Abstract
Periodontitis, a microbiome-driven inflammatory disease of the tooth-attachment apparatus, is epidemiologically linked with other disorders, including cardio-metabolic, cognitive neurodegenerative and autoimmune diseases, respiratory infections, and certain cancers. These associations may, in part, be causal, as suggested by interventional studies showing that local treatment of periodontitis reduces systemic inflammation and surrogate markers of comorbid diseases. The potential cause-and-effect connection between periodontitis and comorbidities is corroborated by studies in preclinical models of disease, which additionally provided mechanistic insights into these associations. This overview discusses recent advances in our understanding of the periodontitis-systemic disease connection, which may potentially lead to innovative therapeutic options to reduce the risk of periodontitis-linked comorbidities.
1. Introduction
Periodontitis is an exemplar of a microbe-driven chronic inflammatory disease that persists in susceptible individuals, in part due to reciprocally reinforced interactions between the dysbiotic microbiome and the host inflammatory response 1,2. In its severe form, periodontitis is the sixth most prevalent condition in the world and afflicts about 10% of the adult population 3,4. If untreated, periodontitis leads to progressive destruction of the tooth-attachment apparatus (gingiva, cementum, periodontal ligament and alveolar bone) and eventual tooth loss, while compromising mastication and esthetics and affecting the quality of life 5–9. Standard-of-care therapy (scaling and root planing often with adjunctive anti-microbial approaches) is not always effective, especially in highly susceptible patients, and thus periodontitis poses as a serious public health and socioeconomic problem 7,10–12. The combined direct and indirect costs (due to loss in productivity) of periodontal disease in the USA and Europe were estimated, respectively, at $154.06 billion and €158.64 billion 13.
Periodontitis is moreover linked epidemiologically with other disorders, including cardiovascular disease, type-2 diabetes, obesity, rheumatoid arthritis, osteoporosis, respiratory infections, inflammatory bowel disease, Alzheimer’s disease, nonalcoholic fatty liver disease, chronic kidney disease and certain cancers 14–18 (Figure 1). From a medical and therapeutic perspective, it is essential to understand whether the association of periodontitis with comorbid disorders is simply of correlative nature or whether arises also from causal interactions. In the latter regard, a possible mechanism contributing to the independent association of periodontitis and inflammatory comorbidities may involve periodontitis-associated low-grade systemic inflammation, which is a common denominator of many chronic conditions 14,19. Conversely, systemic diseases also affect periodontitis, for instance type-2 diabetes may aggravate periodontitis, in part, by augmenting the inflammatory burden on the periodontal tissues and by adversely affecting the composition of the periodontal microbiome 14,20,21.
Figure 1: Periodontitis and comorbidities.
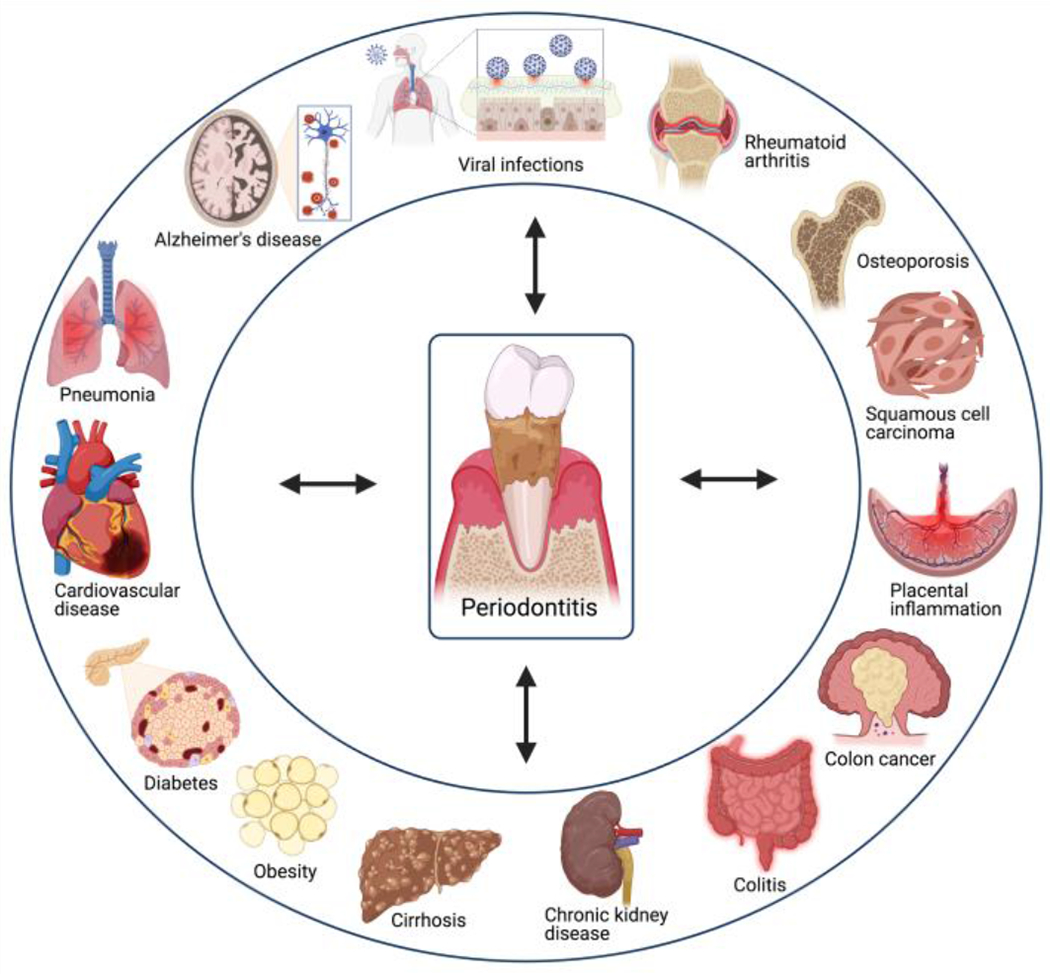
The indicated periodontitis-associated disorders are reviewed in this volume.
