Abstract
Background:
Scleral cross-linking is a potential method to inhibit axial elongation of the eye, preventing the progression of pathologic myopia. Formaldehyde releasers, which are common preservatives found in cosmetics and ophthalmic solutions, have been shown to be not only effective in cross-linking corneal collagen in vitro and in vivo, but also have minimal toxicity effects on the eye. The present study aims to evaluate the efficacy of scleral cross-linking using sodium hydroxymethylglycinate (SMG) to inhibit eye growth using an in vivo rabbit model.
Methods:
A cross-linking solution containing 40mM SMG was delivered to the sub-Tenon’s space behind the equator. The application regimen included a two-quadrant injection performed 5 times over 2 weeks on New Zealand white rabbits (n=5, Group 1), and one-time injection followed for up to 5 days on Dutch-Belted rabbits (n=6, Group 2). Group 1 was monitored serially for axial length changes using B-scan ultrasound (US) for 5-6 weeks. Group 2 was injected with a higher viscosity solution formulation. Both groups were evaluated for thermal denaturation temperature changes of the sclera post-mortem.
Results:
Axial growth was limited by 10-20% following SMG treatment as compared to the untreated eye. Thermal denaturation analysis showed increased heat resistance of the treated eyes in the areas of injection. Overall, the SMG treatment inhibited eye growth with few side effects from the injections.
Conclusions:
Cross-linking solutions delivered via sub-Tenon injection provide a potential method for limiting axial length growth in progressive myopia and could be used as a potential treatment for myopia.
Keywords: Myopia, subtenon injection, scleral cross-linking, sodium hydroxymethylglycinate, axial myopia treatment
Precis
The application of topical cross-linking solutions containing formaldehyde releasers via sub-Tenon’s injection in live rabbits is described. The results indicate that axial growth stunting of the rabbit globe can be achieved without clinical side effects.
BACKGROUND
By most accounts, progressive and severe near-sightedness (high myopia) and pathologic myopia are reaching pandemic proportions. Currently, high and pathologic myopia are leading causes of blindness worldwide. It is estimated that by 2050, 4.8 billion people will be affected by myopia, and 939 million will have high myopia [1]. Sight-threatening pathology associated with high myopia is as high as 70% [2–4]. Particularly in Asia and highly educated industrialized countries, pathologic and high myopia rates are skyrocketing. High myopia in Singapore, for example, occurs in 1 of every 12 persons [5]. The cost of ophthalmic care for patients with myopia, which includes eye specialist visits, optical purchases, contact lenses and LASIK surgery, is estimated at approximately US $755 million per year [6]. While these treatments are able to correct the myopic refractive error to a certain degree, they do not slow the physiological changes associated with excessive axial elongation [7]. There are several sight-threatening complications in the setting of high myopia, such as foveoschisis, retinal detachments, atrophy and choroidal neovascularization [8], and glaucoma [9], which can lead to pathologic myopia and irreversible loss of vision. Staphyloma, which has an extremely high complication rate, occurs with an increasing frequency as refractive error and axial length (AL) increase. Curtin’s landmark paper reported about 5% staphyloma for AL 27.5-28.4mm, with the rate increasing to 33% for AL 29.5-30.4mm [10]. Hsiang et al. have also reported the presence of staphyloma in 90% of patients with high myopia (>-8D) [11]. Thus, increased AL is closely tied to the development of staphyloma (although not a requirement), which carries a significant risk of sight-threatening complications.
Progressive high myopia is potentially treatable through scleral cross-linking, a theory supported by the fact that blockage of enzymatic collagen cross-linking results in increased levels of form-deprivation (FD) induced myopia [12]. El Sheikh and Phillips have recently discussed the feasibility and potential of using riboflavin photochemistry, commonly known as collagen cross-linking (CXL) [as has been used on the cornea for the treatment of keratoconus (KC)], for posterior scleral stabilization to halt axial elongation [13]. Although successfully used for treating destabilization of the anterior globe surface, that is, the corneal ectasia that develops in KC, treatment of the posterior globe with scleral cross-linking requires surgical access to the region, which has its own set of associated issues. Previous attempts at scleral cross-linking (i.e. sTXL=scleral therapeutic tissue cross-linking) using CXL were also reported, although difficulty accessing the posterior sclera with a UV light source was a concern [14], as well as changes in ERG amplitudes following CXL with UV A light in rabbit sclera [15]. More recently, the CXL approach has been successfully used to halt axial elongation in visually form-deprived rabbits (achieved by tarsorrhaphy), although multiple regions of the posterior sclera required separate irradiation zones [16]. Currently, the only clinically-available modality that can stabilize and reduce the axial growth of the eye (at 2 years) with minimal myopic rebound effect is topical atropine [17] [18].
