Abstract
Chronological age is a risk factor for SARS-CoV-2 infection and severe COVID-19. Previous findings indicate that epigenetic age could be altered in viral infection. However, the epigenetic aging in COVID-19 has not been well studied. In this study, DNA methylation of the blood samples from 232 healthy individuals and 413 COVID-19 patients is profiled using EPIC methylation array. Epigenetic ages of each individual are determined by applying epigenetic clocks and telomere length estimator to the methylation profile of the individual. Epigenetic age acceleration is calculated and compared between groups. We observe strong correlations between the epigenetic clocks and individual’s chronological age (r > 0.8, p < 0.0001). We also find the increasing acceleration of epigenetic aging and telomere attrition in the sequential blood samples from healthy individuals and infected patients developing non-severe and severe COVID-19. In addition, the longitudinal DNA methylation profiling analysis find that the accumulation of epigenetic aging from COVID-19 syndrome could be partly reversed at late clinic phases in some patients. In conclusion, accelerated epigenetic aging is associated with the risk of SARS-CoV-2 infection and developing severe COVID-19. In addition, the accumulation of epigenetic aging from COVID-19 may contribute to the post-COVID-19 syndrome among survivors.
Subject terms: DNA methylation, Viral infection, Ageing
Age is a risk factor for SARS-CoV-2 infection and severe disease. Here the authors perform DNA methylation analyses in whole blood from COVID-19 patients using established epigenetic clocks and telomere length estimators, and describing correlations between epigenetic aging and the risk of SARS-CoV-2 infection and severe disease.
Introduction
The ongoing pandemic of coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome-corona virus 2 (SARS-CoV-2) infection is a rapidly developing global emergency which already claimed over 170 million cases and 3.8 million lives worldwide as of June 13rd, 2021 (WHO Coronavirus Situation Report, 2021)1. The incidence of severity and death in COVID-19 patients has been reported to increase with age2–5. Chronological age has been proved to be one of the major risk factors for developing severe COVID-19 and death6, and this risk is independent of other age-related comorbidities like diabetes, cardiovascular diseases, or obesity7. Therefore, understanding the biological aging of COVID-19 in severe cases versus non-severe patients could be quite useful to model and predict the disease progression with other laboratory assays8.
Aging is a biological process related to diseases and mortality. The biological process in aging is reflected by molecular hallmarks, which include epigenetic modifications and telomere attrition9–11. DNA methylation correlates with aging process and can be used to estimate epigenetic aging across tissues12–14. The deviation between DNA methylation age (DNAm age) and chronological age has been proposed as a biomarker for aging and has been related to risk and survival outcomes in age-related diseases15,16. Previous work showed that the epigenetic landscape of host cell is altered during HIV17–19 and coronavirus20–23 infection, including SARS-CoV-2. In addition, telomere attrition in leukocytes is another hallmark of aging and associated with increased risk of aging-related diseases and human life span24–26, and a DNAm-based telomere length estimator (DNAm TL) has been developed27. Interestingly, telomere length and the epigenetic clock do not correlate with one another, suggesting that DNAm age and TL measure different aspects of biological aging28,29. Recently, epigenetic age at the moment of the infection has been reported to be associated with COVID-1930–32. However, little is known about the epigenetic aging during SARS-CoV-2 infection and in COVID-19 survivors and whether this factor might help predict the risk of developing severe COVID-19.
In this study, we estimated the epigenetic age of the whole blood in COVID-19 patients and healthy individuals using the previously established epigenetic clocks (Hannum, Horvath, PhenoAge, skinHorvath and GrimAge clocks)12,33–36 and telomere length estimator27. We defined epigenetic age acceleration for each case by comparing the individual’s epigenetic age with chronological age to assess whether the accelerated or dysfunctional epigenetic aging is associated with SARS-CoV-2 infection and the severity of COVID-19 syndrome.
Results
Assessment of epigenetic age in COVID-19 patients
To study epigenetic aging in the whole blood and its correlation with non-severe and severe COVID-19, we conducted a genome-wide DNA methylation study on whole blood collected from 232 healthy individuals, 194 non-severe and 213 severe COVID-19 patients in the combined set to calculate their DNAm age and TL using five different epigenetic clocks and DNAm TL estimator. The COVID-19 patients were not significantly different from the healthy individuals in terms of age and sex (Supplementary Table 1).
