Abstract
Background.
Included among the significant risk factors for opioid overdose is concomitant use of other central nervous system depressants, particularly alcohol. Given the continued expansion of community pharmacy in the continuum of care, it is imperative to characterize alcohol use among pharmacy patients dispensed opioids in order to establish a foundation for identification and intervention in these settings.
Methods.
This secondary analysis utilized data from a one-time, cross-sectional health assessment conducted among patients dispensed opioid medications in 19 community pharmacies in Indiana and Ohio. Adult, English speaking, patients not receiving cancer care who were dispensed opioid medications were asked to self-report alcohol and substance use, behavioral and physical health, and demographic information. Descriptive and logistic regression analyses were employed to characterize alcohol use/risky alcohol use and patient characteristics associated therewith.
Results.
The analytical sample included 1,494 individuals. Participants were on average 49 years of age (Standard Deviation=14.9)—with 6% being persons of color (n=89). Weekly drinking was reported by 18.1% (n=204) and daily drinking was reported by 6.8% (n=77) of the study sample, with a total of 143 (9.6%) participants reporting moderate/high risk drinking.
Males (Adjusted Odds Ratio [AOR]=1.94, 95% CI=1.3,2.9), those with higher pain interference (AOR=1.44, 95% CI=1.0,2.0), overdose history (AOR=1.93, 95% CI=1.1,3.5), sedative use (AOR=2.11, 95% CI=1.3,3.5), and tobacco use (AOR=2.41, 95% CI=1.6,3.7) had increased likelihood of moderate/high risk alcohol use (all p<0.05).
Conclusions.
Medication labeling and clinical guidelines clearly indicate that patients should abstain from concomitant use of opioids and alcohol. This study has identified rates and associated risk factors of risky alcohol use among a clinical sample of community pharmacy patients dispensed opioid medications. Continuing this line of research and potential clinical service development has the ability to improve patient safety through addressing a significant gap within the current opioid epidemic.
Keywords: Community pharmacy, alcohol use, opioid medication
1.0. Introduction
Notwithstanding important declines in opioid prescribing in recent years (IQVIA Institute, 2018), enough opioids were dispensed in 2018 to supply 51.4 prescriptions per 100 Americans, and 11% of US counties dispensed enough medication to supply every person with an opioid prescription (CDC, 2020). Metanalytic research has documented average rates in the US of opioid medication misuse among those prescribed opioid medications of 21–29%, with individual studies reporting rates 4 times as high (Vowles et al., 2015). Among the most severe repercussions of the ongoing epidemic is overdose, which in 2018, nearly 70% of the more than 67,000 fatal drug overdoses involved an opioid (Wilson et al., 2020). Recent reports have documented spikes in opioid overdose in the face of the COVID-19 pandemic (CDC, 2021).
Among the significant risks for overdose that persist for those prescribed opioid medications is concomitant use of alcohol. This serious risk is based in both alcohol and opioids acting on μ-opioid receptors (Amato et al., 2011)—potentiating analgesic effects of these substances and producing possible severe respiratory depression, sedation (Brands et al., 2008; FDA, 2018a, b, c; Kuerbis et al., 2014), and heightened overdose risk (CDC, 2018; Cochran et al., 2016). Human lab studies clearly document respiratory and sedative effects of alcohol and opioid medications (Darwish et al., 2015; van der Schrier et al., 2017), resulting in not only overdose risk but also heightened abuse liability compared to alcohol or opioids used alone (Zacny and Gutierrez, 2011). Labeling by the Food and Drug Administration of marketed formulations of opioid medications contain strong warnings advising against concomitant use of opioids and any other central nervous system depressants, specifically citing alcohol (FDA, 2016, 2018a, b, c). The Centers for Disease Control and Prevention clearly concludes: “The risk of harm increases with the amount of alcohol consumed, but there is no safe level of alcohol use for people using opioids”(CDC, 2018).
While there is an emerging literature that is attempting to uncover patient motivation for combining alcohol and opioid use, which could be based in desire for greater analgesia or attempts to cope with emotions (Witkiewitz and Vowles, 2018; Zegel et al., 2021), previous research has nevertheless documented that approximately 1 in 5 individuals with alcohol use disorder also have opioid use disorder (OUD)—with some studies showing as high as 2 in 5 having OUD (Hartzler et al., 2010; Hser et al., 2017). Further, among those individuals seeking alcohol treatment, nearly 70% reported opioid medication misuse in the month before treatment initiation (Price et al., 2011), and half of those with binge alcohol use also report opioid medication misuse in the last month (Esser et al., 2019). Prescription opioid misuse has been noted to be 3.5 times higher among binge drinkers compared to non-drinkers/non-binge drinkers (Esser et al., 2019). Risky alcohol use has also been documented among community pharmacy patients dispensed opioid medications, with ~20% reporting current high-risk drinking (Cochran et al., 2016; Cochran et al., 2019). Beyond these descriptive data, as previous authors have noted, little research has been done to document concomitant alcohol and opioid medication use (Witkiewitz and Vowles, 2018). Given the risks associated with concomitant use of alcohol and opioids, further research is imperative to inform clinicians and health care professionals regarding clinical prevalence and opportunities to address this problem.
With the above mentioned continued nationwide misuse of prescribed opioid medications (SAMHSA., 2020), the well-documented multiple physical health concerns; such as pain and poor general health; and behavioral health problems; such as depression, substance use, and overdose risk; among patients with pain/opioid misuse (IsHak et al., 2018; Jones et al., 2012; Prescription Drug Monitoring Program Assist, 2016; Salas et al., 2018; Scherrer et al., 2017; Scherrer et al., 2016; Seal et al., 2012), along with prescribers reporting high levels of burden and burnout from the myriad of clinical duties (Reith, 2018)—it is imperative to explore community pharmacy as a setting for identification and intervention for possible concomitant use of alcohol and opioid medications.
The role of community pharmacy and pharmacists is rapidly expanding in the US, with vaccination services (Isenor et al., 2016), point of care testing (Gubbins et al., 2017; Hohmeier et al., 2018), and medication therapy management (Viswanathan et al., 2015) being examples of this broadening role. As the last healthcare professional encountered before opioid dispensation, the pharmacist is critical in addressing risks of possible concomitant use of alcohol and opioids. Furthermore, community pharmacy is widely accessible in the US, with >93% of those in the US living ≤5 miles (National Association of Chain Drug Stores, 2015) of the >60,000 store settings (CDC, 2013), which includes the vast majority of rural areas where health care access is limited. This service setting represents an unparalleled opportunity to identify and discuss concomitant alcohol and opioid use with patients across the country. However, a paramount first step to devising approaches to address possible concomitant use of opioid medications and alcohol in community pharmacy settings is gaining an understanding of the physical and behavioral health characteristics of the community pharmacy patients engaged in these behaviors. The purpose of this hypothesis-generating study was to describe recent drinking behaviors and to explore psychosocial and demographic factors correlated with moderate/high risk alcohol use among patients dispensed opioid medications in community pharmacy settings.