Periodontitis-associated systemic inflammation is thought to arise from hematogenous translocation of periodontal microorganisms or the spillover of inflammatory cytokines and other mediators from the periodontium to the circulation 17,22 (Figure 2). Although the oral microbiome does not comprise solely bacteria, the association of periodontitis with systemic disease has been primarily investigated in the context of the bacteriome, despite emerging evidence that viruses (and other types of microorganisms) may influence the host periodontal response as well as synergize with periodontal bacterial pathogens 23–26. It has also been suggested that locally activated lymphocytes (from the draining lymph nodes of the periodontium) may disseminate via the lymphatic circulation to extraoral tissues, where they could exacerbate tissue inflammation 27 (Figure 2). Periodontitis-associated systemic inflammation could additionally contribute to maladaptive rewiring of hematopoietic progenitors in the bone marrow, giving rise to increased production of mature myeloid cells with increased inflammatory responsiveness (a process known as ‘trained myelopoiesis’), hence potentially affecting multiple comorbidities 19,28 (Figure 2). Disseminated periodontal microbes may also have direct pathogenic effects in extra-oral tissues, thus becoming involved in lung infections, endothelial dysfunction, gut dysbiosis, and cancer-promoting functions 27,29–33. In the latter regard, certain types of cancer (e.g., colorectal cancer and oral/orodigestive squamous cell carcinoma) are increasingly appreciated as a comorbidity associated with specific periodontal pathogens.
Figure 2: Potential mechanisms connecting periodontitis to systemic inflammation.

For details on the indicated mechanisms (1-4) see text and relevant reviews in this volume.
These mechanistic insights, mostly derived from studies in preclinical models, imply that periodontitis is a modifiable risk factor for comorbidities, a notion that is supported by clinical interventional studies, which showed that treatment of periodontitis attenuates systemic inflammation and surrogate markers of comorbidities 34–36. Accordingly, emerging adjunctive host-modulation therapies, (e.g., complement-targeted intervention with efficacy in phase 2a trial in patients with periodontal inflammation 37,38), to improve periodontal treatment (beyond the level achieved by conventional approaches alone) acquire increased importance, as they could help reduce the risk of systemic comorbidities 39,40.
In this volume, clinical and basic scientists, with expertise in the pathogenesis of periodontitis and associated disorders, review in detail the association of periodontitis with extra-oral comorbidities and its implications for the overall health.
2. Cardiometabolic disorders and endotoxemia
Periodontitis is bidirectionally associated with cardiometabolic disorders. The translocation of bacterial lipopolysaccharide (LPS) into the blood circulation causes endotoxemia, which is associated with increased risk of cardiometabolic disorders. Although the major source of endotoxemia is thought to be the intestinal microbiota, the dysbiotic periodontal microbiota may also contribute to endotoxemia in patients with periodontitis, as discussed by Pussinen et al 41. The authors review the basic biology of LPS and its host receptor complex (Toll-like receptor 4 and co-receptors) as well as the concept of endotoxemia as a plausible molecular mediator between periodontitis and the elevated risk of cardiometabolic conditions. These include cardiovascular disease, obesity, insulin resistance, type 2 diabetes, non-alcoholic fatty liver disease, metabolic syndrome and dyslipidemia. Although these associations may largely involve the ability of LPS to induce systemic inflammation, LPS (and whole bacteria) in the circulation may also have direct effects on the vessel walls (e.g., endothelial dysfunction) and atherosclerotic lesions (e.g., contribution to the formation of fatty streaks and acceleration of plaque maturation and rupture). Moreover, endotoxemia affects metabolism, as evidenced by a dyslipidemic lipoprotein phenotype; specifically, endotoxemia is positively correlated with the concentration of triglycerides, cholesterol, and apolipoprotein B and is negatively correlated with high-density lipoprotein cholesterol concentration. Conversely, a high-fat diet can lead to increased levels of LPS in the circulation; indeed, such diets have been associated with increased intestinal permeability and metabolic endotoxemia. The authors conclude that metabolic endotoxemia may, in part, explain why an unhealthy diet may increase the risk of not only cardiometabolic disorders but also of other inflammatory diseases, including periodontitis. In other words, endotoxemia may be regarded as a mechanistic link between periodontal disease and cardiometabolic disorders.
3. Periodontitis-atherosclerosis connection and dendritic cells
Dendritic cells (DC) contribute to host immune surveillance and link innate immune signals to induction of T cell immunity. Key DC molecules involved in these interactions include C-type lectins and other pattern-recognition receptors that facilitate antigen recognition and uptake. Cutler and colleagues 42 review the basic biology and role of DCs in the pathogenesis of periodontitis and systemic disease, such as atherosclerosis, and how this cell type may contribute to the association of these comorbidities. The authors note that work in humans and preclinical models has shown that myeloid DCs are readily mobilized in both lymphoid and non-lymphoid tissues as well as in the bloodstream, in response to oral microbial challenge. Intriguingly, however, the keystone periodontal pathogen P. gingivalis can invade DCs in the periodontal tissue or in the peripheral blood by means of a distinct fimbrial adhesin that interacts with the C‑type lectin DC‑specific ICAM3-grabbing non-integrin (DC‑SIGN). As DCs are highly migratory cells, the ability of P. gingivalis to invade and survive within DCs may lead to the systemic dissemination of this pathogen including to atherosclerotic plaques. This notion is consistent with clinical observations that periodontitis-associated bacteremias elevate the P. gingivalis content of myeloid DCs in the blood as well as the frequency of the carrier myeloid DCs. Consistently, P. gingivalis has been immuno-colocalized with DCs in atheromatous plaques of patients with periodontitis patients. The microbial cargo of P. gingivalis-carrying myeloid DCs in the blood contains additional oral and non-oral species that may also contribute to systemic inflammation, in part by contributing to pathologic reprogramming of DCs, rendering them pro-inflammatory and pro-atherogenic. The authors suggest that tailored DC-derived exosomes (derived from tolerogenic DCs, i.e., enriched in anti-inflammatory molecules) may be a novel immunotherapeutic strategy to prevent or mitigate DC-mediated inflammatory responses, thereby promoting oral and systemic health.