Despite advancements in photochemical methods for scleral stabilization, a topical approach may provide advantages. In this report, sclera treatment with sodium hydroxymethylglycinate (SMG) was evaluated in live rabbits. This is the first report of using SMG in live animal sclera, as compared to previous literature regarding these compounds for scleral cross-linking which were all performed in ex vivo systems [19 20]. SMG is a compound from a class known as formaldehyde-releasing agents (FARs). FARs are widely used as chemical preservatives in cosmetics and other personal care products such as shampoos and conditioners. As these compounds are often in contact with the human body, their safety profiles have been evaluated prior to their usage. Given the proper conditions, FARs will release formaldehyde to variable degrees [21]. Curiously, although formaldehyde itself is a known carcinogen, the literature also supports the fact that FARs are non-mutagenic and non-carcinogenic [22]. SMG has found significant commercial use as a chemical preservative and is used in a variety of products that include eyedrop formulations for use in dry eye therapy (Blu-Gel A, Sooft, Italy). SMG’s relatively smaller size and potent cross-linking effects justified its selection as a candidate corneal cross-linking compound for evaluation in live animals, as reported herein. SMG is an amine-based N-formal compound, a potent formaldehyde-releasing agent, which consequently introduces significant collagen cross-linking. We have studied the use of a number of different FARs as potential tissue cross-linking agents for both the cornea and sclera and have published several papers on the topic [19 21 23 24].
Thus, the present study was undertaken in order to evaluate the potential of using SMG cross-linking solutions as a potential treatment for progressive myopia due to axial growth lengthening. Specifically, we examine the effect of SMG on eyes undergoing physiologic growth as a proof-of-principle in vivo study.
MATERIALS AND METHODS
Animal care and experimentation
All animal’s experimental procedures were carried out in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and approved by the Institutional Animal Care and Use Committee (IACUC) of Columbia University. Animal experiments were conducted in 2 groups (total of 11 animals). The first group (G1) consisted of 5 rabbits (10 eyes) employed to elucidate the long-term effect of SMG solution on stunting AL and altering the sclera, as assessed by thermal denaturation using differential scanning calorimetry (DSC). The second group (G2) utilized 6 rabbits (12 eyes), to assess the ability of a higher viscosity SMG solution in enhancing the short term, targeted effects on thermal denaturation. Sample size was determined by the researchers and animal availability.
Rabbits were obtained from Covance (Princeton, NJ), aged 14-18 weeks old, weighing 1.0 to 1.5 kg, Dutch Belted (DB) for G2 and approximately 2 months younger New Zealand White (NZW) for G1. After a 1-week acclimatization period, a pre-treatment baseline evaluation was completed in order to ensure that no signs of ocular inflammation or gross abnormalities were present. The animals were housed in standard cages in a light-controlled room at a temperature of 23°C ± 2°C at a relative humidity of 60% ± 10% and a 12-hour light-dark cycle (6 AM to 6 PM). Rabbits were given food and water ad libitum. Rabbits were examined for up to 6 weeks after the treatment for G1 (long-term effect group) and up to 5 days for G2 (short-term, higher viscosity group). NZW and DB rabbit breeds were found to have no discernible difference in terms of scleral properties at baseline or after SMG treatment.
Topical anaesthesia was applied using 0.5 % proparacaine (Akorn, Illinois). Intraocular pressure (IOP) and pre-injection B-mode ultrasound (US) measurements (Quantel CineScan 10MHz, Quantel Medical, France) were obtained with the animals fully awake with topical anaesthetic applied. For the cross-linking treatment procedure, the animals were sedated with an intramuscular injection of ketamine and xylazine. Euthanasia was performed with an overdose of sodium pentobarbital (Euthasol, Virbac, Fort Worth, TX) through the marginal ear vein. All procedures were performed by the same researchers (QVH, QW, RHS, MZ).
sTXL procedure for G1
The rabbits underwent baseline B-scan US AL recordings (range: 13.3 to 14.1 mm) before the start of experiments. The animals were injected in the sub-Tenon (sT) space 5 times over 2 weeks in two quadrants, superonasal (SNQ) and inferotemporal (ITQ) and AL was followed over 5 weeks. The interval between injections was 3-5 days with the last injection administered at day 19 of treatment. ST injections were delivered with a sharp needle (Precision Glide 30 G · 1/2 gauge, Becton Dickinson & Co, Franklin Lakes, NJ). The “max allowed” SMG concentration of 0.5% (39.06mM), according to the European Committee on cosmetic standards [25], was prepared for injection. This number was rounded to 40mM of SMG for ease of computation and communication. Collagen cross-linking agent, SMG solution (Suttocide A, Ashland Inc. Columbus, OH, USA 40 mM, pH 9.0), was injected in the right sT space and an isotonic phosphate buffer solution (identical in composition, pH and osmolarity to the right eye, but without the SMG) in the left eye as a negative control. The total volume administered per sT injection per eye was 600-750 μL. Indirect ophthalmoscopy was performed immediately after the sT injection, the next day and subsequently on a weekly basis. Following the procedure, a pea-sized amount of erythromycin ophthalmic ointment USP 0.5% was applied to the injection site. Slit-lamp biomicroscopic examination of the eyes were performed daily to ensure no conjunctival or tenon’s scarring developed (with all tissue remaining mobile relative to the underlying sclera). US was performed weekly to assess AL up to week 5. Intermediate measurement time points were not identical across animals, but initial and post-injection time points were acquired for all rabbits. The percentage of right eye vs left eye (untreated) growth was calculated using the formula below:
Axial length was measured 4 times (via B-mode US) and was averaged to arrive at the mean axial length for a given eye at each time point. The standard deviation of the mean was less than 1% of the axial length (and on average < 0.04%).
sTXL procedure for G2
Right eyes were injected with the cross-linking (SMG) solution, whereas left eyes were injected with a control solution (identical to the cross-linking solution, except for the absence of SMG). Solutions were prepared using 1.1-4.4% of hydroxymethylcellulose (HPMC, CAS #9004-65-3, 15cP Sigma-Aldrich, St. Louis, MO, USA). Sub-Tenon’s injections were administered as described above.