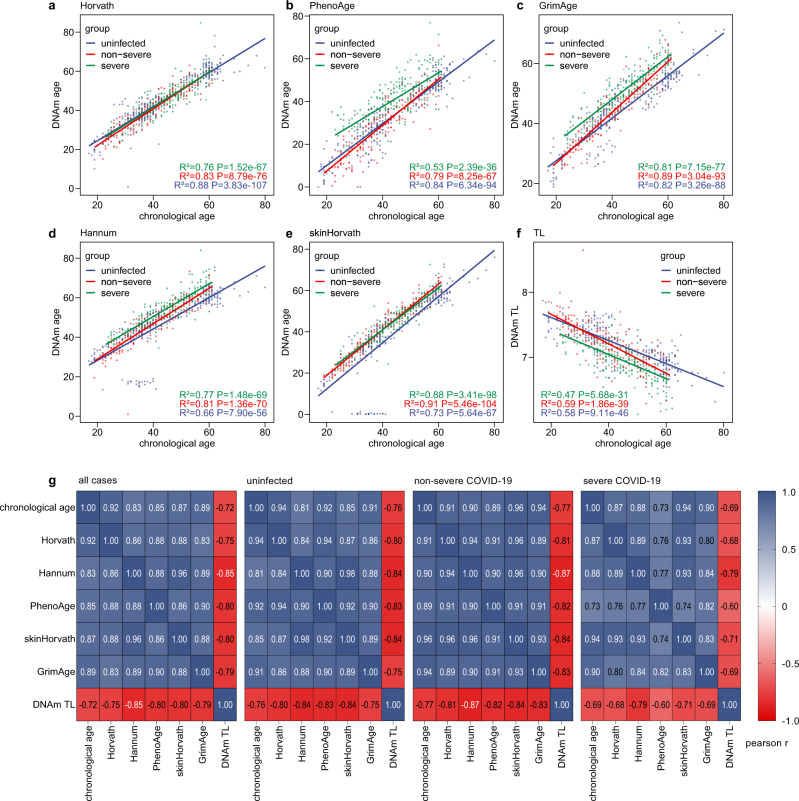
We calculated the Pearson correlation coefficient among individual chronological age, epigenetic clocks and DNAm TL in the combined set of samples. As shown in Fig. 1, all clocks had a strong correlation with the chronological age in the whole set and each sample group (r > 0.8, p < 0.0001), though the PhenoAge clock showed weaker correlations with chronological age in the severe COVID-19 group (r = 0.73, p < 0.0001). In addition, all clocks were strongly correlated with one another in all groups (r > 0.8, p < 0.0001; Fig. 1 and Supplementary Fig. 1), except that PhenoAge clock showed weaker correlations with other clocks in the severe COVID-19 group (r = 0.73 ~ 0.77, p < 0.0001). DNAm TL was negatively correlated with chronological and DNAm ages in the whole set and all groups (r = −0.59 ~ −0.90, p < 0.0001; Fig. 1). Interestingly, DNAm TL showed a stronger linear correlation with DNAm ages compared to chronological age (Fig. 1 and Supplementary Fig. 2).
Fig. 1. Assessment of epigenetic clocks and DNA methylation-based telomere length estimator in patient cohorts.
a–e Correlation of chronological age with five epigenetic clocks (a Horvath; b Hannum; c PhenoAge; d skinHorvath; e GrimAge) and DNA methylation-based telomere length (f TL) estimator in the peripheral blood from 232 healthy individuals, 194 non-severe and 213 severe COVID-19 patients. g Heatmap presents the matrix of Pearson correlation coefficients among chronological age, DNAm ages determined by each epigenetic clock, and DNAm TL in the whole, healthy, non-severe and severe sets. Source data are provided as a Source Data file.
Accelerated epigenetic aging in SARS-CoV-2 infection
We initially assessed the DNAm ages and TLs of the blood samples and found an older DNAm age in the COVID-19 patients for Horvath, Hannum, skinHorvath and GrimAge clocks compared to the healthy individuals (p < 0.05; Supplementary Fig. 3). To adjust for the bias due to individual chronological age, we calculated epigenetic age acceleration for each sample. Individuals with COVID-19 were estimated to have significant DNAm age acceleration for Hannum, PhenoAge, skinHorvath and GrimAge clocks and significant DNAm TL attrition acceleration compared with healthy individuals (all p < 0.0001; Supplementary Fig. 4). The same phenomena were observed in the young (age < 50) and old (age ≥ 50) populations (Supplementary Fig. 5). Together, we found an accelerated epigenetic aging in patients with SARS-CoV-2 infection.
