Abstract
Fear is an extreme form of aversion that underlies pathological conditions such as panic or phobias. Fear conditioning (FC) is the best-understood model of fear learning. In FC the context and a cue are independently associated with a threatening unconditioned stimulus (US). The lateral habenula (LHb) is a general encoder of aversion. However, its role in fear learning remains poorly understood. Here we studied in rats the role of the LHb in FC using optogenetics and pharmacological tools. We found that inhibition or activation of the LHb during entire FC training impaired both cued and contextual FC. In contrast, optogenetic inhibition of the LHb restricted to cue and US presentation impaired cued but not contextual FC. In either case, simultaneous activation of contextual and cued components of FC, by the presentation of the cue in the training context, recovered the conditioned fear response. Our results support the notion that the LHb is required for the formation of independent contextual and cued fear memories, a previously uncharacterized function for this structure, that could be critical in fear generalization.
Subject terms: Fear conditioning, Limbic system
Introduction
Fear is an extreme and uncontrollable reaction to a threatening stimulus. Pavlovian fear conditioning (FC) is probably the most studied and best-understood model of fear learning [1, 2]. FC training normally involves the pairing of a tone (cue) and an electric foot-shock unconditioned stimulus (US). It has long been known that such protocol generates two independent associations relating the cue and the context to the US [3, 4]. Prevailing models postulate that tone-US association takes place in cortical and thalamic auditory inputs to the lateral amygdala [5–8], while context-US association involves context encoding circuits centered in the hippocampus, which sends contextual representation to the amygdala [9, 10].
The lateral habenula (LHb) is a hub for aversive information processing. Aversion-related information reaches the LHb from the basal ganglia and numerous structures of the limbic system [11]. In turn, the LHb projects to the brain stem, where it is one of the few structures that controls both serotoninergic and dopaminergic systems [11]. The LHb and its downstream targets are activated by electric foot-shocks [12–15] and develops responses to cues predicting these aversive stimuli along with the appearance of conditioned responses [14–16]. This suggests the LHb may play a role in FC. However, few studies examined this possibility [17–19]. Here, we investigated the participation of the LHb in the acquisition of contextual and cued FC performing pharmacological and optogenetics manipulations. We show that interfering with the neuronal activity of the LHb during FC training severely impairs the recall of contextual and cued FC. However, when the cue is played in the training context, a conserved FC memory is expressed. Our results support a central role for the LHb in FC learning, critically affecting not only context and cued FC but also, remarkably, the interaction between contextual and cue representations in fear expression.
Materials and methods
Animals
Experiments were performed in male Wistar rats. Animals were 5–6 weeks old at the time of surgery. Experimental procedures were approved by the Animal Care and Use Committee of the University of Buenos Aires (CICUAL), and the Autonomous Community of Madrid (PROEX 167/18). Additional information can be found in the Supplementary Material.
Surgeries
Pharmacology
Rats under deep ketamine/xylazine anesthesia (100 and 5 mg/kg respectively) were bilaterally implanted with 22-Gauge guide cannulae 2.0 mm above the LHb (AP −3.0 mm, ML ± 0.7 mm, DV −3.8 mm from Bregma), or 1.0 mm dorsal, ventral, or lateral to LHb coordinates in specific experiments. Cannulae were fixed to the skull with three surgical steel screws and dental acrylic.
Optogenetics
Rats under deep isoflurane anesthesia (4% induction, 1–2% maintenance, in 0.8 L/minute oxygen) were bilaterally injected with 300 nl of adeno associated viral vector (AAV) per side at the LHb (AP −2.9 mm, ML ± 0.7 mm, DV −5.0 mm from Bregma). Vectors used: AAV8-CamKIIα-ArchT-GFP, AAV8-CamKIIα-GFP, both from UNC Vector Core, or AAV8-hSyn-oChIEF-tdTomato, homemade as described in [20]. Optical fiber implants (200 µm core, 0.39 NA) were bilaterally inserted aiming just above the LHb, with 4 degrees angle from the sagittal plane (AP −3.0 mm, ML ± 1.05 mm, DV −4.5 mm from Bregma), and fixed to the skull with three surgical steel screws and dental acrylic. Behavioral procedures began three weeks after surgery.
During surgeries, animals received a dose of analgesic (meloxicam 0.6 mg/kg) and antibiotic (gentamicin 3 mg/kg).
Behavioral procedures
All behavioral procedures were video recorded and analyzed offline. During training, test, and analysis the experimenter was blind for the treatment. Additional information can be found in the Supplementary Material.
Contextual and cued FC training
Cued FC protocol consisted of a 180 s baseline period followed by 4 tone-shock presentations (17 s, 3 kHz, 80 dB tone followed by 3 s, 0.60 mA) with an inter-stimulus interval of 70 s. For contextual FC training, procedures were equal, but the tone was omitted during conditioning. Additional information can be found in the Supplementary Material.
Pharmacology experiments
Rats received bilateral intra LHb infusions of GABA-A receptor agonist, muscimol (Sigma, 60 ng/µl in saline solution, 0.5 µl/side). Additional information can be found in the Supplementary Material.
Optogenetics experiments
For ArchT experiments, continuous light of 532 nm at 10 mW was delivered with a laser source (CNI, China), starting at tone onset and stopping 5 s after shock termination. For oChIEF experiments, 5 ms pulses at 20 Hz of 447 nm light at 10 mW were delivered with a laser source (Tolket, Argentina) during the whole training. Additional information can be found in the Supplementary Material.
FC tests
For contextual FC test, animals were placed in the training context (Context A) for 180 s and then returned to its home cage. For cued FC test animals were placed in a novel chamber (Context B). Cue test consisted of a pre-tone period of 180 s, followed by a 60 s tone (same as training tone) and a 30 s post-tone period. For Context + Tone test procedure was similar to contextual FC test, but after 180 s in the test cage the tone was presented for 60 s. Additional information can be found in the Supplementary Material.
Freezing analysis
Freezing was manually scored offline. Experimenter was blind for animal´s treatment. Additional information can be found in Supplementary Material.
Statistical analysis
Each animal was taken as an independent measure. Statistical analysis of FC experiments was done by generalized linear mixed modeling (GzLMM, for further details see Supplementary Material). In freezing over time plots, line represents intersubjects’ mean, and shaded area represents +SEM. In bar plots, each dot represents a subject, bars represent mean and error bars +SEM. In figures only significant post-hoc contrasts are informed (*p < 0.05; **p < 0.01, ***p < 0.001).