The precise location of the cannula insertion sites on the sclera was stained with a surgical marker at about 2mm behind the limbus. sT injection was performed as described for G1 in the SNQ or superotemporal quadrant (STQ). Immediately after injection, light reflex and IOP were appreciated 5, 10, 15 and 30 minutes following the injection. At the same time, US scan was performed to confirm the location of the injected solution via identification of a “T-sign”[26]. Both eyes were treated on the same day with a 15 min interval.
Differential scanning calorimetry (DSC)
Following the euthanasia, eye globes were excised. For G2, 6 mm scleral punches were taken from each eye. A minimum of five scleral punches were taken from the superior part of the globe (injection side) and contralateral, non-injected side from each eye. Pieces were processed as previously described [27]. In this way, we determined the denaturation temperature for each scleral punch independently. Averages of thermal denaturation temperatures of scleral punches were compared between corresponding areas from the cross-linked eye and contralateral-paired control eye.
Peak thermal denaturation temperature (Tm) was measured using a Perkin-Elmer DSC 6000 Autosampler (Waltham, MA, USA). The difference in thermal denaturation temperature (ΔTm) between the cross-linked sample and contralateral-paired control sample was determined using the Pyris software (version 11.0; Perkin-Elmer, Waltham, MA, USA) as previously reported [28].
Histology
To further assess for the depth and spread of treatment from a single SMG treatment that might damage surrounding tissues, histology was performed on all rabbits treated in G2. Following euthanasia, the eyes were removed for post-mortem processing. Tissue pieces from the eyelids, extraocular muscles, lacrimal and Harderian glands were fixed in 10% formalin solution, and paraffin-embedded sections were stained with H&E.
Statistical analysis
Analyses were performed with Stata Software (StataCorp LLC, College Station, Texas) The outcome measures are reported as the mean±SD. A Student’s t-test was used for statistical analysis of independent variables. Statistical significance was considered as a p-value of less than 0.05.
RESULTS
Axial length/Thermal denaturation – efficacy evaluation
G1 US measurements demonstrated that the treated eyes, as compared to the contralateral saline-injected control, displayed a lower average amount of AL growth over 5-6 weeks of follow-up, indicating physiologic eye growth was stunted. (Figure 1). Baseline AL values were 13.78 ±0.10 and 13.70 ±0.10 for treated and untreated eyes, respectively (P=0.597, t-test). On average, right eye ALs grew only 81.3 ± 22.2 % (n = 5) at week 2 as compared to the untreated left eye (Table 1). After a treatment was completed at week 2, the right eye AL percentage growth was 92.9 ± 15.2 % (n = 3) that of the left eye at week 3. Further follow-up post-injection showed persistent stunting of right eye physiologic AL growth, which had grown approximately 13 to 15% less than the contralateral control eyes (85.1 ± 2.3 % (n = 4) and 87.8 ± 2.7 % (n = 3) at week 4 and 5 respectively). It should be noted that the rabbits included in this study were not all from the same birth cohort, and therefore rabbits were treated and evaluated at different times (i.e., not simultaneously as a single cohort). Statistical significance was not reached at the intermediate time points but was reached at the week-5 final time point where treated right eyes had grown 1.05 +/− 0.05 mm versus 1.19 +/− 0.05 mm in the control left eye (P=0.018).
Figure 1.
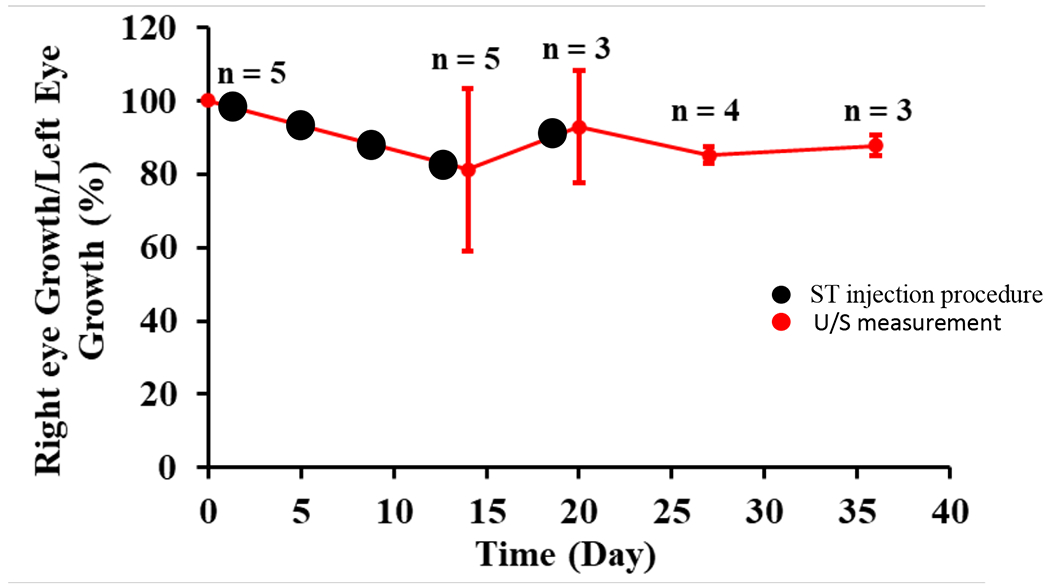
Effect of SMG on G1 physiologic eye growth (as expressed by the ratio of right eye axial length growth as compared to the contralateral control left eye axial length growth) followed over the course of 5-6 weeks after the sT injections. Injection times are represented by black circles and ultrasound measurements by red circles with number of observations for each measurement above.
