Abstract
Asian Americans (AsA), Native Hawaiians, and Pacific Islanders (NHPI) comprise 7.7% of the U.S. population, and AsA have had the fastest growth rate since 2010. Yet the National Institutes of Health (NIH) has invested only 0.17% of its budget on AsA and NHPI research between 1992 and 2018. More than 40 ethnic subgroups are included within AsA and NHPI (with no majority subpopulation), which are highly diverse culturally, demographically, linguistically, and socioeconomically. However, data for these groups are often aggregated, masking critical health disparities and their drivers. To address these issues, in March 2021, the National Heart, Lung, and Blood Institute, in partnership with 8 other NIH institutes, convened a multidisciplinary workshop to review current research, knowledge gaps, opportunities, barriers, and approaches for prevention research for AsA and NHPI populations. The workshop covered 5 domains: 1) sociocultural, environmental, psychological health, and lifestyle dimensions; 2) metabolic disorders; 3) cardiovascular and lung diseases; 4) cancer; and 5) cognitive function and healthy aging. Two recurring themes emerged: Very limited data on the epidemiology, risk factors, and outcomes for most conditions are available, and most existing data are not disaggregated by subgroup, masking variation in risk factors, disease occurrence, and trajectories. Leveraging the vast phenotypic differences among AsA and NHPI groups was identified as a key opportunity to yield novel clues into etiologic and prognostic factors to inform prevention efforts and intervention strategies. Promising approaches for future research include developing collaborations with community partners, investing in infrastructure support for cohort studies, enhancing existing data sources to enable data disaggregation, and incorporating novel technology for objective measurement. Research on AsA and NHPI subgroups is urgently needed to eliminate disparities and promote health equity in these populations.
In 2020, Asian Americans (AsA), Native Hawaiians, and Pacific Islanders (NHPI) comprised 7.7% of the total U.S. population (23.9 million AsA and 1.6 million NHPI) (1). Asian Americans had the fastest growth (38.6%) since 2010 (1) and are projected to surpass 46 million by 2060 (2). These numbers encompass tremendous heterogeneity among the 40 AsA and NHPI ethnic subgroups with respect to indigeneity, nativity or ancestry, culture, immigration patterns, acculturation, educational attainment, income, language, and English proficiency, all of which influence health, health care access, and outcomes (3). However, in most national surveys, health systems data, and studies, these groups are often aggregated, rendering many groups invisible, masking important social and health differences, and skewing health policy and allocation of resources (4). Several novel clues to disease etiology and phenotypic presentations reside within these vastly heterogeneous populations, which would advance disease prevention and clinical care approaches in AsA and NHPI and beyond. The underrepresentation of these populations in health research both undermines health equity and limits important scientific discoveries.
Currently, only 2% of nearly 245 000 participants in the 14 largest National Heart, Lung, and Blood Institute (NHLBI)-supported cohorts have AsA or NHPI ancestry. A review of clinical research funded by the National Institutes of Health (NIH) between 1992 and 2018 found that studies focusing on AsA and NHPI comprised only 0.17% of the total NIH budget over those 26 years (5).
To eliminate health disparities, achieve health equity, and identify untapped scientific potential through the inclusion of AsA and NHPI in future research, the NHLBI, in partnership with 8 other NIH institutes, convened a multidisciplinary workshop, “Identifying Research Opportunities for Asian American, Native Hawaiian, and Pacific Islander Health.” In this paper, we summarize the findings, and synthesize the gap and opportunity areas identified from the workshop to guide future directions of health and prevention research on AsA and NHPI.
Methods
The 3-day workshop (31 March to 1 April 2021) assembled leading experts from multiple disciplines with the following objectives: 1) review the current research in AsA and NHPI populations for common chronic conditions and their risk factors, morbidity, and mortality; 2) uncover knowledge gaps; 3) identify research opportunities; 4) highlight challenges related to conducting health research across and within these groups; and 5) brainstorm effective approaches to engage AsA and NHPI communities in partnership to achieve health equity. Workshop participants are listed in the Appendix (available at Annals.org).
The workshop included 5 major domains that represented common chronic conditions and risk factors among AsA and NHPI populations and priority areas for the 9 participating NIH institutes. These domains included 1) sociocultural, environmental, psychological health, and lifestyle dimensions; 2) metabolic disorders; 3) cardiovascular and lung diseases; 4) cancer; and 5) cognitive function and healthy aging.
For a thorough landscape survey of these 5 domains and to facilitate in-depth discussions, the workshop included 22 presentations with 4 moderated discussion sessions, 2 panel discussions on effective approaches for community engagement and partnerships necessary for the success of longitudinal cohort studies, and a final session devoted to discussing research opportunities.
For the first 2 days, invited experts summarized the current research in AsA and NHPI populations and provided insights on the gaps, opportunities, and barriers. Moderators for each domain facilitated a discussion of the expert panel and summarized key research findings and opportunities. The final day of the workshop centered on brainstorming effective strategies to engage AsA and NHPI community partners for collaboration to advance health research among AsA and NHPI subgroups. A panel of experts on community-engaged research and another panel with longitudinal cohort experience discussed unique challenges and successes related to study design, recruitment, and retention of AsA and NHPI participants. A final moderated session included discussions aimed at synthesizing key opportunities and approaches needed to advance health research for each domain area.
Results
Current Knowledge and Gaps
The Figure shows a heat map matrix depicting the prevalence, incidence, or mortality from chronic health conditions for aggregated and, if available, disaggregated AsA and NHPI groups in comparison to the U.S. White population. Many conditions are more common among AsA and NHPI than in the White population, or data are insufficient. Table 1 summarizes the current health research and corresponding gaps by domain for AsA and NHPI populations. Cross-cutting themes across all domains included: 1) scarce data on prevalence, incidence, risk factors, and outcomes; 2) existing data are not sufficiently disaggregated; and 3) heterogeneity of risk factor prevalence and disease incidence among the diverse AsA and NHPI subgroups. These findings underscore the importance of studying subgroups separately to yield specific and unique insights needed to develop effective and targeted prevention strategies for these groups.
Figure. Heatmap matrix of chronic health conditions in AsA and NHPI compared with White U.S. adults, 2021 NIH Workshop.
Numbers in parentheses are references. AsA = Asian American; AVFA = abdominal visceral fat area; CHD = coronary heart disease; CKD = chronic kidney disease; HCC = hepatocellular carcinoma; HCVA = hemorrhagic cerebrovascular accident; HTN = hypertension; ICVA = ischemic cerebrovascular accident; NAFLD = nonalcoholic fatty liver disease; T2DM = type 2 diabetes mellitus.
* Data from U.S. Census 2020 for each race category alone and in combination with another race group for AsA and NHPI (1); ACS 2019 data are used for estimates of AsA subpopulations (3).
† Prevalence by body mass index criteria.
Table 1.
Current Knowledge and Gaps in Health and Prevention Research Among AsA and NHPI, 2021 NIH Workshop
| Domain | What We Know (Current Knowledge) | What We Need to Know (Gaps) |
|---|---|---|
| Sociocultural, environmental, and psychological health, and lifestyle dimensions | Need longitudinal studies of lifestyle dimensions and mental health effects on health outcomes, focusing on changes over time by immigration and generational status. | |
| Sociocultural influences | AsA and NHPI experience interpersonal and structural discrimination based on race, religion, and color, and it is detrimental to health (79-82). Acculturation is associated with health; this relationship is modified by health outcome, gender, and SES (15, 83). Differences in social network size, composition, and social support exist across AsA and NHPI subgroups (84, 85). Social network size and network behavior are associated with lifestyle factors and CVD (12-14, 86). |
How do immigration patterns, acculturation, discrimination, social networks, and kinship structures intersect and affect health? What is the influence of multiple forms of discrimination (e.g., structural, interpersonal, internalized) on health outcomes? Do acculturation level or patterns differ by ethnic subgroup and gender, and what factors determine these patterns? |
| Environment | Environmental exposure (e.g., air pollution), neighborhood, occupational, and sociocultural context are linked to health behaviors and outcomes (24, 87-89). Ethnic enclaves correlate with health behaviors, including smoking, alcohol use, walking, and diet (90-92). Neighborhoods with a higher proportion of Chinese and Korean Americans have the greatest risk for higher outdoor carcinogenic hazardous pollution, followed by South Asian Americans (92), compared with White neighborhoods. High proportion of the NHPI are exposed to toxic waste (industrial and freeway pollution) (93) than other race/ethnic groups. |
How do neighborhood and other environmental factors affect health behaviors and outcomes? Do ethnic enclaves provide social support or other resilience resources to mitigate poor health outcomes? |
| Psychological health | Mental health disorders are underdiagnosed in all AsA and NHPI subgroups (9). AsA and NHPI have lower admission and utilization rate of mental health services (7). There are large differences in the prevalence of depression, anxiety, stress, and loneliness across AsA and NHPI subgroups (10). Population heterogeneity, cultural tradition, and stigmatization are linked to the prevalence and diagnosis of these mental conditions (11). |
Need culturally appropriate and validated mental health assessment tools for AsA and NHPI. How does mental health change over time and influence other health outcomes? |
| Tobacco | There are substantial differences in adult cigarette smoking within AsA and NHPI populations; Korean, Vietnamese, Filipino, and NHPI men have higher prevalence than White men (33, 76, 94, 95). Women generally have lower smoking rates, but some English-proficient AsA women have similar smoking prevalence to White women (94, 95). E-cigarette use is higher among Filipino and Korean Americans, particularly among younger populations (96). |
Does tobacco use vary by English proficiency status and associate with health outcomes in different subgroups? |
| Diet | AsA and NHPI have diverse diets; they undergo dietary transitions after immigration (18, 97, 98), with an increased Western diet. Diet quality is decreasing over time with longer length of residence in United States (19, 99). |
Need culturally appropriate and validated dietary assessments tools for AsA and NHPI. Does diet influence health outcomes by subgroup? |
| Physical activity | Physical activity is decreasing over time, leading to higher rates of obesity and cardiometabolic risk (100). Relative to White Americans, AsA and NHPI have lower physical activity levels (17). Individual, environmental, and interpersonal factors contribute to low level of physical activity (17). |
Does physical activity affect other risk factors and health outcomes in different subgroups? |
| Sleep | Sleep disturbance are associated with adverse mental and physical health outcomes, including depression, obesity, hypertension, and CVD (20-22). AsA and NHPI are less likely than the White population to experience the recommended sleep duration (23-25). AsA are more likely to have a craniofacial profile prone to develop obstructive sleep apnea, which can result in higher CVD (101). |
Does sleep and sleep apnea affect health outcomes in different subgroups? Need objective assessments of multidimensional sleep (e.g., sleep duration, quality, efficiency) and sleep structure (light and deep sleep and REM sleep) on health outcomes by ethnic subgroup. |
| Metabolic disorders | Why do AsA have higher ectopic fat than other race/ethnic groups, and how is ectopic fat linked to several health outcomes? | |
| Body composition | Body composition (fat distribution and muscle mass) measured by anthropometry and imaging have shown distinct patterns among AsA and NHPI (27, 28, 102). AsA and NHPI have higher amounts of body fat at lower BMI (by 3-4 kg/m2) (28, 103, 104). AsA and NHPI have higher amounts of abdominal visceral fat and hepatic fat as measured by imaging studies (27, 28, 102). Ectopic fat measures have stronger correlations with cardiometabolic disease than BMI or waist circumference in AsA and NHPI (29, 30). |