4. Pneumonia
Pneumonia is a prevalent infectious disease caused by a variety of microbial (bacterial, viral, or fungal) pathogens that can infect the lungs. In a hospital setting, patients on a ventilator may develop ‘ventilator-associated pneumonia’ if microbes in the breathing tube get access to the patients’ lungs. Another type of hospital-associated pneumonia of emerging concern is non-ventilator hospital-acquired pneumonia (NV-HAP). Scannapieco and colleagues 43 review recent evidence that NV-HAP is becoming a leading cause of healthcare-associated infections despite being, at least in principle, a preventable infection. The authors moreover discuss multiple studies that link poor oral health with increased risk of NV-HAP. In this regard, the dental plaque biofilm is considered to be a reservoir for respiratory infections, as oral microbes are commonly isolated from pneumonia patients, most likely reaching the lungs via the oropharyngeal route. Consistent with this notion, periodontitis is epidemiologically associated with increased risk of pneumonia, at least in the elderly. Accumulating clinical evidence supports the importance of oral care as a means to prevent NV-HAP. The authors recommend that the control of the oral biofilm in susceptible populations can decrease the risk for NV-HAP by reducing the burden of potential respiratory pathogens in the salivary secretions that can be potentially aspirated.
5. Alzheimer’s disease
Clinical and microbial markers of periodontal disease have been associated with the incidence and mortality of Alzheimer’s disease. According to the review of the relevant literature by Eick and colleagues 44, this association might, at least in part, be causal and two general mechanisms have been proposed: Direct effects of oral microorganisms infiltrating the brain and the non-mutually exclusive mechanism that periodontitis may aggravate Alzheimer’s disease pathology by increasing systemic inflammation. The authors focus mainly on the first mechanism. Despite the overall evidence that suggests a connection between oral microbial dysbiosis – which is exacerbated in old age – and Alzheimer’s disease, the authors note inconsistencies among different studies. They propose the implementation of methodological consensus guidelines and the reporting of the storage conditions of postmortem brain samples. They also review mechanistic insights derived from animal and in vitro studies on microorganisms in the context of Alzheimer’s disease. Based on both human and animal model-based studies, it seems that a number of different oral bacterial species might be associated with Alzheimer’s disease, despite a predominant focus on P. gingivalis by most studies. The authors also discuss that the modification of the human and mouse gut microbiome by probiotics promotes cognitive health. By the same rationale, they argue, treatments that promote a symbiotic periodontal microbiota (periodontal therapy, probiotics, anti-inflammatory approaches, diets such as the Mediterranean) may also improve cognitive ability in the elderly, a notion that could be tested in future clinical trials.
6. Rheumatoid arthritis
Multiple clinical and epidemiological studies support a bidirectional association between rheumatoid arthritis (RA) and periodontitis. Although RA and periodontitis have different etiology (the former represents autoimmune and the latter dysbiotic inflammation), they appear to share pathophysiological features and genetic risk factors. However, as Koziel and Potempa show 45 in this volume, causal relationships may also link these two inflammatory bone loss disorders. This notion is supported by observations that treatment of RA exerts beneficial effects on the clinical outcome of periodontitis and, conversely, treatment of periodontitis can mitigate disease activity in RA. The authors also discuss mechanistic studies in mouse and rat models that are consistent with a causal relationship between periodontitis and RA. Indeed, pre-existing experimental periodontitis aggravates subsequent experimental arthritis. Moreover, experimental arthritis aggravates periodontitis, suggesting a bidirectional relationship between these two disorders. Furthermore, specific periodontal bacteria can potentially contribute to the formation of altered host epitopes and thus promote autoimmune reactions in rheumatoid arthritis-susceptible individuals. Aggregatibacter actinomycetemcomitans induces host protein citrullination in neutrophils by secreting the pore-forming toxin LtxA, which causes calcium influx and hyperactivation of peptidyl-arginine deiminase (PAD) enzymes, as well as cytolysis, leading to the release of the generated citrullinated autoantigens. P. gingivalis expresses a unique peptidyl-arginine deiminase (PPAD), which can directly citrullinate proteins including host proteins. Thus, both bacterial species may contribute to the generation of the anti-citrullinated protein antibodies (ACPAs) that are rheumatoid arthritis-specific and can promote arthritis in individuals with HLA-DRB1 shared epitope alleles. Antigen mimicry, owing to structural similarities between certain P. gingivalis proteins and host proteins, has also been postulated as a candidate link between periodontitis and development of RA. In conclusion, the authors have presented a strong case that the traditional viewpoint of the immunological processes underlying the pathogenesis of RA is rather limited and needs to integrate the role of bacteria as important environmental risk factors that can contribute to the autoimmune inflammatory reactions in RA.
