Abstract
Background
Clinicians use sex-based kidney function estimating equations, but the appropriate sex modifier for transgender adults undergoing hormone therapy (HT) is undetermined.
Objectives
Compare median estimated creatinine clearance (eCrCL; Cockcroft-Gault) and estimated glomerular filtration rates (eGFRs; Modification of Diet in Renal Disease [MDRD] and Chronic Kidney Disease Epidemiology Study [CKD-EPI]) before and during HT when estimated with and without sex assigned at birth.
Methods
Single-system retrospective cohort study of transgender adults (2007-2017) prescribed ≥90 days HT (index date = first order) and measured serum creatinine ≤6 months pre-index date (baseline) and ≤12 months post-index date. We grouped patients based on testosterone or estrogen treatment and compared eCrCL and eGFRs at baseline up to 6-12 months post-index date using equations based on sex assigned at birth (female or male modifier in testosterone or estrogen groups, respectively) or gender identity (male or female modifier in testosterone or estrogen groups, respectively). We used Wilcoxon signed-rank tests (Bonferroni correction) for all comparisons.
Results
In total, 29 (median age 26 years, follow-up 259 days) and 41 patients (29 years, 250 days) were prescribed testosterone or estrogen, respectively. In the testosterone group, the maximum eCrCL and eGFR changes based on sex assigned at birth were −14%, P = 0.0181; −18%; P = 0.0009, respectively, and based on gender identity were +5%, P > 0.025 and +11%, P = 0.0094, respectively. In the estrogen group, eCrCL or eGFRs based on sex assigned at birth did not change from baseline but based on gender identity were −17%, P < 0.0001 and −26%, P < 0.0001, respectively.
Conclusion and Relevance
Female-based equations may underestimate kidney function in transgender adults undergoing testosterone or estrogen treatment. Prospective cohort studies are needed to confirm the clinical significance of these findings.
Keywords: hormone therapy, transgender adults, estimated creatinine clearance, serum creatinine, estimated glomerular filtration rate
Introduction
Approximately 1 million adults are transgender in the United States, 1 and this population is growing worldwide. 2 Transgender people, individuals whose gender identity differs from their sex assigned at birth, may take testosterone or estrogen treatment to align secondary sex characteristics with their gender identity. 3 Although hormone therapy is associated with improved quality of life among transgender adults, 4 its impact on interpreting sex-based clinical estimates, specifically creatinine-based kidney function estimating equations, 5 is unclear.
In vivo and population-based cohort data describe conflicting effects of sex hormones on kidney function. 6 Although it is unclear whether hormone therapy influences kidney function directly, it typically causes marked physiologic and body composition changes within months after initiation among transgender adults. 7 Increased or decreased percent lean muscle mass, and corresponding increases or decreases in serum creatinine concentrations, may alter creatinine-based kidney function estimating equations.8-12 Because estimating equations include a sex-based modifier to account for average body composition differences between sexes,13-15 clinicians have recommended using equations based on gender identity, rather than one’s sex assigned at birth, among transgender adults undergoing hormone therapy.6,15,16 Despite this recommendation, no investigators have studied the impact of switching between male and female estimating equations on longitudinal kidney function estimates before and during hormone therapy.
Because pharmacists and prescribing clinicians use creatinine-based kidney function estimating equations to guide dose adjustment of several drugs cleared by the kidneys, 17 we conducted a short-term preliminary investigation to compare estimated creatinine clearance (eCrCL) and estimated glomerular filtration rates (eGFR) before and during hormone therapy among transgender adults. Our primary objective was to compare changes in eCrCL and eGFR based on sex assigned at birth before and during hormone therapy. Our secondary objective was to compare changes from baseline when estimated based on gender identity. We hypothesized that when using equations based on sex assigned at birth during hormone therapy, testosterone or estrogen treatment would be associated with decreased or increased estimates, respectively, and the extent of these changes would be decreased when using equations based on gender identity.
Materials and Methods
Study Design
This was a single-system, multicenter, retrospective cohort study of healthy transgender adults (≥18 years of age) receiving medical care at University of Washington (UW) Medicine in Seattle, Washington, USA. UW Medicine is an integrated health system that includes 4 hospitals and affiliated clinics, plus 12 neighborhood clinics. The University of Washington Institutional Review Board (STUDY00009038) reviewed this project.
