Abstract
Falls are a common cause of injury in older adults (OAs), and age-related declines across the sensory systems are associated with increased falls risk. The vestibular system is particularly important for maintaining balance and supporting safe mobility, and aging has been associated with declines in vestibular end-organ functioning. However, few studies have examined potential age-related differences in vestibular perceptual sensitivities or their association with postural stability. Here we used an adaptive-staircase procedure to measure detection and discrimination thresholds in 19 healthy OAs and 18 healthy younger adults (YAs), by presenting participants with passive heave (linear up-and-down translations) and pitch (forward–backward tilt rotations) movements on a motion-platform in the dark. We also examined participants’ postural stability under various standing-balance conditions. Associations among these postural measures and vestibular perceptual thresholds were further examined. Ultimately, OAs showed larger heave and pitch detection thresholds compared to YAs, and larger perceptual thresholds were associated with greater postural sway, but only in OAs. Overall, these results suggest that vestibular perceptual sensitivity declines with older age and that such declines are associated with poorer postural stability. Future studies could consider the potential applicability of these results in the development of screening tools for falls prevention in OAs.
Subject terms: Psychology, Human behaviour, Sensory processing
Introduction
Falls are the most common cause of fatal and non-fatal injuries in adults over the age of 65 years1,2 and age-related declines across sensory systems (e.g., vision, hearing, proprioception, vestibular) contribute to an increased risk of falls. The vestibular system is particularly important for maintaining balance and supporting safe mobility3,4. It is well established that vestibular functioning changes with age as evidenced by physiological changes and changes to peripheral vestibular end-organ measures5–7. There are also well-known age-related behavioral changes, such as declines in postural control, that occur with older age8–14. However, much less is known about age-related changes to vestibular perception (but see 15 for a review) and how vestibular perceptual abilities are associated with postural stability in older adults16–21. In this study, we examine age-related differences in vestibular detection and discrimination thresholds during passive heave and pitch movements in the dark, and explore how these percepts are associated with posturography measures (i.e., center of pressure path length, velocity, root-mean-squared velocity).
Age-related changes in vestibular end-organ structure and functioning
The vestibular system comprises five distinct organs: three semicircular canals that detect rotational motion in all three axes (yaw, pitch, and roll), as well as two otolith organs that detect linear acceleration and gravity in the vertical or heave axis (saccule), and in the horizontal, or surge and sway axes (utricular, and some saccular)22. These vestibular end organs send sensory information to the brain through the vestibulocochlear nerve, which informs the perception of self-motion23 for a review).
Age-related changes to the vestibular system have been evaluated by examining the vestibular end organs histologically and microscopically24–26 (but see 5–7,27 for reviews). These studies have demonstrated that aging is associated with declines in end organ integrity, including deterioration of the otoconia in the otolith organs, particularly in the utricle28 and a loss of Type I29 and Type II hair cells24,29–32, with the semicircular canals being particularly susceptible to Type I hair cell loss32,33. There is also evidence of degeneration of the vestibular ganglion with aging34,35, especially the superior vestibular nerve36 which relays afferent superior (i.e., pitch) and lateral (i.e., yaw) semicircular canal information to the brain.
Within clinical settings, saccular, utricular, and semicircular canal functioning are often assessed using cervical vestibular-evoked myogenic potentials (cVEMP), ocular VEMPs (oVEMP) and video head impulse testing (vHIT), respectively. Reported age-related changes to these measures include decreased cVEMP amplitudes37,38 and increased oVEMP latencies39, which suggest decline in vestibular functioning. There is also evidence of slightly lower vHIT gains in older adults over 85 years of age compared to younger adults37,40,41, although many studies also show that vHIT is resistant to age-related effects42,43. Less, however, is understood about how age-related changes to end-organ functioning, as measured by VEMP and vHIT, are associated with age-related changes in self-motion perception or vestibularly-informed behaviors such as balance and postural control44–47.
Age-related changes in postural control and vestibular perception
A wealth of previous research has characterized age-related changes to postural control48–52. For instance, older adults demonstrate poorer standing balance (e.g., longer center of pressure, or COP, path lengths and greater sway velocity) during static posturography tasks compared to younger adults, particularly with eyes closed9, while standing on compliant or unstable surfaces11,53, or during balance perturbations (e.g., 54).
During passive movements in the dark, self-motion perception is thought to be largely informed through vestibular inputs. Under such conditions younger adults have demonstrated an ability to detect, estimate, and discriminate rotational velocity17,55, linear heading direction20,56–60, distance travelled59,61–63, and target-relative spatial updating64–69. There is emerging, but limited evidence suggesting that vestibular perception changes with older age. For instance several studies have shown that, compared to younger adults, older adults demonstrate higher direction-discrimination thresholds for sway, heave, and roll-tilt15,16,18,19,70,71 but not yaw16,70. These studies have also shown that younger and older adults do not demonstrate differences in their ability to detect movements in yaw, or in their ability discriminate between two passively-applied yaw rotations17. Greater age-related differences in direction-discrimination thresholds have been reported for linear movements compared to rotational movements16. No studies to our knowledge, however, have examined age-related perceptual changes in the pitch direction, although previous studies have suggested that older adults may have a biased perception of verticality (with “backward disequilibrium syndrome”) and exhibit a backwards-tilted bias in their subjective postural vertical, when compared to healthy control participants72–74. As such, examining pitch perception in older adults may be particularly informative given that forward-tilt detection has been described as a potential predictor of falls risk in older adults75,76. Likewise, falls and recovery from falls, especially in older adults, may be associated with motion in the z-plane, as elevating and lowering strategies are the most common strategies used to recover from a fall due to tripping75–78. Therefore, we also investigated the extent to which there may be age-related differences in the ability to detect, and discriminate between, vertical linear (i.e., heave) motions.
Furthermore, it might be expected that less sensitive vestibular perception may have negative implications for behaviors that are informed by self-motion perception, such as standing balance. Yet, few studies16,18,19 have examined the extent to which age-related changes in balance and postural control are associated with measures of vestibular self-motion perception. It has recently been shown that in younger and middle-aged adults, different measures of COP path length are related to vestibular perceptual thresholds for many translational and rotational movements79. The results showed that vestibular perceptual thresholds in the lateral plane were positively associated with COP path length. In older adults specifically, some research has indicated that higher roll-tilt thresholds are associated with a greater risk of failing the most difficult condition of a Romberg Balance Test (i.e., quiet stance on a compliant surface with eyes closed16,18,19). While failure to successfully complete this type of balance test is an important indicator of poor postural control, the binary nature of “pass” and “fail” tasks could conceal subtler differences or declines in postural stability which may, nonetheless, be important predictors of mobility or falls risk80–83. Higher resolution spatial and temporal measures of sway could allow for the examination of finer differences in postural control which may not be severe enough to cause full balance failures. It would also provide the opportunity to relate such differences to vestibular perceptual thresholds in older and younger adults.
To our knowledge, associations between vestibular perceptual thresholds (e.g., direction discrimination, detection, magnitude-discrimination) and more precise spatial or temporal features of postural sway (e.g., COP path length and velocity) have not been examined in healthy older adults. In general, better characterizing vestibular perceptual sensitivities of older adults across a range of motion types and axes, as well as understanding how these perceptual abilities are associated with high resolution measures of posture could help to clarify the extent to which age-related declines in vestibular function (e.g., presbyvestibulopathy84) contribute to balance problems and falls risk.
Current study
In this study, we measured peripheral vestibular end-organ functioning using vHIT and VEMPs in older adults, as well as behavioral balance functioning (posturography during quiet standing) and vestibular perception (two-interval detection and magnitude discrimination thresholds) in younger and older adults. We also examined associations between perceptual thresholds and posturography measures in each age group. Specifically, by passively moving participants using a 6 degrees-of-freedom motion platform we measured movement detection and magnitude discrimination thresholds during heave translation, which stimulates the saccule, and during pitch rotation, which stimulates both anterior and posterior canals, as well as the saccule and utricle. We also used a static posturography task to assess postural stability and performed a series of exploratory correlations between vestibular perceptual thresholds and posturography measures for each age group.
Methods
Participants
19 healthy older adults (Mage = 70.47 years, SD = 5.64, range = 65–89 years, 11 females, 8 males) and 18 younger adults (Mage = 26.00 years, SD = 4.27, range = 20–34 years, 13 females, 5 males) completed the study. All participants gave written informed consent. A subset of the older adult participants were included as a control sample in Gabriel et al.85. Participants were recruited from the community using posters, social media posts, websites, and through an existing participant database. These individuals were eligible to participate if they did not have a history of stroke, seizure, disabling musculoskeletal disorder, acute psychiatric disorder, dementia, mild cognitive impairment, clinically diagnosed vestibular disorders (e.g., Meniere’s disease), hearing loss, or if they were unable to provide informed consent. All older adults obtained above cut-off scores on the Montreal Cognitive Assessment for mild cognitive impairment (MoCA; i.e., 26 points86). All methods in this study were approved by the University Health Network’s Research Ethics Board (Protocol #: 18-6123.0), the University of Toronto Research Ethics Board (Protocol #: 00037394), and The Hospital for Sick Children Research Ethics Board (Protocol #: 1000056920).
Baseline assessment session tests
Older adult participants first underwent a series of baseline sensory (i.e., hearing and vestibular), cognitive, and balance assessments (Table 1), each described in detail in the following sections.
Table 1.
