Abstract
This meta-analysis aimed at investigating the efficacy of high-flow nasal oxygenation (HFNO) against hypoxemia in patients with obesity compared with conventional oxygenation therapy and non-invasive ventilation. Databases were searched from inception to August 2021. Studies involving peri- or post-procedural use of HFNO were included. The primary outcome was risk of hypoxemia, while the secondary outcomes included status of oxygenation and carbon dioxide elimination. Ten randomized controlled trials (RCTs) were included. We found that HFNO prolonged the safe apnea time at induction compared to control group [mean difference (MD) = 73.88 s, p = 0.0004; 2 RCTs] with no difference in risk of peri-procedural hypoxemia [relative risk (RR) = 0.91, p = 0.64; 4 RCTs], minimum SpO2 (MD = 0.09%, p = 0.95; 4 RCTs), PaO2 (MD = − 8.13 mmHg, p = 0.86; 3 RCTs), PaCO2 (MD = − 6.71%, p = 0.2; 2 RCTs), EtCO2 (MD = − 0.28 mmHg, p = 0.8; 4 RCTs) between the two groups. HFNO also did not improve postprocedural PaO2/FiO2 ratio (MD = 41.76, p = 0.58; 2 RCTs) and PaCO2 (MD = − 2.68 mmHg, p = 0.07; 2 RCTs). This meta-analysis demonstrated that the use of HFNO may be associated with a longer safe apnea time without beneficial impact on the risk of hypoxemia, oxygenation, and CO2 elimination in patients with obesity. The limited number of trials warranted further large-scale studies to support our findings.
Subject terms: Health care, Medical research
Introduction
Peri- or post-procedural oxygen supplementation has been a widely accepted approach to increasing pulmonary oxygen reserves and delaying the onset of oxygen desaturation during apnea1–3. Oxygen supplementation is essential for patients at induction of anesthesia or receiving sedation, especially those at risk of difficult intubation4,5 and those undergoing rapid sequence induction6 or with limited oxygen reserves7. Oxygen supplementation is also important following anesthesia or administration of sedatives, as the residual effects of these regimens can lead to hypoxemia, hypoventilation, and loss of airway patency3,8. Patients with obesity are considered at higher risks of difficulty in mask ventilation9 and tracheal intubation10 compared with those in individuals without. Besides, obesity is associated with a reduced functional residual capacity (FRC), atelectasis, and significant shunting in dependent lung regions with an increase in resting metabolic rate, work of breathing, and minute oxygen demand11. Therefore, patients with obesity are at high risk of a rapid drop in arterial oxygen level after the cessation of breathing. Moreover, because coexisting cardiovascular diseases are common in patients with obesity12, hypoxemia-induced cardiovascular complications (e.g., myocardial depression) following sedation remain a major concern13. Consequently, effective oxygen supplementation is crucial to the prevention of peri- and post-procedural pulmonary and cardiovascular complications in this patient population.
High-flow nasal oxygenation (HFNO) refers to the delivery of oxygen at high flow rates without recourse to invasive or non-invasive ventilation. In the critical care setting, pooled evidence has demonstrated the clinical benefits of applying HFNO in patients with acute respiratory failure or those at high risk of post-extubation respiratory failure14–16. A recent meta-analysis recruiting mostly patients without obesity also reported the effectiveness of HFNO for prolonging the duration of safe apnea and elevating minimum SpO2 as well as decreasing the risk of hypoxemia in patients receiving anesthetic induction or sedation17. Because upper airway obstruction due to posterior displacements of oropharyngeal structures (i.e., soft palate, base of tongue, and epiglottis) is a definite risk in patients with obesity after anesthesia induction or sedation18 during which a patent airway remains a key factor for successful oxygenation19, the results of the previous meta-analysis17 may not be applicable to those with obesity. To clarify the benefits of HFNO in this patient population, this meta-analysis aimed at comparing the risk of hypoxemia, oxygenation status, and carbon dioxide elimination between patients with obesity receiving HFNO and those undergoing conventional oxygen therapy (COT) or non-invasive ventilation (NIV) in a variety of clinical settings.
Methods
This meta-analysis was conducted in accordance with the recommendations of the PRISMA statement and registered with the International Prospective Register of Systematic Reviews (CRD42021271777).
Data sources and searches
We searched the Embase, Medline, Google scholar, and the Cochrane Library databases from inception to August 19, 2021, using the following search terms: ("Obesity" or "Obes*" or "Overweight" or "Severe Obesity" or "Morbid Obesity") and ("(high flow or high-flow) ADJ4 (oxygen or cannula* or oxygenation)" or "HFNO" or "HFNC" or "NHF" or "Optiflow" or "THRIVE" or "Transnasal Humidified Rapid Insufflation Ventilatory Exchange") limited to randomized controlled trials (RCTs). No restriction was placed on language, gender, sample size, and study location during literature search. The search strategies for these databases are demonstrated in Supplemental Table 1. Regarding Google scholar, a hand-search strategy was adopted to find the related articles. Once a relevant article was identified, a forward snowballing strategy20,21 was used to optimize the efficiency of the literature search. Additional records identified by reviewing the reference lists of the retrieved studies were also reviewed for eligibility of being included in the current study.
Inclusion criteria
To scrutinize the eligibility of the acquired publications for the present meta-analysis, we adopted the following PICO (population, intervention, comparison, outcomes) criteria: (a) Population: adults patients (age ≥ 18 years) with obesity, (b) Intervention: the use of HFNO as the intervention measure, (c) Comparison: the use of COT [e.g., mask/nasal oxygenation] or NIV as a control, (d) Outcomes: inclusion of at least one of these outcomes: incidence of hypoxemia, minimum O2 saturation, PaO2, safe apnea time, PaCO2 or EtCO2. Only RCTs were included for analysis. The authors of the included articles with missing information were contacted for possible access to the original data.
