Abstract
Objective/Background:
Sleep problems are common in people on the autism spectrum. This study reviews one detailed approach to querying the electronic health record (EHR) in a large tertiary care center.
Patients/Methods:
We developed methods for identifying people on the autism spectrum and defined their sleep problems using the key words, “sleep” or “melatonin”, or International Classification of Diseases (ICD) codes. We examined treatment responses of these individuals to melatonin supplementation.
Results:
Sleep problems were documented in 86% of patients with ages ranging from 6–30 years old. Our specific keyword search yielded more patients with sleep diagnoses than ICD codes alone. About two-thirds of patients who received melatonin supplementation reported benefit from its use.
Conclusions:
Our study provides a framework for using deidentified medical records to characterize sleep, a common co-occurring condition, in people on the autism spectrum. Using specific keywords could be helpful in future work that queries the EHR.
Keywords: Autism Spectrum Disorder, Insomnia, Sleep, Melatonin, Electronic Health Record
1. Introduction
Autism is a heterogenous condition with core features of impairments in social communication and restricted behaviors (“Neurodevelopmental Disorders,” 2013). Sleep problems are highly prevalent in people on the autism spectrum and contribute to challenging daytime behavior and family stress (Herrmann, 2016). Children on the autism spectrum have an increased frequency of sleep problems compared to their typically developing peers (Liu et al., 2006; Reynolds et al., 2019). Carmassi et. al. (2019) reviewed 65 studies and some reported a prevalence of sleep disturbance in people on the autism spectrum between 64% and 93%. There are a wide variety of sleep problems in people on the autism spectrum, with multifactorial causes. Insomnia is one of the most commonly reported sleep problems (Veatch et al., 2015). Insomnia is defined as difficulty initiating or maintaining sleep, with adequate opportunity to sleep, accompanied by daytime consequences (Sateia, 2014). Given their neurodevelopmental disability, the consequences of poor sleep, such as insomnia, may be more profound in people on the autism spectrum.
Prior studies of people on the autism spectrum have evaluated sleep objectively with polysomnography (PSG) or actigraphy, subjectively with validated questionnaires and sleep diaries completed by parents, or a combination of both (Elia et al., 2000; Liu et al., 2006; Malow et al., 2006). These forms of sleep evaluation have limitations with small sample sizes and are often cross-sectional rather than longitudinal. Some people on the autism spectrum cannot tolerate PSG, and PSG is subject to a first-night effect of decreased total sleep time and lower sleep efficiency (Malow et al., 2006). While actigraphy provides reasonable estimates of sleep parameters compared with PSG, it does not capture important aspects of sleep, such as bedtime resistance (Malow et al., 2016; Walia & Mehra, 2019). Collection of PSG or actigraphy data is costly, burdensome, and limits sample size. The EHR can provide information on objective data and is a rich resource for subjective information collected by clinical documentation. One way to characterize sleep problems in large datasets is to take advantage of electronic health records (EHR).
The EHR is a rich resource for characterizing medical conditions, including course over time and response to treatment. EHRs are varied between different health systems but the benefit of them is they can be queried for key terms and ICD codes. At our institution, we have access to a database of de-identified patient information that creates a mirror-image of the EHR. The EHR offers a more complete assessment of a patient’s health history with different clinical notes, communications with parents and medication records listed over time. This is particularly useful when characterizing complex conditions, such as autism spectrum disorder (ASD). The EHR’s advantages include a longitudinal database and a variety of information including past medical history, medication lists, laboratory results, clinic, and hospital documentation.
Prior sleep research in obstructive sleep apnea has utilized EHR by querying International Classification of Diseases (ICD) diagnostic codes (Hinkle & Kaelber, 2021; Keenan et al., 2020). However, identifying sleep problems within the EHR has not been well studied. Using this de-identified health record data, we aimed to assess sleep problems in people on the autism spectrum. The purpose of the study was to determine the feasibility of a detailed chart review process within an EHR to identify people on the autism spectrum and subsequently characterize their sleep problems. We first validated an algorithm for detecting autism cases, using ICD diagnostic coding and chart review to classify patients, as in previous studies (Brooks et al., 2021; Bush et al., 2017). We then assessed sleep problems within the validated sample of people on the autism spectrum using chart review. We utilized a REDCap data form to build our database and generate reports. Here we describe our novel approach and discuss its benefits and challenges.
2. Materials and Methods
2.1. Institutional Electronic Health Records (EHR) database
Approval was obtained through the Institutional Review Board to review the Synthetic Derivative (SD). The SD is a de-identified database of Vanderbilt University Medical Center’s EHR system that contains over 3 million unique individual records (Roden et al., 2008). The SD contains diagnostic and procedure codes, demographics, problem lists, medications, clinic and hospital notes and clinical communications. All charts in the SD had names redacted and dates shifted by up to 1 calendar year consistently within each record but differing across all records to provide anonymity. Dates are altered throughout the SD system as an additional layer of privacy protection. Individual’s age at time of record review is available. However, patient ages in clinical notes are redacted and replaced with an age range, for example “birth-12 year old”.
2.2. Identification of individuals with autism
In the SD, we generated a set of putative ASD cases for manual chart review by deploying an algorithm that required the presence of at least one occurrence of any of the following thirteen ICD codes (ICD 9: 299.0, 299.00, 299.01, 299, 299.8, 299.80, 299.81, 299.90, 299.91; ICD 10: F84.0, F84.8, F84.5, F84.9) AND the presence of at least one of the following keywords; “asperger”, “autism”, “autistic”, “pervasive developmental disorder”(See Table 1). With guidance from experts in the clinical presentation of ASD (AM and BAM), we developed a chart review rubric to facilitate ascertainment of true cases within the SD set. The rubric allowed for the reviewers to stratify cases into low-evidence, medium-evidence, and high-evidence subsets. Cases were defined as follows: Low-evidence cases were required to have at least one affirmative mention of ASD in a chart of any type. Medium-evidence cases were distinguished by the presence of either a psychological evaluation form with an explicit ASD diagnosis OR at least two affirmative mentions of ASD by ASD-specific provider(s) (neurology, psychiatry, developmental pediatric, behavior therapy, occupational therapy, speech language pathology). High-evidence cases were distinguished by the presence of any of the following: a psychology evaluation with confirmatory Autism Diagnostic Observation Schedule (ADOS), a clinic visit by an autism specialist, a clinic visit for medication management for ASD or two mentions of the patient being treated in Treatment and Research Institute for Autism Spectrum Disorder (TRIAD) clinic, even without the actual clinic note. The clinic visits with an autism specialist were within a clinic that used the ADOS for diagnostic purposes and therefore, it was assumed those patients likely had an ADOS even it if was not available for chart review.
