Abstract
Background:
While drinking, one must eventually decide between the immediate rewarding effect of alcohol and the delayed reward of a healthier lifestyle. Individuals differ in their devaluation of a delayed reward based on the time required to receive it, the so called delay discounting. Previous studies showed that adolescents discount steeper than adults and that steeper delay discounting was associated with heavier alcohol use in both groups.
Methods:
In a large-scale longitudinal study, we investigated whether higher rates of delay discounting are a developmental antecedent or a consequence of alcohol use during adolescent development. As part of the IMAGEN project, 2220 adolescents completed the Monetary Choice questionnaire as delay discounting measure and the Alcohol Use Disorders Identification Test as well as the Timeline-Follow-Back interview at the ages 14, 16, 18 and 22. Bivariate latent growth curve models were applied to investigate the relationship between delay discounting and drinking. To further explore the consequences of drinking, we computed the cumulative alcohol consumption to correlate it with the development of discounting. A subsample of 221 participants completed an intertemporal choice task during functional magnetic resonance imaging at ages 14, 16 and 18. Repeated-measures ANOVA was used to differentiate the development of neural processing during intertemporal choices between high-risk and low-risk drinkers.
Results:
Overall, high rates of delay discounting at age 14 predicted a stronger increase of drinking over 8 years. In turn, an average, moderate alcohol use did not affect delay discounting from age 14 to 22. Of note, we found indicators for less brain activity in top-down control areas during intertemporal choices in those participants who drank more.
Conclusions:
Concluding, steep delay discounting is a predictor rather than a consequence of alcohol use in low-level drinking adolescents. Consideration is given to sampling strategies and reliability concerns for future longitudinal studies.
Keywords: adolescence, alcohol, delay discounting, latent growth curve modelling, longitudinal fMRI
Introduction
As a critical period in human development, adolescence is characterized by risky and impulsive behavior, such as excessive alcohol consumption. Impulsivity has been recognized as a multidimensional construct which consists of several facets that are differently associated with alcohol use (Coskunpinar et al., 2013). Here, we focus on delay discounting (DD) or temporal discounting as one dimension of impulsivity (Sharma et al., 2014). DD refers to the devaluation of a reward delivered with a delay compared to its value when delivered immediately. Intertemporal choices such as “5 € now or 10 € in two weeks?” are typically used to assess DD. Intertemporal choices are not just limited to monetary contingencies, though, as subjective reward scenarios can exist within a range of topics or situations. To elaborate, an intertemporal choice relating to alcohol consumption could be “Do I drink another glass of wine or avoid a headache in the morning?” The decision to drink another glass of wine would indicate that the immediate rewarding effect of alcohol intake is worth more than the future benefits of not drinking, such as waking up without a headache or a healthier lifestyle in general. Repeatedly choosing to continue drinking could result in binge drinking behavior, which is highly prevalent among adolescents (Spear, 2018). In the context of the longitudinal IMAGEN project (Schumann et al., 2010), this study aims to further disentangle whether or not increased rates of DD are a developmental antecedent and/or a consequence of adolescent alcohol use. This question addresses a potentially bidirectional relationship between impulsivity and adolescent alcohol use: increased impulsivity in adolescents may promote (binge) drinking behavior, yet increased alcohol use during adolescence might lead to more impulsive decisions by influencing the vulnerable adolescent brain (Bava and Tapert, 2010). To take into account the underlying neurobiological mechanisms, we recruited a subsample to also undergo functional magnetic brain imaging (fMRI) during intertemporal choices.
To decipher the possible interaction of alcohol use and DD, we first need a thorough understanding of the development of DD during adolescence. According to cross-sectional studies, DD is higher in adolescents compared to adults (e.g. de Water et al., 2014, Green et al., 1999, Ripke et al., 2012); however, longitudinal studies have also shown stable DD during early adolescence (from age 12 to 15) (Fernie et al., 2013) and late adolescence (from age 15 to 21) (Audrain-McGovern et al., 2009). Recently, Khurana et al. (2018) showed considerable individual differences in the developmental trajectories of DD between the ages of 11 and 18; some trajectories decreased, while others remained stable or even increased. They reported an increased risk of substance use disorder in participants with high and stable DD (Khurana et al., 2018). The IMAGEN sample, which was recruited at age 14 and followed-up into early adulthood at age 22, is well suited to assess how individual differences in initial DD and trajectories of DD are related to individual differences in the development of alcohol use.
Past studies have shown the adolescent brain to be highly susceptible to external influences, such as alcohol (Chambers et al., 2003, Jacobus and Tapert, 2013). Chambers et al. (2003) offered an explanation for the vulnerable nature of the adolescent brain, citing an imbalance between two motivational circuits resembling the behavioral approach and avoidance systems proposed by Gray (Gray, 1990). The promotional circuit includes brain regions that are innervated by dopaminergic pathways, such as the striatum. In contrast, the inhibitory circuit involves more serotonergic and prefrontal regions, which inhibit suboptimal actions. Structural imaging studies have shown that the cortical (inhibitory) circuit continues to develop until the end of adolescence, whereas the subcortical (approach) circuit matures earlier (Gogtay et al., 2004). Thus, the approach system has relatively more influence on behavioral regulation compared to the inhibitory system. On one hand, the imbalance reinforces the immediate rewarding effect of an action, such as drinking alcohol. On the other hand, it simultaneously reduces the inhibition of a suboptimal action, such as drinking too much alcohol. Hence, the imbalance of the circuits could promote engaging in alcohol use during adolescence. However, one must also consider the neurotoxic effects of alcohol (Jacobus and Tapert, 2013). Alcohol is known to impact relevant neurotransmitter systems; for example, it enhances the dopaminergic circuit (Chastain, 2006). The mesocortical dopamine system with projections in both striatal and frontal regions was also associated with DD (McClure et al., 2004, Peters and Büchel, 2011). Importantly, there were also studies showing altered DD related frontal brain activation in alcohol dependent subjects (Amlung et al., 2014). To conclude, an imbalance between the approach and inhibitory circuits may be a shared mechanism for both alcohol use behavior and DD during adolescence.
In line with the described framework, there is growing evidence for an association between alcohol use and DD. According to a meta-analysis by MacKillop and colleagues (2011), DD was higher in alcohol-dependent users. The effect, however, tends to be weaker in subclinical groups. A dose-dependent relationship was therefore assumed, meaning DD is higher if more alcohol is consumed (MacKillop et al., 2011). Furthermore, DD was correlated with the severity of alcohol-dependence (Reynolds, 2006) and the heaviness of alcohol use (Murphy and MacKillop, 2012). Longitudinal studies indicate a similar pattern: there is stronger evidence for DD predicting addictive behaviors than simple quantity-frequency-measures (Kräplin et al., 2020, Bernhardt et al., 2017). In addition, a longitudinal study in adolescents analyzed a composite score of quantity-frequency measures and self-reported problematic consequences of drinking, positing that DD predicts future behavioral issues related to alcohol consumption (Fernie et al., 2013). The immense efforts of Mischel and colleagues have shown that delay of gratification, a process not equivalent but substantially related to DD (Reynolds and Schiffbauer, 2005), can predict various cognitive and mental health outcomes, even after 40 years (see Mischel et al., 2011 for a review). They also provide evidence that participants who had higher rates of delay of gratification as a child recruited less activity in the inferior frontal gyrus and more activity in the ventral striatum in response to rewarding stimuli during an emotionally-valenced version of a Go/Nogo inhibition task as an adult (Casey et al., 2011). Conversely, evidence about the effects of alcohol use on DD is rare. To our knowledge, there are two longitudinal studies reporting no effect of adolescent alcohol use on future DD (Fernie et al., 2013, Fernández-Artamendi et al., 2018). These studies investigated participants over a two year period. If alcohol actually effects DD, the evidence suggests that a more prolonged alcohol use is needed to cause changes in DD.