Table 1.
Axial Length Changes After Sub-Tenon Injection of Crosslinking Agent
| Right Eye (Treated) Mean +/− SD (n) |
Right Eye (Sham) Mean +/− SD (n) |
P-value | |
|---|---|---|---|
| Baseline Axial Length on Day 0 (in mm) | 13.78 +/− 0.1 (n=5) | 13.7 +/− 0.1 (n=5) | 0.60 |
| Change in Axial length (mm) | |||
| At Day 13 (n=5) | 0.34 +/− 0.14 | 0.40 +/− 0.10 | 0.42 |
| At Day 19 (n=3) | 0.65 +/− 0.12 | 0.73 +/− 0.23 | 0.64 |
| At Day 27 (n=4) | 0.80 +/− 0.12 | 0.94 +/− 0.13 | 0.15 |
| At Day 35 (n=3) | 1.05 +/− 0.05 | 1.19 +/− 0.05 | 0.02* |
Bold font and asterisk denote statistical significance at p < 0.05. Baseline axial length was measured on Day 0 (defined as the first day of treatment). mm = millimeters. n = sample size. SD = standard deviation.
HPMC was added into the SMG solution when treating G2 to evaluate the effect of higher viscosity (with reduced diffusion) on the collagen crosslinking. As shown in Figure 2, Tm shift was calculated based on the intraocular difference of Tm in comparable regions in the SMG-injected treatment eye versus the sham-injected contralateral control eye. DSC data of the non-injected inferior scleral region did not show much Tm shift (−0.2 ± 0.5ºC). In contrast there was a significantly higher Tm shift of 1 ± 0.8° in the injected superior sclera region (P=0.011). Also, the Tm shift for eyes treated with SMG solution containing 1.1-1.4% HPMC (1 ± 0.8°) was greater than that found with SMG solution that did not contain HPMC (0.17 ± 0.11º, P=0.048).
Figure 2.
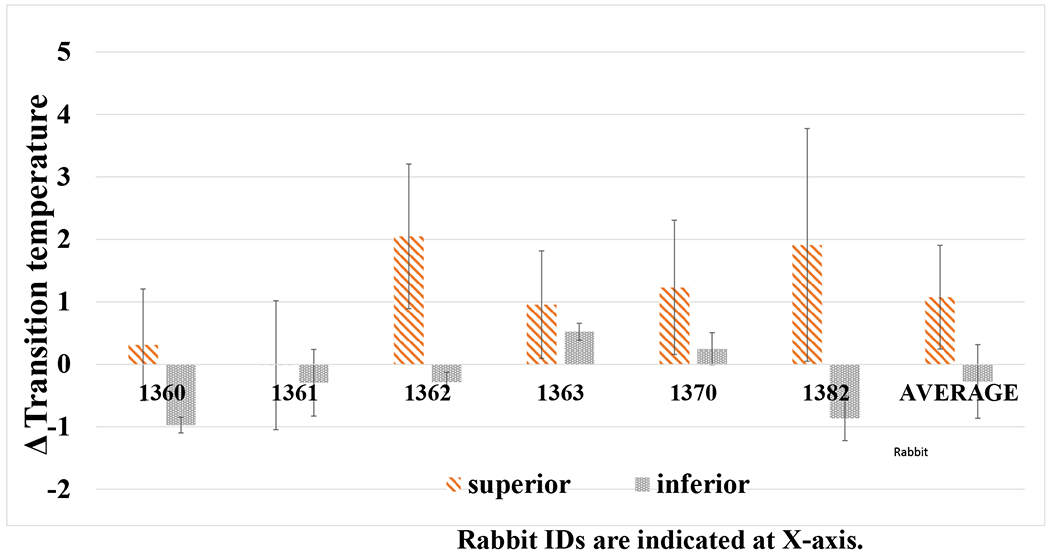
Difference in denaturation temperature (ΔTm) of the difference top versus bottom G2 sclera punches and average ΔTm after treatment with 40 mM SMG containing HPMC (15 cP). Each set of bars represent an individual rabbit (1360, etc., labeled accordingly below) and is the Tm difference between right (treated) and left (control) globes. The average of all 6 animals is shown to the right. Rabbit identification numbers are indicated on the x-axis.