Summary of baseline assessments measured in the older adult participants.
| Baseline measure | M (SD) |
|---|---|
| Hearing | |
| PTA thresholda (dB HL) | 11.29 (5.63) |
| Cognition | |
| MoCAb (/30 total) | 27.39 (1.46) |
| Vestibular end-organ | |
| vHITc (total n) | 10 |
| vHIT (right ear) | 0.96 (0.19) |
| vHIT (left ear) | 0.89 (0.15) |
| cVEMPd (total n) | 14 |
| cVEMP (present, right ear, n) | 87% |
| cVEMP (present, left ear, n) | 67% |
| oVEMPe (n) | 14 |
| oVEMP (present, right ear, n) | 27% |
| oVEMP (present, left ear, n) | 27% |
| Balance | |
| ABCf (/100%) | 94.82 (5.15) |
| Fell in the last year (n) | 3 |
| Near fall(s) in last year (n) | 2 |
| Fear of falling (n) | 2 |
aPTA = Pure Tone Average; frequencies tested: 500, 1000, 2000, and 4000 Hz, inclusive, with a cut-off threshold above 25 dB HL.
bMoCA = Montreal Cognitive Assessment (max score = 30; clinical cut off ≤ 26 pts).
cvHIT = Video Head Impulse Test. 9 participants were not able to come back to complete this session. Median gain for 60 ms reported. One participant obtained a median gain below the 0.7 cut off score at 60 ms (they obtained 0.67, in the left ear).
dcVEMP = 5 participants were not able to come back to complete this session. Cervical Vestibular Evoked Myogenic Potential.
eoVEMP = 5 participants were not able to come back to complete this session. Ocular Vestibular Evoked Myogenic Potential.
fABC = Activities-specific Balance Confidence Scale (max score = 100%).
Hearing
Given that declines in vestibular functioning may be associated with age-related hearing loss85,87–92 older adult participants were screened for hearing abilities. Audiometric testing was completed as per guidelines established by the International Organization of Standardization (ISO; 93). Pure-tone audiometry was used to determine audiometric hearing thresholds using a Grason-Stadler 61 Clinical Audiometer (GSI-61; Grason-Stadler Inc., Eden Prairie, MN) and Telephonics TDH-50P headphones (Telephonics Corporation, Farmindale, NY). Testing was performed in a double-walled sound-attenuating booth (Industrial Acoustics Company, Inc., New York, NY). Frequencies tested were between 250 and 8000 Hz, inclusive. Binaural pure-tone audiometric (PTA) thresholds below 25 dB HL, when averaged across the 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz frequencies, were considered normal. Three older adult participants could not come into the lab to have their hearing tested, but these older adults had self-reported normal hearing and their vestibular threshold and posturography data did not differ significantly from the rest of the older adults.
Cognition
Mild cognitive impairment was screened for using the MoCA. The MoCA is a rapid test designed to screen individuals for mild cognitive impairment. The test assesses general cognitive abilities, by examining several domains of cognitive functioning including attention, executive function, memory, and language and is scored out of a total of 30 points. In this study, level-of-education adjusted scores are reported and all participants obtained a score of 26 or higher (common cut-off for mild cognitive impairment).
Vestibular
Vestibular end-organ functioning was assessed using vHIT and VEMPs to measure semicircular canal and otolith organ functioning respectively. vHIT assesses semicircular canal (in this case lateral canals) functioning by measuring (and here, reporting) participants’ vestibulo-ocular reflex (VOR). A gain less than 0.7 suggests impaired semicircular canal functioning42,94–96.
VEMPs are a measure of otolith organ functioning that exploit the sound-sensitive fibers contained in the saccule and utricle97. cVEMPs were scored as “present”, indicating normal otolith function, if activity presented a P1 peak at 10–25 ms, followed by an N1 trough at 20–40 ms. Absence of this peak was coded as “absent” and indicated potential dysfunction of the otolith organs. oVEMPs were coded as present if the test demonstrated an N1 at 8–20 ms, followed by a P1 between 15 and 30 ms. These latency ranges were based on the possibility that peak latencies may change with older age7,98,99.
Details regarding vHIT and VEMP testing procedures can be found in the online Supplementary Information S.1.
Balance
Self-reported balance function during day-to-day tasks was measured using the Activities-specific Balance Confidence (ABC) scale100. Excellent perceived balance was scored by participants on each item as 100%, and very poor subjective balance as 0%. Posturography tasks were also used to assess standing balance during the experimental session (see details below).
Demographics and health history questionnaire
A questionnaire recording the participants’ demographics and medical background was administered. Items included questions regarding education, dizziness, history or presence of vestibular disorders, fear of falls, history of falls, smoking and drinking habits, subjective cognitive decline, heart disease and other vascular or neurological health problems.
Experimental session
Vestibular psychophysics task
Stimuli and apparatus
The vestibular psychophysics tasks were performed within the KITE—Toronto Rehabilitation Institute’s Challenging Environment Assessment Laboratory (CEAL). CEAL contains a 6.0 m × 5.6 m × 4.1 m enclosed laboratory mounted on a 6-degrees-of-freedom hexapod motion base (i.e., capable of moving in all linear directions and rotating around pitch, yaw, and roll axes), with 60″ actuator arms allowing tilting up to 100 °/s2 in the pitch axis, and 8 m/s2 in the heave direction (see Fig. 1).
Figure 1.
Vestibular psychophysics setup. Schematic of the laboratory setup for the psychophysical task including the 6-degrees-of-freedom motion platform.
For this study, the laboratory was outfitted with a specially constructed chair designed to minimize participants’ head and body movements. The chair was cushioned with foam to reduce vibrotactile feedback. Participants were secured in their seats by means of a four-point harness and had their feet resting on foam mats at the base of the chair to restrict leg-movement and to also reduce vibrotactile cues to the feet during the task. A neck pillow was used to further limit proprioceptive feedback through incidental movement of the head or neck. Finally, participants were blindfolded and wore noise-cancelling headphones that presented white-noise throughout each block to limit the sound created by the hydraulics of the motion base. Lights were also dimmed inside the lab for the duration of the experiment. The experimenter sat inside the lab with the participants but communicated with them through a microphone feeding into the headphones. The goal of this setup was to reduce as many sensory cues to motion as possible to isolate information about the passive movements to those arising primarily from the vestibular system.
Movement specifications
There were four conditions in the main psychophysical task: heave detection, heave discrimination, pitch detection, and pitch discrimination. The point of rotation for pitch movements was at the approximate center of the head. Each trial consisted of (1) a standard movement and (2) a comparison movement. For detection, the platform remained stationary during the “standard movement” (see Table 2 for movement specifications). Magnitudes are stated as peak accelerations for both heave (m/s2) and pitch (°/s2) motions.
Table 2.
Initial peak accelerations used for the psychophysical tasks.
| Movement type | Detection task | Discrimination task |
|---|---|---|
| Heave |
Standard movement = 0 m/s2 Initial comparison movement = 0.5 m/s2 |
Standard movement = 1.0 m/s2 Initial comparison movement = 1.5 m/s2 |
| Pitch |
Standard movement = 0 °/s2 Initial comparison movement = 3 °/s2 |
Standard movement = 20 °/s2 Initial comparison movement = 26 °/s2 |
The initial comparison movements in this table represent the initial acceleration values. These values changed throughout the session as a function of the PEST (Parametric Estimation by Sequential Testing) procedure outlined in the text.
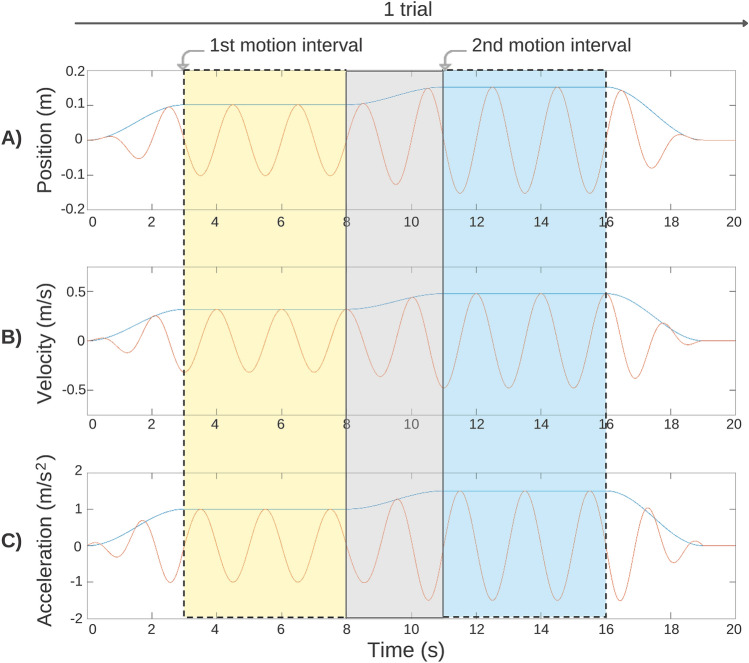
Each full trial consisted of a standard movement and a comparison movement presented in a random order101. The movements all followed the same profile. The platform was oscillated at 0.5 Hz either in pitch or heave, beginning at rest in a central position with the participant sitting upright. The platform was then oscillated sinusoidally around this position with a peak velocity that increased along a raised cosine velocity envelope reaching the desired value after three seconds. The platform then oscillated with this peak velocity, and the corresponding peak accelerations, for five seconds (Fig. 2, yellow shaded area). The peak velocity then changed in magnitude along a raised cosine velocity envelope for three seconds (Fig. 2, grey shaded area) and then oscillated with a second peak velocity and acceleration for another five seconds (i.e., the second movement of the trial; Fig. 2, blue shaded), before returning back to rest for three seconds. The motion base then rested for one second, or until the participant made their response. Each complete trial lasted approximately 20 s (see Fig. 2 for an example of a full heave discrimination trial).
Figure 2.
Diagrammatic representation of a single heave discrimination trial. (A) the position (m) relative to the upright start position, (B) the velocity (m/s) and (C) the acceleration (m/s2) of the motion base. For pitch trials, displacement was measured in degrees, velocity in °/s, and acceleration in °/s2. The yellow shaded area highlights the first movement (5 s; here, standard movement), the grey shaded area represents the fade-in between the first and second movement (3 s), and the blue region represents the second movement (5 s; here, comparison movement). The unshaded white regions represent the fade from no motion to the first movement (3 s), or from the second movement to no motion (3 s), and rest (1 s).
Procedures
Participants completed both a two-interval detection task and a two-interval magnitude discrimination task for each of the two motion types—pitch and heave—resulting in four psychophysical conditions in total. The four conditions (heave detection, heave discrimination, pitch detection, pitch discrimination) were presented in a random order across participants to protect against effects of carryover, practice, or fatigue across conditions. Breaks were provided on an as-needed basis. In total, these four conditions took approximately one hour per participant to complete. Participants also completed a posturography task (described below) to assess their standing balance immediately before completing the vestibular perceptual psychophysics task.