In summary, there is convincing evidence about an association between DD and alcohol use, especially with regard to high-risk or binge drinking or alcohol use disorders. Although studies suggest a dose-dependent association between DD and drinking, studies about low-risk or social drinking are rare. The question remains unclear whether or not DD is a developmental antecedent or a consequence of alcohol use during adolescence and young adulthood – or even both. For a comprehensive understanding of the etiology of alcohol use disorders and the potential role of DD, we believe that it is important to study these associations even for low-level drinking. Therefore, we analyzed data of a large, longitudinal sample of initially 14-year-old adolescents over eight years. By modeling longitudinal changes in DD and alcohol use in latent growth curve models, we assessed whether or not a possible reciprocal relationship exists between the initial values and trajectories of DD and drinking. Regarding the trajectories of DD and drinking, we expect DD to decrease and alcohol use to increase. Concerning the interaction, we propose that higher initial DD at baseline predicts more drinking at age 14 and a steeper increase in drinking. A lower decrease in DD over time should be related to more alcohol consumption in this period, as increased alcohol use hinders brain maturation processes by which DD decreases. To investigate the brain mechanisms underlying the association of DD and drinking, we analyzed a subsample that completed an intertemporal choice task during fMRI. Derived from previous analyses of the task, we used a combination of a-priori and exploratory regions of interest within the mesocortical system to look for association with drinking.
Materials and methods
Participants
The participants in this study were part of the IMAGEN study – a large, longitudinal, multicentre study (Schumann et al., 2010). They were recruited from schools at the age of 14. In Dresden, the participants were also part of the longitudinal study “The adolescent brain”, which was funded by the German Federal Ministry of Education and Research (BMBF). Local ethics research committees approved the study at each site (see Supporting Information for the list of ethic committees). Over the course of the study, the participants completed up to four institute assessments along with online assessments at ages 14, 16, 18 and 22. The institute visits involved a comprehensive assessment of adolescent substance use and decision making in form of questionnaires and computerized tasks in- and outside the scanner. Here, we investigated 2220 participants of the IMAGEN sample, who completed the relevant questionnaires at least for one acquisition wave. 238 participants recruited in Dresden completed an intertemporal choice task (iTeCh) during fMRI at age 14, 16 and 18. Again, participants who completed at least one session were included (see Tables 1 and 2 for sample descriptions). Our group already published work on iTeCh for the first acquisition wave in a cross-sectional comparison with adults (Ripke et al., 2012).
Table 1:
Sample description of 2220 participants, which completed at least one assessment of discounting and alcohol use. 1084 participants were male. Please note that we have missing MCQ data from wave 1 for 18 participants due to incomplete online questionnaires.
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | |
|---|---|---|---|---|
| N | 2202 | 1669 | 1489 | 1350 |
| Age | 14.4 (0.43) | 16.5 (0.63) | 19.0 (0.75) | 22.6 (0.67) |
| MCQ k (sd) | −4.35 (1.46) | −4.42 (1.41) | −4.44 (1.43) | −4.66 (1.51) |
| AUDIT total score (sd) | 1.5 (2.50) | 3.7 (3.48) | 5.6 (4.19) | 6.1 (4.64) |
| Gram alcohol / week (TLFB) | 3.8 (15.66) | -* | 89.8 (12.64) | 95.9 (105.22) |
| Number of cigarettes / week (TLFB) | 2.6 (2.65)*** | -* | 29 (35.3) | 31 (38.9) |
| Number of days with cannabis use / week (TLFB) | 0.91 (1.626) | -* | 0.34 (1.187) | 0.48 (1.483) |
| Verbal comprehension** | 108 (14.6) | − | − | − |
| Perceptual reasoning** | 97 (17.0) | − | − | − |
| Socioeconomic status | 19 (3.8) |
Note: MCQ – Monetary Choice Questionnaire; AUDIT – Alcohol Use Disorder Identification Test; TLFB – Timeline Follow Back interview
TLFB data was not acquired in large IMAGEN sample at age 16
IQ index scores computed from Wechsler Intelligence Scale for children
Number of days with tobacco use (number of cigarettes not acquired)
Table 2:
Sample description of fMRI subsample of 221 participants, which completed at least one fMRI and alcohol assessment. 122 participants were male. Please note that 7 participants did not complete the first MRI session.
| Wave 1 | Wave 2 | Wave 3 | ||||
|---|---|---|---|---|---|---|
| Binge group | Binging | Non-binging | Binging | Non-binging | Binging | Non-binging |
| N | 214 | 202 | 168 | |||
| N complete cases | 148 | |||||
| Binging / Non-binging | 69 / 79 | |||||
| Age (sd) | 14.7 (0.35) | 16.6 (0.41) | 18.7 (0.57) | |||
| 14.6 (0.35) | 14.6 (0.31) | 16.5 (0.38) | 16.5 (0.35) | 18.5 (0.41) | 18.6 (0.39) | |
| iTeCh k (sd) | −3.69 (1.19) | −3.88 (1.44) | −3.87 (1.37) | |||
| −3.92 (1.24) | −3.55 (1.09) | −3.93 (1.38) | −3.72 (1.19) | 3.80 (1.36) | 3.83 (1.41) | |
| MCQ k (sd) | −4.65 (1.58) | −4.66 (1.47) | −4.69 (1.47) | |||
| −4.89 (1.49) | −4.57 (1.47) | −4.78 (1.39) | −4.54 (1.18) | −4.74 (1.31) | −4.53 (1.47) | |
| AUDIT total score (sd) | 2.2 (3.49) | 3.7 (2.79) | 4.3 (2.96) | |||
| 2.2 (3.83) | 1.6 (3.06) | 4.4 (3.12) | 2.7 (2.42) | 6.2 (2.93) | 2.6 (1.57) | |
| Gram alcohol / week (TLFB) | 5.0 (30.51) | 28.5 (45.33) | 49.4 (59.44) | |||
| 3.4 (6.94) | 7.1 (51.85) | 39.8 (54.63) | 16.3 (40.57) | 98.9 (74.4) | 14.7 (13.18) | |
| Number of cigarettes / week (TLFB) | 1 (7.8) | 4 (13.9) | 11 (23.7) | |||
| 1 (5.6) | 1 (11.4) | 3 (8.8) | 4 (15.4) | 15 (26.4) | 8 (21.0) | |
| Days with cannabis use / week (TLFB) | 0.01 (0.057) | 0.05 (0.517) | 0.16 (0.781) | |||
| 0.00 (0.030) | 0.01 (0.087) | 0.11 (0.873) | 0.02 (0.116) | 0.17 (0.602) | 0.22 (1.049) | |
| MFG signal (sd) | 1.24 (1.25) | 1.42 (1.15) | 1.14 (0.90) | |||
| 1.16 (1.41) | 1.35 (1.21) | 1.17 (1.41) | 1.56 (1.06) | 0.97 (0.82) | 1.23 (0.82) | |
| Thalamus signal (sd) | 0.48 (2.69) | 0.79 (2.01) | 0.45 (1.56) | |||
| 0.27 (2.98) | 0.87 (2.69) | 0.89 (2.34) | 0.87 (1.79) | 0.52 (1.50) | 0.48 (1.58) | |
| dlPFC signal (sd) | 0.64 (1.20) | 0.91 (1.09) | 0.65 (1.01) | |||
| 0.70 (1.87) | 0.73 (1.53) | 0.89 (1.65) | 1.06 (1.34) | 0.69 (1.00) | 0.87 (1.14) | |
| VS signal (sd) | 0.009 (0.027) | 0.004 (0.089) | 0.009 (0.020) | |||
| 0.01 (0.018) | 0.01 (0.024) | 0.01 (0.022) | 0.02 (0.044) | 0.01 (0.023) | 0.01 (0.018) | |
| Verbal comprehension** | 114 (13.9) | − | − | |||
| 114 (12.8) | 112 (13.6) | |||||
| Perceptual reasoning** | 95 (15.5) | − | − | |||
| 95 (14.9) | 95 (15.9) | |||||
| Socioeconomic status | 19 (3.8) | − | − | |||
| 18 (3.91) | 19 (3.55) | |||||
Note: MCQ – Monetary Choice Questionnaire; AUDIT – Alcohol Use Disorder Identification Test; TLFB – Timeline Follow Back interview; MFG – medial frontal gyrus; dlPFC – dorsolateral prefrontal cortex; VS – ventral striatum
IQ index scores computed from Wechsler Intelligence Scale for children
Delay Discounting
The Monetary Choice Questionnaire (MCQ) measures DD by asking individuals to choose between rewards available immediately and larger rewards available after a delay (Kirby and Marakovic, 1996). The participants completed the 27-item questionnaire online at age 14, 16, 18 and 22. Additionally, the fMRI subsample completed an intertemporal choice task (iTeCh), during which the participants are asked to choose between a smaller immediate amount and a larger delayed amount of money in 90 trials (Figure 1).