Exclusion criteria
Exclusion criteria were: (1) studies without a control group; (2) those focusing on patients undergoing cardiothoracic surgeries; (3) those in which information regarding outcomes was unavailable, and (4) RCTs published only as letters or abstracts, or (5) those presented as a review, case report, or other forms of publication other than original research.
Study selection
Two authors examined the titles and abstracts of the retrieved RCTs independently for eligibility of being included in the present study. The full texts of the potentially eligible trials were independently reviewed based on the inclusion and exclusion criteria. Differences in opinions about inclusion or exclusion of a particular study were resolved through discussion with a third reviewer.
Data extraction
The following items were retrieved from each trial: first author, year of publication, age, gender, body mass index (BMI), sample size, flow rate of HFNO, type of surgery or procedures, incidence of hypoxemia, level of PaO2, minimum O2 saturation, safe apnea time, EtCO2, and PaCO2. Disagreements were settled by discussion with a third author.
Outcomes and definitions
The primary outcome was the impact of HFNO on the risk of hypoxemia, while the secondary outcomes included minimum SaO2, level of PaO2, PaO2/FiO2 ratio, EtCO2, PaCO2, and safe apnea time. The definition of hypoxemia was in accordance with that of each study. As the efficacy for oxygenation may be different between COT and NIV, subgroup analysis of the impact of choosing either approach as control to assess the therapeutic benefit of HFNO was performed. If a study reported an outcome (e.g., level of PaO2) at different time points, we analyzed the data acquired just before invasive mechanical ventilation.
Assessment of risk of bias
Internal validity of the included RCTs was assessed by two reviewers independently based on the following domains: adequacy of sequence generation, allocation sequence concealment, blinding of participants and caregivers, blinding for outcome assessment, incomplete outcome data, selective outcome reporting, and the other sources of bias22. The risk of bias of each RCT was reported as "low," "unclear", or "high". We regarded the risk of "selective outcome reporting" bias of a study as "unclear" if its protocol was not published or registered. Moreover, the sources of funding were scrutinized for the potential of other biases. Disagreements were resolved by discussion.
Data synthesis and analysis
Cochrane Review Manager (RevMan 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for the present meta-analysis. The pooled risk ratios (RRs) and mean difference (MD) with 95% confidence intervals (CIs) were computed for binary and continuous outcomes, respectively. We assessed heterogeneity with I2 statistics and defined substantial heterogeneity as an I2 over 50%. On the assumption of heterogeneity across the included studies, we adopted a priori a random-effects model for outcome evaluation. The potential publication bias was assessed by visual inspection of a funnel plot on encountering 10 or more trials sharing a particular outcome. Sensitivity analysis was conducted with a leave-one-out approach to weigh the potential influence of the data from an individual trial on the overall outcome. The level of significance was set at < 0.05 for all outcome analyses.
Results
Search results and study characteristics
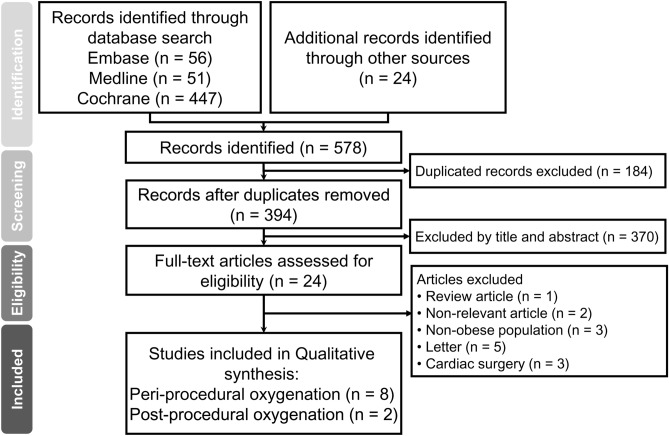
The process of study selection is shown in Fig. 1. First, of the 578 potentially relevant records retrieved from the databases, 184 duplicates were excluded. Second, screening of the titles and abstracts based on the PICO criteria gave 24 potentially eligible studies. Finally, after all text reviews, 10 studies published from 2014 to 2021 involving 564 patients were included in current meta-analysis. Characteristics of the included studies are demonstrated in Table 1. All studies recruited participants of both genders with the proportion of females ranging from 29.7 to 89.5%. The mean BMI in the enrolled patients varied from 33 to 52 kg/m2. The proportion of patients with obstructive sleep apnea (OSA) was between 12.5 and 72.5% in five trials23–27, while five studies did not provide this information28–32. Of the ten studies, two assessed the beneficial effects of HFNO on postoperative pulmonary parameters in patients receiving laparoscopic bariatric surgery23,24, while eight studies evaluated the efficacy of HFNO against peri-procedural hypoxemia or oxygenation status during anesthesia induction (six trials)26–30,32, tracheal intubation in the intensive care unit (one trial)31, and colonoscopy under deep sedation (one trial)25. In the HFNO groups, the flow rate ranged from 50 to 120 L/min. In the control groups receiving COT/NIV, mask oxygenation was adopted in four studies23,24,27,29, while NIV and nasal cannula oxygenation were used in other four26,30–32 and two25,28 RCTs, respectively.
Figure 1.
PRISMA flow diagram of study selection for the current meta-analysis.
Table 1.