Table 1:
Search criteria for defining ASD in the EHR. ICD-9 code 299.0/ICD-10 code F84.0 (autism); ICD-9 code 299.00 (autistic disorder, current or active state); ICD-9 code 299.01 (autistic disorder, residual state); ICD-9 code 299 (pervasive developmental disorders); ICD-9 code 299.8/ICD-10 code F84.8 (other pervasive developmental disorders); ICD-9 code 299.80 (other specified pervasive developmental disorder, current or active state); ICD-9 code 299.81 (other specified pervasive developmental disorder, residual state); ICD-9 code 299.90/ICD-10 code F84.9 (Pervasive developmental disorder, unspecified); ICD-9 code 299.91 (unspecified pervasive developmental disorder, residual state); ICD-10 code F84.5 (Asperger’s Syndrome)
| Filter Documents by Keyword | Search ICD Codes | ASD Forms data | |
|---|---|---|---|
|
ICD-9 CODES
|
ICD-10 CODES
|
|
| 299.0 | F84.0 | ||
| 299.00 | F84.8 | ||
| 299.01 | F84.5 | ||
| 299 | F84.9 | ||
| 299.8 | |||
| 299.80 | |||
| 299.81 | |||
| 299.90 | |||
| 299.91 | |||
Two chart reviewers were instructed to note the presence of the above criteria and to carefully evaluate the available information, making note of conflicting evidence if present, in reaching their determinations. Subjects were excluded if their records did not satisfy the minimum criteria for low-evidence cases, if their records included a psychology evaluation that did not support an ASD diagnosis, or if conflicting evidence placed a diagnosis in doubt.
An initial un-blinded training sample using 65 charts was performed to ensure the reviewers were correctly implementing the chart review rubric. After initial training, the chart reviewers performed a blinded review of a single set of 25 charts. Inter-rater reliability, using a kappa statistic comparing the difference in raw and expected agreement, was estimated at 0.78 (with agreement on 24/25 charts) with regard to inclusion/exclusion. Next, the chart reviewers performed a blinded review of an additional 50 charts with perfect agreement regarding inclusion/exclusion. The remaining charts were divided between the reviewers and were reviewed independently. Each chart reviewer recorded his or her determinations in an Excel spreadsheet and included evidence copied from the record to justify his or her determinations. Upon conclusion of the review, an expert in ASD clinical presentation reviewed the evidence presented by the chart reviewers and confirmed that the evidence presented supported the determinations.
2.3. Chart Selection
This review process generated over 800 patient charts with either low, medium, or high evidence of ASD. The 230 charts with high evidence of ASD were selected to undergo the rigorous review process where we identified sleep problems as described below and in Figure 1.
Figure 1.
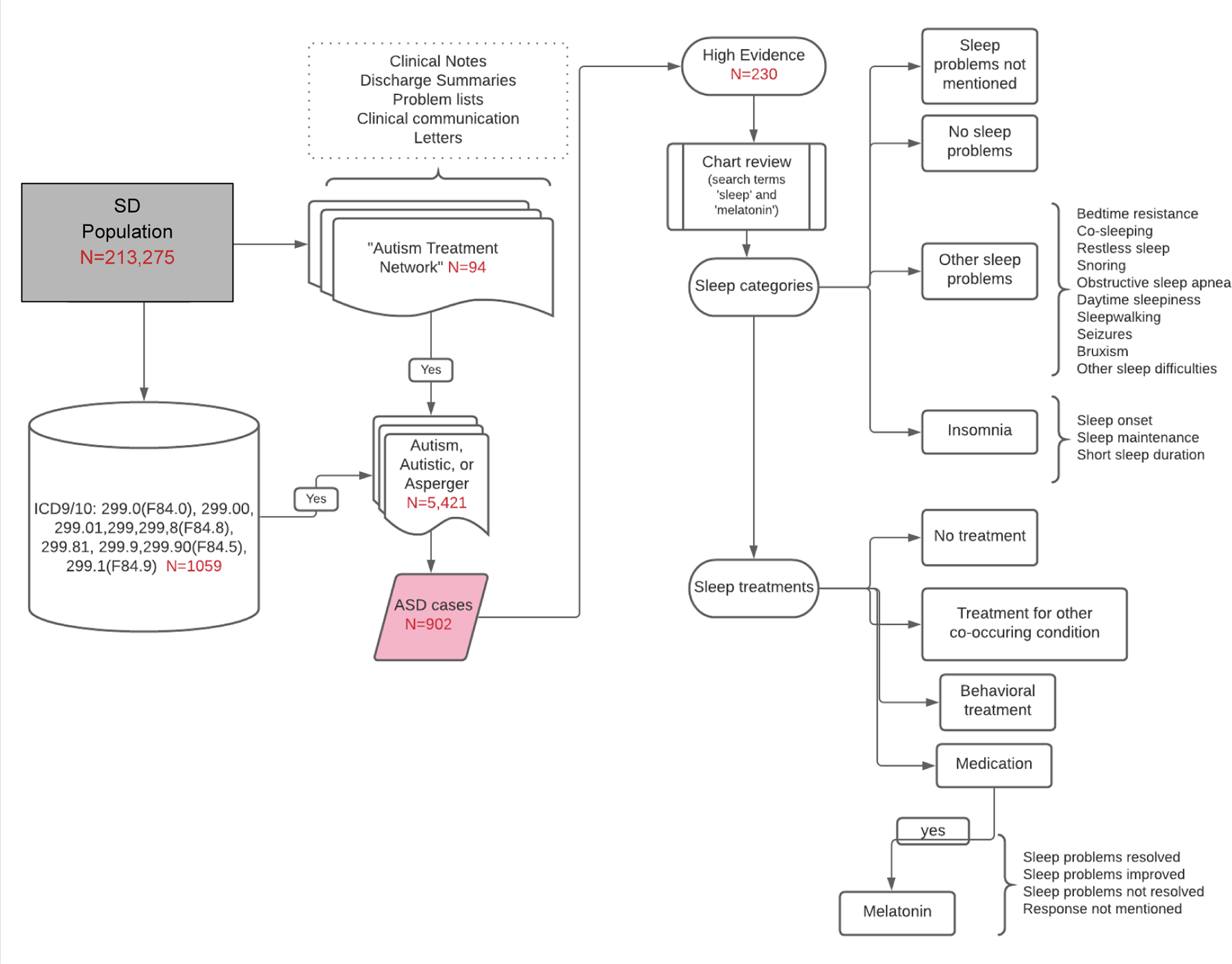
Identification of ASD and patient’s sleep problems and treatments
2.4. Identification of sleep problems in individuals with autism
2.4.1. Pilot Study
Initially, three reviewers (EVS, MN, BAM) each extensively reviewed 10 charts separately and then met to decide on the best strategy to be used to adequately capture the types of sleep problems reported in the EHR. This process allowed us to establish search terms to be used as well as categories for the types of sleep and specific sleep problems to be documented. A REDCap data form was created that was then used to track the required information of each chart. REDCap forms protect sensitive patient health information within a database and are HIPAA (Health Insurance Portability and Accountability Act of 1996)-compliant. They could be access by different individuals at once. To further confirm that we were being inclusive of all sleep problems, each reviewer examined an additional 15 charts with our defined process and again met to analyze them. In this way, we were able to carefully review and then test this process using a total of 25 charts in this pilot study.