Accelerated epigenetic aging and risk of developing severe COVID-19
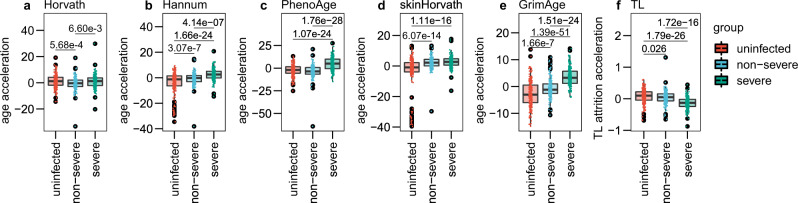
Next, we analyzed the association of COVID-19 severity with epigenetic aging. We found individuals with severe COVID-19 had significant DNAm age acceleration for all epigenetic clocks and DNAm TL attrition acceleration compared with healthy individuals (all p < 0.0001; Fig. 2). In addition, severe COVID-19 patients had significant DNAm age acceleration for Horvath, Hannum, PhenoAge and GrimAge clocks and significant DNAm TL attrition acceleration compared with non-severe COVID-19 patients (all p < 0.05; Fig. 2). Moreover, non-severe COVID-19 patients had significant DNAm age acceleration for Horvath, Hannum, skinHorvath and GrimAge clocks and significant DNAm TL attrition acceleration compared with healthy individuals (all p < 0.05; Fig. 2). Among the five epigenetic clocks, the statistical difference in the pairwise test for GrimAge clock was most significant. In addition, we found that COVID-19 patients developing pneumonia had significantly accelerated epigenetic aging compared with those not developing pneumonia (Supplementary Fig. 6). Together, we found an increasing acceleration of epigenetic aging in the sequential samples of healthy, non-severe and severe groups.
Fig. 2. Accelerated epigenetic aging in non-severe and severe COVID-19 patients.
Distribution of DNAm age acceleration (a–e, five epigenetic clocks) and telomere attrition acceleration (f, TL) in the peripheral blood from 232 healthy individuals, 194 non-severe and 213 severe COVID-19 patients. The y-axis shows the epigenetic age acceleration after adjusting for cell-type fractions (i.e., residual of regressing the epigenetic age acceleration on cell-type fractions). The p value for each t-test is shown above the corresponding line. In the box plots, the lower and upper hinges indicate the 25th and 75th percentiles, and the black line within the box marks the median value. The whiskers extend from the hinges to the largest and smallest values no further than 1.5× inter-quartile range from the hinges, and the points beyond the end of whiskers indicate outliers. Source data are provided as a Source Data file.
Dynamic acceleration of epigenetic aging across COVID-19 disease phases
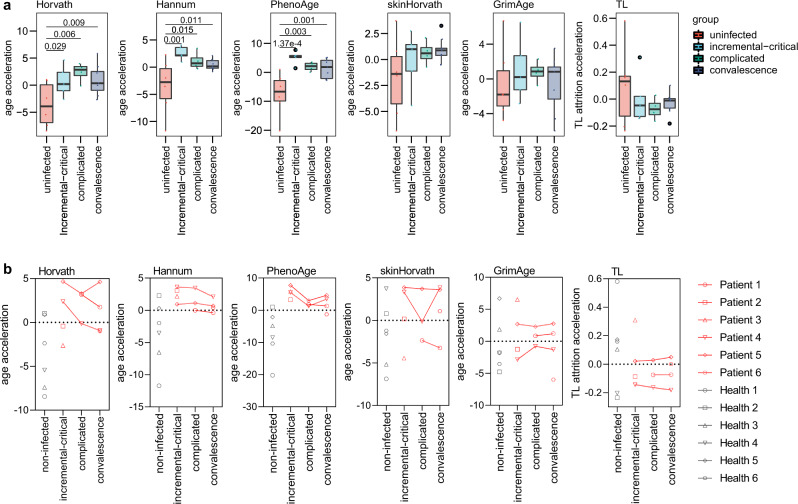
To explore the role of COVID-19 in epigenetic age acceleration, we analyzed the dynamic acceleration of epigenetic age across COVID-19 disease phases in the Bernarde et al’s longitudinal cohort37 with six COVID-19 cases and six uninfected controls. Clinical disease phases were defined based on inflammatory markers and ventilation needs, which reflected temporal disease severity and distinguish between incremental and recovering disease stages, according to the WHO ordinal scale described by Bernardes et al.37.
We assessed the DNAm ages and TLs of the samples collected from each disease phase and found an increasing acceleration of epigenetic aging at the initial phases of COVID-19, and this age acceleration could be partly reversed at later phases (Fig. 3a). Specifically, we found an increasing acceleration of Horvath age at the initial two disease phases and this acceleration was partly reversed in the upcoming convalescence phase. Similarly, Hannum and PhenoAge clocks were accelerated at the initial stage of incremental and critical disease phase and reversed in the upcoming complicated and convalescence phases. In addition, an increasing attrition acceleration of DNAm age at the initial two disease phases was found to be partly reversed in the upcoming convalescence phase, although the differences between every two phases were not statistically significant.