Detection Each individual trial in the detection condition was comprised of two intervals: (1) a motion interval (in pitch or heave, depending on the condition) and (2) a no motion interval. The order of these two motion intervals were randomized across trials within a condition. After both intervals were presented (demarcated by a spoken “one” or “two”, via the headphones, at the point of the peak acceleration), participants were asked to state out loud which of the two intervals was the one in which they had moved (“one” or “two”). The acceleration of the motion presented was varied using a Parametric Estimation by Sequential Testing procedure (PEST102) until the participant’s detection threshold was reached. PEST is an adaptive staircase procedure that uses a set of rules to converge on perceptual thresholds corresponding quickly and efficiently to where participants were 70.7% correct, in this instance103. To vary the values presented logarithmically, the base-10 logarithms of the acceleration values (beginning with the initial comparison movement value; see Table 2) were used by the PEST and the PEST’s log output was exponentiated into peak acceleration values before being fed to the platform’s motors.
Using the logged acceleration values, the PEST decreased the magnitude of the subsequent trial by a single “step” (defined below) if the participant responded correctly on two consecutive trials. If they responded incorrectly once, the value would increase by one step. The initial step sizes were log (0.1) for the heave condition and log (0.2) for the pitch condition. On the third step in the same direction, the size of the step doubled. This was unless the third step in the same direction was before the last reversal, in which case the rule was to wait until the fourth step. A change in direction (e.g., going from an increase to a decrease in peak acceleration, or step) represented a “reversal”. Eight reversals or 60 trials, whichever came first, signaled the end of a block of trials (i.e., vestibular threshold reached). The 70.7% correct threshold was calculated by averaging the accelerations of the last three reversals103.
Discrimination As in the detection condition, the discrimination condition used a similar PEST procedure to determine participants’ movement discrimination thresholds, except with the PEST being applied to the delta relative to the standard movement rather than the comparison amplitude. Participants were required to discriminate (i.e., report which motion felt larger) between two sequentially presented movements of different magnitudes: (1) a standard movement in pitch or heave and (2) a comparison movement, also in pitch or heave. These two movement intervals were separated by a sound file comprised of a spoken “one” or “two” presented through their headphones. Peak accelerations of the comparison movement interval were determined via the above-described PEST procedure using the same initial step sizes and the same termination criteria. The 70.7% correct discrimination thresholds were calculated by averaging the accelerations of the last three reversals103.
Posturography task
Participants also completed a posturography task to assess their standing balance. In this task, participants stood in parallel pose (i.e., feet facing forward, approximately 8″ apart) for 30 s104 on a forceplate (AMTI MSA-6 MiniAmp strain gage amplifier) which captured their center of pressure (COP) path length (cm) and velocity (cm/s). Signals from the forceplate were collected at a sampling rate of 1000 Hz. This was completed for four different trial types: (1) eyes open standing directly on the forceplate (EOF; “firm surface”), (2) eyes open on a piece of high-density foam placed on the forceplate (EOC; “compliant surface”; AIREX, Balance-Pad; 50 × 41 × 6 cm; density = 55 kg/m2), (3) eyes closed on a compliant surface (ECC), (4) eyes closed on a compliant surface while wearing noise-cancelling headphones for sound suppression (ECSS). Participants wore a loose harness during the procedure to protect against falls.
Once collected, the first five seconds of the data were discarded104. The remaining data were passed through a 2nd order zero-lag dual-pass Butterworth filter with a 6 Hz cut-off frequency. Mean COP path lengths, velocity, and velocity root-mean-square (RMS)105–108 were extracted from the data in MATLAB for each of the four trial types (recorded and analyzed separately). COP path length was defined as the absolute length of sway in centimeters produced by the participant during each of the conditions. Increased postural sway was therefore associated with greater COP path lengths. Measures of velocity were obtained by taking the COP excursion and dividing it by trial time, with poorer postural control being related to larger COP velocity. To obtain velocity RMS, the square root of the mean of the squares of the velocity measures were computed. Greater velocity RMS was related to more variable postural sway.
Data analysis
All analyses were run using the threshold values obtained above in R 3.6.0109. All data were winsorized to treat potential outliers using the “DescTools” R package110. If the data are not winsorized the same results are significant. The data were then evaluated for skewness using the “e1071” package111 and evaluated for normality using a generalized Shapiro–Wilk test for normality, then log-transformed to meet the Gaussian assumption (although in-text means and standard deviation are calculated using raw, winsorized data to facilitate comparisons in the literature). To compare the older and younger adults’ vestibular perceptual thresholds in each of the four vestibular perceptual tasks (heave and pitch detection and discrimination) a series of four independent sample t-tests were conducted. Note that the deltas of the discrimination thresholds are reported here (e.g., instead of 22.6 m/s2, we report the delta: 2.6 m/s2). We also calculate and report effect sizes (Cohen’s d). Next, to compare COP path length, velocity, and velocity-RMS across the four posturography tasks, between older and younger adults we ran three separate 2 (Age Group: younger, older) 4 (Condition: EOF, EOC, ECC, ECSS) mixed factorial ANOVA. As with the threshold data, the posturography data were winsorized to treat potential outliers using the “DescTools” R package110 and evaluated for normality using a generalized Shapiro–Wilk test for normality, then square-root transformed to meet the Gaussian assumption. Note that one younger adult participant’s data were not collected due to technical difficulties. Tukey-corrected post-hoc t-tests were used to explore significant interaction effects. Greenhouse–Geisser corrections were applied to correct for violations of sphericity.
We also conducted a series of Bonferroni-corrected Pearson correlations on the raw (i.e., not log-transformed) winsorized data to assess the extent to which COP path length (from each of the four posturography conditions) are associated with each of the four vestibular perceptual thresholds. If the data are not winsorized the same correlations are significant, although the associations are stronger in the older adults and the ECSS condition becomes significantly positively associated with the Heave Detection condition (r = 0.47; p = 0.04). These correlations were conducted separately for the older adult participant group and the younger adult participant group, to compare whether these associations differed as a function of age.
Results
Vestibular psychophysics task
Detection task
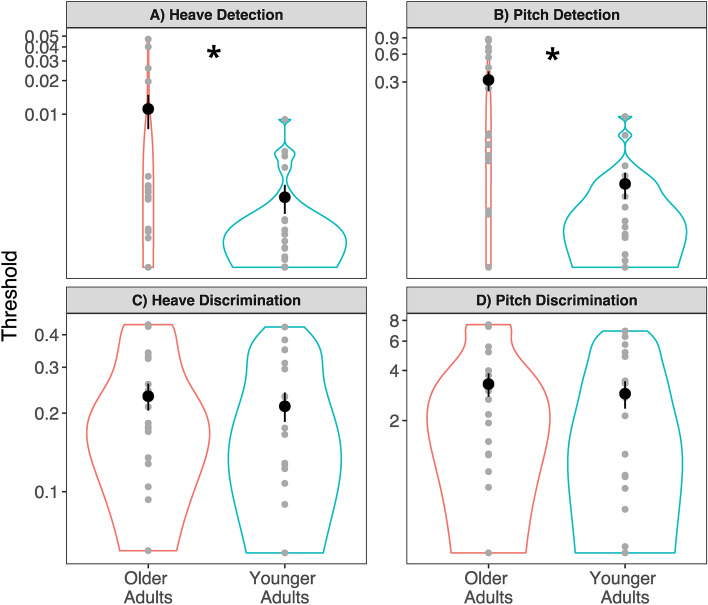
Independent sample t-tests revealed that older adults had significantly greater heave detection thresholds (M = 0.022 m/s2, SD = 0.051) than younger adults (M = 0.002 m/s2, SD = 0.002, t(34.98) = − 2.89, p = 0.007, d = 0.554) (Fig. 3A). Further, older adults had significantly greater pitch detection thresholds (M = 0.329 °/s2, SD = 0.355, d = 1.210) than younger adults (M = 0.024 °/s2, SD = 0.032, t(30.51) = − 4.95, p < 0.001) (Fig. 3B).
Figure 3.
Heave and pitch detection and discrimination thresholds. *p < 0.05. Graphs show data from the, (A) Heave Detection condition, (B) Pitch Detection condition, (C) Heave Discrimination condition, and (D) Pitch Discrimination condition. All data are plotted on logarithmic scales. Thresholds for heave data are in m/s2, and for pitch data in °/s2. Small points represent individual data. The larger black points represent the group means and error bars are standard errors. The width of the borders of the violin plot represents frequency count at each value on the y-axis.
Discrimination task
Independent sample t-tests showed that older adults (M = 0.241 m/s2, SD = 0.137) and younger adults (M = 0.211 m/s2, SD = 0.119) did not have significantly different heave discrimination thresholds (t(34.68) = − 0.61, p = 0.549, d = 0.234) when discriminating from a baseline of 1m/s2 (Fig. 3C). Likewise, there were no significant differences between pitch discrimination thresholds for older (M = 3.41 °/s2, SD = 2.53) and younger adults (M = 2.89 °/s2, SD = 2.25, t(31.23) = − 0.783, p = 0.439, d = 0.217) when discriminating from a baseline of 20°/s2 (Fig. 3D).
