Abstract
Access to clinically relevant small cell lung cancer (SCLC) tissue is limited because surgical resection is rare in metastatic SCLC. Patient-derived xenografts (PDX) and circulating tumor cell-derived xenografts (CDX) have emerged as valuable tools to characterize SCLC. Here, we present a resource of 46 extensively annotated PDX/CDX models derived from 33 patients with SCLC. We perform multi-omic analyses, using targeted tumor next-generation sequencing, RNA-sequencing, and immunohistochemistry to deconvolute the mutational landscapes, global expression profiles, and molecular subtypes of these SCLC models. SCLC subtypes characterized by transcriptional regulators, ASCL1, NEUROD1 and POU2F3 are confirmed in this cohort. A subset of SCLC clinical specimens, including matched PDX/CDX and clinical specimen pairs, confirm that the primary features and genomic and proteomic landscapes of the tumors of origin are preserved in the derivative PDX models. This resource provides a powerful system to study SCLC biology.
Subject terms: Cancer models, Small-cell lung cancer, Cancer genomics
Creating accurate models of small cell lung cancer is essential to ensure the clinical relevance of results. Here, the authors create patient derived xenograft models from 33 patients and show, through multi-omics sequencing, that these models retain the primary features of the original.
Introduction
Lung cancer is the leading cause of cancer death in men and women, responsible for more deaths than colon, breast, and prostate cancers combined1. Small cell lung cancer (SCLC) is an aggressive neuroendocrine (NE) tumor that accounts for ~15% of all lung cancers2,3. Most patients have metastatic disease at the time of diagnosis, a condition associated with a 5-year survival of <5%4. For decades, chemotherapy was the only treatment option for this disease. Immune checkpoint blockade (ICB) has proven effective for only a small subset of patients, whether the administered regimen targets the PD-1/PD-L1 axis alone or is combined with anti-CTLA-45. ICB plus chemotherapy has been FDA-approved for the frontline treatment in SCLC based on the results of phase III ImPower133 trial, which demonstrated a modest but significant increase in overall survival and progression-free survival with the addition of atezolizumab to chemotherapy6. More recently, results from the CASPIAN trial led to the FDA approval of another anti-PD-L1 antibody, durvalumab, based on similarly modest but clinically meaningful survival benefit7,8. Despite the emergence of novel targeted therapies, including inhibitors of PARP, EZH2, and BCL2, their clinical benefits have been limited, and have not led to regulatory approvals. Relative to other types of lung cancer, therapeutic options in SCLC are limited and offer only transient benefits for the large majority of patients.
A major challenge to identifying effective treatments has been the relative lack of model systems that accurately reflect SCLC tumorigenesis. Access to clinically relevant tissue is limited because surgical resection is rare in metastatic SCLC. Until recently this has made researchers heavily reliant on established cell lines and genetically engineered mouse models (GEMM), which both have inherent limitations. Cell lines often carry a biased mutational repertoire and decades of in vitro growth further selects these lines. The available SCLC cell lines were largely generated before the advent of the current standard of care combination therapies and may not accurately represent current resistance mechanisms. GEMMs generally fail to recapitulate the diverse mutational landscape caused by years of tobacco use9. Patient-derived xenografts (PDX) and circulating tumor cell-derived xenografts (CDX) have emerged as valuable tools to interrogate the genomic landscape of SCLC9–12. These patient-derived models have their own limitations, most notably the requirement to be maintained in an immunosuppressed murine host, precluding assessment of novel therapies to stimulate the adaptive immune system. This limitation is counterbalanced by several advantages—notably proximity to primary patient tumors, and avoidance of the selective pressures associated with establishing clonal cell lines able to grow in the in vitro tissue culture environment13. Observed phenotypes in the PDX sample can be directly traced back to the patient and correlated with clinical response to therapies10. SCLC patients typically have a high prevalence of circulating tumor cells, which has been used as a biomarker of tumor burden during treatment14. Invasive SCLC tumor biopsies and surgical resections can be avoided by the collection of circulating tumor cells in the blood for CDX modeling15,16.
SCLC subtypes were recently characterized and defined by the relative expression of genes encoding four major transcriptional regulators, ASCL1, NEUROD1, POU2F3, and YAP1 (defining subtypes SCLC-A, SCLC-N, SCLC-P, and SCLC-Y, respectively)17. Since then, the subtype-defining validity of YAP1 has been questioned, and SCLC-Y is now also described by an “inflamed” gene signature (SCLC-I)12. SCLC-A remains the most common subtype (~50%), followed by SCLC-N (~20%), SCLC-Y (~20%), and SCLC-P (10%)12,18. Reflecting the clinical landscape, all previous publications have only reported data on PDXs/CDXs belonging to SCLC-A and SCLC-N subtypes. The rare non-NE SCLC-P subtype has not been reported as a PDX model and therefore has been less extensively characterized.
Here, we present a resource comprised of a cohort of 46 extensively annotated PDX/CDX models derived from 33 patients with SCLC. We perform multi-omic analyses, using targeted tumor next-generation sequencing (NGS) by MSK-IMPACT, RNA-sequencing, and immunohistochemistry (IHC) to deconvolute the mutational landscapes, global expression profiles, and molecular subtypes of these SCLC models. We directly compare these genomic and proteomic profiles to a subset of SCLC clinical specimens, including matched PDX/CDX and clinical specimen pairs. Moreover, we were able to establish and deeply characterize ten PDX models from one primary and nine metastatic tumors from an individual whose SCLC tumor belongs to the POU2F3 subtype. Therefore, our study provides insight into the genomic, transcriptomic, and protein landscape in PDX/CDX models of the different subtypes of SCLC, including SCLC-P.
Results
Generation of the PDX/CDX panel
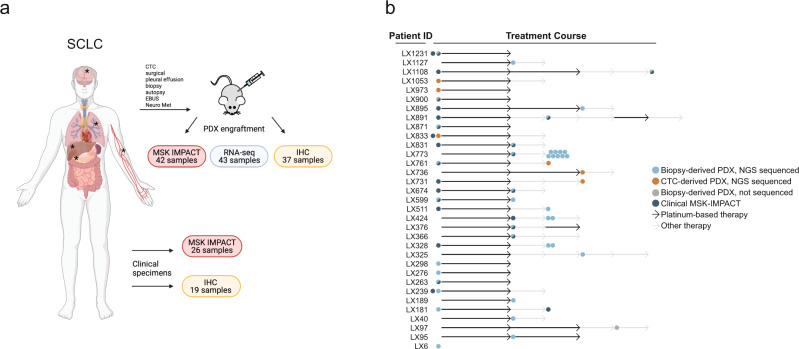
Clinical characteristics of the 46 SCLC PDX/CDX models (33 patient donors) can be found in Supplementary Data 1. World Health Organization’s pathologic classification of SCLC includes two subtypes: SCLC (“pure” SCLC) and combined SCLC, with the latter demonstrating admixture with any histology of non-small cell lung cancer19. Our cohort included five cases representing combined SCLC: three with a large cell NE component, one with squamous cell carcinoma, and one with adenocarcinoma. Of the 46 models, 40 (87%) were tissue-derived PDXs and 6 (13%) were CDXs. The average tumor engraftment time was 112 days, with no appreciable difference between PDXs (110 days) and CDXs (131 days). Out of 198 pure SCLC and 29 mixed histology samples that were initially injected to create a PDX model, 45 samples (23.81%) and 13 samples (44.83%) were successfully engrafted, respectively. IHC was performed on 37 samples, RNA-seq on 43 samples, and NGS via MSK-IMPACT on 42 samples (Fig. 1a). The patient cohort was 55% (18/33) male and 45% female, with an average age of 66 years at diagnosis. Most patient donors, 27 (82%) of 33, were current or former smokers. Nineteen of 33 patients (58%) presented with extensive-stage (ES) disease while the remainder (14/33, 42%) had limited-stage (LS). Treatment history and a depiction of sample collection is shown in Fig. 1b, with most patients receiving cisplatin or carboplatin and etoposide therapy either immediately after, or prior to, sample collection for model generation. IHC and targeted NGS via MSK-IMPACT was available for 19 and 26 of the patient tumors from which PDXs/CDXs were derived, respectively (Fig. 1a).