Patients
We identified patients between January 1, 2007, and January 31, 2017, with at least one transgender health-related clinical visit based on validated diagnosis codes (International Classification of Diseases Ninth or Tenth Revision, ICD-9 or ICD-10).18,19 We extracted demographic, height, weight, clinical laboratory measures (serum creatinine, blood urea nitrogen), concomitant medication orders, and clinical diagnoses within 12 months pre-index and post-index date from the UW Medicine Electronic Data Warehouse, a central repository of electronic medical record data across UW Medicine clinics. Because transgender people may have gender identities outside the binary of man or woman (nonbinary), and not all transgender people want or are able to obtain hormone therapy, we grouped patients based on receipt of either testosterone or estrogen treatment to avoid misrepresenting patients’ gender identities within our cohort. Although we approximated gender identity based on hormone therapy for our secondary objective, this approach was meant to align with availability of binary (male or female) kidney function estimating equations and was not intended to exclude or oversimplify identities of nonbinary adults undergoing hormone therapy.
Eligible patients were prescribed testosterone or estrogen treatment for at least 90 days, with the index date set as the first hormone order date, and at least one creatinine measure within 6 months pre-index date (baseline) and within 12 months post-index date. We selected a 90-day threshold for hormone therapy duration to allow our cohort to primarily include patients receiving maintenance doses of hormone therapy. We excluded patients with documented history of chronic kidney disease, dialysis, kidney transplant, HIV infection, or baseline eGFR <60 mL/min/1.73 m2 (using the re-expressed 4 variable Modification of Diet in Renal Disease [MDRD] equation and sex assigned at birth) to minimize potential sources of nonhormone-related variability in eCrCL and eGFR. We excluded patients with creatinine measures reported within 90 days of the index date only, as clinicians at our institute typically perform follow-up laboratory monitoring 3 months after hormone therapy initiation in healthy patients. 3 We excluded patients with >12 creatinine measures reported within any 6-month period 1 year pre-index or post-index date as a surrogate marker of kidney instability. 20
Primary Outcome Measures
Our primary endpoint was the percent difference in median eCrCL 3-6 months (inclusive) and 6-12 months post-index date compared with baseline using the Cockcroft-Gault (C-G) estimating equation, 21 as 1998 Food and Drug Administration (FDA)-issued industry guidance recommended eCrCL for medication dose adjustment in patients with chronic kidney disease, 22 and most drug product prescribing information includes eCrCL-based dosing guidance for medications requiring kidney dose adjustment. We used ideal body weight, actual body weight, or adjusted body weight at baseline for C-G estimates, 23 and we applied the same body weight estimate used at baseline to all weights reported at follow-up to minimize the influence of potential changes in the body weight estimates between time intervals.
We estimated GFR using the MDRD equation 24 and Chronic Kidney Disease Epidemiology Study (CKD-EPI) equation, 25 as 2020 FDA draft industry guidance recommended using eGFR for medication dosing in pharmacokinetic studies among patients with kidney impairment. 26 For our primary objective, we estimated baseline eCrCL or eGFR using C-G, MDRD, and CKD-EPI equations associated with sex assigned at birth (ie, male-based equations in the estrogen group; female-based equations in the testosterone group). We described the proportion of patients with ≥0.20 mg/dL absolute change in creatinine concentrations and the proportion of patients with ≥25% absolute change in eCrCL and eGFR, as investigators have recommended this threshold for clinically significant decreases in eGFR in the context of acute kidney injury. 27 For our secondary objective, we re-analyzed C-G, MDRD, and CKD-EPI estimates 3-6 months and 6-12 months post-index date using equations associated with gender identity (ie, male-based equations in the testosterone group; female-based equations in the estrogen group) and compared these with baseline estimates using sex assigned at birth.