Relative to other groups, why do AsA have higher ectopic fat deposition, given the same level of BMI? What biological mechanisms drive ectopic adiposity? Why are AsA more prone to low muscle mass and sarcopenia (muscle loss with aging)? Are there reliable nonimaging biomarkers to quantify ectopic fat depots? |
| T2DM | AsA and NHPI have a higher prevalence of T2DM than White Americans (33-36). Within AsA, South Asian and Filipino subgroups have the highest prevalence and incidence of T2DM in the United States, surpassing White, Latino, Black, and Native American populations (32, 34, 36). Among Native Hawaiians, AsA admixture is associated with higher T2DM risk and White admixture confers lower risk (105). Among those with T2DM, compared with White Americans, NHPI have increased risk for myocardial infarction; Chinese, Filipino, and Japanese Americans have elevated risk for ESRD (31). Non-U.S. born Pacific Islander, Chinese, and South, and Southeast Asian Americans have higher risk for gestational diabetes (106). |
Why do South Asian, Filipino, and NHPI groups have higher T2DM incidence than other groups? What biological mechanisms drive T2DM in AsA and NHPI? Do mechanisms differ by weight status? Why is there more glucose intolerance among certain AsA subgroups (Filipino and Japanese American)? Why are gestational diabetes rates higher in immigrant AsA and PI than in second- and third-generation women? |
| NAFLD | NAFLD affects 18% of AsA (107). NAFLD prevalence is higher among U.S.-born AsA, relative to new immigrants (108, 109). Lean NAFLD (among those with BMI <23 kg/m2) is higher among AsA than other race/ethnic groups (110). |
What is the prevalence of NAFLD and lean NAFLD in AsA and NHPI? What are the risk factors for NAFLD and lean NAFLD? What biological mechanisms drive NAFLD and lean NAFLD in AsA and NHPI? |
| Lipoproteins | Chinese and South Asian Americans have high ApoB/ApoA1 and non-HDL-to-HDL cholesterol ratios, which are associated with MI (111). Native Hawaiian and Japanese Americans have lower prevalence of dyslipidemia compared with Filipino Americans in Hawaii (112). |
What biological mechanisms drive dyslipidemia in different AsA and NHPI subgroups? |
| Kidney disease | AsA and NHPI have higher risk for early-stage CKD compared with White Americans (38-40, 113). Risk for and progression of CKD is exacerbated by T2DM, which is higher in NHPI and AsA (114-116). |
What biological mechanisms drive CKD in AsA and NHPI? Why are there differences in diabetes outcomes among AsA subgroups and NHPI with CKD? What factors link diabetes and CKD with CVD in AsA and NHPI? |
| Cardiovascular and lung diseases | Need data on incidence, risk factors, awareness, treatment, and control by subgroup for all heart and lung disorders. What role do genetics and other omics play in CVD? | |
| Hypertension | Hypertension prevalence is higher among some AsA groups and NHPI (33, 42, 117). Among all AsA subgroups, Filipino and South Asian have the highest prevalence of hypertension (41, 76). NHPI have the highest prevalence of hypertension compared with other race/ethnic groups (33). Hypertension awareness, treatment, and control are lower among AsA and NHPI (118, 119). |
What is the prevalence of hypertension, hypertension awareness, and control in different ethnic subgroups? |
| CHD and subclinical atherosclerosis | Prevalence of CHD is often aggregated for AsA and NHPI (120, 121). Health systems data show large differences in incidence of ASCVD among AsA subgroups (122, 123). Incidence of CAC is higher in South Asian men than other race/ethnic groups (43). |
How does CVD (or CHD) risk factor management vary in AsA and NHPI subgroups? How can ethnic disparities be improved for cardiovascular outcomes? Need to incorporate subclinical disease measures (e.g., CT angiographic plaque and carotid plaque) to understand disease progression. What are the most predictive models for ASCVD risk by subgroup? |
| Cardiac function and heart failure | AsA and NHPI have younger age of onset and age at hospitalization for heart failure (46-48). Native Hawaiian ancestry is associated with a higher risk for heart failure (124). |
Need to incorporate subclinical disease measures, such as left ventricular mass/hypertrophy by echo or MRI in future studies. |
| Arrhythmias: AF and SCD | ECG-determined AF data are lacking in AsA and NHPI (125). AsA with AF are less likely to be managed well by rhythm control strategies (126). Data on SCD are very limited in AsA and almost none are available on NHPI (127). Inherited arrhythmic disorders may cause ASCVD and may have varied genetic basis in AsA (128). |
Need serial measurements of ECGs for more valid diagnoses of AF and other arrhythmias. What is the prevalence of SCD by AsA and NHPI? |
| Hemorrhagic and ischemic stroke | Some Asian subgroups have higher risk for hemorrhagic but not ischemic stroke (49-51). AsA and NHPI tend to have more severe clinical presentation and worse outcomes from hemorrhagic stroke (129). There may be heterogeneity in stroke risk factors between AsA and NHPI (130). |
Why do some AsA groups have higher risk or mortality for hemorrhagic stroke? How does this affect anticoagulation guidelines for these subgroups? What is the prevalence and age-adjusted mortality rate for ischemic and hemorrhagic stroke by subgroup? Why is there discordance between high ASCVD risk and low stroke risk in South Asian groups, contrasted with a high hemorrhagic stroke risk but low ASCVD risk in some East Asian groups, contrasted with a high ASCVD and high hemorrhagic stroke risk in Filipino groups? |
| Lung disease and lung function | AsA have relatively lower FEV and FVC (131-134). Some AsA subgroups have a higher risk for asthma but a lower risk for COPD (134). Filipino Americans have a high lifetime prevalence of asthma compared with other groups (134). |
Need improved lung function reference values specifically for AsA and NHPI. What is the prevalence of asthma and COPD by subgroup? How do early life exposomic factors and nativity influence lung function or lung health over the life span? |
| Cancer patterns, risk factors, and screening | Why do AsA and NHPI have much higher risk for certain cancers? What roles do genetics and other omics play in the etiology of cancer? | |
| Patterns and trends | Cancer is the most common leading cause of death in AsA and NHPI overall and in most AsA subgroups (53-58). There are striking disparities in cancer patterns among AsA and NHPI (54, 57-59). Breast and colorectal cancer rates are increasing in most AsA subgroups (54, 57-59). AsA are younger at diagnosis of breast, colorectal, and lung cancer (54, 58, 59, 135-137). Liver cancer rates have been high but are declining; rates remain high among most Southeast Asian groups (62, 63). Gastric cancer rates are persistently high among many East Asian groups, particularly Japanese and Korean Americans (61); Native Hawaiian and American Samoans also have high rates of gastric cancer (64). Lung cancer risk is high among some nonsmoking AsA women (138). American Samoan women more likely to be diagnosed with, and to die of, cervical cancer compared with White women (64). Native Hawaiian groups have the highest cancer mortality rates, higher than White and Black populations (65). |
Need annual population size estimates (denominator) for many AsA and NHPI subgroups to develop reliable incidence and mortality statistics and cancer trends by ethnic subgroup. |
| Risk factors | Most information on etiology on AsA-related cancer come from studies in Asia, less from AsA populations. Risk factors for cancer vary by ethnic subgroup. For example, chronic infection with hepatitis B virus is associated with liver cancer in Chinese and Korean Americans, but hepatitis C is the key risk factor for liver cancer in Vietnamese, Japanese, and Mongolian Americans (62, 63). Pancreatic cancer rates are increasing in most AsA and NHPI (139). Helicobacter pylori infection is the key risk factor for gastric cancer in most AsA and NHPI (61, 140). |
What factors influence cancer etiology and progression within subgroups? |
| Screening | Cancer screening metrics, including that for breast, cervical, lung, and colorectal cancer, in AsA and NHPI is below the Healthy People metrics (141-144). Given unique disparities in risk and prognosis of certain cancers in AsA and NHPI, current screening eligibility criteria, recommendations, and physician practice guidelines do not address effective early detection in these populations (66, 67). |
How can AsA- and NHPI-specific cancer screening guidelines be developed for cancers that are more common in AsA and NHPI, including stomach and liver cancer, and lung cancer among nonsmokers? |
| Domain | What We Know (Current Knowledge) | What We Need to Know (Gaps) |
| Healthy aging, dementia, and AD | Need serial and longitudinal data on health aging and ADRD in AsA and NHPI. What roles do genetics and other omics play in AD and ADRD? | |
| Healthy aging | The main data sources used to monitor healthy aging in the United States (i.e., HRS and NHATS) include very limited AsA and NHPI data (145). | What is the prevalence of age-related conditions among AsA and NHPI? |
| AD and ADRD | Data on AD and ADRD in AsA and NHPI are very limited (68). There are large ethnic differences in cognition in AsA and NHPI, but the data are largely in Chinese and Japanese Americans (69, 70), and lacking for South and Southeast Asian subgroups. Filipino Americans had higher incidence of clinical dementia than Chinese, Japanese, and South Asian subgroups (71). |
Need culturally and linguistically appropriate tools to assess cognitive function and AD/ADRDs. What is the epidemiology of cognitive function in healthy elderly and AD/ADRDs in AsA and NHPI? What mechanisms drive AD and ADRD in AsA and NHPI? Can omics provide greater insight into the development of these disorders? |
AD = Alzheimer disease; ADRD = Alzheimer disease and related dementias; AF = atrial fibrillation; ApoB/ApoA1 = apolipoprotein B/apolipoprotein A1; AsA = Asian American; ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; CAC = coronary artery calcium; CHD = coronary heart disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CT = computed tomography; CVD = cardiovascular disease; ECG = electrocardiography; echo = echocardiography; ESRD = end-stage renal disease; FEV = forced expiratory volume; FVC = forced vital capacity; HRS = Health and Retirement Study; MI = myocardial infarction; MRI = magnetic resonance imaging; NAFLD = nonalcoholic fatty liver disease; NIH = National Institutes of Health; NHATS = National Health and Aging Trends Study; NHPI = Native Hawaiian and Pacific Islander; REM = rapid eye movement; SCD = sudden cardiac death; SES = socioeconomic status; T2DM = type 2 diabetes mellitus.
Sociocultural, Environmental, Psychological Health, and Lifestyle Dimensions
Health and chronic diseases have multilevel drivers, including structural, cultural, environmental, interpersonal, psychological, and individual lifestyle factors. To unravel these complex relationships, it is imperative to investigate these multilevel factors individually and jointly. Because 57% of AsA are immigrants (foreign-born), whereas NHPI are primarily native-born but historically colonized (6, 7), studying changes in psychosocial and lifestyle dimensions by level of immigration patterns, acculturation, and generational status provides unique insights into disease occurrence, prevention, and outcomes. Psychological factors have been associated with a wide array of health conditions and mortality among AsA and NHPI (7). Mental health problems are prevalent and increasing in AsA and NHPI, but stigmatization and a paucity of culturally valid assessment tools contribute to underdiagnosis of psychological disorders and low admission and utilization rates of mental health services (8-11).
Lifestyle dimensions, including tobacco and e-cigarette use, diet, physical activity, and sleep, play an important role in health and chronic diseases. Several studies have found that neighborhood context, social support, and acculturation patterns affect individual lifestyle behaviors across AsA and NHPI subgroups (12-16). There is considerable variation in smoking prevalence, dietary patterns, and physical activity levels in AsA and NHPI. Physical activity levels are lower among AsA and NHPI than the White population (17), and AsA and NHPI undergo dietary transitions after immigration or with a greater exposure to an American diet (18, 19). Sleep deficiency and poor sleep quality among AsA and NHPI populations have been linked to adverse health outcomes, including obesity, diabetes, hypertension, and cardiovascular disease (CVD) (20-22). Compared with the U.S. White population, AsA and NHPI groups tend to have shorter sleep duration, lower sleep efficiency, less time in deep sleep, and a higher risk for obstructive sleep apnea, despite AsA having a lower body mass index (BMI) (23-26).
Metabolic Disorders
Despite lower BMI in most AsA subgroups, many metabolic disorders, including type 2 diabetes mellitus (T2DM), hypertension, nonalcoholic fatty liver disease, and dyslipidemia, are very common conditions in AsA and NHPI. Imaging studies using dual energy X-ray absorptiometry and magnetic resonance imaging for ectopic fat (abdominal visceral fat and hepatic fat) quantification have shown higher amounts of visceral and hepatic fat in several AsA groups compared with U.S. White, Black, and Latino populations (27, 28). Ectopic fat measures have much stronger associations with cardiometabolic disease in AsA and NHPI populations than BMI (28-30).
