)-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), and interferon-gamma (IFN-γ) in the cell, culture media following basil and pneumonia administration were determined using a Luminex Bio-Plex 200 immunoassay kits (Bio-Rad, Hercules, California, USA) according to the manufacturer’s instructions (de Jager, Bourcier, Rijkers, Prakken, & Seyfert-Margolis, 2009; Wen, Dou, Hogaboam, & Kunkel, 2008). Briefly, a 96-well multiplex assay plate was coated with anti-human cytokine 2-plex conjugated beads. Dishes were rinsed 2 times with wash buffer A, and cell-free supernatants, as well as series diluted standard cytokine, were loaded and incubated for 30 minutes at room temperature with a gentle vortex. After 3 items of washing, the biotinylated human cytokine 2-plex detection antibody was added for 30 minutes at room temperature with the gentle vortex. The plates were rewashed, and PE-conjugated streptavidin was added for 10 minutes at room temperature with a gentle vortex. Plates were washed and read using the Luminex Bio-Plex 200 system plate reader. Human stock cytokines of known concentrations, which came together with the kit, were used to generate the standard curves from which the cytokine concentrations present in the samples were calculated.
2.14. Western blot
After treatment, cells were lysed in ice-cold lysis buffer, mixed with a commercial sample buffer (Invitrogen, Carlsbad, CA), and heated at 95 °C for 5 minutes. Samples (10µg) were then electrophoresed on polyacrylamide gels, and the gels were transferred to PVDF membranes. Blots were incubated with rabbit NF-kBp65 (Rabbit mAb (D14E12) Cell Signaling Technology, Inc Danvers, MA) primary antibody (1: 1000 dilution overnight) followed by an anti-rabbit secondary antibody. The western blot membrane was then washed, and the signal was detected using a Super Signal chemiluminescent substrate (Pierce Biotechnology, Waltham, MA)(Suresh et al., 2013).
2.15. Capillary Western blot.
Capillary Western blot was performed with a Western immunoassay system (004–600; Protein Simple, San Jose, CA, USA) according to the manufacturer’s instructions using 12–230 kDa separation modules (Beekman, Janson, Baghat, van Deutekom, & Datson, 2018). Briefly, A549 cells were treated with KP in the presence and absence of basil, and protein samples were diluted 10-fold in sample buffer (100-fold diluted 10× Sample Buffer 2 from the Separation Module), then mixed with Fluorescent Master Mix (Protein Simple, Centennial, CO, USA) and heated at 95°C for 5 min. The samples, blocking reagent, primary antibody (Phospho-NF-κB p65, Rabbit mAb #3033, used 1:100 dilution) and horseradish peroxidase-conjugated anti-rabbit secondary antibody, and chemiluminescent substrate were pipetted onto the plate of the separation module (Suresh, Dolgachev, et al., 2019; Zhang et al., 2019).
2.16. Statistical methods
The experiments were not randomized/blinded, and all the groups in the study are similar, and there are no biased variables included in the study’s outcome. Data are expressed as the mean ± SEM in all figures. Statistical significance of data between 2 groups was analyzed using a 2-tailed, unpaired t-test with Welch’s correction. Data from more than 2 groups were analyzed using 1-way ANOVA with Tukey’s multiple-comparisons test (Graph Pad Prism 8.00). Significance was set at *P < 0.05; **P < 0.01; and ***P < 0.001, cells/basil vs KP and # P < 0.05; ##P < 0.01; and ### P < 0.001, KP vs KP+ basil (Suresh et al., 2012 ; Suresh et al., 2013).
3. RESULTS
3.1. Human lung epithelial cell viability following KP inoculation is better preserved in the presence of Holy basil.
First, we examined the dose-dependent effect of KP on lung cells. Cells were treated with different doses (100–500 CFU) of KP for 24 hours, and cell viability was measured. For this, cells were stained with Trypan blue to distinguish between dead and living cells. The cell number was calculated using an automated cell counter (Countess™, Invitrogen). It was found that the maximum cell death occurred with a dose of 500 CFU bacteria (Figure 1a). For subsequent experiments, we used a concentration of 500 CFU of bacteria per well.
Figure 1: Basil administration increased the cell viability and protected the morphological appearance of the A549 cells following KP.
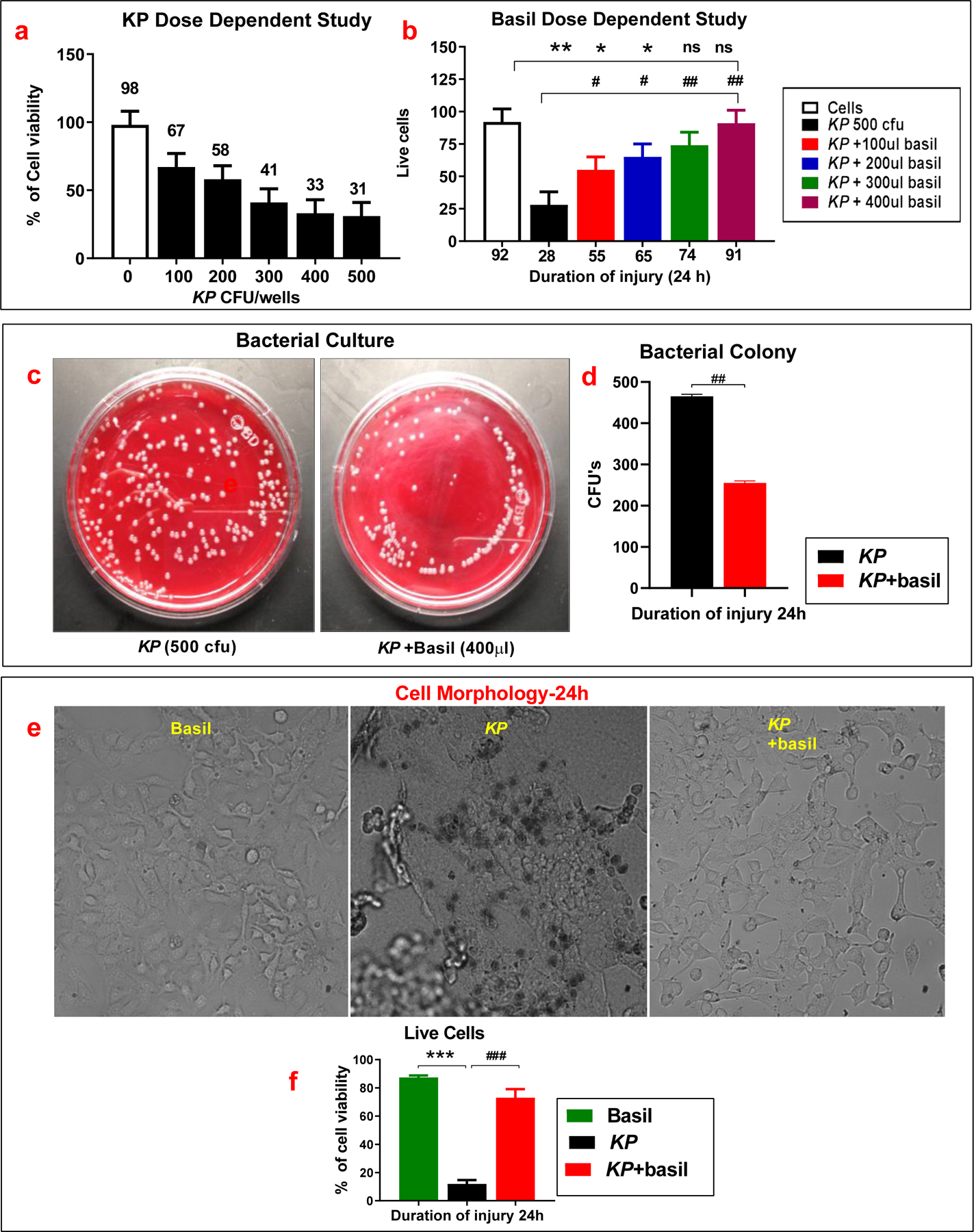
(a). Following the administration of Holy basil in the presence of KP and then examine the percentage of cell viability (b). Bacterial colony-forming units (CFU) on blood agar plates inoculated with KP in the presence and absence of basil extracts were counted (n =5) (c-d). Cells were observed under the microscope, and pictures were taken following KP and holy basil extract administration (e-f) (n =5). Statistical analysis was performed at different time points; paired samples were analyzed using a two-tailed unpaired t-test with Welch’s correction. Data presented in terms of mean ± SEM (n =5). *P< 0.05 vs. cell alone, #P< 0.05 vs. KP, ns= non-significant.