Figure 1:
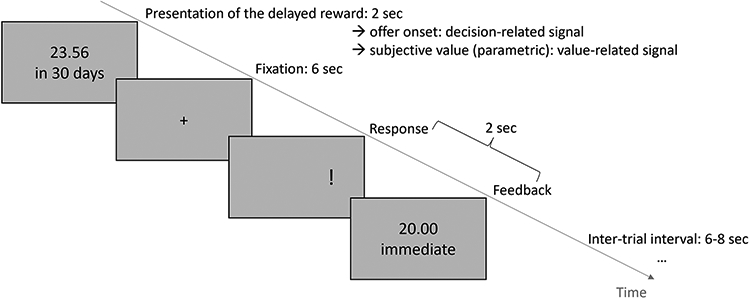
Time course of one trial in the intertemporal choice task (Ripke et al., 2012). Participants have to choose between a delayed amount of money and a fixed immediate amount (here: 20.00 €). Delays ranged from 10 to 180 days and delayed amounts were adapted to each individuals discounting rate (for further details, see Ripke et al., 2012). After the presentation of the delayed reward for 2 seconds, participants have 6 seconds to decide. Then, an exclamation mark indicates which button (left or right) they have to press to select the delayed reward. Each trial was followed by an inter-trial interval (M=7 seconds, range [6-8]). For fMRI analyses, the decision-related signal is based on the onset of offer presentation. The value-related signal refers to a parametric modulator representing the subjective value of an offer based on the individual discounting rate.
For the estimation of the discounting rate k, we used the following equation (see Equation (1)). The temporal discounting rate, k, governs the subjective assignment of value, V, to a monetary amount A when it is delivered after delay D measured in days:
| (1) |
We deliberately decided not to compute different discounting rates for different reward magnitudes, as possible with the MCQ. The overall discounting rate facilitates the comparison with our task-based version. Please refer to the Supporting information for a comparison of task- and questionnaire-based discounting rates.
After computing a discounting rate for each subject, we calculated the consistency, i.e. how many choices are predicted correctly by k? Gray and colleagues (2016) suggest excluding participants with a consistency lower than 70%. When doing so, we excluded 45 data points resulting in four excluded participants, because no MCQ data point was left for those participants. Repeating the LGM without these data revealed similar results with a little lower model fit. Since our aim was to exclude as few data as possible to increase power for model estimation, we decided to use the complete data set.
Drinking behavior
To assess drinking behavior, we used questionnaire and interview data. The Alcohol Use Disorder Identification Test (AUDIT; Saunders et al., 1993) is a 10-item questionnaire. The first three items assess the quantity and the frequency of their drinking. The other items ask about personal experiences or consequences of drinking, such as whether or not they have experienced feelings of guilt or failed to fulfil obligations due to alcohol intake. The total AUDIT score is therefore a combination of a quantity-frequency measure and a measure of the hazardous nature of alcohol use. In addition, we derived the quantity of alcohol use in gram per week from the Timeline Follow Back interview (TLFB), which was acquired at the ages of 14, 18 and 22 in the IMAGEN sample and at the ages of 14, 16 and 18 in the fMRI subsample (Sobell and Sobell, 1992). During the interview, participants were asked how much and what they drank during the last 30 days. Based on the gram-per-week measure, we also estimated the cumulative alcohol consumption by using an area under the curve (linear interpolation). The higher the cumulative alcohol consumption, the more adolescents were exposed to alcohol during the acquisition period.
Latent growth curve modelling
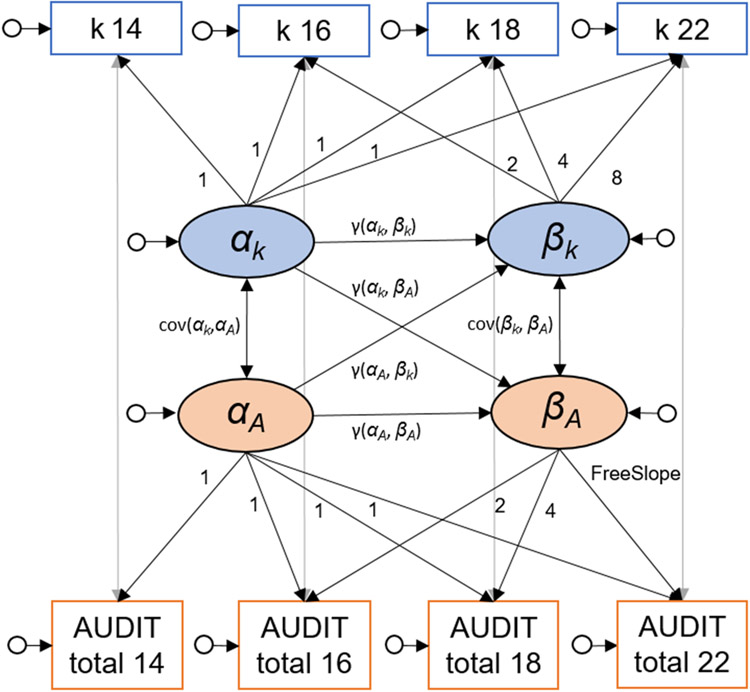
To associate the development of DD and drinking behavior, we computed a bivariate latent growth curve model (LGM; see Figure 2 for a schematic illustration; Duncan and Duncan, 2009, McArdle, 2009). The intercepts (α) represent the baseline, or starting point, for each variable, whereas the slopes (β) represent the development over time.
Figure 2:
Schematic bivariate latent growth curve model for discounting rate k derived from the Monetary Choice Questionnaire (MCQ) and total score of the Alcohol Use Disorder Identification Test (AUDIT). ▭: manifest variables, ○: latent variables, : residual variances, α: intercepts, β: slopes.
Using an LGM for this analysis presents a key advantage compared to a classical analysis of variance (ANOVA); it allows for the examination of individual development and individual differences in development (first level) in addition to group statistics and average development (second level). At the first level, we model the intercept and slope at time (T) for an individual (i) with residual variance (ϵTi) and linear trajectories (λT: factor loadings represent time) for each variable of interest. At the second level, intercepts and slopes are modelled by combining the mean (μα or μϐ; fixed effects) and the variance (Ψα and Ψϐ; random effects), which reflect individual differences in intercepts and slopes (see Equations 2 to 4). For clarity reasons, we report equations for discounting rate k as one variable of interest.
First level:
| (2) |
Second level: Intercept
| (3) |
Second level: Slope
| (4) |
As such, we modelled the developmental trajectories to capture the longitudinal development of DD and alcohol use. Since a previous IMAGEN study showed more drinking from male participants in Germany and France, we additionally ran a model with gender as grouping variable (Kühn et al., 2020). In the bivariate LGMs, we allowed the two intercepts and the two slopes to covary and regressed the slopes against the intercepts to assess the reciprocal associations. Model fits were acceptable (see Supporting information Table S1). The descriptive statistics were calculated using SPSS 27.0. LGMs were computed through the use of the lavaan package in R (Rosseel, 2012). We report standardized estimates. Full information maximum likelihood (FIML) method was used to account for missing values. As reported above, we included each participant who completed at least one wave. Assuming a similar model of development for all participants, each data point is helpful to define the model parameters (Duncan and Duncan, 1994). FIML estimates unbiased parameters of interest by using all available data. Since 98% of the participants who completed only one wave, completed the first wave, the inclusion of those participants mainly improves the estimation of the intercept (Raudenbush and Bryk, 2002).