Posturography
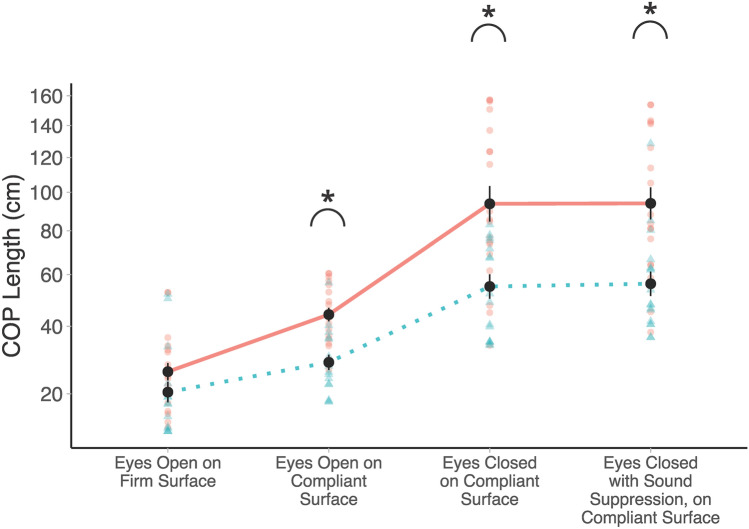
A 2 (Age Group) 4 (Condition: EOF, EOC, ECC, ECSS) mixed factorial ANOVA, on COP path length, revealed a significant main effect of Age Group, F(1, 34) = 14.83, p < 0.001, indicating that older adults (M = 46.8 cm, SD = 33.2) had significantly greater COP path lengths than younger adults (M = 28.1 cm, SD = 17.6) overall. The COP path lengths are shown for all groups in Fig. 4. There was also a significant main effect of Condition, F(1.59, 54.15) = 105.24, p < 0.001, showing significant differences between all conditions (p < 0.001), with the exception of ECC relative to ECSS, t(102) = − 0.266, p = 0.993. Specifically, easier posturography conditions (e.g., EOC) showed smaller COP path lengths than more difficult posturography conditions (e.g., ECC). There was also a significant Age Group Condition interaction (F(1.59, 54.15) = 5.50 p = 0.011). Post-hoc t-tests showed that older adults had significantly greater COP lengths relative to younger adults in the three hardest conditions: EOC (t(72,8) = − 2.452, p = 0.017), ECC (t(72.8) = − 3.958, p < 0.001), and ECSS (t(72.8) = − 4.818, p < 0.001), but not the EOF condition (p > 0.05).
Figure 4.
Posturography data for older and younger adults across all four conditions. *p < 0.05. Mean COP path length (cm) are plotted (older adults = solid red, younger adults = dotted blue). Individual participant data are plotted using single points (older adults = red circles, younger adults = blue triangles). Data are plotted on a square-root scale.
These analyses were repeated with COP velocity (m/s), as well as COP velocity root-mean-square (RMS). Similar results were observed (see Supplementary Information S.2, for full statistical analyses), namely that COP velocity and velocity-RMS were larger in older adults relative to younger adults, with similar differences between the conditions. Likewise, these analyses revealed the same Group Condition interaction, indicating greater velocity as well as velocity-RMS in the three hardest conditions (EOC, ECC, and ECSS) for older adults relative to younger adults.
Correlational analyses between vestibular thresholds and posturography
A series of Bonferroni-corrected Pearson correlations were used to examine the relationship between the four postural task measures for COP path length and the four vestibular perceptual threshold measures in older adults (Fig. 5a) and younger adults (Fig. 5b). Please see the Supplementary Information S.3 for scatterplots, as well as Fisher’s r-to-Z transformation to compare the younger adult and older adults’ correlations. Additional, albeit not significant, Bonferroni-corrected correlations between the four psychophysical measures can be found in the Supplementary Information S.4.
Figure 5.
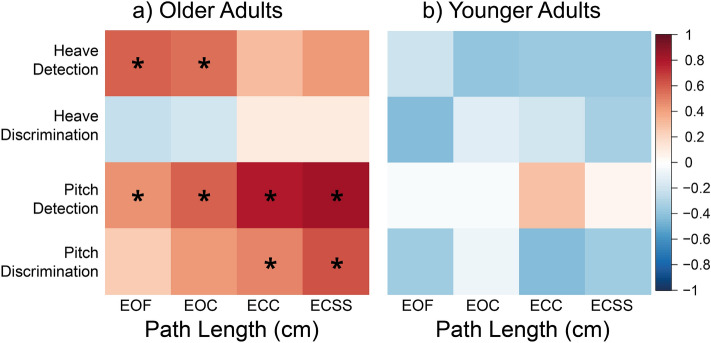
Correlation heatmaps. Graph illustrating the associations between posturography measures (i.e., COP path length, cm) and perceptual thresholds (i.e., m/s2 for heave, and °/s2 for pitch) in, (a) older adults, and (b) younger adults. Blue squares represent negative correlations and red squares represent positive correlations. Lighter squares represent weaker correlations and darker squares represent stronger correlations. Correlations were Bonferroni-corrected. *p < 0.05. EOF Eyes open, firm surface, EOC eyes open, compliant surface, ECC eyes closed, compliant surface, ECSS eyes closed, with sound suppression on a compliant surface.
Perceptual thresholds and posturography
In older adults, heave detection thresholds were positively correlated with COP path length for EOF (r = 0.60, p < 0.007) and EOC (r = 0.56, p = 0.013) conditions, such that higher detection thresholds were associated with greater postural sway (Fig. 5a). Likewise, older adults’ pitch detection thresholds were positively correlated with COP path length for EOF (r = 0.46, p < 0.048), EOC (r = 0.59, p = 0.008), ECC (r = 0.78, p < 0.001) and ECSS (r = 0.82, p < 0.001) conditions. Finally, older adults’ pitch discrimination thresholds were positively associated with COP path length for the ECC (r = 0.49, p < 0.033) and ECSS conditions (r = 0.64, p = 0.004), but there were no correlations between heave discrimination and any measures of COP path length. For younger adults, there were no significant associations observed between any of the posturography measures and vestibular perceptual thresholds (Fig. 5b). We did, however, observe trending negative correlations, similar to 79 who also found trending negative associations between postural sway and rotation direction-discrimination thresholds in younger adults.
In order to account for the continuous effect of age independently influencing postural sway, we also ran multivariate analyses to predict postural sway from the vestibular perceptual thresholds and age (see Supplementary Information S.5). These results show that higher heave detection thresholds significantly predicted larger postural sway in the EOF (p < 0.001) and EOC (p = 0.009) conditions, but that pitch detection predicted ECC (p < 0.001) and ECSS (p < 0.001).
Discussion
We examined age-related changes to vestibular perceptual thresholds for heave and pitch motions. We also explored whether quiet standing balance performance was associated with vestibular perceptual thresholds in older or younger adults. We found that, compared to younger adults, older adults showed significantly higher vestibular detection thresholds in heave, and reported for the first time to our knowledge, in pitch. There were similar patterns of non-significant age-related effects for the heave and pitch discrimination task thresholds, which may be a result of less robust age-related effects and/or insufficient power to observe these effects. Therefore, these results may be a conservative estimation of age-related changes in vestibular thresholds across different motion types and motion tasks.
We also found that older adults had significantly greater COP path lengths relative to younger adults in all conditions except for the easiest condition (i.e., standing on a firm surface with eyes open). Importantly, we also found that, for older adults, but not younger adults, measures of postural sway (COP path length) were positively associated with vestibular perceptual thresholds, such that poorer balance (greater sway) was associated with higher heave and pitch detection thresholds.
Age-related changes in pitch and heave perception
Vestibular detection task
Older adults had higher heave and pitch detection thresholds than younger adults. These results are consistent with current studies in the literature that have also shown older age to be associated with increased direction discrimination (i.e., vestibular perceptual) thresholds in the heave direction15,16,70, as well as in surge, sway, and roll-tilt15,16,18,19,70, but not yaw rotation16,70 (but see 17 who found no age differences in yaw motion perception during a two-interval detection and two-interval magnitude discrimination task). Our study is the first to our knowledge to also examine age-related differences in pitch perception in older and younger adults. Together these results suggest that aging is associated with declines in a range of vestibular perceptual sensitivities across different vestibular perception tasks and movement axes.
Histological, microscopic, and clinical assessments of vestibular functioning have long associated the aging process with vestibular end-organ deterioration. For instance, aging has been linked with significant declines in the number of hair cells, degeneration of the vestibular ganglion, deterioration of the otoconia of the otoliths (see 5,6,27 for reviews), and reduced oVEMP and cVEMP responses7,38,97,99. There is also some evidence of age-related decreases in vestibular cortical network connectivity112, although other studies have shown age-related central gains in vestibular perception, which seem to at least partially compensate for peripheral declines in end-organ responsiveness113.
Despite reporting no diagnosed vestibular impairments or chronic dizziness, some older participants in this study showed evidence of end organ dysfunction of the otoliths (VEMPs), particularly the utricle (oVEMP), which is consistent with prior research5,7,99,114,115. This may have contributed to older adults’ reduced ability to detect pitch and heave motions. It is important to note, however, that the recently described diagnostic criteria for presbyvestibulopathy (PVP; age-related vestibular loss) includes measures of VOR gain (e.g., vHIT measures), but does not include VEMP responses84. It was concluded that, because an absence of VEMP responses is frequently observed in older adults, the significance of these absences for diagnostic purposes is not well understood. In our study, presence, or absence of a VEMP response was not significantly correlated with heave or pitch detection thresholds in our participants (Supplementary Table S.6.1, Supplementary Materials), except for cVEMP presence being associated with reduced heave detection thresholds but only when not correcting for multiple comparisons, (r = − 0.55, p = 0.042). Likewise, we did not observe a significant correlation between VEMP presence and COP path length (Supplementary Table S.6.2, Supplementary Materials). These results are in line with previous findings which showed no associations between cVEMP or oVEMP amplitudes, and vestibular perceptual thresholds in the heave direction, in healthy controls and patients with bilateral vestibulopathy (BV)116. Therefore, the extent to which VEMP responses might relate to observed age-related differences in vestibular perceptual thresholds remains unclear.
Vestibular discrimination task
While we observed age-related differences in pitch and heave detection thresholds, no age-related differences were observed for pitch or heave discrimination tasks. This suggests that while older adults might have a preserved ability to differentiate between two motions of similar magnitudes, their sensitivity to detecting these same motions may be reduced relative to younger adults.
With regards to direction discrimination (which differs from this study’s magnitude discrimination task), previous research has found that older adults tend to demonstrate larger thresholds than younger adults15,16,18,19,70. We suggest a number of possible reasons for this discrepancy.
First, the nature of the task used in this study (magnitude discrimination) is different than those used in the previous aging literature (direction discrimination) where age-related differences were found15,16,18,19,70. To our knowledge, only Chang and colleagues investigated magnitude discrimination in older and younger adults, and also found no significant differences17. Furthermore, these previous direction-discrimination tasks used a one-interval forced choice task, whereas ours used a two-interval forced-choice task, which maybe have further influenced threshold values. For instance, two-interval detection or discrimination tasks such as those used in this current study, are associated with thresholds that are times smaller than 1-interval detection or discrimination tasks103. Such differences in magnitude may have had an impact on the overall thresholds obtained by participants, leading to the null results observed in this condition.
