Abstract
Chronic psychological stress affects brain regions involved in memory such as the hippocampus and accelerates age-related cognitive decline, including in Alzheimer’s disease and vascular dementia. However, little is known about how chronic stress impacts hippocampal vascular function that is critically involved in maintaining neurocognitive health that could contribute to stress-related memory dysfunction. Here, we used a novel experimental rat model that mimics the neuroendocrine and cardiovascular aspects of chronic stress to determine how the neuroendocrine components of the stress response affect hippocampal function. We studied both male and female rats to determine potential sex differences in the susceptibility of the hippocampus and its vasculature to neuroendocrine stress-induced dysfunction. We show that activation of neuroendocrine stress pathways impaired the vasoreactivity of hippocampal arterioles to mediators involved in coupling neuronal activity with local blood flow that was associated with impaired memory function. Interestingly, we found more hippocampal arteriolar dysfunction and scarcer hippocampal microvasculature in male compared to female rats that was associated with greater memory impairment, suggesting the male sex may be at increased risk of neuroendocrine-derived hippocampal dysfunction during chronic stress. Overall, this study revealed the therapeutic potential of targeting hippocampal arterioles to prevent or slow memory decline in the setting of prolonged and/or unavoidable stress.
Keywords: Psychological stress, hippocampal vascular function, neuroendocrine stress response, memory, vascular dementia
Introduction
Cognitive impairment and dementia are leading contributors to disability and are increasing at an alarming rate as the population ages. It is estimated that there are > 55 million people living with dementia worldwide, a startling statistic that is expected to triple by 2050 (Cahill, 2020; Collaborators, 2022). Although aging is the primary non-modifiable risk factor for dementia, many other pathological conditions contribute to dementia that can accelerate age-related cognitive decline. Importantly, it has been estimated that approximately 1/3 of dementia cases may be preventable through changes in modifiable lifestyle factors (Livingston et al., 2017). Psychological stress is emerging as a substantial risk factor for cognitive decline and dementia, including vascular dementia and Alzheimer’s disease (Stuart and Padgett, 2020). Further, frequent/chronic stress occurring in mid-life is associated with a ~ 2.5-fold increased risk for age-related dementias (Johansson et al., 2010; Stuart and Padgett, 2020). There are strategies to lower stress levels throughout life (i.e., psychotherapy, medications, etc.); however, an understanding of how psychological stress affects brain regions critically involved in memory and cognition is essential in order to develop therapeutic strategies to ameliorate memory dysfunction occurring in response to unavoidable psychological stress, such as post-traumatic stress disorder or mood disorders.
The hippocampus is a brain region critical to memory function that is particularly susceptible to injury, including in response to psychological stress (Avila-Villanueva et al., 2020; Kim et al., 2015). Through activation of the hypothalamus-pituitary-adrenal (HPA) axis and subsequent elevated circulating glucocorticoid hormones (e.g., cortisol in humans, corticosterone in rodents), psychological stress has a deleterious effect on the hippocampus. Persistent exposure to elevated corticosterone levels causes changes in dendritic morphology and reduces spine density of rat hippocampal neurons that disrupt synaptic plasticity and impaired memory (Kim et al., 2015; Pavlides et al., 2002; Pereda-Perez et al., 2019; Sousa et al., 2000; Woolley et al., 1990). Further, chronic stress is associated with hippocampal atrophy (decreased hippocampal neuronal volume) in both humans and rats (Li et al., 2019; Lupien et al., 1998; Zimmerman et al., 2016). The underlying mechanism by which chronic stress causes hippocampal neurochemical changes and neuronal cell death thereby affecting learning and memory function is thought to be due to neuronal actions of glucocorticoids (Kim et al., 2015). However, prolonged glucocorticoid treatment and chronic stress have also been shown to reduce hippocampal blood flow that is considered a primary mechanism of hippocampal atrophy (Chao et al., 2010; Endo et al., 1997, 1999; Glodzik et al., 2019), suggesting blood vessels supplying the hippocampus could play a role in stress-induced hippocampal dysfunction.
Perfusion of the hippocampus is maintained by small hippocampal arterioles (HippAs) that are particularly important in neurocognitive health given the high susceptibility of the hippocampus to hypoxia and ischemia. These arterioles are involved in the rapid cascade of events that match local neuronal metabolic demand with appropriate blood flow. The dynamic communication between neurons, glia, and the vasculature, termed neurovascular coupling, is critical to neuronal health, and has been shown to be disrupted in other brain regions during chronic stress (Longden et al., 2014). However, the effect of chronic stress on hippocampal vascular function has yet to be investigated and may be particularly important given the high susceptibility of hippocampal neurons to hypoxic/ischemic injury.
In the current study, we investigated the impact of the neuroendocrine components of chronic stress on hippocampal function using a novel model of stress. This model is induced via vector-mediated brain-derived neurotrophic factor (BDNF) overexpression in the paraventricular nucleus (PVN) of the hypothalamus that causes chronic stimulation of the major hypothalamic stress pathways (e.g., HPA axis and sympathetic nervous system) and hypertension (Erdos et al., 2015; Thorsdottir et al., 2019; Thorsdottir et al., 2021a) without having to subject animals to stressors. We assessed hippocampal-dependent memory and studied the function of isolated and pressurized HippAs to test the hypothesis that stress-associated changes of the neuroendocrine system cause hippocampal vascular dysfunction and ultimately affect memory. To investigate potential mechanisms by which stress disrupts hippocampal function, vascular responses to mediators of neurovascular coupling were determined, including activation of small- and intermediate-conductance calcium-activated potassium (SKCa/IKCa) channels, inward-rectifying potassium (KIR) channels, and nitric oxide (NO). Hippocampal CA1 microvascular density and cell death were also investigated. Importantly, as incidence of dementia is higher in women and the stress response is sexually dimorphic (Levine et al., 2021; Yan et al., 2018), we studied both male and female rats to determine potential sex differences in the susceptibility of the hippocampus and its vasculature to neuroendocrine stress-induced dysfunction.
Materials and Methods
Animals
Experiments were conducted using 24 male and 29 female Sprague Dawley rats (12-16 weeks old) purchased from Charles River, Canada. Rats were housed in pairs of the same sex with environmental enrichment in the University of Vermont Animal Care Facility, an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) accredited facility. Rats acclimated to the animal facility for at least five days prior to handling and were maintained on a 12-h light/dark cycle and allowed access to food and water ad libitum. All procedures occurred at the same time of day and were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All euthanasia was under isoflurane anesthesia according to NIH guidelines.
Surgical procedures
All surgeries were performed using aseptic techniques under isoflurane anesthesia (5% induction, 2-3% maintenance in oxygen). Depth of anesthesia was assured by lack of reflex response to toe pinch of the hind paw. Carprofen (mg/kg/day s.c.) was used for postsurgical analgesia administered at the beginning of the surgery and for two days after surgery.
Viral vector-mediated gene transfer into the PVN.
At eight weeks of age, rats were put under isoflurane anesthesia and placed in a stereotaxic frame, BDNFmyc and green fluorescent protein (GFP) viral vectors (1012 viral particles/ml; 200 nL/side) were injected bilaterally into the PVN using pipettes pulled from thin walled borosilicate glass capillary tubes (OD, 1 mm; ID, 0.58 mm; tip diameter: ~25 μm; World Precision Instruments Inc., Sarasota, FL, USA) at the following stereotactic coordinates: 1.80 mm posterior to bregma; 1.70 mm lateral to the midline; and 7.65 mm ventral from the dorsal surface of the brain, with the micropipette tilted 10° laterally toward the midline. Virus stocks were injected over 5 min using a pneumatic pico pump (World Precision Instruments, Sarasota, FL). The pipette was left in place for an additional 3 min before being withdrawn.
Adeno-associated virus (AAV2) viral vectors were used to elicit the expression of enhanced GFP and BDNFmyc, derived from rat BDNF, constructed and packaged by Vector Biolabs (Philadelphia, PA). The expression of GFP and BDNFmyc was driven by a chicken-β-actin promoter with human cytomegalovirus enhancer, and a woodchuck post-transcriptional regulatory element, which enhanced the expression of transgenes, present downstream of GFP and BDNFmyc. The BDNFmyc plasmid was a generous gift from Dr. Ronald Klein (LSU Health Sciences Center Shreveport) and was used previously to protect retinal ganglion cells in a rat glaucoma model (Martin et al., 2003), and to study cardiovascular effects of BDNF in the PVN (Erdos et al., 2015; Thorsdottir et al., 2019; Thorsdottir et al., 2021b). In addition, full efficacy of BDNFmyc expression driven by the rat neuron-specific enolase promoter was confirmed previously both in vitro and in vivo (Klein et al., 1999). PVN injections of AAV2-GFP and AAV2-BDNFmyc resulted in marked expressions of GFP and BDNFmyc in the PVN as confirmed by fluorescent imaging and an antibody against the myc tag (Fig 1A). Only animals with bilateral GFP or BDNF expression in the PVN were included in the study.