2.0. Methods
2.1. Design, Sites, and Participants
Details of the original study have been described previously elsewhere (Cochran et al., 2021) and are related here in brief. The parent study was a one-time, cross-sectional health assessment conducted among patients filling opioid medications in 19 community pharmacies in Indiana and Ohio from November 2019 to October 2020. Power for the primary outcome analyses estimated a required sample of 1,523 participants, and sample enrollment was achieved in that study (Cochran et al., 2021). Recruitment followed a convenience sampling method (Elfil and Negida, 2017), and patients being dispensed opioid medications were approached by pharmacy staff (pharmacists and technicians) and provided information about possible study participation. Patients interested in learning more about the study were provided a tablet computer where they could enter their contact information, which once submitted, generated an e-consent document. Patients unable to provide contact information at the pharmacy site and caregivers picking up medications on behalf of patients were given an information sheet about how the patient could initiate the survey remotely. Patients who completed the informed consent were directed to a self-screening assessment.
The self-screening assessment asked potential participants to verify they were: 18 years of age or older, English speaking, and not receiving active cancer treatment (given the largely unknown differences in opioid use patterns for this group compared to patients without cancer treatment). In addition, participants were also not included in this study if they had previously completed the survey (verified by study staff), self-reported current or impending involvement with the criminal justice system, or were solely taking buprenorphine or combination products (given some formulations are indicated for pain while others are indicated for opioid use disorder treatment). Eligible participants who completed the survey were provided with a $50 gift card for their time. This project was approved by the University of Cincinnati and University of Utah Institutional Review Boards and was registered on ClinicalTrials.gov (NCT03936985).
2.2. Assessments
The health assessment survey captured alcohol and substance use, behavioral and physical health, and demographic information of patients dispensed opioid medications in community pharmacies. Patients completed 38–113 questions in the survey, dependent on number of substances reported. An important note is that patients were not directly asked if they were consuming alcohol simultaneous to their opioid medication treatment regimen (this was not the intent of the parent study). Rather, these patients with dispensed opioid medications were asked about their recent and regular alcohol use.
Alcohol and drug use.
The primary assessment of alcohol use in this study was the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST). The ASSIST has been found to have criterion, construct, concurrent, and discriminant validity (Humeniuk and Ali, 2006). We likewise utilized additional ASSIST subscales for prescription opioids, street opioids (subscales developed/validated by McNeely, et al. (2016), cannabis, sedatives, cocaine, tobacco, alcohol, methamphetamine, hallucinogens, prescription stimulants, and inhalants (Humeniuk and Ali, 2006) to characterize substance use involvement among participants. A risk score was calculated for each substance subscale and classified into three discrete ASSIST risk categories—low, moderate, and high (Humeniuk and Ali, 2006), with all variables collapsed into low vs. moderate/high use herein.
We also examined individual items from the ASSIST prescription opioid and alcohol subscales, calculating and comparing frequencies and percentages of prescription opioid misuse and alcohol use. These items included: (1) In the past three months, how often have you used the substances you mentioned? (2) During the past three months, how often have you had a strong desire or urge to use [prescription misused or drug]? (3) During the past three months, how often has your use of [prescription misused or drug] led to health, social, legal, or financial problems? (4) During the past three months, how often have you failed to do what was normally expected of you because of your use of [prescription misused or drug] (response categories: never, once or twice, monthly, weekly, daily or almost daily)? (5) Has a friend or relative or anyone else ever expressed concern about your use of [prescription misused or drug]? (6) Have you ever tried and failed to control, cut down or stop using [prescription misused or drug] (response categories: No, never; Yes, in the past 3 months; Yes, but not in the last 3 months)? We included the latter 2 items in our analyses given that participant responses could overlap with the time periods described in the first 4 items, that is to say, the previous 3 months. Regarding prescription opioids, it is important to note that the initial ASSIST instructions regarding use of medications specifically directs patients to:
“...not report medications that are used as prescribed by your doctor. However, please do report use of these medications if you have taken them ‘recreationally’ - which means taking medications that were prescribed to you or to someone else just for the feeling or experience they cause, to get high, or taking them more often or at higher doses than prescribed.”
Thus, we refer to individual level item analyses regarding opioid medication use as “misuse” in our Results and Discussion sections. In addition to substance use characteristics, any history of illicit drug overdose experience was captured using the Overdose Experiences, Self and Witnessed—Drug instrument (Fernandez et al., 2019).
Mental and physical health.
Depression was captured using the 2-item criterion-valid Patient Health Questionnaire (PHQ)-2, with a score of 3 considered as the optimal cut-point for depressive disorders (Kroenke et al., 2003). Participant current pain was assessed using the Brief Pain Inventory, a valid/reliable instrument consisting of pain severity (i.e. intensity of pain) and interference (i.e. interference with daily function) subscales (Keller et al., 2014), scored on a 0–10 scale with higher scores indicating worse pain severity or pain interference. General health status was measured using a 1-item subscale from the construct-valid Short Form-12 (Luo et al., 2003), scored on a 1 to 5 scale with higher scores indicating better health.
Demographics.
Participant demographics assessed included age (years), sex (male vs. female), race (person of color vs. non-person of color [analyzed dichotomously herein given limited sample size amongst racial subgroups], see Limitations), marital status (married vs. not married), employment status (full-time/part-time, disabled, vs. not employed), and insurance status (insured vs. not insured).
2.3. Analyses
Given the descriptive focus of this study, with the exception of the age and pain variables, all other were dichotomized to promote interpretability of the outcomes. We employed descriptive statistical analyses to characterize the study population, comparing demographic, health, and substance use characteristics across low vs. moderate/high risk alcohol use groups and individual alcohol and prescription opioid misuse items. Specifically, we calculated means and standard deviations with accompanying t-tests as well as frequencies and percentages with chi-square tests. Furthermore, we examined exploratory unadjusted associations between moderate/high risk alcohol use with each covariate of interest and a single multivariate logistic regression analysis wherein we assessed associations between demographic, health, and substance use characteristics collected and moderate/high risk alcohol use simultaneously. As a hypothesis-generating study, corrections for multiple comparisons, such as Bonferroni, were not required (Ranstam, 2019). Thus, all p-values were set to 0.05. Given the cross-sectional nature of our data and its limitations, our goal was to provide analyses that would be accessible to clinicians and healthcare professionals. All analyses were conducted using Stata 16.1 (StataCorp, 2021).
3.0. Results
A total of 2,090 patients who came into the pharmacy locations to receive their opioid medication completed the e-consent form, with 1,921 completing the self-screening assessment, of whom 281 were screen-failed. Primary reasons for screen-fails included solely filling buprenorphine medications (n=127), previously completing the survey (n=50), and currently receiving treatment for cancer (n=49). A total of 1,629 and 1,523 participants initiated and completed the survey, respectively. Our current study analytical sample included 1,494 individuals reporting sufficient data to calculate an ASSIST drinking score (i.e., n=29 missing ASSIST data). A total of 143 (9.6%) participants reporting moderate/high risk drinking.
3.1. Demographic Characteristics
Participants were on average 49 years of age (Standard Deviation [SD]=14.9)—with 6% being a person of color (n=89). Just under half of participants were employed (41.5%, n=614), with few being uninsured (5%, n=74). A total of 37.9% (n=564) of participants were male, with a significantly larger proportion of males reporting moderate/high risk alcohol use (52.5%, n=75) compared to low alcohol risk use (36.3%, n=489, p<0.001; Table 1). In multivariate models, males had a nearly two times increased likelihood for engaging in moderate/high risk drinking than females (Adjusted Odds Ratio [AOR]=1.94, 95% CI=1.3,2.9, p=0.001; Table 2).
Table 1.
