Abstract
Background:
Simultaneous or concurrent use (co-use) of alcohol and cannabis is associated with greater use of both substances over time, academic difficulties, more severe substance use consequences, and impacts on cognitive functioning relative to single substance or no substance use. While negative consequences associated with co-use are known, this study examined potential neural mechanisms underlying co-use behaviors versus single substance use, specifically whether alcohol cue-reactivity and stress-cue reactivity differed between co-users reporting frequent same-day use co-use and individuals reporting only alcohol use.
Methods:
Participants included 88 individuals (41 women) reporting only alcohol use and 24 individuals (8 women) reporting co-use of alcohol and cannabis on at least 50% of drinking occasions who completed fMRI stress and alcohol cue reactivity tasks. Because of known sex effects on stress reactivity and alcohol cue reactivity, we tested sex by co-use interactions.
Results:
During alcohol cue presentation, co-users had hypoactivation in thalamus and dorsomedial prefrontal cortex relative to alcohol only users, effects that were driven by differences in responses to neutral cues. Examination of stress cue reactivity revealed sex by co-use interactions in lingual gyrus, with women co-users showing a greater difference between negative and neutral cue reactivity compared to all other groups. In addition, women co-users had elevated connectivity between nucleus accumbens and both medial orbitofrontal cortex and rostral anterior cingulate cortex during negative cue presentation compared to other groups.
Conclusions:
These results provide preliminary evidence of enhanced stress cue reactivity in individuals reporting co-use of alcohol and cannabis, particularly women co-users.
Keywords: Concurrent Alcohol and Cannabis Use, Stress reactivity, Alcohol cue reactivity, Sex effects, psychophysiological interaction
Introduction
Alcohol and cannabis are two of the most commonly used substances in the United States (Substance Abuse and Mental Health Services Administration, 2019). Using alcohol and cannabis together (i.e., co-use) also occurs frequently among adults, with 58% of alcohol users reporting cannabis use and over 75% of cannabis users reporting alcohol use (Barrett et al., 2008). Among users of both alcohol and cannabis, most have used concurrently (the use of each substance on at least one occasion) or simultaneously (use of both substances at the same time during an occasion, such that their effects overlap) (Collins et al., 1998; Earleywine and Newcomb, 1997; Martin et al., 1992; Patrick et al., 2018). Concurrent use is associated with an increased risk for negative outcomes, such as increased frequency and quantity of alcohol and cannabis use, decreased academic performance, and greater likelihood of developing a substance use disorder (Kelly et al., 2015; Meda et al., 2017; Patrick et al., 2018; Subbaraman and Kerr, 2015). Although the mechanisms underlying the link between the co-use of alcohol and cannabis and risk for negative outcomes remains unclear, one potential pathway is the extent to which the co-use of alcohol and cannabis use relates to changes in neural functioning, particularly in stress and reward pathways implicated in addiction (Jacobus et al., 2015b, 2015a; Wade et al., 2020). However, while prior studies have examined alterations in the stress and reward pathways as potential mechanisms underlying risk for alcohol use, these pathways have not been explored in co-users relative to alcohol use alone.
While there is limited research on co-use, previous research has found effects of cannabis or alcohol use alone on stress and reward pathways. Subjective stress is highly predictive of craving for alcohol in all stages of alcohol use disorder (AUD) (Wemm and Sinha, 2019). Laboratory studies demonstrate that acute experiences of stress lead to increases in subjective craving (Kwako et al., 2015; McCaul et al., 2018) and rate of responding for alcohol during an operant task (McCaul et al., 2018), and self-reported stress measured in the laboratory predicted subsequent alcohol use in an AUD sample(Higley et al., 2011). From a neurobiological perspective, engagement of stress circuitry that includes the hypothalamus-pituitary-adrenal (HPA) axis and the extended amygdala plays a key role in the development of cue reactivity responses to alcohol as alcohol shifts from a positive to a negative reinforcer (Koob, 2008; Koob and Volkow, 2010). Frequent alcohol consumption is known to result in blunted neuroendocrine response to stress (Blaine et al., 2019), and over time, stress becomes a cue that elicits responses throughout motivational systems due to the strong connectivity between the amygdala and emotional/motivational circuits that include insula and ventral/dorsal striatum. In fact, dysregulation in neural circuits and neuroendocrine response to stress are the strongest predictor of return to alcohol use in AUDs (Blaine and Sinha, 2017). Although the mechanisms have not been fully explicated, there is evidence of sex differences in the link between stress and alcohol use. For example, females consume alcohol for the negatively reinforcing effects, i.e. negative affect and stress reactivity reduction, more often than males (Peltier et al., 2019). In addition, neuroimaging studies have shown that women with AUD have dysregulation of responses to stress cues in ventromedial prefrontal cortex, a region that has inhibitory inputs to the HPA axis, that have been shown to predict return to alcohol use and cortisol response (Blaine et al., 2017).
Drinking in response to alcohol cues is often reported in anecdotal accounts of relapse (Ludwig, 1988), and has received considerable attention in the literature. Laboratory studies have shown for decades that alcohol cues increase subjective craving in treatment seeking and non-treatment seeking individuals (Litt and Cooney, 1999; Rohsenow and Monti, 1999), and more recent studies have demonstrated a relationship between craving for alcohol after cue exposure and subsequent consumption or administration of alcohol in the laboratory (Bujarski et al., 2018; Kwako et al., 2015; Plebani et al., 2012). Numerous neuroimaging studies of alcohol cue reactivity have consistently found a key set of networked brain regions that preferentially respond to alcohol cues compared to control cues, including anterior cingulate cortex (ACC), ventral and dorsal striatum, orbitofrontal cortex (OFC), insula and brainstem (Claus et al., 2011; Schacht et al., 2013; Vollstädt-Klein et al., 2011). Importantly, studies have demonstrated that responses during alcohol cue reactivity are moderated by factors including AUD severity, treatment seeking status, and sex. For example, we (Claus et al., 2011) showed that males had greater response than females to the taste of alcohol (vs. neutral) cues in left amygdala.
Just as with frequent alcohol consumption (Blaine et al., 2019), frequent cannabis use leads to blunted cortisol and adrenocorticotropic hormone (ACTH) release in response to stress (Cservenka et al., 2018). This is indicative of a dysregulated and decreased ability to respond to stressors. Under non-disorder conditions, endocannabinoids support healthy stress responses. Specifically, they are essential for both fast and slow feedback in synaptic networks to balance excitatory and inhibitory inputs, especially in dopaminergic pathways (Dow-Edwards and Silva, 2017; Parsons and Hurd, 2015). Endocannabinoid signaling in these pathways is sensitive to stress modulation by early life stress and current perceived stress, especially in the presence of genetic risk factors (Filbey et al., 2021). Individuals with cannabis use disorder (CUD) exhibit greater perceived chronic and acute stress(Vujanovic et al., 2016) and those who develop CUD often have higher levels of distress intolerance and negative affect (Macatee et al., 2019). Additionally, those with CUD show greater attentional bias for negative cues and greater stress-reactivity craving than those without CUD (Macatee et al., 2019). Further, participants with AUD who co-use cannabis show attentional bias for cannabis and alcohol cues, and this bias is associated with cue-elicited prefrontal hypoactivation (Müller-Oehring et al., 2019).