Results
Pharmacological inactivation of the LHb impairs contextual fear conditioning
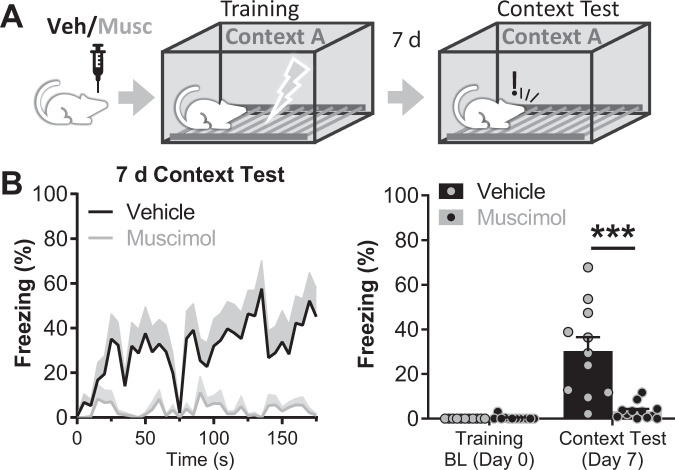
To study the role of the LHb in contextual FC, we analyzed the effect of its inactivation during training. For that purpose, we performed surgeries in rats to implant chronic bilateral intracerebral cannulae aimed at the LHb. After 10 days of recovery time, animals were bilaterally infused with the GABA-A agonist, muscimol, or vehicle in the LHb before FC training. During training, rats were placed in a FC chamber and presented with four unsignaled foot-shocks (Fig. 1A). The infusion of muscimol in the LHb did not modify freezing behavior during FC training (Supplementary Fig. 1). To evaluate contextual FC, animals were placed back in the conditioning chamber 7 days after training and freezing behavior was quantified. Muscimol group displayed significantly lower levels of freezing than the control group (3.32 ± 1.03% for muscimol group vs. 30.31 ± 6.23% for control group, p = 0.0004; nvehicle = 11, nmuscimol = 12; Fig. 1B), suggesting that inactivating the LHb during contextual FC training impairs acquisition of contextual FC.
Fig. 1. Inactivation of the LHb during FC training blocks contextual FC.
A Diagram of the experimental setup: bilateral vehicle/muscimol intra LHb infusions were performed 30 min before FC training. Contextual FC was tested 7 days later. During training, subjects freely explored the cage for a baseline period of 197 s. After that they were exposed to 4 foot-shocks (0.6 mA, 3 s) interspaced by 87 s. In the recall session animals were re-exposed to the training context for 180 s and freezing was quantified. B Test of contextual FC memory. Left panel: freezing over time. Right panel: average baseline freezing during FC training and memory recall session 7 days later. During recall session freezing in the muscimol group was lower than in the control group (t(20) = 4.294, p = 0.0004, nvehicle = 11, nmuscimol = 12). Additional statistics information can be found in the Statistics details section of Supplementary Material.
In a separate cohort of animals, we observed that muscimol infusion in the LHb before exposure to an open field (OF) did not modify locomotion or exploratory behavior (Supplementary Fig. 2). Moreover, upon a subsequent re-exposure to the OF 48 h later, animals infused with muscimol displayed a decrease in exploratory behavior equivalent to control animals (Supplementary Fig. 2). Thus, the inactivation of the LHb by muscimol does not induce a general deficit in exploratory behavior nor in context habituation.
Pharmacological inactivation of the LHb impairs cued fear conditioning
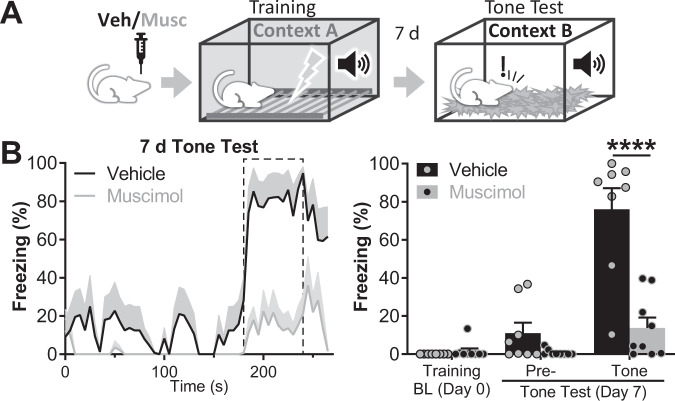
Next, we studied the effect of LHb inactivation during cued FC training. Animals were infused with muscimol or vehicle in the LHb, and 30 min later they were trained in cued FC, in which animals were presented with 4 foot-shocks preceded by a 17-second-long tone (Fig. 2A). Cued FC memory was evaluated 7 days later by exposing the animals to the tone in a novel context (Context B). Inactivation of the LHb did not affect freezing during training (Supplementary Fig. 3A). During memory recall session, muscimol and vehicle groups showed similarly low levels of freezing to Context B (pre-tone freezing: 0.79 ± 0.55% for muscimol group vs. 10.97 ± 5.51% for control group, p = 0.2828; nvehicle = 8, nmuscimol = 9; Fig. 2B). Tone presentation elicited a robust freezing in the vehicle group and significantly lower levels of freezing in the muscimol group (tone freezing: 13.79 ± 5.41% for muscimol group vs. 75.96 ± 11.08% for control group, p < 0.0001; Fig. 2B).
Fig. 2. Inactivation of the LHb during training blocks cued FC.
A Diagram of the experimental setup: bilateral vehicle/muscimol intra LHb infusions were performed 30 min before FC training. Cued FC was tested 7 days later. During training, rats freely explored the cage for a baseline period of 180 s that was followed by 4 tone-shock pairings (17 s of tone followed by 3 s, 0.6 mA shock) interspaced by 70 s. During memory recall, tone was presented for 60 s after a pre-tone period of 180 s. B Left panel: freezing over time. Dashed line delimits tone presentation. Right panel: average freezing for pre- and tone periods. Freezing during pre-tone period was low and not different between vehicle and muscimol groups (pre-tone freezing: 0.79 ± 0.55% for muscimol group vs. 10.97 ± 5.51% for vehicle group; t(28) = 1.469, p = 0.2828). In contrast, during tone presentation, a highly significant reduction in freezing was observed in the muscimol group (t(28) = 5.396, p < 0.0001, nvehicle = 8, nmuscimol = 9). Additional statistics can be found in the Statistics details section.