Population characteristics, overall and stratified by low vs. moderate/high risk alcohol use a
| Total (1,494) | Low risk (n=1,351) | Moderate/high risk (n=143) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | χ2 | p | ||
|
Demographics
| |||||||||
| Age b | 49.4 | 14.9 | 49.6 | 14.9 | 47.7 | 14.4 | 1.47 | 0.14 | |
| Male | 37.9 | 564 | 36.3 | 489 | 52.5 | 75 | 14.30 | <0.001 | |
| Person of Color | 6.0 | 89 | 5.9 | 80 | 6.3 | 9 | 0.03 | 0.86 | |
| Married | 55.2 | 819 | 55.7 | 747 | 50.7 | 72 | 1.28 | 0.26 | |
| >High school | 40.4 | 592 | 41.2 | 546 | 32.4 | 46 | 4.14 | 0.04 | |
| Uninsured | 5.0 | 74 | 4.8 | 64 | 7.1 | 10 | 1.42 | 0.23 | |
| Employment | Full/part time | 41.5 | 614 | 40.5 | 542 | 50.4 | 72 | 5.52 | 0.06 |
| Disabled | 23.6 | 349 | 24.1 | 323 | 18.2 | 26 | |||
|
Health | |||||||||
| General health b | 3.2 | 1. 4 | 3.2 | 1.4 | 3.1 | 1. 3 | 1.15 | 0.25 | |
| Pain severity b | 2.4 | 0.7 | 2.4 | 0.7 | 2.4 | 0.7 | 0.69 | 0.49 | |
| Pain interference b | 2.3 | 0.8 | 2.3 | 0.8 | 2.4 | 0.7 | −1.12 | 0.26 | |
| Depression | 19.7 | 290 | 19.5 | 259 | 21.7 | 31 | 0.39 | 0.53 | |
|
Overdose, opioid medication misuse, and substance use | |||||||||
| Illicit drug overdose history | 10.0 | 149 | 8.7 | 117 | 22.4 | 32 | 27. 10 | <0.001 | |
| Opioid medication misuse | 45.3 | 664 | 44.2 | 588 | 55.9 | 76 | 6.74 | <0.01 | |
| Street opioids c | 1.4 | 20 | 1.7 | 17 | 2.1 | 3 | 0.70 | 0.41 | |
| Cannabis | 11.4 | 168 | 10.3 | 138 | 21.7 | 30 | 16.16 | <0.001 | |
| Sedatives misuse | 17.3 | 257 | 15.8 | 211 | 32.2 | 46 | 24.27 | <0.001 | |
| Cocaine | 2.2 | 32 | 1.9 | 29 | 5.0 | 7 | 5.79 | 0.02 | |
| Tobacco | 37.2 | 549 | 35.2 | 468 | 57.9 | 81 | 27.89 | <0.001 | |
| Methamphetamine c | 1.6 | 23 | 1.4 | 19 | 2.8 | 4 | 1.67 | 0.20 | |
| Stimulant medication misuse c | 4.3 | 64 | 3.9 | 52 | 8.5 | 12 | 6.53 | 0.01 | |
| Hallucinogens c | 0.6 | 9 | 0.5 | 6 | 2.1 | 3 | 5.79 | 0.05 | |
| Inhalants | 0.1 | 2 | 0.2 | 2 | 0.0 | 0 | 0.21 | 1.00 | |
| Alcohol | Weekly drinking | 18.1 | 204 | 15.6 | 154 | 35.0 | 50 | 353.75 | <0.001 |
| Daily drinking | 6.8 | 77 | 2.0 | 20 | 39.9 | 57 | |||
Percentages may not calculate to 100% based on missing responses.
Mean, standard deviation, and t statistic.
p-value based on Fisher exact.
Table 2.
Univariate and multivariate associations between characteristics and moderate/high risk alcohol use
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Demographics | OR | SE | p | 95%CI | OR | SE | p | 95%CI | |
| Age | 0.99 | 0.0 | 0.14 | (1.0–1.0) | 1.00 | 0.0 | 0.81 | (1.0–1.0) | |
| Male (ref. female) | 1.94 | 0.3 | <0.001 | (1.4–2.7) | 1.94 | 0.4 | 0.001 | (1.3–2.9) | |
| Person of color (ref. non-person of color) | 1.07 | 0.4 | 0.86 | (0.5–2.2) | 1.08 | 0.5 | 0.85 | (0.5–2.5) | |
| Married (ref. non-married) | 0.82 | 0.1 | 0.26 | (0.6–1.2) | 0.76 | 0.2 | 0.20 | (0.5–1.2) | |
| ≤High school (ref. >high school) | 0.68 | 0.1 | 0.04 | (0.5–1.0) | 0.66 | 0.1 | 0.06 | (0.4–1.0) | |
| Insured (ref. not ensured) | 1.52 | 0.5 | 0.24 | (0.8–3.0) | 0.99 | 0.4 | 0.99 | (0.4–2.4) | |
| Employment (ref. not employed) | Full/part time | 1.40 | 0.3 | 0.10 | (0.9–2.1) | 1.42 | 0.3 | 0.14 | (0.9–2.3) |
| Disabled | 0.85 | 0.2 | 0.52 | (0.5–1.4) | 0.72 | 0.2 | 0.28 | (0.4–1.3) | |
|
Health | |||||||||
| General health | 0.95 | 0.1 | 0.38 | (0.8–1.1) | 0.98 | 0.1 | 0.74 | (0.8–1.1) | |
| Pain severity | 0.92 | 0.1 | 0.49 | (0.7–1.2) | 0.66 | 0.1 | 0.03 | (0.5–1.0) | |
| Pain interference | 1.14 | 0.1 | 0.26 | (0.9–1.4) | 1.44 | 0.2 | 0.03 | (1.0–2.0) | |
| Depression (ref. not depressed) | 1.14 | 0.2 | 0.54 | (0.8–1.7) | 0.59 | 0.2 | 0.07 | (0.3–1.1) | |
|
Overdose and illicit substance use | |||||||||
| Illicit drug overdose history (ref. no overdose) | 3.04 | 0.7 | <0.001 | (2.0–4.7) | 1.93 | 0.6 | 0.03 | (1.1–3.5 ) | |
| Opioid medication (ref. low risk use) | 1.60 | 0.3 | 0.01 | (1.1–2.3) | 1.25 | 0.3 | 0.33 | (0.8–1.9) | |
| Street opioids (ref. low risk use) | 1.69 | 1.1 | 0.41 | (0.5–5.8) | 1.05 | 1.1 | 0.96 | (0.1–7.5) | |
| Cannabis (ref. low risk use) | 2.41 | 0.5 | <0.001 | (1.6–3.8) | 1.59 | 0.4 | 0.10 | (0.9–2.8) | |
| Sedatives (ref. low risk use) | 2.54 | 0.5 | <0.001 | (1.7–3.7) | 2.11 | 0.5 | 0.003 | (1.3–3.5) | |
| Cocaine (ref. low risk use) | 2.75 | 1.2 | 0.02 | (1.2–6.5) | 0.99 | 0.7 | 0.99 | (0.3–3.8) | |
| Tobacco (ref. low risk use) | 2.53 | 0.5 | <0.001 | (1.8–3.6) | 2.41 | 0.5 | <0.001 | (1.6–3.7) | |
| Methamphetamine (ref. low risk use) | 2.03 | 1.1 | 0.21 | (0.7–6.0) | 0.15 | 0.2 | 0.14 | (0.0–1.8) | |
| Stimulant medication (ref. low risk use) | 2.29 | 0.8 | 0.01 | (1.2–4.4) | 1.67 | 0.7 | 0.21 | (0.7–3.8) | |
| Hallucinogens (ref. low risk use) | 4.74 | 3.4 | 0.03 | (1.2–19.2) | 2.60 | 2.8 | 0.38 | (0.3–22.0) | |
| Inhalants a (ref. low risk use) | -- | -- | -- | -- | -- | -- | -- | -- | |
Could not compute due to insufficient sample
3.2. Mental and Physical Health
No statistically significant differences were found between those with low and moderate/high risk drinking for depression, with just under one-fifth of the sample having a positive screening (n=290). Average general health was 3.2 (SD=1.4) on the 5-point scale among those with low and moderate/high risk drinking, with an average pain severity of 2.4 (SD=0.7) and interference of 2.3 (SD=0.8) on the 10-point scale—with no statistically significant differences detected (Table 1). However, higher pain interference in adjusted models was related to greater odds of moderate/high risk drinking (AOR=1.44, 95% CI=1.0,2.0, p=0.03), but higher pain severity was associated with lower odds of moderate/high risk drinking (AOR=0.66, 95% CI=0.5,1.0, p=0.03; Table 2).