2.4.2. Chart Review
Our chart review proceeded with two of the authors (EVS, MN) reviewing the charts separately and then comparing results to ensure agreement on the types of sleep problems and treatments present in each chart. The two reviewers examined between 20–50 charts at one time. Each reviewer documented reference quotes from the chart to support their choices. Once the two reviewers met if there was disagreement then they would first try to rectify the disagreement by referencing the quotes as supporting evidence. If the disagreement remained, then those charts were sent to a third reviewer. Our third reviewer was a sleep medicine physician with autism experience (BAM) who reviewed the responses and adjudicated the designation. With this method we reviewed 230 charts including those completed during the pilot study.
2.4.3. Defining Sleep Categories
Charts were reviewed using the search terms “sleep” and “melatonin”. Sleep problems were categorized into: (a) sleep onset insomnia; (b) sleep maintenance insomnia; (c) bedtime resistance; (d) short sleep duration; (e) co-sleeping; (f) restless sleep; (g) snoring; (h) obstructive sleep apnea; (i) daytime sleepiness; (j) sleepwalking; (k) seizures; (l) bruxism and (m) sleep difficulties without further detail. To fit into a category the chart reviewer used certain definitions. For example, to qualify as short sleep duration the patient needed to sleep for less than 6 hours per night, which by the National Sleep Foundation’s consensus would be less than recommended at any age and has been used in other studies for adults (Hirshkowitz et al., 2015; Itani et al., 2017). To qualify for sleep onset insomnia the patient had to take greater than 30 minutes to fall asleep (Banaschewski et al., 2021; Smits et al., 2001). For daytime sleepiness, if the sleepiness was secondary to a medical condition or a medication side effect then it was not documented. For obstructive sleep apnea, it was documented if there was a clinical diagnosis of obstructive sleep apnea (OSA) or sleep disordered breathing (SDB) made by pediatric otolaryngology, if they underwent a tonsillectomy and adenoidectomy for the diagnosis of OSA or SDB, or if there was documentation of the patient undergoing a sleep study with results indicative of obstructive sleep apnea (i.e. apnea-hypopnea index of >1) (Roland et al., 2011). Co-sleeping was considered a sleep problem if it was co-sleeping outside of infancy. It was documented if the patient had some type of insomnia (sleep onset, sleep maintenance or short sleep duration) and if the patient had other sleep problems, excluding insomnia. Patients were included if they had a sleep problem at any time within the entirety of their EHR. It was also documented if patients had no sleep problems or if there was no mention of sleep in the record.
2.4.4. Sleep Treatments
If the individual reported sleep problems, we would then seek to identify whether the patient received treatment and what type of treatment the individual received. To be more specific, sleep treatments were classified as (a) hypnotic or psychotropic medication targeted for sleep, (b) behavioral treatment, (c) none documented or (d) other medical/psychiatric conditions treated with expected effects on the patient’s sleep. This last category was created to acknowledge the presence of a co-occurring medical or psychiatric condition that was treated with medications that were either selected or expected to influence the patient’s sleep. Detailed notes were kept on the medications or behavioral treatments that were tried and the date and document name were recorded. It was documented whether the patient had received supplemental melatonin and the response they had to melatonin. The patient’s response was categorized into: (a) sleep problems resolved; (b) sleep problems improved but not completely resolved; (c) minimal improvement or no response; and (d) response not mentioned. Occasionally, the response to melatonin was documented as evolving over time. This evolution was noted and after all available clinical documents were reviewed a decision about the overall response to melatonin was made and placed into one of the four categories. If there was disagreement then adjudication as described above in the chart review process would occur.
2.4.5. ICD codes
We identified individuals from the 230 charts with high evidence of ASD who had at least one sleep-related ICD-9 and ICD-10 code (see table A.1 in appendix), to examine whether the chart review method used (ICD codes versus review of charts for key words) yielded different results.
3. Results
3.1. Description of High Evidence ASD Database
Of the 230 patients with high evidence of ASD, 178 (77.4%) were male and 52 (22.6%) were female, consistent with ASD being more common in males (Brooks et al., 2021). The age range of the patients at the time of our chart review was 6–30 years of age with a mean age of 15.4 years (standard deviation 6.6 years). Most patient charts went back into childhood and many patients had mentions of sleep problems over time. Patient demographics are presented in Table 2. Compared to the general pediatric population, our study of patients with high evidence of ASD included more Whites (84.3% compared to 53.0%). The percentage of American Indians or Alaska Natives with high evidence of ASD were lower than the general pediatric population (0.4% compared to 1.4%) as were Blacks or African Americans (8.7% compared to 13.9)(Bureau, n.d.). The majority of individuals had sleep problems (n= 197; 85.6%) with insomnia being the most commonly reported at 85.2% (n=168) of the patients with sleep problems. About a quarter (25.9%) of people on the autism spectrum with any sleep problem were seen at least once in a sleep medicine clinic.