7. Osteoporosis
Yu and Wang 46 discuss the association of periodontitis with another inflammation-driven bone loss disease, namely osteoporosis. Osteoporosis is an aging-associated bone disease hallmarking deterioration of bone mass, mineral density and architecture, thereby increasing the risk of bone fracture. The authors’ analysis of the relevant literature (clinical studies most of which involved postmenopausal women) indicate a correlation between systemic low bone mineral density (BMD) and alveolar bone loss. Moreover, there is currently modest evidence suggesting an association between systemic BMD and clinical attachment loss in periodontitis. The periodontitis-osteoporosis connection is, in great part, attributed to common risk factors, such as age-related systemic inflammation and oxidative stress, which is a major cause of cellular senescence. These processes contribute to the uncoupling of bone resorption and bone formation, in other words disrupt the balance between osteoclasts and osteoblasts and cause net loss of bone. Vitamin D deficiency and smoking, two other shared risk factors for periodontitis and osteoporosis, also promote net bone loss by adversely affecting the receptor activator of NF-κB ligand (RANKL)/osteoprotegerin (OPG) ratio in both systemic and alveolar bone. Moreover, the complement system plays a key role in the regulation of the overall host inflammatory response and studies in relevant preclinical models showed that complement C3 activation is required for the induction of both periodontal and osteoporotic bone loss. Thus, individuals in whom complement is dysregulated might be predisposed to increased susceptibility to both periodontitis and osteoporosis. The authors conclude that a better understanding of the factors and mechanisms underlying the connection of periodontitis and osteoporosis may lead to an interdisciplinary management of both bone loss disorders through the use of common therapeutics.
8. Chronic kidney disease
The prevalence of severe periodontitis is significantly increased in patients with chronic kidney disease (CKD). Although the two conditions share several risk factors, their relationship might involve additional causes. Parsegian et al 47 comprehensively review potential factors underlying this association, which include host, bacteriological, as well as environmental factors. For instance, CKD-associated uremia leads to increased gingival crevicular fluid levels of urea, thereby generating an alkaline pH environment that is conducive for the growth of periodontal pathogens including P. gingivalis. CKD has also been shown to dysregulate immune and inflammatory responses (e.g., impaired neutrophil recruitment to tissues), which may aggravate microbiome-driven inflammatory conditions such as periodontitis. CKD also exerts adverse effects on skeletal bone that may also affect the alveolar bone. Moreover, compared to non-renal controls, CKD patients have increased abundance of periodontal disease-associated taxa and reduced abundance in certain health-associated taxa. These dysbiotic changes might be the result of uremia, a notion that is consistent with a preclinical model study showing that uremia-associated dysbiosis contributes to increased periodontal bone loss. Specifically, transfer of oral microbiota from uremic mice resulted in increased periodontal bone loss in germ-free recipient mice than in germ-free mice receiving oral microbiota from healthy control mice. Interestingly, some studies indicate that non-surgical periodontal therapy leading to improved clinical periodontal parameters (clinical attachment loss and periodontal pocket depth) may also improve renal parameters (e.g., glomerular filtration rate). However, stronger evidence from randomized controlled trials is required before concluding that periodontitis affects CKD and that periodontal treatment can improve renal disease parameters. In this regard, the authors discuss the limitations regarding the interpretation of current periodontitis-CKD association studies and the effects of non-surgical periodontal therapy on the renal status of CKD patients. Parsegian et al also make recommendations on how future studies can be designed in a manner that can yield enhanced insight into the nature of the association between periodontitis and CKD. The authors conclude that, although periodontitis and CKD are complex conditions with common behavioral, social, and other confounding factors, the two conditions may also be independently connected, a notion that can be strengthened by future studies.
9. Chronic liver disease
Under certain conditions, oral pathogens may translocate and colonize the gastrointestinal tract where they may aggravate dysbiosis and inflammation. These ectopically colonized oral pathogens may also disseminate to the liver where they can exacerbate liver disease. Albuquerque-Souza and Sahingur 48 review this oral–gut–liver microbial and immune axis and its medical implications. If this axis is adequately understood, it could lead to novel insights into the pathogenesis of periodontitis and comorbid diseases, such as chronic liver disease including non-alcoholic fatty liver disease (NAFLD), which is a major focus of these authors’ review. NAFLD is a condition involving excessive fat accumulation (steatosis) in the liver in the absence of significant consumption of alcohol, and may be associated with obesity and metabolic syndrome. NAFLD may progress to nonalcoholic steatohepatitis (NASH), in which fat accumulation together with liver inflammation may result in fibrosis and eventually cirrhosis (advanced scarring/fibrosis), which may be accompanied by hepatocellular carcinoma. Recent epidemiological studies have suggested an association between periodontitis and NAFLD. Although these two diseases share numerous risk factors, a causal link is possible although not yet proven. In support of a direct association between periodontitis and chronic liver disease, periodontal treatment appears to change the composition of the gut microbiota of cirrhotic patients and to modulate their systemic immune response. Albuquerque-Souza and Sahingur also discuss potential mechanisms whereby periodontal pathogens may affect liver pathophysiology. The authors conclude that whereas available evidence (mostly based on preclinical models) supports a possible link between NAFLD and periodontitis, future studies are warranted to strengthen the association, determine if the association is bidirectional, and dissect further the biological mechanisms that link periodontitis and NAFLD.
10. Intestinal inflammation
Clinical observations together with studies in animal models have shown that the oral cavity is a reservoir of resident microbial species that can ectopically colonize the gut and contribute to or exacerbate intestinal pathologies. Although oral microbes cannot readily colonize a healthy gut, they become enriched in the gut microbiota of patients with inflammatory bowel disease, colon cancer, or liver cirrhosis. In general, factors that trigger gut dysbiosis (e.g., inflammation, antibiotics and unhealthy diets) promote the ability of oral pathobionts to colonize the gut. Kitamoto and Kamada 49 review recent mechanistic insights into the connection of periodontitis with intestinal inflammation from both a microbiological and an immunological viewpoint. Swallowed oral pathobionts can reach the gut through the oro-digestive tract and can promote colitis in susceptible hosts, in part, by interacting with local inflammatory macrophages that are thereby induced to secrete proinflammatory cytokines. Translocated oral pathobionts can also exacerbate gut inflammation by promoting gut dysbiosis and impairing intestinal barrier function. Intriguingly, moreover, pathogenic IL-17-secreting CD4+ T cells, which are primed in the oral cavity (e.g., as a result of periodontal disease), can transmigrate through the lymphatic circulation to the gut, where they are reactivated by ectopic oral pathobionts (upon their processing by antigen-presenting cells). In this manner, transmigrated T cells of oral origin can aggravate colitic inflammation. The authors conclude that the recent mechanistic insights into the pathological oral-gut axis can offer new therapeutic options for treating related comorbidities and discuss several potential approaches.