Characteristics of included studies (n = 10).
| Studies | Mean Age (years) H vs. C |
Sample size | Female (%) | BMI (kg/m2) H vs. C |
OSA (%) | Procedure | Setting | Flow (H) | Flow (C) | Country |
|---|---|---|---|---|---|---|---|---|---|---|
| Ferrando 2019 | 46.3 vs. 46.4 | 64 | 66 | 43.1 vs. 45.5 | 12.5 | LBS | PO | 60 L/min; FiO2 = 0.5 | MO at 15 L/min; FiO2 = 0.5 | Spain |
| Fulton 2021 | 48 vs. 46 | 50 | 78 | 43.1 vs. 44.4 | 46 | LBS | PO | 50 L/min; FiO2 = 0.5 | MO 6 L/min | Australia |
| Hamp 2020 | 47 vs. 40 | 40 | 72.5 | 46.3 vs. 45.8 | NA | Bariatric surgery | PPO | 120 L/min | NCO at 10 L/min | Austria |
| Heinrich 2014 | 41 vs. 47† | 22 | 55 | 52 vs. 46 | NA | LBS | PPO | 50 L/min; FiO2 = 1 | MO at 12 L/min; FiO2 = 1 | Germany |
| Jiang 2020 | 47.1 vs. 46.5 | 60 | 48.3 | 33 vs. 33.9 | NA | LC | PPO | 70 L/min, FiO2 = 1 | NIV; FiO2 = 1 | China |
| Riccio 2019 | 54 vs. 59 | 59 | 86.4 | 48 vs. 49 | 16.9 | Colonoscopy¶ | PPO | 60 L/min; FiO2 = 0.36–0.4 | NCO at 4 L/min; FiO2 = 0.36–0.4 | United States |
| Rodriguez 2021 | 66 vs. 66 | 91 | 29.7 | 34 vs. 35 | NA | TI‡ | PPO | 60 L/min; FiO2 = 1 | NIV; PEEP = 5 cmH2O; FiO2 = 1 | French |
| Rosen 2021 | 44 vs. 38.7 | 38 | 89.5 | 39.8 vs. 40 | 13.2 | LBS | PPO | 70 L/min, FiO2 = 1 | NIV; PEEP = 7 cmH2O; FiO2 = 1a | Sweden |
| Vourch 2019 | 51 vs. 46† | 100 | 70 | 42 vs. 41 | NA | Mixed surgery | PPO | 60 L/min, FiO2 = 1 | NIV; PEEP = 5 cmH2O; FiO2 = 1 | France |
| Wong 2019 | 43.1 vs. 44 | 40 | 77.5 | 48.7 vs. 48.8 | 72.5 | NA | PPO | 60 L/min, FiO2 = 1 | MO at 15 L/min; FiO2 = 1 | Canada |
H high-flow nasao oxygenation group, C control group, vs. C †data were presented as median, LBS laparoscopic bariatric surgery, ¶ procedure was performed under deep sedation, MO mask oxygenation, FiO2 fraction of inspired oxygen, NCO nasal cannula oxygenation, MV mask ventilation, BMI body mass index, TI tracheal intubation, ‡ performed in intensive care units, PO post-procedure oxygenation supplementation, PPO peri-procedure oxygenation supplementation, NIV noninvasive ventilation.
Risk of bias assessment
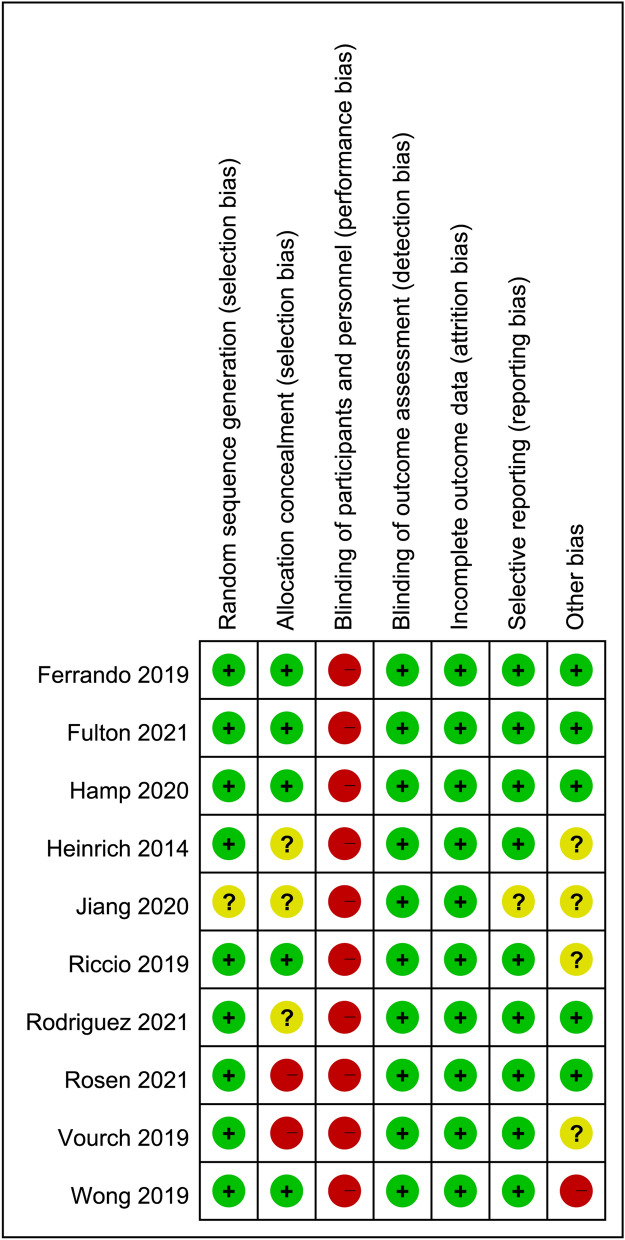
The risks of bias of individual studies are presented in Fig. 2. Regarding selecting bias, the risks of bias were low in most studies. However, one study did not give information about randomization of patients30, three RCTs did not described the methods used to accomplish allocation concealment29–31, and two trials reported that the allocation concealment was not masked26,32. Considering the impossibility of blinding among patients and caregivers in the included trials, performance bias was high in all studies23–32. Despite the lack of blinding, the risk of detection bias was considered low in all studies that used objective indicators (e.g., PaO2) as assessment parameters. The risk of reporting bias was unclear in one study30 that did not declare trial registration, while the risk of other biases was unclear or high in five studies25,27,29,30,32.