Fig. 1. Generation of the PDX/CDX panel.
a Overview of the patient-derived/circulating tumor cell-derived xenograft (PDX/CDX) cohort and clinical specimens. Samples were collected from surgical, pleural effusion, biopsy, autopsy, EBUS, Neuro Met, and CTC isolation and either engrafted into immunodeficient mice to generate PDX/CDX models or directly sent for targeted sequencing (MSK-IMPACT) and Immunohistochemistry (IHC). Created with BioRender.com. b Swimmers Plot depicting sample collection of the PDX/CDX cohort during treatment course (Platinum-based therapy— black arrow, Other therapy—gray arrow). Models derived from biopsies are annotated with a blue or gray dot (with next-generation sequencing [NGS] or not, respectively). Models derived from circulating tumor cells (CTC, sequenced with NGS) are annotated with an orange dot. Dark blue indicates NGS (MSK-IMPACT) for the clinical sample. Arrows are not to scale in terms of treatment duration.
MSK-IMPACT targeted tumor sequencing of matched PDX and clinical SCLC samples
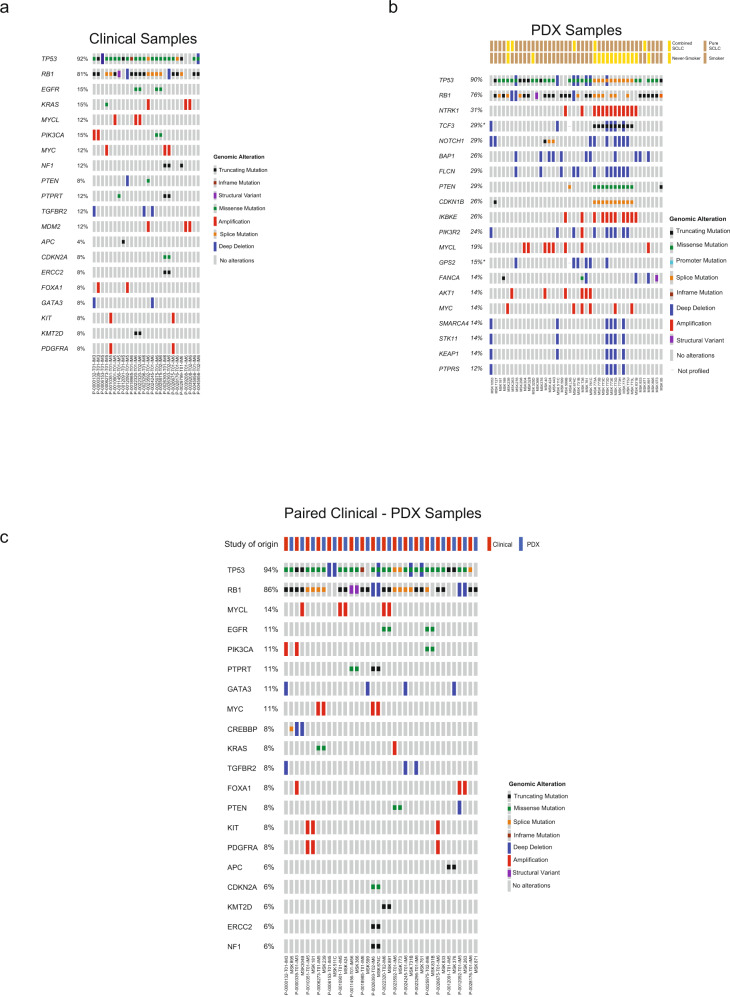
We conducted comparative genomic analyses on our patient tumors versus PDX models to establish the genomic fidelity of the PDX models compared with patient tumor specimens collected at the same time point. A total of 42 PDX samples and 26 clinical samples were profiled by MSK-IMPACT targeted tumor sequencing20–22. Of those, 18 cases included matched pairs, and the top 20 altered genes in these cohorts are presented in Fig. 2. Consistent with known key characteristics of SCLC, NGS revealed mutations (primarily missense and truncating mutations) with the rarer splice and in-frame mutations in TP53 in 24 out of 26 (92%) clinical samples and truncating splice mutations, structural variants, and deep deletions in RB1 in 21 out of 26 (81%) clinical samples (Fig. 2a). Despite the rarity of MAPK pathway mutations in SCLC, two clinical samples (P-0039208-T01-IM6 and P-0039208-T02-IM6) carried KRAS amplifications without TP53 and RB1 mutations23. Notably, these samples also harbored MDM2 amplification, which were only found in one other clinical sample out of 26. MDM2 is a negative regulator of TP53, so amplification-induced overexpression could functionally inactivate TP53, potentially phenocopying missense/truncating TP53 mutations in these samples. Four samples from patients that never smoked (samples P-0022320-T01/2-IM6 and P-0025975-T01/2-IM6,) demonstrated oncogenic mutations in EGFR consistent with a histologic transformation from lung adenocarcinoma24. Alterations in PIK3CA, KMT2D, and SPEN were also observed; and could be traced back to the matched clinical sample.
Fig. 2. Matched PDX and clinical SCLC sample analysis.
Genomic landscape in SCLC (a Clinical Samples, b patient-derived xenograft (PDX) Samples, c paired Clinical-PDX samples) samples representing putative driver alterations (i.e., excludes variants of unknown significance) in the top 20 most frequently altered genes as detected by MSK-IMPACT20 targeted next-generation sequencing.
A higher density of alterations, particularly copy number changes, were identified in the genomic landscapes of the 42 PDX samples than in the clinical samples (Fig. 2b), consistent with the greater purity of human genomic DNA from cancer cells in the PDX (due to the absence of human stromal cells25). Mutations in TP53 and RB1 were largely retained (90% and 76% of samples, respectively). One patient (MSK773) from whom several PDXs were generated had PDX models consistently demonstrating splice site mutations in TP53 and CDKN1B and a missense mutation in PTEN. MSK1053, MSK511c, and MSK773D/E/G/I samples harbored mutations in SMARCA4, STK11, KEAP1, and PTPRS (except MSK511c); mutations more commonly observed in non-small cell lung cancer26-27.
For 18 cases, paired clinical-PDX samples were available for direct comparison to determine whether PDX samples retained features of the original patient tumors (Fig. 2c). Most (89%, 16/18) pairs retained genomic alterations in TP53 and RB1. The PDXs derived from MSK599 and MSK871 both lacked the TP53 alterations found in the corresponding clinical sample. Besides TP53 and RB1, most matched samples harbored the same additional mutations with a few samples exhibiting variability. For example, MSK304B lost PIK3CA and FOXA1 amplification in the PDX sample but gained an MYCL amplification. The observed differences might reflect the intrinsic clonal heterogeneity of the biopsies used for direct sequencing vs. PDX generation or could be due to bottlenecking and selection in PDX generation; these possibilities are not mutually exclusive. Overall, these results demonstrate that primary features of the genomic landscapes of the tumors of origin were preserved in the derivative PDX models.