11. Cancer
Two reviews deal with the association of different forms of cancer with specific periodontal pathogens, the first one involving P. gingivalis and the other with F. nucleatum.
11.1. Porphyromonas gingivalis and orodigestive squamous cell carcinoma
In contrast to the long-established potential of viruses as carcinogenic agents, interest in the role of bacteria in the etiology of cancer is relatively recent, having gained momentum after the association of Helicobacter pylori with gastric cancer in the 1990s. Lamont and colleagues 50 review emerging evidence for an association between periodontal pathogens, such as P. gingivalis, and oral/orodigestive squamous cell carcinoma. Although the discussed studies are correlative and causality remains to be addressed, the authors describe potential carcinogenic mechanisms, established in in vitro models, whereby P. gingivalis may contribute to carcinogenesis. These include promotion of host-cell proliferation and resistance to apoptosis, reduced susceptibility of cancerous cells to chemotherapeutic agents, generation of a dysbiotic inflammatory microenvironment conducive for tumor growth, promotion of angiogenesis and metastasis. The carcinogenic potential of P. gingivalis is furthermore supported by in vivo investigations in relevant animal models and is enhanced in consortia with additional periodontal pathogens, such as Fusobacterium nucleatum. The authors conclude that, whereas large longitudinal and intervention studies are required to establish a causative role for P. gingivalis (and other pathogens) in oral cancer, the current knowledge may pave the way to the discovery of novel targets for early diagnosis.
11.2. Fusobacterium nucleatum role in colorectal and breast cancer
Metagenomic-epidemiological studies and experimental evidence from animal models have linked F. nucleatum with the progression of certain tumor types, including colorectal cancer (CRC) and breast cancer. Bachrach and colleagues 51 initially review the mechanism underlying tumor-specific colonization (tumor tropism) by F. nucleatum, which appears to translocate to colorectal tumors via the hematogenous route, although the contribution of the orodigestive route cannot be formally ruled out. The tropism of F. nucleatum for CRC cells is dependent on its Fap2 lectin, which has affinity for a carbohydrate moiety (Gal-GalNAc) that is overexpressed in CRC as well as in different adenocarcinomas, e.g., of the esophagus, pancreas, prostate, ovary, and breast. Evidence that F. nucleatum is enriched in the breast cancer microbiome further supports the notion that this organism can access tumors via the hematogenous route. Moreover, the authors examine the mechanisms whereby this oral pathobiont contributes to tumor exacerbation. Specifically, they discuss the ability of F. nucleatum to (i) enhance tumor cell proliferation and metastasis; (ii) induce a tumor-permissive immune microenvironment; (iii) inhibit the recruitment of tumor-infiltrating lymphocytes; (iv) promote chemoresistance (at least in CRC); and (v) activate immune checkpoints, thereby suppressing the anti-tumor activity of T cells and natural killer cells. The latter mechanism also involves the participation of the Fap2 lectin which activates the inhibitory immunoreceptor TIGIT (T cell immunoreceptor with immunoglobulin and ITIM domains). The authors conclude that the elucidation of the mechanisms that mediate fusobacterial tumor tropism and tumor progression may facilitate innovative approaches for treating tumors associated with this oral pathobiont. Moreover, on the basis of the overabundance of F. nucleatum in certain tumors, the authors support the notion that this oral pathobiont is a potential diagnostic biomarker at least for CRC.
12. Adverse Pregnancy outcomes
Multiple epidemiological studies indicate an association between periodontitis and adverse pregnancy outcomes, such as preterm birth, low birthweight, miscarriage, preeclampsia, intrauterine growth retardation, neonatal sepsis, and stillbirth. Xu and Han 52 review and discuss the epidemiological evidence as well as mechanistic and interventional/therapeutic studies that could suggest a causal relationship between periodontitis and pregnancy complications. The authors cite a recent case-control study that showed that the prevalence of preterm delivery increased with increasing severity of gingivitis or periodontitis, thus enhancing the plausibility of causality. A cause-and-effect relationship is supported by studies in preclinical models, most of which involved investigation of the effects of F. nucleatum in pregnant mice. Two major mechanisms have been proposed: (i) Direct effects of disseminated oral microorganisms (or their products) in the fetal-placenta unit and (ii) periodontitis-associated systemic inflammation affecting the fetal-placenta unit. With regard to the first mechanism, several oral bacteria, including Bergeyella spp., F. nucleatum, P. gingivalis and A. actinomycetemcomitans, were identified in the amniotic fluid, cord blood and/or placenta in cases with preterm birth, preeclampsia, neonatal sepsis or stillbirth. Regarding the second and non-mutually exclusive mechanism, patients with severe periodontitis have elevated concentrations of pro-inflammatory molecules (e.g., IL-1, IL-6, C-reactive protein and fibrinogen) in the blood; these can presumably stimulate the production in the fetal-placenta unit of inflammatory mediators, such as prostaglandins, thereby causing intrauterine inflammation. However, interventional studies aimed to determine whether periodontal treatment during pregnancy can mitigate the risk for adverse pregnancy outcomes have yielded inconsistent results. Studies in preclinical models have suggested that maternal supplementation of omega-3 fatty acids may protect the fetuses by restraining inflammation; this approach might also be effective in human periodontitis-associated pregnancy complications, given the results of clinical trials that the adjunctive use of omega-3 fatty acids reduces clinical attachment loss and probing depth. The authors conclude that, whereas epidemiological studies unequivocally indicate that periodontal disease is positively correlated with adverse pregnancy outcomes, further studies are warranted to: i) dissect the precise molecular mechanisms involved; and ii) to design more appropriate intervention trials to test for a causality between periodontitis and adverse pregnancy outcomes, ultimately leading to treatments that promote oral health and reduce the risk of pregnancy complications.
