Abstract
Background:
Acute Kidney Disease (AKD) is defined as impaired kidney function present for < 90 days with or without an acute kidney injury (AKI) event. Adults with AKD have an increased risk for progression to chronic kidney disease (CKD) and mortality. There are no data on the epidemiology of AKD in children after transplant. The aim of this study was to evaluate the incidence and risk factors for AKI, AKD, and CKD in children after transplantation.
Methods:
This is a retrospective cohort study of all children undergoing non-kidney solid organ transplant between 2011–2019 at UPMC Children’s Hospital of Pittsburgh. AKI and AKD were defined using the Kidney Disease Improving Global Outcomes criteria. Patients with a new estimated glomerular filtration rate < 60 ml/min/1.73m2 persisting for > 3 months met criteria for new CKD. Variables associated with AKI, AKD, and CKD were analyzed.
Results:
Among 338 patients 37.9% met criteria for severe AKI, 13% for AKD, and 8% for a new diagnosis of CKD. Stage 3 AKI was independently associated with AKD (OR: 5.35; 95% CI: 2.23–12.86). Severe AKI was not associated with new onset CKD whereas AKD was associated with new onset CKD (OR: 29.74; CI: 11.22–78.82).
Conclusion:
AKD may be superior to AKI in predicting risk of CKD in children after non-kidney solid organ transplantation.
Keywords: acute kidney injury, acute kidney disease, transplantation, children
Introduction
There have been over 30,000 pediatric solid organ transplants from 1988 to 2020 and over this time there has been steady improvement in outcomes and patient survival.1 Parallel to this is an increasing global prevalence of chronic kidney disease (CKD) in both children and adults.2 The transplant population has numerous unique risk factors that increase their risk of developing CKD including chronic nephrotoxic medication use, poor diet, minimal physical activity, diabetes mellitus, hypertension, metabolic syndrome, and acute kidney injury (AKI).3,4
Traditionally we define AKI and CKD using glomerular filtration rate (GFR) criteria occurring within 7 days or persisting for more than 90 days, respectively.5,6 The 2012 Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI proposed the term acute kidney disease (AKD) to describe a continuum of AKI through the first 90 days whereafter it can be classified as CKD.5 Defining acute kidney dysfunction as AKI alone may result in missing those patients who have prolonged kidney injury but do not yet meet the diagnosis of CKD. AKD fills this definitional gap. Recently KDIGO published an updated definition of AKD in an effort to combine prior definitions and improve future research efforts.7 Study results have shown that adult patients with cirrhosis8, congenital heart disease9, and after cardiac procedures10 meeting criteria for AKD, with or without AKI, have an increased risk of poor outcomes such as progression to CKD and mortality. Currently there are no studies in pediatric non-kidney solid organ transplant patients specifically exploring the progression of kidney disease as defined by AKI, AKD, and CKD using the most current definitions. Understanding the role of AKD in pediatric transplantation is important because these patients are vulnerable to kidney insults in the early post-transplant period. Therefore, our primary study objective was to determine the incidence of AKI, AKD, and CKD in children after liver, intestine, multivisceral, heart, and lung transplantation at a large pediatric transplant center. Additionally, we sought to elucidate the potential risk factors for AKI, AKD, and CKD in this patient population.
Materials and Methods
Study Design and Population
This study used a retrospective cohort of all recipients of a non-kidney solid organ transplant including liver, intestine, multivisceral, heart, lung, and combined heart-lung who were under the age of 19 at the time of transplant. Data were obtained from the electronic medical record. The study protocol was approved by the Institutional Review Board for Health Sciences Research at the University of Pittsburgh and followed the tenets of the Declaration of Helsinki. All transplants occurred at the UPMC Children’s Hospital of Pittsburgh between the years of 2011 and 2019. Those children who had a history of a prior kidney transplant, received a concurrent kidney transplant, or were dialysis dependent at the time of transplant were excluded from the study. Creatinine values were obtained from both inpatient and outpatient medical records starting on the day of transplant until 2 years post-transplant. Urine output data were obtained starting immediately post-transplant through 7 days after transplant. General post-operative immunosuppression management included the initiation of methylprednisolone and tacrolimus. In the majority of patients, tacrolimus management was organ specific and per protocol: initiated on post-operative day 1 with goal trough levels of 10–15 for the first 6–8 weeks after transplant. Over the first post-operative year, trough levels were generally reduced as tolerated. Deviations from the protocol were on a per patient basis pending rejection, infection, or other untoward immunosuppression side effect. Ganciclovir was started on patients with cytomegalovirus positive donors. Dosing was based on body surface area and creatinine clearance with a maximum dose of 450 mg daily.