Statistical Analysis
We compared the percent change in median eCrCL (C-G) and eGFR (MDRD and CKD-EPI) at 3-6 months post-index date to baseline and 6-12 months post-index date to baseline within each hormone treatment group using Wilcoxon signed-rank tests due to small sample sizes. We summarized continuous variables using median and interquartile range (IQR) or ranges. We used frequencies and percentages to summarize categorical variables. A 2-sided P value <0.025 (Bonferroni correction for multiple testing) was considered statistically significant. We used SAS software version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
Baseline Patient Characteristics
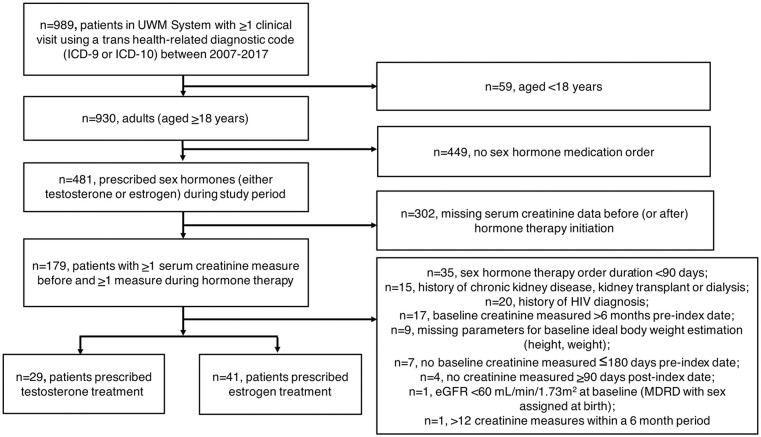
A total of 989 patients had at least one clinical visit for transgender health-related medical care (Figure 1). We analyzed 70 patients, 29 of whom were prescribed testosterone treatment (Table 1). The median follow-up duration in the testosterone group was 259 days (range: 101-357 days). Most patients were prescribed injectable testosterone weekly (n = 20 cypionate or enanthate, median dose: 60.0 mg [32.5-95.0 mg], 6 patients had unknown dosing). Three patients were prescribed 2-4 mg/24-hour testosterone patches daily. Five patients were prescribed topical testosterone gel preparations (median daily dose: 30.5 mg [21.4-43.8 mg]). One patient was prescribed an unknown testosterone preparation.
Figure 1.
Flow diagram of cohort selection.
Abbreviations: eGFR, estimated glomerular filtration rate; ICD-9 or ICD-10, International Classification of Diseases Ninth or Tenth Revision; MDRD, Modification of Diet in Renal Disease; UWM, University of Washington Medicine health system.
Table 1.
Baseline Patient Characteristics.
| Characteristic | Testosterone group, n = 29 a | Estrogen group, n = 41 a |
|---|---|---|
| Age, years | 26 (20-31) | 29 (24-35) |
| Weight, kg | 78.7 (66.1-89.8) | 75.3 (67.0-105.6) |
| Height, cm | 164.0 (160.0-168.0) | 175.1 (171.5-181.0) |
| BMI, kg/m2 | 29.4 (25.0-33.7) | 24.1 (21.1-32.7) |
| Race, ethnicity b | ||
| White, non-Hispanic | 21 (72) | 24 (59) |
| Hispanic, Latino/a | <5 (<17) | 5 (12) |
| Black, non-Hispanic | <5 (<17) | <5 (<12) |
| Asian | <5 (<17) | <5 (<12) |
| American Indian, Alaskan Native | 0 (0) | <5 (<12) |
| Unknown | <5 (<17) | <5 (<12) |
| Systolic blood pressure, mmHg | 121 (112-127), n = 28 | 125 (116-131) |
| Diastolic blood pressure, mmHg | 77 (72-80), n = 28 | 77 (71-83) |
| Any documented tobacco use (current or former) c | 10/28 | 18/37 |
| Past medical history | ||
| Hypertension | 6 (20.7) | 10 (24.4) |
| Diabetes | 4 (13.8) | 4 (9.8) |
| Baseline medications | ||
| ACEi/ARBs | 2 (6.9) | 2 (4.9) |
| Beta blockers | 2 (6.9) | 2 (4.9) |
| Diuretics | 0 (0.0) | 1 (2.4) |
| NSAIDs | 4 (13.8) | 2 (4.9) |
| Aminoglycosides | 0 (0.0) | 0 (0.0) |
| Tenofovir | 0 (0.0) | 2 (4.9) |
| TMP-SMX | 0 (0.0) | 0 (0.0) |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; NSAIDs, nonsteroidal antiinflammatory drugs; TMP-SMX, trimethoprim-sulfamethoxazole.
Data reported as median (interquartile range) and counts (percentages) as appropriate.
To protect patient confidentiality, demographic information with cells <5 patients were reported as such.
Tobacco use was reported as the fraction of the total number of patients with tobacco status data included in the electronic health record.