Diabetes is a recognized health disparity among AsA and NHPI groups (31-33). Among AsA, the South Asian and Filipino subgroups have the highest rates of T2DM, surpassing those of Latino, Black, and Native American groups; East Asian subgroups (Chinese, Japanese, and Korean) also have higher rates than the U.S. White population (34-36). Among NHPI, diabetes is significantly more common (34) and occurs 10 to 15 years earlier (37) than in the U.S. White population. Among AsA and NHPI, chronic kidney disease is usually exacerbated by the high prevalence of T2DM (31, 38); studies have shown a higher prevalence of chronic kidney disease in NHPI groups compared to most U.S. race or ethnic groups (39, 40).
Cardiovascular Disease
Although overall CVD mortality rates have been declining in the United States since 2000, ischemic heart disease mortality rates have stagnated in Asian Indian and Vietnamese men and heart failure mortality rates have increased in Filipino, Asian Indian, and Japanese women and men, Vietnamese women, and Korean men (Shah N. Personal communication.). Hypertension, a major CVD risk factor, is higher among Filipino and South Asian populations than among the U.S. White population (41, 42). Subclinical measures of atherosclerosis (that is, coronary artery calcium), the strongest predictor of CVD, are rarely studied in AsA groups (43, 44), and prognostic data for atherosclerotic cardiovascular disease events exist only for Chinese Americans (45). Compared to the U.S. White population, AsA and NHPI groups have younger age of onset and age at hospitalization for heart failure (46-48). Hemorrhagic stroke is a common problem in AsA and NHPI. Compared to the U.S. White population, many AsA subgroups, in particular Filipino, have a higher incidence or mortality from hemorrhagic stroke (49-51), and NHPI experience hemorrhagic stroke at younger ages with a higher number of risk factors (52).
Cancer
Cancer is the leading cause of death in AsA overall, specifically among Chinese, Korean, Vietnamese, Thai, and Laotian Americans. Native Hawaiians have the highest mortality rates for all types of cancer relative to the U.S. White population; cancer is the most rapidly increasing cause of death among Native Hawaiian and Chamorro subgroups (53-58). There is vast heterogeneity in cancer risk and outcomes across AsA and NHPI populations (54, 57-60): Korean American, Japanese American, Native Hawaiian, and American Samoan populations have higher risk for gastric cancer than the U.S. White population (58, 59, 61); liver cancer incidence has been high among Chinese, Korean, Vietnamese American, Native Hawaiian, and American Samoan populations (62-65). Cancer screening rates in AsA and NHPI are generally low. Currently, no national screening guidelines for early detection of high-risk cancers in AsA and NHPI (gastric, liver, and lung) are available (66, 67).
Cognitive Function and Healthy Aging
Healthy aging, functional ability, and Alzheimer disease and related dementias (ADRD) have not been well studied in most AsA and NHPI groups (68). Few studies of cognitive aging among older Chinese and Japanese American populations are available (69, 70), and 1 study comparing 4 AsA groups reported higher incidence of dementia among Filipino than Chinese, Japanese, and South Asian American subgroups (71).
Knowledge Gaps
Many gaps exist in the epidemiology and risk factors for most chronic conditions in AsA and NHPI subgroups, we have identified potentially addressable gaps based on existing knowledge of unique phenotypes within certain ethnic groups (Table 1).
Opportunities and Barriers
Table 2 highlights major research opportunities that leverage unique phenotypes among AsA and NHPI groups (from Table 1) or are most feasible in advancing science. For example, studying subgroups with divergent body composition (AsA groups with higher visceral and hepatic fat at low BMI vs. NHPI groups with more lean muscle mass and high BMI) who are at higher risk for T2DM can elucidate the underlying biology and the relationships between multilevel factors for a better understanding of disease mechanisms and prevention for many populations. We can also probe unique pathways that link diabetes with heart disease and stroke by studying specific AsA and NHPI groups at higher risk for T2DM but have divergent risk for atherosclerotic CVD and stroke. Given the higher risk for mortality from hemorrhagic stroke in some AsA populations, there is an urgent need to determine the mechanisms underlying risk to prevent stroke for safer use of anticoagulant treatments in these populations.
Table 2.
Opportunities and Potential Barriers for Health and Prevention Research Among AsA and NHPI, 2021 NIH Workshop
| Opportunities and Barriers |
|---|
| Sociocultural, environmental, and psychological health and lifestyle dimensions |
| Sociocultural and environment: The vast diversity in culture and lifestyles offers unique opportunities to carry out mixed-method studies (qualitative and quantitative) to understand the interaction of ethnicity, immigration status, culture, SES, gender roles, and socioecologic determinants on health outcomes. Assess the effects of interpersonal and occupational and/or neighborhood exposures on health outcomes over time among specific AsA and NHPI subgroups with higher disease risk, using an intersectionality framework with demographic and sociocultural factors. |
| Psychological health: Study the incidence, prevalence, risk factors, treatments, and outcomes of mental health conditions among AsA and NHPI and their intersection with other health conditions. |
| Lifestyle dimensions: Address the gender gap in smoking and other tobacco product use, including uptake of smoking cessation medications, and investigate culturally appropriate approaches to quitting. Incorporate change of dietary patterns, physical activity levels, and sleep among AsA and NHPI immigrant populations to examine effects on health outcomes. Barriers: Developing trust in communities for long-term engagement in research studies Lack of existing tools and instruments for individual ethnic subgroups, accounting for cultural and generational status Translations of tools into several commonly spoken languages Overcoming stigma about mental health conditions Geographic and socioeconomic diversity of these populations, with several communities residing in rural areas with limited reach for research participation (e.g., internet connectivity, distance to travel to clinical exams, community health centers without electronic health records) |
| Metabolic disorders |
| Metabolic conditions: Understand the biology underlying higher body fat at lower BMI for specific AsA groups, contrasting with high lean muscle mass and high BMI in Pacific Islanders, and how both body types are associated with higher T2DM risk. Contrast ethnic subgroups with high diabetes prevalence (i.e., Filipino, South Asian, Native Hawaiian, and Pacific Islander) but with disparate CVD outcome (high heart disease risk in all 3 groups, but lower stroke risk in South Asian and NHPI groups). Compare and contrast AsA and NHPI subgroups on phenotypes, epidemiology, disease progression, pathophysiology, and response to therapies for several distinct metabolic and kidney conditions. Determine the cause of isolated postchallenge hyperglycemia among selected AsA and identify biomarkers and/or other diagnostic tools/algorithms for this condition. Determine the cause underlying the higher risk for gestational diabetes for AsA and NHPI, particularly among immigrant women. |
| Imaging: Use objective imaging measurements for body composition (whole-body DXA) and for NAFLD (ultrasound and FibroScan). |
| Omics and microbiome: Use multiple omics, including genomics, social and environmental epigenomics, and the microbiome on metabolic outcomes, to understand the biological underpinnings. |
| CKD: Determine optimal biomarkers to classify CKD at its earliest stages, identify the target site of injury and inflammation, explore biological pathways that promote kidney repair, and promote potential therapeutic development. Barriers: Lack of awareness of diabetes screening and prevention methods among AsA and some NHPI communities Cost and accessibility of imaging for better body composition assessment (MRI, CT, whole-body DXA scans) Inconvenience of fasting blood tests and 2-hour glucose tolerance tests for better T2DM and dyslipidemia classification |
| Cardiovascular and lung diseases |
| Existing databases: Leverage the NIH HeartShare program, All of Us, disease registries, existing cohort studies, electronic health record data, and administrative data sets (e.g., CMS) to better understand the epidemiology of cardiovascular and lung diseases among AsA and NHPI. What biology and risk factors underlie the higher ASCVD risk among certain subgroups (South Asian) vs. higher ischemic and hemorrhagic stroke risk in other subgroups (Chinese). |
| Risk prediction: Develop ASCVD, stroke, AF, and SCD risk prediction models in AsA and NHPI. Develop pharmacogenomic studies of anticoagulation and antiarrhythmic drugs for optimal therapy for AF. |
| Omics: Study the genomic, proteomics, and metabolomics of cardiovascular and lung conditions. |
| Lung disorders: Examine how environmental exposures among the prior generation, changing lifestyle, smoking behaviors, and nutrition influence risk for lung disease and impaired lung structure and function. Leverage existing imaging data from existing studies and clinical data sets to understand pulmonary structure-function relationships and establish normative data on lung function in the United States with diverse AsA and NHPI representation. Barriers: Lack of awareness of CVDs and prevention methods among AsA and NHPI communities Cultural views about prevention of common chronic conditions Cost and accessibility of imaging (CT, MRI, echo), repeat ECGs, and pulmonary function tests Measurement of risk factors preimmigration (environmental and other sociocultural factors) |
| Cancer |
| Multilevel cancer epidemiology cohort: Develop large multilevel integrative cohorts to investigate specific cancer etiology, progression, survival, and survivorship. |
| Invest in statistical methods for population estimates: Invest in infrastructure to support the development of statistical methods for estimating annual AsA and NHPI population sizes in specific subgroups. |
| Link cancer registry data with other databases: Leverage rich data from NCI’s SEER program for secondary data analysis to understand cancer patterns, trends, treatment, and outcomes as well as forecast in subgroups of AsA and NHPI. Link SEER data to existing large data sets (electronic health records, genetic databases, etc.) to generate new knowledge, particularly descriptive epidemiology. |
| Omics and risk prediction for cancer prevention: Investigate multilevel risk and protective factors for cancer and whether multiomic biomarkers can be used for prediction of risk or prognosis in AsA and NHPI. |
| Cancer screening: Identify the reasons and factors underlying lower cancer screening uptake in these populations and develop effective strategies to improve the use of cancer screening. Barriers: Overcoming stigma of a cancer diagnosis Lack of awareness of cancer screening and prevention methods among AsA and NHPI communities |
| Cognitive functioning and healthy aging |
| Healthy aging: Collect nationally representative data on healthy aging, including oversampling of AsA and NHPI groups in national surveys (e.g., NHANES) and in existing longitudinal cohort studies (e.g., HRS, NHATS). |
| Standardized measures: Participate in the international effort to standardize measures of late-life cognition and dementia through the Harmonized Cognitive Assessment Protocol. |
| Functional status: Add functional status, a standard battery of tests, including cognitive tests to ongoing studies (e.g., NHANES, California Health Interview Survey, Multiethnic Cohort study, MESA, MASALA), and focus on identifying gender roles, lifestyle factors, and physical and social environments for healthy aging. |
| Morbidity of ADRD: Conduct research to determine the incidence and prevalence of ADRD, rate of cognitive decline, and mortality, including biomarkers, brain imaging, pathologic features, and the frequency and impact of genetic factors in AsA and NHPI. Barriers: Recruitment of representative samples of each subgroup Lack of validated and translated tools for cognitive function assessment for many subgroups Stigma associated with ADRD |
ADRD = Alzheimer disease and related dementias; AF = atrial fibrillation; AsA = Asian American; ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; CKD = chronic kidney disease; CMS = Centers for Medicare & Medicaid Services; CT = computed tomography; CVD = cardiovascular disease; DXA = dual-energy X-ray absorptiometry; ECG = electrocardiography; echo = echocardiography; HRS = Health and Retirement Study; MASALA = Mediators of Atherosclerosis in South Asians Living in America; MESA = Multi-Ethnic Study of Atherosclerosis; MRI = magnetic resonance imaging; NAFLD = nonalcoholic fatty liver disease; NHANES = National Health and Nutrition Examination Survey; NHATS = National Health and Aging Trends Study; NHPI = Native Hawaiian and Pacific Islander; NIH = National Institutes of Health; SCD = sudden cardiac death; SEER = Surveillance, Epidemiology, and End Results ; SES = socioeconomic status; T2DM = type 2 diabetes mellitus.