Secondly, the frequency of the motion presented during vestibular perception tasks has been found to influence whether age differences are observed. For instance, Roditi and Crane70 found that relative to younger adults, older adults had poorer surge direction-discrimination thresholds if motions were presented at 0.5 Hz, but not if presented at 1 Hz. Indeed, previous studies have found that regardless of age, participants tend to show higher thresholds for lower motion frequencies101,117,118. Once again, smaller thresholds obtained in both groups may have eliminated such previously observed age-related differences.
Finally, the type of motion profile (e.g., single sine waves versus sinusoidal oscillation), may also affect threshold values and whether age-related differences are observed. Repeated sine wave procedures offer a greater number of samples per trial on which to base perceptual estimates, thereby leading to greater sensitivity (Fig. 2). For example, Chang and colleagues17 and Bermùdez Rey and colleagues16 evaluated magnitude discrimination (using repeated sine waves) and direction discrimination (using a single sine wave) respectively, in older adults, for yaw rotation. Chang and colleagues’17 older adults showed thresholds which were almost half (0.81 ( 0.42) °/s) those obtained by Bermùdez Rey and colleagues (16; 1.45 (range 1.14–1.84) °/s).
Ultimately, future studies should more systematically evaluate how different frequencies, motion profiles, and tasks influence the extent to which age-related differences in vestibular perception are observed.
Associations between vestibular perceptual thresholds and postural stability in older and younger adults
Our older adults were less stable than younger adults in all but the easiest (i.e., standing on a firm surface with eyes open) balance conditions. Such age-related differences were observed consistently, regardless of whether we examined COP path length, velocity, or velocity-RMS. These results are consistent with previous literature showing that older adults demonstrate greater COP path length, velocity, and variability compared to younger adults, especially when multiple senses are impoverished or challenged (e.g., vision and proprioception9–11,119. Postural control generally relies on the contributions and integration of visual, vestibular, and somatosensory cues119–122. This process of multisensory integration during standing balance is particularly relevant in the context of aging since older adults tend to show heightened multisensory integration relative to younger adults20,123–127 and may weigh less reliable sensory inputs more than is optimal20,128.
A novel finding of the present study is that poorer vestibular perceptual sensitivity (i.e., higher thresholds) was associated with greater postural sway, but only in older adults. These associations were found particularly for pitch and heave detection (conditions which showed significant age-related effects in the psychophysical tasks), as well as pitch discrimination. Interestingly, for pitch detection and discrimination, the magnitude of the correlations increased systematically as the difficulty of the postural task also increased. One possible reason for this linear association is that the more difficult postural tasks also increase the need to rely on vestibular input for balance maintenance (e.g., in the absence, or limited presence, of other sensory cues). Specifically, standing on a compliant surface reduces the reliability of somatosensory cues, closing the eyes eliminates the availability of visual cues, and auditory cues are reduced with sound suppression. As such, age-related declines in vestibular perceptual sensitivity may be increasingly consequential to posture when other sensory inputs become more impoverished. Importantly, we found fewer significant associations between postural stability and vestibular discrimination thresholds. This lack of association may be due to the nature of the posturography task (i.e., quiet standing). Specifically, given that precise discrimination estimates may be especially important for responding to changes in balance/posture (as opposed to simply maintaining stable balance/posture), using dynamic posturography tasks (e.g., recovery from perturbation) in future may result in even stronger and more consistent associations.
With regards to the association between postural task difficulty and pitch detection and discrimination, a possible explanation for this increased correlation with age might be related to changes in vestibular end organ functioning with age. For instance, semicircular canals—necessary for pitch detection—show more age-related dysfunction in clinical tests than the utricle or saccule38, as well as a more pronounced loss of Type I hair cells29,32,33 and greater degeneration of the superior (relative to the inferior or posterior) vestibular nerve36. Such changes might reflect the extent to which pitch sensitivity contributes to postural stability (e.g., COP path length) as reliance on vestibular information during standing balance increases. Furthermore, it has been postulated that changes in end organ functioning may be associated with increased neural noise18,19,129 resulting in higher perceptual thresholds130 and greater postural sway131–133.
Importantly, no correlations between standing balance and vestibular perception were observed in the younger adults. Future studies should examine whether vestibular perceptual thresholds along other movement axes might be associated with postural sway in younger and older adults. For instance, higher roll-tilt (at 0.2 Hz) vestibular perceptual thresholds have been found to be significantly associated with failing the hardest condition of a modified Romberg balance test, regardless of age18 and previous studies have found lateral translation vestibular thresholds to be significantly associated with postural sway in younger adults79.
Limitations and future directions
Participant sample
Participants in this study underwent rigorous screening, which allowed us to control for certain age-related factors, including for example, cognitive decline133–139, age-related hearing loss and tinnitus85,88,90,98,140, diagnosed vestibular disorders141, and other serious health conditions. However, these common age-related conditions are likely to also influence vestibular perception and postural control and, as such, the sample in this study may not be representative of the typical older adult population. Instead, these results may be a conservative estimate of age-related changes to vestibular perception. Future studies can help disambiguate age-related changes in vestibular perceptual sensitivity thresholds in a more representative sample of older adults by collecting data from participants with a range of sensory, motor, and cognitive abilities. Furthermore, they can also consider collecting additional baseline sensory, cognitive, and motor measures from both samples of participants (older and younger) to determine whether there are associations among these measures and experimentally test outcomes across the lifespan.
Extra-Vestibular cues
It is also important to acknowledge the potential effect of extra-vestibular cues on participants’ detection and discrimination thresholds. Specifically, this study was designed to reduce the influence of non-vestibular cues such as visual (blindfolds and dimmed lights), auditory (white noise, passive noise-suppressing headphones), proprioceptive (four-point harness, inflatable neck-pillow), and vibrotactile (padded seating and foam footrest)—it remains possible that subtle vibration or proprioceptive cues may have still facilitated perceptual judgements during the vestibular psychophysical tasks. Access to these additional non-vestibular sensory cues may account for the very low perceptual thresholds we obtained, particularly in the detection task—although some of these cues (e.g., vibration) could have also been detected by the vestibular system. In fact, previous research has shown that detection thresholds are as much as 31 × smaller than direction-discrimination thresholds142. Such differences in thresholds have been suggested to be due to the influence of vibrotactile cues during detection tasks142. Future studies could consider adding additional vibrational masking noise during the tasks to further control for such additional cues and better account for this variability in our participants.
End-organ testing
In this study, we expected that older adults might demonstrate some evidence of vestibular end organ dysfunction5–7,38,99,114,115 despite the absence of a clinically diagnosed vestibular disorder84. To quantify such dysfunctions, we measured canal and otolith functioning using VEMP and vHIT measures that included a particular set of testing parameters (e.g., VEMP frequency of 500 Hz), and further correlated these metrics to measures of COP and vestibular thresholds. The lack of correlations of vestibular thresholds with vHIT or VEMP suggests that common age-related declines in vestibular perceptual thresholds are not due solely to end organ or afferent deficits (see 84 for a discussion on the lack of association between measures of end organ functioning and presbyvestibulopathy). However, this choice of parameters may affect whether age-related differences are observed. While for many older adults, VEMPs evoked at stimulus frequencies of 500 Hz show the greatest response, many other older adults will only show evoked oVEMP or cVEMP responses at higher tone burst frequencies (e.g., 750 Hz or 1000 Hz). Future studies should more carefully evaluate the associations between vHIT and VEMP measures and vestibular perceptual thresholds using a range of end organ testing parameters. Finally, future studies could also consider evaluating VEMP and vHIT responses in the younger adult participant group to better understand changes in the associations among end organ functioning, vestibular perception, and postural control across the lifespan.
Conclusion
Older adults had higher heave and pitch detection thresholds than younger adults which, together with pitch discrimination thresholds, were associated with increased postural sway (i.e., COP path length) particularly when sensory conditions were impoverished. These results could have implications in the development of screening tools to detect mobility declines in older adults, given that measurable declines in vestibular perceptual sensitivity were associated with poorer postural stability. These convergent perceptual and behavioral measures may therefore allow for better identification of falls risk.
Supplementary Information
Acknowledgements
We would like to acknowledge Dr. Paul Mick, Dr. Peter Grant, and Dr. M. Kathleen Pichora-Fuller for their helpful earlier discussions. We are also grateful for Rebecca Benjamin and Melissa Hazen for their assistance in the collection and interpretation of VEMP and vHIT data. We would also like to thank Susan Gorski, Robert Ramkhalawansingh, and Rob Shewaga for technical assistance in operating the motion platform. J.L.C has received an NSERC Discovery Grant, and G.A.G. has received an NSERC PGS-D.
Author contributions
G.A.G., J.J.G., and J.L.C. conceived the study. L.R.H., J.J.G., S.L.C., K.A.G., B.C.H., and J.L.C. helped designed the study. G.A.G. and J.J.G. collected the data. G.A.G., L.R.H., and J.L.C., analyzed and interpreted the data. G.A.G. and J.L.C. drafted the article, and all authors contributed to critically revising the article.
Competing interests
The authors have no conflict of interest to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-09807-4.
References
- 1.Center for Disease Control. 10 Leading Causes of Injury Deaths by Age Group Highlighting Unintentional Injury Deaths, United States—2018 (2018).
- 2.Center for Disease Control. Leading Causes of Nonfatal Injury Reports (2018).