3.3. Substance Use
Regarding substance use, a greater proportion of persons with moderate/high risk alcohol use than low-risk alcohol use reported a history of illicit drug overdose (moderate/high alcohol=22.4% vs. low alcohol=8.7%; p<0.001). Similarly, a significantly larger proportion of individuals with moderate/high risk alcohol use compared to low-risk alcohol use reported moderate/high risk use of prescription opioid medications (moderate/high alcohol=55.9%, n=76 vs. low alcohol=44.2%, n=588), cannabis (moderate/high alcohol=21.7%, n=30 vs. low alcohol=10.3%, n=138), sedatives (moderate/high alcohol=32.2%, n=46 vs. low alcohol=15.8%, 211), cocaine (moderate/high alcohol=5%, n=7 vs. low alcohol=1.9%, n=29), tobacco (moderate/high alcohol=57.9%, n=81 vs. low alcohol=35.2%, n=468), and stimulant medications (moderate/high alcohol=8.5%, n=12 vs. low alcohol=3.9%, n=52; all p<0.05; Table 1). For adjusted models, overdose history (AOR=1.93, 95% CI=1.1,3.5), sedative (AOR=2.11, 95% CI=1.3,3.5), and tobacco use (AOR=2.41, 95% CI=1.6,3.7) were associated with increased likelihood of moderate/high risk alcohol use (all p<0.05, Table 2).
3.4. Alcohol and Prescription Opioid Use Behaviors
Weekly drinking was reported by 18.1% (n=204) and daily drinking was reported by 6.8% (n=77) of the study sample (i.e., responses to: In the past three months, how often have you used the substances you mentioned). A significantly larger portion of moderate/high risk use participants reported weekly and daily drinking compared to those with low risk use (weekly: moderate/high risk use 35%, n=50 vs. low risk use 15.6%, n=154; daily: moderate/high risk use 39.9%, n=57 vs. low risk use 2%, n=20, p<0.001; Table 1).
For individual level item analyses, Figure 1 shows 44.8% of those who reported daily drinking and 34.4% of those who reported weekly drinking also reported daily opioid medication misuse. Comparisons of proportions of responses to individual subscale items asking about risky alcohol use and prescription opioid misuse behaviors during the past 3 months (i.e., strong desire/urge to use; use leading to problems; failed to do what was expected) and lifetime behaviors (i.e., expressing concern; failing to control/cutdown/stop using) are presented in Figures 2 and 3. Participant reports on these individual items were largely similar. The item with the largest descriptive differences in the last 3 months was participant reported daily desire/urge (alcohol 4.7%, n=54; prescription opioids: 28.7%, n=285), weekly desire/urge (alcohol 10.1%, n=115; prescription opioids: 3.3%, n=33), and monthly desire/urge (alcohol 6.6%, n=75; prescription opioids: 2.5%, n=25) to use.
Figure 1.
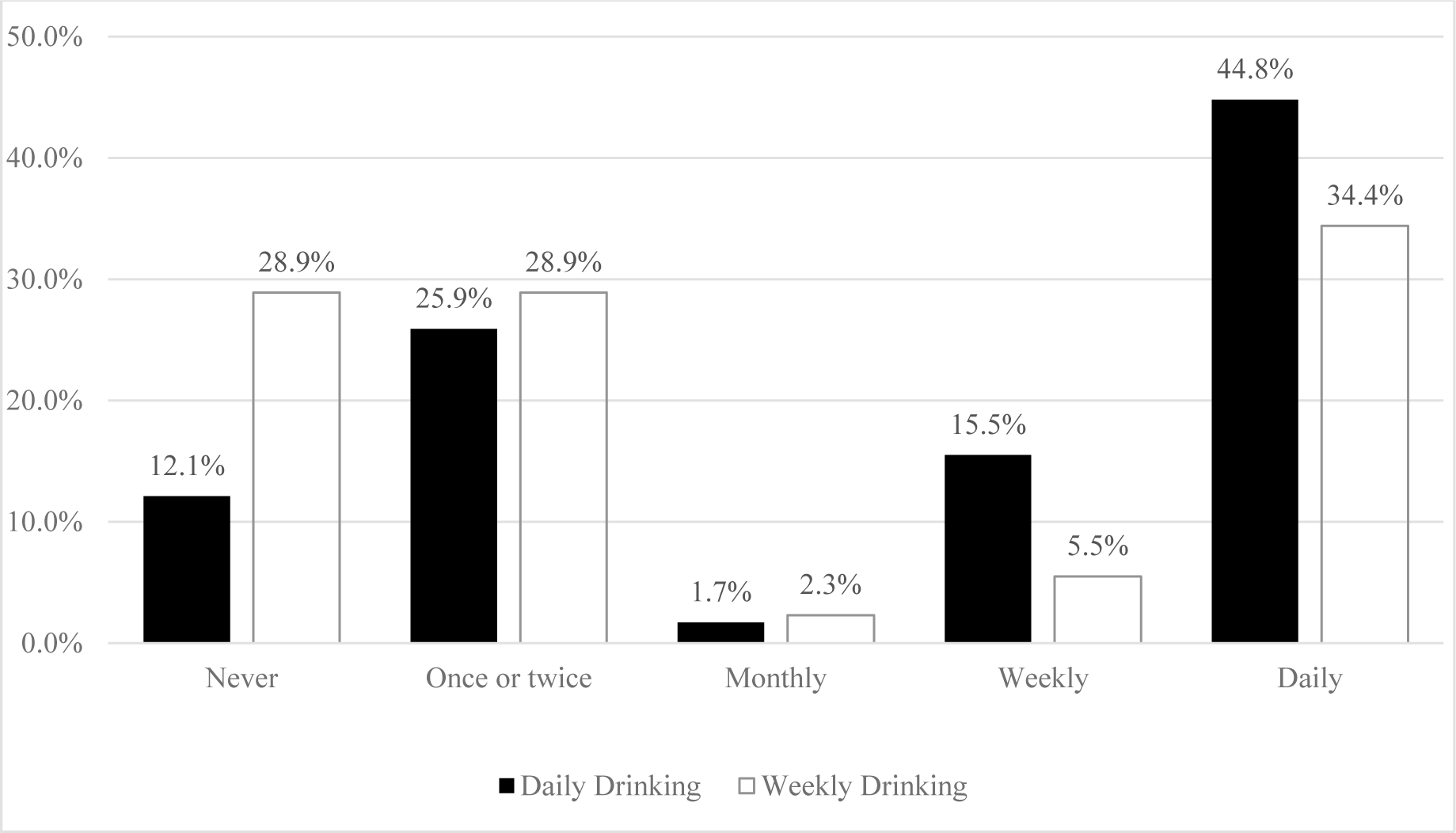
Previous 3 Month Frequency of Prescription Opioid Misuse Use among Participants with Daily (n=58) and Weekly (n=128) Drinking Who Were Dispensed Opioid Pain Medications
Figure 2.