Fig 1. Viral vector-mediated transduction of GFP and BDNFmyc, and assessment of the PVN-BDNF model of chronic neuroendocrine stress.
A. Diagram indicating location of bilateral viral vector injections into the paraventricular nucleus of the hypothalamus (PVN), and representative fluorescent images of coronal brain sections ~1.8 mm posterior to bregma showing PVN expression of GFP and BDNFmyc. B-D. Radiotelemetric recordings of daytime mean arterial pressure (MAP), heart rate and body temperature averaged over the last two weeks of the experiment in male and female rats. E. Fecal corticosterone levels measured from samples collected on the last day of the experiment from male and female rats. F. Body weight gain during the 5 weeks following viral vector injections in male and female rats. Results represent mean ± SD, *p < 0.05, **p < 0.01 vs GFP of the same sex by unpaired Mann-Whitney test.
Telemeter transmitter implantation and recording.
Radiotelemetric transducers (model HD-S10; Data Sciences International, St. Paul, MN) were implanted into the descending aorta of separate groups of rats via a midline abdominal incision. The aorta was isolated and briefly occluded, and the tip of the catheter was inserted using a 21-gauge needle. Surgical glue (3M Vetbond Tissue Adhesive) and a nitrocellulose patch were applied to secure the catheter in place. The transducer was sutured to the abdominal muscle, and the incision closed in layers. Blood pressure, heart rate, and body temperature of the animals were analyzed with Dataquest A.R.T. Analysis software (Data Sciences International). Data were recorded every 10 min for 15 seconds and averaged between 8:00 AM and 4:00 PM to calculate daytime values and between 8:00 PM and 4:00 AM to calculate nighttime values for each animal.
Behavioral tests of memory function
To determine the effect of neuroendocrine stress and hypertension on hippocampal-dependent cognitive function, long-term memory and spontaneous alternation as a test of spatial memory were tested using a novel object recognition (NOR) task and continuous Y maze task, as done previously (Johnson et al., 2021; Johnson et al., 2020). Briefly, groups of male and female rats that received PVN injections of either BDNF or GFP (n=6-8/group) acclimated to the behavioral room for one hour prior to testing. For the NOR task, each rat was allowed to habituate to the open field arena for five minutes, followed by acquisition period exploring two identical objects for 10 minutes (Ballarini et al., 2009). Forty-eight hours later, rats were placed in the same arena containing one familiar and one novel object. Automated live tracking software (ANY-maze, Stoelting Co., Wood Dale, IL, USA) was used to quantify baseline locomotion and exploratory behavior during the habituation phase, as well as the time that each rat spent investigating each object during acquisition and testing phases. The time spent investigating both the novel and familiar objects was used to calculate a recognition index as a measure of long-term memory function. For the continuous Y maze task, rats were allowed to freely explore a Y maze for eight minutes. Y maze tasks were video recorded to allow for quantification of spontaneous alternation behavior (SAB) and total arm entries that were used to calculate an alternation index as a measure of spatial memory (Hughes, 2004; Johnson et al., 2020). All videos were analyzed by a reviewer that was blinded to group.
Experimental protocol for isolated hippocampal arterioles (HippAs)
To understand the potential role of the hippocampal vasculature in hippocampal dysfunction in this neuroendocrine model of stress, HippAs supplying CA1 in the dorsal hippocampus were isolated and studied in a pressurized arteriograph system, as previously described (Johnson and Cipolla, 2016; Johnson et al., 2020). Briefly, rats that underwent behavioral testing (n=6-8/group) were decapitated under deep isoflurane anesthesia (3 % oxygen) and brains immediately removed and placed in cold, oxygenated artificial cerebrospinal fluid (aCSF). To eliminate experimental variability from anesthesia-induced stress, all rats were anesthetized for the same amount of time prior to decapitation. To allow for verification of vector injection, the PVN was removed prior to HippA dissection and immersion-fixed in 4 % ice-cold paraformaldehyde (PFA). HippAs were carefully dissected, mounted on glass cannulas and pressurized in an arteriograph chamber (Living Systems Instrumentation, Burlington, VT, USA) (Johnson and Cipolla, 2016; Johnson et al., 2020). HippAs were equilibrated at 40 mmHg for one hour, after which intravascular pressure was increased to 120 mmHg in a stepwise manner to determine if vessels developed spontaneous myogenic tone and to measure myogenic reactivity. Lumen diameter and wall thickness were recorded at each intravascular pressure. Pressure was then returned to 40 mmHg for the remainder of the experiment. To investigate endothelial and vascular smooth muscle function of HippAs to mediators of neurovascular coupling, reactivity to various pharmacological agents was measured: NS309 (10−8 – 10−5 M), an SKCa/IKCa channel agonist; extracellular KCl (3 – 20 mM) to determine inward rectifying potassium (KIR) channel function; and sodium nitroprusside (SNP, 10−8 – 10−5 M), a NO donor.
At the end of each experiment, aCSF was replaced with aCSF containing zero calcium, papaverine (10−4 M) and diltiazem (10−5 M) to fully relax the vascular smooth muscle, and passive structural measurements made within the pressure range of 5 – 120 mmHg.
Measurement of fecal corticosterone levels
Fecal pellets (1-2 pellets/rat) for corticosterone determination were collected before 8:30 am on week 5 following vector injections. Pellets were dried under the fume hood until dry weight did not change and then homogenized using pestle and mortar. Fecal powder was weighed out into a 2 mL Eppendorf tube. Corticosterone was extracted by incubating with 100 % EtOH (10mL/mg fecal matter) on an Aros 160 orbital shaker for 4 hours. Samples were then centrifuged (5,000 rpm, 15 min, 4°C). The supernatant was transferred into a new Eppendorf tube and EtOH was removed by vacuum centrifugation using Savant ISS110 SpeedVac (medium setting) and the sample was concentrated to 20X. The concentrated samples were kept on ice and closed to avoid evaporation of EtOH. Fecal corticosterone levels were determined using a commercially available DetectX Corticosterone ELISA kit (Arbor Assays, Ann Arbor, MI) following the manufacturer’s protocol.
Assessment of CA1 microvasculature
To determine stress-induced changes and potential sex differences in the microvascular structure of the CA1 region of the hippocampus, some rats were perfused with the dye 1,1'-Didodecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI). The dye solution was prepared by dissolving pronanosome NIO-N nanovesicles (Nanovex Biotech, Llanera, Asturias, Spain) in phosphate buffered saline (PBS) at 1mg/ml at 60° C in a water bath. Then, DiIC12(3) (Thermo Fisher D383, 5mM in ethanol) was added for a final concentration of 50μM and the solution was sonicated for 1 min. Animals were perfused (4 mL/min) with ~ 300 mL of ice-cold PBS followed by 20 mL liposome-encapsulated DiIC12(3) and 200-300 mL ice-cold 4 % PFA. Brains were quickly harvested, sliced into 2-mm coronal slabs and fixed in 4% PFA overnight. Subsequently, brain tissue transparency was increased using the SeeDB protocol (Ke et al., 2013) involving stepwise incubation with solutions containing increasing concentrations of fructose (20-100%) and 0.5% α-thioglycerol. Brain slabs were then imaged using a custom-made microscope slide on a Zeiss LSM-7 two-photon microscope yielding z-stack images of the hippocampal CA1 vasculature up to 1 mm in depth. These z-stacks were processed with Imaris image analysis software as follows: first, raw images were pre-processed using the Histogram Equalize Layers function and Gaussian Blur. Then, an Imaris “Surface object” was created around the vasculature (surface grain size = 2.37μm, diameter of largest sphere=8.9 μm) and used as a 3D mask to clear noise outside of the vasculature. Next, an Imaris “Filament object” (minimum diameter = 3.56 μm; minimum branch length to diameter ratio = 5.0) of the vasculature was created, which was used for statistical analysis of percent vascular density and for creating a 3D Euclidian distance maps (EDMs) representing the distance of every point within the extravascular space of the to the nearest blood vessel. Histograms of these 3D distance maps were generated to demonstrate distribution of “distances to the nearest blood vessel”.
Histology of brain sections to verify vector injections and assess neuronal injury
Animals were perfused with 400 mL of ice-cold PBS followed by 400 mL of ice-cold 4% paraformaldehyde in PBS. Brains were removed and post-fixed for 2 hours in 4% paraformaldehyde and then equilibrated in 30% sucrose solution at 4°C. Coronal sections (40 μm) were cut on a microtome (Leica SM2000R) and mounted on Fisher Superfrost Plus slides. Hematoxylin and eosin (H&E) staining was performed using standard methodology. Images of coronal brain sections containing dorsal hippocampus captured using a Leica-Aperio VERSA whole slide imager (Leica Biosystems Division of Leica Microsystems Inc.) and hippocampal morphology used to confirm consistent location within the dorsal hippocampus prior to doing immunohistochemical assessments of hippocampal neuronal death.