Shetty et al. (2008) reported that Leaves of O. sanctum were dried in the powdered and were undertaken to assess the potential of aqueous and alcoholic extracts in wound healing in Wistar albino rats. They found that aqueous extract possesses a better effect than alcoholic extract at a dose of 800 mg/kg body weight. Next, the same experiment was repeated in different amounts of the three basil extracts employed (Holy basil extract, Holy basil juice, and Thai basil extract) because we need to know the protective nature is the same or not. Various treatments of basil were utilized again (100–400 µl; 1% solution). We have used the dry powder, and the exact molecular weight of the powder is not known. However, based on the molecular weight of 2320 KD reported for Basil seed gum by Naji Tabasi et al. in Food Hydrocolloids, 2016, 52, 350–358, our concentration is only 8.6 µM. Our results demonstrated that increasing the concentration of basil resulted in increased cell viability even with KP infection. Both Holy basil juice and the Thai basil extract were shown to confer protective effects following bacteria treatment (data not shown). As shown in Fig 1b, 400 µl of 1% holy basil solution protected 91% of the cell population from the detrimental effects of KP exposure (compared to 28% viable cells after KP exposure alone). We used this dose of basil for all further experiments.
Similarly, both Holy basil juice and the Thai basil extract were shown to confer protective effects following bacterial treatment (data not shown). Additionally, we quantified the number of bacterial colonies on the blood agar plates in the presence or absence of basil. There were significantly fewer bacterial colonies following basil treatment, suggesting that it actively inhibits the growth of KP (Figure 1c, d).
Next, we performed a morphological examination of the KP-treated cells in the presence or absence of basil and imaged them using a fluorescence microscope. In the Holy basil administered group, cells looked healthier with lower mortality rates than pneumonia alone control group (Figure-1c). The black spots seen in the figure represent cells stained with trypan blue (dead). Cells in the KP alone group were detached and lay loose as they had lost adhesion following infection. The data suggest that cell viability and morphological appearance were preserved upon basil treatment compared to KP alone controls, highlighting the basil’s protective effects (Figure 1e, f).
3.2. Basil administration reduced apoptosis and improved phagocytosis and bacterial clearance following KP infection.
Next, we examined the apoptosis of lung cells using the ApoStat kit that detects the presence of activated caspases (Suresh et al., 2016). Caspases are a known family of proteins that play an essential role in apoptosis. The data shows that basil administration reduced apoptosis in A549 cells. Strikingly, treatment with basil alone protected cells from apoptosis (Figure 2a) compared with cells with no treatment (control group). Here, we found a significant elevation in the levels of activated intracellular caspases 24 hours post-KP infection (Figure 2a-b). Flow cytometry (pan-caspase) data further revealed a significant elevation of inactive caspases following exposure to KP, which was significantly diminished in the presence of basil (Figure 2c). Thus, basil administration protects human lung epithelial cells from cell death due to apoptosis under the KP treatment paradigm.
Figure 2: Basil administration led to less apoptosis and improved phagocytosis in A549 cells.
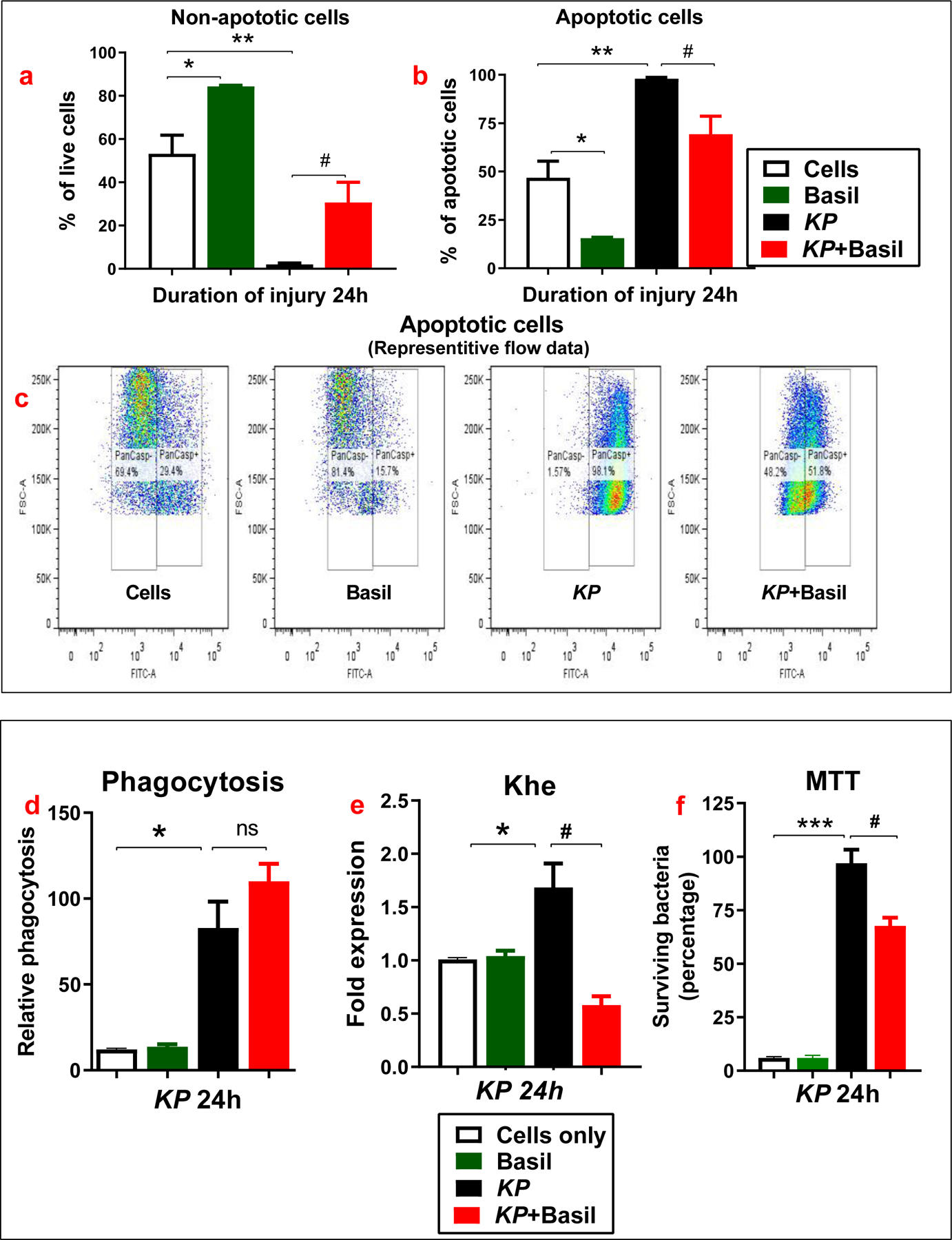
Representative flow cytometry analysis (ApoStat) data from cells inoculated with KP in the presence or absence of basil is provided (a-c). Cells treated with FITC labeled KP were analyzed by a phagocytic assay (d). Khe expression at 24 hours following KP inoculation was assayed using qPCR (e). Bactericidal capacity was measured at 24 hours following inoculation with KP (f). Statistical analysis was performed at different time points; paired samples were analyzed using a two-tailed unpaired t-test with Welch’s correction. Data presented in terms of mean ± SEM (n =3–5). *P< 0.05 vs. cell alone, #P< 0.05 vs. KP, ns= non-significant.