Variable Definitions
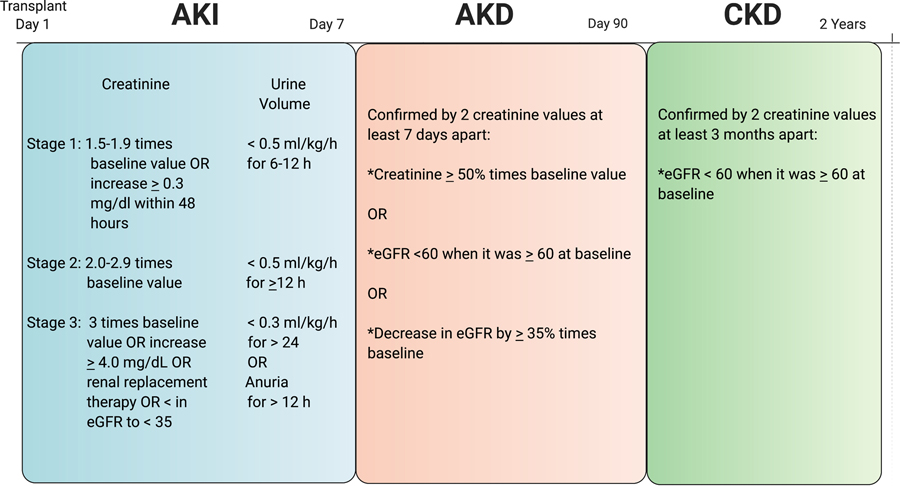
Baseline CKD was defined as an estimated GFR (eGFR) < 60 ml/min per1.73 m2 prior to transplant using the modified Schwartz equation.11,12 Baseline creatinine and eGFR were defined based on the serum creatinine level the day of, but prior to the transplant. AKI was defined based on the maximum KDIGO staging using either serum creatinine or urine output in the immediate 7 days after transplant (Figure 1).5 Stages 2 or 3 AKI were classified as severe AKI. The AKI definition was limited to the first 7 days post-transplant due to the availability of urine output and creatinine data. We defined AKD as a serum creatinine ≥ 50% increase from baseline or an eGFR < 60 ml/min per 1.73 m2 when it was ≥ 60 ml/min per 1.73 m2 at baseline or a decrease in eGFR by ≥ 35% from baseline.7 A patient was categorized as having AKD if they had 2 serum creatinine values that met criteria > 7 days apart within day 0 to 90 post-transplant (Figure 1). Patients did not have to meet AKI criteria in order to meet AKD criteria. A new diagnosis of CKD was defined as an eGFR < 60 ml/min per 1.73 m2 on at least 2 separate time points at least 3 months apart more than 90 days post transplantation when the eGFR was ≥ 60 ml/min per 1.73 m2 prior to transplant (Figure 1).6
Figure 1:
Criteria used to define acute kidney injury (AKI)5, acute kidney disease (AKD)7, and chronic kidney disease (CKD)12. Baseline creatinine (mg/dL) and eGFR (estimated glomerular filtration rate, mL/min per 1.73 m2) are based on the serum creatinine on the day of, but prior to transplant.