To control for the spatial specificity of muscimol infusion we infused muscimol in areas around the LHb before cued FC training (Supplementary Fig. 4A). We found that neither infusion of muscimol 1 mm dorsal, lateral, or ventral to the LHb significantly affected freezing behavior (Supplementary Fig. 4B). Moreover, freezing behavior was unaltered during cued or contextual FC recall in muscimol infused animals with non-voluntarily bilaterally missed infusions (Supplementary Fig. 5). Recall of contextual and cued FC was also impaired if evaluated 24 h after training (Supplementary Fig. 6), suggesting that LHb inactivation impairs formation of cued and contextual long-term memories.
Context + tone test of FC reveals a conserved fear conditioning memory
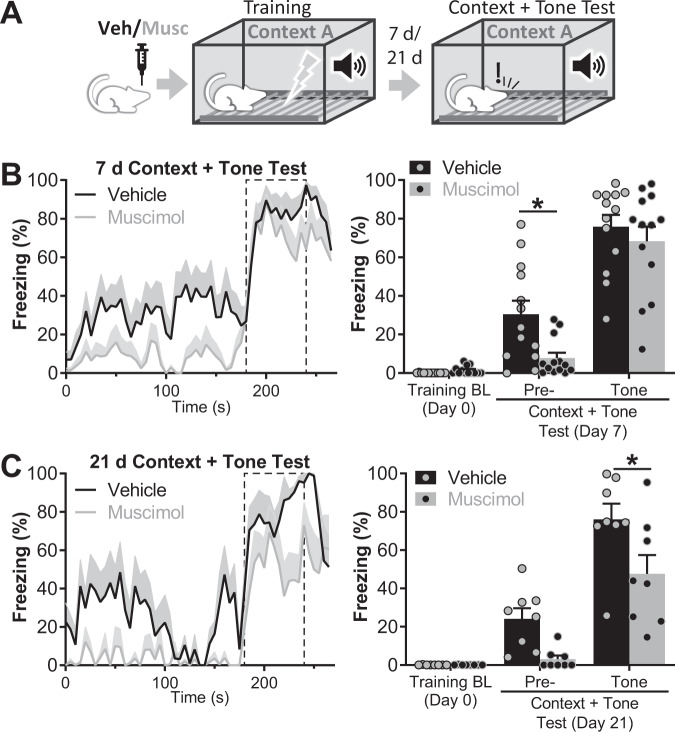
To further investigate the extent to which inactivation of the LHb impairs FC learning, we performed additional experiments in which freezing to the cue was evaluated in the training context (Context A), a condition we called “context + tone” (Fig. 3A and Supplementary Fig. 3). In that test, the pre-tone period is a readout of the contextual component of the FC memory. Indeed, confirming our previous result, during memory recall we observed a reduction of freezing in the muscimol group during the pre-tone period (pre-tone freezing: 7.76 ± 2.74% for the muscimol group vs. 30.44 ± 7.05% for the control group, p = 0.0474; nvehicle = 13, nmuscimol = 13; Fig. 3B). Presenting the tone further increased freezing in the control group and, most notably, it also induced a robust freezing in the muscimol group, which reached freezing values equivalent to the control group (tone freezing: 68.31 ± 7.47% for muscimol group vs. 75.82 ± 6.08% for control group, p = 0.8460; Fig. 3B). Thus, our results show that even under the inactivation of the LHb, a FC memory is formed that could be effectively retrieved when the cue is presented in the conditioning context.
Fig. 3. FC formed under inactivation of the LHb could be retrieved in context + tone conditions.
A Experiment diagram: bilateral intra LHb infusions of vehicle/muscimol were performed 30 min before training. Animals were trained in cued FC. Memory was evaluated 7 or 21 days later in the same context used for training (Context A). The tone was presented for 60 s after a pre-tone period of 180 s. B Freezing during recall session 7 days after training. Left panel: freezing over time. Dashed line delimits tone presentation. Right panel: average freezing for pre- and tone periods. Freezing to the context was higher in vehicle group (t(46) = 2.613, p = 0.0474). Tone elicited a robust freezing in the muscimol group equivalent to the vehicle group (t(46) = 0.899, p = 0.8460, nvehicle = 13, nmuscimol = 13). Additional statistics can be found in the Statistics details section of Supplementary Material. C Freezing during recall session 21 days after FC training. Left panel: freezing over time. Dashed line delimits tone presentation. Right panel: average freezing for pre- and tone periods. 21 days after FC training, freezing in context + tone conditions in the muscimol group was lower than in the vehicle group (t(26) = 2.683, p = 0.0491) indicating a reduced temporal stability of memory elicited by the presentation of the cue in the training context. nvehicle = 8, nmuscimol = 8. Additional statistics can be found in the Statistics details section of Supplementary Material.
In our previous article we showed that the inactivation of the LHb during Inhibitory Avoidance learning accelerates the decay of the memory [21]. To investigate the long-term stability of the FC memory formed under the inactivation of the LHb, we evaluated, in a new set of animals, freezing in “context + tone” conditions 3 weeks after training (Fig. 3A, C). Freezing levels of the muscimol group in the “context + tone” condition was lower than in the vehicle group 3 weeks after training (tone freezing: 47.59 ± 9.82% for the muscimol group vs. 75.97 ± 8.21% for the control group, t(26) = 2.683, p = 0.0491; nvehicle = 8, nmuscimol = 8; Fig. 3C), showing that, under the inactivation of the LHb, FC generates a weakened memory that is harder to retrieve and temporarily less stable.