Fig. 3. Epigenetic aging across COVID-19 disease phases.
a Acceleration of DNAm aging (five epigenetic clocks) and telomere attrition of the blood samples collected from uninfected control and patients at each clinical disease phase. b Dynamic change of epigenetic age acceleration in each individual across COVID-19 disease phases. The p value for each t-test is shown above the corresponding line. In the box plots, the lower and upper hinges indicate the 25th and 75th percentiles, and the black line within the box marks the median value. The whiskers extend from the hinges to the largest and smallest values no further than 1.5 × inter-quartile range from the hinges, and the points beyond the end of whiskers indicate outliers. Source data are provided as a Source Data file.
Furthermore, we tracked the dynamic changes of DNAm ages and TLs during the clinical disease phases in individual patients and observed similar result to the group-wise comparison. The accelerated DNAm ages at initial phases were partly reversed at the later phases in some COVID-19 patients by using the five epigenetic clocks, while the reversible outcome of accelerated attrition of DNAm TL from initial to later phases was found only in one patient (Fig. 3b). Therefore, we speculated that COVID-19 syndrome might accelerate epigenetic aging in SARS-CoV-2 infected patients, and this process would be partly reversible in the clinical disease phases.
Discussion
In this study, we took advantage of prior indication that epigenetic age could be altered in the presence of viral infections17,20,38 and from the fact that shorter telomeres have been associated with the risk of developing COVID-19 with worse outcomes32. We used five epigenetic clocks and one telomere length estimator to determine the epigenetic age of the whole blood in COVID-19 patients and healthy individuals. We observed strong correlation of the epigenetic clocks with the chronological age and increasing acceleration of epigenetic aging in the blood samples of healthy individuals and COVID-19 patients. Our epigenetic study with consistent findings from different epigenetic clocks provides evidence for the association of accelerated epigenetic aging with the risk of SARS-CoV-2 infection and developing severe COVID-19. In addition, we found a reversible influence of COVID-19 syndrome on epigenetic aging in some COVID-19 patients with the longitudinal DNA methylation profiling analysis. Overall, these findings suggest that COVID-19 may perturb the epigenetic clock and telomere length. Although the epigenetic clocks and telomere length are known as independent28,29, DNAm aging was parallelled by a telomere shortening in all the observations.
DNA methylation-based estimator of telomere length was shown to have a relatively weak correlation with PCR-based telomere length that is recognized as an accurate quantification36. Therefore, PCR assays are needed to validate the correlation between telomere length and COVID-19. Mongelli et al. applied PCR assay to quantify telomere length and found a telomere shortening in the post-COVID-19 survivors39. Although some of our findings could be supported by Mongelli, the association of telomere attrition with severe COVID-19 and dynamic change of telomere length across disease phases need to be confirmed in a longitudinal cohort with PCR assays.
Epigenetic changes have been reported to be associated with the SARS-CoV-2 infection and unfavorable COVID-19 outcomes, including the methylation regulation of Angiotensin Converting Enzyme 2 (ACE2)40, interferon-related pathways23, and immune response genes23,41. Considering the DNA methylation changes in SARS-CoV-2 infection that may affect the expression of metabolic process and epigenetic aging of COVID-1941, a further question could be whether the accelerated epigenetic aging exists before the first viral contact, worsening progressively up or coming back to normal during the convalescence phases and post-COVID-19 period. Our analysis of epigenetic clocks and telomere shortening indicates an increasing age acceleration at the initial phases of COVID-19 and this could be partly reversed at later phases. We speculated that COVID-19 syndrome might accelerate epigenetic aging in SARS-CoV-2-infected patients based on these findings. Our longitudinal DNA methylation profiling analysis indicates that the influence of COVID-19 syndrome on epigenetic aging in peripheral blood could be reversed in some patients. However, additional experiments are necessary to validate these findings and elucidate the underlying mechanism.
In the current study, we found accelerated epigenetic aging could serve as a predicting biomarker for severe COVID-19 that requires hospitalization with increased mortality. However, a recent study found that the association of accelerated epigenetic aging with severe COVID-19 varied among different epigenetic clocks and datasets42, which might be attributed to the small sample size in some datasets, unspecified severity of COVID-19 cases, and the different estimation method of age acceleration. Interestingly, the difference between severe COVID-19 patients and other individuals in the GrimAge clock was most significant among the five epigenetic clocks in our cohort. The strong stratification in the severity of COVID-19 may come from the correlation between GrimAge clock and smoking-associated changes that were shown to be a prognostic factor for severe illness and mortality in COVID-19 patients43–45.