Forty-one patients were prescribed estrogen treatment (Table 1). The median follow-up duration in the estrogen group was 250 days (range: 106-354 days). Most patients were prescribed oral estradiol tablets (n = 21, median total daily amount: 4 mg [2-4 mg] divided as once, twice, or thrice daily doses). Eight patients were prescribed transdermal estradiol patches (median weekly amount: 150 μg [100-200 μg] delivered via 1-3 patches applied up to twice weekly). Eleven patients were prescribed injectable estradiol (cypionate or valerate, median weekly dose: 10.0 mg [6.6-15.0 mg], 4 patients had unknown dosing). One patient was prescribed topical estrogen gel (dose unknown). Thirty-eight (90.1%) patients were prescribed oral spironolactone (median total daily amount: 200 mg [100-200 mg] divided as once or twice daily doses). Eleven patients were prescribed adjunctive antiandrogenic agents (dutasteride, finasteride) or progestogens (micronized progesterone or medroxyprogesterone acetate). 3
Laboratory Values and Body Composition Measures at Baseline and Follow-up
In the testosterone group, median body weight and body mass index were lower at 3-6 months and 6-12 months compared with baseline, but this was not statistically significant (Table 2). Median creatinine concentrations at 3-6 months and 6-12 months were statistically increased compared with baseline (P = 0.0020 and P = 0.0006, respectively). Seven (24.1%) patients had ≥0.20 mg/dL increased creatinine concentration at either 3-6 months or 6-12 months compared with baseline (data not shown).
Table 2.
Longitudinal Changes in Laboratory and Physiologic Values During the First Year of Hormone Therapy in Transgender Adults.
| Median parameter | Testosterone group, n = 29
a
|
Estrogen group, n = 41
a
|
||||
|---|---|---|---|---|---|---|
| Baseline b | 3-6 months | 6-12 months | Baseline b | 3-6 months | 6-12 months | |
| Weight, kg | 78.7 (66.1-89.8) | 68.5 (62.1-87.0), n = 9 | 74.5 (65.9-90.5), n = 22 | 75.3 (67.0-105.6) | 75.3 (64.1-114.3), n = 29 | 72.7 (67.6-103.0), n = 37 |
| Δ from baseline | −0.7 (−3.2 to 1.2) | −0.9 (−2.4 to 0.0) | −0.3 (−3.2 to 3.2) | 0.2 (−3.8 to 5.4) | ||
| P value c | 0.5703 | 0.0286 | 0.9329 | 0.4537 | ||
| BMI, kg/m2 | 29.4 (25.0-33.7) | 27.5 (23.5-28.5), n = 9 | 28.6 (25.2-34.0), n = 22 | 24.1 (21.1-32.7) | 25.2 (21.3-34.1), n = 29 | 24.4 (22.2-33.5), n = 37 |
| Δ from baseline | −0.3 (−1.3 to 0.4) | −0.3 (−0.9 to 0.0) | −0.1 (−1.0 to 1.1) | 0.1 (−1.3 to 1.8) | ||
| P value c | 0.4961 | 0.0286 | 0.9412 | 0.4537 | ||
| Serum creatinine, mg/dL | 0.74 (0.64-0.82) | 0.81 (0.77-0.86), n = 17 | 0.84 (0.74-0.94), n = 20 | 0.83 (0.76-0.94) | 0.87 (0.81-0.92), n = 26 | 0.81 (0.74-0.95), n = 29 |
| Δ from baseline | 0.05 (0.02 to 0.14) | 0.12 (0.00 to 0.18) | −0.03 (−0.11 to 0.04) | 0.00 (−0.09 to 0.06) | ||
| P value c | 0.0020 | 0.0006 | 0.5245 | 0.8911 | ||
| BUN, mg/dL | 10.0 (9.0-13.0) | 9.0 (8.0-11.0), n = 17 | 12.0 (10.0-14.0), n = 20 | 12.0 (10.0-14.0) | 13.5 (10.0-16.0), n = 26 | 12.0 (10.0-14.0), n = 29 |
| Δ from baseline | −1.0 (−3.0 to 1.0) | 1.0 (−1.0 to 2.5) | 0.0 (−1.5 to 4.0) | 0.0 (−2.5 to 1.5) | ||
| P value c | 0.1537 | 0.1161 | 0.3403 | 0.6465 | ||
Abbreviations: BMI, body mass index; BUN, blood urea nitrogen.
Data presented as median (interquartile range). For all parameters, each participant had at least one value reported at 3-6 months only, 6-12 months only, or at both 3-6 months and 6-12 months.
Baseline values were measured within 6 months before hormone therapy initiation.
Within-group comparisons at 3-6 months versus baseline and at 6-12 months versus baseline using Wilcoxon signed-rank test (with Bonferroni correction for multiple comparisons).
In the estrogen group, median body weight and body mass index were similar at 3-6 months and 6-12 months compared with baseline (Table 2). Median creatinine concentrations were similar at 3-6 months and 6-12 months compared with baseline. Eight (19.5%) patients had ≥0.20 mg/dL absolute change in creatinine concentrations at either 3-6 months or 6-12 months, 4 of whom had increased values compared with baseline (data not shown).
