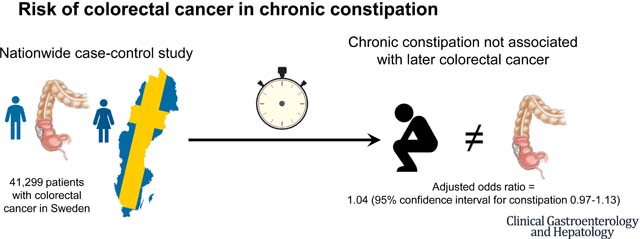
Abstract
Background and Aims:
Prolonged colon transit times may increase contact time between potential carcinogens in the stool and the colonic mucosa. Nonetheless, previous studies have yielded conflicting results connecting chronic constipation with risk of CRC. We examined the association between chronic constipation and later CRC.
Methods:
In this nationwide case-control study, we identified 41,299 CRC cases by colorectal biopsy in Sweden between July 2007 and December 2016 and matched them to 203,181 age- and sex-matched controls from the general population. We compared odds of earlier chronic constipation (defined as ≥2 laxative prescriptions in the Prescribed Drug Register with ≥6 months between first-last prescription) between CRC cases and controls using logistic regression. In separate analyses, we compared odds of earlier constipation between CRC cases and sibling comparators, but also examined earlier risk of having an inpatient/outpatient specialty diagnosis of chronic constipation prior to CRC.
Results:
Overall, 3,943 patients with CRC met our criteria for chronic constipation prior to CRC. The crude proportion of chronic constipation in CRC patients was 9.5% compared to 8.8% in controls. After multivariable adjustment, there was a modest association between chronic constipation and later CRC (OR=1.10, 95% CI=1.06–1.14) that vanished using sibling comparators to control for residual confounding (OR=1.04, 95% CI=0.97–1.13). In a sensitivity analysis of 126,650 CRC patients diagnosed 1989–2016, we found no association with earlier chronic constipation diagnosed in inpatient/outpatient specialty clinics (OR=0.88, 95% CI=0.75–1.04).
Conclusions:
In a nationwide case-control study, chronic constipation was not associated with later CRC.
Keywords: constipation, colon cancer, colorectal cancer, histopathology, colonoscopy
Graphical Abstract
INTRODUCTION
Chronic constipation affects an estimated 15% of the adult population worldwide,1 and is more likely to occur in those of lower socioeconomic status (SES) and increasing age.2 Similarly, colorectal cancer (CRC) is diagnosed in approximately 1 in 10 individuals globally,3 with increased risk in persons of low SES and with advancing age.4 Mechanistically, prolonged colon transit times may increase contact time between potential carcinogens in the stool and the colonic mucosa,5 but whether chronic constipation is a risk factor for CRC remains controversial.2 Moreover, constipation can be a symptom of CRC, keeping the possibility open that previous positive associations may be a result of reverse causation.
Current guidelines do not recommend a colonoscopy for chronic constipation in the absence of alarm symptoms,6 though most groups acknowledge that chronic constipation may portend an increased risk for CRC.7, 8 The evidence suggesting this link comes from many sources of varying methodology,9–15 with far fewer studies suggesting a lack of association between chronic constipation and CRC.16–19 The majority of these studies have not been strictly population-based9, 10, 12, 15, 19 or used bowel movement frequency as a surrogate for chronic constipation.12–14, 17, 20
Understanding the potential link between chronic constipation and CRC could have important implications for population-based screening initiatives given the high prevalence of both conditions. CRC is the second leading global cause of cancer mortality, and incidence rates are four times higher in the developed world compared to developing countries.3 This translates to significant financial considerations; in Sweden alone, 9% of patients with chronic constipation will undergo a colonoscopy per year.21
In a nationwide histopathology register-based study including all CRC cases in Sweden, we examined the potential association between chronic constipation and later CRC.
METHODS
In this nationwide case-control study we matched CRC cases with controls from the Swedish general population to explore if earlier constipation was a risk factor for CRC.