Of note, there is a lack of molecular biomarkers potentially valuable in monitoring post-COVID-19 syndrome that will require long-term assistance among the millions of COVID-19 survivors46. Based on our findings, we speculate that the accumulation of epigenetic aging and telomere attrition after SARS-CoV-2 infection might contribute to the post-COVID-19 syndrome, and irreversible epigenetic aging might be served as a biomarker for the risk of developing post-COVID-19 syndrome.
It is noteworthy that this study has some limitations. First, our longitudinal analysis indicates the recovery of accelerated epigenetic aging occurred in some patients, though none of the post-COVID-19 survivors with post-COVID-19 syndrome was included in our study. In addition, the limited cases were included in the longitudinal analysis, and the findings require validation in a larger and more diverse longitudinal cohort. Next, the causal relationship between COVID-19 and accelerated epigenetic aging remains unanswered in the current study. A longitudinal cohort with sustaining follow-up from the time point before SARS-CoV-2 infection to post-COVID-19 phase would be helpful to address this question, though it would be challenging to conduct a study with this design. A long-term follow-up of crowdsourced populations47,48 may facilitate the longitudinal research and provide valuable evidence. Furthermore, we cannot rule out the potential confounding effects of chronic inflammation, oxidative stress in respiratory failure and prior medication use. Finally, the application of epigenetic aging markers in predicting COVID-19 outcomes is limited by the cost and accessibility of methylation arrays. However, representative age-related CpG methylations could be applied by using qPCR-based assays and pyrosequencing39,49,50. We anticipate that epigenetic aging-related markers would be useful to predict disease progression in COVID-19 with other laboratory assays8,51.
In conclusion, our results indicate that accelerated epigenetic aging is associated with the risk of SARS-CoV-2 infection and developing severe COVID-19. In addition, COVID-19 could exert influence on the epigenetic clock and telomere attrition and accelerate the epigenetic aging, which may contribute to the post-COVID-19 syndrome. However, this influence is reversible in some patients. Together with other laboratory assays and clinical characteristics, it would be helpful to identify the patients with high risk of developing severe COVID-19 and post-COVID-19 syndrome.
Methods
Study design and patients
This study was designed to compare the DNAm age and TL in COVID-19 patients and healthy individuals and determine the dynamic change of DNAm age and TL across COVID-19 disease phases. This study included whole blood samples of 407 COVID-19 patients collected from fourteen hospitals in Spain23, six COVID-19 patients from Bernardes et al’s longitudinal cohort in Germany37 and 232 healthy individuals from previous studies with well-characterized populations52–56. The included COVID-19 patients did not present the risk factors of comorbidities as we previously described23. The whole blood samples of healthy individuals were collected before 2019 to ensure they had never been exposed to SARS-CoV-2. The healthy individuals and COVID-19 patients did not present significant differences in age and gender (Supplementary Table 1). The protocol of this study was approved by the institutional ethics review board of Josep Carreras Leukaemia Research Institute. Written informed consent was obtained from all participants. We conducted this study in compliance with the principles of the Declaration of Helsinki.
Methylation array data processing
DNA from the whole blood samples in all cases were hybridized to the Illumina Infinium MethylationEPIC Beadchip (EPIC array) that interrogates almost 850,000 CpG sites of the human genome. The “minfi” R package57 and Combat tool were used to perform raw data processing, batch effect correction, and data analysis as we previously described15,58–60. The methylation β value for each CpG site in each sample was calculated to represent the methylation level.
DNA methylation age calculation
We determined the DNAm age with the blood-based epigenetic clocks, including the Horvath clock with 353 CpGs based on multiple tissue types12, the Hannum clock with 71 CpGs identified in blood DNA samples33, the PhenoAge clock based on 513 CpGs derived from whole blood34, the skinHorvath clock that integrated the dataset of whole blood with skin, endothelial cells, mouth mucosa and saliva35, and the GrimAge clock using 1030 CpGs associated with smoking packyears and physiological risk factors36,61.
Although the EPIC array lacks some of the CpGs in the Hannum and Horvath clocks due to the difference of EPIC array with HM450 array that these clocks rely on, missing clock CpGs on the EPIC array was demonstrated to not substantially affect the accuracy of the DNAm age estimation using blood sample by McEwen et al.62 or epithelial tissue in our previous work15. The deviation between epigenetic age and chronological age, also known as epigenetic age acceleration, was calculated for every sample based on the residuals from regressing the DNAm age or TL on chronological age, as described by McEwen et al.62.