Fluorescent immunohistochemistry (IHC) was performed to detect BDNFmyc in the PVN and caspase-3 in the hippocampus using the following primary antibodies: mouse anti-c-Myc (Santa Cruz-9E10, 1:200, overnight incubation at 4°C), rabbit anti-caspase-3 (Cell Signaling-9661, 1:200, overnight incubation at 4°C), and mouse anti-NeuN (Abcam, ab177487, 1:200, overnight incubation at 4°C). Secondary antibodies were donkey anti-mouse AF546 (Invitrogen, 1:200, 2-h incubation at room temperature) and donkey anti-rabbit A555 (Invitrogen, 1:200, 2-h incubation at room temperature). GFP and BDNFmyc immunofluorescence in the PVN were detected with a fluorescent microscope (Nikon Eclipse 50i) in all animals involved in the study. The effect of BDNF-induced chronic neuroendocrine stress and hypertension on hippocampal CA1 neuronal apoptosis was determined by assessing the number of caspase-3 positive cells in the pyramidal layer of the CA1 region of the dorsal hippocampus. Z-stack images of caspase-3 and NeuN immunoreactivity were taken using a Nikon A1R confocal laser scanning imaging system and a 20X objective. Images were Z-project averaged using ImageJ software (NIH). To create a region of interest (ROI) of the pyramidal layer, NeuN immunofluorescence was blurred using ImageJ’s Gaussian Blur procedure (sigma=20) and thresholded with the Auto threshold method to create a mask image. Caspase-3 positive cells were counted within this ROI using the Analyze particles method and normalized to volume.
Drugs and solutions
Diltiazem was purchased from MP Biomedicals (Santa Ana, CA, USA), and all other compounds, including NS309, KCl, SNP, papaverine, paraformaldehyde and those used to make aCSF were purchased from Sigma Aldrich (St. Louis, MO, USA). Stock solutions of SNP, papaverine and diltiazem were made weekly and stored at 4° C until use. NS309 stock solution was aliquoted and stored at −20 °C until use. aCSF contained (mM): NaCl 122.0, NaHCO3 26.0, NaH3PO4 1.25, KCl 3.0, MgCl2 1.0, and CaCl2 2.0. aCSF with higher concentrations of KCl (8, 12 and 15 mM) were made with reduced amounts of NaCl to maintain constant osmolality. Buffer solutions were made each week and stored without glucose at 4 °C. Glucose was added (4.0 mM) immediately prior to each experiment. Zero calcium aCSF was made similarly, omitting the addition of CaCl2. aCSF was aerated with 5 % CO2, 10 % O2 and 85 % N2 to maintain pH at 7.40 ± 0.05 and the temperature within the arteriograph chamber was maintained at 37.0 ± 0.1 °C throughout the experiments.
Data calculations
To determine memory function, recognition index was calculated using the following formula: TimeNovel Object/TimeTotal where TimeNovel is the amount of time (sec) a rat spent investigating the novel object and TimeTotal is the total time (sec) that rat spent investigating both objects. Spontaneous alternation index was calculated using the following formula: [# Alternations / (# Arm Entries – 2)].
For isolated HippA experiments, percent myogenic tone was calculated using the following equation: % Tone = [1 – (φactive / (φpassive)] x 100 %; where (φactive is the diameter under active conditions and (φpassive is the diameter under fully relaxed conditions at a specific intravascular pressure. Percent reactivity to NS309, KCl and SNP was calculated from the equation: % Reactivity = [(φdose – (φbaseline) / (φpassive – (φbaseline)] x 100 %; where φdose is the diameter of the vessel after treatment with a specific concentration of drug and φbaseline is the starting diameter before any drug treatment. Outer diameter (φouter) was calculated at each pressure by the equation: φouter = φinner + 2WT; where φinner is the inner diameter of the vessel fully relaxed and WT is the measured wall thickness. Cross-sectional area (CSA) was calculated by the equation: CSA = π(φouter / 2)2 - π(φinner / 2)2 at 5 mmHg and 40 mmHg intravascular pressure. For wall tension, wall stress and wall strain, all diameter and WT measurements were converted to cm. Wall tension was calculated by converting pressure (mmHg) into dynes/cm2 x (φinner / 2). Wall stress was calculated by the equation: Wall Stress = wall tension / WT. Wall strain was calculated by the equation: Wall Strain = (φpassive – φ5) / φ5; where φ5 is the passive diameter at 5 mmHg. Percent distensibility was calculated using the equation: % Distensibility =[φpassive – φ5) / φ5] x 100 %.
Percent vascular density was obtained by first generating a filament model of the vasculature using Imaris software. Volumes of each vascular segment was then exported from Imaris, summed up and divided by the total volume of the z-stack image. Distribution of distances to the nearest blood vessel was analyzed by first generating 3D EDMs using Imaris. EDMs were then exported as .tif images where the value of each pixel encoded the distance of that point in the extravascular space to the nearest blood vessel. These tif images were then further processed by a custom Python algorithm, which generated a histogram of all pixel values representing distances in the 3D EDM. Histograms obtained from individual animals were then averaged for each experimental group. Images obtained from the two hemispheres were handled as individual samples.
Statistical analyses
The number of animals used in each experiment was determined by a statistical power calculation using a two-sided 95 % confidence interval for a single mean and 1 – β of 0.80 based on previous studies using similar methodology (Johnson and Cipolla, 2017; Johnson et al., 2020). We determined that n=6/group was sufficient to detect statistical differences in both vasoreactivity of HippAs to mediators of neurovascular coupling as well as novel object recognition and y maze tasks. All statistical testing was performed using GraphPad Prism 8.0 software (GraphPad Software Inc., La Jolla, CA). Results are presented as mean ± SD and a p-value of < 0.05 was considered significant. To determine differences between two groups, a two-sided non-parametric Mann-Whitney test was used. We determined differences in hippocampal vascular density using a two-way ANOVA with a post-hoc Tukey test to determine the interaction between independent variables (e.g., sex and stress). Rats were randomized to treatment group, and the order of experiments were randomized using an online randomization tool (Random.org). All experiments and data analyses were completed blinded to group.
Data availability
Data will be made available upon reasonable request.
Results
Assessment of the PVN-BDNF chronic neuroendocrine stress model
To investigate the effect of long-term activation of neuroendocrine stress mechanisms on hippocampal vascular function and memory, and whether one sex is more susceptible to stress-induced hippocampal dysfunction, we used a novel neuroendocrine model of stress involving overexpression of BDNF in the PVN of the hypothalamus (or GFP as control) (Erdos et al.,2015; Thorsdottir et al., 2019; Thorsdottir et al., 2021a). Fluorescent microscopy was used to confirm injections, and Fig 1A shows representative images of PVN neurons positively expressing either GFP or BDNF. Importantly, this model activates a neuroendocrine stress response without needing to physically handle or stress the animals, such as is required for other models including the commonly used chronic unpredictable stress paradigm (Sequeira-Cordero et al., 2019). This allows us to focus our investigation on understanding the effects of chronic neuroendocrine stress responses on memory and hippocampal vascular function independently of any hippocampal stimulation that may be associated with other stress models (i.e., learning).
After five weeks, both male and female rats receiving PVN-BDNF treatment demonstrated several aspects of stress-related cardiovascular, metabolic, and endocrine responses. Radiotelemetry measurements indicated elevated mean arterial pressure (Fig 1B), heart rate (Fig 1C), and body temperature (Fig 1D) in male and female PVN-BDNF-treated rats compared to GFP controls. Daily blood pressure, heart rate and body temperature telemetry recordings over the course of the study are presented in Supplementary Fig 1. Fecal levels of the stress hormone corticosterone were elevated in both male and female BDNF-treated rats (Fig 1E), confirming BDNF-mediated activation of the HPA axis. BDNF treatment in male rats also resulted in substantially decreased weight gain compared to GFP controls (Fig 1F), however, the same treatment had no effect in female rats potentially due to female rats gaining less weight at this age compared to males.
Memory was impaired by chronic neuroendocrine stress to a greater extent in males than females
Psychological stress is known to disrupt cognition and contribute to dementia (Avila-Villanueva et al., 2020; Stuart and Padgett, 2020). We assessed locomotion, anxiety-like behavior and hippocampal-dependent memory function using behavioral tasks four weeks after PVN-BDNF or GFP injections. In an open field arena, male BDNF-treated rats exhibited increased locomotion compared to GFP controls, including moving faster (Fig 2A) and further (Fig 2B). However, BDNF treatment did not change locomotion in female rats. The latency to the center of the arena, a measure of anxiety-like behavior, was reduced with BDNF treatment in both sexes compared to GFP controls (Fig 2C). Further, BDNF-treated rats of both sexes also crossed through the center of the arena more times than control counterparts (Fig 2D). These findings suggest that PVN-BDNF-derived neuroendocrine stress exerted an anxiolytic effect that was similar in both sexes, while only inducing hyperactivity, as measured by increased average speed and distance traveled, in male but not female rats.
Fig 2. Assessment of locomotion and exploratory behavior.