SCLC subtype and NE markers
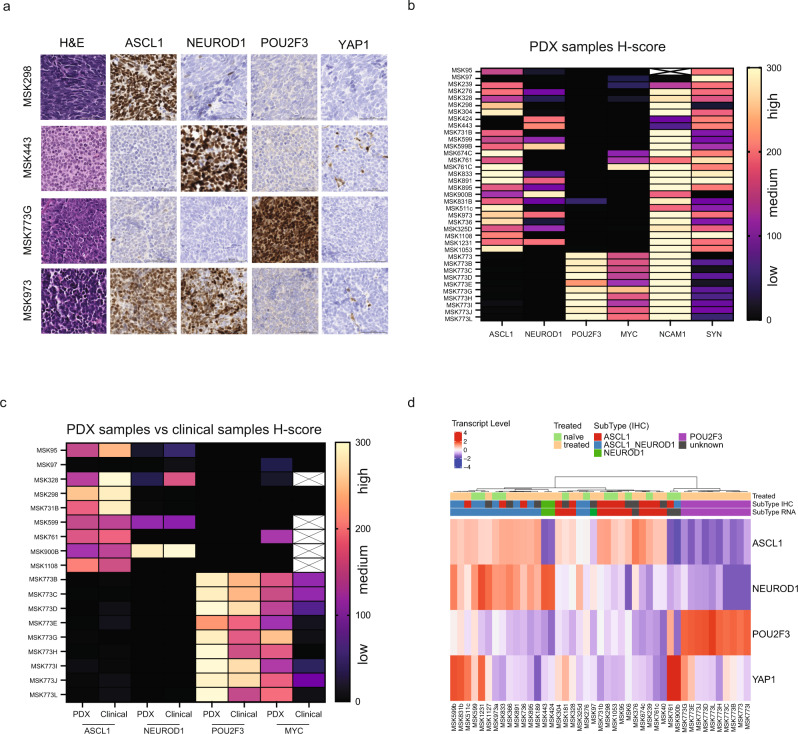
SCLC diagnoses of donor patient biopsies were morphologically confirmed by a pathologist. Selected cases exhibited spindle cell and nesting growth patterns, along with the predominance of sheet-like/solid morphology. Representative examples of SCLC PDX histology by hematoxylin and eosin (H&E) staining are shown in Fig. 3a. SCLC has been divided into biologically distinct subtypes based on the expression of the transcriptional regulators ASCL1, NEUROD1, POU2F3, and YAP117. However, recent reports show only sporadic expression of YAP1 in clinical samples and CDXs28,29, questioning the role of YAP1 in SCLC. We performed IHC on 37 PDX/CDX samples, and a pathologist reviewed and scored tumors for the fraction of positive cells for each protein, as well as staining intensity (H-score). In our prior analysis of primary human tumors by IHC, it was noted that many SCLC tumors co-express ASCL1 and NEUROD1, defining an intermediate between dichotomous expression of these factors28. Figure 3a shows representative staining of four selected cases derived from ASCL1, NEUROD1, POU2F3, and ASCL1/NEUROD1 subtypes (see Supplementary Figs. 1–4 for other cases). Most PDXs showed medium/high H-score expression for ASCL1, NEUROD1, or both. A distinct subset of ten PDXs (MSK773, B-L) derived from one patient (biopsy and different tissue sites post-mortem) were strongly positive for POU2F3 and negative for the other subtype-defining transcription factors (Fig. 3a, b). YAP1 expression was consistently absent or very low across all samples, regardless of intracellular localization (nuclear or cytoplasmic staining, Supplementary Fig. 5a). This parallels the recent studies noted above28,29, further suggesting that YAP1 may not be an optimal marker to distinguish between SCLC subtypes.
Fig. 3. SCLC subtype annotation and neuroendocrine markers.
a H&E and immunohistochemistry images for the indicated proteins are shown. Scale bar, 50 μM. Four representative tumors are shown. Three independent cores per sample were stained for H&E and the indicated protein with a representative example shown here. b Heatmap depicts H-scores derived from 37 scored patient-derived xenograft (PDX) tumors for the fraction of positive cells for each protein shown, as well as staining intensity. White box with a cross (MSK95, NCAM1) indicates staining failed. Source data are provided as a Source Data file. c Heatmap depicts H-scores derived from 18 paired PDX tumors and clinical samples for the fraction of positive cells for each protein shown, as well as staining intensity. White boxes with cross indicate no staining available. Source data are provided as a Source Data file. d Heatmap of gene expression of 43 PDX samples for the selected markers; ASCL1, NEUROD1, POU2F3, and YAP1. Color-coded panel on top depicts treatment status (naïve or treated) and subtype annotation based on immunohistochemistry (IHC) or RNA-sequencing. Source data are provided as a Source Data file.
Consistent with previous literature, all POU2F3-expressing PDXs also expressed MYC (Fig. 3b and Supplementary Figs. 1–4). Most PDXs (with the exception of 5) showed high expression of the NE marker NCAM1; most also expressed synaptophysin, although this was lower or absent in the POU2F3 PDXs (Fig. 3b and Supplementary Figs. 1–4). Paired samples, with analysis of a clinical sample and the corresponding PDX, were available for 18 cases, and H-scores of subtype markers ASCL1, NEUROD1, POU2F3, and YAP1, as well as expression of MYC, were similar or unchanged in most pairs (Fig. 3c and Supplementary Figs. 5B, 6, 7). These data illustrate that the PDX cohort largely retains both the genomic and proteomic features of the original SCLC biopsy samples, increasing confidence in this model system as a platform for studying SCLC.
The expression of subtype markers was further confirmed by RNA-seq (Fig. 3d). Heatmap shows samples clustered based on subtype expression (ASCL1, NEUROD1, POU2F3, and YAP1). Subtype annotation based on IHC and RNA-seq largely overlapped, and no pattern emerged between naïve and treated samples. Interestingly, the strong YAP1 RNA expression seen in some PDXs (MSK599b, MSK831b, MSK511c, MSK773G, MSK1231, MSK761, MSK900b, and MSK773E) was not observed on IHC (Supplementary Fig. 5C). Recent data have suggested that YAP1-driven SCLC cell lines express YAP together with its homologous heterodimeric transcriptional coactivator TAZ to both activate and induce negative feedback regulation of the Hippo pathway30. YAP1 subtype has also been correlated with NOTCH expression (NOTCH1, NOTCH2, and NOTCH3)30,31. We explored YAP/TAZ and their downstream transcriptional targets in our PDX/CDX dataset (Supplementary Fig. 5D). Strikingly, the POU2F3-positive PDX subset highly expressed YAP1 downstream genes, including NOTCH2 and NOTCH3, regardless of whether YAP1 itself was expressed. ASCL1- and NEUROD1-driven PDXs had lower expression of such genes, suggesting this pathway is inactivated in the NE subsets as previously described30. Notably, two samples, SCLC-A/N MSK900b and SCLC-A MSK761, showed relatively high expression of YAP1, similar to the SCLC-P models, however, YAP1 protein staining was absent (Supplementary Fig. 5C, D).