Telomere length estimation
To explore the dynamic change of telomere length in the COVID-19 disease process, we used a DNA methylation-based estimator for telomere length based on the Horvath’s Elastic Net regression model27. The deviation between DNAm TL and chronological age, defined as DNAm TL attrition acceleration, was calculated for every sample based on the residuals from regressing the DNAm TL on chronological age.
Cell composition estimation
Based on the accumulating evidence reporting the change of immune cell composition in severe COVID-19 patients,37,63,64 we estimated the cell counts with the ‘meffil’ tool65 and performed cell composition adjustment to control the impact on epigenetic age acceleration estimation.
Statistical methods
The correlation among DNAm age, DNAm TL and chronological age of the samples was calculated with the Pearson correlation coefficient. A linear regression model was applied to determine the relationship between the epigenetic age and chronological age at the time of sample collection. All statistical analysis was performed using R Statistical Software (version 3.6.1). P values < 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This study was funded by the National Natural Science Foundation of China (81902877, HY), the Natural Science Foundation of Guangdong Province (2022A1515012656, HY; 2018A0303130303, HY), the “La Marató de TV3” Foundation (565/C/2021) to AP and ME, the Program of Introducing Talents of Discipline to Universities, and the National Key Clinical Discipline (2012).
Funding
The funding sources had no involvement in any of the study design, data collection, data analysis and interpretation, writing of the report, and decision to submit the article for publication.
Source data
Author contributions
H.Y. conceived and supervised the study; H.Y., X.C., X.W., W.L., V.D., L.P.S., A.P., and M.E. conducted data collection; V.D., L.P.S., A.P., and M.E. provided the study materials; X.C., X.W., and D.R. assisted with the collection and sorting of metadata; H.Y., T.W. and X.C. analyzed and interpreted the data; H.Y. and X.C. searched the literature and draft the manuscript; V.D., T.W., L.P.S., A.P., M.E., W.L., D.R., and H.Y. critically revised the manuscript; All authors have approved the final manuscript.
Peer review
Peer review information
Nature Communications thanks Maria Cristina Polidori, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Data availability
The complete DNA methylation raw data of the severe and non-severe COVID-19 cases have been deposited on the GEO repository under accession number GSE168739, and the clinical outcomes of these cases are not publicly available for data privacy but are available from Dr. Manel Esteller (mesteller@carrerasresearch.org) upon request for research collaboration. The timeframe for response to data access requests is 30 days. There are no restrictions on the reuse of data. In addition, the DNA methylation raw data of the longitudinal cohort and healthy individuals analyzed in this study were available at GEO with identifiers of GSE161678, GSE149318, GSE123914, GSE145254, GSE118144 and GSE141682. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29801-8.
References
- 1.WHO, 2021. Coronavirus Situation Report. https://cdn.who.int/media/docs/default-source/searo/whe/coronavirus19/sear-weekly-reports/searo-weekly-situation-report-23-2021.pdf?sfvrsn=55403fbf_5.