- 3.Jahn K. The aging vestibular system: Dizziness and imbalance in the elderly. Adv. Oto-Rhino-Laryngol. 2019;82:143–149. doi: 10.1159/000490283. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson GP, McCaslin DL, Grantham SL, Piker EG. Significant vestibular system impairment is common in a cohort of elderly patients referred for assessment of falls risk. J. Am. Acad. Audiol. 2008;19(10):799–807. doi: 10.3766/jaaa.19.10.7. [DOI] [PubMed] [Google Scholar]
- 5.Allen D, Ribeiro L, Arshad Q, Seemungal BM. Age-related vestibular loss: Current understanding and future research directions. Front. Neurol. 2016;7:231. doi: 10.3389/fneur.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anson E, Jeka JJ. Perspectives on aging vestibular function. Front. Neurol. 2016;6:269. doi: 10.3389/fneur.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maheu M, Houde MS, Landry SP, Champoux F. The effects of aging on clinical vestibular evaluations. Front. Neurol. 2015;6:205. doi: 10.3389/fneur.2015.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horak FB. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl. 2):352. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 9.Hytönen M, Pyykkö I, Aalto H, Starck J. Postural control and age. Acta Otolaryngol. 1993;113(1–2):119–122. doi: 10.3109/00016489309135778. [DOI] [PubMed] [Google Scholar]
- 10.Pyykko I, Jantti P, Aalto H. Postural control in elderly subjects. Age Ageing. 1990;19(3):215–221. doi: 10.1093/ageing/19.3.215. [DOI] [PubMed] [Google Scholar]
- 11.Teasdale N, Stelmach GE, Breunig A. Postural sway characteristics of the elderly under normal and altered visual and support surface conditions. J. Gerontol. 1991;46(6):B238–B244. doi: 10.1093/geronj/46.6.B238. [DOI] [PubMed] [Google Scholar]
- 12.Teasdale N, Stelmach GE, Breunig A, Meeuwsen HJ. Age differences in visual sensory integration. Exp. Brain Res. 1991;85(3):691–696. doi: 10.1007/BF00231755. [DOI] [PubMed] [Google Scholar]
- 13.Teasdale N, Simoneau M. Attentional demands for postural control: The effects of aging and sensory reintegration. Gait Posture. 2001;14(3):203–210. doi: 10.1016/S0966-6362(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 14.Anacker SL, Di Fabio RP, Horak FB. Influence of sensory inputs on standing balance in community-dwelling elders with a recent history of falling. Phys. Ther. 1992;72(8):575–584. doi: 10.1093/ptj/72.8.575. [DOI] [PubMed] [Google Scholar]
- 15.Kobel MJ, Wagner AR, Merfeld DM, Mattingly JK. Vestibular thresholds: A review of advances and challenges in clinical applications. Front. Neurol. 2021;12:643634. doi: 10.3389/fneur.2021.643634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM. Vestibular perceptual thresholds increase above the age of 40. Front. Neurol. 2016;7:162. doi: 10.3389/fneur.2016.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang NYN, et al. Vestibular perception and the vestibulo-ocular reflex in young and older adults. Ear Hear. 2014;35(5):565–570. doi: 10.1097/AUD.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beylergil SB, Karmali F, Wang W, Bermúdez Rey MC, Merfeld DM. Vestibular roll tilt thresholds partially mediate age-related effects on balance. Prog. Brain Res. 2019;248:249–267. doi: 10.1016/bs.pbr.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Karmali F, Rey MCB, Clark TK, Wang W, Merfeld DM. Multivariate analyses of balance test performance, vestibular thresholds, and age. Front. Neurol. 2017;8:578. doi: 10.3389/fneur.2017.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramkhalawansingh R, Butler JS, Campos JL. Visual-vestibular integration during self-motion perception in younger and older adults. Psychol. Aging. 2018;33(5):798–813. doi: 10.1037/pag0000271. [DOI] [PubMed] [Google Scholar]
- 21.Crane BT. Human visual and vestibular heading perception in the vertical planes. JARO J. Assoc. Res. Otolaryngol. 2014;15(1):87–102. doi: 10.1007/s10162-013-0423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day BL, Fitzpatrick RC. The vestibular system. Curr. Biol. 2005;15(15):583. doi: 10.1016/j.cub.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 23.Angelaki DE, Cullen KE. Vestibular system: The many facets of a multimodal sense. Annu. Rev. Neurosci. 2008;31(1):125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- 24.Rosenhall U. Degenerative patterns in the aging human vestibular neuro-epithelia. Acta Otolaryngol. 1973;76(1–6):208–220. doi: 10.3109/00016487309121501. [DOI] [PubMed] [Google Scholar]
- 25.Rosenhall U, Rubin W. Degenerative changes in the human vestibular sensory epithelia. Acta Otolaryngol. 1975;79(1–2):67–80. doi: 10.3109/00016487509124657. [DOI] [PubMed] [Google Scholar]
- 26.Ross MD, Johnsson LG, Peacor D, Allard LF. Observations on normal and degenerating human otoconia. Ann. Otol. Rhinol. Laryngol. 1976;85(3):310–326. doi: 10.1177/000348947608500302. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki S, Yamasoba T. Dizziness and imbalance in the elderly: Age-related decline in the vestibular system. Aging Dis. 2015;6(1):38–47. doi: 10.14336/AD.2014.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnsson LG, Hawkins JE. Sensory and neural degeneration with aging, as seen in microdissections of the human inner ear. Ann. Otol. Rhinol. Laryngol. 1972;81(2):179–193. doi: 10.1177/000348947208100203. [DOI] [PubMed] [Google Scholar]
- 29.Rauch SD, Velazquez-Villaseñor L, Dimitri PS, Merchant SN. Decreasing hair cell counts in aging humans. Ann. N. Y. Acad. Sci. 2001;942(1):220–227. doi: 10.1111/j.1749-6632.2001.tb03748.x. [DOI] [PubMed] [Google Scholar]
- 30.Anniko M. The aging vestibular hair cell. Am. J. Otolaryngol. Neck Med. Surg. 1983;4(3):151–160. doi: 10.1016/s0196-0709(83)80037-4. [DOI] [PubMed] [Google Scholar]
- 31.Gleeson M, Felix H. A comparative study of the effect of age on the human cochlear and vestibular neuroepithelia. Acta Otolaryngol. 1987;104(sup436):103–109. doi: 10.3109/00016488709124982. [DOI] [PubMed] [Google Scholar]
- 32.Merchant SN, Velazquez-Villaseñor L, Tsuji K, Glynn RJ, Wall C, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative vestibular hair cell data. Ann. Otol. Rhinol. Laryngol. Suppl. 2000;109(5_suppl):3–13. doi: 10.1177/00034894001090S502. [DOI] [PubMed] [Google Scholar]
- 33.Matheson AJ, Darlington CL, Smith PF. Dizziness in the elderly and age-related degeneration of the vestibular system. NZ. J. Psychol. 1999;28:10. [PubMed] [Google Scholar]
- 34.Velazquez-Villaseñor L, Merchant SN, Tsuji K, Glynn RJ, Wall C, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative Scarpa’s ganglion cell data. Ann. Otol. Rhinol. Laryngol. Suppl. 2000;109:14–19. doi: 10.1177/00034894001090S503. [DOI] [PubMed] [Google Scholar]
- 35.Park JJ, Tang Y, Lopez I, Ishiyama A. Age-related change in the number of neurons in the human vestibular ganglion. J. Comp. Neurol. 2001;431(4):437–443. doi: 10.1002/1096-9861(20010319)431:4<437::AID-CNE1081>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 36.Richter E. Quantitative study of human scarpa’s ganglion and vestibular sensory epithelia. Acta Otolaryngol. 1980;90(1–6):199–208. doi: 10.3109/00016488009131716. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen KD, Welgampola MS, Carey JP. Test–retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2010;31(5):793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agrawal Y, et al. Decline in semicircular canal and otolith function with age. Otol. Neurotol. 2012;33(5):832–839. doi: 10.1097/MAO.0b013e3182545061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Layman AJ, Li C, Simonsick E, Ferrucci L, Carey JP, Agrawal Y. Association between saccular function and gait speed: Data from the Baltimore Longitudinal study of aging. Otol. Neurotol. 2015;32(2):260–266. doi: 10.1097/MAO.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Layman AJ, Carey JP, Agrawal Y. Epidemiology of vestibular evoked myogenic potentials: Data from the Baltimore Longitudinal Study of Aging. Clin. Neurophysiol. 2015;126(11):2207–2215. doi: 10.1016/j.clinph.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matiño-Soler E, Esteller-More E, Martin-Sanchez JC, Martinez-Sanchez JM, Perez-Fernandez N. Normative data on angular vestibulo-ocular responses in the yaw axis measured using the video head impulse test. Otol. Neurotol. 2015;36(3):466–471. doi: 10.1097/MAO.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 42.McGarvie LA, MacDougall HG, Halmagyi GM, Burgess AM, Weber KP, Curthoys IS. The video head impulse test (vHIT) of semicircular canal function—Age-dependent normative values of VOR gain in healthy subjects. Front. Neurol. 2015;6:1. doi: 10.3389/fneur.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Figtree WVC, Menant JC, Chau AT, Hübner PP, Lord SR, Migliaccio AA. Prevalence of vestibular disorders in independent people over 50 that experience dizziness. Front. Neurol. 2021;12:709. doi: 10.3389/fneur.2021.658053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anson E, Bigelow RT, Studenski SA, Deshpande N, Agrawal Y. Failure on the foam eyes closed test of standing balance associated with reduced semicircular canal function in healthy older adults. Ear Hear. 2019;40(2):340–344. doi: 10.1097/AUD.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anson E, et al. Loss of peripheral sensory function explains much of the increase in postural sway in healthy older adults. Front. Aging Neurosci. 2017;9:202. doi: 10.3389/fnagi.2017.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal Y, Davalos-Bichara M, Zuniga MG, Carey JP. Head impulse test abnormalities and influence on gait speed and falls in older individuals. Otol. Neurotol. 2013;34(9):1729–1735. doi: 10.1097/MAO.0b013e318295313c. [DOI] [PubMed] [Google Scholar]