Collectively, these studies point to the potential for concurrent co-users to differ from alcohol only users in the functional neural responses and connectivity. A recent study examining structural integrity in a community sample of adolescents found that more frequent past month concurrent binge drinking and cannabis use was associated with lower white matter integrity across frontolimbic tracts, such as the cingulum/cingulate gyrus, relative to cannabis or binge drinking alone (Wade et al., 2020). However, no other studies, to our knowledge, have specifically examined concurrent use of cannabis and heavy drinking on stress and reward related responses. In addition, no other research has examined potential sex differences in concurrent alcohol and cannabis use effects on the brain.
The current study is a secondary analysis of an existing dataset that collected stress and alcohol cue reactivity tasks in heavy drinkers, some of whom reported frequent cannabis use on days in which alcohol was consumed. We hypothesized group differences between those who use alcohol and cannabis on the same day (i.e., co-users) and alcohol only users during alcohol and stress cue reactivity in key circuits associated with alcohol and stress cue processing, and further hypothesized a moderating effect of sex on activation patterns. Specifically, we hypothesized that co-users would show medial prefrontal cortex (mPFC) hyperactivation in the neutral state relative to alcohol only users, given that each substance alone has been shown to dysregulate basal HPA and ANS activity and this partially driven by loss of mPFC inhibition of these circuits (Blaine et al., 2019, Cservenka et al., 2018). mPFC neutral state hyperactivity is a strong predictor of shortened time to relapse (Blaine et al., 2019, Blaine et al., 2020). This hyperactivity might be related to changes in known mPFC functions, including attentional bias (Clarke et al., 2014) and emotional awareness (Smith et al., 2014). Similarly, we hypothesized that co-users would show greater thalamic activation in the neutral state than alcohol only users. Altered thalamic activation is a central feature of AUDs that has been linked to greater craving (Volkow et al., 1997). Moreover, the thalamus not only sends, but also receives and modulates, cortical responses to sensory input (Sherman, 2016) and is linked to poorer executive function in those with AUDs (Savage et al., 2020). As a result, we further hypothesized that relative to neutral, both alcohol and stress cues would be associated with hypoactivation relative to neutral cues in these regions (Blaine et al., 2018, Blaine et al., 2020, Seo et al., 2013). Finally, altered cortico-striatal connectivity is another hallmark of AUDs (Lee et al., 2013) which is linked to impaired inhibitory control (Meyer and Bucci 2016) and craving (Bracht et al., 2021). Thus, we hypothesized that co-users would show increased connectivity between the OFC and nucleus accumbens in response to alcohol cues (Klenowski, 2018) and stressful images (Christoffel et al., 2011).
Methods and Materials
Participants
Participants for this study were selected from a larger sample of heavy drinkers that were recruited for a longitudinal study of self-change in alcohol use. Details of the recruiting methods are presented in (Al-Khalil et al., 2021). Briefly, advertisements were focused on individuals who self-identified as a “moderate to heavy drinker,” “binge drinker,” or “weekly drinker,” which were the phrases used in advertisements, flyers, and at recruitment events. To be eligible for the study, participants had to be between the ages of 22 and 55, report “harmful and hazardous drinking” as determined by the Alcohol Use Disorder Identification Test (AUDIT; Saunders et al., 1993) with scores > 7 for men and > 6 for women, have a breath alcohol concentration of 0.000 g% at the screening appointment, and be right-handed, with handedness assessed using the Edinburgh handedness questionnaire (Oldfield, 1971). Potential participants were excluded if they reported receiving treatment for or diagnosis of a psychiatric illness (not including mood disorders), prior head injury, use of medications that impact the central nervous system, or any contraindications for participating in an MRI study (e.g., pregnancy, non- removable metallic implants), history of non-alcohol substance use disorder (not including cannabis, nicotine), estimated IQ < 80, or were seeking treatment for AUD. Institutional Review Board-approved written informed consent was obtained for all participants.
For the current analysis, participants were classified into groups based on substance use behavior that was reported on the 90-day Timeline Follow-back at the baseline assessment (TLFB; (Sobell and Sobell, 2004)). Each day was identified as an alcohol day, cannabis day, or a day in which both substances were used (i.e., co-use days). The total proportion of co-use days was computed for each individual by dividing the number of co-use days by the total number of days on which alcohol was consumed. Individuals who only consumed alcohol and never reported cannabis use in the previous 90 days were identified as alcohol-only users. To be identified as a frequent co-user, participants had to report co-use on at least 50% of days in which alcohol was consumed; all other participants were excluded from the current analysis. Of the 190 participants that were enrolled in the study, 154 completed the imaging task and 148 had usable imaging data (see Supplement for imaging exclusions). Of these 148 with usable imaging data, 24 were classified as frequent co-users, 88 as alcohol-only users, and 36 as other (i.e., infrequent co-users, cannabis use with no co-use). This analysis focused only on frequent co-users and alcohol-only users. With a sample of 112 participants, we had 80% power to detect an interaction effect size of f = 0.27 with an alpha level of 0.05.
Materials
Questionnaires/Interviews
Participants completed a battery of psychological and neuropsychological assessments at the baseline visit including a 90-day TLFB assessment (Sobell and Sobell, 2004), which was used to acquire quantity of alcohol and other substances on each day, the Penn Alcohol Craving Scale (PACS; (Flannery et al., 1999)), and the NIH Toolbox Emotion Battery (Salsman et al., 2013). For the purposes of this analysis, we focused on the Negative Affect and Stress domains of the Emotion Battery which includes the following measures: Anger affect, Anger hostility, Anger physical aggression, Fear affect, Fear somatic, Sadness, Perceived Stress, and Self-efficacy. PACS scores range from 0–30, and NIH Emotion Battery scores are T Scores with a mean of 50 and a standard deviation of 10.
Stress and Alcohol Cue Task
Participants completed two 12.5-min runs with pseudorandom presentations of 150 visual cues (i.e., 50 pictures of each category in each run: alcohol, negative, neutral). Participants were instructed to identify each picture as alcohol, negative, or neutral by pressing the appropriate button - alcohol (index), negative (middle), and neutral (ring) - on a custom-built hand device at the Mind Research Network. Each picture was presented for 2300 milliseconds (ms). Between pictures, a variable duration fixation cross (1380, 1840, or 2300 ms) was presented in order to introduce temporal jitter into the design (Jezzard et al., 2001). Sequences of six stimuli (2 from each category) were presented in mini-blocks, and between each mini-block was an extended fixation period ranging from 4600 ms to 8280 ms. Stimuli were presented using EPrime v2.0 (Psychology Software Tools, PA, USA) and with a rear projection mirror system.