- 2.Gupta S, et al. Factors associated with death in critically Ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin AT, et al. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 2020;35:1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, et al. Epidemiological feature, viral shedding, and antibody seroconversion among asymptomatic SARS-CoV-2 carriers and symptomatic/presymptomatic COVID-19 patients. J. Infect. Public Health. 2021;14:845–851. doi: 10.1016/j.jiph.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu, Y. et al. Association between age and clinical characteristics and outcomes of COVID-19. Eur. Respir. J. 55, (2020). [DOI] [PMC free article] [PubMed]
- 6.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people. Aging (Albany NY) 2020;12:9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogata, A. F. et al. Ultra-sensitive serial profiling of SARS-CoV-2 antigens and antibodies in plasma to understand disease progression in COVID-19 patients with severe disease. Clin. Chem. (2020). [DOI] [PMC free article] [PubMed]
- 9.Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184:306–322. doi: 10.1016/j.cell.2020.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 14.Bell CG, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019;20:249. doi: 10.1186/s13059-019-1824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, et al. Dysfunctional epigenetic aging of the normal colon and colorectal cancer risk. Clin. Epigenetics. 2020;12:5. doi: 10.1186/s13148-019-0801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu M, Hazelton WD, Luebeck GE, Grady WM. Epigenetic aging: more than just a clock when it comes to cancer. Cancer Res. 2020;80:367–374. doi: 10.1158/0008-5472.CAN-19-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteban-Cantos A, et al. Epigenetic age acceleration changes 2 years after antiretroviral therapy initiation in adults with HIV: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV. 2021;8:e197–197e205. doi: 10.1016/S2352-3018(21)00006-0. [DOI] [PubMed] [Google Scholar]
- 18.Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J. Infect. Dis. 2015;212:1563–1573. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horvath S, et al. Perinatally acquired HIV infection accelerates epigenetic aging in South African adolescents. AIDS. 2018;32:1465–1474. doi: 10.1097/QAD.0000000000001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corley, M. J. et al. Genome-wide DNA methylation profiling of peripheral blood reveals an epigenetic signature associated with severe COVID-19. J. Leukoc. Biol., (2021). [DOI] [PMC free article] [PubMed]
- 21.Schäfer, A. & Baric, R. S. Epigenetic Landscape during Coronavirus Infection. Pathogens6, (2017). [DOI] [PMC free article] [PubMed]
- 22.Menachery VD, et al. MERS-CoV and H5N1 influenza virus antagonize antigen presentation by altering the epigenetic landscape. Proc. Natl Acad. Sci. USA. 2018;115:E1012–1012E1021. doi: 10.1073/pnas.1706928115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro de Moura M, et al. Epigenome-wide association study of COVID-19 severity with respiratory failure. EBioMedicine. 2021;66:103339. doi: 10.1016/j.ebiom.2021.103339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbeev KG, et al. Association of leukocyte telomere length with mortality among adult participants in 3 longitudinal studies. JAMA Netw. Open. 2020;3:e200023. doi: 10.1001/jamanetworkopen.2020.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haycock PC, et al. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demanelis, K. et al. Determinants of telomere length across human tissues. Science369, (2020). [DOI] [PMC free article] [PubMed]
- 27.Lu AT, et al. DNA methylation-based estimator of telomere length. Aging (Albany NY) 2019;11:5895–5923. doi: 10.18632/aging.102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marioni RE, et al. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int J. Epidemiol. 2018;45:424–432. doi: 10.1093/ije/dyw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banszerus, V. L., Vetter, V. M., Salewsky, B., König, M. & Demuth, I. Exploring the relationship of relative telomere length and the epigenetic clock in the lipidcardio cohort. Int. J. Mol. Sci.20, (2019). [DOI] [PMC free article] [PubMed]
- 30.Kuo, C. L. et al. COVID-19 severity is predicted by earlier evidence of accelerated aging. medRxiv: the preprint server for health sciences, (2020).
- 31.Polidori MC, Sies H, Ferrucci L, Benzing T. COVID-19 mortality as a fingerprint of biological age. Ageing Res. Rev. 2021;67:101308. doi: 10.1016/j.arr.2021.101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Froidure A, et al. Short telomeres increase the risk of severe COVID-19. Aging (Albany NY) 2020;12:19911–19922. doi: 10.18632/aging.104097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hannum G, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine ME, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horvath S, et al. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging (Albany NY) 2018;10:1758–1775. doi: 10.18632/aging.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu AT, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11:303–327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernardes JP, et al. Longitudinal multi-omics analyses identify responses of megakaryocytes, erythroid cells, and plasmablasts as hallmarks of severe COVID-19. Immunity. 2020;53:1296–1314.e9. doi: 10.1016/j.immuni.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gindin, Y. et al. DNA Methylation and immune cell markers demonstrate evidence of accelerated aging in patients with chronic HBV or HCV, with or without HIV co-infection. Clin. Infect. Dis. (2020). [DOI] [PMC free article] [PubMed]