13. Viruses and the oral cavity
Two reviews deal with the potential role of viruses in periodontitis and associated comorbidities, focusing predominantly on herpesviruses and the novel coronavirus, SARS-CoV-2, the cause of COVID-19.
13.1. Viruses, periodontitis, and comorbidities
Besides bacteria, the oral mucosal tissues harbor also other microorganisms, including archaea, fungi, and viruses; however, the connection of periodontitis with systemic disease is predominantly studied in the context of the bacteriome. Teles and colleagues 53 review the literature and explore the biologically plausible hypothesis that viruses may have an analogous role in the connection between periodontal diseases and systemic comorbidities. First, they discuss the connection of viruses with periodontitis. Although a number of studies have shown a correlation between oral viral infection (e.g., herpes herpesviruses) and periodontitis, it is uncertain whether this association is causal (viruses directly contributing to periodontal disease pathogenesis) or consequential, i.e., viral replication and thus detection results from the ability of certain inflammatory mediators to support viral replication. In either case, however, an active viral infection may exacerbate periodontitis through several plausible mechanisms: Viruses may potentially promote the pathogenicity of bacteria, may have direct cytopathic effects on stromal cells of the periodontium, and can also either enhance inflammation or suppress immune responses, both of which can disrupt periodontal tissue homeostasis. The authors suggest that longitudinal and interventional clinical studies are warranted to conclusively determine whether viruses indeed contribute to the pathogenesis of periodontitis. Viruses of the oral mucosal tissues may, at least in principle, contribute to diseases in extra-oral sites. In this regard, the oral cavity is a site of transmission of viruses, either through the saliva (e.g., transmission of Epstein–Barr virus, cytomegalovirus, and herpes simplex virus) or through the blood circulation, where viruses can gain access and contribute to systemic disorders. In this context, viruses found in periodontitis, such as cytomegalovirus, herpes simplex virus, type-2, and Epstein-Barr virus, have been associated with cardiovascular disease and pre-eclampsia. The periodontium may also be a reservoir for two recently described viruses, the novel coronavirus SARS-CoV-2 and the small circular DNA virus redondovirus. Observations that the oral cavity is a site of SARS-CoV-2 infection and likely a reservoir and site of transmission for the virus, even more so in the presence of periodontitis, might in part explain the recent association of periodontitis with COVID-19. The newly established association of redondovirus sequences with both periodontitis and critical respiratory illness (increased levels levels of redondoviruses in oropharyngeal samples of critically ill patients and in the lungs of intubated patients), suggests that this virus might be implicated in oropharyngeal aspiration pneumonia. The authors conclude that open-ended approaches (metagenomics and metatranscriptomics) can be used to better study the relationship of viruses within the overall microbiome (bacterial, viral and fungal communities). The ultimate goal would be to develop strategies for diagnosis and treatment of systemic diseases in which periodontal viruses play a contributing role.
13.2. COVID-19
The coronavirus disease 2019 (COVID-19), a viral pandemic infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is associated with an overexuberant inflammatory response and can potentially affect multiple organs in susceptible individuals. COVID-19 and periodontitis share several risk factors, such as, diabetes, hypertension, and obesity among others. However, even after adjusting for potential confounders, periodontitis still appears to be associated with COVID-19; in particular, a recent case-control study of 568 participants showed that periodontitis is significantly associated with increased risk of intensive care unit admission, necessity of assisted ventilation, and death of patients with COVID-19. Tamimi and colleagues 54 discuss this study and review possible factors that may explain the association of periodontal disease and COVID-19. Among factors that could, at least in principle, predispose to increased susceptibility to COVID-19 are alterations in the inflammatory responsiveness of patients with periodontitis. In other words, a chronic inflammatory condition, such as periodontitis, could aggravate the course of COVID-19. In this regard, periodontitis is associated with systemic inflammation and immunometabolic alterations (in systemic tissues including the bone marrow), which in turn could prime the immune system to an exaggerated inflammatory response following COVID-19 infection in susceptible patients. Clinical observations also indicate that the oral cavity may be an important site for SARS-CoV-2 infection. If the oral mucosa, including the periodontal pockets, is a reservoir of SARS-CoV-2, this could provide partial explanation for the periodontitis-COVID-19 connection. Additional future studies are warranted to confirm and strengthen the currently limited evidence for a direct periodontitis–COVID-19 connection. More research is also required to dissect the exact mechanisms underlying this association. From a medical and therapeutic viewpoint, it is important to discover whether the link between periodontitis and COVID-19 is simply a correlative one or due to causal mechanisms that can be controlled, thus helping reduce the risk of COVID-19 complications.
14. Bone marrow and periodontitis-associated comorbidities
Oral health is reciprocally related to systemic health and, not surprisingly, a bidirectional relationship often exists between periodontitis and linked comorbidities. Periodontitis and other chronic inflammatory diseases are moreover associated with aging-related elevation of systemic inflammation, known as ‘inflamm-aging’, and their severity and prevalence increases with advanced age. However, little has been achieved in our understanding of reciprocal causal relationships between periodontitis and comorbidities and why the susceptibility to these disorders increases with aging. Hajishengallis and colleagues 55 review the literature on two recently emerged concepts, trained innate immunity (TII) and clonal hematopoiesis of indeterminate potential (CHIP) that could offer novel insights into these questions. Given that chronic diseases are largely driven by the action of inflammatory immune cells, pro-proliferative and pro-inflammatory alterations to their precursors in the bone marrow, i.e., the hematopoietic stem and progenitor cells (HSPCs), may affect multiple disorders that emerge as comorbidities. Alterations to HSPCs that can give rise to myeloid progeny cells with increased inflammatory capacity may result from two non-mutually exclusive phenomena; (i) TII, an epigenetically based memory state of enhanced immune responsiveness to future challenges and (ii) CHIP, the age-related acquisition of somatic mutations that confer clonal expansion advantage to affected HSPCs and heightened inflammatory activity to their mutant myeloid progeny. The authors review the relevant literature which suggests that (i) maladaptive TII may causally link periodontitis and comorbidities, whereas CHIP, the prevalence of which increases with aging, can aggravate the severity of these disorders. However, more evidence is required to establish the underlying inflammatory axis between the bone marrow and peripheral tissue inflammation. Such mechanistic insights into the comorbid connection of periodontitis and systemic diseases may pave the way to novel diagnostic and therapeutic targets for their holistic treatment.