Procedures
fMRI Acquisition
All scans were acquired on a Siemens 3T Trio scanner located at the Mind Research Network. An echo-planar gradient-echo pulse sequence with a simultaneous multi-slice encoding (https://www.cmrr.umn.edu/multiband) was used (TR/TE = 460/29 ms; flip angle = 44 degrees; multi-band acceleration factor = 8, matrix size = 82×82×56 slices, voxel size = 3 mm isotropic, phase encoding direction = AP, bandwidth = 2772 Hz/Px, 1615 images, scan duration = 12.5 min) with a 32-channel head coil to acquire slices parallel to the ventral surface of a participant’s OFC to reduce signal dropout and distortion in this region (Deichmann et al., 2003). Structural MRI was acquired using a high-resolution five-echo T1-weighted MP-RAGE anatomical image (TR/TE/TI = 2530/5.36/1200 ms; flip angle = 7 degrees; matrix size = 256×256×192 sagittal slices; voxel size = 1 mm isotropic).
fMRI Analysis
MRI Quality Control. Imaging data quality was assessed by computing Image Quality Metrics (IQMs) using Magnetic Resonance Imaging Quality Control (MRIQC) (Esteban et al., 2017). Thirty-one runs of data were excluded for quality issues (see details in Supplement).
fMRI Preprocessing
Preprocessing of MRI/fMRI data was performed using fMRIPrep v. 1.5.4 (Esteban et al., 2019) (see Supplement). Briefly, functional images were corrected for distortion, slice-time corrected, motion corrected, and normalized to the MNI 152 template. Functional data were resampled to 2mm isotropic voxels, smoothed with a 6mm Gaussian kernel, and ICA-AROMA (Independent Component Analysis Automated Removal of Motion Artifacts) (Pruim et al., 2015) was used to identify and remove motion artifacts.
Individual and Group Level Analyses
NIH Toolbox.
The eight negative affect and stress measures from the NIH Toolbox were first analyzed using a 2 (Group: Alcohol + Cannabis vs. Alcohol only) x 2 (Sex: Male vs. Female) multivariate analysis of variance (MANOVA), and separate univariate ANOVAs were used to examine each measure independently. In addition, A 2 (Group: Alcohol + Cannabis vs. Alcohol only) x 2 (Sex: Male vs. Female) ANOVA was used to examine group differences in self-reported craving on the PACS.
fMRI Preprocessing
Activation Analysis.
The FMRI Expert Analysis Tool (FEAT) Version 6.00, of the (FMRIB’s Software Library (FSL; www.fmrib.ox.ac.uk/fsl) was used to conduct a general linear model (GLM) analysis on fMRI time series following removal of first five volumes and application of a high pass filter cutoff of 90s. For each run, a GLM was conducted to predict the variance of the BOLD signal according to three explanatory variables (EVs): the presentation of alcohol, neutral, and negative cues, and their temporal derivatives; runs also included 14 confound regressors: framewise displacement, six motion parameters and their derivatives, and the principal component of the aCompCor (i.e., white matter and Cerebrospinal Fluid signal confounds). Individual runs within a subject were analyzed and then combined using a fixed effects model in FEAT that computed mean and pooled variance maps for each contrast of interest.
Psychophysiological Interaction Analysis.
Generalized psychophysiological interaction (gPPI) analyses were conducted to examine how connectivity between brain regions changed as a function of the task condition (McLaren et al., 2012). An anatomical mask was created from the MNI-152_2mm_brain template for left nucleus accumbens (NAcc). The anatomical mask was thresholded with using a 60 percent probabilistic location threshold, binarized, and then resampled onto each participant’s native space (3.024mm x 3.024mm x 3mm). ANTs applytransform was used for native space resampling with the participant’s normalized and preprocessed BOLD timeseries as the reference template and nearest neighbor interpolation. A level 1 GLM was performed including the time-series data with the same task and confound regressors mentioned previously. In addition, the gPPI level-1 GLMs included three interaction EVs, created from the mean-centered seed time-course and each of the three zero-centered task regressors. The interaction terms modeled FC within the left NAcc seed region based on the task condition (seed*alcohol, seed*negative, seed*neutral). Subject and group level models executed with the threshold parameters, also using Mixed FLAME 1, yielded two contrasts of interest mapping the whole brain connectivity with the left NAcc seed region based on presentation of alcohol cues and negative cues after removing the seed connectivity associated with the neutral cues (seed*alcohol-seed*neutral; seed*negative-seed*neutral).
Group Analysis.
For higher-level analysis, stage 1 of the FMRIB’s Local Analysis of Mixed Effects (Woolrich, 2008) was used to model the fixed and random effects for the entire sample. To correct for multiple comparisons, group level statistic images were thresholded using Gaussian Random Field theory with a 2-tailed voxel-wise threshold of Z > 2.81 (p < 0.005) and a cluster significance threshold (Worsley, 2001) of p < .05.
Results
Sample Characterization
Table 1 displays demographics and quantity/frequency of substance use for each group.
Table 1.
Sample characterization
| Frequent co-users | Alcohol only | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| N | 8 | 16 | 41 | 47 |
| Age | 30.8 (8.2) | 30.9 (7.2) | 34.1 (10.8) | 36.1 (10.2) |
| Income | ||||
| $0–29,999 | 50.0% | 37.5% | 43.9% | 57.4% |
| $30,000–59,999 | 50.0% | 50.0% | 36.6% | 17.0% |
| Over $60,000 | 0% | 12.5% | 19.5% | 25.5% |
| Percent Hispanic | 75.0% | 50.0% | 46.3% | 46.8% |
| Race | ||||
| White | 50.0% | 56.3% | 63.4% | 63.8% |
| Black | 0.0% | 6.3% | 4.9% | 6.4% |
| Asian | 12.5% | 12.5% | 4.9% | 4.3% |
| American Indian | 25.0% | 12.5% | 26.8% | 23.4% |
| Native Hawaiian | 0.0% | 0.0% | 0.0% | 2.1% |
| Number drinking days | 49.1 (23.8) | 55.3 (23.0) | 46.1 (23.9) | 44.8 (27.1) |
| Drinks per drinking day | 5.9 (2.8) | 6.4 (2.4) | 5.7 (3.5) | 6.5 (4.8) |
| Drinks per day | 3.7 (3.6) | 3.9 (2.3) | 3.2 (3.7) | 3.4 (3.8) |
| Percent heavy drinking days | 38% (37%) | 37% (34%) | 32% (26%) | 30% (31%) |
| Percent heavy days while drinking | 60% (34%) | 58% (37%) | 59% (31%) | 56% (36%) |
| Drinks per drinking day: Co-use day | 6.0 (2.7) | 6.5 (2.5) | - | - |
| Drinks per drinking day: Alcohol only-use day | 5.3 (1.7) | 6.2 (2.8) | 5.7 (3.5) | 6.5 (4.8) |
| Percent nicotine users | 50% | 75%* | 27%* | 19%* |
A larger proportion of male co-users used nicotine than both females and males who used alcohol only. Drinks per drinking day was computed by totaling the number of drinks consumed over the measured interval (90 days) and dividing by the total number of days in which alcohol was consumed, whereas drinks per day was computed by dividing the total number of drinks by the total number of days in the measured interval (i.e., 90). Similarly, percent heavy drinking days was computed by dividing the total number of days on which individuals had a binge episode and dividing by 90, whereas percent heavy days while drinking was computed by dividing the number of heavy drinking days by the number of days that a participant reported alcohol consumption.
Self-report of Craving and Negative Affect
The Group x Sex interaction was significant for self-reported craving for alcohol (F(1,108) = 4.98, p=0.03), such that female co-users reported higher craving than the other three groups. The main effects of group and sex were also significant (Group: F(1,108) = 9.22, p=0.003; Sex: F(1,08) = 7.68, p=0.007), with co-users reporting higher craving than alcohol-only users and women reporting higher craving than men.