Our previous study determined the phagocytosis and bactericidal activity in isolated alveolar macrophages from naive and KP-administered mice (Suresh, Dolgachev, et al., 2019). We tried to determine if basil could increase phagocytosis in human lung cells following KP infection in a separate set of experiments. The data revealed that the relative phagocytic activity was significantly higher at 24 hours in the basil + KP group than KP alone suggesting that basil treatment increased phagocytosis of the bacteria KP within A549 cells (Figure 2d). Finally, the expression of the Khe gene was evaluated for its role in the survival of the A549 cell population following KP treatment. As expected, Khe’s appearance increased following inoculation with KP but was significantly attenuated in basil treatment (Figure 2e).
Moreover, the bactericidal capacity of isolated lung epithelial cells from KP and KP + basil groups was evaluated using the MTT assay. Intracellular bacterial clearance of A549 cells was significantly higher in the KP + basil administered group than KP alone controls (Figure 2f). There was only 60 % survival of bacteria in the basil treated group compared to 100% within the KP alone group. These data suggest that A549 cells drive bacterial clearance via a combination of enhanced bactericidal activity and increased phagocytic clearance capability triggered by basil treatment.
3.3. Basil reduces pro-inflammatory gene expression following pneumonia administration.
We have previously reported that the pro-inflammatory and hypoxia-related genes drive the acute inflammatory response following lung contusion and acid-induced aspiration in mice (Suresh, Balijepalli, et al., 2019; Suresh, Dolgachev, et al., 2019). In a separate set of experiments, we examined gene expression levels in cell lysates following basil treated and pneumonia alone groups at a 24-hour time following infection. The levels of multiple pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), Interferon-gamma (IFN-γ), macrophage inflammatory proteins (MIP-2), IL-10, VEGF-A, NOS-2, and Arginase were measured. The levels of IL-1β and IL-6 were significantly elevated in the KP administered groups compared to the KP + basil group (Figure 3a, b). The TNF- α in the cells was significantly elevated at a 24-hour time point in the KP alone group compared to the KP + basil treated group (Figure 3c). MIP-2 was also significantly elevated at the 24-hour interval in KP-treated cells compared to the KP + basil group (Figure 3d). Here we found no significant difference in the levels of IL-10, VEGF-A, NOS-2, and Arginase in the KP and KP + basil administered groups (Figure 3e-h). These data suggest that the presence of basil confers a protective role, evidenced by the decreased inflammatory response following pneumonia.
Figure 3: Basil administration reduced inflammatory gene expression following KP.
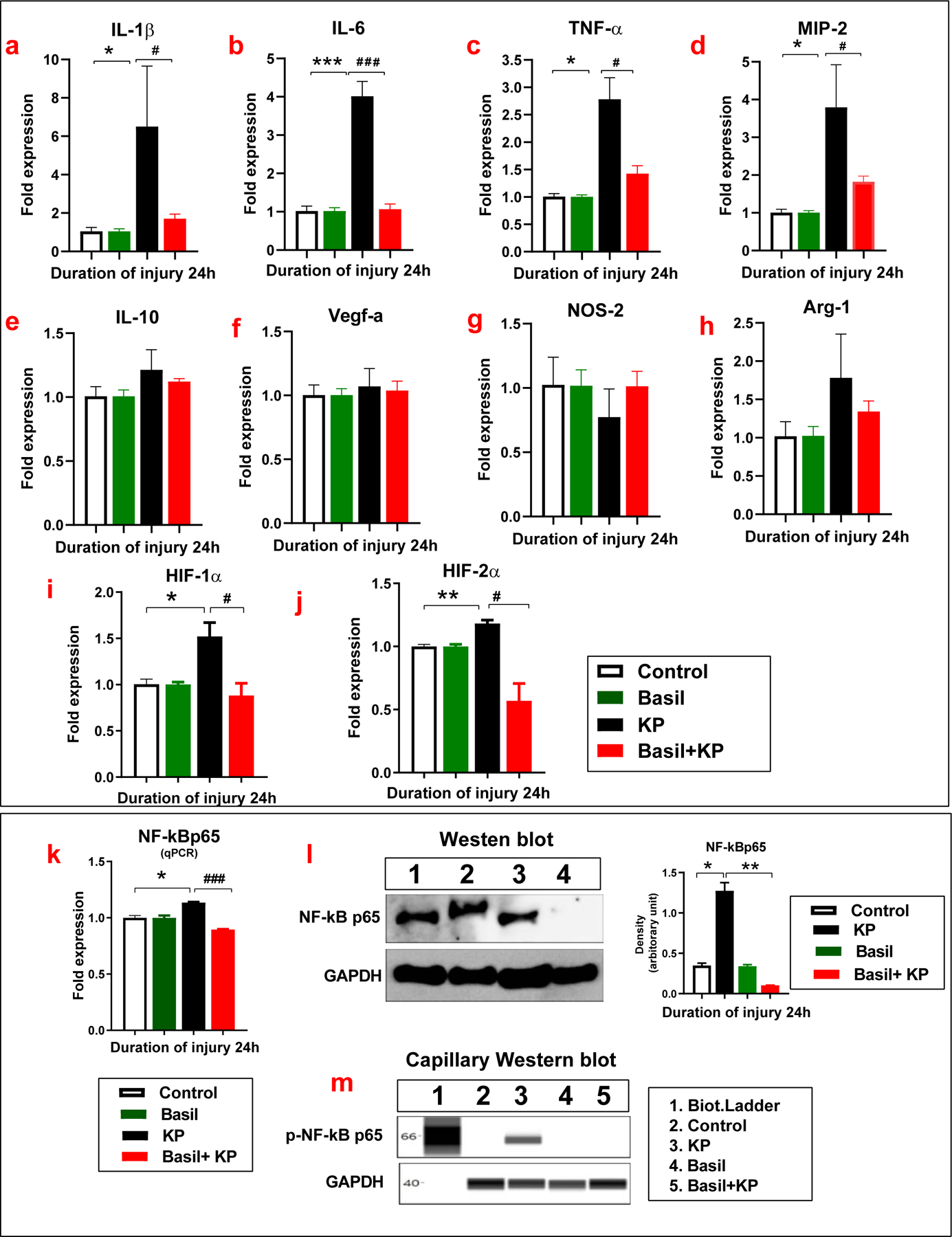
Following pneumonia, cell lysate was collected at 24 hours. Gene expression was determined by qPCR. The levels of IL-1β, IL-6(a-b), TNF-α(c), MIP-2(d), IL-10(e), VEGF-A (f), NOS-2(g), and Arginase-1(h) were measured (n =3). Basil diminished hypoxic gene (HIF-1/2α) expressions following KP. Expressions of HIF-1α and HIF-2α with basil administration following KP infection were determined using Real-time PCR (i-j). The gene levels of NF-kB (k) were measured by qPCR, and the protein level of expression was determined by Western blot (l). The phosphorylation of NF-kBp65 expression was measured by Capillary Western blot (m) (n =3). Statistical analysis was performed at different time points; paired samples were analyzed using a two-tailed unpaired t-test with Welch’s correction. Data presented in terms of mean ± SEM (n =3). *P< 0.05 vs. cell alone, #P< 0.05 vs. KP, ns= non-significant.