Percent Changes in eCrCL and eGFR Using Estimating Equations Based on Sex Assigned at Birth
Testosterone group
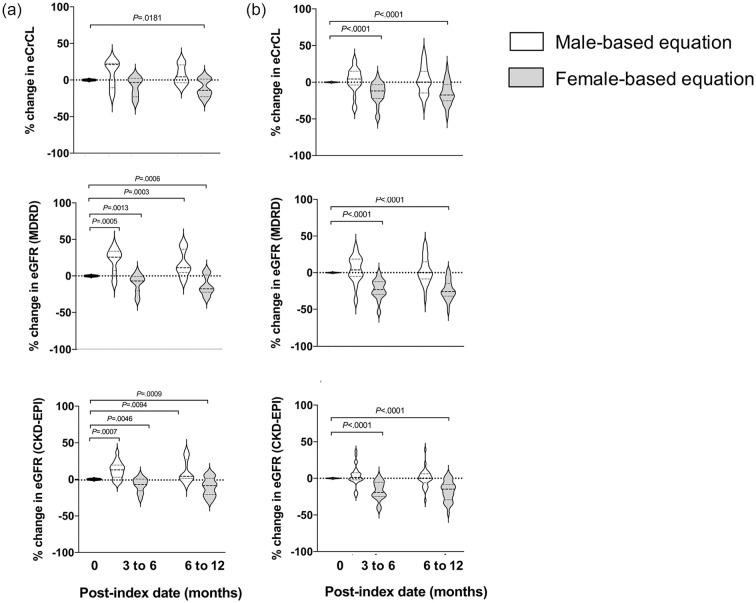
Using female-based equations at baseline and follow-up in the testosterone group, C-G estimates decreased from baseline, although this was statistically significant at 6-12 months only (Figure 2a, gray plots): baseline, 120 (97-143) mL/min; 3-6 months (n = 5), 94 (93-114) mL/min, −3%, P = 0.3125; 6-12 months (n = 15), 93 (83-119) mL/min, −14%, P = 0.0181. Median MDRD and CKD-EPI estimates statistically decreased at both 3-6 months (n = 17) and 6-12 months (n = 20) compared with baseline (Figure 2a, gray plots): MDRD at baseline, 99 (83-120) mL/min/1.73 m2; 3-6 months, 89 (81-96) mL/min/1.73 m2, −7%, P = 0.0013; 6-12 months, 80 (71-100) mL/min/1.73 m2, −18%, P = 0.0006; CKD-EPI at baseline, 116 (97-124) mL/min/1.73 m2; 3-6 months, 105 (94-112) mL/min/1.73 m2, −7%, P = 0.0046; 6-12 months, 94 (83-113) mL/min/1.73 m2, −9%, P = 0.0009. Seven (24.1%) patients had ≥25% decrease in C-G, MDRD, or CKD-EPI estimates at 3-6 months or 6-12 months (data not shown).
Figure 2.
Percent changes in median eCrCL and eGFR based on sex assigned at birth or gender identity. All baseline (0 months) estimates used equations based on sex assigned at birth. White plots = male-based estimating equation. Gray plots = female-based estimating equation. (a) Estimated creatinine clearance based on the Cockcroft-Gault equation, eGFR based on MDRD equation, and eGFR based on CKD-EPI equation within the testosterone group (n = 29). (b) Estimated creatinine clearance based on the Cockcroft-Gault equation, eGFR based on MDRD equation, and eGFR based on CKD-EPI equation within the estrogen group (n = 41).
Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Study equation; eCrCL, estimated creatinine clearance; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
Estrogen group
Using male-based equations at baseline and follow-up in the estrogen group, C-G estimates decreased at 3-6 months (n = 21) and increased at 6-12 months (n = 28), although neither was statistically significant (Figure 2b, white plots): C-G at baseline, 129 (112-153) mL/min; 125 (116-144) mL/min, 5%, P = 0.2842; 145 (105-163) mL/min, 0%, P = 0.6567. Changes in median MDRD and CKD-EPI estimates were within +5% at 3-6 months (n = 26) and 6-12 months (n = 29) compared with baseline (Figure 2b, white plots): MDRD at baseline, 111 (94-125) mL/min/1.73 m2; 3-6 months, 105 (101-120) mL/min/1.73 m2, 4%, P = 0.2451; 6-12 months, 117 (90-133) mL/min/1.73 m2, 0%, P = 0.6476; CKD-EPI baseline, 117 (104-126) mL/min/1.73 m2; 116 (109-126), 1%, P = 0.2348; 6-12 months, 122 (101-130) mL/min/1.73 m2, 0%, P = 0.9705. Ten (24.4%) patients had ≥25% absolute change in C-G, MDRD, or CKD-EPI estimates at 3-6 months or 6-12-months from baseline (n = 6 increased, n = 4 decreased, data not shown).