15. Conclusion and outlook
The epidemiological, clinical interventional and animal model-based studies discussed in the various chapters of this volume collectively indicate that the association of periodontitis and linked comorbidities is quite complex, involving both common risk factors and pathophysiology as well as bidirectional causal relationships (independent of known confounding factors). Despite the enormous progress made in the field 17,34,35,56–63, unequivocal evidence that effective treatment of periodontitis can ameliorate the risk or incidence of epidemiologically-lined comorbidities conditions is not currently available. In this regard, multi-center randomized controlled clinical trials are required to implicate periodontitis as a modifiable risk factor for linked comorbidities. Further improvement of local periodontal treatment via innovative adjunctive host-modulation approaches 39,40, such as by modulating complement with the C3-targeted drug AMY-101, which showed efficacy in a recent phase 2a trial in patients with periodontal inflammation 37,38, may greatly contribute to prevent systemic inflammation and promote overall health.
Achieving a holistic and mechanistic understanding of periodontitis-associated comorbidities may lead to new therapeutic options for the treatment of periodontitis and associated comorbidities. Some of these novel approaches may be ‘central’ rather than ‘local’, for instance the targeting of maladaptive training of hematopoietic progenitors in the bone marrow as a central hub linking distinct comorbidities. It is therefore important to accelerate the transfer of research findings from basic and clinical studies into routine clinical practice.
Acknowledgements
The author’s research is supported by grants from the U.S. National Institutes of Health (DE024153, DE029436, DE026152, DE028561 and DE031206). The figure was created using Biorender.com.
References
- 1.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams DW, Greenwell-Wild T, Brenchley L, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021;184(15):4090–4104.e4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol. 2017;44 (S18):S94–S105. [DOI] [PubMed] [Google Scholar]
- 4.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brauchle F, Noack M, Reich E. Impact of periodontal disease and periodontal therapy on oral health-related quality of life. Int Dent J. 2013;63(6):306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapple IL. Time to take periodontitis seriously. BMJ. 2014;348:g2645. [DOI] [PubMed] [Google Scholar]
- 7.Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. The Lancet. 2019;394(10194):249–260. [DOI] [PubMed] [Google Scholar]
- 8.Saito A, Hosaka Y, Kikuchi M, et al. Effect of initial periodontal therapy on oral health-related quality of life in patients with periodontitis in Japan. J Periodontol. 2010;81(7):1001–1009. [DOI] [PubMed] [Google Scholar]
- 9.Collaborators GDaIIaP. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonetti MS, Chapple IL, Working Group 3 of Seventh European Workshop on P. Biological approaches to the development of novel periodontal therapies--consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38 Suppl 11:114–118. [DOI] [PubMed] [Google Scholar]
- 11.Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol. 2017;44(5):456–462. [DOI] [PubMed] [Google Scholar]
- 12.Righolt AJ, Jevdjevic M, Marcenes W, Listl S. Global-, Regional-, and Country-Level Economic Impacts of Dental Diseases in 2015. J Dent Res. 2018;97(5):501–507. [DOI] [PubMed] [Google Scholar]
- 13.Botelho J, Machado V, Leira Y, Proença L, Chambrone L, Mendes JJ. Economic burden of periodontitis in the United States of America and Europe – an updated estimation. J Periodontol. 2021;Online ahead of print: doi: 10.1111/jper.10813. [DOI] [PubMed] [Google Scholar]
- 14.Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontol 2000. 2020;83(1):7–13. [DOI] [PubMed] [Google Scholar]
- 15.Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(10):606–620. [DOI] [PubMed] [Google Scholar]
- 16.Acharya C, Sahingur SE, Bajaj JS. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 2017;2(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. 2020;83(1):90–106. [DOI] [PubMed] [Google Scholar]
- 18.Hajishengallis G Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21 Online ahead of print: doi: 10.1038/s41577-41020-00488-41576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao E, Mattos M, Vieira GHA, et al. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe. 2017;22(1):120–128 e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teles F, Wang Y, Hajishengallis G, Hasturk H, Marchesan JT. Impact of systemic factors in shaping the periodontal microbiome. Periodontol 2000. 2021;85(1):126–160. [DOI] [PubMed] [Google Scholar]
- 22.Teles R, Wang C-Y. Mechanisms involved in the association between peridontal diseases and cardiovascular disease. Oral Diseases. 2011;17(5):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Feng P, Slots J. Herpesvirus-bacteria synergistic interaction in periodontitis. Periodontol 2000. 2020;82(1):42–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slots J Life-threatening pathogens in severe/progressive periodontitis: Focal infection risk, future periodontal practice, role of the Periodontology 2000. Periodontol 2000. 2020;84(1):215–216. [DOI] [PubMed] [Google Scholar]
- 25.Taylor LJ, Dothard MI, Rubel MA, et al. Redondovirus diversity and evolution on global, individual, and molecular scales. J Virol. 2021:JVI0081721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz PI. Subgingival fungi, Archaea, and viruses under the omics loupe. Periodontol 2000. 2021;85(1):82–89. [DOI] [PubMed] [Google Scholar]