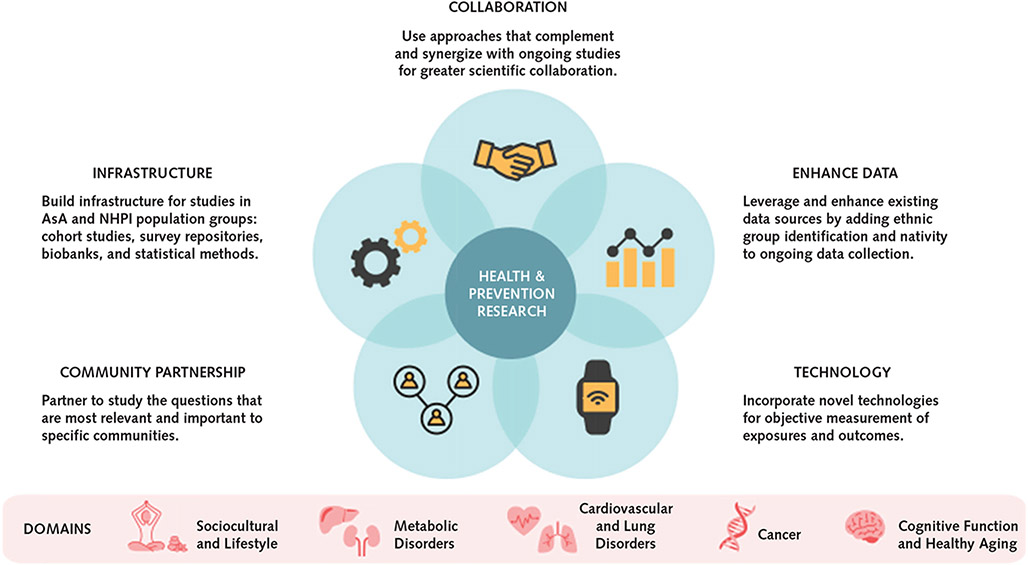
A critical opportunity is to enhance electronic health records and claims data by collecting detailed (standardized) AsA and NHPI ethnicity, nativity and birthplace, language, generational status, length of residence, and other social determinants of health. Used in conjunction with data from existing surveillance and behavioral risk factor surveys, these data could further our understanding of multilevel determinants of a wide range of common diseases in AsA and NHPI and other U.S. groups.
A cost-effective and scientifically sound approach is to leverage existing infrastructure in ongoing prospective cohort studies integrating recruitment of additional AsA and NHPI groups. For example, studies of aging using the standardized Health and Retirement Survey could expand recruitment to include specific AsA and NHPI subgroups. Such expansion would facilitate measurement standardization and comparative studies with other race or ethnic groups.
Barriers to AsA and NHPI research corresponding to opportunities in each domain are summarized in Table 2. One of the key challenges is building trust within each community to establish authentic partnership and long-term engagement with academic investigators for collaborations. Another major challenge is the lack of validated tools and survey instruments that are linguistically and culturally appropriate for these diverse populations.
Practical Approaches
We propose specific complementary and integrated strategies for future AsA and NHPI research in Table 3 and the Appendix Figure (available at Annals.org). An optimal design would use a community-engaged and participatory framework that builds capacity and trust among academic researchers and community stakeholders to establish mutually beneficial research partnerships. Within this framework, an effective strategy is to employ a populomics approach (that is, measuring the complex interplay of sociobehavioral, community, and biological factors within the context of the health care system) (72) or an exposome approach (that is, measuring all exposures of an individual across the lifespan) for prospective cohort studies designed with adequate sample sizes by ethnic subgroup. Such cohorts can include immigrants and subsequent generations to examine the intersections of sociocultural and multilevel drivers on health and outcomes. It would be highly efficient to leverage ongoing data collection for existing cohorts or surveillance studies by expanding sampling frames to capture more ethnic subgroups, in addition to adding detailed ethnicity and nativity variables to enable data disaggregation. Investments in basic infrastructure support, such as repositories of validated instruments, statistical tools, and biobanks that are linked to phenotypic data sets, would also enable cross-ethnic research. Lastly, incorporation of modern technology, such as imaging, digital, mobile, and biosensing tools, is critical for capturing more objective phenotypic data.
Table 3.
Suggested Approaches for Investigators to Improve the Quality and Impact of Health and Prevention Research Among AsA and NHPI, 2021 NIH Workshop*
| Approaches and Specific Steps |
|---|
|
I. Engage and build sustainable partnership with AsA and NHPI communities. Partner with AsA and NHPI community-based organizations and community leaders to build sustainable partnership to enhance participation, engagement, recruitment, retention, study implementation, capacity building, and outcome dissemination. |
|
Community-engaged partnership: Develop a community-engaged approach focusing on patient-centered outcomes within a community context, partnering with community organizations and community leaders to build capacity for research. Forge strong and sustainable relationships and build research coalitions with community-based organizations before initiating the study and hire culturally and linguistically competent staff and community health workers to improve recruitment materials, engagement methods, results interpretation, and dissemination. |
|
Capacity building: Recognize that not all communities have the ability or expertise to conduct research; help build their capacity through communication and training in study design, operation, and implementation to enhance sustainability and long-term relationships. Facilitate study participation by incorporating flexible clinic hours, use community-based settings for in-person exams, provide transportation assistance and adequate remuneration. Focus on patient-centered outcomes, within a community context aimed at practical benefits (e.g., technical and material assistance, education of youth), and commit to data equity and data ownership for the community. |
|
Probability samples: Develop a system or methods for probability sampling (covering all socioeconomic strata and other relevant demographic strata) for more valid population-specific representation with community outreach efforts for recruitment. |
|
II. Build infrastructure for studies in AsA and NHPI population groups. Develop a state-of-the-art research infrastructure to serve as the platform to collaborate, coordinate, and implement innovative research in AsA and NHPI populations. |
|
AsA and NHPI prospective cohorts: Develop multiple prospective cohort studies with novel approaches, deep phenotyping, serial measurements, and follow-up. Include sufficient sample size in distinct AsA and NHPI subgroups for valid comparison of exposures, genotype, phenotype, and outcomes for common chronic conditions in these groups. Include multigenerational cohorts, hybrid cohorts (traditional epidemiologic cohorts supplemented by data from electronic health records), and immigrant cohorts. Use a populomics approach (72) with multilevel, multicentered, multidisciplinary methods to investigate the risk factors, progression, and outcomes of multiple conditions and aging and their underlying biological mechanisms with a socioecological model framework. Develop multilevel conceptual frameworks to study the intersection of ethnicity, culture, socioeconomic position, gender roles, and socioecological determinants on health. Use an exposome approach to study environmental influences and biological response across the life span. Incorporate early-life and cumulative measures of occupational and neighborhood exposures, including those in the country of origin for more recent immigrants. |
|
Develop a survey repository: Establish centralized repositories of standard or novel surveys and instruments (in multiple languages). These include tools for measuring acculturation, immigration factors, and social/interpersonal measures (discrimination, social support, neighborhood cohesion). (Examples of existing repositories include the NIH PhenX toolkit or the Common Data Elements repository.) Develop, test, and validate dietary assessment tools, mental health instruments, and cognitive function measures in specific AsA and NHPI ethnic groups. |
|
Biobank: Create biobanks that are linked to phenotypic and clinical data (EHR) to enable genomic and other omics to advance biological discoveries unique to AsA and NHPI populations. |
|
Methods studies in AsA and NHPI: Build statistical methods for estimating annual AsA and NHPI population sizes to generate annual rates for monitoring of trends and patterns to inform prevention, resource allocation, and patient care. |
|
III. Leverage and enhance existing data sources. Expand AsA and NHPI inclusion and ethnic group identification in ongoing cohorts, EHRs, national and state surveys, and other data sources, with appropriate data disaggregation. |
|
Coding standardization: Standardize coding methods for AsA and NHPI subgroups (expanding ethnic categorization and nativity) in federal and state data sets, EHR data, disease registries, and claims data sets. Leverage electronic data resources for screening and recruitment of study participants, and link data with various data sets for exposure and outcome measures. |
|
Secondary data analysis: Conduct secondary analyses of existing data sets (e.g., national cancer registry systems, California Health Interview Survey, NHANES, NHPI National Health Interview Survey); carry out pooled analyses from existing cohorts and ongoing cohorts; add ancillary studies to ongoing cohorts. |
|
IV. Incorporate novel technologies for exposure assessment and clinical evaluation. Leverage modern imaging techniques and digital technology for exposure and outcome assessment to advance health research in AsA and NHPI populations. Imaging: Use the most appropriate imaging technologies: DXA, MRI, or CT, to accurately quantify body composition; ultrasonography and FibroScan for NAFLD; advanced low-dose CT for lung disease and cardiac plaque; echo and MRI for cardiac function. Sensor technology: Deploy wearable biosensing and mobile health technology to capture objective, real-time and continuous data for more accurate and reliable measurement of exposures. Omics: Use modern omics approaches to combine genomic, transcriptomic, proteomic, and metabolomic data with rich phenotypic, environmental, and behavioral data. |
AsA = Asian American; CT = computed tomography; DXA = dual-energy X-ray absorptiometry; echo = echocardiography; EHR = electronic health record; MRI = magnetic resonance imaging; NAFLD = nonalcoholic fatty liver disease; NHANES = National Health and Nutrition Examination Survey; NHPI = Native Hawaiian and Pacific Islander.
The proposed methods and specific steps are not mutually exclusive and may be complementary and synergistic with other ongoing studies.
Discussion
Asian Americans, Native Hawaiians, and Pacific Islanders are heterogenous populations with diverse multilevel risk profiles, phenotypic presentations, and health disparities in many chronic diseases. For many conditions, AsA and NHPI are more likely to be diagnosed at a younger age, or a later stage, and have a worse prognosis, although specific data on morbidity and mortality for many subgroups are lacking. The key recurring themes across all domains were the paucity of AsA and NHPI–focused research for many lifestyle behaviors and health conditions and that most of the existing data on AsA and NHPI are not appropriately disaggregated. Furthermore, the NIH investment in research of this growing American subpopulation has been woefully low to date (5).
Due to striking phenotypic differences within and across AsA and NHPI populations, their expansion and inclusion in health research will provide crucial and unique opportunities to uncover novel leads into cause and outcomes. For example, the paradoxical relationship between low or normal BMI yet high prevalence of T2DM and other metabolic disorders among specific AsA populations is distinct and has promising potential for clinical and biological mechanism discovery. Given the low BMI yet high ectopic fat and low muscle mass often observed among AsA, imaging methods should be incorporated into future studies to more accurately characterize body adiposity and muscle mass to clarify the biological underpinnings of this paradox and provide mechanistic insights into the development and progression of many metabolism-related diseases.
Community engagement is vital to improve research participation and quality. To successfully address the health needs of AsA and NHPI and leverage opportunities to advance health research, it is imperative to build representative research teams and a workforce within AsA and NHPI communities. Studies should integrate social, behavioral, environmental, and biological factors within a multilevel populomicsframework (72) to address the determinants of health and health disparities as proposed by the National Institute on Minority Health and Health Disparities (73). This successful approach has also been the cornerstone of the NHLBI cohort study (the Hispanic Community Health Study/Study of Latinos) and could serve as a model for future NIH investments into AsA and NHPI prospective cohorts.