3.4. Basil administration reduces hypoxic gene HIF-1/2 alpha and NF-kB p65 expression following KP
We have previously reported that the hypoxia-inducible factor (HIF-1) alpha is a crucial mediator of the acute inflammatory response following lung contusion, a sterile lung injury(Baliga et al., 2013; Singh et al., 2001). Here, we further pursued defining the role of HIF-1/2α and NF-κB in A549 cells following KP exposure. The expression of HIF-1/2α was significantly increased after inoculation with KP; however, this was significantly reduced in the presence of basil (Figure 3i-j). This data suggests that basil dampens the acute inflammatory response to KP via alteration of hypoxia signaling pathways. We also examined NF-κB-p65 gene expression in A549 cells in the presence or absence of basil following KP administration. There was a profound change in the expression of NF-κBp65 following KP infection. Basil administration led to significant attenuation of the NF-Κbp65 expression (Figure 3 k).
Additionally, we examined NFκB-p65 activation from whole-cell lysates using western blot analysis. We found that NF-kB expression was significantly higher in KP-treated cells compared to the basil and KP administered group (Figure 3 l). Moreover, we examined the phosphorylation of the NF-kB subunit from whole-cell lysates following KP in the presence and absence of holy basil using Capillary western blot analysis. The data show that the basil inhibits the NF-KB phosphorylation in the human lung epithelial cells treated with KP (Figure 3 m). These results suggest that the acute inflammatory responses depend on NF-κBp65 expression.
3.5. Basil administration reduces cell injury and inflammation following KP
In a new set of experiments, we evaluated cytokine expression changes following basil treatment to determine the role of basil administration in cytokine production during KP infection. The release of cytokines like interleukins (IL-2, IL-4, IL-6, IL-18, and IL-10), tumor necrosis factor (TNF-α), Granulocyte-macrophage colony-stimulating factor (GM-CSF), and Interferon-gamma (IFN-γ) into the cell culture medium was analyzed using Enzyme-Linked Immunosorbent Assay (ELISA). The cell culture medium from KP-administered cells showed higher IL-2, IL-4, IL-6, and TNF-α compared to the group with basil treatment (Figure 4a-d). Similarly, the levels of GM-CSF and IL-18 were also considerably reduced in the basil-administered group compared to KP alone (Figure 4e-f). Here we found no significant difference in the IL-10 levels following basil administration compared to KP alone (Figure 4g). In contrast, the level of IFN-γ was increased with basil treatment irrespective of bacterial infection (Figure 4h). Moreover, in the cell lysates, IFN-γ gene expression was significantly higher following basil treatment (Figure 4i). Overall, these data demonstrate that basil moderates the cytokine response, possibly in part via activation of IFN-γ and inhibition of NF-κB activity.
Figure 4: Basil reduced production of pro-inflammatory cytokines in A549 cells following KP.
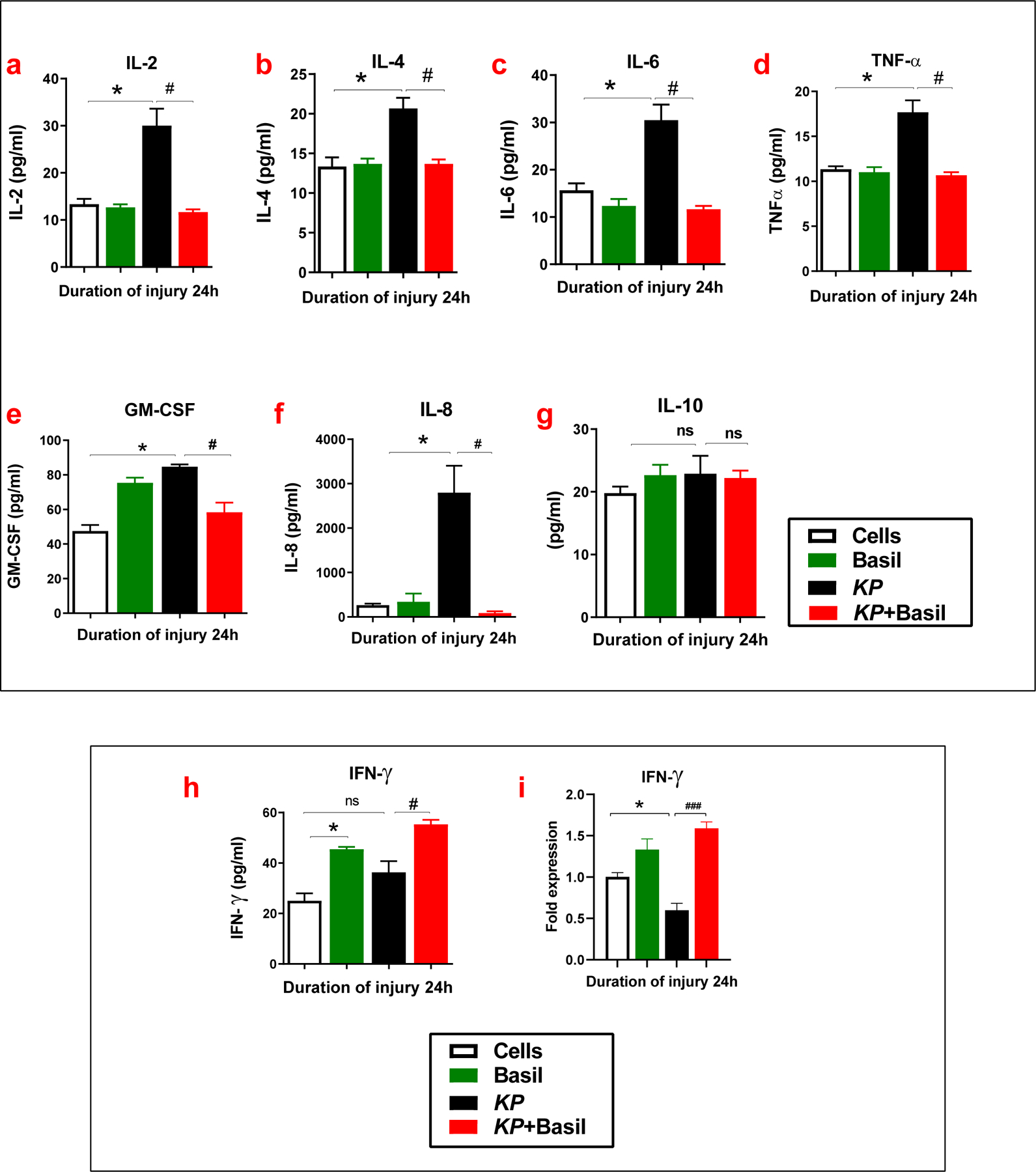
Following pneumonia, cell culture media was collected at 24 hours. The levels of IL-2 (a), IL-4 (b), IL-6 (c), TNF-α (d), GM-CSF (e), IL-8 (f), and IL-10 (g) and IFN-γ (h) were measured in the media using ELISA (n =3). Expressions of IFN-γ with basil administration following KP infection was determined using Real-time qPCR (i) (n =3). Statistical analysis was performed at different time points; paired samples were analyzed using a two-tailed unpaired t-test with Welch’s correction. Data presented in terms of mean ± SEM (n= 3). *P< 0.05 vs. cell alone, #P< 0.05 vs. KP, ns= non-significant.