Recently, SCLC lacking high expression of ASCL1, NEUROD1, and POU2F3 have been re-categorized on the basis of their inflamed gene signature, including immune checkpoint genes and human leukocyte antigens (HLAs), rather than solely based on the expression of YAP112. This prompted us to look at the expression of HLAs and antigen-presenting gene signatures12 in our PDX cohort. Seven of 43 models (MSK831b, MSK511c, MSK325d, MSK773B, MSK773H, MSK761, and MSK891) showed high expression of HLAs and related antigen presentation genes such as HLA-DRA or HLA-DBP1 (Supplementary Fig. 5e); these models were diverse and were not clearly attributed to a distinct subtype or treatment status (naïve/treated).
Transcriptional analysis of SCLC tumors of different subtypes
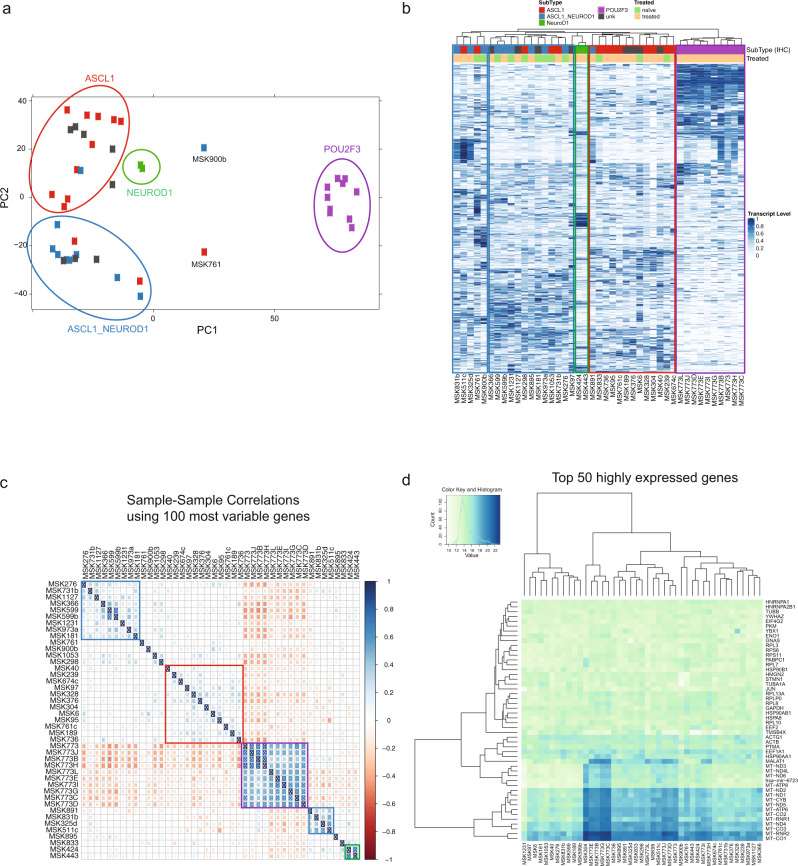
To compare the SCLC tumors of different subtypes, we applied principal component analysis (PCA) to the top 500 genes ranked by variance over all samples (Fig. 4a). SCLC-P tumors clustered closely together and were distinct from the non-SCLC-P tumors. SCLC-A and SCLC-A/N formed separate clusters with some overlap. The two SCLC-N tumors lacking ASCL1 clustered closely together, and tumors with no subtype annotation based on IHC clustered near SCLC-A or SCLC-A/N cohorts. Notably, the two samples (SCLC-A/N MSK900b and SCLC-A MSK761, annotated in Fig. 4a) with YAP1 downstream gene expression patterns similar to SCLC-P samples were positioned in an intermediate space between non-SCLC-P and SCLC-P samples, in relatively close proximity to the SCLC-P samples. These two PDXs also clustered closely together, but distinct from the SCLC-P samples, in hierarchical clustering (Fig. 4b). SCLC-A/N MSK900b and SCLC-A MSK761 were both chemonaïve, and all SCLC-P samples were treated. Overall, treatment status did not appear to be a determinant of clustering (Fig. 4b).
Fig. 4. Transcriptional profile analysis of SCLC tumors of different subtypes.
a Principal component analysis plot showing 43 patient-derived xenograft (PDX) samples color-coded based on immunohistochemistry (IHC) subtype annotation. Circles indicate four separate clusters (ASCL1-driven cluster, red; ASCL1/NEUROD1-driven cluster, blue; NEUROD1-driven cluster, green; POU2F3-driven cluster, purple). Source data are provided as a Source Data file. b Unbiased hierarchical clustering of 43 PDX samples. Color-coded panel on top depicts treatment status (naïve or treated) and subtype annotation based on IHC. Boxes indicate five separate clusters (two ASCL1/NEUROD1-driven clusters, blue; one NEUROD1-driven cluster, green; one ASCL1-driven cluster, red; one POU2F3-driven cluster, purple). Source data are provided as a Source Data file. c Sample-sample correlation plot using the top 100 highest variance genes. The Spearman correlation was used and the samples were ordered using hierarchical clustering with complete linkage. Five clusters emerged and are boxed (two ASCL1/NEUROD1-driven clusters, blue; one NEUROD1-driven cluster, green; one ASCL1-driven cluster, red; one POU2F3-driven cluster, purple). Source data are provided as a Source Data file. d Heatmap of the top 50 highly expressed genes in 43 PDX samples. Blue indicates high expression and green indicates low expression. Color key and histogram represents the normalized log 2-transformed counts. Source data are provided as a Source Data file.
Unbiased hierarchical clustering revealed distinct clusters, suggesting one clear cluster to be of POU2F3 origin (purple box), one cluster of mostly ASCL1 origin (red box), one cluster of NEUROD1 origin (green box), and two separate clusters of mostly ASCL1/NEUROD1 or ASCL1 origin (blue boxes) based on IHC (Fig. 4b). These suggested clusters were largely preserved when the analysis was based on the 100 most variable genes (Fig. 4c), with a few samples not falling into any cluster or into a different cluster than shown in Fig. 4b. However, clusters changed slightly when analyzing samples based on the top 50 highly expressed genes with only a subset of the SCLC-P samples forming a clear cluster (Fig. 4d). This subset of SCLC-P was notable for highly expressed mitochondrial genes such as MT-RNR2 or MT-CO3/1. In summary, PCA analysis and hierarchical clustering revealed distinct subtypes that were primarily reflective of the relative expression of ASCL1, NEUROD1, and POU2F3, including a substantial population with co-expression of ASCL1 and NEUROD1.