fMRI data acquisition and analysis
Functional data were acquired with a 3T whole-body MR tomograph (Magnetom TRIO, Siemens, Erlangen, Germany) equipped with a 12-channel head coil at the Neuroimaging Center Dresden. A standard echo planar imaging (EPI) sequence was used for the functional images [repetition time (TR): 2410 ms; echo time (TE): 25 ms; flip angle: 80°]. fMRI scans were obtained from 42 transversal slices, oriented 30° clockwise to the anterior commissure – posterior commissure line, with a thickness of 2 mm (1 mm gap), a field of view (FOV) of 192x192 mm2 and an in-plane resolution of 64x64 pixels, resulting in a voxel size of 3x3x3 mm3. For structural images, a 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) image was acquired (TR: 1900 ms, TE: 2.26 ms, FOV: 256x256 mm2, 176 slices, voxel size: 1x1x1 mm3, flip angle: 9°) Images were presented via NNL goggles (Nordic Neurolab, Bergen, Norway). The task presentation and recording of the behavioral responses was performed using Presentation® software (version 11.1, Neurobehavioral Systems, Inc., Albany, CA).
fMRI data analysis was performed using SPM12 (Wellcome Department of Neuroimaging, London, United Kingdom) and MATLAB R2015a (Mathworks, Inc., Sherborn, MA). The preprocessing followed a standard pipeline including slice-time correction, realignment, coregistration to the respective structural image of the participant, normalization to the standard EPI template [Montreal Neurological Institute (MNI)] and smoothing with an isotropic Gaussian kernel (8 mm full-width at half-maximum). The first-level regressors included one regressor representing the offer onset and the corresponding parametric modulator. The parameter represents the subjective value of the presented offer, which was calculated via Equation 1 using the discounting rate determined at age 14. The discounting rate at age 14 was used first because the task was adapted to it and second to ensure comparability between acquisition waves. At the end of each trial, an exclamation mark appeared at one side of the screen, indicating where participants had to press to select the presented (delayed) offer. To separate the corresponding motor responses, we included two regressors representing the onsets of button presses with the left and right hand, respectively. We included the six realignment parameters as nuisance regressors.
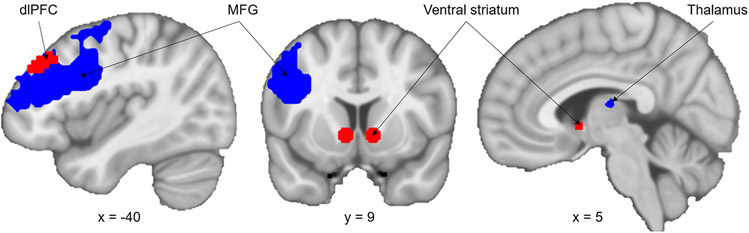
As introduced in previous analyses of the iTeCh data, we focus on the phase of presenting the delayed reward. The signal elicited by the reward presentation can be separated into two components. The first component represents a general, decision-related signal and is modeled by the onset of reward presentation (intercept). The second component represents a value-related signal, which is modeled as parametric modulation (slope) by the subjective value of the delayed reward presented (computed by the individual discounting rate; see Figure 1). The decision-related signal is represented in a large network including parietal and frontal regions, such as the anterior cingulate cortex or the dorsolateral prefrontal cortex (dlPFC) (Ripke et al., 2015). We see value-related signal in the prominent valuation-network of the ventral striatum (VS), posterior cingulate cortex and ventral-medial prefrontal cortex (Ripke et al., 2015). Since we assumed an imbalance within the mesolimbic system as shared mechanism of adolescent drinking and DD, we defined two a-priori regions of interest (ROIs) derived from our previous studies of the iTeCh task: dlPFC and VS (Ripke et al., 2015, Ripke et al., 2012).
Lately, we reported the surprisingly low reliability of the value-related signal (Fröhner et al., 2019). Therefore, we performed a novel, exploratory approach to fMRI analysis. We extracted signal from reliable ROIs in the offer contrast by using our toolbox fmreli (https://github.com/nkroemer/reliability). By setting the minimal threshold for between-session Spearman’s correlation to 0.35 and the minimal cluster size to 10, we identified eight moderately reliable clusters (Taylor, 1990). To identify a meaningful ROI, we looked for overlap with the group statistics of the offer contrast and found the left medial frontal gyrus (MFG; including parts of the inferior frontal gyrus) and parts of the thalamus. Thus, we investigated four ROIs: the two a-priori ROIs VS and left dlPFC and the two reliable ROIs left MFG and thalamus (see Figure 3). Please refer to an online repository for group statistics and correlation maps of the value-related and decision-related signal, as well as the below depicted ROIs (https://neurovault.org/collections/AEWGYGKQ/).
Figure 3:
Regions of interest (ROI) for fMRI analyses. Red: a-priori ROIs ventral striatum and dorsolateral prefrontal cortex (dlPFC) from previous analyses. Blue: reliable ROIs medial frontal gyrus (MFG) and Thalamus defined by minimal Spearman’s correlation of 0.35 and the minimal cluster size to 10.
First, we correlated the signal of each ROI with discounting and drinking. Afterwards, we planned to compute bivariate LGMs for each ROI and drinking. However, we could not estimate a model with an acceptable model fit. Therefore, we grouped the participants based on their binge drinking behavior (binging or not-binging) as outlined in their responses in the Timeline Follow Back interview. We defined binge drinking as consumption of at least five standard drinking units (SDU) per occasion for male participants and four SDUs for female participants. Participants were categorized as binging, if they reported at least one binge occasion during the last 30 days. We used the TLFB for grouping to avoid additional missing values, because it was acquired at the same date as the MRI. Afterwards, we ran a repeated-measures ANOVAs with “binge-group” as a between-subject factor and the signal in each ROI as dependent variable.
As proposed during the review of the manuscript we conducted additional whole-brain analyses. Madhyastha et al. (2018) propose a new approach to use voxel-wise fMRI data. By means of their package neuropointillist (https://github.com/IBIC/neuropointillist), bivariate LGMs can be conducted to associate intercepts and slopes of signal in each voxel with intercept and slope of a variable of interest, e.g. drinking (Madhyastha et al., 2018). We planned to do so for both the decision-related and the value-related signals. To ensure that the LGMs would converge, we needed large enough voxel values and hence a sufficient amount of variance to be explained. As in our ROI analyses, the LGMs did not converge for the value signal. In contrast, for the decision-related signal the LGMs converged and ran within a mask of the main effect/group statistics. We thus looked for associations between intercepts and slopes of decision-related signal and gram of alcohol drunk per week acquired by the TLFB.
Results
Development of delay discounting
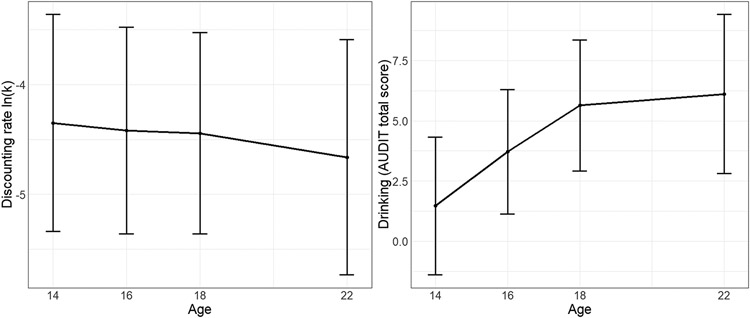
First, we characterized the development of DD (represented by discounting rate ln(k)) during adolescence and early adulthood. We hypothesized DD would decrease over time, which would align with previous findings of higher DD in adolescents compared to adults (Ripke et al., 2012). The average, ln(k) decreased slowly from −4.35 d−1 at age 14 to −4.66 d−1 at age 22, which resembles the small decrease found in other longitudinal studies (e.g. Fernie et al., 2013). Within the LGM, the decrease of ln(k) was significantly different from zero (μβk = −1.302, p = 1.740e-07; see Figure 4).
Figure 4:
Left panel: Average development for discounting rate ln(k) measured by the Monetary Choice Questionnaire. Right panel: Average development of drinking measured by the Alcohol Use Disorder Identification Test (AUDIT). According to the latent growth curve model, discounting is decreasing and drinking is increasing over time. Error bars represent standard deviations.