3.6. Schematic diagram of how to protect basil A549 cells from pneumonia
Basil has anti-microbial potential against gram-negative bacteria. Basil does not destroy all the bacteria in the culture and does not increase the bacterial phagocytosis by lung cells. Basil inhibits the release of some inflammatory cytokines and increases the cells’ release of a beneficial cytokine during bacterial infection. Thus, basil protects the cells by maintaining a healthy balance of the cytokines essential for the body’s proper immunological response (Figure-5).
Figure. 5.
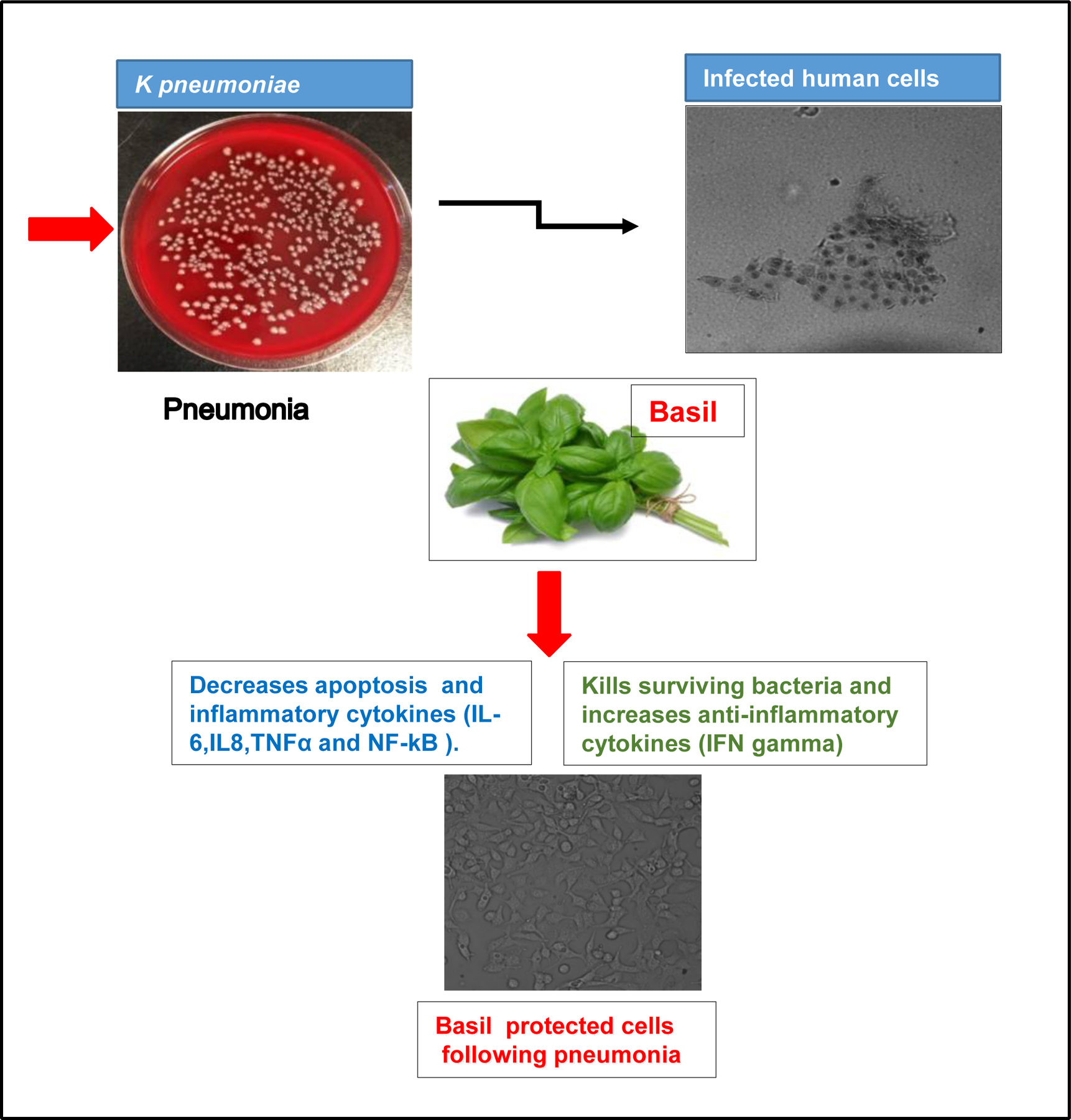
A schematic diagram for how holy basil protected human lung epithelial cells from Klebsiealla pneumoniae.
4. Discussion
Pneumonia is one of the important causes of infectious morbidity and mortality in the United States of America (Craven, Barber, Steger, & Montecalvo, 1990; Leu, Kaiser, Mori, Woolson, & Wenzel, 1989; E. Wang et al., 2001). We have specifically chosen to study KP-mediated infection because it accounts for a significant proportion of community- and hospital-acquired pneumonia. KP is particularly virulent because it causes substantial alveolar necrosis and has the potential to cause septicemia. The same result was found for the gram-positive strain of Streptococcus pneumoniae bacteria that causes pneumonia in young children. Ocimum sanctum (Basil/Tulasi) has been shown to have many medicinal properties in Ayurveda (traditional Indian medicine) (Saharkhiz et al., 2015). Previous studies have demonstrated that Holy basil has multiple anti-inflammatory effects, likely via bioactive secondary metabolites that could act alone or in synergy (Ahmad, Abuzinadah, Alkreathy, Banaganapalli, & Mujeeb, 2018; Jamshidi & Cohen, 2017; Khan et al., 2010).