Ocular reactions – Toxicity evaluation
All rabbits were monitored for 5 days after injections for any signs of treatment toxicity. Eye examination performed right after the injection revealed chemosis in all eyes regardless of the injected solution. Conjunctival redness was routinely observed on both eyes as well. These findings persisted on the treated eye for one day after treatment and subsided subsequently. Direct and indirect ophthalmoscopy findings did not indicate any change in any rabbits during follow-up observations.
As shown in Figure 3, IOP was found to be higher in treated and control eyes of all rabbits right after the injection and persisted for up to 30 min (day 0 on the graph), although with a significant variation between rabbits. IOP was also measured for the G1 group (data not shown) with no sustained IOP elevations observed. IOP subsided by the next day to values slightly lower than the baseline and returned to the pre-treatment measurements after the second day. To examine if there was any cellular damage in the vicinity of an SMG injection area, we carried out histology with adnexal tissues found no significant defference in the appearance of the SMG-treated tissues (Figure 4) compared to sham-treated controls (not shown).
Figure 3.
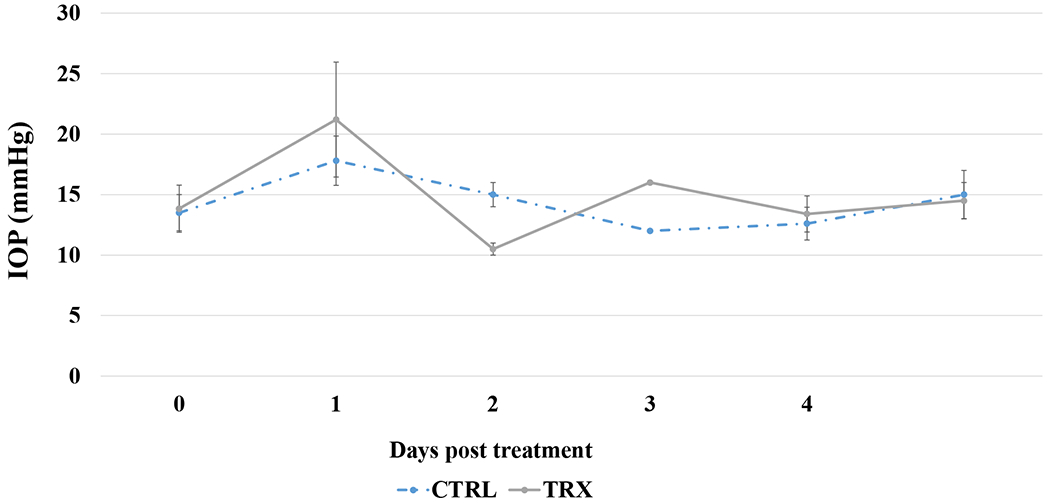
IOP of the G2 treated right eye (TRX) as compared to the untreated left eye (CTRL) after the 40mM SMG containing HPMC (15cP) treatment.
Figure 4.
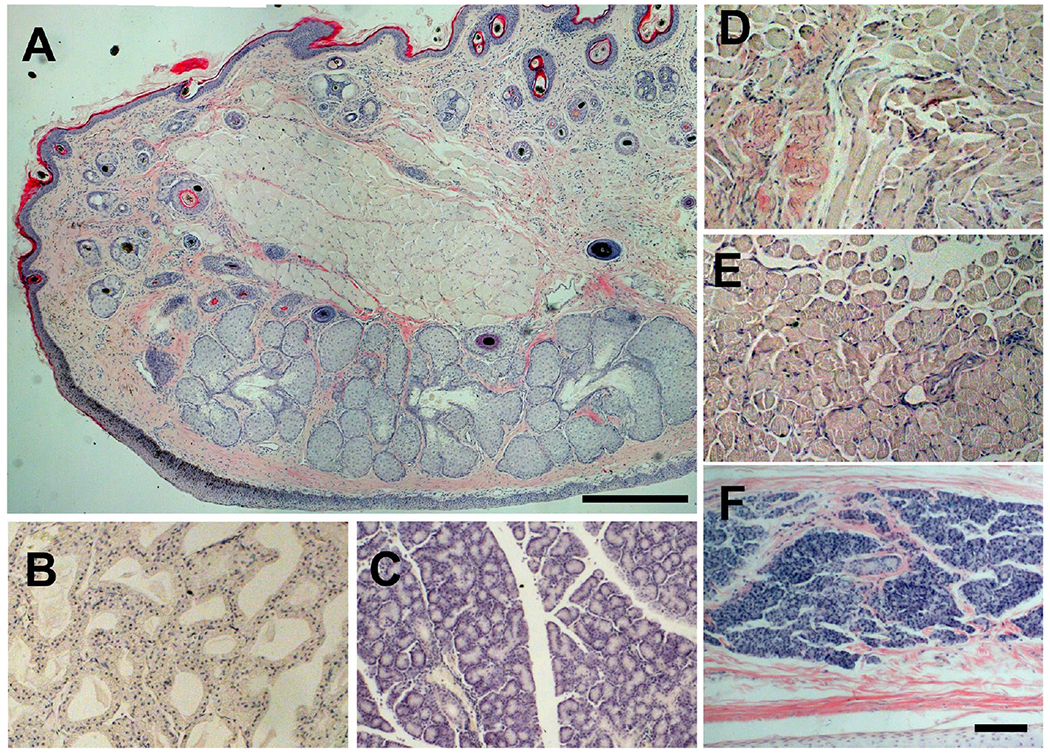
Histology of adnexal tissues after SMG injection. SMG at 40mM was injected into the sT and the eye fixed at 5 days after the injection. Paraffin sections of adnexal tissues with H/E staining are shown. A, eyelid, B, Harderian gland, C, lacrimal gland, D, superior oblique muscle, E, superior rectus muscle, F, nictitating membrane, Bar, 200 μm in panel A, 100 μm in all other panels.