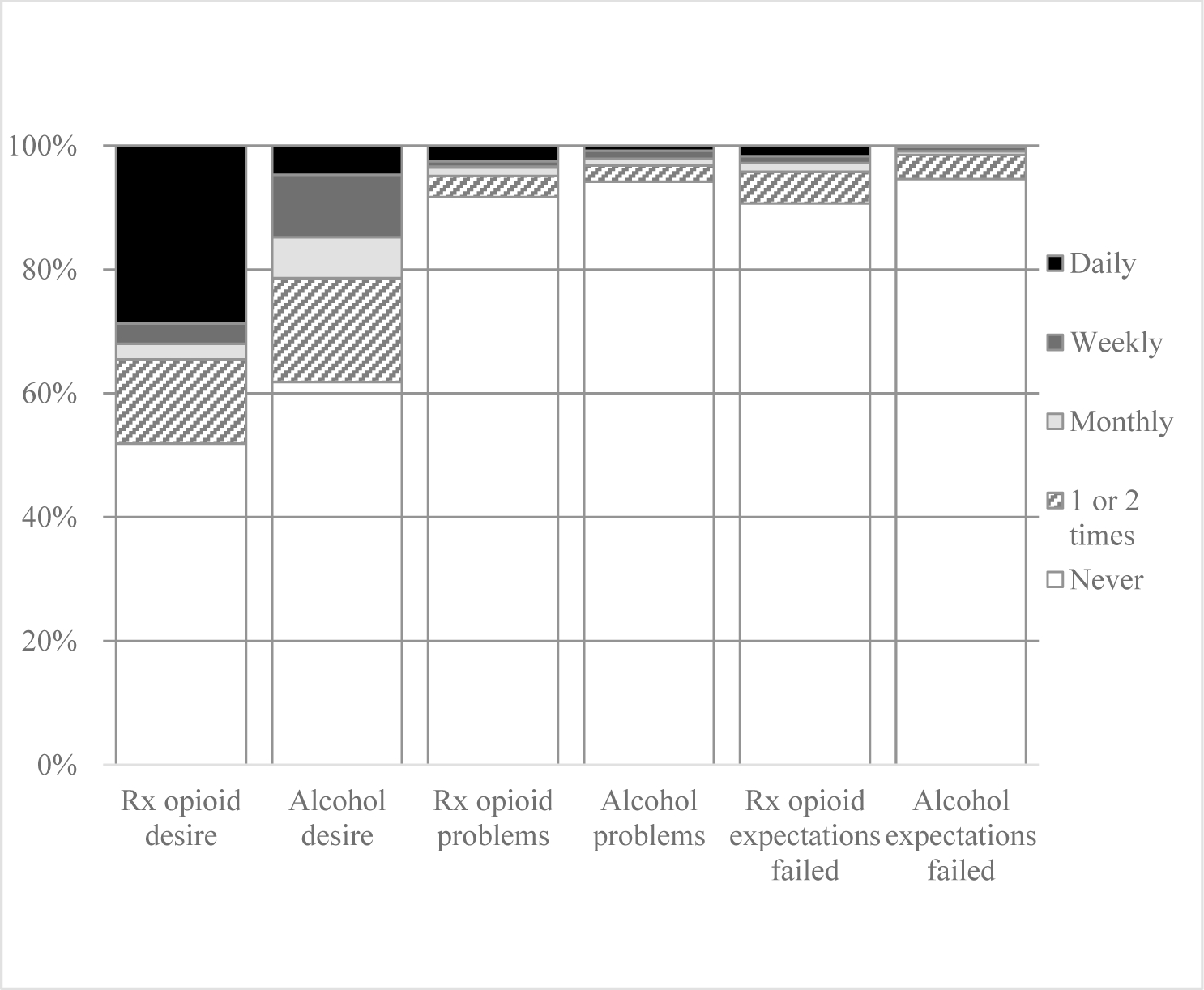
Opioid Medication Misuse and Alcohol Use Behaviors in the Past 3 Months Among Patients Dispensed Opioid Medications
Figure 3.
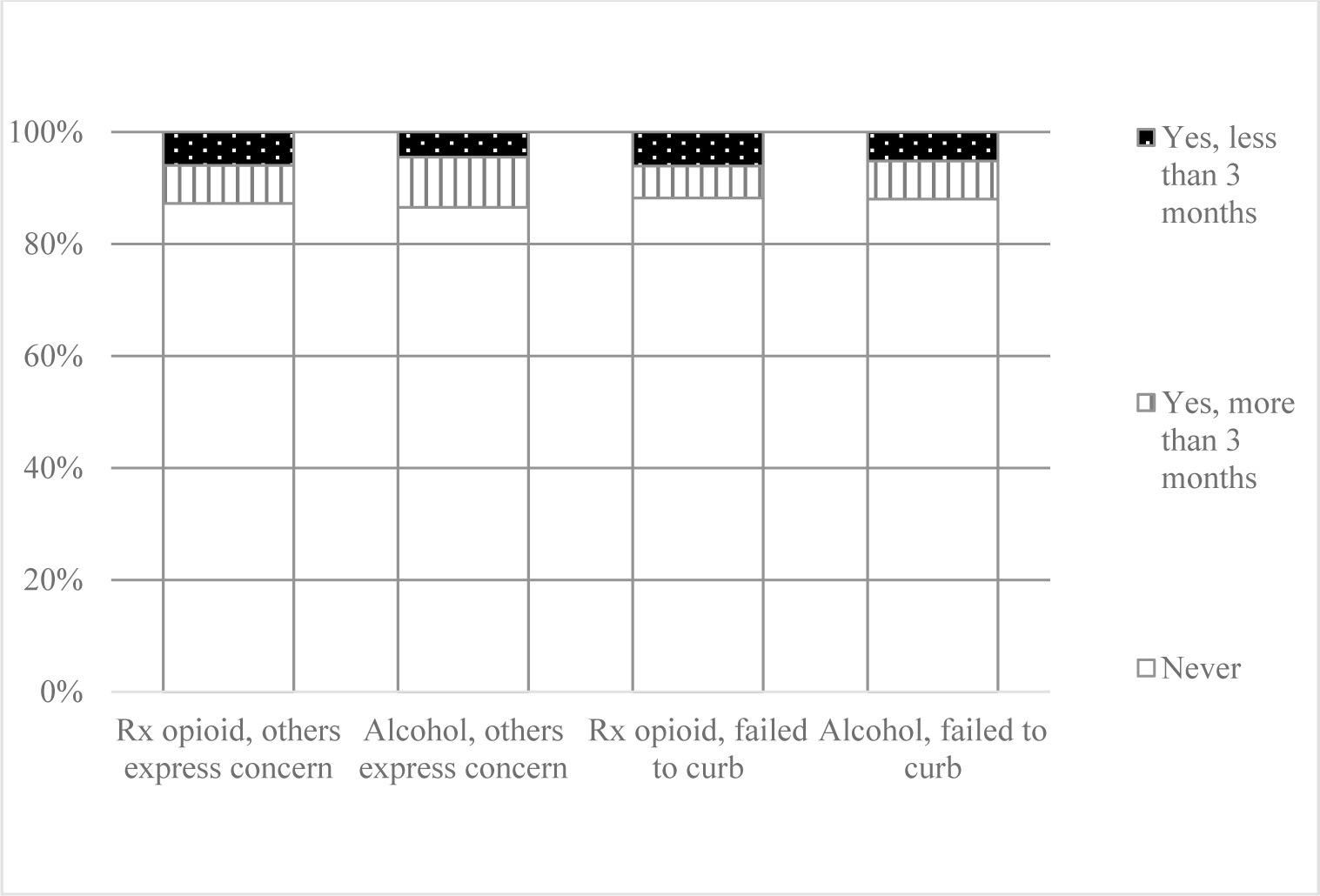
Opioid Medication Misuse and Alcohol Use Behaviors during Lifetime among Patients Dispensed Opioid Medications
4.0. Discussion
This study characterized recent drinking behaviors and explored psychosocial and demographic correlates of moderate/high risk alcohol use among patients dispensed opioid medications in community pharmacy settings. These results provide potentially useful clinical information to advance the understanding in the field regarding patient attributes associated with risky drinking among patients dispensed opioid medications, engaged in moderate/high risk opioid use, and/or opioid misuse. Results reported herein highlight important implications for advancing the field in three areas: the clinical prevalence of alcohol use and related behaviors among patients filling opioid medications, patient-level demographic and health characteristics associated with increased likelihood of risky drinking, and information that supports intervention development and pilot testing.