- 47.Karmali F, Whitman GT, Lewis RF. Bayesian optimal adaptation explains age-related human sensorimotor changes. J. Neurophysiol. 2018;119(2):509–520. doi: 10.1152/jn.00710.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osoba MY, Rao AK, Agrawal SK, Lalwani AK. Balance and gait in the elderly: A contemporary review. Laryngosc. Investig. Otolaryngol. 2019;4(1):143–153. doi: 10.1002/lio2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maki BE, McIlroy WE. Postural control in the older adult. Clin. Geriatr. Med. 1996;12(4):635–658. doi: 10.1016/S0749-0690(18)30193-9. [DOI] [PubMed] [Google Scholar]
- 50.Mahoney JR, Cotton K, Verghese J, Newman A. Multisensory integration predicts balance and falls in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019;74(9):1429–1435. doi: 10.1093/gerona/gly245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahoney JR, Verghese J. Does cognitive impairment influence visual-somatosensory integration and mobility in older adults? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020;75(3):581–588. doi: 10.1093/gerona/glz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maylor EA, Wing AM. Age differences in postural stability are increased by additional cognitive demands. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 1996;51(3):143–154. doi: 10.1093/geronb/51b.3.p143. [DOI] [PubMed] [Google Scholar]
- 53.Woollacott MH, Shumway-Cook A, Nashner LM. Aging and posture control: Changes in sensory organization and muscular coordination. Int. J. Aging Hum. Dev. 1986;23(2):97–114. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]
- 54.Manchester D, Woollacott MH, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J. Gerontol. 1989;44(4):M118–M127. doi: 10.1093/geronj/44.4.M118. [DOI] [PubMed] [Google Scholar]
- 55.Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp. Brain Res. 2008;186(4):677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- 56.Butler JS, Campos JL, Bülthoff HH. Optimal visual–vestibular integration under conditions of conflicting intersensory motion profiles. Exp. Brain Res. 2015;233(2):587–597. doi: 10.1007/s00221-014-4136-1. [DOI] [PubMed] [Google Scholar]
- 57.Butler JS, Campos JL, Bülthoff HH, Smith ST. The role of stereo vision in visual-vestibular integration. Seeing Perceiving. 2011;24(5):453–470. doi: 10.1163/187847511X588070. [DOI] [PubMed] [Google Scholar]
- 58.Butler JS, Smith ST, Campos JL, Bülthoff HH. Bayesian integration of visual and vestibular signals for heading. J. Vis. 2010;10(11):23–23. doi: 10.1167/10.11.23. [DOI] [PubMed] [Google Scholar]
- 59.Campos JL, Butler JS, Bülthoff HH. Multisensory integration in the estimation of walked distances. Exp. Brain Res. 2012;218(4):551–565. doi: 10.1007/s00221-012-3048-1. [DOI] [PubMed] [Google Scholar]
- 60.Crane BT. Direction specific biases in human visual and vestibular heading perception. PLoS One. 2012;7(12):e51383. doi: 10.1371/journal.pone.0051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris LR, Jenkin M, Zikovitz DC. Visual and non-visual cues in the perception of linear self motion. Exp. Brain Res. 2000;135(1):12–21. doi: 10.1007/s002210000504. [DOI] [PubMed] [Google Scholar]
- 62.Jaekl PM, Jenkin MR, Harris LR. Perceiving a stable world during active rotational and translational head movements. Exp. Brain Res. 2005;163(3):388–399. doi: 10.1007/s00221-004-2191-8. [DOI] [PubMed] [Google Scholar]
- 63.Fetsch CR, Turner AH, DeAngelis GC, Angelaki DE. Dynamic reweighting of visual and vestibular cues during self-motion perception. J. Neurosci. 2009;29(49):15601–15612. doi: 10.1523/JNEUROSCI.2574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frissen I, Campos JL, Souman JL, Ernst MO. Integration of vestibular and proprioceptive signals for spatial updating. Exp. Brain Res. 2011;212(2):163–176. doi: 10.1007/s00221-011-2717-9. [DOI] [PubMed] [Google Scholar]
- 65.Campos JL, Siegle JH, Mohler BJ, Bülthoff HH, Loomis JM. Imagined self-motion differs from perceived self-motion: Evidence from a novel continuous pointing method. PLoS One. 2009;4(11):e7793. doi: 10.1371/journal.pone.0007793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siegle JH, Campos JL, Mohler BJ, Loomis JM, Bülthoff HH. Measurement of instantaneous perceived self-motion using continuous pointing. Exp. Brain Res. 2009;195(3):429–444. doi: 10.1007/s00221-009-1805-6. [DOI] [PubMed] [Google Scholar]
- 67.Pfeiffer C, Serino A, Blanke O. The vestibular system: A spatial reference for bodily self-consciousness. Front. Integr. Neurosci. 2014;8:31. doi: 10.3389/fnint.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matthews BL, Ryu JH, Bockaneck C. Vestibular contribution to spatial orientation. Acta Otolaryngol. 1989;108(S468):149–154. doi: 10.3109/00016488909139036. [DOI] [PubMed] [Google Scholar]
- 69.Angelaki DE, Klier EM, Snyder LH. A vestibular sensation: Probabilistic approaches to spatial perception. Neuron. 2009;64(4):448–461. doi: 10.1016/j.neuron.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roditi RE, Crane BT. Directional asymmetries and age effects in human self-motion perception. JARO J. Assoc. Res. Otolaryngol. 2012;13(3):381–401. doi: 10.1007/s10162-012-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seemungal BM, Gunaratne IA, Fleming IO, Gresty MA, Bronstein AM. Perceptual and nystagmic thresholds of vestibular function in yaw. J. Vestib. Res. 2004;14(6):461–466. doi: 10.3233/VES-2004-14604. [DOI] [PubMed] [Google Scholar]
- 72.Barbieri G, Gissot AS, Pérennou D. Ageing of the postural vertical. Age (Omaha) 2010;32(1):51–60. doi: 10.1007/s11357-009-9112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manckoundia P, Mourey F, Pérennou D, Pfitzenmeyer P. Backward disequilibrium in elderly subjects. Clin. Interv. Aging. 2008;3(4):667. doi: 10.2147/CIA.S3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manckoundia P, Mourey F, Pfitzenmeyer P, Van Hoecke J, Pérennou D. Is backward disequilibrium in the elderly caused by an abnormal perception of verticality? A pilot study. Clin. Neurophysiol. 2007;118(4):786–793. doi: 10.1016/j.clinph.2006.11.274. [DOI] [PubMed] [Google Scholar]
- 75.Van den Bogert AJ, Pavol MJ, Grabiner MD. Response time is more important than walking speed for the ability of older adults to avoid a fall after a trip. J. Biomech. 2002;35(2):199–205. doi: 10.1016/S0021-9290(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 76.Roos PE, Dingwell JB. Using dynamic walking models to identify factors that contribute to increased risk of falling in older adults. Hum. Mov. Sci. 2013;32(5):984–996. doi: 10.1016/j.humov.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Boer T, Wisse M, Van Der Helm FCT. Mechanical analysis of the preferred strategy selection in human stumble recovery. J. Biomech. Eng. 2010;132:7. doi: 10.1115/1.4001281. [DOI] [PubMed] [Google Scholar]
- 78.Roos PE, McGuigan MP, Trewartha G. The role of strategy selection, limb force capacity and limb positioning in successful trip recovery. Clin. Biomech. 2010;25(9):873–878. doi: 10.1016/j.clinbiomech.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 79.Karmali F, Goodworth AD, Valko Y, Leeder T, Peterka RJ, Merfeld DM. The role of vestibular cues in postural sway. J. Neurophysiol. 2021;125(2):672–686. doi: 10.1152/jn.00168.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prosperini L, Pozzilli C. The clinical relevance of force platform measures in multiple sclerosis: A review. Mult. Scler. Int. 2013;2013:1–9. doi: 10.1155/2013/756564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baloh RW, Jacobson KM, Beykirch K, Honrubia V. Static and dynamic posturography in patients with vestibular and cerebellar lesions. Arch. Neurol. 1998;55(5):649–654. doi: 10.1001/archneur.55.5.649. [DOI] [PubMed] [Google Scholar]
- 82.Wolter NE, et al. BalanCI: Head-referenced cochlear implant stimulation improves balance in children with bilateral cochleovestibular loss. Audiol. Neurotol. 2020;25(1–2):60–71. doi: 10.1159/000503135. [DOI] [PubMed] [Google Scholar]
- 83.Wolter NE, et al. Unilateral hearing loss is associated with impaired balance in children: A pilot study. Otol. Neurotol. 2016;37(10):1589–1595. doi: 10.1097/MAO.0000000000001218. [DOI] [PubMed] [Google Scholar]
- 84.Agrawal Y, et al. Presbyvestibulopathy: Diagnostic criteria Consensus document of the classification committee of the Bárány Society. J. Vestib. Res. Equilib. Orientat. 2019;29(4):161–170. doi: 10.3233/VES-190672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gabriel GA, et al. Vestibular perceptual thresholds in older adults with and without age-related hearing loss. Ear Hear. 2021;43:420–435. doi: 10.1097/AUD.0000000000001118. [DOI] [PubMed] [Google Scholar]
- 86.Nasreddine ZS, et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 87.Campos JL, Ramkhalawansingh R, Pichora-Fuller MK. Hearing, self-motion perception, mobility, and aging. Hear. Res. 2018;369:42–55. doi: 10.1016/j.heares.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 88.Carpenter MG, Campos JL. The effects of hearing loss on balance: A critical review. Ear Hear. 2020;41:107S–119S. doi: 10.1097/AUD.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 89.Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch. Intern. Med. 2012;172(4):369–371. doi: 10.1001/archinternmed.2011.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lubetzky AV, Gospodarek M, Arie L, Kelly J, Roginska A, Cosetti M. Auditory input and postural control in adults: A narrative review. JAMA Otolaryngol. Head Neck Surg. 2020;146:E1–E8. doi: 10.1001/jamaoto.2020.0032. [DOI] [PubMed] [Google Scholar]
- 91.Viljanen A, et al. Hearing as a predictor of falls and postural balance in older female twins. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009;64(2):312–317. doi: 10.1093/gerona/gln015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zuniga MG, et al. Association between hearing loss and saccular dysfunction in older individuals. Otol. Neurotol. 2012;33(9):1586–1592. doi: 10.1097/MAO.0b013e31826bedbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.ISO 8253-1. Acoustics-Audiometric Test Methods-Part 1: Basic Pure Tone Air and Bone Conduction Threshold Audiometry. (International Organization for Standardization, 1989).