The limited investment by the NIH in AsA and NHPI health and prevention research may have stemmed from the continuing myth of the “model minority” stereotype for AsA or a misconception that AsA are a homogenous demographic group, further perpetuated by the continuing practice of aggregating these diverse groups in federally funded research and publications (4). There are ample opportunities to improve data availability, including development of statistical approaches for analyses of data for numerically smaller populations and determining annual population denominators. Other sources of data, such as electronic health records, administrative claims, and admissions or encounters data should consistently collect information regarding specificAsA and NHPI ethnic identity (including mixed ancestral backgrounds), language, and immigration factors. Some health systems have included ethnic subgroup data, enabling the creation of highly useful clinical and administrative data sets to yield novel findings about disease epidemiology and outcomes among several AsA and NHPI groups and several other race or ethnic groups in the United States (31, 34, 74-76). While these health systems include data on race and ethnicity, there remain areas of necessary data collection, including country of birth, length of U.S. residence, generational status, and English language proficiency. These immigration-related variables comprise a range of barriers as well as opportunities for improving health care access and lifestyle behaviors, and ultimately for mitigating health disparities (77, 78).
To achieve health equity, we need broader policy changes, including more tangible directed research funding, grant review panels that include members representing these diverse communities and researchers with expertise in working with these populations, and mandates to governmental and health systems that require data disaggregation by ethnic origin groups. The goal is to support representation, inclusion, and equity and to leverage the unparalleled diversity of the United States for scientific gain.
In summary, this report presents the relevant findings and highlights knowledge gaps, research opportunities, potential barriers, and actionable approaches that can yield new leads for disease cause and prognostic factors to inform prevention and clinical care among AsA and NHPI. Integrating scientific goals with the priorities of AsA and NHPI community partners is vital to ensuring participation and impact when conducting epidemiologic, clinical, and prevention research to reduce the burden of chronic diseases and health disparities. Ultimately, addressing these gaps and barriers will inform how public health and health care providers prevent and manage chronic diseases among AsA and NHPI. This report serves as a blueprint to advance health justice for all AsA and NHPI populations.
Key Summary Points.
Asian Americans (AsA), Native Hawaiians, and Pacific Islanders (NHPI) comprise 7.7% of the U.S. population and include more than 40 ethnic groups that are highly diverse culturally, demographically, linguistically, and socioeconomically. Much of the existing data about AsA and NHPI are aggregated across groups, which obscures important disparities in health and results in knowledge gaps about common health conditions among specific AsA and NHPI groups.
Approximately 57% of AsA and 17% of NHPI are foreign-born. Collecting and integrating data about each group's ancestry and nativity, immigration patterns, generational status, length of residence, and acculturation level will provide unique insights into novel etiologic factors, phenotypic patterns, and prognostic factors that influence health.
There is limited or no data on the incidence, prevalence, and risk factors of many common chronic conditions (diabetes, kidney disease, cardiovascular disease, lung disorders, cancer, and cognitive impairment) in specific AsA and NHPI subgroups.
There is a critical need to capture specific and accurate AsA and NHPI ethnic identification and nativity and ancestry, using standardized coding methods, in existing and ongoing data collection sources to facilitate data disaggregation and pooling across data sources.
There is an urgent need to develop an innovative research infrastructure to support well-powered and representative prospective cohorts to understand mechanisms of disease with appropriate data disaggregation across AsA and NHPI subgroups. Equally important are the development of culturally and linguistically appropriate instruments and measures and the creation of survey and other data repositories linked to biobanks.
It is essential to use community-engaged approaches and partner with community organizations and trusted gatekeepers to effectively study health issues that reflect community priorities. These partnerships promote development of culturally relevant strategies, enhance workforce diversity, recruitment and retention efforts, and ultimately evidence-based preventive services and clinical care tailored for AsA and NHPI groups.
Future studies should incorporate the latest technologies, including bioimaging techniques, biosensing and digital platforms, and -omics, to facilitate objective assessment and real-time data capture to understand different phenotypes and causal mechanisms underlying exposures and outcomes among AsA and NHPI populations.
Acknowledgment:
The authors would like to thank Lori Whitten for preparing meeting notes and Rashaad Houston for his expert informational technology support for the workshop.
Financial Support:
The National Heart, Lung, and Blood Institute, National Institutes of Health, provided administrative support for the planning committee conference calls and the workshop. C.L. Jackson's contribution was funded, in part, by the Intramural Program at the NIH, National Institute of Environmental Health Sciences (Z1A ES103325-01).
Appendix: Workshop Participants (31 March to 1 April 2021)
Co-Chairs:
Alka M. Kanaya, MD, University of California, San Francisco
Ann Hsing, PhD, MPH, Stanford University
Members:
Maria Rosario G. Araneta, PhD, MPH, University of California, San Diego
Sonali Bose, MD, MPH, Icahn School of Medicine at Mount Sinai, New York
Martha L. Daviglus, MD, PhD, University of Illinois College of Medicine
Scarlett Lin Gomez, PhD, MPH, University of California, San Francisco
Frank B. Hu, MD, PhD, MPH, Harvard T.H. Chan School of Public Health
Nadia S. Islam, PhD, New York University Grossman School of Medicine
Chandra L. Jackson, PhD, MS, National Institute of Environmental Health Sciences, NIH
Namratha R. Kandula, MD, MPH, Northwestern University
John S.K. Kauwe, PhD, Brigham Young University
Marjorie K.L. Mala Mau, MD, University of Hawai’i at Mānoa
Jinkook Lee, PhD, University of Southern California
Kiang J. Liu, PhD, Northwestern University
Simin Liu, MD, MPH, ScD, Brown University
Grace X. Ma, PhD, Temple University
K.M. Venkat Narayan, MD, MSc, MBA, Emory University
Tung Nguyen, MD, University of California, San Francisco
Latha Palaniappan, MD, MS, Stanford University
Sela V. Panapasa, PhD, University of Michigan
Ninez Ponce, MPP, PhD, University of California, Los Angeles
Raynald Samoa, MD, City of Hope Comprehensive Cancer Center
V. Wendy Setiawan, MS, PhD, University of Southern California
Sono Shah, PhD, Pew Research Center
Svati H. Shah, MD, MHS, Duke University School of Medicine
Chau Trinh-Shevrin, DrPH, New York University Grossman School of Medicine
Daichi Shimbo, MD, Columbia University Irving Medical Center
Janice Y. Tsoh, PhD, University of California, San Francisco
Dhananjay Vaidya, MBBS, PhD, MPH, Johns Hopkins University
Barbara Vickrey, MD, MPH, Icahn School of Medicine at Mount Sinai, New York
Paul J. Wang, MD, Stanford University
Thomas J. Wang, MD, University of Texas Southwestern Medical Center
Nathan D. Wong, PhD, MPH, University of California, Irvine
Merle Kataoka-Yahiro, DrPH, MS, APRN, University of Hawai’i at Mānoa
NIH Staff
National Heart, Lung, and Blood Institute
Division of Cardiovascular Sciences
Yuling Hong, MD, MSc, PhD (Workshop Co-Leader)
Sean Coady, MA, MS (Workshop Co-Leader)
David Goff, MD, PhD
Meagan G. Grant, PhD
Mona Puggal, MPH, MBA, PMP
Jared P. Reis, PhD
Clinical Applications and Prevention Branch
Alison Brown, PhD, MS
Division of Lung Diseases
Marishka Brown, PhD
Qing Lu, DVM, PhD
Aruna Natarajan, MD, PhD
Koyeli Banerjee, PhD
National Cancer Institute
Tram Kim Lam, PhD, MPH, Division of Cancer Control and Population Sciences
Pothur Srinivas, PhD, Division of Cancer Control and Population Sciences
National Eye Institute
Mary Frances Cotch, PhD, Office of Vision Health and Population Sciences
National Human Genome Research Institute
Teri Manolio, MD, PhD, Division of Genomic Medicine
Renee Rider, JD, MS, CGC, Division of Genomic Medicine
National Institute of Aging
Dallas Anderson, PhD, Division of Neuroscience
Marilyn M. Miller, PhD, Division of Neuroscience
National Institute of Diabetes and Digestive and Kidney Diseases
Beena Akolkar, PhD, Division of Diabetes, Endocrinology, & Metabolic Diseases
Barbara Linder, PhD, Division of Diabetes, Endocrinology, & Metabolic Diseases
Susan Yanovski, PhD, Office of Obesity Research and Division of Digestive Diseases & Nutrition
National Institute of Mental Health
Dawn Morales, PhD, Office of Rural Mental Health Research
National Institute of Minority Health and Health Disparity
Larissa Avilés-Santa, MD, MPH, Division of Scientific Programs
Rina Das, PhD, Division of Extramural Scientific Programs
Tilda Farhat, PhD, MPH, Office of Science Policy, Planning, Evaluation, and Reporting
National Institute of Neurological Disorders and Stroke
Richard T. Benson, MD, PhD, Office of Global Health and Health Disparities
Stacey D. Chambers, MS, Office of Global Health and Health Disparities
Naomi E. Booker, MPH, Office of Global Health and Health Disparities
Erica L. Littlejohn, PhD, Office of Global Health and Health Disparities
Appendix Figure.
Key approaches needed to improve prevention research in AsA and NHPI populations, 2021 NIH Workshop.
Footnotes
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Disclosures: Disclosures can be found at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M21-3729.
Author contributions are available at Annals.org.
Contributor Information
Alka M. Kanaya, University of California, San Francisco, San Francisco, California.
Ann W. Hsing, Stanford University, Stanford, California.
Sela V. Panapasa, University of Michigan, Ann Arbor, Michigan.
Namratha R. Kandula, Northwestern University, Chicago, Illinois.
Maria Rosario G. Araneta, University of California, San Diego, San Diego, California.
Daichi Shimbo, Columbia University Irving Medical Center, New York, New York.
Paul Wang, Stanford University, Stanford, California.
Scarlett L. Gomez, University of California, San Francisco, San Francisco, California.
Jinkook Lee, University of Southern California, Los Angeles, California.
K.M. Venkat Narayan, Emory University, Atlanta, Georgia.
Marjorie K.L. Mala Mau, University of Hawai’i at Mānoa, Honolulu, Hawaii.
Sonali Bose, Icahn School of Medicine at Mount Sinai, New York, New York.
Martha L. Daviglus, University of Illinois at Chicago, Chicago, Illinois.
Frank B. Hu, Harvard T.H. Chan School of Public Health, Boston, Massachusetts.
Nadia Islam, New York University Grossman School of Medicine, New York, New York.
Chandra L. Jackson, National Institute of Environmental Health Sciences, National Institutes of Health, Bethesda, Maryland.
Merle Kataoka-Yahiro, University of Hawai’i at Mānoa, Honolulu, Hawaii.
John S.K. Kauwe, Brigham Young University, Salt Lake City, Utah.
Simin Liu, Brown University, Providence, Rhode Island.
Grace X. Ma, Temple University, Philadelphia, Pennsylvania.
Tung Nguyen, University of California, San Francisco, San Francisco, California.
Latha Palaniappan, Stanford University, Stanford, California.
V. Wendy Setiawan, University of Southern California, Los Angeles, California.
Chau Trinh-Shevrin, New York University Grossman School of Medicine, New York, New York.
Janice Y. Tsoh, University of California, San Francisco, San Francisco, California.
Dhananjay Vaidya, Johns Hopkins University, Baltimore, Maryland.
Barbara Vickrey, Icahn School of Medicine at Mount Sinai, New York, New York.
Thomas J. Wang, University of Texas Southwestern Medical Center, Dallas, Texas.
Nathan D. Wong, University of California, Irvine, Irvine, California.
Sean Coady, National Heart, Lung, and Blood Institute, Bethesda, Maryland.
Yuling Hong, National Heart, Lung, and Blood Institute, Bethesda, Maryland.
References
- 1.U.S. Census Bureau. Race and Ethnicity in the United States: 2010 Census and 2020 Census. 2021. Accessed at www.census.gov/library/visualizations/interactive/race-and-ethnicity-in-the-united-state-2010-and-2020-census.html on 12 August 2021.