Figure 2.
Risks of bias of the included studies.
Outcomes analyses
Primary outcome: impact of HFNO on risk of hypoxemia
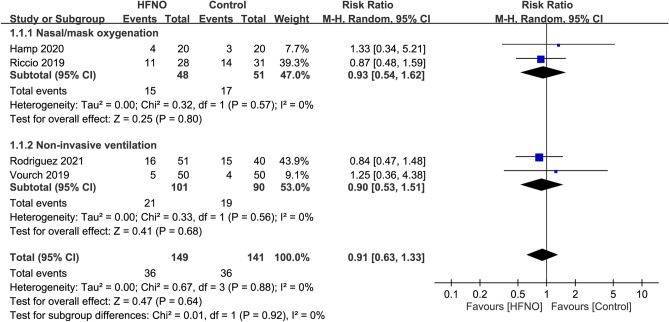
Of the four RCTs included in the present meta-analysis, hypoxemia was defined as an SaO2 < 90% in three studies25,28,32, and < 80% in one trial31 which was conducted in the intensive care unit. The incidence of hypoxemia was 24.2% and 25.5% in the HFNO and control group, respectively. Pooled results revealed no significant difference in the risk of hypoxemia between patients receiving HFNO and those undergoing COT/NIV (RR = 0.91, 95% CI 0.63 to 1.33, p = 0.64; I2 = 0%; 4 RCTs; n = 290) (Fig. 3). Consistently, subgroup analysis showed no significant impact of the choice of different approaches (i.e., COT or NIV) on the risk of hypoxemia (p = 0.92) (Fig. 3). Sensitivity analysis demonstrated a consistent finding when the four trials were removed one at a time.
Figure 3.
Forest plot comparing the risk of hypoxemia between HFNO and control groups. HFNO, high-flow nasal oxygenation; M-H, Mantel–Haenszel; CI, confidence interval.
Secondary outcome: impact of HFNO on peri-procedural oxygenation-related parameters
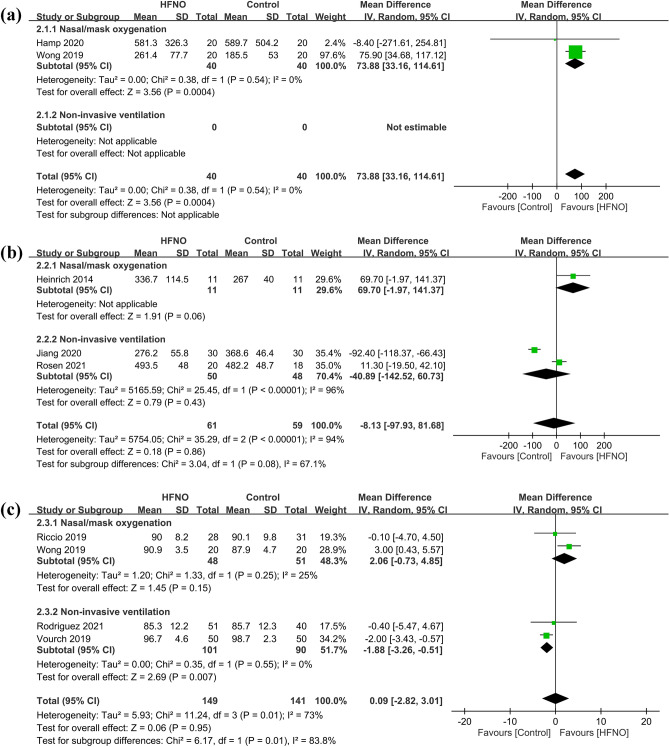
The safe apnea time was defined in two of the included studies either as the time taken for the SpO2 to drop below 95%27,28 or the maximum time of observation before invasive mechanical ventilation, which was six minutes in one study27 and 15 min in the other28. Our results demonstrated that the use of HFNO significantly increased the safe apnea time compared to the use of COT (MD = 73.88 s, 95% CI 33.16–114.61, p = 0.0004; I2 = 0%; 2 RCTs; n = 80) (Fig. 4a)27,28. However, forest plot showed no significant difference in PaO2 (MD = − 8.13 mmHg, 95% CI − 97.93 to 81.68, p = 0.86; I2 = 94%; 3 RCTs; n = 120) between the two groups (Fig. 4b)26,29,30. Sensitivity analysis demonstrated a consistent finding when one trial was removed one at time. In addition, forest plot also revealed no significant difference in minimum SpO2 (MD = 0.09%, 95% CI − 2.82 to 3.01, p = 0.95; I2 = 73%; 4 RCTs; n = 290) between the two groups (Fig. 4c)25,27,31,32. Sensitivity analysis indicated that the minimum SpO2 was lower in the HFNO group compared to that in the control group when one study27 was removed.
Figure 4.
Forest plot comparing (a) safe apnea time, (b) PaO2, and (c) minimum SpO2 between HFNO and control groups. HFNO, high-flow nasal oxygenation; IV, inverse variance; CI, confidence interval.
Subgroup analysis showed no significant impact of the choice of different approaches (i.e., COT or NIV) on PaO2 (p = 0.08) (Fig. 4b). For minimum SpO2, subgroup analysis revealed a significantly lower minimum SpO2 associated with the use of HFNO compared to that with NIV (MD = − 1.88%, 95% CI − 3.26 to − 0.51, p = 0.007; I2 = 0%; 2 RCTs; n = 191), while there was no difference between HFNO and COT (p = 0.15).