Registers and covariates
Demographic data (dates of birth and death, immigration/emigration, sex, age, county of residence, and education) from all study participants were retrieved from the Total Population Register22 and the longitudinal integrated database for health insurance and labor market studies (LISA) using an individual’s PIN.23 Data were linked to the Swedish Patient Register24 to obtain data on inpatient and outpatient medical encounters.
Within the study population we determined medical comorbidities in the last five years prior to study entry using relevant ICD-8, ICD-9, and ICD-10 codes (Table S2) prospectively recorded in routine clinical practice to determine presence of cardiovascular disease (CVD), diabetes, and neurologic disease (Table S2) both at any point up to CRC diagnosis and within 5 years of CRC diagnosis date or index date. Cancer diagnoses (excluding CRC) were determined from the Swedish Cancer Register.25
Source database
Patients with a colorectal biopsy consistent with CRC were identified through the Epidemiology Strengthened by histopathology Reports in Sweden (ESPRESSO) study, a histopathology-based cohort consisting of 6.1 million gastrointestinal (GI) pathology reports collected across Sweden’s 28 pathology departments from 1965–2017.26 Between 2015 and 2017, the cohort was assembled by collecting all Swedish histopathology data from GI tract sites accompanied by information on date of biopsy, topography (location of biopsy), and morphology. Each biopsy sample is categorized according to an individual’s personal identity number (PIN), and includes information on date of birth and sex. Through linkage with individual PINs, it is possible to follow individuals over time across available Swedish registers (see below), and link data to information on healthcare diagnoses according to International Classification of Diseases (ICD) codes. Individuals with histopathology data are matched with up to five controls from the general population (without GI histopathology) as well as all siblings.
Definition of CRC
CRC was defined according to relevant SNOMED codes (Table S1) in the ESPRESSO cohort between July 2007 and December 2016. Patients were excluded from analyses if they were diagnosed with inflammatory bowel disease (since patients with this disease often undergo screening for CRC and this may bias the results) or precancerous polyps (since their presence may indicate that constipation is secondary to the lesion rather than preceding it) at any time before the biopsy/CRC diagnosis date or if diagnosed with CRC ≥3 months before the biopsy date using the Cancer Register (Figure 1; for definitions see Appendix). CRC patients were also excluded if they had emigrated out of Sweden before the biopsy date or had immigrated to Sweden <2 years before their biopsy (and thus would not have complete data on constipation). Identical exclusion criteria were applied to matched reference controls.
Figure 1:
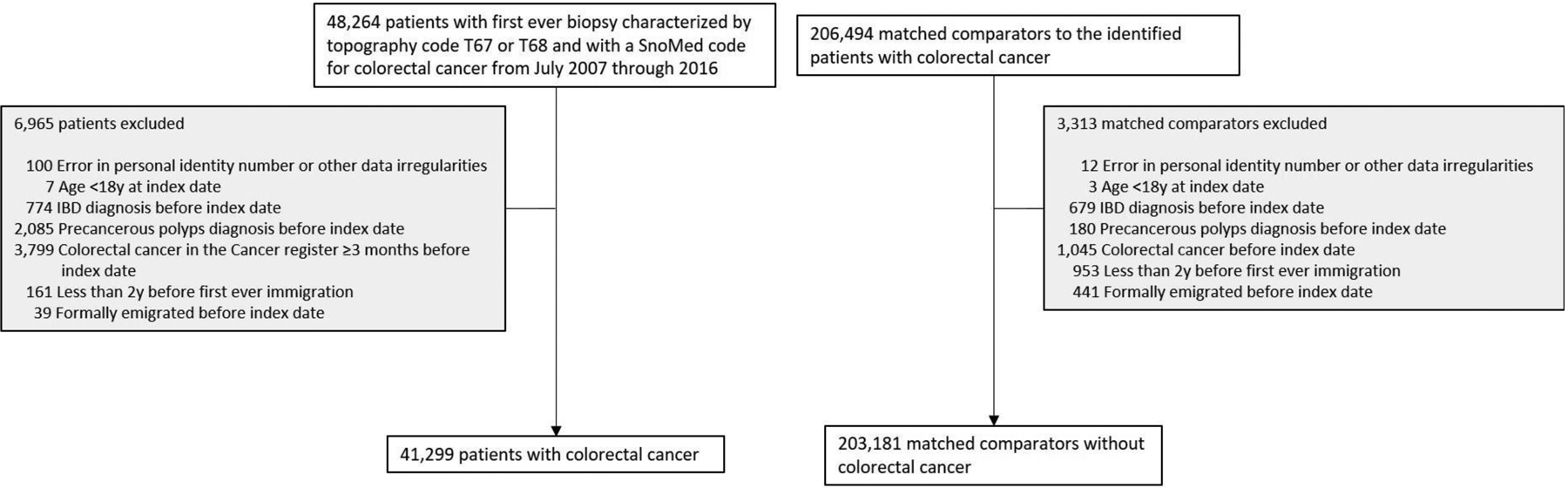
Flow chart of identified patients and their matched comparators
Definition of Chronic Constipation
Because no ICD code for chronic constipation exists (as opposed to “any constipation”), we defined chronic constipation as:
Having ≥2 earlier records of laxatives (Anatomical Therapeutic Chemical (ATC) code A06A*) with ≥6 months between first-last prescription plus
The first prescription ≥2 years before the CRC diagnosis (or matching date for controls) plus
The second laxative prescription needed to occur ≥6 months before the CRC diagnosis or matching date.
We required the first laxative prescription to occur ≥2 years before the CRC diagnosis (or matching date for controls) and the second prescription ≥6 months before the CRC diagnosis or matching date in order to account for potential reverse causation where constipation could be a presenting symptom of CRC. The former requirement (1st prescription ≥2 years) explains why we started our inclusion of CRC in July 2007. The Swedish Prescribed Drug Register began in July 2005, so only two years later could any CRC patient fulfill our exposure requirements.
We performed several sensitivity analyses making use of the Swedish Patient Register to test several stricter definitions of constipation: (a) ⩾2 visits ever for any constipation using the ICD-9 code 564.xx or the ICD-10 code K59.xx in inpatient or non-primary outpatient care starting in 1987 (when ICD-9 was introduced in Sweden). Similar to the main analysis, we required the first constipation diagnosis to occur ⩾2 years before the CRC diagnosis (or matching date for controls), and the second constipation diagnosis needed to occur ⩾6 months before the CRC diagnosis or matching date (main sensitivity analysis). (b) ⩾2 visits for constipation with ⩽12 months between any 2 constipation diagnoses (must not be the 1st and 2nd constipation diagnosis). (c) ⩾2 visits for constipation within 12 month period (as in #1) + two prescriptions of laxatives (ATC code A06A*) during an 18-month period starting 6 months before the index date (the date of the first health care contact with constipation as a primary diagnosis) as previously described.21 “Two dispatches” corresponds to 6 months of constipation medication, which is sufficient to satisfy the chronicity condition of the Rome III/IV criteria.27
Controls
Index individuals with CRC were matched at the time of diagnosis/biopsy on age, sex, calendar year and county with up to five controls from the Swedish general population22 with index date set as the date of CRC diagnosis (Figure 1).
Siblings
CRC patients were also compared to their siblings. Siblings were identified through the Total Population Register.22 Sibling comparisons allowed us to assess the potential association between chronic constipation and later CRC with further control for unmeasured confounding (siblings share more genetic and early environmental factors than matched controls).
Statistical Analysis
We used conditional logistic regression to estimate unadjusted and multivariable-adjusted odds ratios (OR) and 95% confidence intervals (CI) for having an earlier record of chronic constipation.
Although data were collected on comorbidities as above, we did not adjust for medical comorbidities in the regression; since matching occurred at the time of CRC diagnosis, matching on comorbidities would mean adjusting for comorbidities at the time of CRC diagnosis and therefore after the onset of constipation—thus potentially over-adjusting any association between chronic constipation and CRC.