Gene expression signatures in SCLC
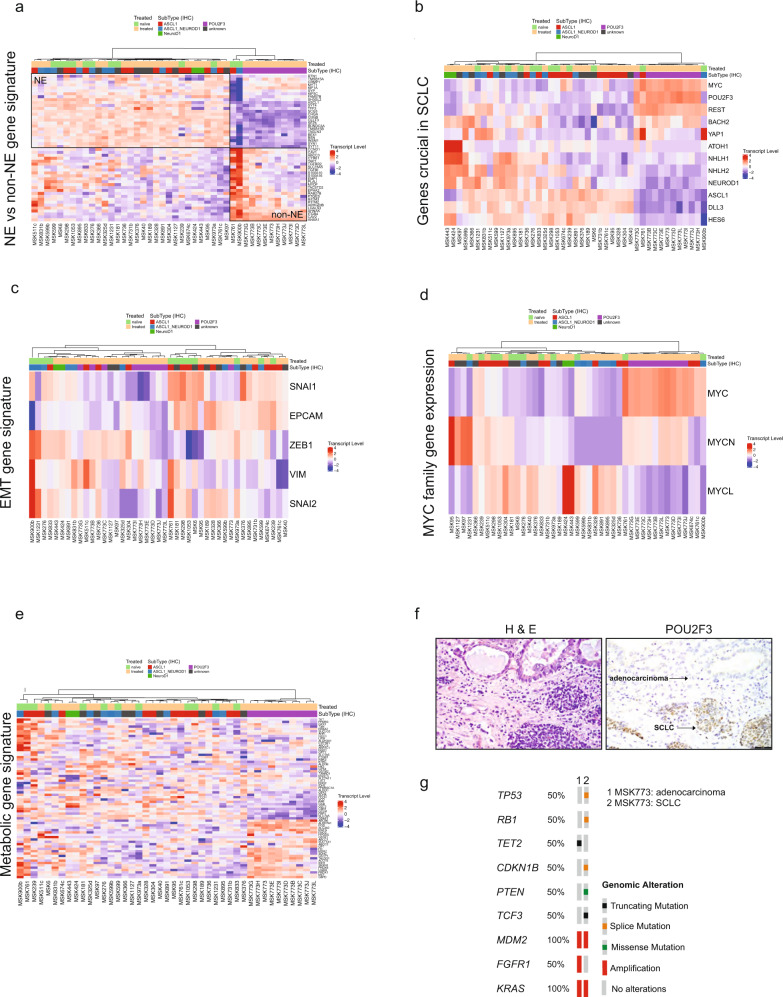
To differentiate lung NE cells from non-NE cells, we assessed these PDX/CDX models using a previously defined 50-gene NE signature tool32. As expected, NE subtypes (SCLC-A, SCLC-N, and SCLC-A/N) were markedly different from non-NE (SCLC-P) subtypes (Fig. 5a). The two non-SCLC-P samples that clustered closest to the SCLC-P group in PCA (Fig. 4), MSK761 (SCLC-A) and MSK900b (SCLC-A/N), here showed the most distinctive expression of genes in the cohort, with high expression of the non-NE determinant genes and strikingly low transcription levels of NE genes. Genes that were highly expressed in the NE-expressing subset included SYN1, INSM1, and CHGA. Drilling down on a few genes highlighting NE transcriptional regulators also revealed high expression of REST and BACH2 and low expression of DLL3 and ATOH1 in the non-NE SCLC-P subtype (Fig. 5b).
Fig. 5. Gene expression signatures in SCLC.
Heatmap of gene expression of 43 patient-derived xenograft (PDX) samples for the selected gene signatures; neuroendocrine (NE) vs. non-NE gene signature32 (a), genes crucial in SCLC (b), EMT gene signature (c), MYC family gene expression (d), and metabolic gene signature42 (e). Color-coded panel on top depicts treatment status (naïve or treated) and subtype annotation based on IHC. Source data are provided as a Source Data file. f H&E and immunohistochemistry image for POU2F3 are shown for the clinical MSK773. Scale bar as indicated, 20 μM. g Genomic landscape in MSK773A (adenocarcinoma) vs. MSK773A (SCLC) samples representing putative driver alterations (i.e. excludes variants of unknown significance) in altered genes as detected by MSK-IMPACT20 targeted next-generation sequencing assay.
While SCLC is primarily an epithelial disease, epithelial to mesenchymal transition (EMT) has been associated with the non-NE subset of SCLC and is defined by expression of vimentin (encoded by VIM) and loss of epithelial cell adhesion molecule (EPCAM)32,33. SCLC-P samples did not express detectable EPCAM but showed variable (low to medium) expression of VIM as well as low expression of EMT transcription factors SNAI1/2 and ZEB1 (Fig. 5c). EPCAM and SNAI1 expression was heterogenous but tended to be highest in SCLC-A. No clear SCLC subtype associations emerged from these genes or a more extensive EMT gene signature34 (Fig. 5c and Supplementary Fig. 8). These findings are consistent with a recent report describing low VIM expression in three CDXs of the non-NE subtype, suggesting that EMT is not always a hallmark of non-NE cells in SCLC9.
MYC gene family member expression
MYC, MYCL, and MYCN define a gene family implicated in SCLC oncogenesis and subtype differentiation35–37. To determine the relative expression of MYC family genes, we analyzed bulk RNA-sequencing for MYC, MYCN, and MYCL and also compared this to MYC IHC (validated antibodies are unavailable for MYCN and MYCL)9,38 data in Fig. 3. Paralleling protein assessment of MYC, SCLC-P samples highly expressed MYC at the RNA level (Fig. 5d). These samples expressed MYCN to a lesser degree and MYCL was low or absent. Not surprisingly, SCLC-A/N MSK900b and SCLC-A MSK761, which have consistently clustered with the SCLC-P cohort, also expressed high levels of MYC but did not express MYCN or MYCL. Most of the SCLC-A samples showed expression of MYCL, consistent with the previous reports39. Interestingly, the two SCLC-N samples (MSK424 and MSK443) in the cohort had the highest MYCL transcript expression levels (Fig. 5d). MSK-IMPACT targeted tumor sequencing20 revealed an MYCL amplification for some samples that harbored high MYCL expression, including MSK424, MSK304, MSK40, MSK599b, MSK891, and MSK736. Recent reports highlight that SCLC subtypes expressing different MYC family members are metabolically distinct40,41. We proceeded to look at a metabolic gene signature42 and interestingly, SCLC-P samples showed high expression of a subset of metabolic genes in comparison to the non-SCLC-P samples (Fig. 5e) such as ABCB6, PGD, or G6PD. While a clear subset of genes was overexpressed in the MYChi SCLC-P samples, no clear set of metabolic genes was regulated in the MYCNhi or MYCLhi SCLC samples, highlighting some degree of metabolic heterogeneity in our SCLC sample set.
Origin of the SCLC-P models
About 10% of SCLC cases are driven by the transcription factor POU2F3; however, to date, there are no reports of an animal model representing this subtype. We were fortunate to obtain a set of POU2F3-driven PDXs, derived from a male patient in his 70s with ES-SCLC. This patient was a life-long never-smoker, unusual for SCLC, but he did have a history of Hodgkin’s disease over 40 years earlier, for which he had received mantle field radiation. The patient presented with a lung mass, bone metastases, and a large volume of pleural effusion, diagnosed by pleural fluid cytology as SCLC. He was initially treated with carboplatin and etoposide, and after initial response had radiological progression within 6 months of initial diagnosis. He underwent a percutaneous lung biopsy at that time, from which an initial PDX was established (MSK773). This biopsy showed an admixed SCLC and lung adenocarcinoma (Fig. 5f). RB immune expression was lost in the SCLC and retained in the adenocarcinoma. The resulting PDX demonstrated SCLC histology, strongly positive for POU2F3 (Fig. 3). The patient was subsequently treated with ipilimumab and nivolumab, without response. Prior to death, the patient elected to participate in our institutional medical donation program, allowing us to perform an immediate post-mortem autopsy, enabling the establishment of nine additional PDXs from recurrent and metastatic sites (lung, chest, liver, diaphragm, rib). As shown in Fig. 3, all ten samples from this patient were POU2F3-positive by IHC and RNA-sequencing and expressed MYC.