Optogenetic inactivation of the LHb impairs cued fear conditioning
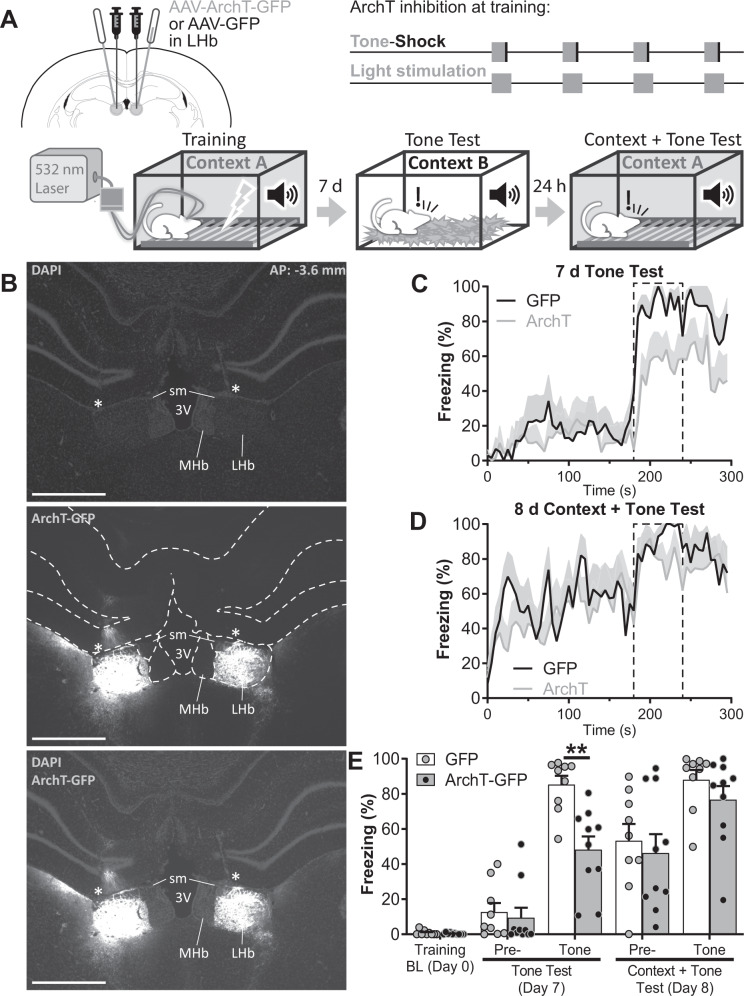
Previous articles have described that the LHb signals cue and US as increases in neuronal activity [14, 15]. To study if that increase in neuronal activity is required for FC we inactivated the LHb during tone and US presentation by means of optogenetic tools. We injected at the LHb an AAV encoding the inhibitory light-dependent proton pump Archaerhodopsin (ArchT) fused to the fluorescent protein GFP, or GFP as control, and implanted optic fiber cannulae bilaterally in the LHb with the tip placed immediately above the LHb (Fig. 4A, B). In whole cell patch-clamp recordings we confirmed that LHb neurons could be inhibited by ArchT (Supplementary Fig. 7). It has been reported that foot-shocks induce an increase in neuronal activity at the LHb that lasts for seconds [14, 15], therefore during training we inhibited the LHb from tone onset to 5 s after shock termination (Fig. 4A). We did not observe differences in freezing between ArchT and GFP groups during training (Supplementary Fig. 8). Seven and eight days later, animals underwent two recall sessions. First, a pure cued memory recall session, in which the cue was played in a novel context, followed, the next day, by a context + tone session in which cued and contextual memories were evaluated by playing the cue in the training context. On cued memory recall session we found that freezing to the tone was lower in the ArchT than in the GFP group (freezing to the tone during tone test session: 48.26 ± 7.54% for ArchT group vs. 85.32 ± 4.99% for GFP group, t(66) = 3.410, p = 0.0089; nGFP = 9, nArchT = 10; Fig. 4C, E). On the other hand, on context + tone session, ArchT group showed levels of freezing equivalent to GFP group during the pre-tone period, indicating a conserved contextual FC memory (Fig. 4D, E; pre-tone freezing during context + tone session: 46.29 ± 10.71% for ArchT group vs. 53.32 ± 9.51% for GFP group, t(66) = 0.178, p = 1.0000). In addition, in the context + tone session freezing levels in the ArchT group during tone presentation were higher than in the previous session (t(66) = 3.366, p = 0.0089), and not different from the GFP group, (tone freezing during context + tone session: 76.64 ± 7.85% for ArchT group vs. 87.99 ± 9.51% for GFP group, t(66) = 1.145, p = 0.8742, Fig. 4E), indicating again that LHb inhibition during FC training induces context-dependent deficiencies in cued FC. Importantly, ArchT optogenetic inhibition of the LHb for the same amount of time, but during inter stimulus period did not affect cued memory (Supplementary Fig. 9).
Fig. 4. Optogenetic inactivation of the LHb during cue and US, impaired cued but not contextual FC.
A Experiment diagram: Top-Left: animals were bilaterally transfected with AAV-ArchT-GFP or AAV-GFP in the LHb and implanted with optic fibers above the LHb 4 weeks before training. Top-Right: during training, optogenetic light stimulation was delivered starting with the tone and stopping 5 s after the shock (tone and shock presentations were as previously described for cued FC). Bottom: diagram of training and memory tests. Cued memory was tested 7 days after training in Context B. The same animals were re-tested the following day in the training context to evaluate contextual FC memory and tone freezing in context + tone condition. B Microphotographs of the AAV-ArchT-GFP infection at ~3.6 mm posterior to Bregma (top, middle, bottom: DAPI, GFP, and merge respectively). Dashed white lines in the middle panel delimitates brain structures. * indicates the optic fiber tract. MHb: medial habenula, sm: stria medullaris, 3V: third ventricle. Scale bars: 1 mm. Freezing over time during tone test at day 7 (C) and during context + tone test at day 8 (D). Dashed line delimits tone presentation. E Average freezing on tone test, and context + tone test sessions. During tone test ArchT group displayed lower levels of freezing to the tone than GFP group (t(66) = 3.410, p = 0.0078). The following day, during context + tone session, freezing levels of the ArchT group to both the context and the tone were equivalent to those of the GFP group (t(66) = 0.178, p = 1.0000 and t(66) = 1.145, p = 0.8742 respectively; nGFP = 9, nArchT = 10). Additional statistics can be found in the Statistics details section of Supplementary Material.