Table 2:
Demographics of high evidence ASD database
| Demographics | ||
|---|---|---|
| Age | 15.4 years (SD 6.6 yrs) | |
| Sex | % | |
| Male | 77.4 | |
| Female | 22.6 | |
| Race | % | |
| White | 84.3 | |
| American Indian or Alaska Native | 0.4 | |
| Asian | 3 | |
| Black or African American | 8.7 | |
| Native Hawaiian or Other Pacific Islander | 0.4 | |
| Other | 0.4 | |
| Not available | 2.2 | |
| Ethnicity | % | |
| Hispanic or Latino | 7.4 | |
| Not Hispanic or Latino | 90.9 | |
| Not available | 1.3 | |
2.2. Insomnia Types
In the children and young adults who had insomnia, 72.6% had sleep onset insomnia, 81% had sleep maintenance insomnia, and 36.3% had short sleep duration. The three types of insomnia (sleep onset, sleep maintenance and short sleep duration) commonly overlapped. In 46 cases (27.4%) all three types of insomnia were found. In 50 cases (29.8%) sleep maintenance insomnia and sleep onset insomnia were co-existing. It was rarer for sleep onset insomnia and short sleep duration to co-exist with only two (1.2%) cases having those two types of insomnia. This was also true for sleep maintenance insomnia and short sleep duration. Six (or 3.6%) cases had only these two types of insomnia. When looking at each type of insomnia in isolation, 34 (20%) patients with insomnia had only sleep maintenance insomnia. In those individuals the most common coexisting sleep problem was snoring with 52.9% (n=18). In individuals with only sleep onset insomnia (n=24) the most common coexisting sleep problem was restless sleep in 45.8% (n=11). It was also more common for patients with sleep onset insomnia to have bedtime resistance than individuals with sleep maintenance insomnia (16.7% vs 5.9%). Lastly, just 7 patients had only short sleep duration.
Other sleep problems excluding insomnia were found in 29 patients (14.7% of patients with a sleep problem). For patients without insomnia, 13.8% had bedtime resistance, 7.9% had co-sleeping, 13.8% had restless sleep, 38% had snoring, 17.2% had OSA, 6.9% had daytime sleepiness, 0 patients had sleepwalking, 6.9% had seizures, 3.4% had bruxism and 34.5% had sleep difficulties not further specified.
For all patients with a sleep problem including ones with co-existing insomnia, 16.5% had bedtime resistance, 29.5% had co-sleeping, 33.5% had restless sleep, 43.5% had snoring, 20% had OSA, 26% had daytime sleepiness, 6.5% had parasomnias, 4% had seizures, 8% had bruxism and 5% had sleep difficulties not further specified. When comparing the existence of other sleep problems in patients with and without insomnia, a higher proportion of patients with insomnia had coexisting daytime sleepiness, restless sleep and co-sleeping. The highest prevalence of insomnia with co-sleeping was short sleep duration. The types of sleep problems with and without insomnia are compared in Figure 2.
Figure 2.
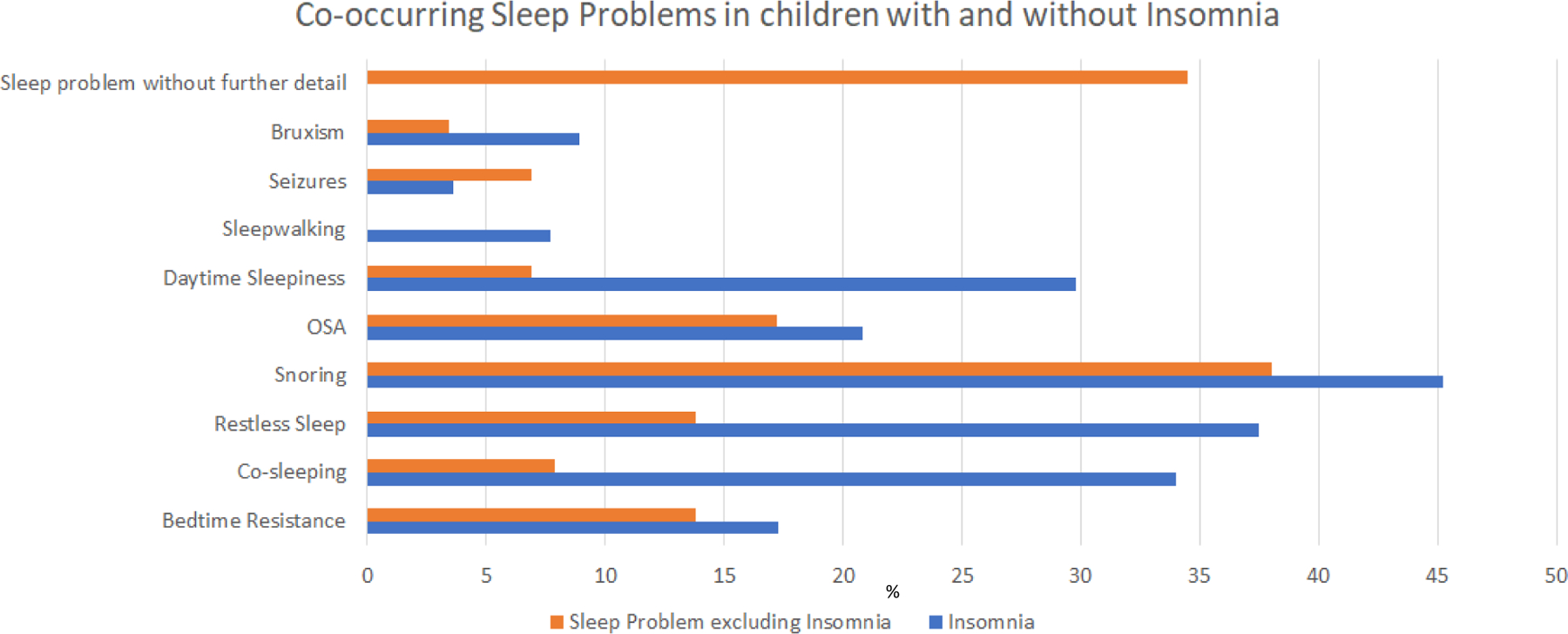
Co-occurring sleep problems in children with and without insomnia
Only 11% of patients were sleeping well. Four percent of patients had no mention of sleep in their electronic health record.
In terms of treatments for disordered sleep, 138 patients (69%) received a medication for sleep. By far the most common medication for sleep was melatonin with 92% of the patients who received a medication getting melatonin. Behavioral treatments were documented as being used in 101 patients (50.5%). Eighty patients (40%) of patients with sleep problems received both a sleep medication and behavioral treatment. A treatment for a co-occurring medical condition that had the side effect of helping with sleep was documented in 45 patients (23%). Finally, 59 patients (30%) had no documented treatment for their sleep problem.
3.3. Response to Supplemental Melatonin
Of the patient charts that had a documented response to melatonin, the largest percentage at 67% had some benefit to its initiation with either resolution of sleep problems (n=15, 18.1%) or sleep problems partially improved (n=41, 49.4%). Twenty-seven patients (32.5%) were documented to have no response to initiation of melatonin. Finally, 44 (35%) of the 127 patients with sleep problems and documented treatment with melatonin had no documentation in the chart on the response of melatonin. These patients commonly had melatonin on their medication list but no mention of taking the medication in their medical record.