POU2F3 expression in the lung is normally tightly restricted to tuft cells, rare chemosensory epithelial cells43. The high expression and strong dependence on POU2F3 suggested that the SCLC-P subtype might arise through a malignant transformation of tuft cells44. Lung adenocarcinoma arises from the much more common type II pneumocyte or related progenitor cells45. It has also been extensively described that lung adenocarcinoma can undergo histologic transformation to NE SCLC, either de novo or as a mechanism of acquired resistance to targeted inhibitors46–48. The admixture of lung adenocarcinoma with this POU2F3-positive SCLC (Fig. 5f) implies that the type II pneumocyte might also serve as a precursor for SCLC-P. The mixed histology of this case suggested single cancer with differentiation into two lineage fates, or a “collision” tumor derived from independent transformation events in two distinct cells of origin. To distinguish between these possibilities, we performed MSK-IMPACT sequencing separately on the adenocarcinoma and SCLC components. Shared somatic mutations and copy number changes confirm clonality between these distinct histologies, with RB1 and TP53 loss specific to the SCLC (Fig. 5g). These data support that SCLC-P can derive from the same cell of origin as lung adenocarcinoma. The set of 10 MSK773 PDX from various anatomically distinct sites will serve as a particularly informative resource for understanding the biology and evolution of the rare SCLC-P subtype.
Discussion
Existing model systems used to functionally interrogate the SCLC landscape include cell lines and GEMMs. Both model systems come with limitations; cell lines harbor a strong selection bias in the successful establishment of in vitro clonal growth, and GEMMs lack the diversity of mutations caused by long-term tobacco use. As such, other model systems that accurately recapitulate SCLC biology are needed. Here, we present in detail a large cohort of SCLC PDX/CDX models, representing different disease stages and treatment histories. We characterized molecular subtypes and the global gene expression landscapes of SCLC in our cohort using a multi-omic approach, including IHC, RNA-sequencing, and targeted tumor next-generation sequencing (by MSK-IMPACT).
All models presented were successful engraftments with an average time to first-generation tumor growth of 112 days. Models were derived from a variety of sources (e.g., biopsy, pleural effusion, blood) and tissue sites (e.g., lung, lymph node, liver, brain). PDX tumors largely retained clinical features of the original biopsy, including the hallmarks of SCLC, inactivation of TP53 and RB1. The significance of the few differences we did observe in genomic spectra of primary tumors and their derivative models remains to be elucidated. A caveat is that these PDX/CDX models are maintained in immune-incompetent mice: the absence of an intact adaptive immune system and replacement of human stromal elements by murine cells may alter tumor-intrinsic features of antigen presentation and immune profiling.
Current first-line standards of care for SCLC include platinum-based chemoimmunotherapy for ES disease and chemoradiotherapy for LS disease. Recent reports have shown SCLC cell lines respond differently to drug treatment based on subtype; for example, SCLC-P cell lines appear to be sensitive to PARP inhibition12. Similarly, SCLC GEMM models suggest that MYC-high SCLC may have selective sensitivity to Aurora kinase inhibition49. With new therapies emerging from early clinical trials, it will be important to identify the SCLC subtypes of patients to validate these observations and to inform future treatment strategies. Consistent with the known distribution of subtypes, most of our samples express ASCL1 or both ASCL1 and NEUROD1. We also identified ten POU2F3-driven SCLC samples, derived from one donor. Characteristic features of these models include high POU2F3 and MYC expression by IHC and RNA-seq and low expression of NE markers. Unbiased hierarchical clustering by global gene expression largely recapitulates subtype assignments based on the primary transcription factors. Interestingly, there were two samples, MSK761 and MSK900b, that were assigned to ASCL1 or ASCL1/NEUROD1 groups by transcription factor expression, but which also clustered closer to POU2F3-driven PDXs by PCA and were notable for expression of YAP1 and its downstream genes such as LATS2 or CAV1. As mentioned earlier, the role of YAP1 as a defining subtype marker in SCLC has recently been questioned, and a new subtype defined by an inflamed gene signature was proposed12. While MSK761 showed high expression of antigen-presenting genes, MSK900b did not. MSK761 was derived from a liver biopsy performed at the initial diagnosis. MSK761c, a CDX sample derived from the blood of the same patient after chemoimmunotherapy and combination immunotherapy, showed high expression of ASCL1 by IHC which was further confirmed by RNA-seq. The high YAP1 expression seen in the treatment-naïve MSK761 liver PDX was not found in the subsequent MSK761c CDX. Moreover, MSK761c did not cluster with MSK761 in the PCA, but with the other ASCL1-driven samples, further demonstrating the heterogeneity of tumor cell characteristics found in this patient. Whether these observed differences might be attributable to the site of origin of these samples is unclear. Setting this case aside, the transcriptional analysis did not reveal any major differences between treated and treatment-naïve samples. Bigger sample size and definition of gene expression signatures that correlate with disease progression and drug resistance are needed.
A primary strength of PDX models is the close linkage to the patient. PDX samples allow us to generate multiple models from patients at diagnosis and subsequently from different stages during treatment, establishing a powerful resource for the analysis of disease progression and shifting drug sensitivities. We anticipate our cohort of 46 PDX/CDX samples, derived from 33 donors, to be of substantial use to other research groups studying SCLC. This resource, and others like it, offers a powerful system to characterize SCLC biology and inform clinical research treatment strategies for patients with SCLC.
Methods
Patient samples
Patient samples for the generation of PDX models and subsequent analyses were collected with written informed consent from patients under protocols approved by the MSKCC Institutional Review Board/Privacy Board (IRB Protocols 12-245, 06-107, and 14-091). Metadata for each patient can be found in Supplementary Data 1. Tumor DNA extracted from formalin-fixed paraffin-embedded tissue or frozen and normal DNA was used for genomic sequencing with Memorial Sloan Kettering-Integrated Mutational Profile of Actionable Cancer Targets (MSK-IMPACT)20. After PicoGreen quantification and quality control by Agilent BioAnalyzer, 3–143 ng of DNA were used to prepare libraries using the KAPA Hyper Prep Kit (Kapa Biosystems KK8504) with eight cycles of PCR. About 50–500 ng of each barcoded library were captured by hybridization in equimolar pools of 7–26 samples using the IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets) assay (Nimblegen SeqCap), designed to capture all protein-coding exons and select introns of 410 or 468 commonly implicated oncogenes, tumor suppressor genes, and members of pathways deemed actionable by targeted therapies. Captured pools were sequenced on a HiSeq 2000, HiSeq 2500, or HiSeq 4000 in a PE100 or PE125 run using the HiSeq SBS Kit v4, TruSeq Rapid SBS Kit – HS, HiSeq Rapid SBS Kit v2, or HiSeq 3000/4000 SBS Kit (Illumina) producing an average of 574X coverage per tumor and 418X per normal.
Three iterative versions of the MSK-IMPACT next-generation sequencing panel that screens for mutations, translocations, and copy number alterations were used across this study, consisting of 341, 410, or 468 cancer-associated genes20. For PDX/CDX samples, MuTect, Pindel, and VarDict were used to detect single nucleotide variants (SNVs) and insertions and deletions (Indels) (https://github.com/mskcc/roslin-variant/wiki/Roslin-Methods-v2.5). Copy number alterations (CAN) was called using the FACETS50 EM algorithm. For clinical samples, MuTect, Vardict, Pindel, and Somatic Indel Detector were used to detect single nucleotide variants (SNVs) and insertions and deletions (Indels)51, with variant allele frequency thresholds set to 5% for non-hotspot alterations and 2% for hotspot sites. Copy number alterations (CAN) were identified using a custom algorithm20. Structural variants were detected using Delly52. All identified variants were filtered against the patient-matched normal sample and manually reviewed to retain only true positive somatic calls.