Secondary outcome: impact of HFNO on peri-procedural carbon dioxide level
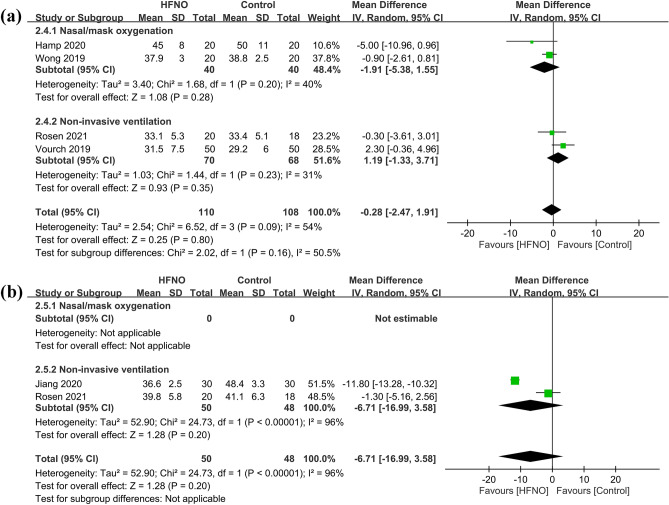
Our results demonstrated no significant difference in EtCO2 (MD = − 0.28 mmHg, 95% CI − 2.47 to 1.91, p = 0.8; I2 = 54%; 4 RCTs; n = 218) (Fig. 5a)26–28,32 between the HFNO and control groups. Sensitivity analysis verified a consistent finding when one trial was removed one at time. Forest plot showed no significant difference in the PaCO2 (MD = − 6.71%, 95% CI − 16.99 to 3.58, p = 0.2; I2 = 96%; 2 RCTs; n = 98) (Fig. 5b)26,30 between the HFNO and control groups. The limited availability of trials (i.e., only two) precluded the conduction of a sensitivity analysis of this outcome. Subgroup analysis indicated no significant impact of choosing different approaches of conventional oxygenation (i.e., COT or NIV) on EtCO2 (p = 0.16) (Fig. 5a).
Figure 5.
Forest plot comparing (a) EtCO2 and (b) PaCO2 between HFNO and control groups. HFNO, high-flow nasal oxygenation; IV, inverse variance; CI, confidence interval.
Secondary outcome: impact of HFNO on postprocedural respiratory parameters
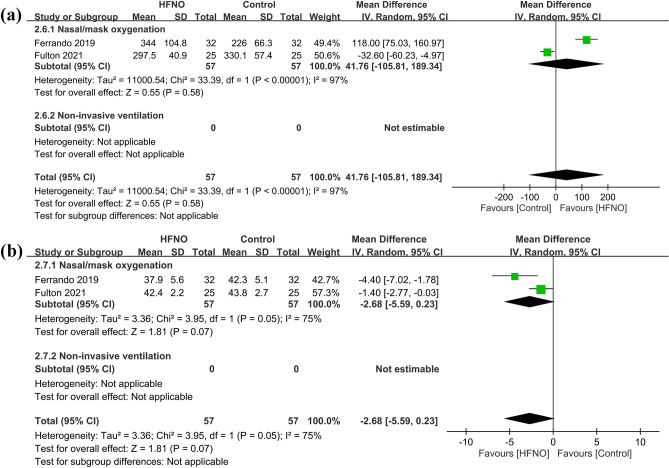
Two RCTs provided information on postprocedural respiratory parameters at three hours23,24. This time point was chosen based on the observation that patients undergoing bariatric surgery usually spend three hours in the postanesthesia care unit before discharge23. Forest plot revealed comparable respiratory parameters, namely, PaO2/FiO2 ratio (MD = 41.76, 95% CI − 105.81 to 189.34, p = 0.58; I2 = 97%; 2 RCTs; n = 114) (Fig. 6a) and PaCO2 (MD = − 2.68 mmHg, 95% CI − 5.59 to 0.23, p = 0.07; I2 = 75%; 2 RCTs; n = 114) between the HFNO and control groups (Fig. 6b). Sensitivity analysis was not performed because only two trials were available for outcome comparison.
Figure 6.
Forest plot comparing (a) PaO2/FiO2 ratio and (b) PaCO2 level between HFNO and control groups. HFNO, high-flow nasal oxygenation; IV, inverse variance; CI, confidence interval.
Discussion
Despite oxygen supplementation, patients with obesity may still experience significant hypoxemia after anesthesia-induced apnea because of a reduced FRC and an increased minute oxygen demand11 that highlight the importance of implementing appropriate postprocedural oxygenation strategy for improving patient safety. Accordingly, the current meta-analysis focused on a comparison between HFNO and COT/NIV in this particular patient population. Our meta-analysis demonstrated that the use of HFNO prolonged the apnea time without a beneficial impact on the risk of hypoxemia, minimum SpO2, PaO2, EtCO2, and PaCO2 in patients with obesity receiving peri-procedural oxygenation. After tracheal extubation, the application of HFNO was also not associated with an elevated PaO2/FiO2 ratio and a decreased PaCO2 level at postoperative three hours.