Development of alcohol use
Second, we analyzed the development of alcohol use as measured by the total AUDIT score. We predicted an increase in drinking over the course of adolescence into young adulthood. At the beginning of the study, 48% of the 14-year-old participants had not yet been drinking. The average total AUDIT score increased from 1.5 at age 14 to 6.1 at age 22. Within the LGM, the increase of alcohol use was significant (μβA = 1.486, p < .001; see Figure 4). Although 97% of the participants drank at age 22, only 8.4% of them reported a total AUDIT score of 8 or higher. Since a total AUDIT score higher than 8 represents hazardous alcohol use, we consider our sample to be low-level drinkers (Saunders et al., 1993). In addition, the average cumulative alcohol consumption over the 8-year study period was 21,675 gram alcohol. Assuming 416 weeks for eight study years, this equals 52 gram alcohol per week, which approximates 1.3 l beer or 0.5 l wine; see Figure S3 for the distribution of cumulative alcohol consumption).
Delay discounting and alcohol use
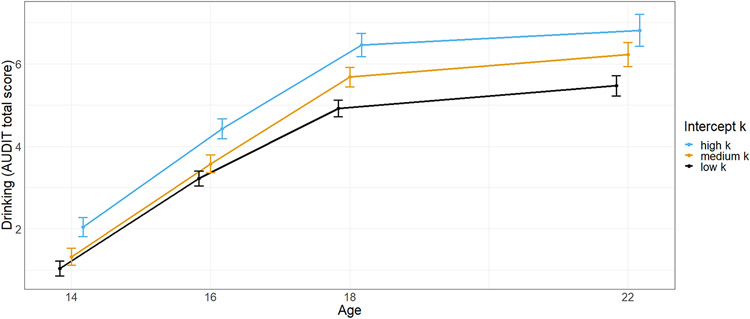
By modeling a bivariate LGM, we estimated the association of initial levels and trajectories of DD and alcohol use. Through these means, we investigated whether or not alcohol use is a cause and/or a consequence of increased rates of DD. There was a trend for a relation between a higher intercept of ln(k) with a higher intercept of drinking (cov(αk, αA) = 0.170, p = .062). The association of slopes was not significant (cov(βk, βA) = −0.010, p = .830). Notably, a high intercept of ln(k) was found to be related to a higher slope of drinking (γ(αk, βA) = 0.099, p = .011, see Figure 5), which means 1% of variance in drinking development were explained by ln(k). Concurrently, the initial discounting rate correlated with the cumulative alcohol consumption (r(1449) = 0.086, p = .001)), which has to be considered a very weak correlation (Cohen, 1988). Thus, a higher initial discounting rate preceded a more pronounced increase of alcohol use and the cumulative alcohol consumption during adolescence into young adulthood. Vice versa, the intercept of drinking was not associated with the slope of ln(k) (γ(αA, βk) = 0.013, p = .809). Thus, initial drinking did not influence the development of DD, which seems not surprising given the very low alcohol consumption at age 14. In addition, the cumulative alcohol consumption did not correlate with the development of the discounting rate (r(1449) = 0.004, p = .874). Please note that adding gender as a grouping variable did not change the reported associations (see Supporting information).
Figure 5:
Development of drinking measured by total score of the Alcohol Use Disorder Identification Test (AUDIT). For illustration purposes grouped by discounting rate k at baseline (intercept) measured by the Monetary Choice Questionnaire. According to the latent growth curve model, drinking is increasing more the higher the intercept of k. Error bars represent a 95% confidence interval.
fMRI results
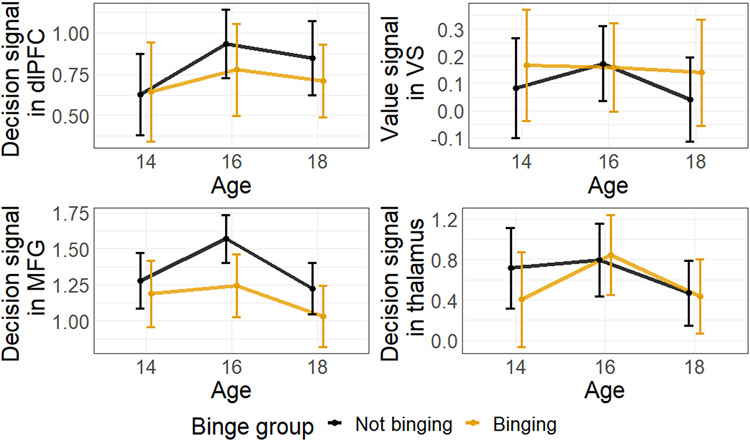
For a subsample, we acquired fMRI data during an intertemporal choice task (see Supporting information for the behavioral results of the fMRI subsample [Table S5] and a comparison of the samples [Figure S2]). We used two a-priori ROIs, the left dlPFC for the decision-related signal and the VS for the value-related signal. Additionally, we defined two reliable ROIs for the decision-related signal, the left MFG and the thalamus. In Figure 6, we depicted development of decision- and value-related signal from age 14 to 18.
Figure 6:
The development of decision-related signal in the a-priori ROI left dorsolateral prefrontal cortex (PFC) as well the reliable ROIs left medial frontal gyrus (MFG) and thalamus. The development of value-related signal in ventral striatum (VS). Participants were grouped by binging or not-binging at age 18 according to their responses in the Timeline Follow Back interview (Standard drinking units => 5 for male participants and Standard drinking units => 4 four female participants). Error bars represent 95% confidence interval.
We correlated signal for each ROI signal with the discounting rate k. We found hardly any correlation between ln(k) and the extracted signals, which was due to the adaptive nature of the task (Ripke et al., 2012). It was adapted to the individual discounting rates to elicit similar brain activity in participants. We found a cross-sectional correlation between ln(k) and value-related signal in the ventral striatum at age 14 (r = −0.165, p = .020) and a longitudinal correlation between striatal signal at age 16 and ln(k) at age 18 (r = 0.192, p < .001; see Tables S8/9 for complete correlation results).
fMRI and drinking
To check whether or not there is a link between the extracted signal and the drinking behavior, we computed correlations. More drinking at age 16 was associated with less decision signal in dlPFC at age 16 (r = −0.163, p = .025) as well as at age 18 (r = −0.170, p = .019). Concurrently, higher decision signal in dlPFC at age 16 was associated with less drinking at age 18 (r = −0.150, p = .032; see Tables S8/9 for the complete correlation results). Next, we could not identify a LGM with an acceptable model fit for the fMRI data. We concluded three acquisition waves and the comparably small sample size were not adequate to model the mostly quadratic trajectories of the decision- and the value-related signal. Therefore, we performed a repeated-measures ANOVA that involved grouping participants into binging (N = 69) and not-binging (N = 79) based on their responses in the TLFB interview at age 18 Figure. Notably, only participants with a complete data set could be included in the following analysis (N = 148). A repeated-measures ANOVA with signal as dependent variable, age as within-subject factor and binging as between-subject factor was computed for each ROI. To sum it up, we could neither identify significant change nor an association with binge group for the thalamus, dlPFC and VS ROI. We found a trend for a change in MFG activity, which did not interact with binge group. However, MFG signal was higher in non-bingers over all measurements. Combined with the correlation results for the dlPFC, adolescents that drink less seem to recruit more cognitive control during intertemporal choices.
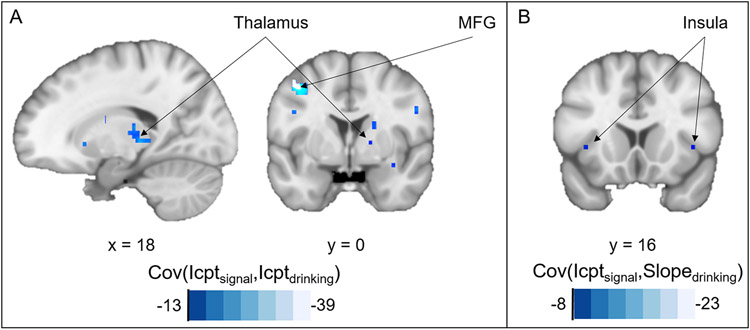
By means of the R-package neuropointillist (https://github.com/IBIC/neuropointillist), we computed voxel-wise LGMs for all voxels with a significant decision-related signal in the second-level statistic. We found mainly negative correlations between the intercepts of decision-related signal and drinking (measured by gram alcohol drunk per week). This applied amongst others for a large cluster in the Thalamus (cluster size = 787 voxel) and a smaller cluster of the medial frontal gyrus (cluster size = 51; see Figure 7A). Interestingly, we identified both regions as reliable in our analyses above. We found only single voxels with comparably low correlation for the association of slopes of decision-related signal and drinking. The same goes for the association of the intercept of decision-related signal and slope of drinking. However, we identified a potentially interesting pattern: negative correlation between the intercept of bilateral decision-related signal in small parts of the insula (right: cluster size = 15, left: cluster size = 18) and slope of drinking (see Figure 7B). Concluding, participants with higher decision signal during the intertemporal choice task drank less at age 14 and showed less drinking increase during the study. The correlation between intercept of drinking and slope of decision-related signal was positive in some parts of the brain. However, this needs to be interpreted with caution, given that we found barely significant change (slopes) in these voxels. We added the maps with the voxel-wise correlations to our neurosynth repository (https://neurovault.org/collections/AEWGYGKQ/). Please note that LGMs for other voxels of the decision-related signal or the value-related signal did not converge, which we will discuss in the limitations.