3.4. ICD codes versus chart review process
Table 3 shows the number of individuals identified through chart review versus the number of individuals identified by a sleep-related ICD code in their medical record. Our results indicate that, by using the chart review process, we were able to identify 50% more individuals with insomnia (111 charts compared to 56 charts) and 68% more individuals with sleep problems other than insomnia (26 charts compared to 6 charts). The chart review process was also accurate in identifying charts without sleep problems or no mention of sleep. There was only one individual who had a sleep-related ICD code and where sleep was not mentioned specifically in this individual’s chart.
Table 3.
ICD code search for sleep problems compared to chart review
| ICD code for sleep problem | Chart review documents insomnia Yes |
Chart review mentions sleep problem other than insomnia Yes |
Chart review mentions “no sleep problem” Yes |
Chart review does not mention sleep Yes |
|---|---|---|---|---|
| No | 111 | 26 | 23 | 9 |
| Yes | 56 | 6 | 1 | 0 |
4. Discussion
Our study, using de-identified patient records within an EHR at one tertiary care hospital, provides a framework for characterizing sleep problems in people on the autism spectrum. We found similar rates of sleep problems in individuals with autism as demonstrated in prior studies. For example, Liu and colleagues found 86% of their cohort of 167 children had daily sleep problems. They reported higher rates of bedtime resistance than our study, but we found higher rates of insomnia (85% compared to 54%). These differences may be due to differences in the sleep categories and the ways sleep problems were defined in the respective studies. Our study utilizing EHR may not represent the prevalence of people on the autism spectrum with sleep problems in the community as there may be a subset of people on the autism spectrum with comorbid disorders who are more likely to then be screened for and have sleep problems (Mannion et al., 2013).
Many neurodevelopmental disorders have comorbid sleep disturbances, although the type of sleep problem ranges between individual neurodevelopmental disorders. For example, children with Angelman Syndrome (AS) have a similar presentation of sleep problems to people on the autism spectrum with 62% with night awakenings in one study by Bruni et al. (2004). Our study found 62% of people on the autism spectrum with sleep maintenance insomnia. Children with AS were more prone to short sleep duration with 70% of children getting less than 8 hours of sleep compared to our study which found short sleep duration in 28% of people on the autism spectrum, although our criteria for short sleep duration was different and included only 6 hours of sleep or less. Fewer children with AS took more than 30 minutes to fall asleep (32%) compared to our study which found sleep onset insomnia in 55% of people on the autism spectrum. Children with Down Syndrome (DS) are particularly at risk for OSA. One study using polysomnography in patients with DS found 79% of patients in their study had OSA(Dyken et al., 2003). In one study of DS children and adolescents, insomnia was less common compared to people on the autism spectrum in our study (18.3% compared to 76%) (Fucà et al., 2021).
There is limited research into OSA and restless leg syndrome (RLS) or restless sleep disorder (RSD) in people on the autism spectrum. We found over half of all patients with high evidence of ASD (55%) had either snoring, OSA or both. The Liu et al. study found fewer sleep disordered breathing problems compared to our study with one-fourth of parents reporting a sleep disordered breathing problem in their child. In terms of restless sleep, our study indicated that 29% of individuals with high evidence of ASD experienced restless sleep. A previous small pilot study found that 77% of children with autism had restless sleep and showed improvement of restless sleep with iron supplementation (Dosman et al., 2007). These findings suggest that sleep disordered breathing and restless sleep could be significant factors in the disruption of sleep for people on the autism spectrum and merit future research.
Our categorization of co-sleeping outside infancy to be a sleep problem is controversial as co-sleeping can be a cultural tradition and therefore not a problem. We decided to include it as a problem as several studies support the presence of co-sleeping to be related to sleep problems in both typically developing children and children with neurodevelopmental disabilities. Köse et al. (2017) found that co-sleeping with a parent increases the risk of sleep disorder in the child thirteen-fold. Another study of children with autism found correlation between sleep problems and co-sleeping (Liu et al., 2006). Furthermore, since co-sleeping was being brought up in a clinical setting within the EHR and not simply disclosed on a standardized parental questionnaire this presentation suggested that it was more likely to be a problem.
A unique aspect of our study was the detail that was put into the chart review and categorization of sleep problems. Every chart was reviewed in its entirety by two separate reviewers for 13 unique sleep problems. Furthermore, sleep treatments, both medical and behavioral, were assessed with particular attention to melatonin and patients’ response to it. Charts commonly contained information about sleep problems and treatments over many years, thus allowing our review to assess whether a response to melatonin was short-lived or more permanent. To our knowledge this study is one of the first to utilize multiple facets of the EHR to characterize many different types of sleep problems.
We utilized a novel approach to the characterization of ASD within the EHR. Previous studies have utilized keyword searches as we utilized (Brooks et al., 2021). However, our algorithm further delineated the evidence of ASD in the chart as low, medium or high. These criteria allow for further certainty in a condition that can be challenging to diagnosis given its heterogenous presentation. Our large study of 230 patients with high evidence of ASD were each evaluated with detailed reviews of sleep problems and treatment. In undertaking this review of medical records, we realized the importance of reviewing all notes and communications that were available in the EHR. As sleep problems can be brought up in many different settings and can change over time, this was necessary to get a complete understanding of the patient’s sleep issues. This method of thorough review was also helpful when trying to understand patients’ response to initiation of melatonin.
Sleep treatments within the EHR were varied. It was interesting to note that 22% of people on the autism spectrum received a medication to treat a co-occurring medical condition but had the expected side effect of helping with sleep. Given that people on the autism spectrum commonly have co-occurring medical conditions utilizing medications that can treat multiple problems could help reduce polypharmacy in this population. Half of the patients who had documented sleep problems had documentation that they received behavioral treatments. It is possible that more patients received behavioral treatment but that this was not as well documented compared to medication treatments.
Overall, we found melatonin response to be challenging to discern in the EHR. A prior study looked at melatonin response in children with autism and found 25% no longer had sleep concerns, 60% had improved sleep, 13% had no response and only 1% had an undetermined response (Andersen et al., 2008). In our review, for patients who had a documented response to melatonin, we found more patients who did not improve with melatonin- 32.5% compared to only 13% in the Andersen study. This difference may be related to our higher percentage of patients with undocumented response to melatonin, which was 40% compared to only 1% in the Andersen study that did not have a documented response. Our study population of patients through a tertiary hospital EHR could also be different from the patients presenting to a pediatrician’s clinic, which was the case for the Andersen study. Sleep problems in our population may be more complex and multifaceted and thus have a less robust response to melatonin supplementation. Additionally, it is important to note that in the Andersen study all patients were recommended behavioral treatments alongside melatonin administration. This may account for some of the differences in responsiveness of melatonin and for the lack of information about melatonin response. Finally, since melatonin is a dietary supplement, it was not always documented in the medication list of a patient but was mentioned in the chart that the patient was taking it. During the review process, charts might report a patient was “sleeping well” but upon further review the patient would have melatonin on their medication list. To avoid contaminating the “sleeping well” control group, those charts would be marked as “other sleep problems” even though there was no explicit mention of a sleep problem.