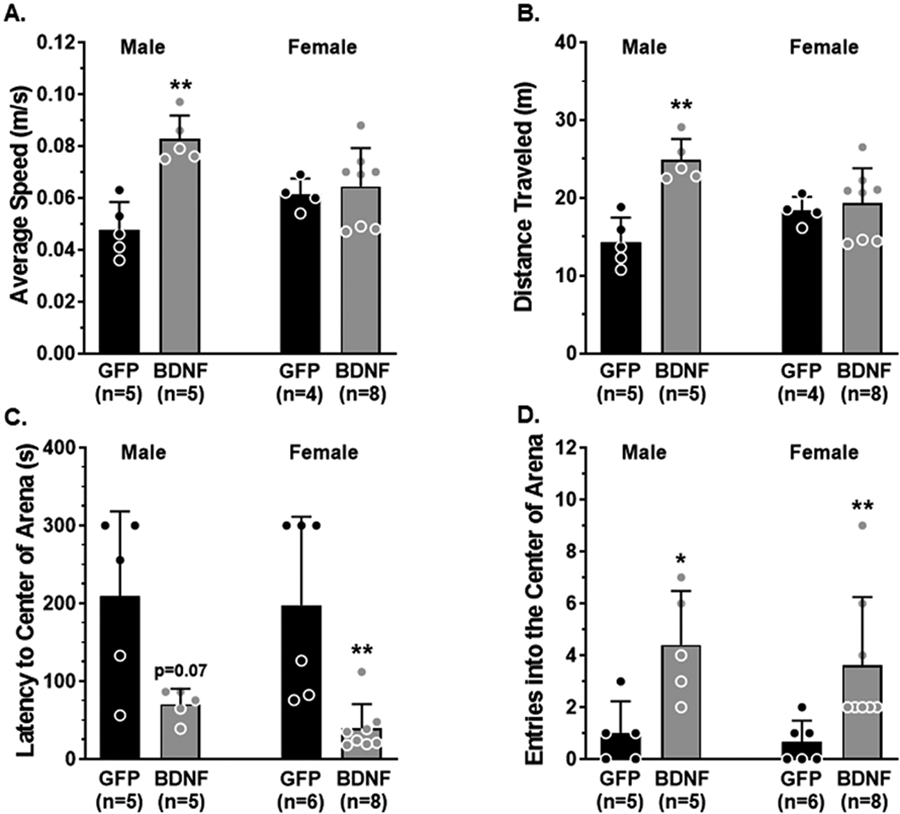
A-B. During the habituation phase of the NOR task male BDNF-treated rats traveled at an increased speed and a greater distance compared to male GFP controls whereas average speed and distance traveled were similar between groups of female rats. C-D. BDNF-treated male and female rats had a decreased latency to cross through the center of the arena and entered the center more times compared to GFP controls. Data are mean ± SD, * p < 0.05, **p<0.01 vs. GFP of the same sex by unpaired Mann-Whitney test.
An object recognition task was used to quantify the amount of time spent investigating a novel and a familiar object and a recognition index was calculated as a measure of long-term memory function. A rodent that is cognitively intact will spend the majority of the time investigating the novel object and therefore have a recognition index > 0.50. However, a rat with impaired memory function will not remember the familiar object and spend closer to equal amounts of time investigating both objects (recognition index ~ 0.50) (Broadbent et al., 2010; Quillfeldt, 2016). Fig 3A shows that PVN-BDNF treatment in both male and female rats significantly reduced recognition indices compared to GFP controls of the same sex, indicating impaired memory function following long-term neuroendocrine stress. Given the predominant role of the hippocampus in spatial navigation, we also used a continuous Y maze task to calculate alternation index and assess spatial memory function. Rats with intact spatial memory will exhibit spontaneous alternation behavior: they remember the arm of the maze they were recently in and naturally alternate between maze arms. A rat with impaired spatial memory will reverse directions and return to the maze arm they just visited (Fig 3B) (Hughes, 2004; Lalonde, 2002). BDNF-treated rats entered the Y maze arms a similar number of times as controls (22 ± 6 vs. 18 ± 5 entries in males; 28 ± 7 vs. 23 ± 7 entries in females; p > 0.05). Interestingly, BDNF-treated male rats performed worse in the Y maze task and had a reduced alternation index compared to GFP controls that did not occur in BDNF-treated female rats. Together, these data suggest memory function in male rats was more susceptible to disruption from neuroendocrine stress than in female rats, although both sexes demonstrated substantially impaired long-term memory function.
Fig 3. Behavioral assessments of long-term memory via a novel object recognition (NOR) task and spatial memory via a continuous Y maze task.
A. Illustration of the NOR task paradigm consisting of a 48-hour consolidation period to allow for assessment of long-term memory function. Recognition Index was decreased in male and female BDNF-treated rats compared to GFP controls. B. Illustration of the inherent spontaneous alternation behavior of cognitively intact rodents preferring to alternate between maze arms (left) and arm reversals occurring in error as an assessment of spatial memory function. Alternation Index was lower in male BDNF-treated rats compared to GFP controls but was unaffected by neuroendocrine stress in female rats. Data are mean ± SD, * p < 0.05 vs. GFP of the same sex by unpaired Mann-Whitney test.
Chronic neuroendocrine stress impaired reactivity of isolated HippAs
Hippocampal neurons are particularly susceptible to hypoxic/ischemic injury (Kawahara et al., 2004; Kirino, 1982; Nikonenko et al., 2009), highlighting the importance of the arterioles perfusing the hippocampus in maintaining neuronal health. To determine if hippocampal vascular function was disrupted by long-term neuroendocrine stress potentially contributing to impaired memory function, freshly isolated arterioles that supply the CA1 region of the dorsal hippocampus were studied in an arteriograph chamber (Johnson and Cipolla, 2017; Johnson et al., 2020). We investigated vasoreactivity of HippAs to mediators involved in neurovascular coupling, including SKCa/IKCa channels, KIR channels, and NO at physiological intraluminal pressure.
HippAs developed pressure-induced myogenic tone at 40 mmHg intravascular pressure that was similar between PVN-BDNF and -GFP treated male (31 ± 5 vs. 30 ± 8 %; p > 0.05) and female rats (32 ± 12 vs 24 ± 7 %; p > 0.05). Fig 4A shows representative lumen diameter traces and reactivity of HippAs to SKCa/IKCa channel activation with NS309 as an assessment of endothelial function. Percent reactivity was significantly reduced in BDNF-treated male and female rats compared to controls (Fig 4A), indicating that neuroendocrine stress disrupted endothelial function in the hippocampus in both sexes. Elevated extracellular K+ up to ~ 15 mM causes vasodilation of cerebral intraparenchymal arterioles, including HippAs (Johnson and Cipolla, 2017), via activation of K+ channels and subsequent vascular smooth muscle hyperpolarization (Filosa et al., 2006; Knot et al., 1996; Longden and Nelson, 2015). Interestingly, vasodilation in response to elevated extracellular KCl was substantially blunted in HippAs from both male and female BDNF-treated rats compared to same sex controls (Fig 4B). In fact, 15 mM KCl, a concentration known to activate KIR channels that caused robust vasodilation of HippAs from GFP control rats, caused vasoconstriction in PVN-BDNF-treated rats of both sexes such that lumen size returned to baseline diameters, demonstrated by ~ 0 % vasoreactivity (Fig 4B). These findings indicate that long-term neuroendocrine stress impaired KIR channel function in the hippocampal vasculature in both male and female rats.
Fig 4. Reactivity of hippocampal arterioles (HippAs) to small- and intermediate-conductance calcium-activated (SKCa/IKCa) channel and inward rectifying potassium (KIR) channel activation.