Patient-derived xenografts
All animal experiments were approved by the Memorial Sloan Kettering Cancer Center (MSKCC) Animal Care and Use Committee (IACUC Protocol 04-03-009) and mice were housed in accredited facilities under pathogen-free conditions. Female NOD.Cg-Prkdc<scid> Il2rg<tm1Wjl>/SzJ (Stock #: 005557) (6–8 weeks old) were obtained from Jackson Laboratory. PDX and CDX models were generated from primary tumors and whole blood samples as described previously13. Briefly, cells were injected s.c. in the flank of one mouse per model and subsequently passaged multiple times. Tumor size was measured using a caliper once to twice weekly and the following equation was used to calculate volume: V = π/6 × L × W2 (L length; W width). Tumors were harvested when the tumor volume reached 1500 mm3. Maximal tumor size was not exceeded.
RNA-sequencing
Sample preparation for RNA-sequencing and subsequent analysis was performed as in ref. 26. Briefly, Illumina HiSeq instrument (4000 or equivalent; a 2x150 bp paired-end (PE)) according to manufacturer’s instructions was used for RNA-sequencing in collaboration with Genewiz.
RNA-seq analysis
The output data (FASTQ files) are mapped to the target genome using the rnaStar aligner53 that maps reads genomically and resolves reads across splice junctions. We use the two-pass mapping method outlined in ref. 54 in which the reads are mapped twice. The first mapping pass uses a list of known annotated junctions from Ensemble. Novel junctions found in the first pass are then added to the known junctions and a second mapping pass is done (on the second pass the RemoveNoncanoncial flag is used). After mapping, we post-process the output SAM files using the PICARD tools to add read groups, AddOrReplaceReadGroups which in addition sort the file and coverts it to the compressed BAM format.
We then compute the expression count matrix from the mapped reads using HTSeq (www-huber.embl.de/users/anders/HTSeq) and one of several possible gene model databases. The raw count matrix generated by HTSeq are then be processed using the R/Bioconductor package DESeq (www-huber.embl.de/users/anders/DESeq) which is used to both normalize the full dataset and analyze differential expression between sample groups.
A PCA analysis was done using the top 500 highest variance genes and the first two PCA components were plotted. Heatmaps were generated using both the log-transformed absolute normalized intensity of counts and also of the Z-score of log normalized intensity over each gene. The Z-score heatmaps were plotted for specific genes sets and the samples were clustered using standard hierarchical clustering with the Manhattan distance metric and Ward.D linkage from the hclust function in R. The intensity heatmaps were generated using both the top differentially expressed genes or the top variable expression genes as measured by the gene standard deviation. A sample/sample correlation plot was done by computing the Spearman correlation of the top 100 most variable genes. The correlation matrix was plotted using the corrplot function from the R corrplot package using the hclust function with complete linkage to order the samples. See Supplementary Table 1 for a list of program versions used in RNA-seq analysis.
Data files
Human:
GENOME: UCSC HG19
GTF: gencode.v18.annotation
Tissue microarray construction
For the immunohistochemical evaluation of PDX samples, tissue microarray blocks were constructed containing 3 × 1 mm cores per case. Slides from these blocks were subsequently stained with H&E and reviewed by a pathologist for quality assessment.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissues (standard paraffin blocks for donor samples and TMA blocks for PDX samples) were used to obtain 4 µm sections for immunohistochemistry (IHC). IHC was performed using standard immuno-peroxidase methods and manufacturer instructions (See Table). All slides were scanned in Aperio AT2 digital whole slide scanner and reviewed with Aperio ImageScope v12.4.3.7005 and/or under a light microscope by an experienced pathologist. Nuclear staining was considered positive for ASCL1, MYC, NEUROD1, and POU2F3; whereas cytoplasmic for synaptophysin, cytoplasmic and membranous for CD56, and nuclear ± cytoplasmic for YAP1. Percentage of positive cells and staining intensity (0/1 + /2 + /3+) were recorded. H scores were calculated by multiplying the percentage of positive cells by the staining intensity. For PDX samples, three independent cores per sample were stained for the indicated protein, and the H-score was derived from the average of the three cores. For clinical samples, one core per sample was stained for the indicated protein and H-score derived from this single core. See Supplementary Table 2 for a list of antibodies used for immunohistochemistry.
Statistics and reproducibility
RNA-seq was performed once for 43 PDX samples and IHC was performed once for 37 PDX samples and 19 clinical samples. MSK-IMPACT was performed once for 42 PDX samples and 26 clinical samples. No statistical method to predetermine sample size was used. SCLC patient samples that were successfully engrafted were used without further selection criteria. The experiments were not randomized. The Investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Information
Acknowledgements
Supported by NCI 1 R35CA263816-01, R01 CA197936, and U24 CA213274 (C.M.R.), the SU2C/VAI Epigenetics Dream Team (C.M.R.), NCI 1R01CA258784 (T.S.), the Druckenmiller Center for Lung Cancer Research (C.M.R. and T.S.), Parker Institute for Cancer Immunotherapy grant (T.S.); International Association for the Study of Lung Cancer grant (T.S.), LC190161 Congressionally Directed Medical Research Programs (DOD-IITRA) (T.S.). We acknowledge the use of the Integrated Genomics Operation Core (special thanks to Cassidy C Cobbs), funded by the NCI Cancer Center Support Grant (CCSG, P30 CA08748), Cycle for Survival, and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology (special thanks to Ritika Kundra and Yichao Sun) and the National Cancer Institute Cancer Center Core Grant No. P30-CA008748.The PPBC Biobank and Precision Pathology Center are supported by the NCI Cancer Center Support Grant P30-CA008748. We gratefully acknowledge the members of the Molecular Diagnostics Service in the Department of Pathology, in particular Rose Brannon. We wish to acknowledge assistance from the Bioinformatics Core which is funded in part through the NIH/NCI Cancer Center Support Grant P30-CA008748. We thank all members of the MSKCC Antitumor Assessment Core Facility (core grant: P30-CA008748 S5). We thank all members of the Rudin laboratory for their collaborative efforts.
Source data
Author contributions
Conceptualization: R.C., C.M.R., and T.S.; Methodology: R.C., J.V.E., S.C., N.D.S., C.B.J., F.E.K., M.A., N.S.S., V.A., P.M., S.E.T., E.d.S., J.T.P., C.M.R., and T.S.; Investigation: R.C., J.V.E., N.D.S., C.B.J., F.E.K., M.A., M.H.R., N.S.S., V.A., A.K., M.M., C.A.I-D., W.V.L., U.B., M.K.B., N.R., T.J.H, E.d.S., J.T.P., C.M.R., and T.S.; Validation: R.C., J.V.E., and N.D.S.; Formal Analysis: R.C., J.V.E., S.C., N.D.S., F.E.K., C.M.R., and T.S.; Writing: R.C., T.S., and C.M.R.; Supervision: T.S. and C.M.R.; Funding acquisition: T.S. and C.M.R. All authors read and approved the final version of the manuscript.