All analyses were conditioned on matching factors (sex, age, county, year of biopsy), except in siblings where we conditioned on matching set within family instead of adjusted for the above four matching variables. We further adjusted for level of education (<9, 9–12, >12 years or missing). Statistics were carried out using SAS statistical software v9.4. A p-value <0.05 was considered statistically significant. The study was approved by the Stockholm Ethics Review Board. Informed consent was waived by the board since the study was strictly register-based.28
RESULTS
We identified 48,264 cases of CRC from 2007–2016 with a biopsy report demonstrating CRC (Figure 1). After exclusions there remained 41,299 CRC patients who were matched to 203,181 controls (Figure 1).
Both CRC patients and matched controls had a mean age in the 71.8 with slight male predominance (Table 1). Compared to matched controls without CRC, CRC patients were more likely to be diagnosed with CVD, non-CRC cancers, and diabetes.
Table 1.
Baseline characteristics of study cohort
| Characteristic | Colorectal cancer (n=41,299) | Matched comparators (n=203,181) |
|---|---|---|
| Female, no. (%) | 19 508 (47.2%) | 95 951 (47.2%) |
| Male, no. (%) | 21 791 (52.8%) | 107 230 (52.8%) |
| Age | ||
| Mean (SD) | 71.8 (11.5) | 71.8 (11.5) |
| Median (IQR) | 72.9 (64.8–80.3) | 72.8 (64.8–80.3) |
| Range, min-max | 18.1–100.0 | 18.3–99.9 |
| Categories, no. (%) | ||
| 18y – <30y | 75 (0.2%) | 337 (0.2%) |
| 30y – <40y | 372 (0.9%) | 1 799 (0.9%) |
| 40y – <50y | 1 452 (3.5%) | 7 084 (3.5%) |
| 50y – <60y | 4 119 (10.0%) | 20 294 (10.0%) |
| 60y – <70y | 10 708 (25.9%) | 52 952 (26.1%) |
| ≥70y | 24 573 (59.5%) | 120 715 (59.4%) |
| Country of birth, no. (%) | ||
| Nordic country | 38 314 (92.8%) | 187 630 (92.3%) |
| Other | 2 985 (7.2%) | 15 549 (7.7%) |
| Missing | 0 | 2 (0.0%) |
| Level of education, no. (%) | ||
| ≤9 years | 15 919 (38.5%) | 77 794 (38.3%) |
| 10–12 years | 15 979 (38.7%) | 77 552 (38.2%) |
| >12 years | 8 961 (21.7%) | 44 752 (22.0%) |
| Missing | 440 (1.1%) | 3 083 (1.5%) |
| Year of colon cancer/Index year, no. (%) | ||
| July 2007–2010 | 16 412 (39.7%) | 80 830 (39.8%) |
| 2011–2013 | 13 746 (33.3%) | 67 637 (33.3%) |
| 2014–2016 | 11 141 (27.0%) | 54 714 (26.9%) |
| Disease history ever before index date, no. (%) | ||
| Cardiovascular disease (CVD) | 22 949 (55.6%) | 94 208 (46.4%) |
| Myocardial infarction (MI) | 3 278 (7.9%) | 16 319 (8.0%) |
| Cardiovascular disease in inpatient care | 20 120 (48.7%) | 78 927 (38.8%) |
| Cancer (excluding CRC) | 13 418 (32.5%) | 41 775 (20.6%) |
| Cancer (excluding CRC) using the Cancer register only | 8 794 (21.3%) | 33 802 (16.6%) |
| Diabetes | 5 516 (13.4%) | 19 427 (9.6%) |
| Neurologic disease | 8 306 (20.1%) | 40 351 (19.9%) |
| None of the above | 11 161 (27.0%) | 77 205 (38.0%) |
| Disease history within 5 years before index date, no. (%) | ||
| Cardiovascular disease (CVD) | 19 031 (46.1%) | 66 229 (32.6%) |
| Myocardial infarction (MI) | 1 450 (3.5%) | 6 244 (3.1%) |
| Cardiovascular disease in inpatient care | 15 941 (38.6%) | 49 553 (24.4%) |
| Cancer (excluding CRC) | 10 026 (24.3%) | 24 237 (11.9%) |
| Cancer (excluding CRC) using the Cancer register only | 3 862 (9.4%) | 12 562 (6.2%) |
| Diabetes | 5 030 (12.2%) | 15 998 (7.9%) |
| Neurologic disease | 4 606 (11.2%) | 21 103 (10.4%) |
| None of the above | 15 824 (38.3%) | 113 770 (56.0%) |
CRC, colorectal cancer.