Race was classified as black/other as compared to white as specified in the medical record. Nephrotoxin medication days were defined as the number of days where at least 1 dose of 3 or more distinct nephrotoxic medications13,14 were administered in the first 7 days after transplant. A list of nephrotoxic medications is provided in Supplemental Table 1. Adjustment for illness severity was performed using predicted mortality calculated from a calibrated, modified, encounter-level electronic Pediatric Logistic Organ Dysfuntion-2 (ePELOD-2) score. This score was constructed by incorporating 9 measures of organ dysfunction during the admission of interest, excluding creatinine which is typically used in the calculation of PELOD-2, and was recalibrated using mortality data from the study center as previously described.15
Statistical Analysis
Continuous variables are presented as medians with interquartile ranges. Categorical variables are presented as numbers (percentages). Variables identified by univariable regression models with a p-value < 0.2 were included in the multivariable logistic regression model to determine factors independently associated with AKI and AKD separately for the overall patient cohort and for the larger individual transplant groups (liver and heart). To determine variables associated with a new diagnosis of CKD, patients with an eGFR prior to surgery meeting criteria for CKD, were excluded from the analysis. Results are shown as odds ratios (OR) with 95% confidence intervals (CI) for multivariable logistic regression. Discrimination ability of each of the logistic regression models were evaluated using the area under the curve of the receiver operating characteristic plot. Due to the small number of new CKD events in the liver and heart transplant groups, rather than logistic regression, potential associations of severe AKI and new CKD as well as AKD and new CKD were evaluated using the chi-square test. Statistical significance was set at a p-value of ≤ 0.05. All statistical analyses were done using Stata 16.0 (Stata Corporation, College Station, TX, USA).
Results
Baseline Characteristics
A total of 346 children received a liver, heart, lung, intestine, multivisceral, or heart-lung transplant during the study period. After excluding patients that received a prior kidney transplant (n=3), were dialysis dependent at the time of transplant (n=1) and those that received a concurrent kidney transplant (n=4), there were 338 children remaining in the cohort. Liver transplantation was the most common (n= 199), followed by heart (n=87), multivisceral (n=19), lung (n=17), intestine (n=13), and heart-lung (n=3) transplantation. Baseline demographics and clinical characteristics are summarized in Table 1. Prior to transplant 3 patients in the liver transplant group, 4 in the heart transplant group, and 5 patients in the multivisceral transplant group had a diagnosis of CKD according to their eGFR.
Table 1:
Baseline Demographics
| Characteristicsa | All patients | Liver | Heart | Multivisceral | Lung | Intestine | Heart-Lung |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Number of patients | 338 | 199 | 87 | 19 | 17 | 13 | 3 |
|
| |||||||
| Race | |||||||
| White | 275 (81.4) | 168 (84.5) | 64 (73.6) | 16 (84.2) | 15 (88.2) | 11 (84.6) | 3 (100) |
| Black | 29 (8.6) | 12 (6) | 13 (14.9) | 1 (5.3) | 1 (5.9) | 2 (15.4) | 0 (0) |
| Other | 34 (10) | 19 (9.5) | 10 (11.5) | 2 (1.1) | 1 (5.9) | 0 (0) | 0 (0) |
|
| |||||||
| Sex | |||||||
| Male | 173 (51.2) | 93 (46.7) | 53 (61) | 11 (57.9) | 6 (35.3) | 7 (53.8) | 3 (100) |
| Female | 165 (48.8) | 106 (53.3) | 34 (39) | 8 (42.1) | 11 (64.7) | 6 (46.2) | 0 (0) |
|
| |||||||
| Age (months) | 57 (15–158.5) | 34 (12–126) | 70 (11–164) | 58 (25.5–137) | 169 (147–187) | 68 (55–105) | 175 (137–179) |
|
| |||||||
| BMI | 17.7 (15.6–20.8) | 18.1 (16.1–20.8) | 16.7 (14.8–21.5) | 18.3 (15.4–20.8) | 17.4 (16.4–18.1) | 14.8 (14.3–17.8) | 15.5 (15.2–16.5) |
|
| |||||||
| Pre-existing CKD | 12 (3.6) | 3 (1.5) | 4 (4.6) | 5 (26.3) | 0 (0) | 0 (0) | 0 (0) |
|
| |||||||
| e-PELOD-2 Predicted Mortality | 0.028 (0.011–0.065) | 0.022 (0.007–0.024) | 0.034 (0.016–0.165) | 0.018 (0.014–0.044) | 0.03 (0.010–0.047) | 0.041 (0.017–0.074) | 0.026 (0.020–0.394) |
Data expressed as median (interquartile range) or N (%)
Abbreviations: e-PELOD-2 electronic Pediatric Logistic Organ Dysfunction-2
Incidence of AKI, AKD, and new CKD after Transplantation
A total of 186 patients (55%) met criteria for AKI within 7 day of transplant. There were 11 patients that met criteria for AKI based on urine output only (Supplemental Table 2). Table 2 shows the incidence of AKI, severe AKI, AKD, and new CKD in our study population. Recipients of a heart transplant had the highest rates of AKI, severe AKI, AKD, and CKD when compared to the entire cohort. Interestingly, the lung transplant group had a high rate of AKI (70.6%) but no cases of AKD. Supplemental Figure 1 depicts the relationships among patients meeting criteria for severe AKI, AKD, and new CKD. Within the entire cohort, 17% who met criteria for severe AKI also met criteria for AKD and 9% met criteria for new CKD after transplant. Of the 49 patients who received heart transplantation who developed severe AKI, 20% also met criteria for AKD (Supplemental Figure 2). Of note, a total of 23 patients in the entire cohort died within 2 years after transplant (9 within the first 3 months). Of the patients that died 14 met criteria for AKI, 4 for AKD, and 0 for CKD. No deaths were directly attributed to kidney failure.