Limitations of our design were the retrospective nature of the study and the challenges related to use of EHR data. Varied documentation by many different providers led to some difficulties acquiring consistent information. These variations limited our ability to define sleep problems by American Academy of Sleep Medicine (AASM) guidelines or Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria. It also led to missing data, for example, response to treatment, particularly behavioral treatments were often absent. While most charts had mention of sleep, there were a few charts that made no mention of sleep. It is possible those patients had sleep problems that were not asked about or documented.
Our study included a wide age range of patients from 6–30 years old with a mean age of 15.4 years. This study gives support to the long-standing nature of sleep problems in people on the autism spectrum. However, it also made it difficult to define sleep categories as sleep evolves over the lifespan. We chose definitions that would be clinically significant at any age, for example, short sleep duration as any person sleeping less than 6 hours per night.
Our study only looked at patients with high evidence of ASD. While this is an advantage in terms of confidence in their diagnosis, it may have caused ascertainment bias in terms of their sleep problems. All the patients with high evidence of ASD had documentation from physicians with expertise in autism and may have been more likely to discuss sleep problems in that population. Finally, due to the lengthy review process, we limited our review to the high evidence for ASD charts.
5. Conclusions
Our study can be used to guide future work using the EHR. We propose a method for differentiating ASD evidence and for evaluating challenging areas of study such as pediatric sleep problems. Our study includes patients seen in many different clinical settings with about a quarter of them seen in a sleep clinic. This fact may allow for a more diverse group of people on the autism spectrum with sleep problems to be included in sleep research. Our approach to sleep problems in people on the autism spectrum improved the identification of patients with sleep problems who were identified in the EHR compared to only using ICD codes to identify patients with sleep problems. To better understand the impacts of sleep disorders on people on the autism spectrum, it would be helpful to apply this methodology to other neurodevelopmental disorders.
In terms of feasibility, this study included a lengthy review process especially when compared to using ICD codes to identify patients. From this review process we were able to identify keywords that if used, could generate necessary information for determining sleep problems, sleep treatments and their responses. These keywords can be incorporated into future work with natural language processing to automate the process and allow for application on a larger scale.
Supplementary Material
Highlights:
Children and adolescents with autism spectrum disorder have many sleep problems
Methods for qualifying the evidence for autism spectrum disorder
The electronic medical record provides insight into sleep problems
Melatonin is a commonly used treatment in children with autism spectrum disorder
Acknowledgements: Synthetic Derivative
The project described was supported by the National Center for Research Resources, Grant UL1 RR024975–01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445–06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- AASM
American Academy of Sleep Medicine
- ADOS
autism diagnostic observation schedule
- AS
Angelman Syndrome
- ASD
autism spectrum disorder
- DS
Down Syndrome
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders
- EHR
electronic health record
- ICD
international classification of diseases
- OSA
obstructive sleep apnea
- PSG
polysomnography
- RLS
restless leg syndrome
- RSD
restless sleep disorder
- SD
synthetic derivative
- TRIAD
Treatment and Research Institute for Autism Spectrum Disorder
Appendix
Table A.1.
ICD-9 and ICD-10 codes associated with sleep disorders or problems
| ICD-9 Code | Disorder |
|---|---|
| 307.4 | Specific disorders of sleep of nonorganic origin |
| 307.4 | Nonorganic sleep disorder, unspecified |
| 307.41 | Transient disorder of initiating or maintaining sleep |
| 307.42 | Persistent disorder of initiating or maintaining sleep |
| 307.43 | Transient disorder of initiating or maintaining wakefulness |
| 307.44 | Persistent disorder of initiating or maintaining wakefulness |
| 307.45 | Circadian rhythm sleep disorder of nonorganic origin |
| 307.46 | Sleep arousal disorder |
| 307.47 | Other dysfunctions of sleep stages or arousal from sleep |
| 307.48 | Repetitive intrusions of sleep |
| 307.49 | Other specific disorders of sleep of nonorganic origin |
| 327 | Organic sleep disorders |
| 327 | Organic disorders of initiating and maintaining sleep [Organic insomnia] |
| 327 | Organic insomnia NOS |
| 327.01 | Insomnia due to medical condition classified elsewhere |
| 327.02 | Insomnia due to mental disorder |
| 327.09 | Other organic insomnia |
| 327.1 | Organic disorder of excessive somnolence [Organic hypersomnia] |
| 327.1 | Organic hypersomnia NOS |
| 327.11 | Idiopathic hypersomnia with long sleep time |
| 327.12 | Idiopathic hypersomnia without long sleep time |
| 327.13 | Recurrent hypersomnia |
| 327.14 | Hypersomnia due to medical condition classified elsewhere |
| 327.15 | Hypersomnia due to mental disorder |
| 327.19 | Other organic hypersomnia |
| 327.2 | Organic sleep apnea |
| 327.2 | Organic sleep apnea NOS |
| 327.21 | Primary central sleep apnea |
| 327.22 | High altitude periodic breathing |
| 327.23 | Obstructive sleep apnea |
| 327.24 | Idiopathic sleep related nonobstructive alveolar hypoventilation |
| 327.25 | Congenital central alveolar hypoventilation syndrome |
| 327.26 | Sleep related hypoventilation/hypoxemia in conditions classifiable elsewhere |
| 327.27 | Central sleep apnea in conditions classified elsewhere |
| 327.29 | Other organic sleep apnea |
| 327.3 | Circadian rhythm sleep disorder |
| 327.3 | Circadian rhythm sleep disorder, unspecified |