Examination of the negative affect and stress scales revealed several important differences. For sadness, fear affect, and perceived stress, the two-way Group x Sex interactions were significant (sadness: F(1, 106) = 5.56, p = 0.02; fear affect: F(1, 106) = 8.63, p = 0.004; perceived stress: F(1, 106) = 4.27, p = 0.04). For sadness, fear affect, and perceived stress, women co-users had significantly higher scores than the other subgroups. In addition, the main effect of sex was significant for all three (sadness: F(1, 106) = 5.41, p = 0.02; fear affect: F(1, 106) = 7.91, p = 0.006; perceived stress: F(1, 106) = 5.63, p = 0.02), with women reporting higher scores than men. The main effect of group was also significant for all three (sadness: F(1, 106) = 8.21, p = 0.005; fear affect: F(1, 106) = 9.36, p = 0.003; perceived stress: F(1, 106) = 7.22, p = 0.008), with the co-use group reporting greater scores on each measure. The Group x Sex interaction and main effect of sex were not significant for anger affect, but there was a significant group effect (F(1, 106) = 4.29, p = 0.04), with the co-using group reporting higher levels of anger affect than the alcohol-only group. Finally, while the Group x Sex interaction for self-efficacy was not significant (F(1, 106) = 2.32, p = 0.13), both main effects were significant (Group: F(1,106) = 4.61, p = 0.03; Sex: F(1, 106) = 8.22, p = 0.005), with alcohol-only users and men reporting higher self-efficacy than co-users and women, respectively. All other scales failed to show any significant interactions or main effects. Means and standard deviations for each group are presented in Table 2.
Table 2.
Penn Alcohol Craving Scale Scores and NIH Emotions Toolbox Negative Affect and Stress by Co-use status and Sex
| Frequent co-users | Alcohol only | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Cravinga | 16.6 (7.3) | 9.8 (6.3) | 9.6 (6.0) | 9.1 (5.1) |
| Anger Affectb | 59.6 (13.2) | 51.6 (10.4) | 51.9 (9.8) | 53.0 (9.3) |
| Anger Hostility | 56.4 (8.5) | 53.5 (11.7) | 53.1 (9.4) | 52.8 (11.2) |
| Anger Physical Aggression | 53.6 (10.4) | 52.2 (11.0) | 57.0 (10.7) | 56.9 (11.6) |
| Sadnessa | 64.2 (10.2) | 50.3 (13.3) | 51.6 (10.4) | 52.0 (12.9) |
| Fear Affecta | 66.4 (9.4) | 53.5 (11.9) | 53.1 (9.2) | 55.7 (10.7) |
| Fear Somatic | 50.4 (9.2) | 51.1 (10.3) | 48.5 (7.6) | 49.8 (9.0) |
| Perceived Stressa | 64.9 (8.5) | 53.7 (10.6) | 53.8 (10.8) | 53.4 (11.3) |
| Self-efficacyc | 40.1 (9.0) | 47.5 (7.1) | 51.1 (10.0) | 52.0 (9.8) |
A significant interaction between Group and Sex was observed, with women co-users reporting greater craving, sadness, fear affect, and perceived stress than the other three groups. In addition, there were main effects of Sex and Group with women having higher scores than men and co-users having scores craving than alcohol-only users.
A main effect of Group was observed, such that co-users reported higher anger affect than alcohol-only users.
Significant main effects of Sex and Group were observed for self-efficacy, with men scoring higher than women and alcohol-only users scoring higher than co-users.
Alcohol Cue Reactivity
The analysis of the alcohol vs. neutral contrast revealed significant main effects of co-use status and sex, but no Group x Sex interaction. Specifically, participants reporting alcohol use only had greater BOLD response in dorsomedial prefrontal cortex and right thalamus than participants reporting co-use of cannabis and alcohol (see Figure 1). Examination of the nature of the main effects revealed that alcohol-only users and individuals reporting co-use had similar levels of response to the alcohol cues, but the co-using group had significantly higher response to the neutral cues in both dmPFC and thalamus.
Figure 1.
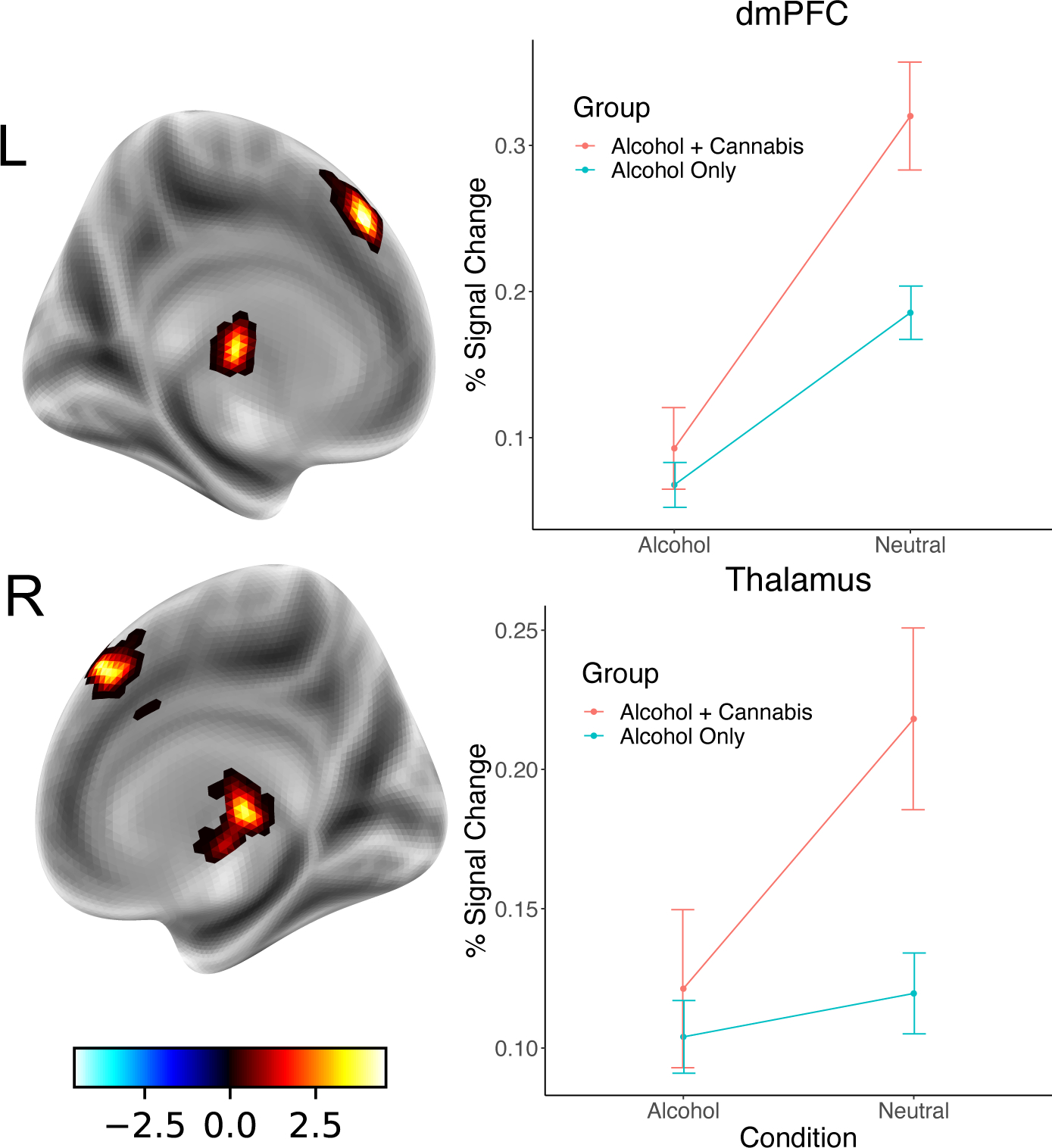
Regions in which individuals reporting only alcohol use had greater BOLD response in the Alcohol vs. Neutral contrast than participants reporting co-use of cannabis and alcohol. In both the thalamus and dorsomedial prefrontal cortex (dmPFC), co-users had greater response to neutral cues than individuals reporting only alcohol use; responses to alcohol cues were similar across both groups.