Although a previous meta-analysis showed that the use of HFNO could reduce the risk of hypoxemia in patients receiving sedation or anesthetic induction17, only one of the included trials recruited patients with obesity; therefore, its findings may not be applicable to patients with obesity. Indeed, our results did not support a superior beneficial effect of HFNO against hypoxemia compared to that in the control group. Consistently, the levels of PaO2 and minimum SpO2 were comparable between the two groups, indicating no significant association between the use of HFNO and a reduced risk of hypoxemia. Therefore, one of the striking clinical implications of the present study was that this patient population, who are at risk of hypoxemia, may not benefit from the use of HFNO. In concert with our finding, a closed claims analysis on the management of difficult tracheal intubation showed that a delay in alternative airway intervention and judgment errors may contribute to brain ischemia and mortality33. Besides, the use of HFNO may be associated with an elevated risk of delayed airway management (e.g., tracheal intubation)34 possibly because of a false sense of security that loosens the alert for potential airway problems. In this regard, we suggest that hypoxemia in patients with obesity receiving oxygen supplementation with HFNO should be promptly managed without exposing the patients to unnecessary risks.
A recent international multicenter trial comparing HFNO with standard facemask pre-oxygenation for rapid sequence induction in patients with a normal body build (i.e., mean BMI around 25 kg/m2)35 demonstrated no difference in the incidence of hypoxemia (i.e., SpO2 < 93%) between pre-oxygenation using HFNO or tight facemask. Our findings were consistent with those in that study35. The lack of efficacy of HFNO for the prevention of hypoxemia compared to COT/NIV may be attributable to inadequate positive airway pressure associated with HFNO. First, although a previous study suggested the need for an adequate airway patency to achieve effective oxygenation32, the limited positive airway pressure generated by HFNO (e.g., 2.7 cmH2O) may be unable to relieve airway obstruction after anesthesia or sedation in patients with obesity36. Moreover, although a previous study has demonstrated a positive correlation between the flow rate of HFNO and nasopharyngeal pressure, which could reach over 3 cmH2O at a flow rate of 50 L/min37, whether a higher flow could improve the risk of hypoxemia in patients with obesity remains unclear. In the current study, there were four trials that provided the outcome of hypoxemia. While three of the trials25,31,32 used a flow rate of 60 L/min, the other28 adopted a flow rate of 120 L/min. Despite the obvious difference, our sensitivity analysis demonstrated that removal of the study using a higher flow rate28 had no significant impact on the risk of hypoxemia. Nevertheless, since the number of trials included in the current meta-analysis was relatively small to arrive at a robust conclusion. Second, maintenance of an adequate FRC and avoidance of alveolar collapse is also important for efficient oxygenation32. Although a previous small-scale study with 20 participants reported an increased lung volume and FRC as another potential benefit of HFNO particularly in patients with higher BMIs38, that study included only two patients with BMI > 40 kg/m2. In contrast, all of our included studies focused on patients with BMI > 30 kg/m2. Therefore, our findings implicated that the low-level positive airway pressure generated by HFNO may not be able to increase the lung volume in our patient population.
Apart from the lack of a beneficial influence of HFNO on hypoxemia, the present study also showed no positive impact of HFNO on peri-procedural CO2 clearance. Although a previous study demonstrated that the enhanced CO2 clearance associated with the use of HFNO may be flow-dependent39, our results (flow: 50–120 L/min) and those of a recent study (flow: 70 L/min)35 did not support this finding that the use of HFNO was associated with a low CO2 clearance. Regarding the impact of HFNO on postprocedural CO2 clearance, our results were derived from two trials that recruited patients undergoing laparoscopic operations in which CO2 was used for abdominal CO2 insufflation. Although CO2 clearance may be modified by anesthesiologists immediately after laparoscopic surgery, the present study focused on postoperative three hours so that such an impact would be minimal. Nevertheless, our findings may not be extrapolated to patients receiving non-laparoscopic procedures. Further studies are needed to address this issue.
One recent meta-analysis of three clinical trials enrolling 160 patients with or without obesity reported a safe extension of apnea time by 33.4 s in participants receiving HFNO versus those subjected to COT at anesthesia induction17. In spite of the demonstration of a HFNO-associated prolongation of safe apnea time compared to COT in the current study, the finding should be interpreted with caution. First, in spite of our finding of a significant prolongation of safe apnea time, the result was based on two trials enrolling only 80 patients27,28. Second, a prolongation of merely 73 s may not be of clinical significance in patients with obesity who usually present with a difficult airway9,10. Third, notwithstanding the inclusion of patients with similar BMI and age, there were wide variations between the two studies27,28 in mean apnea time in both the HFNO (i.e., 581.328 vs. 261.427 s) and COT (i.e., 589.728 vs. 185.527 s) groups. Because of the heterogeneity, more studies are required to explore the efficacy of HFNO for prolonging safe apnea time. Overall, our results are in line with those of a recent study35 that reported comparable incidences of hypoxemia between patients with or without HFNO despite a prolonged safe apnea in those receiving HFNO35.
In patients undergoing bariatric surgery, the prevalence of postoperative atelectasis could be as high as 37%40. Not only does obesity predispose to postoperative atelectasis but atelectasis in this patient population also resolves more slowly than in those with normal body build41,42. Despite the recommendation of oxygen supplementation in patients at high risk of postoperative atelectasis43, we found that the use of HFNO was unable to improve oxygenation parameters in patients with obesity both during the periprocedural period and at three hours after surgery. Consistent with our findings, another meta-analysis investigating patients with obesity undergoing cardiac surgery demonstrated no significant improvements in atelectasis score, dyspnea score, PaO2/FiO2 ratio, and reintubation rate in patients receiving HFNO compared with those undergoing COT44. The lack of benefits of HFNO in patients with obesity during the postoperative period underscores the need for timely intervention (e.g., reintubation) in case of respiratory distress after tracheal extubation.