Table 2:
Comparison of the Incidence of AKI, AKD, and CKD Among All Patients and Within Each Transplant Group
| Transplant type | AKI | Severe AKI | AKD | New CKD |
|---|---|---|---|---|
| All transplant patients | 186 (55.0%) | 128 (37.9%) | 44 (13.0%) | 27 (8%) |
| Liver | 90 (45.2%) | 60 (30.1%) | 18 (9.0%) | 7 (3.5%) |
| Heart | 64 (73.6%) | 49 (56.3%) | 21 (24.1%) | 14 (16.1%) |
| Multivisceral | 10 (52.6%) | 7 (36.8%) | 3 (15.8%) | 2 (10.5%) |
| Intestine | 8 (61.5%) | 4 (30.8%) | 2 (15.4%) | 1 (7.7%) |
| Lung | 12 (70.6%) | 6 (35.3%) | 0 (0%) | 3 (5.9%) |
| Heart-Lung | 2 (66.7%) | 1 (33.3%) | 0 (0%) | 0 (0%) |
Abbreviations: AKI Acute Kidney Injury, AKD Acute Kidney Disease, CKD Chronic Kidney Disease
Risk Factors for AKI, AKD, and new CKD
Among the total study population, age (p<0.01), severity of illness (p=0.01), and nephrotoxin medication days (p<0.01) were independently associated with AKI (Table 3 and Supplemental Table 3). The analysis was repeated individually in each of the two largest patient cohorts, patients that received a liver or heart transplant. Nephrotoxic medication days (OR: 2.54, 95% CI: 1.69–3.82, p<0.01) and female sex (OR: 0.15, 95% CI: 0.03–0.86; p=0.03) were significantly associated with AKI after heart transplantation. Nephrotoxic medication days (OR: 1.44, 95% CI: 1.09–1.90, p=0.01) as well as age at the time of admission to the intensive care unit (OR: 1.01, 1.00–1.01, p<0.01) were significantly associated with meeting criteria for AKI in patients after liver transplantation.
Table 3:
Multivariable logistic regression of risk factors for acute kidney injury compared to those without acute kidney injury in all transplant patients
| Characteristic | OR (95% CI) | P-Value |
|---|---|---|
| Age | 1.01 (1.00–1.01) | <0.01 |
| e-PELOD-2 Predicted Mortality | 7.45 (1.55–35.83) | 0.01 |
| Nephrotoxin Medication Days | 1.71 (1.42–2.05) | <0.01 |
Abbreviations: e-PELOD-2 electronic Pediatric Logistic Organ Dysfunction-2
Area under the curve of the receiver operating characteristic plot: 0.73
There were 13 patients that met criteria for AKD that did not meet criteria for AKI within 7 days of transplant (Supplemental Figure 2). In the overall transplant population age (p=0.02), race (p=0.03), and stage 3 AKI (p<0.01) were associated with AKD (Table 4 and Supplemental Table 4). The odds of developing AKD was 5 times greater in patients with stage 3 AKI compared to patients without stage 3 AKI. When the same potential risk factors were evaluated in the liver transplant group, age (OR: 1.01, 95% CI: 1.00–1.02, p<0.01) and stage 3 AKI (OR: 5.37, 95% CI: 1.44–20.05, p=0.01) were associated with AKD. AKI was not associated with AKD in patients after heart transplant (Supplemental Table 5).