- 94.MacDougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP. Application of the video head impulse test to detect vertical semicircular canal dysfunction. Otol. Neurotol. 2013;34(6):974–979. doi: 10.1097/MAO.0b013e31828d676d. [DOI] [PubMed] [Google Scholar]
- 95.Halmagyi GM, Chen L, MacDougall HG, Weber KP, McGarvie LA, Curthoys IS. The video head impulse test. Front. Neurol. 2017;8:1. doi: 10.3389/fneur.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Janky KL, Patterson JN, Shepard NT, Thomas MLA, Honaker JA. Effects of device on video head impulse test (vHIT) gain. J. Am. Acad. Audiol. 2017;28(9):778–785. doi: 10.3766/jaaa.16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brantberg K. Vestibular evoked myogenic potentials (VEMPs): Usefulness in clinical neurotology. Semin. Neurol. 2009;29(5):541–547. doi: 10.1055/s-0029-1241042. [DOI] [PubMed] [Google Scholar]
- 98.Li C, Zuniga MG, Nguyen KD, Carey JP, Agrawal Y. How to interpret latencies of cervical and ocular vestibular-evoked myogenic potentials: Our experience in fifty-three participants. Clin. Otolaryngol. 2014;39(5):297–301. doi: 10.1111/coa.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Piker EG, Jacobson GP, Burkard RF, McCaslin DL, Hood LJ. Effects of age on the tuning of the cVEMP and oVEMP. Ear Hear. 2013;34(6):e65–e73. doi: 10.1097/AUD.0b013e31828fc9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995;50A(1):M28–M34. doi: 10.1093/gerona/50A.1.M28. [DOI] [PubMed] [Google Scholar]
- 101.Naseri AR, Grant PR. Human discrimination of translational accelerations. Exp. Brain Res. 2012;218(3):455–464. doi: 10.1007/s00221-012-3035-6. [DOI] [PubMed] [Google Scholar]
- 102.Taylor MM, Creelman CD. PEST: Efficient estimates on probability functions. J. Acoust. Soc. Am. 1967;41(4A):782–787. doi: 10.1121/1.1910407. [DOI] [Google Scholar]
- 103.Merfeld DM. Signal detection theory and vestibular thresholds: I. Basic theory and practical considerations. Exp. Brain Res. 2011;210(3–4):389–405. doi: 10.1007/s00221-011-2557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scoppa F, Capra R, Gallamini M, Shiffer R. Clinical stabilometry standardization. Basic definitions—Acquisition interval—Sampling frequency. Gait Posture. 2013;37(2):290–292. doi: 10.1016/j.gaitpost.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 105.Paillard, T. & Noé, F. Techniques and methods for testing the postural function in healthy and pathological subjects. BioMed. Res. Int.2015, 1–15 (2015). [DOI] [PMC free article] [PubMed]
- 106.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 1996;43(9):956–966. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 107.Li Z, Liang YY, Wang L, Sheng J, Ma SJ. Reliability and validity of center of pressure measures for balance assessment in older adults. J. Phys. Ther. Sci. 2016;28(4):1364–1367. doi: 10.1589/jpts.28.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moghadam M, et al. Reliability of center of pressure measures of postural stability in healthy older adults: Effects of postural task difficulty and cognitive load. Gait Posture. 2011;33(4):651–655. doi: 10.1016/j.gaitpost.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 109.R Core Team. A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2017).
- 110.Signorell, A. et al. DescTools: Tools for descriptive statistics. (2019).
- 111.Meyer, D., Hornik, K., Weingessel, A., & Leisch, F. e1071: Misc Functions of the Department of Statistics. Probability Theory Group (Formerly: E1071), TU Wien (2018).
- 112.Cyran CAM, Boegle R, Stephan T, Dieterich M, Glasauer S. Age-related decline in functional connectivity of the vestibular cortical network. Brain Struct. Funct. 2016;221(3):1443–1463. doi: 10.1007/s00429-014-0983-6. [DOI] [PubMed] [Google Scholar]
- 113.Peters RM, Blouin JS, Dalton BH, Inglis JT. Older adults demonstrate superior vestibular perception for virtual rotations. Exp. Gerontol. 2016;82:50–57. doi: 10.1016/j.exger.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 114.Su HC, Huang TW, Young YH, Cheng PW. Aging effect on vestibular evoked myogenic potential. Otol. Neurotol. 2004;25(6):977–980. doi: 10.1097/00129492-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 115.Tseng CL, Chou CH, Young YH. Aging effect on the ocular vestibular-evoked myogenic potentials. Otol. Neurotol. 2010;31(6):959–963. doi: 10.1097/MAO.0b013e3181e8fb1a. [DOI] [PubMed] [Google Scholar]
- 116.Agrawal Y, Bremova T, Kremmyda O, Strupp M, MacNeilage PR. Clinical testing of otolith function: Perceptual thresholds and myogenic potentials. JARO J. Assoc. Res. Otolaryngol. 2013;14(6):905. doi: 10.1007/s10162-013-0416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat. Space. Environ. Med. 1986;57(11):1088–1096. [PubMed] [Google Scholar]
- 118.Gundry AJ. Thresholds of perception for periodic linear motion. Aviat. Space. Environ. Med. 1978;49(5):679–686. [PubMed] [Google Scholar]
- 119.Peterka RJ. Sensorimotor integration in human postural control. J. Neurophysiol. 2002;88(3):1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 120.Bronstein, A. M. Multisensory integration in balance control. In Handbook of Clinical Neurology, vol. 137 57–66 (Elsevier B.V., 2016). [DOI] [PubMed]
- 121.Assländer L, Peterka RJ. Sensory reweighting dynamics in human postural control. J. Neurophysiol. 2014;111(9):1852–1864. doi: 10.1152/jn.00669.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ivanenko Y, Gurfinkel VS. Human postural control. Front. Neurosci. 2018;12:171. doi: 10.3389/fnins.2018.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Dieuleveult AL, Siemonsma PC, van Erp JBF, Brouwer A-M. Effects of aging in multisensory integration: A systematic review. Front. Aging Neurosci. 2017;9:80. doi: 10.3389/fnagi.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mozolic, J. L., Hugenschmidt, C. E., Peiffer, A. M., & Laurienti, P. J. Multisensory integration and aging. In The Neural Bases of Multisensory Processes 381–392 (CRC Press, 2011).
- 125.DeLoss DJ, Pierce RS, Andersen GJ. Multisensory integration, aging, and the sound-induced flash illusion. Psychol. Aging. 2013;28(3):802–812. doi: 10.1037/a0033289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Laurienti PJ, Burdette JH, Maldjian JA, Wallace MT. Enhanced multisensory integration in older adults. Neurobiol. Aging. 2006;27(8):1155–1163. doi: 10.1016/j.neurobiolaging.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 127.Lupo J, Barnett-Cowan M. Impaired perceived timing of falls in the elderly. Gait Posture. 2018;59:40–45. doi: 10.1016/j.gaitpost.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 128.Berard JR, Fung J, Lamontagne A. Impact of aging on visual reweighting during locomotion. Clin. Neurophysiol. 2012;123(7):1422–1428. doi: 10.1016/j.clinph.2011.11.081. [DOI] [PubMed] [Google Scholar]
- 129.Diaz-Artiles A, Karmali F. Vestibular precision at the level of perception, eye movements, posture, and neurons. Neuroscience. 2021;468:282–320. doi: 10.1016/j.neuroscience.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley; 1966. [Google Scholar]
- 131.Kuo AD. An optimal state estimation model of sensory integration in human postural balance. J. Neural Eng. 2005;2(3):S235–S249. doi: 10.1088/1741-2560/2/3/S07. [DOI] [PubMed] [Google Scholar]
- 132.Rosenberg MJ, Galvan-Garza RC, Clark TK, Sherwood DP, Young LR, Karmali F. Human manual control precision depends on vestibular sensory precision and gravitational magnitude. J. Neurophysiol. 2018;120(6):3187–3197. doi: 10.1152/jn.00565.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van Der Kooij H, Peterka RJ. Non-linear stimulus-response behavior of the human stance control system is predicted by optimization of a system with sensory and motor noise. J. Comput. Neurosci. 2011;30(3):759–778. doi: 10.1007/s10827-010-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carr S, Pichora-Fuller MK, Li KZH, Phillips N, Campos JL. Multisensory, multi-tasking performance of older adults with and without subjective cognitive decline. Multisens. Res. 2019;32(8):797–829. doi: 10.1163/22134808-20191426. [DOI] [PubMed] [Google Scholar]
- 135.Carr S, Li KZ, Pichora-Fuller MK, Campos JL. Effects of age on listening and postural control during realistic multitasking conditions. Hum. Mov. Sci. 2020;73:102664. doi: 10.1016/j.humov.2020.102664. [DOI] [PubMed] [Google Scholar]
- 136.Nieborowska V, Lau ST, Campos JL, Pichora-Fuller MK, Novak A, Li KZH. Effects of age on dual-task walking while listening. J. Mot. Behav. 2019;51(4):416–427. doi: 10.1080/00222895.2018.1498318. [DOI] [PubMed] [Google Scholar]
- 137.Moylan KC, Binder EF. Falls in older adults: Risk assessment, management and prevention. Am. J. Med. 2007;120(6):493.e1–e6. doi: 10.1016/j.amjmed.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 138.Leandri M, et al. Balance features in Alzheimer’s disease and amnestic mild cognitive impairment. J. Alzheimer’s Dis. 2009;16(1):113–120. doi: 10.3233/JAD-2009-0928. [DOI] [PubMed] [Google Scholar]
- 139.Li KZH, Lindenberger U. Relations between aging sensory/sensorimotor and cognitive functions. Neurosci. Biobehav. Rev. 2002;26(7):777–783. doi: 10.1016/S0149-7634(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 140.Abdel-Salam GMS. The association between age-related sensorineural hearing loss and saccular dysfunction in the elderly. Saudi J. Otorhinolaryngol. Head Neck Surg. 2020;22(1):7–12. doi: 10.4103/SJOH.SJOH_19_19. [DOI] [Google Scholar]
- 141.Agrawal Y, Smith PF, Rosenberg PB. Vestibular impairment, cognitive decline and Alzheimer’s disease: Balancing the evidence. Aging Ment. Health. 2020;24(5):705–708. doi: 10.1080/13607863.2019.1566813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chaudhuri SE, Karmali F, Merfeld DM. Whole body motion-detection tasks can yield much lower thresholds than direction-recognition tasks: Implications for the role of vibration. J. Neurophysiol. 2013;110(12):2764–2772. doi: 10.1152/jn.00091.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.