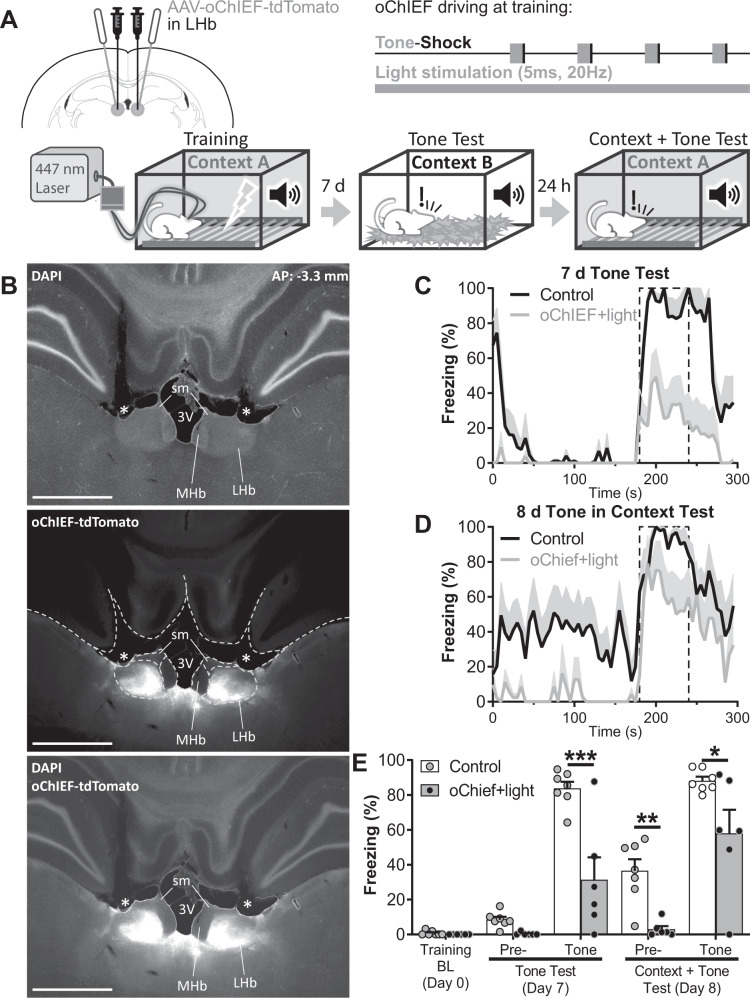
Optogenetic activation of the LHb impairs cued and contextual fear conditioning
The previous experiment showed that, in contrast to pharmacological inactivation, optogenetic inhibition of the LHb during cue and US did not affect freezing to the context, suggesting that inhibition of the LHb during the entire training is required to impair the contextual component of FC. It has been proposed elsewhere that the LHb participates in context encoding through a dynamic synchronization with the hippocampus [22, 23]. If this mechanism links the LHb to contextual FC, any arbitrary whole-training disruption of the endogenous pattern of activity of the LHb, regardless of its sign, should disrupt contextual FC. To investigate this possibility, we assayed the effect on FC of a sustained arbitrary optogenetic excitation of the LHb during training. Based on published reports, we assayed the fast excitatory opsin oChIEF, which has a fast recovery kinetics that allows sustained stimulation [24]. It has been shown that optogenetic stimulation of the LHb with oChIEF with 5 ms pulses delivered at 20 Hz does not to generate place aversion [25], thus we chose that pattern of stimulation during FC training. This light stimulation pattern implies a 10% duty cycle that, in contrast with the sustained illumination that would be required for constant ArchT activation, would result in minimal temperature changes in the illuminated tissue (Supplementary Fig. 10). In patch-clamp experiments, we verified that neurons of the LHb could be reliably activated by 5 ms light pulses at 20 Hz for the same amount of time of our training protocol (≈ 8 min, Supplementary Fig. 11), demonstrating the feasibility of our approach. To achieve sustained stimulation of the LHb during FC training we infused an AAV encoding oChIEF and implanted optic fibers bilaterally immediately above the LHb (Fig. 5A, B). Three weeks later, subjects were trained in cued FC under a sustained 20 Hz optogenetic excitation. Light stimulation did not modify freezing during FC training (Supplementary Fig. 12). Seven and eight days later, memory was evaluated in a pure cued memory recall session at day 7, followed the next day by a context + tone session. During pure cued memory recall session, oChIEF stimulated animals showed lower levels of freezing to the tone than the control group (freezing to the tone during tone test: 31.4 ± 12.85% for oChIEF + light group vs. 83.76 ± 3.87% for control group, t(42) = 4.454, p = 0.0004; nControl = 7, noChIEF + light = 6; Fig. 5C, E). The next day, during “context + tone” session, oChIEF stimulated animals showed a pronounced reduction in contextual freezing (pre-tone freezing during context + tone test: 2.97 ± 1.84% for oChIEF + light group vs. 36.51 ± 6.61% for control group, t(42) = 3.874, p = 0.0026; Fig. 5D, E). Notably, following cue presentation, oChIEF + light group displayed freezing levels higher than in pure cued memory recall session performed the previous day, replicating the profile of memory deficits generated by pharmacological and optogenetic inhibition of the LHb (tone freezing in the oChIEF + light group: Day 7 vs. Day 8: t(42) = 3.810, p = 0.0031; Fig. 5D, E). However, freezing levels of the oChIEF + light group were still lower than in the control group (tone freezing during context + tone test: 57.97 ± 13.55% for oChIEF + light group vs. 88.1 ± 2.37% for control group, t(42) = 2.995, p = 0.0317; Fig. 5D, E).
Fig. 5. Optogenetic excitation of the LHb during complete FC training impairs contextual and cued FC.