Peer review
Peer review information
Nature Communications thanks Ryohei Katayama and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Data availability
The Gene expression data generated in this study has been deposited in the Arrayexpress database under accession code E-MTAB-11230. The genomic sequencing data generated in this study underlying Figs. 2 and 5g are publicly available via the cBioPortal for cancer genomics https://www.cbioportal.org/study/summary?id=lung_pdx_msk_2021. The raw data were available under restricted access, access can be obtained by contacting the corresponding author Charles Rudin at rudinc@mskcc.org. The remaining data were available within the Article, Supplementary Information, or Source Data File. Source data are provided with this paper.
Competing interests
C.M.R. has consulted regarding oncology drug development with Amgen, Daiichi Sankyo, Genentech/Roche, Ipsen, Jazz, Merck, Pfizer, Syros, and Vavotek. C.M.R. serves on the scientific advisory boards of Bridge Medicines, Earli, and Harpoon Therapeutics. T.S. has research funding from Jazz Pharmaceuticals. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Charles M. Rudin, Email: rudinc@mskcc.org
Triparna Sen, Email: sent@mskcc.org.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29794-4.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hann C., Wu, M. A., Rekhtman, N. & Rudin, C. M. In Cancer Principles and Practice of Oncology (Wolters Kluwer, 2019).
- 3.Bernhardt EB, Jalal SI. Small cell lung cancer. Cancer Treat. Res. 2016;170:301–22. doi: 10.1007/978-3-319-40389-2_14. [DOI] [PubMed] [Google Scholar]
- 4.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–72. doi: 10.1002/cncr.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonia SJ, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–95. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 6.Horn L, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018;379:2220–9. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 7.Goldman JW, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–39. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 9.Simpson KL, et al. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity. Nat. Cancer. 2020;1:437–451. doi: 10.1038/s43018-020-0046-2. [DOI] [PubMed] [Google Scholar]
- 10.Drapkin BJ, et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Disco. 2018;8:600–15. doi: 10.1158/2159-8290.CD-17-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner EE, et al. Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 axis. Cancer Cell. 2017;31:286–99. doi: 10.1016/j.ccell.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gay CM, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39:346–60. doi: 10.1016/j.ccell.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel VC, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–73. doi: 10.1158/0008-5472.CAN-08-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naito T, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J. Thorac. Oncol. 2012;7:512–9. doi: 10.1097/JTO.0b013e31823f125d. [DOI] [PubMed] [Google Scholar]
- 15.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 2011;192:373–82. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter L, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 2017;23:114–9. doi: 10.1038/nm.4239. [DOI] [PubMed] [Google Scholar]
- 17.Rudin CM, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat. Rev. Cancer. 2019;19:289–97. doi: 10.1038/s41568-019-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George J, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis WD, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10:1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 20.Cheng DT, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 2015;17:251–64. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Disco. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byers LA, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Disco. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quintanal-Villalonga A, et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 2020;17:360–71. doi: 10.1038/s41571-020-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneeberger VE, Allaj V, Gardner EE, Poirier JT, Rudin CM. Quantitation of murine stroma and selective purification of the human tumor component of patient-derived xenografts for genomic analysis. PLoS ONE. 2016;11:e0160587. doi: 10.1371/journal.pone.0160587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohlhieter CA, et al. Concurrent mutations in STK11 and KEAP1 promote ferroptosis protection and SCD1 dependence in lung cancer. Cell Rep. 2020;33:108444. doi: 10.1016/j.celrep.2020.108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan EJ, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Disco. 2017;7:596–609. doi: 10.1158/2159-8290.CD-16-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baine MK, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J. Thorac. Oncol. 2020;15:1823–35. doi: 10.1016/j.jtho.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearsall SM, et al. The rare YAP1 subtype of SCLC revisited in a biobank of 39 circulating tumor cell patient derived explant models: a brief report. J. Thorac. Oncol. 2020;15:1836–43. doi: 10.1016/j.jtho.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tlemsani C, et al. SCLC-CellMiner: a resource for small cell lung cancer cell line genomics and pharmacology based on genomic signatures. Cell Rep. 2020;33:108296. doi: 10.1016/j.celrep.2020.108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018;20:888–99. doi: 10.1038/s41556-018-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, et al. Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Transl. Lung Cancer Res. 2018;7:32–49. doi: 10.21037/tlcr.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calbo J, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell. 2011;19:244–56. doi: 10.1016/j.ccr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Byers LA, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 2013;19:279–90. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson BE, et al. myc family oncogene amplification in tumor cell lines established from small cell lung cancer patients and its relationship to clinical status and course. J. Clin. Invest. 1987;79:1629–34. doi: 10.1172/JCI112999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little CD, Nau MM, Carney DN, Gazdar AF, Minna JD. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983;306:194–6. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- 37.Nau MM, et al. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985;318:69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- 38.Bragelmann J, et al. Family matters: how MYC family oncogenes impact small cell lung cancer. Cell Cycle. 2017;16:1489–98. doi: 10.1080/15384101.2017.1339849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato F, et al. MYCL is a target of a BET bromodomain inhibitor, JQ1, on growth suppression efficacy in small cell lung cancer cells. Oncotarget. 2016;7:77378–88. doi: 10.18632/oncotarget.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang F., et al. Guanosine triphosphate links MYC-dependent metabolic and ribosome programs in small-cell lung cancer. J Clin Invest. 131, e139929 (2021). [DOI] [PMC free article] [PubMed]
- 41.Chalishazar MD, et al. MYC-driven small-cell lung cancer is metabolically distinct and vulnerable to arginine depletion. Clin. Cancer Res. 2019;25:5107–21. doi: 10.1158/1078-0432.CCR-18-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galan-Cobo A, et al. LKB1 and KEAP1/NRF2 pathways cooperatively promote metabolic reprogramming with enhanced glutamine dependence in KRAS-mutant lung adenocarcinoma. Cancer Res. 2019;79:3251–67. doi: 10.1158/0008-5472.CAN-18-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita J, Ohmoto M, Yamaguchi T, Matsumoto I, Hirota J. Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PLoS ONE. 2017;12:e0189340. doi: 10.1371/journal.pone.0189340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YH, et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 2018;32:915–28. doi: 10.1101/gad.314815.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu X, et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc. Natl Acad. Sci. USA. 2012;109:4910–5. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Offin M, et al. Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J. Thorac. Oncol. 2019;14:1784–93. doi: 10.1016/j.jtho.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quintanal-Villalonga, A. et al. Multi-omic analysis of lung tumors defines pathways activated in neuroendocrine transformation. Cancer Discov. 11, 3028–3047 (2021). [DOI] [PMC free article] [PubMed]
- 49.Mollaoglu G, et al. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell. 2017;31:270–85. doi: 10.1016/j.ccell.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 2016;44:e131. doi: 10.1093/nar/gkw520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zehir A, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017;23:703–13. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rausch T, et al. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics. 2012;28:i333–i9. doi: 10.1093/bioinformatics/bts378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engstrom PG, et al. Systematic evaluation of spliced alignment programs for RNA-seq data. Nat. Methods. 2013;10:1185–91. doi: 10.1038/nmeth.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Information
Data Availability Statement
The Gene expression data generated in this study has been deposited in the Arrayexpress database under accession code E-MTAB-11230. The genomic sequencing data generated in this study underlying Figs. 2 and 5g are publicly available via the cBioPortal for cancer genomics https://www.cbioportal.org/study/summary?id=lung_pdx_msk_2021. The raw data were available under restricted access, access can be obtained by contacting the corresponding author Charles Rudin at rudinc@mskcc.org. The remaining data were available within the Article, Supplementary Information, or Source Data File. Source data are provided with this paper.