Table 4:
Multivariable logistic regression of risk factors for acute kidney disease compared to those without acute kidney disease in all transplant patients
| Characteristic | OR (95% CI) | P-Value |
|---|---|---|
|
| ||
| Age | 1.01 (1.00–1.01) | 0.02 |
|
| ||
| Race | 2.49 (1.09–5.68) | 0.03 |
|
| ||
| Acute Kidney Injury | ||
| Stage 1 | 2.29 (0.90–5.86) | 0.08 |
| Stage 2 | 0.96 (0.31–2.92) | 0.94 |
| Stage 3 | 5.35 (2.23–12.86) | <0.01 |
Area under the receiver operating characteristic curve: 0.71
For the overall transplant cohort as well as individually in the patients after liver and heart transplantation AKI (severe and all stages of AKI) was not associated with a new diagnosis of CKD during the 2 year study period. In contrast, AKD was significantly associated with a new diagnosis of CKD in the overall patient cohort (Table 5 and Supplemental Table 6). Similarly, AKD was significantly associated with a new diagnosis of CKD in patients after liver (p<0.01) and heart (p<0.01) transplantation.
Table 5:
Multivariable logistic regression of risk factors for chronic kidney disease compared to those without chronic kidney disease in all transplant patients
| Characteristic | OR (95% CI) | P-Value |
|---|---|---|
| Age | 1.00 (0.99–1.01) | 0.57 |
| Race | 2.54 (0.81–7.96) | 0.11 |
| Acute Kidney Disease | 29.74 (11.22–78.82) | <0.01 |
Area under the receiver operating characteristic curve: 0.84
Discussion
In pediatric non-kidney solid organ transplant recipients at a large transplant center, AKD occurred in 13% and a new diagnosis of CKD was found in 8% of patients. Among the patients with a new diagnosis of CKD, 70% had a preceding diagnosis of AKD whereas AKI within the first 7 days following transplant was not associated with an increased risk of a new diagnosis of CKD. A 30-fold increased odds of a new diagnosis of CKD for those with a prior diagnosis of AKD, suggests that a diagnosis of AKD may offer an important opportunity to delay the progression of CKD. This is the first study exploring the incidence of AKD and its association with a new diagnosis of CKD in pediatric transplant patients.
We identified AKD as a risk factor for CKD in this special population. For this reason careful kidney monitoring during this potentially vulnerable time 3 months following transplant is important. We should not rely on AKI alone defined by either serum creatinine or urine output in the first 7 days post-transplant to exclusively identify patients who are at risk for CKD. Similar results were seen in a study by Williams et al. who analyzed the incidence rates of AKI and CKD after non-kidney solid organ transplant in 303 children at the Hospital for Sick Children.16 They did not include urine output for defining AKI but reported strikingly similar rates of AKI (67%) and CKD (8%) when compared to our study results. The investigators did not define AKD but found that patients with an AKI episode by 3 months after transplant had a greater risk of CKD. There was no association with meeting criteria for AKI in the first week after transplant and CKD both in our study and in the results reported by Williams and colleagues. The transplant population is at unique risk for repeated, prolonged kidney injury in the months to years after transplantation due to medication use and infection risks. We advocate for regular monitoring of kidney function during routine post-transplant care as well as the involvement of pediatric nephrologists in the care of transplant patients meeting criteria for AKD.
Each type of solid organ transplantation has its own unique risk factors. We identified pediatric patients after heart transplantation as a patient group requiring particularly close attention to kidney health after transplant. Interestingly, we found male sex to be protective for AKI in our cohort of patients after heart transplant. Multiple studies in adult patients have shown that being a woman is a risk factor for cardiac surgery associated AKI.17 When compared to the other patient cohorts, patients that received a heart transplant had the highest rates of AKI, AKD, and a new diagnosis of CKD in our study. Compared to abdominal transplant, patients undergoing heart transplantation have unique risk factors for AKI including intraoperative bypass exposure, low cardiac output, and cyanosis due to congenital heart disease. In 88 patients under 20 years of age after heart transplant Hollander and colleagues also reported a high AKI incidence of 72%.18 Similar to our study results, they found no association between AKI in the immediate post-operative period and CKD. These findings argue for close monitoring of kidney function even after the immediate post-transplant period.