DISCUSSION
In this study, the “max allowed” concentration of SMG was used to investigate the effect of SMG in sTXL on the inhibition of physiologic eye growth. There were no apparent clinical side effects on the retina, sclera and adnexal tissues in any SMG-treated rabbits as judged by gross observation, slit-lamp biomicroscopy, and an ophthalmoscope by trained ophthalmologists (MZ, QVH), although its effects on human patients need to be evaluated thoroughly.
SMG treatment resulted in about 15% stunting of the physiologic eye growth that persisted for at least 5 to 6 weeks of life (approximately 3 to 4 weeks post-treatment). Although the short-term effect was pronounced and seen just after the end of the treatment period, there were also long-term effects noted as well, which may reflect alterations in the natural scleral remodelling that occurs during physiologic growth [29].
Wollensak previously described the use of a sT injection using glyceraldehyde (a chemical cross-linking agent similar in concept to the FARs used in the present work) for stiffening the rabbit sclera [30], and genipin has been shown to limit AL in FD guinea pigs [23 28 30 31]. These investigators have demonstrated the advantage of using a chemical agent over the CXL technique for the purpose of stabilizing the posterior sclera. The thermal denaturation of scleral collagen at different locations of the eye was determined using the phase transition temperature from DSC measurement. The denaturation temperature is correlated with the stiffness of the tissue or the extent of the collagen cross-linking (from SMG or other external factors). Specifically, in the DSC analysis for G2 rabbits, right eyes were treated with SMG in the superior posterior region. Thus, several different locations in the posterior region were measured using DSC. The sclera of the treated eye had a higher transition temperature compared to fellow control eyes in all rabbits. While this effect was minimal, it suggests that the increased collagen resistance to heat was due to a cross-linking effect.
As shown in our previous work, SMG it is a potent cross-linking compound that is capable, at the right concentration, to stiffen collagenous tissue with minimal toxicity effect. The spectrum of the potential application of SMG as a tissue cross-linker is wide and expanding. One of the clinical uses of SMG is on ectatic corneal diseases such as keratoconus, where it can be used in a variety of moadilities such as topical drops, gel, and a corneal reservoir [32]. In addition, the natural function of SMG as a preservative also allows it to be used to treat a variety of bacterial pathogens [33 34].
In this study, SMG was able to inhibit the growth of the eye’s AL without any apparent side effects or obstruction of the tissue remodeling processes, suggesting that SMG is a potential collagen cross-linking agent to prevent the progression of myopia. Also, a posterior sT injections of the chemical cross-linking agent is a readily-accessible, potentially localized, and minimally invasive procedure. Injection via sT’s space of a soluble and diffusible agent is advantageous as it can contact the scleral surface uniformly. sT’s injection is a well-known technique used extensively in clinical eye surgery as an anaesthetic application. It has several advantages over other similar types of regional anaesthetic ocular blocks, such as subconjunctival and retrobulbar techniques. Injection of fluid into sT space tracks behind the globe to open up the posterior sT space [35] and thus, such an injection could be ideal for inducing a change in the post-equatorial sclera, the location of axial elongation and remodeling. Another major advantage of an injection approach (versus photochemical) is the relative ease of re-application if repeated treatments are required. However, future studies that optimize various factors such as reagent formulation, dosage, and injection frequency, with an animal model are needed to assess the feasibility of SMG for myopic treatment. It should be noted that employing an injection method for treatment does expose the eye to non-trivial risks that are associated with sT injections, such as an injection into the choroidal or retinal circulation, globe perforation, conjunctival hemorrhage, and infection. A risk-benefit ratio will have to be evaluated carefully before any clinical application is considered.
There are a few limitations of the study, including a low number of observation and inconsistent and relatively short follow-up (including only n=3 at the 5 week time point for G1 rabbits), potentially influencing the significance of the results. Moreover, a thermal denaturation assay is an indirect method of assessing cross-linking efficacy. The present study examined the effect of SMG on eyes undergoing physiological growth, as a proof-of-principle in vivo study. The results did not invalidate the potential of SMG treatment with myopia, and future experiments are planned with employing established myopia models (e.g. form-deprivation myopia) to evaluate the effect of SMG on myopia-induced eye growth under controlled conditions, in order to further test the clinical potential of SMG [16].
The effect of SMG in our study was evaluated with AL measurements and thermal denaturation temperature difference. Both methods indirectly assess the effect of cross-linking, with DSC being the most accepted and reliable method to measure cross-linking effects in collagenous tissues. We have previously used thermal denaturation (as thermal shrinkage temperature) to reliably measure chemically and UVA-riboflavin-induced cross-linking of collagenous tissue
In summary, administration of a collagen cross-linker, SMG, demonstrated a possibility of stunting of physiologic eye growth in growing rabbits with no apparent clinical side effects, suggesting that SMG is a promising candidate modality for scleral stiffening to prevent myopic progression
Acknowledgements
We thank John Peregrin for help in histology.