Figure 7:
Neuropointillist results of voxel-wise latent growth curve analysis (Madhyastha et al., 2018). Voxels with significant correlation between (A) intercept of decision-related signal and intercept of drinking and (B) intercept of decision-related signal and slope of drinking.
Discussion
In a large longitudinal study, we investigated whether increased DD is a developmental antecedent and/or a consequence of alcohol use during adolescence and early adulthood. In addition, we looked for neural correlates of intertemporal choices to further elucidate the shared brain mechanisms that may underlie the development of DD and alcohol use. Therefore, we assessed a large sample of adolescents from age 14 until age 22. As expected, DD decreased during adolescence, while alcohol use increased. Steeper DD at age 14 was associated with a more pronounced increase of alcohol use and a higher cumulative alcohol consumption over 8 years. Concluding, higher DD precedes alcohol use in our adolescent, low-level drinkers. In turn, we found no evidence for an effect of moderate alcohol use on DD during adolescence into young adulthood. An fMRI analysis revealed higher decision signals in frontal top-down control regions in adolescents with less alcohol use.
In our data, higher initial rates of DD precede a steeper increase in alcohol use. Given the small effect size (0.016), we propose DD as minor, but one causing factor of alcohol use during adolescence and young adulthood. This conclusion is in line with the vulnerability concept, stating impulsivity as a predisposing factor of more pronounced alcohol use leading to alcohol use disorders (Verdejo-García et al., 2008). Similarly, the Substance Use Risk Profile Scale measures impulsivity as one out of four risk factor for substance use (Woicik et al., 2009, Jurk et al., 2015). Recently, a study concluded that DD predicts future addictive symptoms (Kräplin et al., 2020). Data from the IMAGEN study showed that DD predicted cannabis use, but cannabis use did not predict DD (Mackey et al., 2017; https://doi.org/10.1016/j.biopsych.2017.02.604). Thus, the reported studies suggest DD as a transdiagnostic predisposing factor of substance use in general, not alcohol use in particular (Amlung et al., 2019). In addition, the role of family risk for substance use was statistically moderated by DD (Kim-Spoon et al., 2019). Hence, DD is a potential target for the prevention of pathological drinking. Moreover, a meta-analysis demonstrated that some clinical interventions already decrease DD without explicitly targeting it (Rung and Madden, 2018). Future research is needed to investigate whether or not interventions targeting DD can affect drinking behavior in the long term, given that in our study only 1% of variance in alcohol use is explained by DD.
According to our results, the moderate alcohol consumption does not affect development of DD. This is in line with the longitudinal study from Fernie and colleagues (2013) who report no prediction of DD by alcohol involvement over two years in early adolescence. Notably, the initial alcohol use at age 14 is especially low, as half of the participants did not yet drink at age 14. Even for the 22-year-old participants, the average AUDIT score is below what is categorized as hazardous alcohol use. Thus, the early, low-level drinking patterns do not cause behavioral or neurobiological changes (Fernández-Artamendi et al., 2018). Likewise, we could not show an impact of low-level alcohol use on the development of cognitive abilities in a previous analysis of our sample (Jurk et al., 2016). We believe that it is important to collect evidence about actual effects of moderate alcohol use given that the adolescent brain is vulnerable to neurotoxic effects as described earlier (Bava and Tapert, 2010). Nevertheless, future studies need to explicitly recruit adolescents with a higher probability to develop heavier alcohol use, e.g. due to family history. By this means, they could check whether a more pronounced alcohol use effects DD.
Moreover, we provide some insight regarding the disparity between longitudinal studies showing stable DD during adolescence (Audrain-McGovern et al., 2009, Fernie et al., 2013) and cross-sectional studies showing higher DD in adolescents compared to adults (Green et al., 1999). The decrease in DD appears to be a slow process as part of the development from an impulsive adolescent to a more thoughtful and controlled adult. While DD has been shown to decrease for the majority of adolescents, there is great variance between the starting points and trajectories between individuals, which also stresses the importance of initial sample characteristics (Khurana et al., 2018). Subjectively, longitudinal studies need almost a decade to cover the entire span of adolescence. Thus, more long-term projects are needed to monitor adolescents into young adulthood. Only then it is possible to consider meaningful individual differences in starting points, direction, and steepness of developmental trajectories for certain variables of interest and correlate them with each other.
Through our fMRI analyses we found some indication for heightened activation in frontal control regions in participants that drink less. Concerning the left MFG, we found increased decision-related signal in non-binging adolescents. Similarly, we found medial frontal voxels, where decision-related signal was negatively associated with drinking at age 14. In addition, decision signal in the left dlPFC at age 16 correlated negatively with alcohol use at age 16 and 18. Caution is warranted interpreting these rather small effects. However, in reference to the previously discussed imbalance of promotional and inhibitory systems (Chambers et al., 2003), higher frontal activity in less drinking participants might reflect their increased top-down control to compensate the dominant promotional system. Hence, when faced with the decision of whether or not to drink more alcohol, they may also invest more control resources in deciding against drinking. In our low-level drinking sample and due to the resulting low cut-off for binge-drinking, the described imbalance might not be as strong and therefore harder to detect. Previously, brain activation in general and prefrontal dysfunction in particular have been suggested as one predisposing factor for increased alcohol use (Stephens and Duka, 2008, Squeglia et al., 2017). Lately, DD was shown to mediate the relationship between adverse childhood experiences and substance use (Levitt et al., 2021). Levitt and colleagues (2021) suggest that growing-up in such an environment causes brain changes that in turn promote DD and substance use. The study exemplifies the complexity of the etiology of substance use, e.g. alcohol use, and emphasizes the need of neurobiological and longitudinal evidence. Here, we reported longitudinal fMRI and DD measures and their associations with drinking. However, we will close with limitations of our study and an extensive outlook about open questions and considerations for future studies.
Limitations
As in many other longitudinal studies, we had to deal with missing data in the sample. We decided to include all participants, although we have 25% to 39% missing data points from the second to the fourth acquisition wave. The inclusion of all participants leads to a more appropriate estimation of the intercept (Raudenbush and Bryk, 2002). However, the estimation of the developmental trajectories (slopes) might be impacted by an attrition bias. As we report in detail in the Supporting information, female, well-educated, more patient and low-level drinking participants remained in the study longer. Thus, participants who drank more and discounted steeper dropped-out more often, which decreased variance in our sample and might lead to an underestimation of the respective slopes. Therefore, our results might underestimate the association between discounting at baseline and the drinking slope as well as the association between slopes. Concluding, we would expect stronger associations in a less-biased, more drinking sample, which is in line with previous studies that propose a dose-dependent relationship between discounting and drinking (MacKillop et al., 2011).
In the fMRI subsample, the LGM did not reveal an association between initial DD and drinking development, supposing that a certain sample size and power is needed to detect the small effect. According to Bayesian comparisons, participants recruited in Dresden were initially less impulsive and showed less drinking and slower increase in drinking over the course of adolescence, which in combination with the lower sample size and less assessment points might lead to different results (see Supporting information).
Regarding the fMRI results, we could only show weak associations which demonstrated less frontal activity in those participants who drink more. It is important to note that we chose a liberal binge criterion of at least one episode to keep group sizes similar. However, the cut-off might be too low to detect differences in “heavy” drinkers. From a developmental perspective, we could not show substantial changes in the brain activity between the ages 14 and 18. As described above, we doubt that the four years between 14 and 18 are sufficient to acquire the relatively slow changing processes over the course of adolescence. Given the low reliability of the a-priori ROIs, the results in general and the trajectories in particular have to be interpreted with caution. However, to our knowledge, we were the first to use reliable ROIs to look for behavioral associations. Even though this type of analysis is still in its infancy, we consider it a valuable outcome that we were able to identify regions which are relevant based on the literature.