Certainly, there are many instances where organ transplantation can lead to improvements in kidney function, such as in patients with decreased cardiac function or hepatorenal syndrome prior to transplant. However, the time period following a transplant can be a particularly at-risk period for kidney injury not just from the surgery itself, but also the need for frequent nephrotoxic medication dose adjustments, infection, and rapid changes in fluid status. We know that the development of end stage kidney disease after pediatric non-kidney solid organ transplants significantly increases the risk of mortality after transplant.19 Early recognition of patients with AKD prior to a CKD diagnosis may provide an opportunity for interventions to prevent CKD and end stage renal disease. The impact of the structured implementation of renal protective strategies when patients are at increased risk for AKI/AKD such as nephrology referral, strict blood pressure control and avoidance of hyperglycemia should be studied prospectively.
In our study cohort, 13 patients met criteria for AKD that did not meet criteria for AKI within 7 days of transplant, representing about a third of our study cohort with AKD. We speculate that patients not meeting criteria for AKI by serum creatinine or urine output, but positive for AKD may have had subclinical AKI during the 7 day post-operative period in which biomarker positivity can occur in the absence of changes in urine output or serum creatinine.20,21 Alternatively, these patients may have had no AKI in the 7 days after transplant, but an AKI event 7–90 days after transplant. In a study of over 1 million adult non-hospitalized adult Canadian residents, the investigators found that individuals that met criteria for AKD without AKI when compared to those without AKD had significantly higher risks of developing new CKD, progression of pre-existing CKD, kidney failure, and death.22 Unfortunately, we do not have data on biomarkers of kidney damage or stress available for our study. Future prospective investigations should include biomarker testing to determine potential associations of urine or serum biomarkers with AKD and the development of new CKD in the pediatric transplant population.
Our study has limitations. Given the retrospective nature of the study we were limited to available data in the medical record, however, all patients had ≥ 2 individual serum creatinine values 90 days after transplant and >90 days-2 years after transplant. We exclusively used creatinine to define AKD and CKD and did not use albuminuria, which may have changed the incidence of new CKD or AKD in our results.6,7 Due to the small event rates of CKD, we were unable to perform multivariable regression within the individual transplant groups. Additionally, we did not have data available regarding prior exposure to cardiopulmonary bypass or the use of ventricular assist device support, which are important potential risk modifiers for kidney injury in patients undergoing heart transplantation.23,24 It is possible that death was a competing risk factor, in particular for AKD and CKD. Lastly, greater than 50% of our study population were liver transplant patients which limits the generalizability of our results to other solid organ transplant recipients.
In conclusion, AKD which can develop with or without AKI increases risk of developing CKD by 30-fold 3 months-2 years after transplant. These results highlight the importance of minimizing kidney toxicity and monitoring kidney function closely both immediately post-transplant as well as in the long term care of transplant patients. Our results underscore the importance of future studies examining the impact of structured prevention strategies targeted at children that meet AKI and AKD criteria after transplant.
Supplementary Material
Supplemental Figure 2: A Venn diagram depicting the number of patients with and relationships of acute kidney injury (AKI), acute kidney disease (AKD), and new chronic kidney disease (CKD) in the entire patient cohort.
Supplemental Figure 1: Venn diagram showing the number of patients with and relationships of severe acute kidney injury (AKI), acute kidney disease (AKD), and chronic kidney disease (CKD) in the a) entire cohort, b) liver transplant patients, c) heart transplant patients, and d) multivisceral transplant patients.
Acknowledgments/Funding:
K23DK116973 (DYF)
1K23HD099331-01A1 (CMH)
Abbreviations:
- AKD
Acute Kidney Disease
- AKI
Acute Kidney Injury
- CKD
Chronic Kidney Disease
- GFR
Glomerular Filtration Rate
- KDIGO
Kidney Disease: Improving Global Outcomes
- eGFR
estimated Glomerular Filtration Rate
- ePELOD-2
electronic Pediatric Logistic Organ Dysfuntion-2
- OR
Odds Ratio
- CI
Confidence Interval
Data Availability Statement:
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy restrictions.