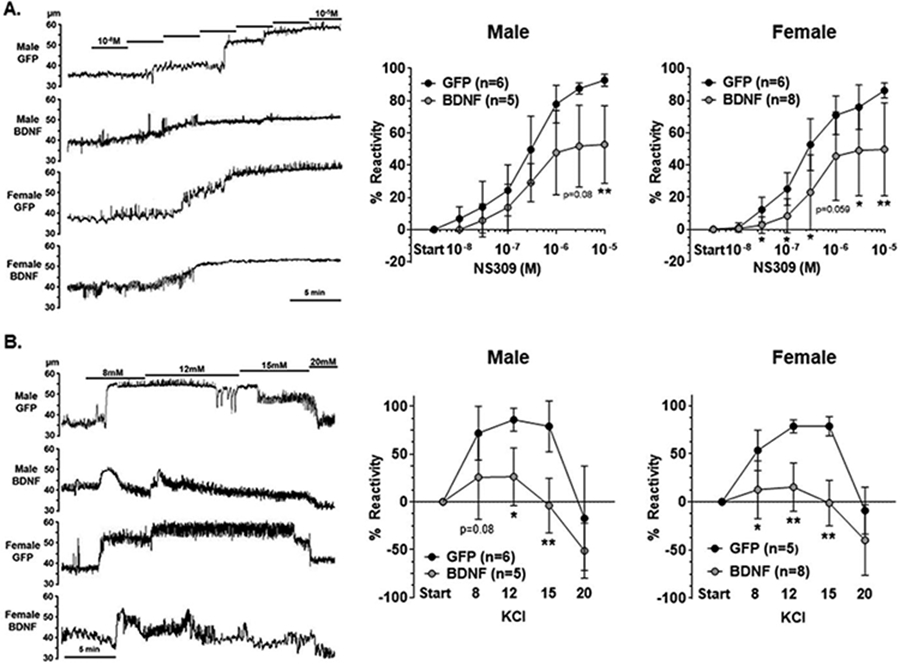
A. Representative lumen diameter tracings of HippAs from male and female BDNF and GFP treated rats during the concentration response curve to the SKCa/IKCa channel agonist NS309. Solid lines represent cumulative doses of NS309 from 10−8M to 10−5M (left panel). Increasing concentrations of NS309 caused vasodilation of HippAs from all groups, however, HippAs from male (middle panel) and female (right panel) BDNF-treated rats had decreased reactivity at maximum doses compared to controls. B. Representative lumen diameter tracing of HippAs from male and female BDNF and GFP treated rats during exposure to increased extracellular K+ concentrations (left panel). Vasodilation occurred in response to increased KCl up to 15mM K+ in HippAs from GFP controls of male (middle panel) and female (right panel) rats that then constricted back to baseline diameters at 20mM KCl. However, HippAs from BDNF-treated rats of both sexes had blunted vasodilation in response to 8, 12 and 15mM KCl compared to controls of the same sex. Data are mean ± SD, * p < 0.05, ** p < 0.01 vs. GFP of the same sex by unpaired Mann-Whitney test.
Sodium nitroprusside (SNP), an endothelial-independent NO donor that relaxes vascular smooth muscle caused vasodilation of HippAs from all groups, as shown in representative lumen diameter traces in Fig 5A. However, SNP had less of a vasodilatory effect in HippAs from male rats treated with BDNF compared to control vessels (Fig 5B). Interestingly, this impairment in reactivity was not present in HippAs from female PVN-BDNF treated rats (Figure 5B), suggesting stress impaired vascular smooth muscle function in the hippocampus in a sex-dependent manner, and demonstrated greater vascular dysfunction in the hippocampus in male compared to female rats.
Fig 5. Reactivity of hippocampal arteriole (HippA) vascular smooth muscle to nitric oxide.

A. Representative lumen diameter tracings of HippAs from male and female BDNF and GFP treated rats during the concentration response curve to NO donor SNP. B. Increasing doses of SNP caused vasodilation of HippAs from male (left panel) and female (right panel) rats. HippAs from male BDNF-treated rats had blunted vasoreactivity at lower concentrations of SNP compared to GFP controls, whereas HippAs from female rats dilated similarly. Data are mean ± SD, * p < 0.05, vs. GFP of the same sex by unpaired Mann-Whitney test.
There were no stress-induced structural changes in HippAs, as lumen diameter, vessel wall thickness and cross-sectional area under fully relaxed conditions were similar between groups (Supplementary Fig 2A-C). Further, biomechanical properties of HippAs were also unaffected by stress, as there were no differences in stress-strain curves of arterioles from BDNF-treated and GFP control rats of either sex, indicating no changes in vessel stiffness in response to neuroendocrine stress (Supplementary Fig 3).
Hippocampal vascular density differed by sex but was unaffected by neuroendocrine stress
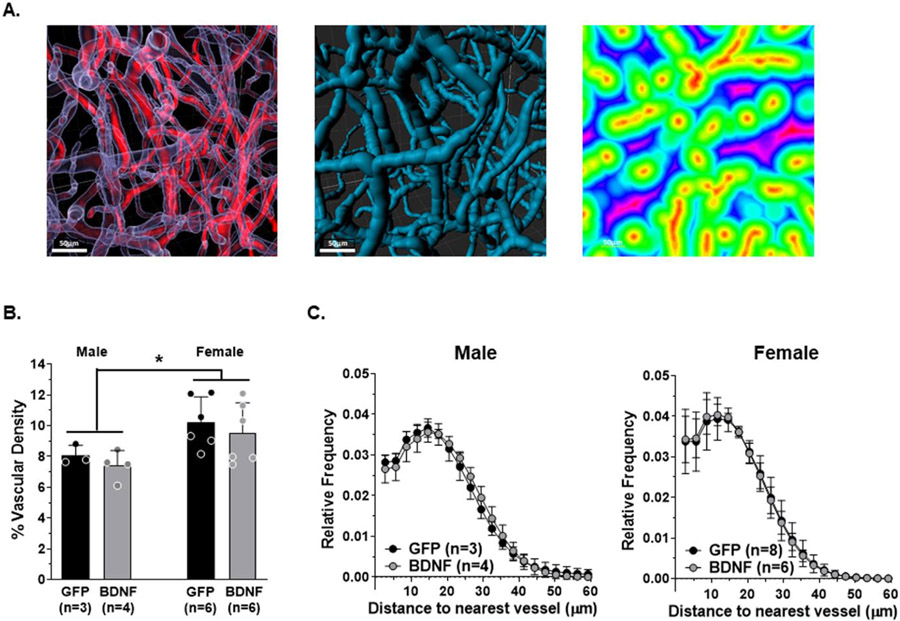
Vascular density is known to be lower in the hippocampus compared to regions of the cerebral cortex that is thought to increase its susceptibility to hypoxia/ischemia (Cavaglia et al., 2001; Nair et al., 1960; Shaw et al., 2021). In addition, chronic stress and hypertension are known to reduce capillary density in the brain (Burrage et al., 2018; Sokolova et al., 1985; Ungvari et al., 2021). However, little is known about the effect of neuroendocrine components of the stress response on hippocampal vascular density that could result in hypoxic/ischemic neuronal injury/death and potentiate memory dysfunction. Therefore, vascular density within the hippocampal CA1 region of separate groups of PVN-BDNF and -GFP-treated male and female rats was analyzed from 3D images using Imaris image analysis software. Vascular density was quantified in two ways: The percentage of intravascular volume was determined using a “filament” object representing the fluorescently labeled vascular network that was generated with the Imaris software. In addition, 3D EDMs were created to analyze the extravascular space and determine the distribution of distances to nearest blood vessels. Fig 6A shows representative images of the fluorescently labeled hippocampal blood vessels (left panel in red), the “filament” object representing the vascular network (left panel outlined in white and middle panel in blue), and a 2D slice of the 3D EDM of the extravascular space (right panel). Interestingly, BDNF treatment had no effect on these parameters in either male or female rats; however, male rats in general had lower vascular density in CA1 compared to female rats (two-way ANOVA, F1,15 = 7.96, p = 0.013; Fig 6B). There were no differences in the distribution of distances to nearest blood vessels with BDNF treatment in either male or female rats compared to GFP controls (Fig 6C). However, when males and females were compared regardless of treatment, there was a significant effect of sex on distribution of distances to nearest blood vessels with a rightward shift towards longer distances in males compared to females, and a significantly reduced frequency of distances less than 15 μm from the nearest blood vessel (two-way ANOVA, F1,17 = 8.83, p = 0.009). These findings suggest that the hippocampus of male rats may be more susceptible to hypoxia/ischemia due to more sparse vascularization in CA1 compared to that of female rats.
Fig 6. Analysis of vascular density in the hippocampal CA1 region.
A. Representative images showing the raw vascular image (red, left panel) and the “filament” object generated by Imaris software representing the vascular network (transparent on left panel, and blue on middle panel). Two-dimensional slice of a three-dimensional Euclidian distance map (EDM) indicating color-coded distances of every point in the extravascular space to the nearest blood vessel as represented by the “filament” object (right panel). B. Percent vascular density and C. distribution of distances to the nearest blood vessel indicated no statistical differences between BDNF and GFP animals; however, vascular density was lower in males compared to females. Data are mean ± SD, * two-way ANOVA, F1,15 = 7.96, p = 0.013; scale bars = 50 μm.
Long-term neuroendocrine stress increased apoptosis in CA1
To determine if stress-induced changes in hippocampal vascular function and memory of PVN-BDNF-treated rats were associated with neuronal cell death, neuronal apoptosis was assessed immunohistochemically by counting caspase 3 positive cells in the CA1 pyramidal layer. Fig 7A shows representative photomicrographs of caspase 3, NeuN and DAPI staining from male and female rats receiving either PVN-BDNF or -GFP vector injections. In CA1 (region sampled outlined in red in Fig 7B), BDNF treatment significantly increased the number of caspase 3 positive cells in both male and female rats compared to same-sex PVN-GFP controls (Fig 7C), suggesting that chronic neuroendocrine stress caused apoptosis in the hippocampus.
Fig 7. Detection of neuronal apoptosis in the hippocampal CA1 region by assessing the density of caspase-3 positive cells in the pyramidal layer.