Concerning the voxel-wise LGM analyses, models did only converge for regions being significantly activated in the group-statistic of decision-related signal. For other voxels and the value-related signal, the LGMs did not converge. Again, this raises awareness for the complexity of longitudinal (fMRI) analyses. Although there are now a variety of novel methods available, our study was designed and initiated before we knew what might be needed for such analyses. In our case, we think that the value-related signal does not provide enough variance to be explained in a model such as the LGM. Task-based fMRI was often tailored to elicit reliable group activity in specific regions. Tasks were not designed to find individual markers of brain activity that might even correlate with other variables of interest. Lastly, even if we would expect longitudinal brain-behavior correlations, the expected effect sizes are low. Thus, although our sample of 148 completed cases is comparably large, it might still not be sufficient to detect the effects.
Conclusions and Outlook
In a large longitudinal study, adolescents with higher DD at age 14 showed a stronger increase in alcohol use over the following eight years. The amount of alcohol consumed in this period did not affect the development of DD but drinking levels in our sample were mostly moderate. Imaging results showed small evidence for higher decision signal in medial and prefrontal regions indicating a stronger top-down control in adolescents who drink less. Moreover, we showed a slow decrease in DD during adolescence into early adulthood, which closes a gap between existing longitudinal and cross-sectional studies. Concluding, higher DD is rather a developmental antecedent than a consequence of drinking in adolescent, low-level drinkers.
Besides the scientific evidence, our study raises some important methodological issues, which might be considered in future studies. Here, a sample of adolescents was recruited at age 14, some of them had already started to drink alcohol, others not. Future studies should consider recruiting alcohol-naïve adolescent to have a methodological more robust ground for analyses of causes and consequences of alcohol use. Over the course of the study, most of the participants engaged in low-level drinking, whereas some did not drink at all, and only some showed hazardous alcohol use. For investigations with a specific focus on alcohol-use disorders, we suggest the consideration of risk factors, e.g. family history or adverse childhood events. By oversampling participants under risk, the sample will potentially include more (future) heavy drinkers. Concluding, longitudinal studies need to consider potential biases due to attrition when they define their sampling strategy. Recruitment of a representative sample does not guarantee a representative sample over the course of the study. Finally, before starting a longitudinal study about inter- and intraindividual differences, the reliability of the measures of interest should be investigated. Especially in fMRI studies, evidence is still rare about which paradigms and contrasts elicit reliable brain activity. In the future, evidence about reliability in fMRI should be systematically collected to facilitate study preparation.
Supplementary Material
Table 3:
Summary of results for repeated-measurements ANOVA
| Effect of age | Effect of group | Age x group interaction | |
|---|---|---|---|
| dlPFC |
F(2, 364) = 1.603 p = .203 partial η2 = 0.013 |
F(1,182) = 0.429 p = .513 partial η2 = 0.002 |
F(2,364) = 0.285 p = .752 partial η2 = 0.002 |
| Ventral striatum |
F(2, 364) = 0.377 p = .686 partial η2 = 0.002 |
F(1,182) = 0.526 p = .469 partial η2 = 0.003 |
F(2,364) = 0.248 p = .781 partial η2 = 0.001 |
| Thalamus |
F(1.89, 275.49) = 1.861 p = .160 partial η2 = 0.013 |
F(1,146) = 0.427 p = .515 partial η2 = 0.003 |
F(1.56, 275.49) = 1.558 p = .214 partial η2 = 0.011 |
| MFG |
F(2,292) = 2.984 p = .052 partial η2 = 0.020 → quadratic F(1,146) = 4.617, p = .033, partial η2 = 0.031 |
F(1,146) = 4.542 p = .035 partial η2 = 0.030 |
F(2, 292) = 0.444 p = .642 partial η2 = 0.003 |
Note: MFG – medial frontal gyrus, dlPFC: dorsolateral prefrontal cortex
Acknowledgements
This study was supported by the Deutsche Forschungsgemeinschaft (DFG project numbers 402170461 [TRR 265] and 178833530 [SFB 940] and [NE 1383/14-1]) and the German Ministry of Education and Research (BMBF Grants 01GS08152; 01EV0711; 01EE1406A, 01EE1406B, 01EE1406D [Forschungsnetz AERIAL]; 01GL1745B [Forschungsnetz IMAC-Mind]). JHF received a PhD-scholarship from the SFB 940 „Volition and Cognitive Control: mechanisms, modulators and dysfunctions”. Other sources included the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behavior in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), Human Brain Project (HBP SGA 2, 785907, and HBP SGA 3, 945539), the Medical Research Council Grant 'c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the National Institute of Health (NIH) (R01DA049238, A decentralized macro and micro gene-by-environment interaction analysis of substance use behavior and its brain biomarkers), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Medical Research Foundation and Medical Research Council (grants MR/R00465X/1 and MR/S020306/1), the National Institutes of Health (NIH) funded ENIGMA (grants 5U54EB020403-05 and 1R56AG058854-01). Further support was provided by grants from: - the ANR (ANR-12-SAMA-0004, AAPG2019 - GeBra), the Eranet Neuron (AF12-NEUR0008-01 - WM2NA; and ANR-18-NEUR00002-01 - ADORe), the Fondation de France (00081242), the Fondation pour la Recherche Médicale (DPA20140629802), the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012, the Fondation de l’Avenir (grant AP-RM-17-013 ), the Fédération pour la Recherche sur le Cerveau; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence; ImagenPathways "Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways" is a collaborative project supported by the European Research Area Network on Illicit Drugs (ERANID). This paper is based on independent research commissioned and funded in England by the National Institute for Health Research (NIHR) Policy Research Programme (project ref. PR-ST-0416-10001). The views expressed in this article are those of the authors and not necessarily those of the national funding agencies or ERANID.
We thank the participants for their impressive encouragement and participation over the years. We thank Matthew J. Belanger for language editing of the manuscript. We thank Tara Madhyastha for her help in setting up the neuropointillist analyses.