| 327.31 | Circadian rhythm sleep disorder, delayed sleep phase type |
| 327.32 | Circadian rhythm sleep disorder, advanced sleep phase type |
| 327.33 | Circadian rhythm sleep disorder, irregular sleep-wake type |
| 327.34 | Circadian rhythm sleep disorder, free-running type |
| 327.35 | Circadian rhythm sleep disorder, jet lag type |
| 327.36 | Circadian rhythm sleep disorder, shift work type |
| 327.37 | Circadian rhythm sleep disorder in conditions classified elsewhere |
| 327.39 | Other circadian rhythm sleep disorder |
| 327.4 | Organic parasomnia |
| 327.4 | Organic parasomnia NOS |
| 327.41 | Confusional arousals |
| 327.42 | REM sleep behavior disorder |
| 327.43 | Recurrent isolated sleep paralysis |
| 327.44 | Parasomnia in conditions classified elsewhere |
| 327.49 | Other organic parasomnia |
| 327.5 | Organic sleep related movement disorders |
| 327.51 | Periodic limb movement disorder |
| 327.52 | Sleep related leg cramps |
| 327.53 | Sleep related bruxism |
| 327.59 | Other organic sleep related movement disorders |
| 327.8 | Other organic sleep disorders |
| ICD-10 code | Disorder |
| A81.83 | Fatal familial insomnia |
| G47.8 | Other sleep disorders |
| F51.9 | Sleep disorder not due to a substance or known physiological condition, unspecified |
| G47.9 | Sleep disorder, unspecified |
| G47 | Sleep disorders |
| F51.8 | Other sleep disorders not due to a substance or known physiological condition |
| G47.12 | Idiopathic hypersomnia without long sleep time |
| G47.14 | Hypersomnia due to medical condition |
| F51.11 | Primary hypersomnia |
| F51.13 | Hypersomnia due to other mental disorder |
| G47.11 | Idiopathic hypersomnia with long sleep time |
| G47.19 | Other hypersomnia |
| F51.12 | Insufficient sleep syndrome |
| F51.19 | Other hypersomnia not due to a substance or known physiological condition |
| G47.10 | Hypersomnia, unspecified |
| G47.1 | Hypersomnia |
| G47.13 | Recurrent hypersomnia |
| F51.1 | Hypersomnia not due to a substance or known physiological condition |
| G47.30 | Sleep apnea, unspecified |
| G47.39 | Other sleep apnea |
| G47.3 | Sleep apnea |
| G47.32 | High altitude periodic breathing |
| G47.31 | Primary central sleep apnea |
| G47.37 | Central sleep apnea in conditions classified elsewhere |
| G47.36 | Sleep related hypoventilation in conditions classified elsewhere |
| G47.35 | Congenital central alveolar hypoventilation syndrome |
| G47.34 | Idiopathic sleep related nonobstructive alveolar hypoventilation |
| G47.33 | Obstructive sleep apnea (adult) (pediatric) |
| G47.09 | Other insomnia |
| F51.05 | Insomnia due to other mental disorder |
| G47.0 | Insomnia |
| G47.00 | Insomnia, unspecified |
| G47.01 | Insomnia due to medical condition |
| F51.02 | Adjustment insomnia |
| F51.04 | Psychophysiologic insomnia |
| F51.01 | Primary insomnia |
| F51.09 | Other insomnia not due to a substance or known physiological condition |
| F51.03 | Paradoxical insomnia |
| G47.59 | Other parasomnia |
| F51.5 | Nightmare disorder |
| F51.3 | Sleepwalking [somnambulism] |
| G47.52 | REM sleep behavior disorder |
| G47.50 | Parasomnia, unspecified |
| G47.54 | Parasomnia in conditions classified elsewhere |
| G47.51 | Confusional arousals |
| F51.4 | Sleep terrors [night terrors] |
| G47.53 | Recurrent isolated sleep paralysis |
| G47.5 | Parasomnia |
| G47.29 | Other circadian rhythm sleep disorder |
| G47.21 | Circadian rhythm sleep disorder, delayed sleep phase type |
| G47.22 | Circadian rhythm sleep disorder, advanced sleep phase type |
| G47.27 | Circadian rhythm sleep disorder in conditions classified elsewhere |
| G47.2 | Circadian rhythm sleep disorders |
| G47.25 | Circadian rhythm sleep disorder, jet lag type |
| G47.20 | Circadian rhythm sleep disorder, unspecified type |
| G47.26 | Circadian rhythm sleep disorder, shift work type |
| G47.23 | Circadian rhythm sleep disorder, irregular sleep wake type |
| G47.24 | Circadian rhythm sleep disorder, free running type |
| G47.61 | Periodic limb movement disorder |
| G47.6 | Sleep related movement disorders |
| G47.69 | Other sleep related movement disorders |
| G47.63 | Sleep related bruxism |
| G47.62 | Sleep related leg cramps |
| R06.83 | Snoring |
| P28.3 | Primary sleep apnea of newborn |
| P28.4 | Other apnea of newborn |
| Z73.811 | Behavioral insomnia of childhood, limit setting type |
| Z72.821 | Inadequate sleep hygiene |
| Z73.81 | Behavioral insomnia of childhood |
| Z72.82 | Problems related to sleep |
| Z72.820 | Sleep deprivation |
| Z73.812 | Behavioral insomnia of childhood, combined type |
| Z73.810 | Behavioral insomnia of childhood, sleep-onset association type |
| Z73.819 | Behavioral insomnia of childhood, unspecified type |
| F15.282 | Other stimulant dependence with stimulant-induced sleep disorder |
| F11.182 | Opioid abuse with opioid-induced sleep disorder |
| F14.182 | Cocaine abuse with cocaine-induced sleep disorder |
| F19.982 | Other psychoactive substance use, unspecified with psychoactive substance-induced sleep disorder |
| F13.982 | Sedative, hypnotic or anxiolytic use, unspecified with sedative, hypnotic or anxiolytic-induced sleep disorder |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None
References
- Andersen IM, Kaczmarska J, McGrew SG, & Malow BA (2008). Melatonin for Insomnia in Children With Autism Spectrum Disorders. Journal of Child Neurology, 23(5), 482–485. 10.1177/0883073807309783 [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Bruni O, Fuentes J, Hill CM, Hvolby A, Posserud M-B, & Schroder C (2021). Practice Tools for Screening and Monitoring Insomnia in Children and Adolescents with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders 10.1007/s10803-021-05236-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JD, Bronskill SE, Fu L, Saxena FE, Arneja J, Pinzaru VB, Anagnostou E, Nylen K, McLaughlin J, & Tu K (2021). Identifying Children and Youth With Autism Spectrum Disorder in Electronic Medical Records: Examining Health System Utilization and Comorbidities. Autism Research, 14(2), 400–410. 10.1002/aur.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau, U. C. (n.d.). 2020 Census Illuminates Racial and Ethnic Composition of the Country. Census.Gov Retrieved February 21, 2022, from https://www.census.gov/library/stories/2021/08/improved-race-ethnicity-measures-reveal-united-states-population-much-more-multiracial.html