References
- 1.National Data Organ Procurement and Transplantation Network, June 1, 2020, https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed July 10, 2021.
- 2.GBD Chronic Kidney Disease Collaborative. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–940. [DOI] [PubMed] [Google Scholar]
- 4.Ruebner RL, Reese PP, Denburg MR, Rand EB, Abt PL, Furth SL. Risk factors for end-stage kidney disease after pediatric liver transplantation. Am J Transplant. 2012;12(12):3398–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 7.Lameire N, Levin A, Kellum JA, et al. Harmonizing Acute and Chronic Kidney Disease Definition and Classification: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021. [DOI] [PubMed] [Google Scholar]
- 8.Tonon M, Rosi S, Gambino CG, et al. Natural history of acute kidney disease in patients with cirrhosis. J Hepatol. 2020. [DOI] [PubMed] [Google Scholar]
- 9.Fuhrman DY, Nguyen L, Joyce EL, Priyanka P, Kellum JA. Outcomes of adults with congenital heart disease that experience acute kidney injury in the intensive care unit. Cardiol Young. 2020:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuura R, Iwagami M, Moriya H, et al. The Clinical Course of Acute Kidney Disease after Cardiac Surgery: A Retrospective Observational Study. Sci Rep. 2020;10(1):6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein SL, Mottes T, Simpson K, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016;90(1):212–221. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein SL, Kirkendall E, Nguyen H, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013;132(3):e756–767. [DOI] [PubMed] [Google Scholar]
- 15.Horvat CM, Ogoe H, Kantawala S, et al. Development and Performance of Electronic Pediatric Risk of Mortality and Pediatric Logistic Organ Dysfunction-2 Automated Acuity Scores. Pediatr Crit Care Med. 2019;20(8):e372–e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams C, Borges K, Banh T, et al. Patterns of kidney injury in pediatric nonkidney solid organ transplant recipients. Am J Transplant. 2018;18(6):1481–1488. [DOI] [PubMed] [Google Scholar]
- 17.Neugarten J, Sandilya S, Singh B, Golestaneh L. Sex and the Risk of AKI Following Cardio-thoracic Surgery: A Meta-Analysis. Clin J Am Soc Nephrol. 2016;11(12):2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollander SA, Montez-Rath ME, Axelrod DM, et al. Recovery From Acute Kidney Injury and CKD Following Heart Transplantation in Children, Adolescents, and Young Adults: A Retrospective Cohort Study. Am J Kidney Dis. 2016;68(2):212–218. [DOI] [PubMed] [Google Scholar]
- 19.Ruebner RL, Reese PP, Denburg MR, Abt PL, Furth SL. End-stage kidney disease after pediatric nonrenal solid organ transplantation. Pediatrics. 2013;132(5):e1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang F, Hu X, Dai X, et al. Subclinical acute kidney injury is associated with adverse outcomes in critically ill neonates and children. Crit Care. 2018;22(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James MT, Levey AS, Tonelli M, et al. Incidence and Prognosis of Acute Kidney Diseases and Disorders Using an Integrated Approach to Laboratory Measurements in a Universal Health Care System. JAMA Netw Open. 2019;2(4):e191795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toth R, Breuer T, Cserep Z, et al. Acute kidney injury is associated with higher morbidity and resource utilization in pediatric patients undergoing heart surgery. Ann Thorac Surg. 2012;93(6):1984–1990. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Krawczeski CD, Zappitelli M, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39(6):1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2: A Venn diagram depicting the number of patients with and relationships of acute kidney injury (AKI), acute kidney disease (AKD), and new chronic kidney disease (CKD) in the entire patient cohort.
Supplemental Figure 1: Venn diagram showing the number of patients with and relationships of severe acute kidney injury (AKI), acute kidney disease (AKD), and chronic kidney disease (CKD) in the a) entire cohort, b) liver transplant patients, c) heart transplant patients, and d) multivisceral transplant patients.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy restrictions.