Footnotes
Disclosure/Conflicts of Interest
Dr Banaschewski served in an advisory or consultancy role for ADHS digital, Infectopharm, Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Roche, and Takeda. He received conference support or speaker’s fee by Medice and Takeda. He received royalities from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press; He received royalties from Hogrefe, Kohlhammer, CIP Medien, and Oxford University Press. The present work is unrelated to the above grants and relationships. Dr Barker has received honoraria from General Electric Healthcare for the teaching of scanner programming courses. Dr Poustka served in an advisory or consultancy role for Roche and Viforpharm and received speaker’s fee by Shire. She received royalties from Hogrefe, Kohlhammer and Schattauer. The present work is unrelated to the above grants and relationships. The other authors report no biomedical financial interests or potential conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Amlung M, Marsden E, Holshausen K, Morris V, Patel H, Vedelago L, Naish KR, Reed DD, McCabe RE (2019) Delay Discounting as a Transdiagnostic Process in Psychiatric Disorders: A Meta-analysis. JAMA Psychiatry 76:1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, Sweet LH, Acker J, Brown CL, MacKillop J (2014) Dissociable brain signatures of choice conflict and immediate reward preferences in alcohol use disorders. Addict Biol 19:743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP (2009) Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend 103:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Tapert SF (2010) Adolescent Brain Development and the Risk for Alcohol and Other Drug Problems. Neuropsychol Rev 20:398–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt N, Nebe S, Pooseh S, Sebold M, Sommer C, Birkenstock J, Zimmermann US, Heinz A, Smolka MN (2017) Impulsive Decision Making in Young Adult Social Drinkers and Detoxified Alcohol-Dependent Patients: A Cross-Sectional and Longitudinal Study. Alcohol Clin Exp Res 41:1794–1807. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Jonides J, Berman MG, Wilson NL, Teslovich T, others (2011) Behavioral and neural correlates of delay of gratification 40 years later. Proc Natl Acad Sci 108:14998–15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN (2003) Developmental Neurocircuitry of Motivation in Adolescence: A Critical Period of Addiction Vulnerability. Am J Psychiatry 160:1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain G (2006) Alcohol, Neurotransmitter Systems, and Behavior. J Gen Psychol 133:329–335. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical power analysis for the behavioral sciences, Routledge. [Google Scholar]
- Coskunpinar A, Dir AL, Cyders MA (2013) Multidimensionality in Impulsivity and Alcohol Use: A Meta-Analysis using the UPPS Model of Impulsivity. Alcohol Clin Exp Res 37:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Water E, Cillessen AHN, Scheres A (2014) Distinct Age-Related Differences in Temporal Discounting and Risk Taking in Adolescents and Young Adults. Child Dev:1881–1897. [DOI] [PubMed] [Google Scholar]
- Duncan SC, Duncan TE (1994) Modeling Incomplete Longitudinal Substance Use Data Using Latent Variable Growth Curve Methodology. Multivariate Behavioral Research 29:313–338. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC (2009) The ABC’s of LGM: An Introductory Guide to Latent Variable Growth Curve Modeling. Soc Personal Psychol Compass 3:979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Artamendi S, Martínez-Loredo V, Grande-Gosende A, Simpson IC, Fernández-Hermida JR (2018) What Predicts What? Self-Reported and Behavioral Impulsivity and High-Risk Patterns of Alcohol Use in Spanish Early Adolescents: A 2-Year Longitudinal Study. Alcohol Clin Exp Res 42:2022–2032. [DOI] [PubMed] [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, Field M (2013) Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction 108:1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhner JH, Teckentrup V, Smolka MN, Kroemer NB (2019) Addressing the reliability fallacy in fMRI: Similar group effects may arise from unreliable individual effects. Neuroimage 195:174–189. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA (1990) Brain Systems that Mediate both Emotion and Cognition. Cogn Emot 4:269–288. [Google Scholar]
- Green L, Myerson J, Ostaszewski P (1999) Discounting of delayed rewards across the life span: age differences in individual discounting functions. Behav Processes 46:89–96. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF (2013) Neurotoxic Effects of Alcohol in Adolescence. Annu Rev Clin Psychol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk S, Kuitunen-Paul S, Kroemer NB, Artiges E, Banaschewski T, Bokde AL, Büchel C, Conrod P, Fauth-Bühler M, Flor H (2015) Personality and substance Use: psychometric evaluation and validation of the Substance Use Risk Profile Scale (SURPS) in English, Irish, French, and German adolescents. Alcohol Clin Exp Res 39:2234–2248. [DOI] [PubMed] [Google Scholar]
- Jurk S, Mennigen E, Goschke T, Smolka MN (2016) Low-level alcohol consumption during adolescence and its impact on cognitive control development. Addict Biol 23:313–326. [DOI] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Hurt H (2018) Modeling Trajectories of Sensation Seeking and Impulsivity Dimensions from Early to Late Adolescence: Universal Trends or Distinct Sub-groups? J Youth Adolesc 47:1992–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Lauharatanahirun N, Peviani K, Brieant A, Deater-Deckard K, Bickel WK, King-Casas B (2019) Longitudinal pathways linking family risk, neural risk processing, delay discounting, and adolescent substance use. J Child Psychol Psychiatry 60:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Marakovic NN (1996) Delay-discounting probabilistic rewards: Rates decrease as amounts increase. Psychon Bull Rev 3:100–104. [DOI] [PubMed] [Google Scholar]
- Kräplin A, Höfler M, Pooseh S, Wolff M, Krönke K-M, Goschke T, Bühringer G, Smolka MN (2020) Impulsive decision-making predicts the course of substance-related and addictive disorders. Psychopharmacology (Berl) 237:2709–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Lisofsky N, Banaschewski T, Barker G, Bokde ALW, Bromberg U, Büchel C, Brühl R, Quinlan EB, Desrivières S, Flor H, Grigis A, Garavan H, Gowland P, Heinz A, Ittermann B, Martinot J-L, Martinot M-LP, Nees F, Orfanos DP, Paus T, Poustka L, Fröhner JH, Smolka MN, Walter H, Whelan R, Schumann G, Gallinat J (2020) Hierarchical associations of alcohol use disorder symptoms in late adolescence with markers during early adolescence. Addict Behav 100:106130. [DOI] [PubMed] [Google Scholar]
- Levitt EE, Amlung MT, Gonzalez A, Oshri A, MacKillop J (2021) Consistent evidence of indirect effects of impulsive delay discounting and negative urgency between childhood adversity and adult substance use in two samples. Psychopharmacology (Berl) 238:2011–2020. [DOI] [PubMed] [Google Scholar]
- Mackey S, Chaarani B, Duffy C, Garavan H, Consortium I (2017) 879. Temporal Discounting at Age 14 Predicts Cannabis Use at Ages 16 and 18. Biol Psychiatry 81:S355. [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR (2011) Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 216:305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhyastha T, Peverill M, Koh N, McCabe C, Flournoy J, Mills K, King K, Pfeifer J, McLaughlin KA (2018) Current methods and limitations for longitudinal fMRI analysis across development. Dev Cogn Neurosci 33:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ (2009) Latent Variable Modeling of Differences and Changes with Longitudinal Data. Annu Rev Psychol 60:577–605. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD (2004) Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science 306:503–507. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, Kross E, Teslovich T, Wilson NL, Zayas V, Shoda Y (2011) ‘Willpower’ over the life span: decomposing self-regulation. Soc Cogn Affect Neurosci 6:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, MacKillop J (2012) Living in the here and now: interrelationships between impulsivity, mindfulness, and alcohol misuse. Psychopharmacology (Berl) 219:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C (2011) The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci 15:227–239. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS (2002) Hierarchical linear models: Applications and data analysis methods, sage. [Google Scholar]
- Reynolds B (2006) A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol 17:651–667. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R (2005) Delay of Gratification and Delay Discounting: A Unifying Feedback Model of Delay-Related Impulsive Behavior. The Psychological Record 55:439–460. [Google Scholar]
- Ripke S, Hübner T, Mennigen E, Müller KU, Li S-C, Smolka MN (2015) Common neural correlates of intertemporal choices and intelligence in adolescents. J Cogn Neurosci 27:387–399. [DOI] [PubMed] [Google Scholar]
- Ripke S, Hübner T, Mennigen E, Müller KU, Rodehacke S, Schmidt D, Jacob MJ, Smolka MN (2012) Reward processing and intertemporal decision making in adults and adolescents: The role of impulsivity and decision consistency. Brain Res 1478:36–47. [DOI] [PubMed] [Google Scholar]
- Rosseel Y (2012) lavaan: An R Package for Structural Equation Modeling. 48. [Google Scholar]
- Rung JM, Madden GJ (2018) Experimental reductions of delay discounting and impulsive choice: A systematic review and meta-analysis. J Exp Psychol Gen 147:1349–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, Conrod PJ, Dalley JW, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Mallik C, Mann K, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Reed L, Smolka M, Spanagel R, Speiser C, Stephens DN, Ströhle A, Struve M (2010) The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry 15:1128–1139. [DOI] [PubMed] [Google Scholar]
- Sharma L, Markon KE, Clark LA (2014) Toward a theory of distinct types of “impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychol Bull 140:374–408. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline Follow-Back, in Measuring Alcohol Consumption: Psychosocial and Biochemical Methods, Measuring Alcohol Consumption: Psychosocial and Biochemical Methods (LITTEN RZ, ALLEN JP eds), pp 41–72, Humana Press, Totowa, NJ. [Google Scholar]
- Spear LP (2018) Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci 19:197–214. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Ball TM, Jacobus J, Brumback T, McKenna BS, Nguyen-Louie TT, Sorg SF, Paulus MP, Tapert SF (2017) Neural predictors of initiating alcohol use during adolescence. Am J Psychiatry 174:172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Duka T (2008) Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond, Ser B: Biol Sci 363:3169–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R (1990) Interpretation of the Correlation Coefficient: A Basic Review. J Diagn Med Sonogr 6:35–39. [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L (2008) Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32:777–810. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Stewart SH, Pihl RO, Conrod PJ (2009) The substance use risk profile scale: A scale measuring traits linked to reinforcement-specific substance use profiles. Addict Behav 34:1042–1055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.