Main results
Overall, 3,943 patients with CRC met our criteria for chronic constipation. The crude proportion of chronic constipation in CRC patients was 9.5% compared to 8.8% in matched controls. Conditioning on matching set, and adjusting for education there was a modest association between chronic constipation and later CRC (OR=1.10, 95% CI=1.06–1.14)(Table 2). The association between chronic constipation and later CRC did differ according to age category (p for interaction <0.001), level of education (p for interaction <0.001), and year of colon cancer diagnosis (p for interaction 0.013), though the effect size differences were small.
Table 2.
Prevalence and odds ratio of constipation in patients with colorectal cancer and matched comparators
| N exposed (% with constipation) | Odds ratio* (95%CI) | Odds ratio** (95%CI) | |
|---|---|---|---|
| CRC with constipation | Comparators with constipation | ||
| 3 943 (9.5%) | 17 887 (8.8%) | 1.10 (1.06–1.14) | 1.10 (1.06–1.14) |
| 2 376 (12.2%) | 10 604 (11.1%) | 1.12 (1.07–1.18) | 1.13 (1.07–1.18) |
| 1 567 (7.2%) | 7 283 (6.8%) | 1.06 (1.00–1.13) | 1.07 (1.01–1.13) |
| 5 (6.7%) | 0 | - | - |
| 6 (1.6%) | 17 (0.9%) | 1.83 (0.70–4.80) | 2.08 (0.78–5.58) |
| 43 (3.0%) | 111 (1.6%) | 1.93 (1.34–2.77) | 1.91 (1.33–2.75) |
| 159 (3.9%) | 542 (2.7%) | 1.51 (1.26–1.81) | 1.51 (1.25–1.81) |
| 661 (6.2%) | 2 276 (4.3%) | 1.47 (1.34–1.61) | 1.47 (1.34–1.61) |
| 3 069 (12.5%) | 14 941 (12.4%) | 1.01 (0.97–1.05) | 1.01 (0.97–1.05) |
| 3 630 (9.5%) | 16 342 (8.7%) | 1.10 (1.06–1.14) | 1.10 (1.06–1.14) |
| 313 (10.5%) | 1 545 (9.9%) | 1.24 (0.95–1.64) | 1.25 (0.95–1.64) |
| 1 621 (10.2%) | 8 275 (10.6%) | 0.95 (0.89–1.02) | 0.95 (0.89–1.02) |
| 1 487 (9.3%) | 6 028 (7.8%) | 1.25 (1.16–1.35) | 1.25 (1.16–1.35) |
| 765 (8.5%) | 3 116 (7.0%) | 1.18 (1.04–1.34) | 1.18 (1.04–1.34) |
| 1 008 (6.1%) | 4 943 (6.1%) | 1.00 (0.93–1.08) | 1.01 (0.94–1.08) |
| 1 480 (10.8%) | 6 431 (9.5%) | 1.16 (1.09–1.23) | 1.16 (1.09–1.23) |
| 1 455 (13.1%) | 6 513 (11.9%) | 1.12 (1.05–1.19) | 1.12 (1.05–1.19) |
| 2 629 (13.8%) | 10 283 (15.5%) | 0.87 (0.82–0.92) | 0.87 (0.83–0.92) |
| 1 210 (12.1%) | 3 395 (14.0%) | 0.93 (0.83–1.04) | 0.93 (0.83–1.04) |
| 745 (14.8%) | 2 513 (15.7%) | 0.90 (0.76–1.08) | 0.91 (0.76–1.09) |
| 823 (17.9%) | 4 046 (19.2%) | 0.78 (0.67–0.91) | 0.78 (0.67–0.91) |
| 763 (4.8%) | 5 188 (4.6%) | 1.23 (1.12–1.35) | 1.23 (1.12–1.35) |
CRC, colorectal cancer.