Funding
Supported in part by: a Research to Prevent Blindness grant to the Columbia University Department of Ophthalmology, and National Institutes of Health Grants, NEI R01EY020495 (dcp), NEI P30 EY019007, and NCRR UL1RR024156.
Abbreviations
- AL
axial length
- CXL
corneal collagen cross-linking = riboflavin UVA photochemical cross-linking
- DB
Dutch-belted rabbits
- ECM
extracellular matrix
- ERG
electroretinogram
- FAR
formaldehyde releaser
- FD
form-deprivation
- H&E
hematoxylin and eosin
- KC
keratoconus
- IOP
intraocular pressure
- IT
inferotemporal
- LASIK
laser-assisted in situ keratomileusis
- NZW
New Zealand white rabbits
- PP
posterior pole
- sCXL
scleral cross-linking (riboflavin-UVA photochemical technique)
- SMG
sodium hydroxymethylglycinate
- SNQ
superonasal quandrant
- STQ
superotemporal quandrant
- ITQ
inferotemporal
- sT
sub-Tenon’s injection
- Tm
thermal denaturation (melting) temperature
- ΔTm
difference in thermal denaturation temperature
- sTXL
scleral therapeutic tissue cross-linking
- TXL
therapeutic tissue cross-linking
- US
ultrasound (B-mode)
- UVA
ultraviolet A irradiation
Footnotes
Competing interests
Patents issued through Columbia University: U.S. Patent Nos. 8,466,203; 9,125,856; 10,105,350; and 10,292,967. The authors declare that they have no competing interest
References
- 1.Holden BA, Fricke TR, Wilson DA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016;123(5):1036–42 doi: 10.1016/j.ophtha.2016.01.006[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina 1992;12(2):127–33 doi: 10.1097/00006982-199212020-00009[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Celorio JM, Pruett RC. Prevalence of lattice degeneration and its relation to axial length in severe myopia. Am J Ophthalmol 1991;111(1):20–3 doi: 10.1016/s0002-9394(14)76891-6[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 4.Group TEDC-CS. Risk factors for idiopathic rhegmatogenous retinal detachment. The Eye Disease Case-Control Study Group. Am J Epidemiol 1993;137(7):749–57 [PubMed] [Google Scholar]
- 5.Pan CW, Zheng YF, Anuar AR, et al. Prevalence of refractive errors in a multiethnic Asian population: the Singapore epidemiology of eye disease study. Invest Ophthalmol Vis Sci 2013;54(4):2590–8 doi: 10.1167/iovs.13-11725[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 6.Zheng YF, Pan CW, Chay J, Wong TY, Finkelstein E, Saw SM. The economic cost of myopia in adults aged over 40 years in Singapore. Invest Ophthalmol Vis Sci 2013;54(12):7532–7 doi: 10.1167/iovs.13-12795 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt 2005;25(5):381–91 doi: 10.1111/j.1475-1313.2005.00298.x[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol 2014;157(1):9–25 e12 doi: 10.1016/j.ajo.2013.08.010[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 9.Chang RT. Myopia and glaucoma. Int Ophthalmol Clin 2011;51(3):53–63 doi: 10.1097/IIO.0b013e31821e5342[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 10.Curtin BJ. The posterior staphyloma of pathologic myopia. Trans Am Ophthalmol Soc 1977;75:67–86 [PMC free article] [PubMed] [Google Scholar]
- 11.Hsiang HW, Ohno-Matsui K, Shimada N, et al. Clinical characteristics of posterior staphyloma in eyes with pathologic myopia. Am J Ophthalmol 2008;146(1):102–10 doi: 10.1016/j.ajo.2008.03.010 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 12.McBrien NA, Norton TT. Prevention of collagen crosslinking increases form-deprivation myopia in tree shrew. Exp Eye Res 1994;59(4):475–86 doi: 10.1006/exer.1994.1133[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Elsheikh A, Phillips JR. Is scleral cross-linking a feasible treatment for myopia control? Ophthalmic Physiol Opt 2013;33(3):385–9 doi: 10.1111/opo.12043[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 14.Wollensak G, lomdina E. Long-term biomechanical properties of rabbit sclera after collagen crosslinking using riboflavin and ultraviolet A (UVA). Acta Ophthalmol 2009;87(2):193–8 doi: 10.1111/j.1755-3768.2008.01229.x[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Zhang F, Liu K, Zhao X. Safety evaluation of rabbit eyes on scleral collagen cross-linking by riboflavin and ultraviolet A. Clin Exp Ophthalmol 2015;43(2):156–63 doi: 10.1111/ceo.12392[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.Dotan A, Kremer I, Livnat T, Zigler A, Weinberger D, Bourla D. Scleral cross-linking using riboflavin and ultraviolet-a radiation for prevention of progressive myopia in a rabbit model. Exp Eye Res 2014;127:190–5 doi: 10.1016/j.exer.2014.07.019[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 17.