Shetty et al. reported that Leaves of O. sanctum were dried in the powdered and were undertaken to assess the potential of aqueous and alcoholic extracts in wound healing in Wistar albino rats. Moreover, Shetty et al. also studied acute toxicity in healthy rats (n = 6), and they orally fed with increasing doses (400 mg/kg to 6 g/kg body weight) of extracts for 14 days. They found both the alcoholic and aqueous extract doses significantly increased wound breaking strength and decreased the percentage of wound contraction and lipid peroxidation compared to the control group. Moreover, the aqueous extract possesses a better effect than alcoholic extract at a dose of 800 mg/kg body weight. The doses up to 4 g/kg body weight did not produce any toxicity or mortality (Shetty et al., 2008).
Another study from Gautam and Goel (2014) reported that, in an oral acute toxicity study, a high dose of Ocimum sanctum at 2000 mg/kg did not show any observable toxic effects in the mice in terms of any deaths or abnormal symptoms, which confers its nontoxicity and safety in mice. Additionally, a Subacute toxicity study in rats which were administered 200, 400, and 800 mg/kg of Ocimum sanctum for 28 days did not indicate any mortality, change in food and water intake, body, and organ weights, or histopathological changes in the organs like liver, kidney, spleen, heart, testis or ovary which further strengthen the safety profile of Ocimum sanctum (Gautam & Goel, 2014).
Raina et al. (2015) reported that, in a subacute toxicity study, animals received O. sanctum extract by oral gavage at the doses of 250, 500, and 1000 mg/kg/day for 28 days. At the end of the study, the animals were sacrificed and evaluated for the effect of O. sanctum extract on clinical, hematological, biochemical, and histopathological parameters. They reported that that oral administration of OSE was not toxic to male and female Wistar rats up to the highest dose tested, thereby suggesting its clinical safety (Raina, Chandrasekaran, Deepak, Agarwal, & Ruchika, 2015).
Here, we endeavored to define the role of basil in a clinically relevant model of gram-negative bacterial pneumonia in human lung epithelial cells to assess its potential to lessen injury and decrease cell death following pneumonia. We found that the basil treatment significantly reduced cell death following pneumonia. We also found that pro-apoptotic caspases’ expression was significantly reduced in cells treated with basil before inoculation with KP. Apoptosis is an active form of cell death and has been suggested to be a mechanism underlying several lung diseases’ pathogenesis (Kaiser & Offermann, 2005; Martin, Hagimoto, Nakamura, & Matute-Bello, 2005; Suresh et al., 2016).
It has been previously reported that alveolar macrophages are associated with the resolution or progression of KP infection in animal models of Streptococcus pneumonia (Rijneveld et al., 2002; Suresh, Dolgachev, et al., 2019). Here we evaluated the bactericidal and phagocytic capacity in isolated human lung epithelial cells in the presence and absence of basil. The administration of basil in the lung epithelial cells showed increased phagocytosis and bactericidal activity, as we confirmed by an MTT bacterial viability assay. Further evaluation of Khe, indicative of surviving KP within the lung epithelial cells, supported these findings (Figure-2). These results confirm the hypothesis that the administration of basil results in enhanced bacterial clearance of KP.
Bacterial lung infection is characterized by pro-inflammatory cytokines within the alveolar space, damaging the alveolar epithelial cells (R. M. Strieter, Kunkel SM, et al., 1999). Pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, are produced due to the up-regulation of inflammatory reactions following KP infection (Zhang et al., 2019). Here, we show that pro-inflammatory cytokine expression in A549 cells in response to KP infection is diminished upon treatment with basil extract.
Previous studies show that the Hypoxia-inducible factor (HIF) regulates the adaptive cellular response to hypoxia by stimulating the transcription of a series of hypoxia-inducible genes when the tissue is deprived of adequate oxygen supply. It also governs target genes’ expression, which is important in angiogenesis, energy metabolism, and cell survival (van Uden, Kenneth, & Rocha, 2008; G. L. Wang, Jiang, Rue, & Semenza, 1995; G. L. Wang & Semenza, 1993). Furthermore, HIF and NF-κB are known to cooperatively regulate the response to hypoxia and inflammation (Taylor et al., 2011). Here, the expression of both HIF-1/2a and NF-κBp65 (Total/Phospho) was reduced following basil treatment, suggesting that it likely attenuates inflammation following bacterial pneumonia via modulation of the interconnected HIF and NF-κB pro-inflammatory pathways (Figure 3 l -m).
Additionally, pro-inflammatory mediators have also been suggested to characterize bacterial lung infection in the alveolar space with damage to the alveolar epithelial cells (Cai, Batra, Lira, Kolls, & Jeyaseelan, 2010; R. M. Strieter & Kunkel, 1994). Cytokines are small secreted proteins released by cells that regulate cell interaction and communication (Standiford & Huffnagle, 1997; Yoshizaki et al., 1990). These include both pro and anti-inflammatory cytokines. Pro-inflammatory cytokines are produced predominantly by activated macrophages. They are involved in the up-regulation of inflammatory reactions, while anti-inflammatory cytokines are immunoregulatory molecules that counter-regulate the pro-inflammatory cytokine response (Walley, Lukacs, Standiford, Strieter, & Kunkel, 1996; Winter et al., 2007). Our results show that increased expression of pro-inflammatory cytokines, such as the specific interleukin family of proteins in the A549 cells, is inhibited in the presence of basil. Basil, therefore, potentially attenuates inflammation following bacterial pneumonia via modulation of cytokine-mediated pro-inflammatory pathways. The anti-inflammatory effects of Holy Basil have been previously documented in many in vitro and in vivo studies (Ahmad et al., 2018; Jamshidi & Cohen, 2017; Khan et al., 2010). It is plausible that it activates multiple bioactive secondary metabolites that act alone or synergistically to inhibit inflammatory pathway signaling.
Basil also increased the release of another cytokine, interferon-gamma (IFN-γ), during bacterial infection. IFN- γ is a protein critical for innate and adaptive immunity against viral, bacterial, and protozoal diseases(Cavalcanti et al., 2012; Zeng et al., 2005). Studies show that deficiency of IFN-γ leads to unhealthy inflammatory cytokines and inadequate immunological response (Cavalcanti et al., 2012; Zeng et al., 2005). IFN-γ is essential for cell-mediated immunity against fungi and other intracellular microorganisms that can potentially cause chronic pneumonia (Dalton et al., 1993; N. M. Miller, Wang, Tan, & Dittel, 2015). It is also considered to play a pivotal role in host defense against several infectious diseases. It is implicated for its role as a mediator of host defense against various respiratory pathogens (Docke et al., 1997; Rijneveld et al., 2002).
The Ayurvedic tradition of consuming Holy basil daily may be an active lifestyle choice that could be adopted to address respiratory problems observed in susceptible populations. We think this is the first study that strongly associates the benefits of applying basil extract on cell survival using a lethal model of gram-negative pneumonia.
5. CONCLUSION.
This study demonstrated that Holy basil significantly reduced A549 cell death following Klebsiella pneumoniae infection. The basil’s effect on cell viability is potentially mediated by upregulation of IFN-γ with either subsequent or independent reduction of pro-inflammatory cytokines. These results will inspire further in vivo studies to better characterize and evaluate Holy basil’s mechanism and role in bacterial pneumonia and other respiratory ailments in general. Holy basil is a promising therapeutic agent for controlling and healing bacterial pneumonia. It is promising due to its strength in vitro, absence of any significant side effects to the cells, low cost for purification, use, and ease of access.