- Bush RA, Connelly CD, Pérez A, Barlow H, & Chiang GJ (2017). Extracting autism spectrum disorder data from the electronic health record. Applied Clinical Informatics, 08(3), 731–741. 10.4338/ACI-2017-02-RA-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosman CF, Brian JA, Drmic IE, Senthilselvan A, Harford MM, Smith RW, Sharieff W, Zlotkin SH, Moldofsky H, & Roberts SW (2007). Children With Autism: Effect of Iron Supplementation on Sleep and Ferritin. Pediatric Neurology, 36(3), 152–158. 10.1016/j.pediatrneurol.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Dyken ME, Lin-Dyken DC, Poulton S, Zimmerman MB, & Sedars E (2003). Prospective Polysomnographic Analysis of Obstructive Sleep Apnea in Down Syndrome. Archives of Pediatrics & Adolescent Medicine, 157(7), 655–660. 10.1001/archpedi.157.7.655 [DOI] [PubMed] [Google Scholar]
- Elia M, Ferri R, Musumeci SA, Del Gracco S, Bottitta M, Scuderi C, Miano G, Panerai S, Bertrand T, & Grubar JC (2000). Sleep in subjects with autistic disorder: A neurophysiological and psychological study. Brain & Development, 22(2), 88–92. 10.1016/s0387-7604(99)00119-9 [DOI] [PubMed] [Google Scholar]
- Fucà E, Costanzo F, Celestini L, Mandarino A, & Vicari S (2021). Characterization of Sleep Disturbances in Children and Adolescents with Down Syndrome and Their Relation with Cognitive and Behavioral Features. International Journal of Environmental Research and Public Health, 18(9), 5001. 10.3390/ijerph18095001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann S (2016). Counting Sheep: Sleep Disorders in Children With Autism Spectrum Disorders. Journal of Pediatric Health Care, 30(2), 143–154. 10.1016/j.pedhc.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Hinkle J, & Kaelber D (2021). Utilizing Big Data to Identify the Prevalence of and Risk Factors for Obstructive Sleep Apnea in a Pediatric Population https://pediatrics.aappublications.org/content/147/3_MeetingAbstract/25
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Adams Hillard PJ, Katz ES, Kheirandish-Gozal L, Neubauer DN, O’Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, & Ware JC (2015). National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health, 1(4), 233–243. 10.1016/j.sleh.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Itani O, Jike M, Watanabe N, & Kaneita Y (2017). Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Medicine, 32, 246–256. 10.1016/j.sleep.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Keenan BT, Kirchner HL, Veatch OJ, Borthwick KM, Davenport VA, Feemster JC, Gendy M, Gossard TR, Pack FM, Sirikulvadhana L, Teigen LN, Timm PC, Malow BA, Morgenthaler TI, Zee PC, Pack AI, Robishaw JD, & Derose SF (2020). Multisite validation of a simple electronic health record algorithm for identifying diagnosed obstructive sleep apnea. Journal of Clinical Sleep Medicine, 16(2), 175–183. 10.5664/jcsm.8160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hubbard JA, Fabes RA, & Adam JB (2006). Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry and Human Development, 37(2), 179–191. 10.1007/s10578-006-0028-3 [DOI] [PubMed] [Google Scholar]
- Malow BA, Connolly HV, Weiss SK, Halbower A, Goldman S, Hyman SL, Katz T, Madduri N, Shui A, Macklin E, & Reynolds AM (2016). The Pediatric Sleep Clinical Global Impressions Scale—A New Tool to Measure Pediatric Insomnia in Autism Spectrum Disorders. Journal of Developmental & Behavioral Pediatrics, 37(5), 370–376. 10.1097/DBP.0000000000000307 [DOI] [PubMed] [Google Scholar]
- Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, & Stone WL (2006). Characterizing Sleep in Children with Autism Spectrum Disorders: A Multidimensional Approach. Sleep, 29(12), 1563–1571. 10.1093/sleep/29.12.1563 [DOI] [PubMed] [Google Scholar]
- Mannion A, Leader G, & Healy O (2013). An investigation of comorbid psychological disorders, sleep problems, gastrointestinal symptoms and epilepsy in children and adolescents with Autism Spectrum Disorder. Research in Autism Spectrum Disorders, 7(1), 35–42. 10.1016/j.rasd.2012.05.002 [DOI] [Google Scholar]
- Neurodevelopmental Disorders. (2013). In Diagnostic and Statistical Manual of Mental Disorders American Psychiatric Association. 10.1176/appi.books.9780890425596.dsm01 [DOI] [Google Scholar]
- Reynolds AM, Soke GN, Sabourin KR, Hepburn S, Katz T, Wiggins LD, Schieve LA, & Levy SE (2019). Sleep Problems in 2- to 5-Year-Olds With Autism Spectrum Disorder and Other Developmental Delays. Pediatrics, 143(3). 10.1542/peds.2018-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, & Masys DR (2008). Development of a Large-Scale De-Identified DNA Biobank to Enable Personalized Medicine. Clinical Pharmacology & Therapeutics, 84(3), 362–369. 10.1038/clpt.2008.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PS, Rosenfeld RM, Brooks LJ, Friedman NR, Jones J, Kim TW, Kuhar S, Mitchell RB, Seidman MD, Sheldon SH, Jones S, Robertson P, & American Academy of Otolaryngology—Head and Neck Surgery Foundation. (2011). Clinical practice guideline: Polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngology--Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery, 145(1 Suppl), S1–15. 10.1177/0194599811409837 [DOI] [PubMed] [Google Scholar]
- Sateia MJ (2014). International Classification of Sleep Disorders-Third Edition. Chest, 146(5), 1387–1394. 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- Smits MG, Nagtegaal EE, van der Heijden J, Coenen AML, & Kerkhof GA (2001). Melatonin for Chronic Sleep Onset Insomnia in Children: A Randomized Placebo-Controlled Trial. Journal of Child Neurology, 16(2), 86–92. 10.1177/088307380101600204 [DOI] [PubMed] [Google Scholar]
- Veatch OJ, Maxwell-Horn AC, & Malow BA (2015). Sleep in Autism Spectrum Disorders. Current Sleep Medicine Reports, 1(2), 131–140. 10.1007/s40675-015-0012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia HK, & Mehra R (2019). Practical aspects of actigraphy and approaches in clinical and research domains. Handbook of Clinical Neurology, 160, 371–379. 10.1016/B978-0-444-64032-1.00024-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