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology 2006;113(12):2285–91 doi: 10.1016/j.ophtha.2006.05.062[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Fang YT, Chou YJ, Pu C, et al. Prescription of atropine eye drops among children diagnosed with myopia in Taiwan from 2000 to 2007: a nationwide study. Eye (Lond) 2013;27(3):418–24 doi: 10.1038/eye.2012.279[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babar N, Kim M, Cao K, et al. Cosmetic preservatives as therapeutic corneal and scleral tissue cross-linking agents. Invest Ophthalmol Vis Sci 2015;56(2):1274–82 doi: 10.1167/iovs.14-16035[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zyablitskaya M, Takaoka A, Munteanu EL, Nagasaki T, Trokel SL, Paik DC. Evaluation of Therapeutic Tissue Crosslinking (TXL) for Myopia Using Second Harmonic Generation Signal Microscopy in Rabbit Sclera. Invest Ophthalmol Vis Sci 2017;58(1):21–29 doi: 10.1167/iovs.16-20241 [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takaoka A, Cao K, Oste EM, Nagasaki T, Paik DC. Topical therapeutic corneal and scleral tissue cross-linking solutions: in vitro formaldehyde release studies using cosmetic preservatives. Biosci Rep 2019;39(5) doi: 10.1042/BSR20182392[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Groot AC, Flyvholm MA, Lensen G, Menne T, Coenraads PJ. Formaldehyde-releasers: relationship to formaldehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde-releasers. Contact Dermatitis 2009;61(2):63–85 doi: 10.1111/j.1600-0536.2009.01582.x[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 23.Kim M, Takaoka A, Hoang QV, Trokel SL, Paik DC. Pharmacologic alternatives to riboflavin photochemical corneal cross-linking: a comparison study of cell toxicity thresholds. Invest Ophthalmol Vis Sci 2014;55(5):3247–57 doi: 10.1167/iovs.13-13703[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta J, Takaoka A, Zyablitskaya M, Nagasaki T, Paik DC. Development of a topical tissue cross-linking solution using sodium hydroxymethylglycinate (SMG): viscosity effect. Biosci Rep 2020;40(1) doi: 10.1042/BSR20191941 [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Scientific Committee on Costmetic Products and Non-Food Products Intended for Consumers. Opinion concerning the determination of certain formaldehyde releasers in cosmetic products. . SCCNFP; 2002 [Google Scholar]
- 26.Winder S, Walker SB, Atta HR. Ultrasonic localization of anesthetic fluid in sub-Tenon’s, peribulbar, and retrobulbar techniques. J Cataract Refract Surg 1999;25(1):56–9 doi: 10.1016/s0886-3350(99)80011-x[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 27.Zyablitskaya M, Munteanu EL, Nagasaki T, Paik DC. Second Harmonic Generation Signals in Rabbit Sclera As a Tool for Evaluation of Therapeutic Tissue Cross-linking (TXL) for Myopia. J Vis Exp 2018(131) doi: 10.3791/56385[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoang Q Wen Q Chang S, Trokel S, Silverman R, Paik D. In Vivo Crosslinking of Scleral Collagen in the Rabbit Using Sub-Tenon Injection of Nitroalcohol. Investigative Ophthalmology & Visual Science 2013;54(15):5169–6923821202 [Google Scholar]
- 29.Lin X, Wang BJ, Wang YC, et al. Scleral ultrastructure and biomechanical changes in rabbits after negative lens application. Int J Ophthalmol 2018; 11 (3):354–62 doi: 10.18240/ijo.2018.03.02[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wollensak G, Iomdina E. Crosslinking of scleral collagen in the rabbit using glyceraldehyde. J Cataract Refract Surg 2008;34(4):651–6 doi: 10.1016/j.jcrs.2007.12.030[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Corpuz CC. Effects of scleral cross-linking using genipin on the process of form-deprivation myopia in the guinea pig: a randomized controlled experimental study. BMC Ophthalmol 2015;15:89 doi: 10.1186/s12886-015-0086-z[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zyablitskaya M, Jayyosi C, Takaoka A, et al. Topical Corneal Cross-Linking Solution Delivered Via Corneal Reservoir in Dutch-Belted Rabbits. Transl Vis Sci Technol 2020;9(9):20 doi: 10.1167/tvst.9.9.20[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amponin DE, Przybek-Skrzypecka J, Zyablitskaya M, et al. Ex vivo anti-microbial efficacy of various formaldehyde releasers against antibiotic resistant and antibiotic sensitive microorganisms involved in infectious keratitis. BMC Ophthalmol 2020;20(1):28 doi: 10.1186/s12886-020-1306-8[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapuano PB, Scanameo AH, Amponin DE, et al. Antimicrobial Studies Using the Therapeutic Tissue Cross-Linking Agent, Sodium Hydroxymethylglycinate: Implication for Treating Infectious Keratitis. Invest Ophthalmol Vis Sci 2018;59(1 ):332–37 doi: 10.1167/iovs.17-23111 [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar CM, McNeela BJ. Ultrasonic localization of anaesthetic fluid using sub-Tenon’s cannulae of three different lengths. Eye (Lond) 2003; 17(9):1003–7 doi: 10.1038/sj.eye.6700501 [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]