Our study had several limitations. First, the limited number of trials not only blemished the reliability of our findings but also precluded our analysis of some clinical outcomes such as the risk of atelectasis or hypoxemia-associated complications. Second, the heterogeneity across the included trials in study settings (e.g., anesthetic induction vs. sedation, intensive care unit vs. operating theater) and approaches (i.e., COT or NIV) may introduce bias to our results. Nevertheless, a comparison between HFNO and other widely accepted clinical approaches to oxygen supplementation (e.g., COT and NIV) is still of clinical significance. Third, our findings on the association between HFNO and conventional respiratory parameters may not reflect clinical outcomes. Fourth, all included studies in the current meta-analysis investigated patients with BMI > 30 kg/m2, the efficacy of HFNO against hypoxemia in patients with less severe obesity remains to be elucidated.
Conclusion
The current study showed that, compared with conventional oxygen therapy or non-invasive ventilation, the use of high-flow nasal oxygenation was unable to provide additional peri- or post-procedural respiratory benefits for patients with obesity (i.e., BMI > 30 kg/m2) except for a prolongation of safe apnea time. Further large-scale studies are warranted to validate our findings.
Supplementary Information
Author contributions
Conceptualization and literature search: K.C.H. and C.C.K.; methodology: C.C.K.; Trial selection: K.F.W. and P.C.C.; Data analysis: I.C.T.; Data extraction: I.C.T. and C.H.L.; Writing—original draft preparation: K.C.H. and P.W.H.; Writing—review and editing: K.C.H. and C.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kuo-Chuan Hung and Ching-Chung Ko.
These authors jointly supervised this work: Ping-Wen Huang and Cheuk-Kwan Sun.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-10396-5.
References
- 1.Bouroche G, Bourgain JL. Preoxygenation and general anesthesia: a review. Minerva Anestesiol. 2015;81:910–920. [PubMed] [Google Scholar]
- 2.Kung MC, Hung CT, Ng KP, Au TK, Lo R, Lam A. Arterial desaturation during induction in healthy adults: should preoxygenation be a routine? Anaesth. Intensive Care. 1991;19:192–196. doi: 10.1177/0310057X9101900206. [DOI] [PubMed] [Google Scholar]
- 3.Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: physiologic basis, benefits, and potential risks. Anesth. Analg. 2017;124:507–517. doi: 10.1213/ANE.0000000000001589. [DOI] [PubMed] [Google Scholar]
- 4.Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2013;118:251–270. doi: 10.1097/ALN.0b013e31828604c6. [DOI] [PubMed] [Google Scholar]
- 5.Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br. J. Anaesth. 2015;115:827–848. doi: 10.1093/bja/aev371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weingart SD. Preoxygenation, reoxygenation, and delayed sequence intubation in the emergency department. J. Emerg. Med. 2011;40:661–667. doi: 10.1016/j.jemermed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Benumof JL. Preoxygenation: best method for both efficacy and efficiency. Anesthesiology. 1999;91:603–605. doi: 10.1097/00000542-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson LI. The effects of residual neuromuscular blockade and volatile anesthetics on the control of ventilation. Anesth. Analg. 1999;89:243–251. doi: 10.1213/00000539-199907000-00045. [DOI] [PubMed] [Google Scholar]
- 9.Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–1236. doi: 10.1097/00000542-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Juvin P, Lavaut E, Dupont H, et al. Difficult tracheal intubation is more common in obese than in lean patients. Anesth. Analg. 2003;97:595–600. doi: 10.1213/01.ANE.0000072547.75928.B0. [DOI] [PubMed] [Google Scholar]
- 11.Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth. Analg. 1998;87:654–660. doi: 10.1213/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO. Obesity and cardiovascular diseases. Minerva Med. 2017;108:212–228. doi: 10.23736/S0026-4806.17.05022-4. [DOI] [PubMed] [Google Scholar]
- 13.Laporta ML, Sprung J, Weingarten TN. Respiratory depression in the post-anesthesia care unit: Mayo clinic experience. Bosn. J. Basic Med. Sci. 2021;21:221–228. doi: 10.17305/bjbms.2020.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Yin H, Zhang R, Ye X, Wei J. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients after planned extubation: a systematic review and meta-analysis. Crit. Care. 2019;23:180. doi: 10.1186/s13054-019-2465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Yin H, Zhang R, Wei J. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients with acute respiratory failure: a systematic review and meta-analysis of randomized controlled trials. BMC Pulm. Med. 2017;17:201. doi: 10.1186/s12890-017-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Yin H, Zhang R, Wei J. High-flow nasal cannula oxygen therapy vs conventional oxygen therapy in cardiac surgical patients: a meta-analysis. J. Crit. Care. 2017;38:123–128. doi: 10.1016/j.jcrc.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Spence EA, Rajaleelan W, Wong J, Chung F, Wong DT. The effectiveness of high-flow nasal oxygen during the intraoperative period: a systematic review and meta-analysis. Anesth. Analg. 2020;131:1102–1110. doi: 10.1213/ANE.0000000000005073. [DOI] [PubMed] [Google Scholar]