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health Grant HL102013 to KR.
Footnotes
AUTHOR DISCLOSURE
None of the authors has a financial relationship with a commercial company.
COMPETING INTERESTS
The authors declare no competing interests.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- Ahmad A, Abuzinadah MF, Alkreathy HM, Banaganapalli B, & Mujeeb M (2018). Ursolic acid rich Ocimum sanctum L leaf extract loaded nanostructured lipid carriers ameliorate adjuvant induced arthritis in rats by inhibition of COX-1, COX-2, TNF-alpha and IL-1: Pharmacological and docking studies. PLoS One, 13(3), e0193451. doi: 10.1371/journal.pone.0193451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliga MS, Jimmy R, Thilakchand KR, Sunitha V, Bhat NR, Saldanha E, . . . Palatty PL (2013). Ocimum sanctum L (Holy Basil or Tulsi) and its phytochemicals in the prevention and treatment of cancer. Nutr Cancer, 65 Suppl 1, 26–35. doi: 10.1080/01635581.2013.785010 [DOI] [PubMed] [Google Scholar]
- Ballinger MN, Paine R 3rd, Serezani CH, Aronoff DM, Choi ES, Standiford TJ, . . . Moore BB (2006). Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol, 34(6), 766–774. doi: 10.1165/rcmb.2005-0246OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman C, Janson AA, Baghat A, van Deutekom JC, & Datson NA (2018). Use of capillary Western immunoassay (Wes) for quantification of dystrophin levels in skeletal muscle of healthy controls and individuals with Becker and Duchenne muscular dystrophy. PLoS One, 13(4), e0195850. doi: 10.1371/journal.pone.0195850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R 3rd, & Standiford TJ (1997). Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun, 65(4), 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Batra S, Lira SA, Kolls JK, & Jeyaseelan S (2010). CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol, 185(10), 6214–6225. doi: 10.4049/jimmunol.0903843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti YV, Brelaz MC, Neves JK, Ferraz JC, & Pereira VR (2012). Role of TNF-Alpha, IFN-Gamma, and IL-10 in the Development of Pulmonary Tuberculosis. Pulm Med, 2012, 745483. doi: 10.1155/2012/745483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Huang Y, Yang Y, & Qiu H (2013). Acidinduced cell injury and death in lung epithelial cells is associated with the activation of mitogenactivated protein kinases. Mol Med Rep, 8(2), 565–570. doi: 10.3892/mmr.2013.1537 [DOI] [PubMed] [Google Scholar]
- Cilloniz C, Martin-Loeches I, Garcia-Vidal C, San Jose A, & Torres A (2016). Microbial Etiology of Pneumonia: Epidemiology, Diagnosis and Resistance Patterns. Int J Mol Sci, 17(12). doi: 10.3390/ijms17122120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM (2014). Tulsi - Ocimum sanctum: A herb for all reasons. J Ayurveda Integr Med, 5(4), 251–259. doi: 10.4103/0975-9476.146554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven DE, Barber TW, Steger KA, & Montecalvo MA (1990). Nosocomial pneumonia in the 1990s: update of epidemiology and risk factors. Semin Respir Infect, 5, 157–172. [PubMed] [Google Scholar]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, & Stewart TA (1993). Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science, 259(5102), 1739–1742. [DOI] [PubMed] [Google Scholar]
- de Jager W, Bourcier K, Rijkers GT, Prakken BJ, & Seyfert-Margolis V (2009). Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol, 10, 52. doi: 10.1186/1471-2172-10-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, . . . Kox W (1997). Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med, 3, 678–681. [DOI] [PubMed] [Google Scholar]
- Dolgachev V, Panicker S, Balijepalli S, McCandless LK, Yin Y, Swamy S, . . . Machado-Aranda D (2018). Electroporation-mediated delivery of FER gene enhances innate immune response and improves survival in a murine model of pneumonia. Gene Ther, 25(5), 359–375. doi: 10.1038/s41434-018-0022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgachev VA, Yu B, Reinke JM, Raghavendran K, & Hemmila MR (2012). Host susceptibility to gram-negative pneumonia after lung contusion. J Trauma Acute Care Surg, 72(3), 614–622; discussion 622–613. doi: 10.1097/TA.0b013e318243d9b101586154-201203000-00010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam MK, & Goel RK (2014). Toxicological Study of Ocimum sanctum Linn Leaves: Hematological, Biochemical, and Histopathological Studies. J Toxicol, 2014, 135654. doi: 10.1155/2014/135654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, & Cheng Y (2018). MicroRNA-1247 inhibits lipopolysaccharides-induced acute pneumonia in A549 cells via targeting CC chemokine ligand 16. Biomed Pharmacother, 104, 60–68. doi: 10.1016/j.biopha.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Jamshidi N, & Cohen MM (2017). The Clinical Efficacy and Safety of Tulsi in Humans: A Systematic Review of the Literature. Evid Based Complement Alternat Med, 2017, 9217567. doi: 10.1155/2017/9217567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, & Offermann MK (2005). Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol, 174(8), 4942–4952. [DOI] [PubMed] [Google Scholar]
- Khan A, Ahmad A, Akhtar F, Yousuf S, Xess I, Khan LA, & Manzoor N (2010). Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res Microbiol, 161(10), 816–823. doi: 10.1016/j.resmic.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Leu HS, Kaiser DL, Mori M, Woolson RF, & Wenzel RP (1989). Hospital-acquired pneumonia. Attributable mortality and morbidity. Am J Epidemiol, 129(6), 1258–1267. [DOI] [PubMed] [Google Scholar]
- Martin TR, Hagimoto N, Nakamura M, & Matute-Bello G (2005). Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc, 2(3), 214–220. doi:2/3/214 [pii]10.1513/pats.200504–031AC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NM, Wang J, Tan Y, & Dittel BN (2015). Anti-inflammatory mechanisms of IFN-gamma studied in experimental autoimmune encephalomyelitis reveal neutrophils as a potential target in multiple sclerosis. Front Neurosci, 9, 287. doi: 10.3389/fnins.2015.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PR, Croce MA, Bee TK, Qaisi WG, Smith CP, Collins GL, & Fabian TC (2001). ARDS after pulmonary contusion: accurate measurement of contusion volume identifies high-risk patients. J Trauma, 51(2), 223–228; discussion 229–230. [DOI] [PubMed] [Google Scholar]
- Mondal S, Mirdha BR, & Mahapatra SC (2009). The science behind sacredness of Tulsi (Ocimum sanctum Linn.). Indian J Physiol Pharmacol, 53(4), 291–306. [PubMed] [Google Scholar]
- Pattanayak P, Behera P, Das D, & Panda SK (2010). Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn Rev, 4(7), 95–105. doi: 10.4103/0973-7847.65323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina P, Chandrasekaran CV, Deepak M, Agarwal A, & Ruchika KG (2015). Evaluation of subacute toxicity of methanolic/aqueous preparation of aerial parts of O. sanctum in Wistar rats: Clinical, haematological, biochemical and histopathological studies. J Ethnopharmacol, 175, 509–517. doi: 10.1016/j.jep.2015.10.015 [DOI] [PubMed] [Google Scholar]