A. Representative images showing expression of the neuronal marker NeuN (green) and caspase-3 (red) as well as DAPI (blue) from male and female GFP and BDNF rats. The mask used for counting caspase-3 positive cells was generated from NeuN immunofluorescence using Gaussian blurring and is outlined in yellow. B. Diagram indicating the area where images were taken. C. Average densities of caspase-3 positive cells in the pyramidal layer in male and female GFP and BDNF rats. Results are represented as mean ± SD; **p < 0.01 vs GFP of the same sex analyzed by unpaired Mann-Whitney test; scale bars = 100 μm, D3V: dorsal 3rd ventricle, LV: lateral ventricle.
Discussion
Dementia is growing in incidence as the world’s population ages, affecting the majority of people over 70 years of age and often involving hippocampal dysfunction (Driscoll et al., 2003; Harada et al., 2013). Chronic stress, which commonly associates with mood disorders or post-traumatic stress disorder, is known to cause cognitive dysfunction and contribute to dementia (Avila-Villanueva et al., 2020; Stuart and Padgett, 2020; Yan et al., 2018). While stress is not an underlying etiology of Alzheimer’s disease, it is thought to accelerate Alzheimer’s pathology and worsen cognitive decline (Avila-Villanueva et al., 2020; Stuart and Padgett, 2020). The majority of investigation of the relationship between chronic stress and cognition has focused on the impact of glucocorticoids on neuronal function, including in the hippocampus (Kim et al., 2015; Pavlides et al., 2002; Pereda-Perez et al., 2019; Sousa et al., 2000; Woolley et al., 1990), with much less emphasis on understanding how chronic stress may be affecting the vasculature perfusing the hippocampus. Here, we used a novel experimental model that mimics the neuroendocrine and cardiovascular aspects of chronic stress without the psychological effects of subjecting animals to actual stressors, and report that activation of these stress-related mechanisms impaired hippocampal vascular function. Thus, vascular dysfunction may represent a novel underlying mechanism by which chronic stress affects hippocampal function to contribute to dementia. Interestingly, there was more hippocampal arteriolar dysfunction and scarcer hippocampal microvasculature in male compared to female rats that was associated with greater memory impairment, suggesting the male sex may be at increased risk of neuroendocrine-derived hippocampal dysfunction during chronic stress. This is the first study that we are aware of to provide direct evidence of hippocampal vascular mechanisms of stress-induced memory dysfunction independently of neuronal and glial influences. These findings broaden our understanding of the impact of chronic stress on vulnerable brain regions and highlight the importance of long-term effective stress management in ameliorating stress-induced cognitive burden.
The control of local blood flow occurs at the level of pre-capillary arterioles and upstream penetrating arterioles, making the function of HippAs critical in maintaining basal perfusion of the hippocampus and protecting neurocognitive health (Fernandez-Klett et al., 2010). Pre-capillary arterioles are also centrally involved in neuronal activity-dependent changes in local blood flow through their involvement in neurovascular coupling (Fernandez-Klett et al., 2010; Iadecola and Gottesman, 2019). In cortical brain regions, arteriole dysfunction impairs neurovascular coupling and contributes to cognitive decline in models of Alzheimer’s disease and after ischemic stroke (Girouard and Iadecola, 2006; Povlsen et al., 2016), but less is known about the vasculature in the hippocampus. In the current study, exposure to chronic neuroendocrine stress resulted in impaired vasodilatory responses of HippAs to activation of SKCa/KCa and KIR channels in both male and female rats. These potassium channels are involved in conducting local vasodilation to upstream arterial segments to reduce vascular resistance and increase local blood flow (Guerra et al., 2018; Hannah et al., 2011; Longden and Nelson, 2015; Povlsen et al., 2016). This mechanism is critical to matching neuronal metabolic demand with appropriate blood flow, and KIR channel dysfunction in other brain regions impairs neurovascular coupling (Dabertrand et al., 2021; Longden et al., 2014; Longden and Nelson, 2015; Mughal et al., 2021; Povlsen et al., 2016). In fact, neurovascular coupling was compromised in the amygdala of rats after 7 days of unpredictable stress that was due to glucocorticoid-induced KIR channel dysfunction (Longden et al., 2014). Although neurovascular coupling was not directly measured in the current study, our finding of impaired KIR channel function in HippAs suggests neurovascular un-coupling occurs in the hippocampus in response to neuroendocrine stress that could cause neuronal dysfunction and contribute to memory loss and dementia.
Another important aspect of neurovascular coupling is the initiation of the local vasodilation in response to neuronal activity. In the hippocampus, neuronally-derived NO diffuses to surrounding microvasculature to relax vascular smooth muscle and is considered the primary mechanism by which local vasodilation occurs during hippocampal neuronal activity and is distinct from other brain regions (Lourenco et al., 2014; Lovick et al., 1999). In the current study, the vasodilatory response of HippAs to the NO donor SNP was blunted only in arterioles from male rats experiencing neuroendocrine stress but remained unaffected in female rats. Together with the KIR channel dysfunction, these findings suggest that two critical aspects of neurovascular coupling are impaired in the hippocampus in response to neuroendocrine stress in males: the ability for neuronal activity to generate a local vasodilation, and the ability of that vasodilation to be conducted upstream to increase local blood flow. Interestingly, only the latter seems to be impaired in females. The additional vascular dysfunction in response to neuroendocrine stress in the hippocampus of males suggests neurovascular uncoupling occurs to a greater extent that could impair neuronal function and explain the more robust memory dysfunction in male compared to female rats, despite there being similar levels of hippocampal apoptosis. The mechanism(s) by which the female sex may be modestly protected from hippocampal vascular dysfunction in response to neuroendocrine stress remain unclear from the current study, but may involve differences in the central regulation and magnitude of neuroendocrine and cardiovascular stress responses, and/or differential local protective mechanisms in the hippocampus against chronic neuroendocrine stress.
After five weeks of neuroendocrine stress, neuronal apoptosis in CA1 of the hippocampus occurred similarly in both sexes; however, whether the greater vascular dysfunction in males causes more neuronal injury at later time points remains unknown. Although we did not determine the underlying mechanism of CA1 neuronal apoptosis in the current study, we speculate that hippocampal vascular dysfunction occurring in response to neuroendocrine stress impairs neurovascular coupling that, over time, causes neuronal cell death. Alternatively, neuronal cell death could occur due to non-vascular mechanisms associated with neuroendocrine stress, such as neuroinflammation. However, a primary mechanism of neuroinflammation-induced hippocampal neuronal cell death is through elevated proinflammatory cytokines and tumor necrosis factor alpha-mediated necroptosis (Vieira et al., 2014; Yang et al., 2017), not caspase-3-mediated apoptotic cell death as demonstrated in the current study. It is also possible that changes in hippocampal neuronal network function and neuroplasticity underlie the greater memory disruption in males than females independently of neuronal cell death. Future studies are needed to determine if the vascular dysfunction in this model impairs neurovascular coupling and/or neuroplasticity to a greater extent in males, and whether hippocampal injury occurs in response to vascular dysfunction and whether it progresses with time in a sex-dependent manner.
Women are more likely to develop pathologies involving stress as a central etiology (i.e., mood disorders) that is thought to contribute to the higher incidence of dementia, including Alzheimer’s disease in women (Levine et al., 2021; Yan et al., 2018). However, adult female rodents perform better in hippocampal-dependent learning and memory tasks in response to chronic stress compared to males (Luine, 2016; Luine et al., 2017). In the cerebrovasculature, the effect of sex-specific steroids (i.e., estrogens and androgens) are complex, but in general female-specific sex hormones are considered protective against pathological states affecting the cerebral circulation including hypertension and ischemic stroke (Abi-Ghanem et al., 2020; Ibrahim et al., 2006; Robison et al., 2019). The findings in the current study are in support of this concept, with female rats demonstrating less neuroendocrine stress-induced memory and hippocampal vascular dysfunction than males. However, aging is the primary non-modifiable risk factor for dementia. When female sex hormones decrease with menopause, the sex-dependent prevalence of cerebrovascular pathology and dementia that favors disease in adult men reverses and occurs more often in older women (Dichgans and Leys, 2017; Gannon et al., 2019; Iadecola et al., 2019). Here, we studied young adult, gonadally intact female and male rats to understand how stress impacted memory and hippocampal vascular function in adulthood, as even mid-life stress has been shown to increase the risk for dementia (Johansson et al., 2010; Stuart and Padgett, 2020). Additional studies are necessary to understand the potential compounding effects of age and neuroendocrine stress on memory and HippA function, and whether this occurs in a sex-dependent manner in the post-menopausal state. Regardless, this is the first study that we are aware of to investigate the impact of chronic stress mechanisms on hippocampal vascular function, and how one sex may be more susceptible to hippocampal dysfunction in response to the neuroendocrine mediators of chronic stress.