- 39.Mongelli A, et al. Evidence for biological age acceleration and telomere shortening in COVID-19 survivors. Int J. Mol. Sci. 2021;22:6151. doi: 10.3390/ijms22116151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sang, E. R., Tian, Y., Miller, L. C. & Sang, Y. Epigenetic evolution of ACE2 and IL-6 genes: non-canonical interferon-stimulated genes correlate to COVID-19 susceptibility in vertebrates. Genes (Basel)12, (2021). [DOI] [PMC free article] [PubMed]
- 41.Li, S. et al. Metabolic reprogramming and epigenetic changes of vital organs in SARS-CoV-2-induced systemic toxicity. JCI Insight6, (2021). [DOI] [PMC free article] [PubMed]
- 42.Franzen, J. et al. Epigenetic clocks are not accelerated in COVID-19 patients. Int. J. Mol. Sci.22, (2021). [DOI] [PMC free article] [PubMed]
- 43.Kuderer NM, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clift, A. K. et al. Smoking and COVID-19 outcomes: an observational and Mendelian randomisation study using the UK Biobank cohort. Thorax, (2021). [DOI] [PMC free article] [PubMed]
- 45.Leung, J. M. et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur. Respir. J. 55, (2020). [DOI] [PMC free article] [PubMed]
- 46.Oronsky, B. et al. A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol, 1–9 (2021). [DOI] [PMC free article] [PubMed]
- 47.Yu H, Luo Y. Decentered crowdfunded clinical studies-open a new era of medical research. JAMA Oncol. 2019;5:9–10. doi: 10.1001/jamaoncol.2018.4650. [DOI] [PubMed] [Google Scholar]
- 48.Persson, J., Parie, J. F. & Feuerriegel, S. Monitoring the COVID-19 epidemic with nationwide telecommunication data. Proc. Natl. Acad. Sci. USA 118, (2021). [DOI] [PMC free article] [PubMed]
- 49.Xiao, C., Yi, S. & Huang, D. Genome-wide identification of age-related CpG sites for age estimation from blood DNA of Han Chinese individuals. Electrophoresis, (2021). [DOI] [PubMed]
- 50.Yu H, et al. Novel assay for quantitative analysis of DNA methylation at single-base resolution. Clin. Chem. 2019;65:664–673. doi: 10.1373/clinchem.2018.298570. [DOI] [PubMed] [Google Scholar]
- 51.Yang HS, et al. Routine laboratory blood tests predict SARS-CoV-2 infection using machine learning. Clin. Chem. 2020;66:1396–1404. doi: 10.1093/clinchem/hvaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Åsenius F, et al. The DNA methylome of human sperm is distinct from blood with little evidence for tissue-consistent obesity associations. PLoS Genet. 2020;16:e1009035. doi: 10.1371/journal.pgen.1009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaimi I, et al. Variation in DNA methylation of human blood over a 1-year period using the Illumina MethylationEPIC array. Epigenetics. 2018;13:1056–1071. doi: 10.1080/15592294.2018.1530008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vyas CM, et al. Pilot study of DNA methylation, molecular aging markers and measures of health and well-being in aging. Transl. Psychiatry. 2019;9:118. doi: 10.1038/s41398-019-0446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsai MH, et al. S100A6 promotes B lymphocyte penetration through the blood-brain barrier in autoimmune encephalitis. Front Genet. 2019;10:1188. doi: 10.3389/fgene.2019.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeung KS, et al. Cell lineage-specific genome-wide DNA methylation analysis of patients with paediatric-onset systemic lupus erythematosus. Epigenetics. 2019;14:341–351. doi: 10.1080/15592294.2019.1585176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aryee MJ, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price EM, Robinson WP. Adjusting for Batch Effects in DNA Methylation Microarray Data, a Lesson Learned. Front Genet. 2018;9:83. doi: 10.3389/fgene.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Z, et al. Genome-wide analysis identifies critical DNA methylations within NTRKs genes in colorectal cancer. J. Transl. Med. 2021;19:73. doi: 10.1186/s12967-021-02740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou, Q. et al. DNA methylation-based signature of CD8+ tumor-infiltrating lymphocytes enables evaluation of immune response and prognosis in colorectal cancer. J. Immunother. Cancer9, (2021). [DOI] [PMC free article] [PubMed]
- 61.Li, X. et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. Elife9, (2020). [DOI] [PMC free article] [PubMed]
- 62.McEwen LM, et al. Systematic evaluation of DNA methylation age estimation with common preprocessing methods and the Infinium MethylationEPIC BeadChip array. Clin. Epigenetics. 2018;10:123. doi: 10.1186/s13148-018-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilk AJ, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Min JL, Hemani G, Davey Smith G, Relton C, Suderman M. Meffil: efficient normalization and analysis of very large DNA methylation datasets. Bioinformatics. 2018;34:3983–3989. doi: 10.1093/bioinformatics/bty362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete DNA methylation raw data of the severe and non-severe COVID-19 cases have been deposited on the GEO repository under accession number GSE168739, and the clinical outcomes of these cases are not publicly available for data privacy but are available from Dr. Manel Esteller (mesteller@carrerasresearch.org) upon request for research collaboration. The timeframe for response to data access requests is 30 days. There are no restrictions on the reuse of data. In addition, the DNA methylation raw data of the longitudinal cohort and healthy individuals analyzed in this study were available at GEO with identifiers of GSE161678, GSE149318, GSE123914, GSE145254, GSE118144 and GSE141682. Source data are provided with this paper.