The main effect of sex was also significant, with women demonstrating greater response than men in bilateral postcentral gyrus/precuneus, left middle frontal gyrus, and rostromedial prefrontal cortex. In all cases except in the postcentral gyrus, women had slightly reduced BOLD response compared to men during alcohol cue presentation, but much lower levels of response during neutral cues than men; these differences resulted in negative contrasts for men and less negative or positive contrasts for women.
Neither the Group x Sex interaction nor main effects were significant for connectivity with the left NAc during alcohol cue reactivity.
Stress Cue Reactivity
The analysis of the negative vs. neutral contrast showed a significant Group x Sex interaction, but no main effects were significant. In the interaction, there was a significant cluster in right fusiform/lingual gyrus. In this region, women co-users had elevated levels of BOLD response during negative compared to neutral cues, a difference that was greater than women alcohol-only users. Men showed the opposite pattern, such that co-users had greater response to neutral stimuli compared to negative cues and alcohol-only users had greater response to negative cues compared to neutral stimuli (see Figure 2).
Figure 2.
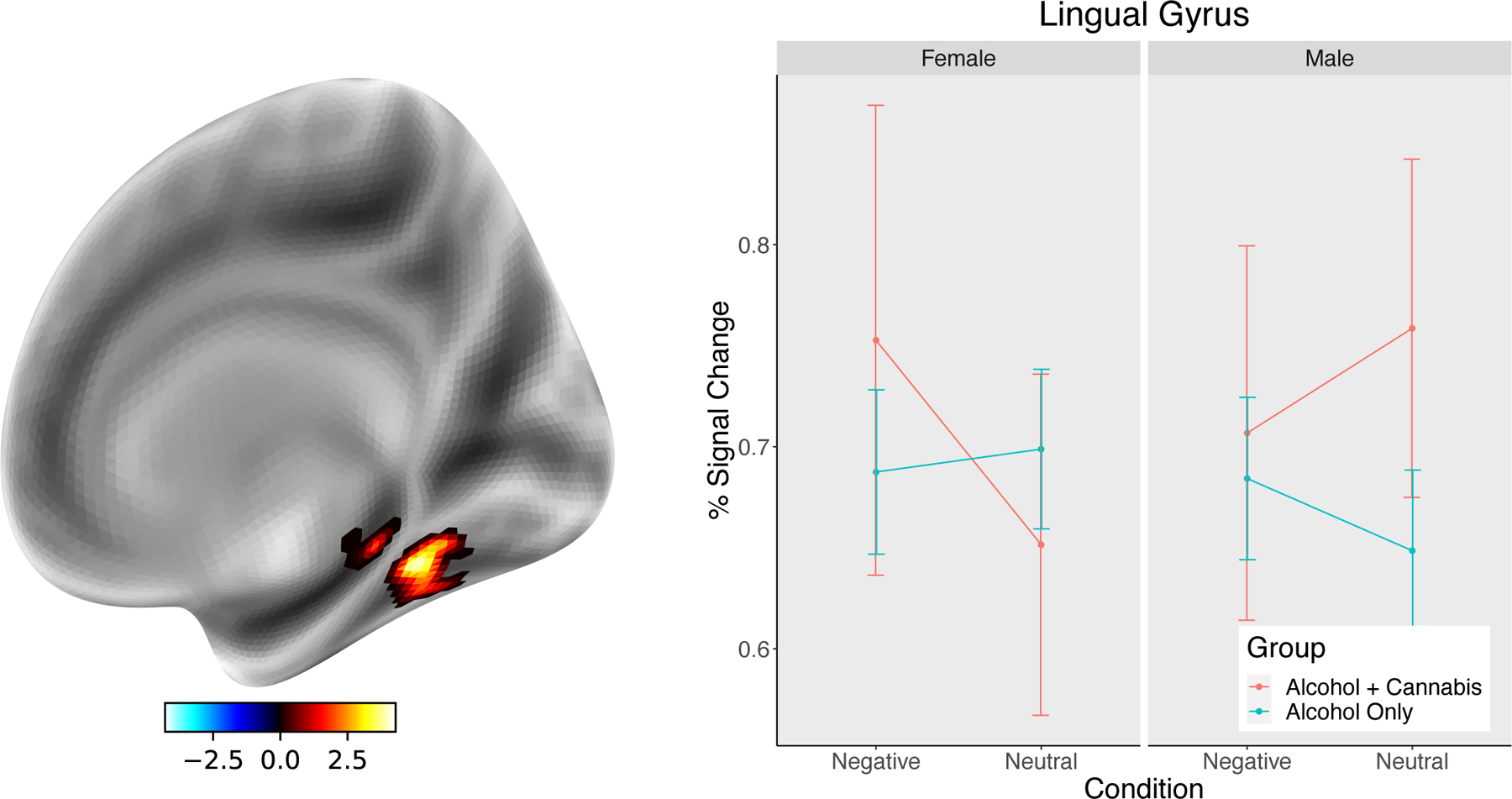
Region in right lingual gyrus showing a sex by co-use interaction in signal change within the Negative vs. Neutral contrast. Whereas female co-users tended to show elevated levels of signal change to negative pictures compared to neutral, male co-users had the opposite pattern with greater response to neutral compared to negative stimuli. Participants using only alcohol had relatively minimal differences between negative and neutral stimuli.
The Group x Sex interaction was significant for left NAc connectivity in two clusters within medial prefrontal cortex/rostral anterior cingulate cortex. In both clusters, women who reported co-use had significantly greater connectivity with left NAc during negative compared to neutral cues compared to the other three groups (see Figure 3).
Figure 3.