Chronic hypertension is associated with hippocampal atrophy, capillary rarefaction and is known to accelerate cognitive decline in humans and rats (Brown and Thore, 2011; Iadecola and Gottesman, 2019; Korf et al., 2004; Yano et al., 2017). In the current study, it is possible that the persistently elevated blood pressure in this neuroendocrine model of stress contributed to the hippocampal vascular dysfunction present. This could explain, at least in part, why male rats that had higher blood pressure elevation to PVN-BDNF treatment than female rats had more HippA dysfunction and greater impairment of hippocampal-dependent memory. However, there was no evidence in the current study of hypertension-induced hippocampal vascular remodeling that is present in other models of chronic hypertension (Johnson and Cipolla, 2017; Johnson et al., 2020). Further, while male rats had decreased hippocampal microvascular density than females, there was no effect of neuroendocrine stress suggesting hypertension associated with the stress response did not cause capillary rarefaction in this brain region. This could be due to the limited duration and/or magnitude of hypertension being insufficient to induce hippocampal vascular remodeling. Alternatively, it could be that the dysfunction of HippAs in response to neuroendocrine stress occurred independently of elevations in blood pressure. Underlying mechanisms by which chronic stress causes HippA and memory dysfunction, including roles for elevated blood pressure and/or elevated glucocorticoids, remain to be determined.
Conclusions
The findings of the current study provide new insight into how the chronic neuroendocrine stress response affects the hippocampus representing a novel vascular mechanism by which cognitive decline and dementia are exacerbated by stress. Chronic psychological stress has now emerged as a modifiable risk factor for dementia that has been persistently overlooked given the relatively “silent” nature of psychological symptoms (Avila-Villanueva et al., 2020). As there are currently no approved disease-modifying drugs, the approach to combating dementia lies solely in preventative strategies, particularly in mitigating modifiable risk factors and co-morbidities that exacerbate age-related cognitive decline and underlying pathologies such as Alzheimer’s disease (Cahill, 2020; Iadecola et al., 2019). This study revealed the therapeutic potential of targeting hippocampal arterioles to prevent or slow memory decline in the setting of prolonged and/or unavoidable psychological stress. This may be particularly important given the recent and evidently long-lasting stressful challenges associated with the COVID-19 global pandemic, not only in aging populations of people already afflicted with mild-to-moderate/severe cognitive dysfunction, but people of all ages.
Supplementary Material
Highlights.
Chronic stress is a substantial risk factor for cognitive decline and dementia
How neuroendocrine aspects of stress affect the memory-centric hippocampus is unclear
Hippocampal vascular function and memory were impaired by neuroendocrine stress
There was greater vascular and memory dysfunction in male rats than female rats
Acknowledgements
We thank Nicole DeLance of the Microscopy Imaging Center at the University of Vermont for her technical expertise in performing histology.
Funding
This work was supported by the American Heart Association Career Development Award 20CDA35310239 to ACJ, the National Institutes of Health National Heart, Lung, and Blood Institute R01HL133211 to BE, National Institute on Aging R03AG072016 to BE, and National Center for Research Resources 1S10OD025030 to the Microscopy Imaging Center at the University of Vermont, and the Cardiovascular Research Institute of Vermont Early Career Research Awards UVM037415 and UVM036071 to ACJ.
Abbreviations
- AAALAC
Association for Accreditation of Laboratory Animal Care
- AAV2
adeno-associated virus
- aCSF
artificial cerebrospinal fluid
- ANOVA
Analysis of variance
- BDNF
brain-derived neurotrophic factor
- CA1
Cornu Ammonis-1
- EDM
Euclidian distance map
- GFP
green fluorescent protein
- H&E
hematoxylin and eosin
- HippA
hippocampal arteriole
- HPA axis
hypothalamic-pituitary-adrenal axis
- IHC
immunohistochemistry
- IKCa channel
intermediate-conductance calcium-activated potassium channel
- KIR channel
inward rectifying potassium channel
- ΝΓΗ
National Institutes of Health
- NO
nitric oxide
- NOR
novel object recognition
- PBS
phosphate buffered saline
- PFA
paraformaldehyde
- PVN
paraventricular nucleus
- ROI
region of interest
- SAB
spontaneous alternation behavior
- SD
standard deviation
- SKCa channel
small-conductance calcium-activated potassium channel
- SNP
Sodium nitroprusside
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement
Abbie C. Johnson, PhD: Conceptualization, Methodology, Investigation, Formal analysis, Resources, Project administration, Supervision, Funding acquisition, Visualization, Writing- Original Draft, Writing- Review & Editing
Friederike Uhlig, PhD: Investigation, Methodology, Writing- Review & Editing
Zachary Einwag: Investigation, Methodology, Writing- Review & Editing
Noelle Cataldo: Investigation, Methodology, Writing- Review & Editing
Benedek Erdos, PhD: Conceptualization, Methodology, Validation, Investigation, Formal analysis, Resources, Project administration, Supervision, Funding acquisition, Visualization, Writing- Review & Editing
References
- Abi-Ghanem C, Robison LS, Zuloaga KL, 2020. Androgens' effects on cerebrovascular function in health and disease. Biol Sex Differ 11, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Villanueva M, Gomez-Ramirez J, Maestu F, Venero C, Avila J, Fernandez-Blazquez MA, 2020. The Role of Chronic Stress as a Trigger for the Alzheimer Disease Continuum. Front Aging Neurosci 12, 561504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarini F, Moncada D, Martinez MC, Alen N, Viola H, 2009. Behavioral tagging is a general mechanism of long-term memory formation. Proc Natl Acad Sci U S A 106, 14599–14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE, 2010. Object recognition memory and the rodent hippocampus. Learn Mem 17, 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Thore CR, 2011. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathology and applied neurobiology 37, 56–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrage E, Marshall KL, Santanam N, Chantler PD, 2018. Cerebrovascular dysfunction with stress and depression. Brain Circ 4, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill S, 2020. WHO's global action plan on the public health response to dementia: some challenges and opportunities. Aging Ment Health 24, 197–199. [DOI] [PubMed] [Google Scholar]
- Cavaglia M, Dombrowski SM, Drazba J, Vasanji A, Bokesch PM, Janigro D, 2001. Regional variation in brain capillary density and vascular response to ischemia. Brain Res 910, 81–93. [DOI] [PubMed] [Google Scholar]
- Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW, 2010. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord 24, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GBDDF, 2022. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabertrand F, Harraz OF, Koide M, Longden TA, Rosehart AC, Hill-Eubanks DC, Joutel A, Nelson MT, 2021. PIP2 corrects cerebral blood flow deficits in small vessel disease by rescuing capillary Kir2.1 activity. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichgans M, Leys D, 2017. Vascular Cognitive Impairment. Circ Res 120, 573–591. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ, 2003. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex 13, 1344–1351. [DOI] [PubMed] [Google Scholar]
- Endo Y, Nishimura JI, Kobayashi S, Kimura F, 1997. Long-term glucocorticoid treatments decrease local cerebral blood flow in the rat hippocampus, in association with histological damage. Neuroscience 79, 745–752. [DOI] [PubMed] [Google Scholar]
- Endo Y, Nishimura JI, Kobayashi S, Kimura F, 1999. Chronic stress exposure influences local cerebral blood flow in the rat hippocampus. Neuroscience 93, 551–555. [DOI] [PubMed] [Google Scholar]
- Erdos B, Backes I, McCowan ML, Hayward LF, Scheuer DA, 2015. Brain-derived neurotrophic factor modulates angiotensin signaling in the hypothalamus to increase blood pressure in rats. Am J Physiol Heart Circ Physiol 308, H612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U, 2010. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A 107, 22290–22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT, 2006. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9, 1397–1403. [DOI] [PubMed] [Google Scholar]
- Gannon OJ, Robison LS, Custozzo AJ, Zuloaga KL, 2019. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem Int 127, 38–55. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C, 2006. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. Journal of applied physiology 100, 328–335. [DOI] [PubMed] [Google Scholar]
- Glodzik L, Rusinek H, Tsui W, Pirraglia E, Kim HJ, Deshpande A, Li Y, Storey P, Randall C, Chen J, Osorio RS, Butler T, Tanzi E, McQuillan M, Harvey P, Williams SK, Ogedegbe G, Babb JS, de Leon MJ, 2019. Different Relationship Between Systolic Blood Pressure and Cerebral Perfusion in Subjects With and Without Hypertension. Hypertension 73, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra G, Lucariello A, Perna A, Botta L, De Luca A, Moccia F, 2018. The Role of Endothelial Ca(2+) Signaling in Neurovascular Coupling: A View from the Lumen. International journal of molecular sciences 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah RM, Dunn KM, Bonev AD, Nelson MT, 2011. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31, 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada CN, Natelson Love MC, Triebel KL, 2013. Normal cognitive aging. Clin Geriatr Med 29, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN, 2004. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev 28, 497–505. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M, 2019. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. Journal of the American College of Cardiology 73, 3326–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Gottesman RF, 2019. Neurovascular and Cognitive Dysfunction in Hypertension. Circ Res 124, 1025–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim J, McGee A, Graham D, McGrath JC, Dominiczak AF, 2006. Sex-specific differences in cerebral arterial myogenic tone in hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 290, H1081–1089. [DOI] [PubMed] [Google Scholar]
- Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, Skoog I, 2010. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain 133, 2217–2224. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Cipolla MJ, 2016. Altered hippocampal arteriole structure and function in a rat model of preeclampsia: Potential role in impaired seizure-induced hyperemia. J Cereb Blood Flow Metab, 271678X16676287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Cipolla MJ, 2017. Altered hippocampal arteriole structure and function in a rat model of preeclampsia: Potential role in impaired seizure-induced hyperemia. J Cereb Blood Flow Metab 37, 2857–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Li Z, Orfila JE, Herson PS, Cipolla MJ, 2021. Hippocampal network dysfunction as a mechanism of early-onset dementia after preeclampsia and eclampsia. Progress in neurobiology 199, 101938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Miller JE, Cipolla MJ, 2020. Memory impairment in spontaneously hypertensive rats is associated with hippocampal hypoperfusion and hippocampal vascular dysfunction. J Cereb Blood Flow Metab 40, 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara N, Wang Y, Mukasa A, Furuya K, Shimizu T, Hamakubo T, Aburatani H, Kodama T, Kirino T, 2004. Genome-wide gene expression analysis for induced ischemic tolerance and delayed neuronal death following transient global ischemia in rats. J Cereb Blood Flow Metab 24, 212–223. [DOI] [PubMed] [Google Scholar]
- Ke MT, Fujimoto S, Imai T, 2013. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci 16, 1154–1161. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Pellman B, Kim JJ, 2015. Stress effects on the hippocampus: a critical review. Learn Mem 22, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T, 1982. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239, 57–69. [DOI] [PubMed] [Google Scholar]
- Klein RL, Muir D, King MA, Peel AL, Zolotukhin S, Moller JC, Kruttgen A, Heymach JV Jr., Muzyczka N, Meyer EM, 1999. Long-term actions of vector-derived nerve growth factor or brain-derived neurotrophic factor on choline acetyltransferase and Trk receptor levels in the adult rat basal forebrain. Neuroscience 90, 815–821. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Zimmermann PA, Nelson MT, 1996. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol 492 ( Pt 2), 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ, 2004. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension 44, 29–34. [DOI] [PubMed] [Google Scholar]
- Lalonde R, 2002. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26, 91–104. [DOI] [PubMed] [Google Scholar]
- Levine DA, Gross AL, Briceno EM, Tilton N, Giordani BJ, Sussman JB, Hayward RA, Burke JF, Hingtgen S, Elkind MSV, Manly JJ, Gottesman RF, Gaskin DJ, Sidney S, Sacco RL, Tom SE, Wright CB, Yaffe K, Galecki AT, 2021.Sex Differences in Cognitive Decline Among US Adults. JAMA Netw Open 4, e210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qin J, Yan J, Zhang N, Xu Y, Zhu Y, Sheng L, Zhu X, Ju S, 2019. Differences of physical vs. psychological stress: evidences from glucocorticoid receptor expression, hippocampal subfields injury, and behavioral abnormalities. Brain Imaging Behav 13, 1780–1788. [DOI] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N, 2017. Dementia prevention, intervention, and care. Lancet 390, 2673–2734. [DOI] [PubMed] [Google Scholar]
- Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT, 2014. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A 111, 7462–7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longden TA, Nelson MT, 2015. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 22, 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco CF, Santos RM, Barbosa RM, Cadenas E, Radi R, Laranjinha J, 2014. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic Biol Med 73, 421–429. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Brown LA, Key BJ, 1999. Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience 92, 47–60. [DOI] [PubMed] [Google Scholar]
- Luine V, 2016. Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. The Journal of steroid biochemistry and molecular biology 160, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Gomez J, Beck K, Bowman R, 2017. Sex differences in chronic stress effects on cognition in rodents. Pharmacol Biochem Behav 152, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M,McEwen BS, Hauger RL, Meaney MJ, 1998. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1, 69–73. [DOI] [PubMed] [Google Scholar]
- Martin KR, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW, 2003. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci 44, 4357–4365. [DOI] [PubMed] [Google Scholar]
- Mughal A, Harraz OF, Gonzales AL, Hill-Eubanks D, Nelson MT, 2021. PIP2 Improves Cerebral Blood Flow in a Mouse Model of Alzheimer's Disease. Function (Oxf) 2, zqab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V, Palm D, Roth LJ, 1960. Relative vascularity of certain anatomical areas of the brain and other organs of the rat. Nature 188, 497–498. [DOI] [PubMed] [Google Scholar]
- Nikonenko AG, Radenovic L, Andjus PR, Skibo GG, 2009. Structural features of ischemic damage in the hippocampus. Anat Rec (Hoboken) 292, 1914–1921. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS, 2002. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus 12, 245–257. [DOI] [PubMed] [Google Scholar]
- Pereda-Perez I, Valencia A, Baliyan S, Nunez A, Sanz-Garcia A, Zamora B, Rodriguez-Fernandez R, Esteban JA, Venero C, 2019. Systemic administration of a fibroblast growth factor receptor 1 agonist rescues the cognitive deficit in aged socially isolated rats. Neurobiol Aging 78, 155–165. [DOI] [PubMed] [Google Scholar]
- Povlsen GK, Longden TA, Bonev AD, Hill-Eubanks DC, Nelson MT, 2016. Uncoupling of neurovascular communication after transient global cerebral ischemia is caused by impaired parenchymal smooth muscle Kir channel function. J Cereb Blood Flow Metab 36, 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillfeldt JA 2016. Behavioral Methods to Study Learning and Memory in Rats. In: Rodent Model as Tools in Ethical Biomedical Research. Eds. Andersen M, Tufik S. Springer, Cham. [Google Scholar]
- Robison LS, Gannon OJ, Salinero AE, Zuloaga KL, 2019. Contributions of sex to cerebrovascular function and pathology. Brain Res 1710, 43–60. [DOI] [PubMed] [Google Scholar]
- Sequeira-Cordero A, Salas-Bastos A, Fornaguera J, Brenes JC, 2019. Behavioural characterisation of chronic unpredictable stress based on ethologically relevant paradigms in rats. Sci Rep 9, 17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K, Bell L, Boyd K, Grijseels DM, Clarke D, Bonnar O, Crombag HS, Hall CN, 2021. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat Commun 12, 3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova IA, Manukhina EB, Blinkov SM, Koshelev VB, Pinelis VG, Rodionov IM, 1985. Rarefication of the arterioles and capillary network in the brain of rats with different forms of hypertension. Microvascular research 30, 1–9. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM, 2000. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 97, 253–266. [DOI] [PubMed] [Google Scholar]
- Stuart KE, Padgett C, 2020. A Systematic Review of the Association Between Psychological Stress and Dementia Risk in Humans. J Alzheimers Dis 78, 335–352. [DOI] [PubMed] [Google Scholar]
- Thorsdottir D, Cruickshank NC, Einwag Z, Hennig GW, Erdos B, 2019. BDNF downregulates beta-adrenergic receptor-mediated hypotensive mechanisms in the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 317, H1258–H1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsdottir D, Einwag Z, Erdos B, 2021a. BDNF shifts excitatory-inhibitory balance in the paraventricular nucleus of the hypothalamus to elevate blood pressure. Journal of neurophysiology 126, 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsdottir D, Einwag Z, Erdos B, 2021b. BDNF shifts excitatory-inhibitory balance in the paraventricular nucleus of the hypothalamus to elevate blood pressure. Journal of neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A, 2021. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol 17, 639–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira M, Fernandes J, Carreto L, Anuncibay-Soto B, Santos M, Han J, Fernandez-Lopez A, Duarte CB, Carvalho AL, Santos AE, 2014. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol Dis 68, 26–36. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS, 1990. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res 531, 225–231. [DOI] [PubMed] [Google Scholar]
- Yan Y, Dominguez S, Fisher DW, Dong H, 2018. Sex differences in chronic stress responses and Alzheimer's disease. Neurobiol Stress 8, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Hu K, Chen J, Zhu S, Li L, Lu H, Li P, Dong R, 2017. Necrostatin-1 protects hippocampal neurons against ischemia/reperfusion injury via the RIP3/DAXX signaling pathway in rats. Neuroscience letters 651, 207–215. [DOI] [PubMed] [Google Scholar]
- Yano Y, Reis JP, Levine DA, Bryan RN, Viera AJ, Shimbo D, Tedla YG, Allen NB, Schreiner PJ, Bancks MP, Sidney S, Pletcher MJ, Liu K, Greenland P, Lloyd-Jones DM, Launer LJ, 2017. Visit-to-Visit Blood Pressure Variability in Young Adulthood and Hippocampal Volume and Integrity at Middle Age: The CARDIA Study (Coronary Artery Risk Development in Young Adults). Hypertension 70, 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Ezzati A, Katz MJ, Lipton ML, Brickman AM, Sliwinski MJ, Lipton RB, 2016. Perceived Stress Is Differentially Related to Hippocampal Subfield Volumes among Older Adults. PloS one 11, e0154530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request.