- 27.Kitamoto S, Nagao-Kitamoto H, Jiao Y, et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell. 2020;182(2):447–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavakis T, Mitroulis I, Hajishengallis G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat Immunol. 2019;20(7):802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrugia C, Stafford GP, Potempa J, et al. Mechanisms of vascular damage by systemic dissemination of the oral pathogen Porphyromonas gingivalis. FEBS J. 2020;Epub ahead of print: doi.org/10.1111/febs.15486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arimatsu K, Yamada H, Miyazawa H, et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4:4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mammen MJ, Scannapieco FA, Sethi S. Oral-lung microbiome interactions in lung diseases. Periodontol 2000. 2020;83(1):234–241. [DOI] [PubMed] [Google Scholar]
- 34.D’Aiuto F, Gkranias N, Bhowruth D, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018;6(12):954–965. [DOI] [PubMed] [Google Scholar]
- 35.Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9):911–920. [DOI] [PubMed] [Google Scholar]
- 36.Kaneoka A, Pisegna JM, Miloro KV, et al. Prevention of Healthcare-Associated Pneumonia with Oral Care in Individuals Without Mechanical Ventilation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Infect Control Hosp Epidemiol. 2015;36(8):899–906. [DOI] [PubMed] [Google Scholar]
- 37.Hajishengallis G, Hasturk H, Lambris JD, Contributing a. C3-targeted therapy in periodontal disease: moving closer to the clinic. Trends Immunol. 2021;42(10):856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasturk H, Hajishengallis G, Lambris JD, Mastellos DC, Yancopoulou D. Phase 2a clinical trial of complement C3 inhibitor AMY-101 in adults with periodontal inflammation. J Clin Invest. 2021;Online ahead of print. doi: 10.1172/JCI152973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balta MG, Papathanasiou E, Blix IJ, Van Dyke TE. Host Modulation and Treatment of Periodontal Disease. J Dent Res. 2021;100 Online ahead of print: doi: 10.1177/0022034521995157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajishengallis G, Chavakis T, Lambris JD. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol 2000. 2020;84(1):14–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pussinen PJ, Kopra E, Pietiäinen M, et al. Periodontitis and cardiometabolic disorders: the role of lipopolysaccharide and endotoxemia. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Awady AR, Elashiry M, Morandini AC, Meghil M, Cutler CW. Dendritic cells: Critical Link to Alveolar Bone loss and Systemic Disease Risk in Periodontitis. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scannapieco FA, Giuliano KK, Baker D. Oral Health Status and the Etiology and Prevention of Non-ventilator - Hospital Associated Pneumonia. Periodontol 2000. 2022;In press. [DOI] [PubMed] [Google Scholar]
- 44.Jungbauer G, Stähli A, Zhu X, Alberi LA, Anton Sculean A, Sigrun Eick S. Periodontal microorganisms and Alzheimer’s disease – a causative relationship? Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koziel J, Potempa J. Pros and Cons of Causative Association Between Periodontitis and Rheumatoid Arthritis. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu B, Wang C-Y. Osteoporosis and Periodontal Diseases – An Update on Their Association and Mechanistic Links. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parsegian K, Randall D, Curtis M, Ioannidou E. Association between periodontitis and chronic kidney disease. Periodontol 2000. 2022;In press. [DOI] [PubMed] [Google Scholar]
- 48.Albuquerque-Souza E, Sahingur SE. Periodontitis, Chronic Liver Diseases and the Emerging Oral-Gut-Liver Axis. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitamoto S, Kamada N. Periodontal connection with intestinal inflammation: Microbiological and immunological mechanisms. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamont RJ, Fitzsimonds Z, Wang H, Gao S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alon-Maimon T, Mandelboim O, Bachrach G. Fusobacterium nucleatum and cancer. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu B, Han YW. Oral Bacteria, Oral Health and Adverse Pregnancy Outcomes. Periodontol 2000. 2022;In press. [DOI] [PubMed] [Google Scholar]
- 53.Teles T, Collman RG, Mominkhan D, Wang Y. Viruses, periodontitis, and comorbidities. Periodontol 2000. 2022;In press. [DOI] [PubMed] [Google Scholar]
- 54.Tamimi F, Altighania S, Sanz M. Periodontitis and COVID-19. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hajishengallis G, Li X, Divaris K, Chavakis T. Maladaptive trained immunity and clonal hematopoiesis as potential mechanistic links between periodontitis and inflammatory comorbidities. Periodontol 2000. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desvarieux M, Demmer RT, Jacobs DR, Papapanou PN, Sacco RL, Rundek T. Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology study. J Am Heart Assoc. 2013;2(6):e000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Clin Periodontol. 2013;40 Suppl 14:S85–105. [DOI] [PubMed] [Google Scholar]
- 58.Bokhari SA H, Khan AA, Butt AK, et al. Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J Clin Periodontol. 2012;39(11):1065–1074. [DOI] [PubMed] [Google Scholar]
- 59.Tonetti MS. Periodontitis and risk for atherosclerosis: an update on intervention trials. J Clin Periodontol. 2009;36 Suppl 10:15–19. [DOI] [PubMed] [Google Scholar]
- 60.Türer ÇC, Durmuş D, Balli U, Güven B. Effect of Non-Surgical Periodontal Treatment on Gingival Crevicular Fluid and Serum Endocan, Vascular Endothelial Growth Factor-A, and Tumor Necrosis Factor-Alpha Levels. J Periodontol. 2017;88(5):493–501. [DOI] [PubMed] [Google Scholar]
- 61.Bajaj JS, Matin P, White MB, et al. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2018;315(5):G824–G837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD. Control of Periodontal Infection Reduces the Severity of Active Rheumatoid Arthritis. J Clin Rheumatol 2007;13(3):134–137. [DOI] [PubMed] [Google Scholar]
- 63.Khare N, Vanza B, Sagar D, Saurav K, Chauhan R, Mishra S. Nonsurgical Periodontal Therapy decreases the Severity of Rheumatoid Arthritis: A Case-control Study. J Contemp Dent Pract. 2016;17(6):484–488. [DOI] [PubMed] [Google Scholar]