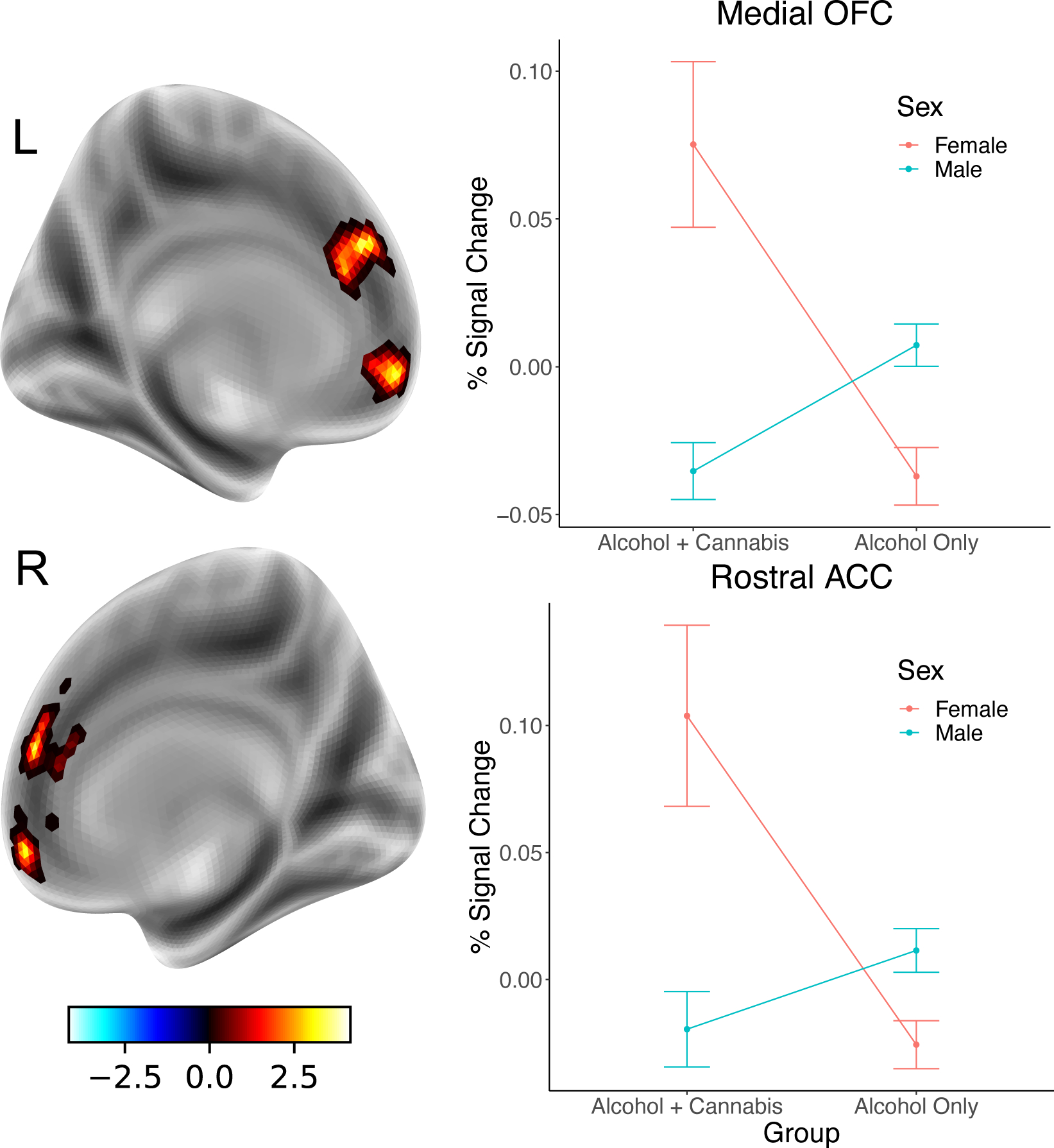
The difference in connectivity between the left nucleus accumbens and medial orbitofrontal cortex (mOFC) and rostral anterior cingulate cortex (rACC) was greatest for women co-users compared to all other groups. Connectivity was determined using a generalized psychophysiological interaction approach with left nucleus accumbens as the seed region, and differences in connectivity measured during the presentation of negative stimuli and neutral stimuli.
Discussion
Overall, this study found that co-use of cannabis and alcohol was associated with differential neural response to alcohol and negative affect pictures, and that sex moderated these co-use effects such that women co-users showed greater responsivity to negative cues and greater engagement of neural circuits implicated in stress. Additionally, self-report assessments of negative affect and stress indicated a similar set of findings, with women co-users demonstrating elevated levels of negative affect and stress compared to women who reported only alcohol use and compared to men. These findings further support literature suggesting increased negative consequences in individuals who report co-use (Cummings et al., 2019). This extends those findings to suggest that women who co-use may have even greater levels of risk than co-using men.
In the alcohol cue reactivity contrast, we observed that participants reporting alcohol use only had greater BOLD response in thalamus and dorsomedial PFC than those who reported co-use of cannabis and alcohol. Importantly, this difference was driven by increased response to neutral cues in the co-using participants rather than an alcohol-related effect; co-using participants had greater positive response to neutral cues in both regions. The dmPFC is involved in monitoring ongoing performance, a process that relies on increased attentional resources (Sajad et al., 2019), and appears to be sensitive to deliberation and response time (Grinband et al., 2011). In the current study, response times to alcohol cues were faster than neutral cues, but there was no difference between co-users and alcohol-only users, suggesting that the different activation to neutral cues was not likely related to a difference in response selection. However, some studies have shown that this region is more active with greater uncertainty (Volz et al., 2004), so it may be possible that co-users had greater uncertainty about the category of the neutral pictures compared to alcohol-only users. The thalamus acts to gate sensory information to cortical regions and the elevated response during neutral cues could be the result of additional visual processing that is necessary to identify the relevant cue type. While the neural responses to alcohol cues alone did not show any differences between the groups, co-users did report higher levels of craving for alcohol over the past week compared to alcohol-only users, and women reported greater craving than men. This finding is only preliminary, but it may suggest that co-use acts to sensitize circuits implicated in cue-reactivity, which may lead to greater basal levels of craving.
Of most interest in the current study are the findings on stress reactivity, particularly in women co-users. First, while several previous studies have reported increased negative affect and stress in women who engage in heavy drinking (Peltier et al., 2019), less work has examined these constructs in co-users. The elevated levels of fear affect and perceived stress in women are consistent with use that may occur to reduce negative affect. It may be that women are more likely to co-use alcohol and cannabis, which may be more likely to occur with increased levels of overall psychological distress.
Our brain imaging results also provide evidence of greater reactivity and connectivity during the presentation of stressful cues in women who were co-users. Specifically, we found increased differences in BOLD response to negatively valenced pictures compared to neutral pictures, which we had not hypothesized, in right lingual gyrus among women co-users compared to the other groups. The right lingual gyrus has been implicated in early visual processing, particularly in the process of recognizing and differentiating visual scenes, and is linked to amygdala activity in the processing of emotional scenes (Kehoe et al., 2012). Enhanced response in this region among women co-users may represent increased attention to and processing of the negative affective pictures displayed during the task. This is consistent with prior reports of attentional bias towards stressful cues in CUD (Macatee et al., 2019) and greater reactivity to moderately negative stimuli in women (Yuan et al., 2009). In addition, our connectivity analyses demonstrated that women co-users had greater connectivity between left NAc and two regions within medial frontal cortex: medial OFC and rostral ACC. The NAc has major efferent and afferent connections throughout medial frontal cortex (George and Koob, 2010), which have been implicated in the regulation of emotional responses. The connections with the left NAc may be key in the links between stress responses and subjective craving in individuals with AUD or other substance use disorders (Jasinska et al., 2014). The enhanced connectivity in women co-users when presented with negative images may suggest a potential mechanism that leads to increased likelihood of co-use in this group compared to alcohol-only groups and male co-users (Peltier et al., 2019). Future studies that more precisely assess co-use and/or simultaneous use, as well as reactions to stressful events (e.g., through techniques such as daily diaries or ecological momentary assessment) will be important to more fully understand the link between neural responses and increased risk for co-use of cannabis and alcohol.