Percent Changes in eCrCL and eGFR Using Estimating Equations Based on Gender Identity
Using male-based equations at follow-up in the testosterone group, the percent changes in median MDRD and CKD-EPI estimates were statistically significantly higher at 3-6 months and 6-12 months, respectively, compared with baseline (Figure 2a, white plots): MDRD: +26%, P = 0.0005 and +11%, P = 0.0003; CKD-EPI: +13%, P = 0.0007 and +4%, P = 0.0094. The percent change in C-G estimates was numerically higher at both 3-6 months and 6-12 months compared with baseline, but this was not statistically significant: +21% and +5%, both P > 0.025. Using female-based equations at follow-up in the estrogen group, the percent changes in median C-G, MDRD, and CKD-EPI estimates were statistically lower at 3-6 months and 6-12 months, respectively, compared with baseline (Figure 2b, gray plots): C-G: −12% and −17%, both P < 0.0001; MDRD: −23% and −26%, both P < 0.0001; CKD-EPI: −19% and −15%, both P < 0.0001.
Discussion
To our knowledge, this preliminary study is the first to describe eCrCL or eGFR with or without the estimating equation based on sex assigned at birth in a cohort of transgender adults. Using C-G, MDRD, or CKD-EPI estimating equations based on sex assigned at birth, we observed decreased eCrCL and eGFR estimates 3-6 months and 6-12 months during testosterone treatment (vs baseline) and no change in eCrCL or eGFR estimates 3-6 months and 6-12 months during estrogen treatment. Furthermore, most patients in each treatment group had creatinine concentration changes within 0.2 mg/dL and eCrCL or eGFR changes within 25% of baseline estimates. This finding suggests on average, neither testosterone nor estrogen treatment were likely associated with clinically significant changes in estimated kidney function and aligns with serum creatinine measures and kidney function estimates reported by several retrospective8-10 and prospective11,12 cohort studies among transgender adults. Our study adds to the body of evidence suggesting that increased serum creatinine concentrations are associated with testosterone treatment.
Assuming our observations in the testosterone group were likely related to nonkidney-based changes in creatinine concentrations,11,15 we explored estimating equations based on gender identity during hormone therapy, rather than sex assigned at birth, in an attempt to minimize the percent difference in kidney function estimates between baseline and follow-up. Using male-based C-G, MDRD, and CKD-EPI equations in the testosterone group, we observed percent changes between +13% and +26% at 3-6 months (compared with −9% to −3% based on sex assigned at birth, ie, female-based equations) and between 0% and +5% at 6-12 months (compared with −14% to −9% based on sex assigned at birth). This finding supports the recommendation by Webb et al 15 suggesting clinicians use male-based equations after at least 6 months of testosterone therapy.
We also used female-based estimating equations at follow-up in the estrogen group and observed percent changes between −23% and −12% at 3-6 months (vs 1%-5% using sex assigned at birth, ie, male-based equations) and between −26% and −15% at 6-12 months (compared with 0% using sex assigned at birth). Thus, female-based estimating equations may underestimate eCrCL and eGFR in transgender adults within the first year of estrogen treatment. This finding is unsurprising, as MDRD and CKD-EPI equations include a sex-specific covariate to adjust for average differences in serum creatinine between sexes,24,25 and the C-G equation includes an arbitrary female modifier to decrease eCrCL by 15% compared with male-based equations. 21 Because female-based estimating equations cause systematically lower kidney function estimates than male-based equations, and because we observed no change in serum creatinine within our estrogen treatment group, the female-based estimating equations likely underestimated kidney function during estrogen treatment. If kidney function is underestimated, then certain medications with kidney-based dose adjustments may be underdosed, 22 although reports of subtherapeutic dosing among transgender adults are lacking. Thus, female-based equations may be inappropriate for medication dosing during the first year of testosterone or estrogen treatment.