4.1. Clinical Prevalence
Rates of alcohol and opioid use reported appear to generally be in-line with previous research of possible concomitant use (Cochran et al., 2016; Cochran et al., 2019; Esser et al., 2019; Hartzler et al., 2010; Hser et al., 2017; Vowles et al., 2015). Noted in the methods, patients were not directly asked if they were consuming alcohol simultaneous to their opioid medication treatment regimen. Nevertheless, the alcohol assessment period included the time-frame in which the patient had an opioid prescription. Within these circumstances, we identified approximately 25% of patients prescribed opioid medications with weekly or daily drinking patterns (weekly drinking 18.1%; daily drinking 6.8%). Of those reporting drinking in the last 3 months, 44.8% reported daily drinking and 34.4% weekly drinking while simultaneously reporting daily opioid medication misuse (i.e., responses to: In the past three months, how often have you used the substances you mentioned). Moreover, nearly 10% of our sample endorsed moderate/high risk drinking behaviors. One noted difference from some previous research in community pharmacy and the current study was that risky alcohol use was measured in previous research using the Alcohol Use Disorders Identification Test-C (AUDIT-C; Cochran et al., 2016; Cochran et al., 2019), which identified ~20% with risky alcohol use. This difference in prevalence of risk use may be based in the AUDIT-C focusing more on quantity and frequency (Bush et al., 1998) of use compared to the ASSIST that focuses on specific behaviors related to possible use disorders such as desire to use, problems experienced, and failure to do what was expected.
Therefore, these results illustrate that despite decreased opioid prescribing rates noted over the last years—a non-trivial subset of patients may continue to engage in moderate/high risk alcohol use or regular drinking patterns that increase chances for adverse events. A question thus arises if misinformed norms among patients or health care professionals exist that suggest concomitant use may be acceptable. Such continued behaviors raise the possible concern whether it may be necessary for pharmacists and prescribers to do more to communicate the risks of possible concomitant use of alcohol and pain medications to patients. Future research would benefit from establishing an in-depth understanding of patients’ motivations for these behaviors, such as possible patient hope for increased analgesia, and what messages come from health care professionals about possible concomitant use.
We also observed that individual subscale items for prescription opioid and alcohol use were similar in proportion of patients’ endorsement. The exception in item level endorsement was for desire/urge to use. For this item, prescription opioids appear to have a larger portion of patients who reported daily desire/urge to use—but with alcohol having higher proportions for desire/urges at weekly and monthly intervals. This finding could be indicative of greater physical dependence on opioids for participants compared to alcohol with associated acute withdrawal, which could be related to the fact all study participants were prescribed opioid treatment. It may point to the fact, nevertheless, that pharmacists could have greater success targeting alcohol use compared to opioids for initial use reductions as interventions are designed.
4.2. Characteristics Associated with Risk Alcohol Use
Results of this study showed that individuals engaged in possible concomitant opioid and alcohol use had a number of demographic and health related characteristics associated with moderate/high level risk use. In spite of trends in opioid prescribing that have been shown to have higher rates among women (Ganem et al., 2016; Glanz et al., 2019; Lo-Ciganic et al., 2019; Salas et al., 2018), in both descriptive and regression analyses, males prescribed opioid medications were more likely to be engaged in moderate/high risky alcohol use. This finding is potentially valuable in that it demonstrates that despite more women being prescribed opioid medications nationally, drinking patterns showing men outpacing women (SAMHSA., 2020) remain constant within that broader trend. Thus, efforts to identify risk likely need to be equally distributed, rather than only focusing on a single sex.
Pain interference (interference with daily function) was also noted to be marginally associated with moderate/high risky alcohol use among participants. Previous research among patients living with HIV and/or chronic pain has shown that risky alcohol use is associated with higher levels of pain interference (Larance et al., 2016; Ngo et al., 2021) and opioid medication misuse (Ngo et al., 2021). Future longitudinal research should work to untangle causal ordering of these associations, such that may expose antecedental factors that could be tenable targets for early intervention. Conversely, pain severity was associated with lower risk of moderate/high risk alcohol use. This finding may be related to previous research that has shown inconsistent associations between different levels of alcohol use, pain severity, and opioid medication misuse (Paulus et al., 2019), which requires further research to clarify possible alcohol-related dose effects on these relationships.
We likewise noted that other illicit substance use involvement, specifically moderate/high risk sedative and tobacco use, were associated with moderate/high risk alcohol use. The association of sedative misuse is of particular importance given the clearly documented central nervous system depressant effects already potentially at play with opioids combined with alcohol (Armoon et al., 2021). Given these associations, it therefore comes as little surprise that illicit overdose history was also associated with risky drinking within our sample. Future research should seek to ascertain the multiplicative risk produced by these substances and possible methods for risk attenuation.
4.3. Needed Intervention Development and Testing
Given the continued prevalence along with identified risk factors associated with possible concomitant use of alcohol and opioids, it is likely necessary to work to develop strategies for community pharmacists to engage patients with these behaviors. While underpinning motivations for combined use of alcohol and opioids, such as distress tolerance (Zegel et al., 2021), may not be possible to be addressed by pharmacy clinicians, it may be reasonable to universally screen patients picking up opioid medications for current and recent alcohol use in order to set the foundation for motivation-based interventions about eliminating alcohol use during opioid therapy or potentially reconnecting patients with prescribers to consider substituting non-opioid pain treatments. Further, it also seems reasonable to include auxiliary assessments of possible use of additional psychoactive substances, particularly those that could increase respiratory depressive effects of concomitant use—such as sedative medications. In all cases, however, pharmacists should ensure patients are dispensed naloxone and are trained in its administration. Such practice focused education could be effectively disseminated through continuing education programs to pharmacy professionals—and monitored through dispensing records to assess impact of training.