- Rijneveld AW, Lauw FN, Schultz MJ, Florquin S, Te Velde AA, Speelman P, . . . Van Der Poll T (2002). The role of interferon-gamma in murine pneumococcal pneumonia. J Infect Dis, 185(1), 91–97. doi: 10.1086/338122 [DOI] [PubMed] [Google Scholar]
- Saharkhiz MJ, Kamyab AA, Kazerani NK, Zomorodian K, Pakshir K, & Rahimi MJ (2015). Chemical Compositions and Antimicrobial Activities of Ocimum sanctum L. Essential Oils at Different Harvest Stages. Jundishapur J Microbiol, 8(1), e13720. doi: 10.5812/jjm.13720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty S, Udupa S, & Udupa L (2008). Evaluation of Antioxidant and Wound Healing Effects of Alcoholic and Aqueous Extract of Ocimum sanctum Linn in Rats. Evid Based Complement Alternat Med, 5(1), 95–101. doi: 10.1093/ecam/nem004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivananjappa MM, Mhasavade D, & Joshi MK (2013). Aqueous extract of Terminalia arjuna attenuates tert-butyl hydroperoxide-induced oxidative stress in HepG2 cell model. Cell Biochem Funct, 31(2), 129–135. doi: 10.1002/cbf.2867 [DOI] [PubMed] [Google Scholar]
- Simamura E, Hirai KI, Shimada H, & Koyama J (2001). Apoptosis and epithelial phagocytosis in mitomycin C-treated human pulmonary adenocarcinoma A549 cells. Tissue Cell, 33(2), 161–168. [DOI] [PubMed] [Google Scholar]
- Singh S, Rehan HM, & Majumdar DK (2001). Effect of Ocimum sanctum fixed oil on blood pressure, blood clotting time and pentobarbitone-induced sleeping time. J Ethnopharmacol, 78(2–3), 139–143. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, & Huffnagle GB (1997). Cytokines in host defense against pneumonia. J Investig Med, 45, 335–345. [PubMed] [Google Scholar]
- Strieter RM, & Kunkel SL (1994). Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med, 42(4), 640–651. [PubMed] [Google Scholar]
- Strieter RM, Kunkel SM, et al. (1999). Chemokines in Lung Injury: Thomas A. Neff lecture. chest, 116(1), 103S–110S. [DOI] [PubMed] [Google Scholar]
- Suresh MV, Balijepalli S, Zhang B, Singh VV, Swamy S, Panicker S, . . . Raghavendran K (2019). Hypoxia-Inducible Factor (HIF)-1alpha Promotes Inflammation and Injury Following Aspiration-Induced Lung Injury in Mice. Shock, 52(6), 612–621. doi: 10.1097/SHK.0000000000001312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh MV, Dolgachev VA, Zhang B, Balijepalli S, Swamy S, Mooliyil J, . . . Raghavendran K (2019). TLR3 absence confers increased survival with improved macrophage activity against pneumonia. JCI Insight, 4(23). doi: 10.1172/jci.insight.131195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh MV, Thomas B, Machado-Aranda D, Dolgachev VA, Kumar Ramakrishnan S, Talarico N, . . . Raghavendran K (2016). Double-Stranded RNA Interacts With Toll-Like Receptor 3 in Driving the Acute Inflammatory Response Following Lung Contusion. Crit Care Med, 44(11), e1054–e1066. doi: 10.1097/CCM.0000000000001879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh MV, Wagner MC, Rosania GR, Stringer KA, Min KA, Risler L, . . . Reddy RC (2012). Pulmonary administration of a water-soluble curcumin complex reduces severity of acute lung injury. Am J Respir Cell Mol Biol, 47(3), 280–287. doi: 10.1165/rcmb.2011-0175OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh MV, Yu B, Lakshminrusimha S, Machado-Aranda D, Talarico N, Zeng L, . . . Raghavendran K (2013). The protective role of MnTBAP in oxidant-mediated injury and inflammation in a rat model of lung contusion. Surgery, 154(5), 980–990. doi: 10.1016/j.surg.2013.05.023 S0039–6060(13)00268–7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, & Shah YM (2011). Hypoxia-inducible factor-2alpha mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology, 140(7), 2044–2055. doi: 10.1053/j.gastro.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uma Devi P, Ganasoundari A, Vrinda B, Srinivasan KK, & Unnikrishnan MK (2000). Radiation protection by the ocimum flavonoids orientin and vicenin: mechanisms of action. Radiat Res, 154(4), 455–460. [DOI] [PubMed] [Google Scholar]
- van Uden P, Kenneth NS, & Rocha S (2008). Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. The Biochemical journal, 412(3), 477–484. doi: 10.1042/BJ20080476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthakal R, Suresh MV, & Kowluru A (2009). Down-regulation of expression and function of nucleoside diphosphate kinase in insulin-secreting beta-cells under in vitro conditions of glucolipotoxicity. Mol Cell Biochem, 329(1–2), 121–129. doi: 10.1007/s11010-009-0113-6 [DOI] [PubMed] [Google Scholar]
- Walley KR, Lukacs NW, Standiford TJ, Strieter RM, & Kunkel SL (1996). Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun, 64(11), 4733–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Ouellet N, Simard M, Fillion I, Bergeron Y, Beauchamp D, & Bergeron MG (2001). Pulmonary and systemic host response to Streptococcus pneumoniae and Klebsiella pneumoniae bacteremia in normal and immunosuppressed mice. Infect Immun, 69(9), 5294–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, & Semenza GL (1995). Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A, 92(12), 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, & Semenza GL (1993). Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem, 268(29), 21513–21518. [PubMed] [Google Scholar]
- Wen H, Dou Y, Hogaboam CM, & Kunkel SL (2008). Epigenetic regulation of dendritic cell-derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood, 111(4), 1797–1804. doi: 10.1182/blood-2007-08-106443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Blainey AD, Harrison KJ, & Clarke SK (1981). Causes of pneumonia presenting to a district general hospital. Thorax, 36(8), 566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C, Taut K, Srivastava M, Langer F, Mack M, Briles DE, . . . Maus UA (2007). Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2-CCR2 axis. J Immunol, 178(9), 5828–5838. doi:178/9/5828 [pii] [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Nishimoto N, Matsumoto K, Tagoh H, Taga T, Deguchi Y, . . . et al. (1990). Interleukin 6 and expression of its receptor on epidermal keratinocytes. Cytokine, 2, 381–387. [DOI] [PubMed] [Google Scholar]
- Zeng X, Moore TA, Newstead MW, Deng JC, Kunkel SL, Luster AD, & Standiford TJ (2005). Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect Immun, 73(12), 8226–8236. doi:73/12/8226 [pii] 10.1128/IAI.73.12.8226-8236.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Swamy S, Balijepalli S, Panicker S, Mooliyil J, Sherman MA, . . . Suresh MV (2019). Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia. FASEB J, 33(12), 13294–13309. doi: 10.1096/fj.201901047RR [DOI] [PMC free article] [PubMed] [Google Scholar]