Mechanisms underpinning potential hormone therapy–mediated changes in estimated kidney function are unclear. Testosterone treatment increased proinflammatory and profibrotic signaling in animal models of kidney obstruction 6 ; however in humans, low total testosterone concentrations were associated with increased cardiovascular risk among cisgender men with chronic kidney disease.6,28 In animal models, estradiol treatment was protective against age-related mechanisms of kidney decline, including diminished nitric oxide synthesis and fibrosis formation. 28 Conversely, large community-based studies among cisgender women observed an association between estrogen-containing medication exposure and increased odds of microalbuminuria, a marker of early kidney disease, and decreased eGFRs. 6 Based on these data, testosterone or estrogen treatment may be associated with altered kidney function. Prospective, interventional studies using standardized hormone regimens are needed to determine the effect of hormone therapy on kidney function at doses used for gender-affirming medical care. 3
Nonkidney-related changes in body composition likely influenced the observed decreases in eCrCL and eGFR in the testosterone group, although this potential influence was slight in the estrogen group. Serum creatinine, an endogenous biomarker of glomerular filtration, is produced from muscle metabolism and it is either increased or decreased, respectively, by increased or decreased muscle mass. 29 Thus, it is unclear whether creatinine-based estimating equations reliably estimate kidney function in transgender adults undergoing hormone therapy. 5 Because serum creatinine must be at steady state to estimate kidney function accurately, 29 prospective reference interval studies using exogenous filtration markers (eg, inulin or iohexol) are needed to determine appropriate kidney function reference intervals for transgender adults undergoing hormone therapy. Furthermore, people who identify as nonbinary may take lower doses of either testosterone or estrogen treatment, with unclear implications on body composition changes and kidney function estimating equations. Future prospective studies, particularly reference interval studies, should include nonbinary patients and develop appropriate reference intervals for these patient populations.
This study has several strengths. Our longitudinal design allowed each patient to serve as their own control before and during hormone therapy. We used validated ICD-9 and ICD-10 codes to identify adults who received transgender health-related services within the UW Medicine system.18,19 However, this small hypothesis-generating study had certain limitations. We used a convenience sample based on electronic medical record data, which may have introduced selection bias that limited generalizability of our findings to transgender adults receiving medical care within the UW Medicine system only. Although we used validated ICD-9 and ICD-10 codes to identify transgender patients, some clinicians document transgender-related medical visits using nonspecific ICD diagnostic codes that are not validated for identifying transgender patients in the electronic medical record (eg, E34.9 endocrine disorder, unspecified). 30 We did not have access to clinic notes and were unable to comment on our cohort’s self-reported gender identity. Our analyzable sample was small, limiting our ability to control for potential confounding factors (eg, concomitant medication orders).
We did not have access to measured creatinine clearance (eg, 24-hour urinary creatinine clearance), measured GFRs using exogenous filtration markers (eg, inulin or iohexol), or body composition measures (eg, lean muscle mass), limiting our ability to compare measured (rather than estimated) changes in kidney function during hormone therapy. Because clinicians use creatinine-based kidney function estimates for chronic kidney disease staging, transplant eligibility determination, and dosing of certain medications cleared by the kidney,13,15 future studies should examine the association between altered estimates and these outcomes.
Conclusion and Relevance
Whether sex-based or gender identity-based kidney function estimating equations are accurate for transgender adults remains unclear, although female-based estimating equations may underestimate eCrCL or eGFR among transgender adults undergoing either testosterone or estrogen treatment. Larger prospective studies with measured GFRs are needed to determine the impact of hormone therapy on kidney function in transgender adults during short-term (<1 year) and long-term (≥1 year) treatment. Blanket application female-based estimating equations may underestimate kidney function in transgender adults undergoing hormone therapy.