4.4. Limitations
While this study has many strengths, it nevertheless possesses limitations that should be taken into account when considering its findings. Our study results have demonstrated relationships between assessed indicators in anticipated directions, which provides assurance of a degree of validity. However, the study and analyses were all conducted within a cross-sectional design. Thus, causal inference is not possible, and future longitudinal research would be needed to make inferences about causal relationships observed herein. In addition, this study relied on self-report measures, which can be associated with response biases. Further, while this study boasts a somewhat large sample size, it was nevertheless conducted within two midwestern US states, and respondents were primarily White—thus all results may only represent the sample recruited with limited external validity. Future research should seek to replicate this study within other geographic locations that would include greater racial/ethnic diversity to examine if findings extend to other pharmacy populations. We also acknowledge that despite the well validated measures employed in this study for alcohol use, frequency and quantity of alcohol use may not be well depicted by our assessments in relation to opioid consumption. More detailed examination of alcohol use would enhance the understanding of the extent to which patients are engaging in risky alcohol use and opioid medication use. To this important point, this hypothesis-generating study is a first step in characterizing risk among community pharmacy patients who may use alcohol and opioid medications. Future research should seek to replicate and expand upon these findings, including leveraging more advanced transdiagnostic or approaches to phenotyping that could yield further insight into these patients, their behaviors, and possible needs. However, should the goal of such approaches be more prognostic (e.g., predicting weekly or daily drinking), this may necessitate time series data to allow for greater predictive potential.
5.0. Conclusion
The opioid epidemic continues to negatively affect public health in the US, with its most severe consequences, including overdose, increasing. Among the most significant risk factors for opioid overdose include concomitant use of other substances that are central nervous system depressants. Alcohol is included among such substances—which has stimulated labeling and clinical guidelines to encourage patients to abstain from concomitant use of opioids and alcohol. This study has identified rates and associated risk factors of possible concomitant alcohol use among a clinical sample of community pharmacy patients dispensed opioid medications. Findings herein provide important future steps for research— including needs for further characterization as well as possible development and testing of clinical intervention pathways. Continuing this line of research and clinical service development has the potential to have an important impact on improving patient safety, addressing a significant gap within the current opioid epidemic.
Highlights.
Concomitant alcohol/opioid use is a significant issue among pharmacy patients
Risky alcohol and opioid medication use is associated with other substance use
Pharmacies may be a screening/intervention point for concomitant alcohol/opioid use
Role of Funding Source
This project was supported by a grant from the National Institute on Drug Abuse (UG1DA013732; UG1DA049444). The funder played no part in the conceptualization, execution, or authorship of this project.
Footnotes
Conflict of Interest
No conflicts declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 References
- Amato L, Minozzi S, Davoli M, Vecchi S, 2011. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev (9), Cd005031. [DOI] [PubMed] [Google Scholar]
- Armoon B, SoleimanvandiAzar N, Rostami M, Higgs P, Bayani A, Bayat AH, Mohammadi R, Ahounbar E, Fattah Moghaddam L, 2021. Drug type and risk behaviors associated with non-fatal overdose among people who use drugs: a systematic review and meta-analysis. J Addict Dis, 1–12. [DOI] [PubMed] [Google Scholar]
- Brands B, Blake J, Marsh DC, Sproule B, Jeyapalan R, Li S, 2008. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis 27(3), 37–48. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, 1998. The AUDIT Alcohol Consumption Questions (AUDIT-C): An effective brief screening test for problem drinking. Arch Intern Med 158(16), 1789–1795. [DOI] [PubMed] [Google Scholar]
- CDC, 2013. Select features of state pharmacist collaborative practice laws. US Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- CDC, 2018. Alcohol screening and brief intervention for people who consume alcohol and Use opioids. Centers for Diease Control and Prevention; Atlanta, GA. [Google Scholar]
- CDC, 2020. U.S. Opioid Prescribing Rate Maps. https://www.cdc.gov/drugoverdose/maps/rxratemaps.html#:~:text=The%20overall%20national%20opioid%20prescribing%20rate%20declined%20from%202012%20to,168%20million%20total%20opioid%20prescriptions). (Accessed July 9 2020).
- CDC, 2021. Provisional drug overdose death counts. (Accessed September 13 2021).
- National Association of Chain Drug Stores N, 2015. Improve patients’ access to pharmacist services co-sponsor h.r. 592/s. 314 the pharmacy and medically underserved areas enhancement act. National Association of Chain Drug Stores, Alexandria, VA. [Google Scholar]
- Cochran G, Bacci JL, Ylioja T, Hruschak V, Miller S, Seybert AL, Tarter R, 2016. Prescription opioid use: Patient characteristics and misuse in community pharmacy. J Am Pharm Assoc 56(3), 248–256.e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran G, Brown J, Yu Z, Frede S, Bryan MA, Ferguson A, Bayyari N, Taylor B, Snyder ME, Charron E, Adeoye-Olatunde OA, Ghitza UE, Winhusen T, 2021. Validation and threshold identification of a prescription drug monitoring program clinical opioid risk metric with the WHO Alcohol, Smoking, and Substance Involvement Screening Test. Drug Alcohol Depend, 109067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran G, Chen Q, Field C, Seybert AL, Hruschak V, Jaber A, Gordon AJ, Tarter R, 2019. A community pharmacy-led intervention for opioid medication misuse: A small-scale randomized clinical trial. Drug Alcohol Depend 205, 107570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran G, Gordon AJ, Lo-Ciganic WH, Gellad WF, Frazier W, Lobo C, Chang CH, Zheng P, Donohue JM, 2016. An Examination of Claims-based Predictors of Overdose from a Large Medicaid Program. Med Care 55(3):291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish M, Bond M, Yang R, Tracewell W, Robertson P Jr., 2015. Assessment of alcohol-induced dose dumping with a hydrocodone bitartrate extended-release tablet formulated with CIMA(®) abuse deterrence technology. Clin Drug Investig 35(10), 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfil M, Negida A, 2017. Sampling methods in clinical research; an educational review. Emerg 5(1), e52–e52. [PMC free article] [PubMed] [Google Scholar]
- Esser MB, Guy GP Jr., Zhang K, Brewer RD, 2019. Binge drinking and prescription opioid misuse in the U.S., 2012–2014. Am J Prev Med 57(2), 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2016. FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. Food and Drug Adminisitration; Washington, DC. [Google Scholar]
- FDA, 2018a. Highlights of prescribing information: oxycodone. US Food and Drug Administration. Silver Spring, MD. [Google Scholar]
- FDA, 2018b. Highlights of prescribing information: oxycontin. US Food and Drug Administration. Silver Spring, MD. [Google Scholar]
- FDA, 2018c. Highlights of prescribing information: percocet. US Food and Drug Administration. Silver Spring, MD. [Google Scholar]
- Fernandez AC, Bush C, Bonar EE, Blow FC, Walton MA, Bohnert ASB, 2019. Alcohol and drug overdose and the influence of pain conditions in an addiction treatment sample. J Addict Med 13(1), 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem VJ, Mora AG, Nnamani N, Bebarta VS, 2016. A 3-Year comparison of overdoses treated in a military emergency department-complications, admission rates, and health care resources consumed. Mil Med 181(10), 1281–1286. [DOI] [PubMed] [Google Scholar]