- 2.Budiman A, Ruiz NG. Asian Americans are the fastest-growing racial or ethnic group in the U.S. Pew Research Center; 2021. Accessed at www.pewresearch.org/fact-tank/2021/04/09/asian-americans-are-the-fastest-growing-racial-or-ethnic-group-in-the-u-s/ on 14 August 2021.
- 3.Budiman A, Ruiz NG. Key facts about Asian origin groups in the U.S. Pew Research Center; 2021. Accessed at www.pewresearch.org/fact-tank/2021/04/29/key-facts-about-asian-origin-groups-in-the-u-s/ on 14 August 2021.
- 4.Yi SS, Kwon SC, Sacks R, et al. Commentary: persistence and health-related consequences of the model minority stereotype for Asian Americans. Ethn Dis. 2016;26:133–8. [PMID: 26843806] doi: 10.18865/ed.26.1.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ðoán LN, Takata Y, Sakuma KK, et al. Trends in clinical research including Asian American, Native Hawaiian, and Pacific Islander participants funded by the US National Institutes of Health, 1992 to 2018. JAMA Netw Open. 2019;2:e197432. [PMID: 31339543] doi: 10.1001/jamanetworkopen.2019.7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Community Survey: Table B02018: Asian alone or in any combination by selected groups. 2019. Accessed at https://data.census.gov/cedsci/table?q=B02018&tid=ACSDT1Y2019.B02018&hidePreview=true on 12 August 2021.
- 7.Sue S, Yan Cheng JK, Saad CS, et al. Asian American mental health: a call to action. Am Psychol. 2012;67:532–44. [PMID: 23046304] doi: 10.1037/a0028900 [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Matejkowski J. Mental health service utilization among noncitizens in the United States: findings from the National Latino and Asian American Study. Adm Policy Ment Health. 2012;39:406–18. [PMID: 21755392] doi: 10.1007/s10488-011-0366-8 [DOI] [PubMed] [Google Scholar]
- 9.Sue S, Consolacion TB. Clinical psychology issues among Asian/Pacific Islander Americans. Routledge; 2013. [Google Scholar]
- 10.Kalibatseva Z, Leong FT. Depression among Asian Americans: review and recommendations. Depress Res Treat. 2011;2011:320902. [PMID: 21961060] doi: 10.1155/2011/320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim G, Aguado Loi CX, Chiriboga DA, et al. Limited English proficiency as a barrier to mental health service use: a study of Latino and Asian immigrants with psychiatric disorders. J Psychiatr Res. 2011;45:104–10. [PMID: 20537658] doi: 10.1016/j.jpsychires.2010.04.031 [DOI] [PubMed] [Google Scholar]
- 12.Kroenke CH, Le GM, Conroy SM, et al. Egocentric social networks, lifestyle behaviors, and body size in the Asian Community Health Initiative (CHI) cohort. PLoS One. 2020;15:e0232239. [PMID: 32374741] doi: 10.1371/journal.pone.0232239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sentell TL, Agner JL, Davis J, et al. Social networks in patients hospitalized with preventable conditions for heart disease and diabetes in Hawai’i by health literacy. Chronic Illn. 2021:1742395320987892. [PMID: 33497289] doi: 10.1177/1742395320987892 [DOI] [PubMed] [Google Scholar]
- 14.Tsoh JY, Burke NJ, Gildengorin G, et al. A social network family-focused intervention to promote smoking cessation in Chinese and Vietnamese American male smokers: A feasibility study. Nicotine Tob Res. 2015;17:1029–38. [PMID: 26180229] doi: 10.1093/ntr/ntv088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Sofiani ME, Langan S, Kanaya AM, et al. The relationship of acculturation to cardiovascular disease risk factors among U.S. South Asians: findings from the MASALA study. Diabetes Res Clin Pract. 2020;161:108052. [PMID: 32113027] doi: 10.1016/j.diabres.2020.108052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young MC, Gerber MW, Ash T, et al. Neighborhood social cohesion and sleep outcomes in the Native Hawaiian and Pacific Islander National Health Interview Survey. Sleep. 2018;41. [PMID: 29771373] doi: 10.1093/sleep/zsy097 [DOI] [PubMed] [Google Scholar]
- 17.Yi SS, Roberts C, Lightstone AS, et al. Disparities in meeting physical activity guidelines for Asian-Americans in two metropolitan areas in the United States. Ann Epidemiol. 2015;25:656–660.e2. [PMID: 26065343] doi: 10.1016/j.annepidem.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satia JA. Dietary acculturation and the nutrition transition: an overview. Appl Physiol Nutr Metab. 2010;35:219–23. [PMID: 20383236] doi: 10.1139/H10-007 [DOI] [PubMed] [Google Scholar]
- 19.Talegawkar SA, Kandula NR, Gadgil MD, et al. Dietary intakes among South Asian adults differ by length of residence in the USA. Public Health Nutr. 2016;19:348–55. [PMID: 25990446] doi: 10.1017/S1368980015001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. [PMID: 23589584] doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redline S, Foody J. Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? [Editorial]. Circulation. 2011;124:2049–51. [PMID: 22064955] doi: 10.1161/CIRCULATIONAHA.111.062190 [DOI] [PubMed] [Google Scholar]
- 22.Matthews EE, Li C, Long CR, et al. Sleep deficiency among native Hawaiian/Pacific islander, black, and white Americans and the association with cardiometabolic diseases: analysis of the National Health Interview Survey data. Sleep Health. 2018;4:273–283. [PMID: 29776622] doi: 10.1016/j.sleh.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38:877–88. [PMID: 25409106] doi: 10.5665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson CL, Kawachi I, Redline S, et al. Asian-White disparities in short sleep duration by industry of employment and occupation in the US: a cross-sectional study. BMC Public Health. 2014;14:552. [PMID: 24894508] doi: 10.1186/1471-2458-14-552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Wheaton AG, Chapman DP, et al. Prevalence of healthy sleep duration among adults–United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:137–41. [PMID: 26890214] doi: 10.15585/mmwr.mm6506a1 [DOI] [PubMed] [Google Scholar]
- 26.Johnson DA, Jackson CL, Williams NJ, et al. Are sleep patterns influenced by race/ethnicity–a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep. 2019;11:79–95. [PMID: 31440109] doi: 10.2147/NSS.S169312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah AD, Kandula NR, Lin F, et al. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA studies. Int J Obes (Lond). 2016;40:639–45. [PMID: 26499444] doi: 10.1038/ijo.2015.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maskarinec G, Raquinio P, Kristal BS, et al. Body fat distribution, glucose metabolism, and diabetes status among older adults: the Multiethnic Cohort Adiposity Phenotype Study. J Epidemiol. 2021. [PMID: 33642515] doi: 10.2188/jea.JE20200538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimoto WY, Jablonski KA, Bray GA, et al. ; Diabetes Prevention Program Research Group. Body size and shape changes and the risk of diabetes in the Diabetes Prevention Program. Diabetes. 2007;56:1680–5. [PMID: 17363740] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gujral UP, Narayan KMV, Kandula NR, et al. Incidence of diabetes and prediabetes and predictors of glycemic change among South Asians in the USA: the MASALA study. BMJ Open Diabetes Res Care. 2020;8. [PMID: 32646924] doi: 10.1136/bmjdrc-2019-001063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanaya AM, Adler N, Moffet HH, et al. Heterogeneity of diabetes outcomes among Asians and Pacific Islanders in the US: the diabetes study of northern California (DISTANCE). Diabetes Care. 2011;34:930–7. [PMID: 21350114] doi: 10.2337/dc10-1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maskarinec G, Erber E, Grandinetti A, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes. 2009;58:1732–8. [PMID: 19258435] doi: 10.2337/db08-1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galinsky AM, Zelaya CE, Simile C, et al. Health conditions and behaviors of Native Hawaiian and Pacific Islander persons in the United States, 2014. Vital Health Stat 3. 2017:1–99. [PMID: 30248010] [PubMed] [Google Scholar]
- 34.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in pacific islanders and asian subgroups: the diabetes study of northern California (DISTANCE). Diabetes Care. 2013;36:574–9. [PMID: 23069837] doi: 10.2337/dc12-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. JAMA. 2019;322:2389–2398. [PMID: 31860047] doi: 10.1001/jama.2019.19365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanaya AM, Herrington D, Vittinghoff E, et al. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care. 2014;37:1621–8. [PMID: 24705613] doi: 10.2337/dc13-2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aluli NE, Jones KL, Reyes PW, et al. Diabetes and cardiovascular risk factors in Native Hawaiians. Hawaii Med J. 2009;68:152–7. [PMID: 19653416] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi AI, Karter AJ, Liu JY, et al. Ethnic differences in the development of albuminuria: the DISTANCE study. Am J Manag Care. 2011;17:737–45. [PMID: 22084893] [PMC free article] [PubMed] [Google Scholar]
- 39.Kataoka-Yahiro MR, Davis J, Rhee CM, et al. Racial/ethnic differences in early detection and screening for chronic kidney disease among adults in Hawaii: A 10-year population health study. Prev Chronic Dis. 2020;17:E84. [PMID: 32816667] doi: 10.5888/pcd17.200011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang J, Morgenstern H, Li Y, et al. Incidence of ESKD among Native Hawaiians and Pacific Islanders living in the 50 US states and Pacific Island territories. Am J Kidney Dis. 2020;76:340–349.e1. [PMID: 32387021] doi: 10.1053/j.ajkd.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 41.Zhao B, Jose PO, Pu J, et al. Racial/ethnic differences in hypertension prevalence, treatment, and control for outpatients in northern California 2010–2012. Am J Hypertens. 2015;28:631–9. [PMID: 25352230] doi: 10.1093/ajh/hpu189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon SS, Carroll MD, Fryar CD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PMID: 26633197] [PubMed] [Google Scholar]
- 43.Kanaya AM, Vittinghoff E, Lin F, et al. Incidence and progression of coronary artery calcium in south Asians compared with 4 race/ethnic groups. J Am Heart Assoc. 2019;8:e011053. [PMID: 30630376] doi: 10.1161/JAHA.118.011053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araneta MR, Barrett-Connor E. Subclinical coronary atherosclerosis in asymptomatic Filipino and white women. Circulation. 2004;110:2817–23. [PMID: 15505100] [DOI] [PubMed] [Google Scholar]
- 45.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur Heart J. 2018;39:2401–2408. [PMID: 29688297] doi: 10.1093/eurheartj/ehy217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaul P, McAlister FA, Ezekowitz JA, et al. Ethnic differences in 1-year mortality among patients hospitalised with heart failure. Heart. 2011;97:1048–53. [PMID: 21508417] doi: 10.1136/hrt.2010.217869 [DOI] [PubMed] [Google Scholar]
- 47.Savitz ST, Leong T, Sung SH, et al. Contemporary reevaluation of race and ethnicity with outcomes in heart failure. J Am Heart Assoc. 2021;10:e016601. [PMID: 33474975] doi: 10.1161/JAHA.120.016601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durstenfeld MS, Ogedegbe O, Katz SD, et al. Racial and ethnic differences in heart failure readmissions and mortality in a large municipal healthcare system. JACC Heart Fail. 2016;4:885–893. [PMID: 27395346] doi: 10.1016/j.jchf.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klatsky AL, Friedman GD, Sidney S, et al. Risk of hemorrhagic stroke in Asian American ethnic groups. Neuroepidemiology. 2005;25:26–31. [PMID: 15855802] [DOI] [PubMed] [Google Scholar]
- 50.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [PMID: 28122885] doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jose PO, Frank AT, Kapphahn KI, et al. Cardiovascular disease mortality in Asian Americans. J Am Coll Cardiol. 2014;64:2486–94. [PMID: 25500233] doi: 10.1016/j.jacc.2014.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakagawa K, Koenig MA, Seto TB, et al. Racial disparities among Native Hawaiians and Pacific Islanders with intracerebral hemorrhage. Neurology. 2012;79:675–80. [PMID: 22815551] doi: 10.1212/WNL.0b013e3182608c6f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asian American Center for Advancing Justice. A Community of Contrasts. Asian Americans, Native Hawaiians and Pacific Islanders in California. 2013. Accessed at www.advancingjustice-alc.org/news_and_media/a-community-of-contrasts-asian-americans-native-hawaiians-and-pacific-islanders-in-california/ on 16 August 2021.