Acknowledgments
Sarah Fadich is an openly transgender woman and uses she/her pronouns. We thank Dr Jessica E. Long, MPH, PhD (Department of Epidemiology, University of Washington) for useful discussions regarding data analysis for transgender patient cohorts.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR002319. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Lauren R. Cirrincione  https://orcid.org/0000-0002-9115-1060
https://orcid.org/0000-0002-9115-1060
References
- 1. Meerwijk EL, Sevelius JM. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health. 2017;107(2):e1-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arcelus J, Bouman WP, Van Den Noortgate W, Claes L, Witcomb G, Fernandez-Aranda F. Systematic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry. 2015;30(6):807-815. doi: 10.1016/j.eurpsy.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 3. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;11:3869-3903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 4. Baker KE, Wilson LM, Sharma R, Dukhanin V, McArthur K, Robinson KA. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;25:bvab011. doi: 10.1210/jendso/bvab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldstein Z, Corneil TA, Greene DN. When gender identity doesn’t equal sex recorded at birth: the role of the laboratory in providing effective healthcare to the transgender community. Clin Chem. 2017;63(8):1342-1352. doi: 10.1373/clinchem.2016.258780. [DOI] [PubMed] [Google Scholar]
- 6. Collister D, Saad N, Christie E, Ahmed S. Providing care for transgender persons with kidney disease: a narrative review. Can J Kidney Health Dis. 2021;8. doi: 10.1177/2054358120985379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cirrincione LR, Huang KJ. Sex and gender differences in clinical pharmacology: implications for transgender medicine. Clin Pharmacol Ther. 2021;110(4):897-908. doi: 10.1002/cpt.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez JD, Tannock LR. Metabolic effects of hormone therapy in transgender patients. Endocr Pract. 2016;22(4):383-388. doi: 10.4158/EP15950.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allen AN, Jiao R, Day P, Pagels P, Gimpel N, SoRelle JA. Dynamic impact of hormone therapy on laboratory values in transgender patients over time. J Appl Lab Med. 2021;6(1):27-40. doi: 10.1093/jalm/jfaa192. [DOI] [PubMed] [Google Scholar]
- 10. Humble RM, Imborek KL, Nisly N, Greene DN, Krasowski MD. Common hormone therapies used to care for transgender patients influence laboratory results. J Appl Lab Med. 2018;3:799-814. doi: 10.1373/jalm.2018.027078. [DOI] [PubMed] [Google Scholar]
- 11. Wierckx K, Van Caenegem E, Schreiner T, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11(8):1999-2011. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- 12. Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. 1973;1(5851):449-451. doi: 10.1136/bmj.1.5851.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whitley CT, Greene DN. Transgender man being evaluated for a kidney transplant. Clin Chem. 2017;63(11):1680-1683. doi: 10.1373/clinchem.2016.268839. [DOI] [PubMed] [Google Scholar]
- 14. Jue JS, Alameddine M, Ciancio G. Kidney transplantation in transgender patients. Curr Urol Rep. 2020;21(1):1. doi: 10.1007/s11934-020. [DOI] [PubMed] [Google Scholar]
- 15. Webb AJ, McManus D, Rouse GE, Vonderheyde R, Topal JE. Implications for medication dosing for transgender patients: a review of the literature and recommendations for pharmacists. Am J Health Syst Pharm. 2020;6:427-433. doi: 10.1093/ajhp/zxz355 [DOI] [PubMed] [Google Scholar]
- 16. Cheung AS, Lim HY, Cook T, et al. Approach to interpreting common laboratory pathology tests in transgender individuals. J Clin Endocrinol Metab. 2021;106(3):893-901. doi: 10.1210/clinem/dgaa546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang DL, Abrass IB, Young BA. Medication safety and chronic kidney disease in older adults prescribed metformin: a cross-sectional analysis. BMC Nephrol. 2014;15:86. doi: 10.1186/1471-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roblin D, Barzilay J, Tolsma D, et al. A novel method for estimating transgender status using electronic medical records. Ann Epidemiol. 2016;26(3):198-203. doi: 10.1016/j.annepidem.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blosnich JR, Cashy J, Gordon AJ, et al. Using clinician text notes in electronic medical record data to validate transgender-related. J Am Med Inform Assoc. 2018;25:905-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmed SB, Culleton BF, Tonelli M, et al. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int. 2008;74(3):370-376. doi: 10.1038/ki.2008.205. [DOI] [PubMed] [Google Scholar]
- 21. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;1:31-41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22. Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009;54(1):33-42. doi: 10.1053/j.ajkd.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winter MA, Guhr KN, Berg GM. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft-Gault equation. Pharmacotherapy. 2012;32(7):604-612. doi: 10.1002/j.1875-9114.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;4:247-254. doi: 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;9:604-612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vondracek SF, Teitelbaum I, Kiser TH. Principles of kidney pharmacotherapy for the nephrologist: core curriculum 2021. Am J Kidney Dis. 2021;78(3):442-458. doi: 10.1053/j.ajkd.2021.02. [DOI] [PubMed] [Google Scholar]
- 27. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151-164. doi: 10.1038/nrneph.2017.181. [DOI] [PubMed] [Google Scholar]
- 29. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;23:2473-2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 30. Agron RT, Gale S, Neavins TM, et al. Evaluation of healthcare for transgender veterans. Endocrine Metabolic Sci. 2021;2:100072. doi: 10.1016/j.endmts.2020.100072 [DOI] [Google Scholar]