It is important to consider a few potential limitations when interpreting the results from this study. First, the sample that was recruited for the primary study were recruited based on heavy alcohol use, and thus the grouping of participants in the current study are limited in the lower range of alcohol use, as compared to treatment seekers with alcohol use disorder, and there is an imbalance in the number of participants per group. Future studies that select based on cannabis and alcohol use will be necessary to determine whether the results reported here are generalizable to more diverse samples. This will be particularly important for women who use both substances, as this seems to be a group that may be at highest risk for experiencing negative affect and stress, which could lead to greater substance use in the future. In addition, it will be critical in future studies to include cannabis-only and control groups to identify the mechanisms that are unique to co-users, as the current design cannot differentiate the relative contributions of alcohol and cannabis to alcohol or stress cue reactivity. Another limitation is the use of retrospective reports of daily alcohol and cannabis use, which were used to identify co-use days. Future studies that incorporate daily diaries or ecological momentary assessment methods will be more likely to accurately capture co-use or simultaneous use days, the temporal sequence of use, and precipitating events including stress and/or craving. While the timeline follow-back method does have limitations, this is the first study to our knowledge that was able to identify the proportion of co-use days and select individuals who are frequent co-users, a methodological advance over prior investigations. Another limitation that must be considered is variability of where female participants were in their menstrual cycle during the time of the scan, given known influences of the menstrual cycle on stress-induced BOLD responses (e.g., Goldstein et al., 2010). Future investigations should carefully track menstrual cycle to use as a covariate or restrict scanning to a single phase (e.g., first half of follicular phase). Next, our groups differed in their use of nicotine, with the co-using group having a higher proportion of tobacco use than the alcohol-only group. Future studies should determine whether this important confound is a population defining feature of co-users, and whether the effects of nicotine use can be partialed out in future analyses. In the current study, controlling for nicotine use reduced the cluster size of all findings below our a priori alpha level. Finally, the voxelwise threshold used to form clusters (i.e., z = 2.81) is below the current recommendation in the field (i.e., z= 3.1; Eklund et al., 2016), which could lead to spurious findings. However, only the medial OFC connectivity finding is no longer significant when increasing the voxelwise threshold to z = 3.1, suggesting that our findings are generally robust.
In conclusion, the current study examined alcohol and stress cue reactivity among individuals reporting co-use of cannabis and alcohol and found differences in neural circuits implicated in the experience of stress as well as in sensory and motor processing. Women co-users appeared to be particularly susceptible to these effects. While prior research has characterized negative effects of co-use, future work should focus on sex differences both in terms of consequences and potential mechanisms that lead to greater levels of risk in women co-users. Careful characterization of co-users and single drug users will be particularly important, as prior neuroimaging studies and the current study have relied on retrospective reports of concurrent or simultaneous use (Bedillion et al., 2021). Identifying mechanisms underlying increased risk will be critical to understanding the interactive effects of cannabis and alcohol, and for identifying targets for prevention-based interventions.
Supplementary Material
Funding:
This work was supported by NIH grant R01 AA023665 (EDC, KW). SKB was supported by R00 AA25401 and EBA was supported by R01 DA039924. The authors declare no competing interests.
References
- Al-Khalil K, Vakamudi K, Witkiewitz K, Claus ED (2021) Neural correlates of alcohol use disorder severity among nontreatment-seeking heavy drinkers: An examination of the incentive salience and negative emotionality domains of the alcohol and addiction research domain criteria. Alcohol Clin Exp Res 45:1200–1214. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Pihl RO, Benkelfat C, Brunelle C, Young SN, Leyton M (2008) The role of dopamine in alcohol self-administration in humans: Individual differences. Eur Neuropsychopharmacol 18:439–447. [DOI] [PubMed] [Google Scholar]
- Bedillion MF, Blaine SK, Claus ED, Ansell EB (2021) The Effects of Alcohol and Cannabis Co-Use on Neurocognitive Function, Brain Structure, and Brain Function. Curr Behav Neurosci Rep 8:134–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, Sinha R (2019) Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict Biol 24:1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Seo D, Sinha R (2017) Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict Biol 22:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Sinha R (2017) Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Wemm S, Fogelman N, Lacadie C, Seo D, Scheinost D, Sinha R. (2020). Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation. Am J Psychiatry 177:1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T, Soravia L, Moggi F, Stein M, Grieder M, Federspiel A, Tschümperlin R, Batschelet HM, Wiest R, Denier N (2021) The role of the orbitofrontal cortex and the nucleus accumbens for craving in alcohol use disorder. Transl Psychiatry 11:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, David Jentsch J, Roche DJO, Ramchandani VA, Miotto K, Ray LA (2018) Differences in the subjective and motivational properties of alcohol across alcohol use severity: Application of a novel translational human laboratory paradigm. Neuropsychopharmacology 43:1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Russo SJ (2011) Structural and synaptic plasticity in stress-related disorders. Rev Neurosci 22:535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JF, Browning M, Hammond G, Notebaert L, MacLeod C (2014) The Causal Role of the Dorsolateral Prefrontal Cortex in the Modification of Attentional Bias: Evidence from Transcranial Direct Current Stimulation. Biol Psychiatry 76:946–952. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SWF, Filbey FM, Sabbineni A, Hutchison KE (2011) Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 36:2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RL, Ellickson PL, Bell RM (1998) Simultaneous polydrug use among teens: Prevalence and predictors. J Subst Abuse 10:233–253. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Lahanas S, Dotson-Bossert J (2018) Marijuana use and hypothalamic pituitary-adrenal axis functioning in humans. Front Psychiatry 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings C, Beard C, Habarth JM, Weaver C, Haas A (2019) Is the Sum Greater than its Parts? Variations in Substance-Related Consequences by Conjoint Alcohol-Marijuana Use Patterns. J Psychoactive Drugs 51:351–359. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R (2003) Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage 19:430–441. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D, Silva L (2017) Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res 1654:157–164. [DOI] [PubMed] [Google Scholar]
- Earleywine M, Newcomb MD (1997) Concurrent versus simultaneous polydrug use: Prevalence, correlates, discriminant validity, and prospective effects on health outcomes. Exp Clin Psychopharmacol 5:353–364. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016) Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ (2017) MRIQC: Advancing the Automatic Prediction of Image Quality in MRI from Unseen Sites. PLoS One 12:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ (2019) fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Beaton D, Prashad S (2021) The contributions of the endocannabinoid system and stress on the neural processing of reward stimuli. Prog Neuro-Psychopharmacology Biol Psychiatry 106:110183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM (1999) Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res 23:1289–1295. [PubMed] [Google Scholar]