- 54.Thompson CA, Gomez SL, Hastings KG, et al. The burden of cancer in Asian Americans: A report of national mortality trends by Asian ethnicity. Cancer Epidemiol Biomarkers Prev. 2016;25:1371–1382. [PMID: 27694108] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Accessed at www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf on 6 September 2021.
- 56.Hastings KG, Jose PO, Kapphahn KI, et al. Leading causes of death among Asian American subgroups (2003–2011). PLoS One. 2015;10:e0124341. [PMID: 25915940] doi: 10.1371/journal.pone.0124341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez SL, Noone AM, Lichtensztajn DY, et al. Cancer incidence trends among Asian American populations in the United States, 1990–2008. J Natl Cancer Inst. 2013;105:1096–110. [PMID: 23878350] doi: 10.1093/jnci/djt157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L, Noone AM, Gomez SL, et al. Cancer incidence trends among Native Hawaiians and Other Pacific Islanders in the United States, 1990–2008. J Natl Cancer Inst. 2013;105:1086–95. [PMID: 23878354] doi: 10.1093/jnci/djt156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomez SL, Glaser SL, Horn-Ross PL, et al. Cancer research in Asian American, Native Hawaiian, and Pacific Islander populations: accelerating cancer knowledge by acknowledging and leveraging heterogeneity. Cancer Epidemiol Biomarkers Prev. 2014;23:2202–5. [PMID: 25368394] doi: 10.1158/1055-9965.EPI-14-0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trinh QD, Nguyen PL, Leow JJ, et al. Cancer-specific mortality of Asian Americans diagnosed with cancer: a nationwide population-based assessment. J Natl Cancer Inst. 2015;107:djv054. [PMID: 25794888] doi: 10.1093/jnci/djv054 [DOI] [PubMed] [Google Scholar]
- 61.Shah SC, McKinley M, Gupta S, et al. Population-based analysis of differences in gastric cancer incidence among races and ethnicities in individuals age 50 years and older. Gastroenterology. 2020;159:1705–1714.e2. [PMID: 32771406] doi: 10.1053/j.gastro.2020.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin H, Ha NB, Ahmed A, et al. Both HCV and HBV are major causes of liver cancer in Southeast Asians. J Immigr Minor Health. 2013;15:1023–9. [PMID: 23864445] doi: 10.1007/s10903-013-9871-z [DOI] [PubMed] [Google Scholar]
- 63.Han SS, Kelly SP, Li Y, et al. Changing landscape of liver cancer in California: a glimpse into the future of liver cancer in the United States. J Natl Cancer Inst. 2019;111:550–556. [PMID: 30544184] doi: 10.1093/jnci/djy180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pacific Island Partnership for Cancer Health Equity. Guam Cancer Facts & Figure 2008–2012, Table 10. Univ Guam; 2015. Accessed at https://u54.guamcrc.org/reports/ on 29 August 2021. [Google Scholar]
- 65.Hawai’i Health Data Warehouse. Health Reports & Data, Mortality, Vital Statistics Reports. Hawaii Health Initiative; 2021. Accessed at http://hhdw.org/health-reports-data/category/cancer/mortality/ on 29 August 2021. [Google Scholar]
- 66.Shah SC, Canakis A, Peek RM Jr, et al. Endoscopy for gastric cancer screening is cost effective for Asian Americans in the United States. Clin Gastroenterol Hepatol. 2020;18:3026–3039. [PMID: 32707341] doi: 10.1016/j.cgh.2020.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang HR, Cho JY, Lee SH, et al. Role of low-dose computerized tomography in lung cancer screening among never-smokers. J Thorac Oncol. 2019;14:436–444. [PMID: 30445189] doi: 10.1016/j.jtho.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 68.2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327–406. [PMID: 33756057] doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 69.Li LW, Ding D, Wu B, et al. Change of cognitive function in U.S. Chinese older adults: a population-based study. J Gerontol A Biol Sci Med Sci. 2017;72:S5–S10. [PMID: 28575265] doi: 10.1093/gerona/glx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armstrong JJ, Godin J, Launer LJ, et al. Changes in frailty predict changes in cognition in older men: The Honolulu-Asia aging study. J Alzheimers Dis. 2016;53:1003–13. [PMID: 27314525] doi: 10.3233/JAD-151172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mayeda ER, Glymour MM, Quesenberry CP Jr, et al. Heterogeneity in 14-year dementia incidence between Asian American subgroups. Alzheimer Dis Assoc Disord. 2017. Jul-Sep;31:181–186. [PMID: 28406845] doi: 10.1097/WAD.0000000000000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibbons MC. Populomics. Stud Health Technol Inform. 2008;137:265–8. [PMID: 18560087] [PubMed] [Google Scholar]
- 73.National Institute on Minority Health and Health Disparities. NIMHD Research Framework. 2017. Accessed at www.nimhd.nih.gov/about/overview/research-framework/nimhd-framework.html on 17 August 2021.
- 74.Zhao B, Jose PO, Pu J, et al. Racial/ethnic differences in hypertension prevalence, treatment, and control for outpatients in northern California 2010–2012. Am J Hypertens. 2015;28:631–9. [PMID: 25352230] doi: 10.1093/ajh/hpu189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DeRouen MC, Thompson CA, Canchola AJ, et al. Integrating electronic health record, cancer registry, and geospatial data to study lung cancer in Asian American, Native Hawaiian, and Pacific Islander ethnic groups. Cancer Epidemiol Biomarkers Prev. 2021;30:1506–1516. [PMID: 34001502] doi: 10.1158/1055-9965.EPI-21-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gordon NP, Lin TY, Rau J, et al. Aggregation of Asian-American subgroups masks meaningful differences in health and health risks among Asian ethnicities: an electronic health record based cohort study. BMC Public Health. 2019;19:1551. [PMID: 31760942] doi: 10.1186/s12889-019-7683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson CL, Walker JR, Brown MK, et al. A workshop report on the causes and consequences of sleep health disparities. Sleep. 2020;43. [PMID: 32154560] doi: 10.1093/sleep/zsaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eder M, Henninger M, Durbin S, et al. Screening and interventions for social risk factors: technical brief to support the US Preventive Services Task Force. JAMA. 2021;326:1416–1428. [PMID: 34468710] doi: 10.1001/jama.2021.12825 [DOI] [PubMed] [Google Scholar]
- 79.Chen AC, Szalacha LA, Menon U. Perceived discrimination and its associations with mental health and substance use among Asian American and Pacific Islander undergraduate and graduate students. J Am Coll Health. 2014;62:390–8. [PMID: 24779453] doi: 10.1080/07448481.2014.917648 [DOI] [PubMed] [Google Scholar]
- 80.Cooc N, Kim GM. The roles of racial discrimination and English in civic outcomes for Asian Americans and Pacific Islanders. Cultur Divers Ethnic Minor Psychol. 2021;27:483–494. [PMID: 33719470] doi: 10.1037/cdp0000443 [DOI] [PubMed] [Google Scholar]
- 81.Ogbenna BT, Ryu S, Lee S, et al. Discrimination and sleep among Asians and Pacific Islanders adults. Sleep. 2021;44. [PMID: 33912974] doi: 10.1093/sleep/zsab109 [DOI] [PubMed] [Google Scholar]
- 82.Spencer MS, Chen J, Gee GC, et al. Discrimination and mental health-related service use in a national study of Asian Americans. Am J Public Health. 2010;100:2410–7. [PMID: 20299649] doi: 10.2105/AJPH.2009.176321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salas-Wright CP, Lee S, Vaughn MG, et al. Acculturative heterogeneity among Asian/Pacific Islanders in the United States: associations with DSM mental and substance use disorders. Am J Orthopsychiatry. 2015;85:362–70. [PMID: 26167805] doi: 10.1037/ort0000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kandula NR, Cooper AJ, Schneider JA, et al. Personal social networks and organizational affiliation of South Asians in the United States. BMC Public Health. 2018;18:218. [PMID: 29402246] doi: 10.1186/s12889-018-5128-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pokhrel P, Fagan P, Cassel K, et al. Social network characteristics, social support, and cigarette smoking among Asian/Pacific islander young adults. Am J Community Psychol. 2016;57:353–65. [PMID: 27297612] doi: 10.1002/ajcp.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shah NS, Huffman MD, Schneider JA, et al. Association of social network characteristics with cardiovascular health and coronary artery calcium in south Asian adults in the United States: the MASALA cohort study. J Am Heart Assoc. 2021;10:e019821. [PMID: 33759541] doi: 10.1161/JAHA.120.019821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tam E, Miike R, Labrenz S, et al. Volcanic air pollution over the Island of Hawai’i: emissions, dispersal, and composition. Association with respiratory symptoms and lung function in Hawai’i Island school children. Environ Int. 2016;92:543–52. [PMID: 27197039] doi: 10.1016/j.envint.2016.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quach T, Liu R, Nelson DO, et al. Disaggregating data on Asian American and Pacific Islander women to provide new insights on potential exposures to hazardous air pollutants in California. Cancer Epidemiol Biomarkers Prev. 2014;23:2218–28. [PMID: 25368397] doi: 10.1158/1055-9965.EPI-14-0468 [DOI] [PubMed] [Google Scholar]
- 89.Hoshiko S, Pearl M, Yang J, et al. Differences in prenatal tobacco exposure patterns among 13 race/ethnic groups in California. Int J Environ Res Public Health. 2019;16. [PMID: 30764487] doi: 10.3390/ijerph16030458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee HK, Han B, Gfroerer JC. Differences in the prevalence rates and correlates of alcohol use and binge alcohol use among five Asian American subpopulations. Addict Behav. 2013;38:1816–23. [PMID: 23254233] doi: 10.1016/j.addbeh.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 91.Li S, Kwon SC, Weerasinghe I, et al. Smoking among Asian Americans: acculturation and gender in the context of tobacco control policies in New York City. Health Promot Pract. 2013;14:18S–28S. [PMID: 23667057] doi: 10.1177/1524839913485757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grineski S, Morales DX, Collins T, et al. The burden of carcinogenic air toxics among Asian Americans in four US metro areas. Popul Environ. 2019;40:257–282. [PMID: 31485094] doi: 10.1007/s11111-018-0308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morey BN. Environmental justice for Native Hawaiians and Pacific Islanders in Los Angeles County. Environ Justice. 2014;7:9–17. [Google Scholar]
- 94.Nguyen AB. Disaggregating Asian American and Native Hawaiian and Other Pacific Islander (AANHOPI) adult tobacco use: findings from wave 1 of the Population Assessment of Tobacco and Health (PATH) study, 2013–2014. J Racial Ethn Health Disparities. 2019;6:356–363. [PMID: 30610569] doi: 10.1007/s40615-018-00532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martell BN, Garrett BE, Caraballo RS. Disparities in adult cigarette smoking - United States, 2002–2005 and 2010–2013. MMWR Morb Mortal Wkly Rep. 2016;65:753–8. [PMID: 27491017] doi: 10.15585/mmwr.mm6530a1 [DOI] [PubMed] [Google Scholar]
- 96.Shi M, Gette JA, Gissandaner TD, et al. E-cigarette use among Asian Americans: a systematic review. J Ethn Subst Abuse. 2020:1–34. [PMID: 33346722] doi: 10.1080/15332640.2020.1861495 [DOI] [PubMed] [Google Scholar]