- 18.de Raaff CAL, Gorter-Stam MAW, de Vries N, et al. Perioperative management of obstructive sleep apnea in bariatric surgery: a consensus guideline. Surg. Obes. Relat. Dis. 2017;13:1095–1109. doi: 10.1016/j.soard.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Akbas S, Ozkan AS. Comparison of effects of low-flow and normal-flow anesthesia on cerebral oxygenation and bispectral index in morbidly obese patients undergoing laparoscopic sleeve gastrectomy: a prospective, randomized clinical trial. Wideochirurgia Inne Tech. Maloinwazyjne. 2019;14:19–26. doi: 10.5114/wiitm.2018.77265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331:1064–1065. doi: 10.1136/bmj.38636.593461.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassar M, Atakpo P, Kash MJ. Manual search approaches used by systematic reviewers in dermatology. J. Med. Libr. Assoc. 2016;104:302–304. doi: 10.3163/1536-5050.104.4.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrando C, Puig J, Serralta F, et al. High-flow nasal cannula oxygenation reduces postoperative hypoxemia in morbidly obese patients: a randomized controlled trial. Minerva Anestesiol. 2019;85:1062–1070. doi: 10.23736/S0375-9393.19.13364-0. [DOI] [PubMed] [Google Scholar]
- 24.Fulton R, Millar JE, Merza M, et al. Prophylactic postoperative high flow nasal oxygen versus conventional oxygen therapy in obese patients undergoing bariatric surgery (OXYBAR study): a pilot randomised controlled trial. Obes. Surg. 2021;13:13. doi: 10.1007/s11695-021-05644-y. [DOI] [PubMed] [Google Scholar]
- 25.Riccio CA, Sarmiento S, Minhajuddin A, Nasir D, Fox AA. High-flow versus standard nasal cannula in morbidly obese patients during colonoscopy: a prospective, randomized clinical trial. J. Clin. Anesth. 2019;54:19–24. doi: 10.1016/j.jclinane.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Rosen J, Frykholm P, Fors D. High-flow nasal cannula versus face mask for preoxygenation in obese patients: a randomised controlled trial. Acta Anaesthesiol. Scand. 2021;26:26. doi: 10.1111/aas.13960. [DOI] [PubMed] [Google Scholar]
- 27.Wong DT, Dallaire A, Singh KP, et al. High-flow nasal oxygen improves safe apnea time in morbidly obese patients undergoing general anesthesia: a randomized controlled trial. Anesth. Analg. 2019;129:1130–1136. doi: 10.1213/ANE.0000000000003966. [DOI] [PubMed] [Google Scholar]
- 28.Hamp T, Prager G, Baron-Stefaniak J, Muller J, Bichler C, Plochl W. Duration of safe apnea in patients with morbid obesity during passive oxygenation using high-flow nasal insufflation versus regular flow nasal insufflation, a randomized trial. Surg. Obes. Relat. Dis. 2020 doi: 10.1016/j.soard.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Heinrich S, Horbach T, Stubner B, Prottengeier J, Irouschek A, Schmidt J. Benefits of heated and humidified high flow nasal oxygen for preoxygenation in morbidly obese patients undergoing bariatric surgery: a randomized controlled study. J. Obes. Bariatr. 2014;1:7. [Google Scholar]
- 30.Jiang W, Shi L, Zhao Q, et al. Ultrasound assessment of gastric insufflation in obese patients receiving transnasal humidified rapid-insufflation ventilatory exchange during general anesthesia induction. [Chinese] Nan fang yi ke da xue xue bao = J. South. Med. Univ. 2020;40:1543–9. doi: 10.12122/j.issn.1673-4254.2020.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez M, Ragot S, Coudroy R, et al. Noninvasive ventilation vs. high-flow nasal cannula oxygen for preoxygenation before intubation in patients with obesity: a post hoc analysis of a randomized controlled trial. Ann. Intensive Care. 2021;11:1–14. doi: 10.1186/s13613-021-00892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vourc'h M, Baud G, Feuillet F, et al. High-flow nasal cannulae versus non-invasive ventilation for preoxygenation of obese patients: the PREOPTIPOP randomized trial. EClinicalMedicine. 2019;13:112–119. doi: 10.1016/j.eclinm.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joffe AM, Aziz MF, Posner KL, Duggan LV, Mincer SL, Domino KB. Management of difficult tracheal intubation: a closed claims analysis. Anesthesiology. 2019;131:818–829. doi: 10.1097/ALN.0000000000002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am. J. Respir. Crit. Care Med. 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 35.Sjöblom A, Broms J, Hedberg M, et al. Pre-oxygenation using high-flow nasal oxygen vs. tight facemask during rapid sequence induction. Anaesthesia. 2021;76:1176–83. doi: 10.1111/anae.15426. [DOI] [PubMed] [Google Scholar]
- 36.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br. J. Anaesth. 2009;103:886–890. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir. Care. 2013;58:1621–1624. doi: 10.4187/respcare.02358. [DOI] [PubMed] [Google Scholar]
- 38.Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br. J. Anaesth. 2011;107:998–1004. doi: 10.1093/bja/aer265. [DOI] [PubMed] [Google Scholar]
- 39.Hermez LA, Spence CJ, Payton MJ, Nouraei SAR, Patel A, Barnes TH. A physiological study to determine the mechanism of carbon dioxide clearance during apnoea when using transnasal humidified rapid insufflation ventilatory exchange (THRIVE) Anaesthesia. 2019;74:441–449. doi: 10.1111/anae.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baltieri L, Peixoto-Souza FS, Rasera-Junior I, Montebelo MI, Costa D, Pazzianotto-Forti EM. Analysis of the prevalence of atelectasis in patients undergoing bariatric surgery. Braz. J. Anesthesiol. 2016;66:577–582. doi: 10.1016/j.bjan.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Damia G, Mascheroni D, Croci M, Tarenzi L. Perioperative changes in functional residual capacity in morbidly obese patients. Br. J. Anaesth. 1988;60:574–578. doi: 10.1093/bja/60.5.574. [DOI] [PubMed] [Google Scholar]
- 42.Eichenberger A, Proietti S, Wicky S, et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth. Analg. 2002;95:1788–1792. doi: 10.1097/00000539-200212000-00060. [DOI] [PubMed] [Google Scholar]
- 43.Hedenstierna G, Edmark L. Mechanisms of atelectasis in the perioperative period. Best Pract. Res. Clin. Anaesthesiol. 2010;24:157–169. doi: 10.1016/j.bpa.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Zhu J, Wang X, et al. Comparison of high-flow nasal cannula (HFNC) and conventional oxygen therapy in obese patients undergoing cardiac surgery: a systematic review and meta-analysis. In Vivo. 2021;35:2521–2529. doi: 10.21873/invivo.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.