- George O, Koob GF (2010) Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev 35:232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N (2010) Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 30:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J (2011) The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage 57:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ (2011) Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology (Berl) 218:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Alejandra Infante M, Castro N, Brumback T, Meruelo AD, Tapert SF (2015a) Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: A three-year longitudinal study. Neuropsychology 29:829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, Tapert SF (2015b) Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci 16:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y (2014) Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neurosci Biobehav Rev 38:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzard P, Matthews PM, Smith SM (2001) Functional magnetic resonance imaging: An introduction to methods, Functional Magnetic Resonance Imaging: An Introduction to Methods. Oxford University Press. [Google Scholar]
- Kehoe EG, Toomey JM, Balsters JH, Bokde AL (2012) Healthy aging is associated with increased neural processing of positive valence but attenuated processing of emotional arousal: an fMRI study. Neurobiol Aging 34:809–821. [DOI] [PubMed] [Google Scholar]
- Kelly AB, Chan GCK, Mason WA, Williams JW (2015) The relationship between psychological distress and adolescent polydrug use. Psychol Addict Behav 29:787–793. [DOI] [PubMed] [Google Scholar]
- Klenowski PM (2018) Emerging role for the medial prefrontal cortex in alcohol-seeking behaviors. Addict Behav 77:102–106. [DOI] [PubMed] [Google Scholar]
- Koob GF (2008) A Role for Brain Stress Systems in Addiction. Neuron 59:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Sells JR, Ramchandani VA, Hommer DW, George DT, Sinha R, Heilig M (2015) Methods for inducing alcohol craving in individuals with co-morbid alcohol dependence and posttraumatic stress disorder: Behavioral and physiological outcomes. Addict Biol 20:733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee E, Ku J, Yoon KJ, Namkoong K, Jung YC (2013) Disruption of orbitofronto-striatal functional connectivity underlies maladaptive persistent behaviors in alcohol-dependent patients. Psychiatry Investig 10:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Cooney NL (1999) Inducing craving for alcohol in the laboratory. Alcohol Res Heal 23:174–178. [PMC free article] [PubMed] [Google Scholar]
- Ludwig AM (1988) Understanding the alcoholic’s mind: The nature of craving and how to control it., Understanding the alcoholic’s mind: The nature of craving and how to control it. New York, NY, US, Oxford University Press. [Google Scholar]
- Macatee RJ, Okey SA, Albanese BJ, Schmidt NB, Cougle JR (2019) Distress intolerance moderation of motivated attention to cannabis and negative stimuli after induced stress among cannabis users: an ERP study. Addict Biol 24:717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Clifford PR, Clapper RL (1992) Patterns and predictors of simultaneous and concurrent use of alcohol, tobacco, marijuana, and hallucinogens in first-year college students. J Subst Abuse 4:319–326. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Weerts EM, Xu X (2018) A paradigm for examining stress effects on alcohol-motivated behaviors in participants with alcohol use disorder. Addict Biol 23:836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012) A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Gueorguieva RV, Pittman B, Rosen RR, Aslanzadeh F, Tennen H, Leen S, Hawkins K, Raskin S, Wood RM, Austad CS, Dager A, Fallahi C, Pearlson GD (2017) Longitudinal influence of alcohol and marijuana use on academic performance in college students. PLoS One 12:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Bucci DJ (2016) Imbalanced Activity in the Orbitofrontal Cortex and Nucleus Accumbens Impairs Behavioral Inhibition. Curr Biol 26:2834–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Berre AP Le, Serventi M, Kalon E, Haas AL, Padula CB, Schulte T (2019) Brain activation to cannabis- and alcohol-related words in alcohol use disorder. Psychiatry Res - Neuroimaging 294:111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971) The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL (2015) Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16:579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Kloska DD, Terry-McElrath YM, Lee CM, O’Malley PM, Johnston LD (2018) Patterns of simultaneous and concurrent alcohol and marijuana use among adolescents. Am J Drug Alcohol Abuse 44:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, McKee SA (2019) Sex differences in stress-related alcohol use. Neurobiol Stress 10:100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, Mackillop J, Amlung M, King AC (2012) Human Laboratory Paradigms in Alcohol Research. Alcohol Clin Exp Res 36:972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF (2015) ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112:267–277. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM (1999) Does urge to drink predict relapse after treatment? Alcohol Res Heal 23:225–232. [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Bludau S, Palomero-Gallagher N, Caspers S, Mohlberg H, Eickhoff SB, Seitz RJ, Amunts K (2018) Cytoarchitecture, probability maps, and functions of the human supplementary and pre-supplementary motor areas. Brain Struct Funct 223:4169–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajad A, Godlove DC, Schall JD (2019) Cortical microcircuitry of performance monitoring. Nat Neurosci 22:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, Kupst MJ, Kelly MAR, Bode RK, Choi SW, Lai JS, Griffith JW, Stoney CM, Brouwers P, Knox SS, Cella D (2013) Emotion assessment using the NIH Toolbox. Neurology 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Savage LM, Nunes PT, Gursky ZH, Milbocker KA, Klintsova AY (2021) Midline Thalamic Damage Associated with Alcohol-Use Disorders: Disruption of Distinct Thalamocortical Pathways and Function. Neuropsychol Rev 31:447–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H (2013) Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addict Biol 18:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R (2011) Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp 32:1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (2016) Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci 19:533–541. [DOI] [PubMed] [Google Scholar]
- Smith R, Fass H, Lane RD (2014) Role of medial prefrontal cortex in representing one’s own subjective emotional responses: A preliminary study. Conscious Cogn 29:117–130. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (2004) Measuring Alcohol Consumption Assessing Alcohol Problems: A Guide for Clinicians and Researchers. Niaaa Nih 75–100. [Google Scholar]
- Subbaraman MS, Kerr WC (2015) Simultaneous versus concurrent use of alcohol and cannabis in the national alcohol survey. Alcohol Clin Exp Res 39:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2019) Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. HHS Publ No PEP19–5068, NSDUH Ser H-54 170:51–58. [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N (1997) Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386:830–833. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Bhler M, Von Der Goltz C, Hermann D, Mann K, Kiefer F (2011) Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: A randomized trial. Biol Psychiatry 69:1060–1066. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, Von Cramon DY (2004) Why am I unsure? Internal and external attributions of uncertainty dissociated by fMRI. Neuroimage 21:848–857. [DOI] [PubMed] [Google Scholar]
- Vujanovic AA, Bonn-Miller MO, Petry NM (2016) Co-Occurring Posttraumatic Stress and Substance Use: Emerging Research on Correlates, Mechanisms, and Treatments - Introduction to the Special Issue. Psychol Addict Behav 30:713–719. [DOI] [PubMed] [Google Scholar]
- Wade NE, Thomas AM, Gruber SA, Tapert SF, Filbey FM, Lisdahl KM (2020) Binge and Cannabis Co-Use Episodes in Relation to White Matter Integrity in Emerging Adults. Cannabis Cannabinoid Res 5:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemm SE, Sinha R (2019) Drug-induced stress responses and addiction risk and relapse. Neurobiol Stress 10:100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M (2008) Robust group analysis using outlier inference. Neuroimage 41:286–301. [DOI] [PubMed] [Google Scholar]
- Worsley KJ (2001) Statistical analysis of activation images In: Functional Magnetic Resonance Imaging: An Introduction to Methods.
- Yuan J, Luo Y, Yan JH, Meng X, Yu F, Li H (2009) Neural correlates of the females’ susceptibility to negative emotions: An insight into gender-related prevalence of affective disturbances. Hum Brain Mapp 30:3676–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