- 97.Lesser IA, Gasevic D, Lear SA. The association between acculturation and dietary patterns of South Asian immigrants. PLoS One. 2014;9:e88495. [PMID: 24558396] doi: 10.1371/journal.pone.0088495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenmöller DL, Gasevic D, Seidell J, et al. Determinants of changes in dietary patterns among Chinese immigrants: a crosssectional analysis. Int J Behav Nutr Phys Act. 2011;8:42. [PMID: 21592378] doi: 10.1186/1479-5868-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–57. [PMID: 21617109] doi: 10.2337/dc11-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalra R, Patel N, Arora P, et al. Cardiovascular health and disease among Asian-Americans (from the National Health and Nutrition Examination Survey). Am J Cardiol. 2019;124:270–277. [PMID: 31128733] doi: 10.1016/j.amjcard.2019.04.026 [DOI] [PubMed] [Google Scholar]
- 101.Sutherland K, Lee RW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology. 2012;17:213–22. [PMID: 21992683] doi: 10.1111/j.1440-1843.2011.02082.x [DOI] [PubMed] [Google Scholar]
- 102.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13:1458–65. [PMID: 16129729] [DOI] [PubMed] [Google Scholar]
- 103.Gujral UP, Vittinghoff E, Mongraw-Chaffin M, et al. Cardiometabolic abnormalities among normal-weight persons from five racial/ethnic groups in the United States: a cross-sectional analysis of two cohort studies. Ann Intern Med. 2017;166:628–636. [PMID: 28384781] doi: 10.7326/M16-1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6. [PMID: 12164465] [DOI] [PubMed] [Google Scholar]
- 105.Sun H, Lin M, Russell EM, et al. ; Samoan Obesity, Lifestyle, and Genetic Adaptations (OLaGA) Study Group. The impact of global and local Polynesian genetic ancestry on complex traits in Native Hawaiians. PLoS Genet. 2021;17:e1009273. [PMID: 33571193] doi: 10.1371/journal.pgen.1009273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010;24:441–8. [PMID: 20670225] doi: 10.1111/j.1365-3016.2010.01140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Golabi P, Paik J, Hwang JP, et al. Prevalence and outcomes of non-alcoholic fatty liver disease (NAFLD) among Asian American adults in the United States. Liver Int. 2019;39:748–757. [PMID: 30597715] doi: 10.1111/liv.14038 [DOI] [PubMed] [Google Scholar]
- 108.Setiawan VW, Stram DO, Porcel J, et al. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology. 2016;64:1969–1977. [PMID: 27301913] doi: 10.1002/hep.28677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lim U, Monroe KR, Buchthal S, et al. Propensity for intra-abdominal and hepatic adiposity varies among ethnic groups. Gastroenterology. 2019;156:966–975.e10. [PMID: 30445012] doi: 10.1053/j.gastro.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weinberg EM, Trinh HN, Firpi RJ, et al. Lean Americans with nonalcoholic fatty liver disease have lower rates of cirrhosis and comorbid diseases. Clin Gastroenterol Hepatol. 2021;19:996–1008. e6. [PMID: 32629123] doi: 10.1016/j.cgh.2020.06.066 [DOI] [PubMed] [Google Scholar]
- 111.McQueen MJ, Hawken S, Wang X, et al. ; INTERHEART study investigators. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–33. [PMID: 18640459] doi: 10.1016/S0140-6736(08)61076-4 [DOI] [PubMed] [Google Scholar]
- 112.Grandinetti A, Chang HK, Theriault A, et al. Metabolic syndrome in a multiethnic population in rural Hawaii. Ethn Dis. 2005;15:233–7. [PMID: 15825969] [PubMed] [Google Scholar]
- 113.Kataoka-Yahiro M, Davis J, Gandhi K, et al. Asian Americans & chronic kidney disease in a nationally representative cohort. BMC Nephrol. 2019;20:10. [PMID: 30626357] doi: 10.1186/s12882-018-1145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wong LL, Kalantar-Zadeh K, Page V, et al. Insights from screening a racially and ethnically diverse population for chronic kidney disease. Am J Nephrol. 2017;45:200–208. [PMID: 28125810] doi: 10.1159/000455389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Go AS, Yang J, Tan TC, et al. ; Kaiser Permanente Northern California CKD Outcomes Study. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol. 2018;19:146. [PMID: 29929484] doi: 10.1186/s12882-018-0942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kataoka-Yahiro MR, Munet-Vilaro F. Barriers to preventive health care for young children. J Am Acad Nurse Pract. 2002;14:66–72. [PMID: 11892538] [DOI] [PubMed] [Google Scholar]
- 117.Young DR, Fischer H, Arterburn D, et al. Associations of overweight/obesity and socioeconomic status with hypertension prevalence across racial and ethnic groups. J Clin Hypertens (Greenwich). 2018;20:532–540. [PMID: 29432662] doi: 10.1111/jch.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ostchega Y, Zhang G, Hughes JP, et al. Factors associated with hypertension control in US adults using 2017 ACC/AHA guidelines: National Health and Nutrition Examination Survey 1999–2016. Am J Hypertens. 2018;31:886–894. [PMID: 29617894] doi: 10.1093/ajh/hpy047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. [PMID: 32902588] doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Centers for Disease Control and Prevention. Diagnosed Cases of Coronary Heart Disease. Summary Health Statistics: National Health Interview Survey, 2018. Accessed at www.cdc.gov/nchs/nhis/shs/tables.htm on 5 September 2021. [Google Scholar]
- 121.Centers for Disease Control and Prevention. Death Rate. National Vital Statistics Report; 2021:Table 10. Accessed at www.cdc.gov/nchs/data/nvsr/nvsr69/nvsr69-13-508.pdf on 5 September 2021. [Google Scholar]
- 122.Pursnani S, Merchant M. South Asian ethnicity as a risk factor for coronary heart disease. Atherosclerosis. 2020;315:126–130. [PMID: 33317714] doi: 10.1016/j.atherosclerosis.2020.10.007 [DOI] [PubMed] [Google Scholar]
- 123.Rodriguez F, Chung S, Blum MR, et al. Atherosclerotic cardiovascular disease risk prediction in disaggregated Asian and Hispanic subgroups using electronic health records. J Am Heart Assoc. 2019;8:e011874. [PMID: 31291803] doi: 10.1161/JAHA.118.011874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davis J, Tam E, Taira D. Disparate rates of utilization and progression to combined heart failure and chronic obstructive pulmonary disease among Asians and pacific islanders in Hawai’i. Hawaii J Med Public Health. 2016;75:228–34. [PMID: 27563499] [PMC free article] [PubMed] [Google Scholar]
- 125.Heckbert SR, Austin TR, Jensen PN, et al. Differences by race/ethnicity in the prevalence of clinically detected and monitor-detected atrial fibrillation: MESA. Circ Arrhythm Electrophysiol. 2020;13:e007698. [PMID: 31934795] doi: 10.1161/CIRCEP.119.007698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gu K, Mahtta D, Kaneria A, et al. Racial disparities among Asian Americans with atrial fibrillation: an analysis from the NCDR PINNACLE registry. Int J Cardiol. 2021;329:209–216. [PMID: 33412180] doi: 10.1016/j.ijcard.2020.12.064 [DOI] [PubMed] [Google Scholar]
- 127.Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–63. [PMID: 11684624] [DOI] [PubMed] [Google Scholar]
- 128.Milman A, Andorin A, Postema PG, et al. Ethnic differences in patients with Brugada syndrome and arrhythmic events: new insights from survey on arrhythmic events in Brugada syndrome. Heart Rhythm. 2019;16:1468–1474. [PMID: 31284050] doi: 10.1016/j.hrthm.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 129.Song S, Liang L, Fonarow GC, et al. Comparison of clinical care and in-hospital outcomes of Asian American and white patients with acute ischemic stroke. JAMA Neurol. 2019;76:430–439. [PMID: 30667466] doi: 10.1001/jamaneurol.2018.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakagawa K, Koenig MA, Asai SM, et al. Disparities among Asians and native Hawaiians and Pacific Islanders with ischemic stroke. Neurology. 2013;80:839–43. [PMID: 23365055] doi: 10.1212/WNL.0b013e3182840797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davis AM, Kreutzer R, Lipsett M, et al. Asthma prevalence in Hispanic and Asian American ethnic subgroups: results from the california healthy kids survey. Pediatrics. 2006;118:e363–70. [PMID: 16882779] [DOI] [PubMed] [Google Scholar]
- 132.Fulambarker A, Copur AS, Javeri A, et al. Reference values for pulmonary function in Asian Indians living in the United States. Chest. 2004;126:1225–33. [PMID: 15486386] [DOI] [PubMed] [Google Scholar]
- 133.Hankinson JL, Kawut SM, Shahar E, et al. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) lung study. Chest. 2010;137:138–45. [PMID: 19741060] doi: 10.1378/chest.09-0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tran HN, Siu S, Iribarren C, et al. Ethnicity and risk of hospitalization for asthma and chronic obstructive pulmonary disease. Ann Epidemiol. 2011;21:615–22. [PMID: 21414801] doi: 10.1016/j.annepidem.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 135.Gomez SL Von Behren J, McKinley M, et al. Breast cancer in Asian Americans in California, 1988–2013: increasing incidence trends and recent data on breast cancer subtypes. Breast Cancer Res Treat. 2017;164:139–147. [PMID: 28365834] doi: 10.1007/s10549-017-4229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gomez SL, Yao S, Kushi LH, et al. Is breast cancer in Asian and Asian American women a different disease? [Editorial]. J Natl Cancer Inst. 2019;111:1243–1244. [PMID: 31093671] doi: 10.1093/jnci/djz091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tuan AW, Davis Lynn BC, Chernyavskiy P, et al. Breast cancer incidence trends by estrogen receptor status among Asian American ethnic groups, 1990–2014. JNCI Cancer Spectr. 2020;4:pkaa005. [PMID: 33392441] doi: 10.1093/jncics/pkaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeRouen MC, Canchola AJ, Thompson CA, et al. Incidence of lung cancer among never-smoking Asian American, Native Hawaiian, and Pacific Islander females. J Natl Cancer Inst. 2021. [PMID: 34345919] doi: 10.1093/jnci/djab143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu L, Zhang J, Deapen D, et al. Differences in pancreatic cancer incidence rates and temporal trends across Asian subpopulations in California (1988–2015). Pancreas. 2019;48:931–933. [PMID: 31180980] doi: 10.1097/MPA.0000000000001337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim Y, Park J, Nam BH, et al. Stomach cancer incidence rates among Americans, Asian Americans and native Asians from 1988 to 2011. Epidemiol Health. 2015;37:e2015006. [PMID: 25687951] doi: 10.4178/epih/e2015006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chawla N, Breen N, Liu B, et al. Asian American women in California: a pooled analysis of predictors for breast and cervical cancer screening. Am J Public Health. 2015;105:e98–e109. [PMID: 25521898] doi: 10.2105/AJPH.2014.302250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thompson CA, Gomez SL, Chan A, et al. Patient and provider characteristics associated with colorectal, breast, and cervical cancer screening among Asian Americans [Editorial]. Cancer Epidemiol Biomarkers Prev. 2014;23:2208–17. [PMID: 25368396] doi: 10.1158/1055-9965.EPI-14-0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Babey SH, Ponce NA, Etzioni DA, et al. Cancer screening in California: racial and ethnic disparities persist. Policy Brief UCLA Cent Health Policy Res. 2003:1–6. [PMID: 14503536] [PubMed] [Google Scholar]
- 144.Sentell T, Braun KL, Davis J, et al. Health literacy and meeting breast and cervical cancer screening guidelines among Asians and whites in California. Springerplus. 2015;4:432. [PMID: 26306294] doi: 10.1186/s40064-015-1225-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.White House Initiative on Asian Americans & Pacific Islanders. Advancing Economic Empowerment for Asian Americans and Pacific Islanders: A Call to Action. Report of the President's Advisory Commission on Asian Americans and Pacific Islanders. U.S. Department of Commerce; 2020. [Google Scholar]