A Experiment diagram: Top-Left: animals were bilaterally infected with AAV-oChIEF-tdTomato in the LHb and implanted with optic fibers above the LHb 4 weeks before training. Top-Right: during whole-training session the LHb was stimulated with 5 ms light pulses at 20 Hz to disrupt endogenous neuronal activity. Bottom: diagram of training and tests. Cued memory was tested 7 days after training in Context B. The same animals were re-tested the following day in the training context to evaluate contextual FC memory and tone freezing in a context + tone session. B Microphotographs of the AAV-oChIEF-tdTomato infection at approximately 3.3 mm posterior to bregma (top, middle, bottom: DAPI, tdTomato fluorescence, and merge respectively). Dashed white lines in the middle panel delimitates brain structures. * indicates the optic fiber tract. MHb: medial habenula, sm: stria medullaris, 3V: third ventricle. Scale bars: 1 mm. Freezing over time during cued test at day 7 (C) and during context + tone test at day 8 (D). Dashed line delimited area indicates tone presentation. E Average freezing of tone test and context + tone test sessions. Disruption of endogenous LHb activity during training by optogenetic stimulation-induced deficits in cued (tone freezing during Tone Test: control vs oChIEF + light: t(42) = 4.454, p = 0.0004; tone freezing during Context + Tone test: control vs oChIEF + light: t(42) = 2.995, p = 0.0317 respectively), and contextual memories (pre-tone freezing during context + tone test: control vs oChIEF + light: t(42) = 3.874, p = 0.0026). nControl = 7, noChIEF + light = 6. Additional statistics can be found in the Statistics details section of Supplementary Material.
Discussion
There is a paucity of information regarding the role of the LHb in fear learning [17–19]. Here we investigated this subject and defined a new role for the LHb in FC, while simultaneously highlighting previously overlooked aspects of the paradigm. Our data indicate that, if conditioning takes place without proper activity of the LHb, neither context nor cued memories could be independently expressed. However, memory expression is evident when both contextual and cued components of FC are reactivated by the presentation of the cue in the conditioning context. Hence, if activity of the LHb is disrupted during FC learning, memory retrieval requires the synergy of contextual and cue information. To the best of our knowledge, that observation turns the LHb into the first brain structure implicated in the regulation of the independent expression of contextual and cued components of FC. Thus, our observation assigns a critical and previously undescribed role to the LHb within the brain circuits implicated in fear learning.
The LHb during fear learning
Three different strategies were used here to study the role of the LHb in FC. These were: pharmacological inhibition, optogenetic inhibition during cue and US, and optogenetic excitation during the entire training. Neither of them affected freezing behavior during training. Previous reports have shown that the LHb regulates the balance between active and passive coping in aversive tasks such as the forced swim test [20, 26], the tail suspension test [27], or the looming task [13]. Thus, our results indicate that the participation of the LHb in the development of passive coping strategies might be a fine-tuned process related to task-specific variables. In contrast, those manipulations affected fear memory recall. A closer examination of those results could provide information about the involvement of the LHb in contextual and cued FC.
The behavioral outcomes of sustained optogenetic excitation and pharmacological inhibition of the LHb during training were, to a great extent, similar. The equivalence of those two manipulations could seem odd, but it is totally predicted if their effects are interpreted as a disruption of LHb encoding during fear acquisition. Indeed, many examples of equivalent behavioral effects of excitation and inhibition of brain structures are present in the literature [28–34]. However, optogenetic excitation and pharmacological inhibition markedly differ in their temporal resolution. While muscimol-mediated inhibition of the LHb extends for at least 2 h beyond the training period [35, 36], optogenetic excitation was precisely limited to it. Hence, the similar outcome of both manipulations suggests that the participation of the LHb in FC is limited to the learning/acquisition phase and does not extend over the consolidation period. This interpretation is in agreement with our previous work describing an acquisition-limited role of the LHb in the Inhibitory Avoidance paradigm of aversive learning [21].
Neuronal activity of the LHb increases during presentation of the cue and the US [14–16]. Furthermore, the LHb is a source of aversive prediction error in monkeys [37] and rats [38]. Thus, it is conceivable that, during cued conditioning, the LHb encodes, as a transient increase in neuronal activity, a signal informing about the aversive value of the US and the cue [39]. Consistent with this hypothesis, we found that optogenetic inhibition of the LHb during tone and shock presentation was sufficient to impair cued memory, while the same amount of inhibition unpaired from cue and US had no effect. The relevance of this phasic signaling of the LHb for cued FC could be inferred from our work. While it would not be required for the cue-US association to be formed, without it the predictive value of the cue would be degraded to a context-specific threat signal.
In contrast to pharmacological inhibition and optogenetic excitation, optogenetic inhibition of the LHb during cue and US did not affect contextual FC. This discrepancy suggests that the contribution of the LHb to context-US association extends beyond its activation by the cue and the US [14–16]. It has been previously proposed that the LHb influences context encoding through a functional interaction with the hippocampus [22, 23]. This hypothesis has received support from physiological and behavioral data generated by us and other authors showing that firing of LHb neurons is synchronized with hippocampal theta rhythm [23, 40, 41] and that LHb-innervated brain-stem neurons regulates hippocampal theta and contextual FC [12, 42]. Our results are partially compatible with that hypothesis since interfering with neuronal activity of the LHb during context encoding, either by pharmacological inactivation or sustained optogenetic activation, severely impaired contextual FC, while cue-US temporally limited inactivation of the LHb did not. On the other hand, we found that, although context by itself did not elicit freezing, it was required for cue evoked freezing, evidencing the retention of some contextual information. Further work is needed in to gain insight into this subject.
In summary, our results suggest a scenario in which the LHb would regulate contextual and cued FC by separate mechanisms related to context encoding and aversion signaling respectively. Both mechanisms have been already proposed separately by other authors [17, 22, 23, 39].
Circuits of the LHb related to FC
The neuronal circuits linking the LHb to FC remain to be elucidated and could not be unequivocally sketched based on the current knowledge. The LHb projects directly to both dopaminergic and serotoninergic systems as well as indirectly through its projection to the Rostromedial Tegmental Nucleus (RMTg [43]). Importantly, both neuromodulators regulate fear learning [44–47] and the RMTg itself has been shown to regulate FC [43]. In addition, many structures upstream of the LHb have been implicated in FC, most notably the Central Amygdala, which sends a dense projection to the LHb [48]. However, the LHb receives sensory and motivational information relevant to FC from multiple structures, such as the Lateral Hypothalamus [14, 16], the Entopeduncular Nucleus [49], the Medial Septum [50], the Median Raphe [12], or the Lateral Preoptic Area [51]. Thus, the LHb could control FC by gating the transmission of aversive signals from several of its inputs to several of its main downstream targets.
Recently, transcriptional profiling of LHb neurons identified a specific cluster of neurons in which foot-shocks modulate expression of immediate early genes [52]. That cluster of LHb cells would be a suitable candidate to participate in FC. Further characterization of their connectivity would allow to define a LHb centered circuit participating in FC.