- Glanz JM, Binswanger IA, Shetterly SM, Narwaney KJ, Xu S, 2019. Association between opioid dose variability and opioid overdose among adults prescribed long-term opioid therapy. JAMA Netw Open 2(4), e192613–e192613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbins PO, Klepser ME, Adams AJ, Jacobs DM, Percival KM, Tallman GB, 2017. Potential for pharmacy-public health collaborations using pharmacy-based point-of-care testing services for infectious diseases. J Public Health Manag Pract 23(6), 593–600. [DOI] [PubMed] [Google Scholar]
- Hartzler B, Donovan DM, Huang Z, 2010. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat 39(2), 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmeier KC, Loomis B, Gatwood J, 2018. Consumer perceptions of and willingness-to-pay for point-of-care testing services in the community pharmacy. Res Social Adm Pharm 14(4), 360–366. [DOI] [PubMed] [Google Scholar]
- Hser Y-I, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Huang D, 2017. Chronic pain among patients with opioid use disorder: results from electronic health records data. J Subst Abuse Treat 77, 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, 2006. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) and Pilot Brief Intervention: A technical report of phase ii findings of the WHO ASSIST project. WHO, Geneva. [Google Scholar]
- Institute IQVIA, 2018. Medicine Use and Spending in the U.S. A review of 2017 and outlook to 2022. IQVIA Institute; Durham, NC. [Google Scholar]
- Isenor JE, Edwards NT, Alia TA, Slayter KL, MacDougall DM, McNeil SA, Bowles SK, 2016. Impact of pharmacists as immunizers on vaccination rates: a systematic review and meta-analysis. Vaccine 34(47), 5708–5723. [DOI] [PubMed] [Google Scholar]
- IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, Dascal J, Marcia L, Gohar Y, Eskander L, Yadegar J, Hanna S, Sadek A, Aguilar-Hernandez L, Danovitch I, Louy C, 2018. Pain and depression: a systematic review. Harv Rev Psychiatry 26(6), 352–363. [DOI] [PubMed] [Google Scholar]
- Jones JD, Mogali S, Comer SD, 2012. Polydrug abuse: A review of opioid and benzodiazepine combination use. Drug Alcohol Depend 125(1–2), 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS, 2014. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 20(5), 309–319. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW, 2003. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 41(11), 1284–1292. [DOI] [PubMed] [Google Scholar]
- Kuerbis A, Sacco P, Blazer DG, Moore AA, 2014. Substance abuse among older adults. Clin Geriatr Med 30(3), 629–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larance B, Campbell G, Peacock A, Nielsen S, Bruno R, Hall W, Lintzeris N, Cohen M, Degenhardt L, 2016. Pain, alcohol use disorders and risky patterns of drinking among people with chronic non-cancer pain receiving long-term opioid therapy. Drug Alcohol Depend 162, 79–87. [DOI] [PubMed] [Google Scholar]
- Lo-Ciganic WH, Huang JL, Zhang HH, Weiss JC, Wu Y, Kwoh CK, Donohue JM, Cochran G, Gordon AJ, Malone DC, Kuza CC, Gellad WF, 2019. Evaluation of machine-learning algorithms for predicting opioid overdose risk among medicare beneficiaries with opioid prescriptions. JAMA netw open 2(3), e190968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, George ML, Kakouras I, Edwards CL, Pietrobon R, Richardson W, Hey L, 2003. Reliability, validity, and responsiveness of the short form 12-item survey (SF-12) in patients with back pain. Spine 28(15), 1739–1745. [DOI] [PubMed] [Google Scholar]
- McNeely J, Strauss SM, Rotrosen J, Ramautar A, Gourevitch MN, 2016. Validation of an audio computer-assisted self-interview (ACASI) version of the alcohol, smoking and substance involvement screening test (ASSIST) in primary care patients. Addiction 111(2), 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo B, Liebschutz JM, Cheng DM, Colasanti JA, Merlin JS, Armstrong WS, Forman LS, Lira MC, Samet JH, Del Rio C, Tsui JI, 2021. Hazardous alcohol use is associated with greater pain interference and prescription opioid misuse among persons living with HIV and chronic pain. BMC public health 21(1), 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus DJ, Rogers AH, Bakhshaie J, Vowles KE, Zvolensky MJ, 2019. Pain severity and prescription opioid misuse among individuals with chronic pain: the moderating role of alcohol use severity. Drug Alcohol Depend 204, 107456. [DOI] [PubMed] [Google Scholar]
- Prescription Drug Monitoring Program Assist, 2016. State Profiles West Virginia. Brandeis University Prescription Drug Monitoring Program Training and Technical Assistance Center, Boston MA. [Google Scholar]
- Price AM, Ilgen MA, Bohnert AS, 2011. Prevalence and correlates of nonmedical use of prescription opioids in patients seen in a residential drug and alcohol treatment program. J Subst Abuse Treat 41(2), 208–214. [DOI] [PubMed] [Google Scholar]
- Ranstam J, 2019. Hypothesis-generating and confirmatory studies, Bonferroni correction, and pre-specification of trial endpoints. Acta Orthop 90(4), 297–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith TP, 2018. Burnout in United States healthcare professionals: a narrative review. Cureus 10(12), e3681–e3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas J, Scherrer JF, Ahmedani BK, Copeland LA, Bucholz KK, Sullivan MD, Burroughs T, Schneider FD, Lustman PJ, 2018. Gender and the Association between long-term prescription opioid use and new-onset depression. J Pain 19(1), 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA., 2020. Key substance use and mental health indicators in the United States: results from the 2019 national survey on drug use and health (HHS Publication No. SMA 18–5068, NSDUH Series H-53). Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; Rockville, MD. [Google Scholar]
- Scherrer JF, Salas J, Schneider FD, Bucholz KK, Sullivan MD, Copeland LA, Ahmedani BK, Burroughs T, Lustman PJ, 2017. Characteristics of new depression diagnoses in patients with and without prior chronic opioid use. J Affect Disord 210, 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer JF, Salas J, Sullivan MD, Schneider FD, Bucholz KK, Burroughs T, Copeland L, Ahmedani B, Lustman PJ, 2016. The influence of prescription opioid use duration and dose on development of treatment resistant depression. Prev Med 91, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC, 2012. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA 307(9), 940–947. [DOI] [PubMed] [Google Scholar]
- StataCorp, 2021. Stata Statistical Software: Release 16.1. StataCorp LP, College Station, TX. [Google Scholar]
- van der Schrier R, Roozekrans M, Olofsen E, Aarts L, van Velzen M, de Jong M, Dahan A, Niesters M, 2017. Influence of ethanol on oxycodone-induced respiratory depression: a dose-escalating study in young and elderly individuals. Anesthesiology 126(3), 534–542. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Kahwati LC, Golin CE, et al. , 2015. Medication therapy management interventions in outpatient settings: A systematic review and meta-analysis. JAMA Intern Med 175(1), 76–87. [DOI] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN, 2015. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156(4), 569–576. [DOI] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H.t., Davis NL, 2020. Drug and opioid-involved overdose deaths - United States, 2017–2018. MMWR Morb Mortal Wkly Rep 69(11), 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE, 2018. Alcohol and opioid use, co-use, and chronic pain in the context of the opioid epidemic: a critical review. Alcohol Clin Exp Res 42(3), 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S, 2011. Subjective, psychomotor, and physiological effects of oxycodone alone and in combination with ethanol in healthy volunteers. Psychopharmacology (Berl) 218(3), 471–481. [DOI] [PubMed] [Google Scholar]
- Zegel M, Rogers AH, Vujanovic AA, Zvolensky MJ, 2021. Alcohol use problems and opioid misuse and dependence among adults with chronic pain: The role of distress tolerance. Psychol Addict Behav 35(1), 42–51. [DOI] [PubMed] [Google Scholar]