The LHb and memory strength
How easy a memory is retrieved, or how long it lasts, are parameters of memory strength [53]. The FC memory formed under inactivation of the LHb could be considered weaker in those two aspects. It is harder to retrieve, since matching between training and testing conditions should be increased for effective retrieval, and is temporally less stable (Fig. 3C). We have previously described that inactivation of the LHb before training in the Inhibitory Avoidance generates a memory that is normally expressed 24 h after training but could not be evidenced 7 days later [21]. Taken together, our two works suggest that the LHb could also be conceptualized as a regulator of aversive memory strength.
Concluding remarks
Cued and contextual FC have been mostly considered two independent associative learnings that take place in parallel during a single experience. This assumption, which has deeply influenced the interpretation of fear learning, has been mostly unchallenged. Here we described for the first time that LHb signaling during conditioning determines that those associations could be independently expressed. Future work on the mechanisms underlying this observation will be critical to understand how different brain regions involved in fear learning interact to regulate the formation, strength, and generalization of fear memories.
Supplementary information
Acknowledgements
We would like to thank Dr. Roberto Malinow for his help in the generation of AAV vectors, to Yamila Paez for her technical assistance, and to Sadegh Nabavi, Dario Lemos, Cecilia Martinez, Mariano Belluscio, Sebastian Giusti and Paula Ospital for their helpful comments.
Author contributions
TES: conceptualization, methodology, software, formal analysis, investigation, data curation, writing-original draft, writing-review & editing, visualization; MRI: validation, formal analysis, investigation, data curation, writing-original draft; CP: validation, pilot experiments, writing-review & editing; DEP: investigation, JHM: resources, writing-review & editing, supervision, funding acquisition; PM: conceptualization, formal analysis, investigation, resources, writing-review & editing, visualization, supervision, funding acquisition; JP: conceptualization, methodology, resources, writing-original draft, writing-review & editing, supervision, project administration, funding acquisition.
Funding
This work was supported by the following grants: PICT 2016-0034 from the National Agency for Scientific and Technological Promotion of Argentina (ANPCyT) to JM; I-COOP + (CSIC) ref COOPA20198 to PM; NARSAD, Young Investigator Award 2015 (#23861) from the Brain and Behavior Foundation, PICT 2015-2609 and PICT 2017-4594 from the ANPCyT to JP. TS was supported by a predoctoral fellowship from the CONICET. CDP is supported by the Canadian Institutes of Health Research grant PJT169117 and the Natural Science and Engineering Research Council of Canada grant RGPIN-2017-06131, and receives Fonds de Recherche en Santé du Québec (FRQS) Junior-1 salary support.
Data availability
Data needed to evaluate the conclusions in the paper is available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01294-5.
References
- 1.Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–6. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- 2.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 3.Phillips RG, Ledoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. [DOI] [PubMed]
- 4.Kim J, Fanselow M. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 5.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–52. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 7.Sah P, Westbrook RF, Lüthi A. Fear conditioning and long-term potentiation in the amygdala. Ann N. Y Acad Sci. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- 8.Bocchio M, Nabavi S, Capogna M. Synaptic plasticity, engrams, and network oscillations in amygdala circuits for storage and retrieval of emotional memories. Neuron. 2017;94:731–43. [DOI] [PubMed]
- 9.Kim WB, Cho JH. Encoding of contextual fear memory in hippocampal–amygdala circuit. Nat Commun. 2020;11:1382. doi: 10.1038/s41467-020-15121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maren S, Phan KL, Liberzon I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–28. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci. 2020;21:277–95. doi: 10.1038/s41583-020-0292-4. [DOI] [PubMed] [Google Scholar]
- 12.Szőnyi A, Zichó K, Barth AM, Gönczi RT, Schlingloff D, Török B, et al. Median raphe controls acquisition of negative experience in the mouse. Science. 2019;366:eaay8746. [DOI] [PubMed]
- 13.Lecca S, Namboodiri VMK, Restivo L, Gervasi N, Pillolla G, Stuber GD, et al. Heterogeneous habenular neuronal ensembles during selection of defensive behaviors. Cell Rep. 2020;31:107752. doi: 10.1016/j.celrep.2020.107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trusel M, Nuno-Perez A, Lecca S, Harada H, Lalive AL, Congiu M, et al. Punishment-predictive cues guide avoidance through potentiation of hypothalamus-to-habenula synapses. Neuron. 2019;102:120–127.e4. doi: 10.1016/j.neuron.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Li Y, Feng Q, Guo Q, Zhou J, Luo M. Learning shapes the aversion and reward responses of lateral habenula neurons. Elife. 2017;19:1–16. doi: 10.7554/eLife.23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazaridis I, Tzortzi O, Weglage M, Märtin A, Xuan Y, Parent M, et al. A hypothalamus-habenula circuit controls aversion. Mol Psychiatry. 2019;24:1351–68. [DOI] [PMC free article] [PubMed]
- 17.Durieux L, Mathis V, Herbeaux K, Muller M–A, Barbelivien A, Mathis C, et al. Involvement of the lateral habenula in fear memory. Brain Struct Funct. 2020;225:2029–44. [DOI] [PubMed]
- 18.Song M, Jo YS, Lee YK, Choi JS. Lesions of the lateral habenula facilitate active avoidance learning and threat extinction. Behav Brain Res. 2017;318:12–7. doi: 10.1016/j.bbr.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Velazquez-Hernandez G, Sotres-Bayon F. Lateral habenula mediates defensive responses only when threat and safety memories are in conflict. ENeuro. 2021;8:1–14. doi: 10.1523/ENEURO.0482-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proulx CD, Aronson S, Milivojevic D, Molina C, Loi A, Monk B. A neural pathway controlling motivation to exert effort. Proc Natl Acad Sci USA. 2018;115:5792–7. [DOI] [PMC free article] [PubMed]
- 21.Tomaiuolo M, Gonzalez C, Medina JH, Piriz J. Lateral Habenula determines long-term storage of aversive memories. Front Behav Neurosci. 2014;8:1–9. doi: 10.3389/fnbeh.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker PM, Rao Y, Rivera ZMG, Garcia EM, Mizumori SJY. Selective functional interaction between the lateral habenula and hippocampus during different tests of response flexibility. Front Mol Neurosci. 2019;12:245. doi: 10.3389/fnmol.2019.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goutagny R, Loureiro M, Jackson J, Chaumont J, Williams S, Isope P, et al. Interactions between the lateral habenula and the Hippocampus: Implication for spatial memory processes. Neuropsychopharmacology. 2013;38:2418–26. doi: 10.1038/npp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–14. [DOI] [PMC free article] [PubMed]
- 25.Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–22. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 26.Coffey KR, Marx RG, Vo EK, Nair SG, Neumaier JF. Chemogenetic inhibition of lateral habenula projections to the dorsal raphe nucleus reduces passive coping and perseverative reward seeking in rats. Neuropsychopharmacology. 2020;45:1115–24. doi: 10.1038/s41386-020-0616-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerniauskas I, Winterer J, de Jong JW, Lukacsovich D, Yang H, Khan F, et al. Chronic stress induces activity, synaptic, and transcriptional remodeling of the lateral habenula associated with deficits in motivated behaviors. Neuron. 2019;104:899–915.e8. doi: 10.1016/j.neuron.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tecuapetla F, Jin X, Lima SQ, Costa RM. Complementary contributions of striatal projection pathways to action initiation and execution. Cell. 2016;166:703–15. doi: 10.1016/j.cell.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 29.Hart EE, Blair GJ, O’Dell TJ, Blair HT, Izquierdo A. Chemogenetic modulation and single-photon calcium imaging in anterior cingulate cortex reveal a mechanism for effort-based decisions. J Neurosci. 2020;40:5628–43. doi: 10.1523/JNEUROSCI.2548-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krueger JN, Wilmot JH, Teratani-Ota Y, Puhger KR, Nemes SE, Crestani AP, et al. Amnesia for context fear is caused by widespread disruption of hippocampal activity. Neurobiol Learn Mem. 2020;175:107295. doi: 10.1016/j.nlm.2020.107295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahn JB, Port RG, Anderson SA, Coulter DA. Modular, circuit-based interventions rescue hippocampal-dependent social and spatial memory in a 22q11.2 deletion syndrome mouse model. Biol Psychiatry. 2020;88:710–8. doi: 10.1016/j.biopsych.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander GM, Riddick NV, McCann KE, Lustberg D, Moy SS, Dudek SM. Modulation of CA2 neuronal activity increases behavioral responses to fear conditioning in female mice. Neurobiol Learn Mem. 2019;163:107044. doi: 10.1016/j.nlm.2019.107044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron. 2018;97:670–83.e6. doi: 10.1016/j.neuron.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77:955–68. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arikan R, Blake NMJ, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods. 2002;118:51–57. doi: 10.1016/S0165-0270(02)00143-7. [DOI] [PubMed] [Google Scholar]
- 36.Edeline JM, Hars B, Hennevin E, Cotillon N. Muscimol diffusion after intracerebral microinjections: a reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiol Learn Mem. 2002;78:100–24. doi: 10.1006/nlme.2001.4035. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Vento PJ, Parrilla-Carrero J, Pullmann D, Chao YS, Eid M, et al. Three rostromedial tegmental afferents drive triply dissociable aspects of punishment learning and aversive valence encoding. Neuron. 2019;104:987–999.e4. doi: 10.1016/j.neuron.2019.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Pullmann D, Cho JY, Eid M, Jhou TC. Generality and opponency of rostromedial tegmental (RMTg) roles in valence processing. Elife. 2019;8:1–19. [DOI] [PMC free article] [PubMed]
- 40.Aizawa H, Yanagihara S, Kobayashi M, Niisato K, Takekawa T, Harukuni R, et al. The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. J Neurosci. 2013;33:8909–21. doi: 10.1523/JNEUROSCI.4369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertone-Cueto NI, Makarova J, Mosqueira A, García-Violini D, Sánchez-Peña R, Herreras O, et al. Volume-conducted origin of the field potential at the lateral habenula. Front Syst Neurosci. 2020;13:78. [DOI] [PMC free article] [PubMed]
- 42.Szőnyi A, Sos KE, Nyilas R, Schlingloff D, Domonkos A, Takács VT, et al. Brainstem nucleus incertus controls contextual memory formation. Science. 2019;364:eaaw0445. doi: 10.1126/science.aaw0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta A, Holmes A. A discrete dorsal raphe to basal amygdala 5-HT circuit calibrates aversive memory. Neuron. 2019;103:489–505.e7. doi: 10.1016/j.neuron.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burghardt NS, Bauer EP. Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: Implications for underlying fear circuits. Neuroscience. 2013;247:253–72. doi: 10.1016/j.neuroscience.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 46.Bissieére S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–92. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- 47.Kwon OB, Lee JH, Kim HJ, Lee S, Lee S, Jeong MJ, et al. Dopamine regulation of amygdala inhibitory circuits for expression of learned fear. Neuron. 2015;88:378–89. doi: 10.1016/j.neuron.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci. 2019;22:1649–58. [DOI] [PubMed]
- 49.Li H, Pullmann D, Jhou TC. Valence-encoding in the lateral habenula arises from the entopeduncular region. Elife. 2019;8:1–17. doi: 10.7554/eLife.41223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang GW, Shen L, Zhong W, Xiong Y, Zhang LI, Tao HW. Transforming Sensory Cues into Aversive Emotion via Septal-Habenular Pathway. Neuron. 2018; 99:1016–28.e5. [DOI] [PMC free article] [PubMed]
- 51.Barker DJ, Miranda-Barrientos J, Zhang S, Root DH, Wang HL, Liu B, et al. Lateral preoptic control of the lateral habenula through convergent glutamate and GABA transmission. Cell Rep. 2017;21:1757–69. doi: 10.1016/j.celrep.2017.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashikawa Y, Hashikawa K, Rossi MA, Basiri ML, Liu Y, Johnston NL, et al. Transcriptional and spatial resolution of cell types in the mammalian habenula. Neuron. 2020;106:743–758.e5. doi: 10.1016/j.neuron.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medina JH, Bekinschtein P, Cammarota M, Izquierdo I. Do memories consolidate to persist or do they persist to consolidate? Behav Brain Res. 2008;192:61–69. doi: 10.1016/j.bbr.2008.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data needed to evaluate the conclusions in the paper is available upon request.