Conditioned on matching set (age, sex, county, and calendar period);
Conditioned on matching set and further adjusted for education
When comparing 17,818 CRC patients to their siblings (n=37,610)(Tables 3 and 4), the crude proportion of chronic constipation in CRC patients was 7.0% compared to 6.6% in siblings. CRC patients were no more likely to have chronic constipation than their siblings (OR=1.04, 95%CI=0.97–1.13).
Table 3.
Baseline characteristics of CRC patients compared to siblings
| Characteristic | Colorectal cancer (n=17,818) | Matched siblings (n=37,610) |
|---|---|---|
| Female, no. (%) | 8 007 (44.9%) | 18 444 (49.0%) |
| Male, no. (%) | 9 811 (55.1%) | 19 166 (51.0%) |
| Age | ||
| Mean (SD) | 65.8 (9.6) | 64.4 (9.8) |
| Median (IQR) | 67.4 (60.7–72.7) | 65.7 (58.8–71.4) |
| Range, min-max | 18.1–84.8 | 18.1–84.5 |
| Categories, no. (%) | ||
| 18y – <30y | 52 (0.3%) | 155 (0.4%) |
| 30y – <40y | 275 (1.5%) | 557 (1.5%) |
| 40y – <50y | 969 (5.4%) | 2 583 (6.9%) |
| 50y – <60y | 2 808 (15.8%) | 7 428 (19.8%) |
| 60y – <70y | 6 994 (39.3%) | 15 285 (40.6%) |
| ≥70y | 6 720 (37.7%) | 11 602 (30.8%) |
| Country of birth, no. (%) | ||
| Nordic country | 17 655 (99.1%) | 37 294 (99.2%) |
| Other | 163 (0.9%) | 315 (0.8%) |
| Missing | 0 | 1 (0.0%) |
| Level of education, no. (%) | ||
| ≤9 years | 5 570 (31.3%) | 12 152 (32.3%) |
| 10–12 years | 7 526 (42.2%) | 16 083 (42.8%) |
| >12 years | 4 671 (26.2%) | 8 841 (23.5%) |
| Missing | 51 (0.3%) | 534 (1.4%) |
| Year of colon cancer/Index year, no. (%) | ||
| July 2007–2010 | 6 121 (34.4%) | 13 329 (35.4%) |
| 2011–2013 | 6 100 (34.2%) | 12 762 (33.9%) |
| 2014–2016 | 5 597 (31.4%) | 11 519 (30.6%) |
| Disease history ever before index date, no. (%) | ||
| Cardiovascular disease (CVD) | 8 227 (46.2%) | 13 845 (36.8%) |
| Myocardial infarction (MI) | 941 (5.3%) | 1 857 (4.9%) |
| Cardiovascular disease in inpatient care | 6 872 (38.6%) | 10 800 (28.7%) |
| Cancer (excluding CRC) | 5 255 (29.5%) | 7 577 (20.1%) |
| Cancer (excluding CRC) using the Cancer register only | 3 289 (18.5%) | 6 541 (17.4%) |
| Diabetes | 2 084 (11.7%) | 2 817 (7.5%) |
| Neurologic disease | 3 513 (19.7%) | 7 568 (20.1%) |
| None of the above | 5 957 (33.4%) | 16 568 (44.1%) |
| Disease history within 5 years before index date, no. (%) | ||
| Cardiovascular disease (CVD) | 6 575 (36.9%) | 8 620 (22.9%) |
| Myocardial infarction (MI) | 399 (2.2%) | 616 (1.6%) |
| Cardiovascular disease in inpatient care | 5 202 (29.2%) | 5 764 (15.3%) |
| Cancer (excluding CRC) | 3 991 (22.4%) | 3 774 (10.0%) |
| Cancer (excluding CRC) using the Cancer register only | 1 475 (8.3%) | 2 144 (5.7%) |
| Diabetes | 1 891 (10.6%) | 2 089 (5.6%) |
| Neurologic disease | 1 891 (10.6%) | 3 304 (8.8%) |
| None of the above | 8 161 (45.8%) | 24 872 (66.1%) |
CRC, colorectal cancer.
Table 4.
Prevalence and odds ratio of constipation in patients with colorectal cancer and siblings
| N exposed (% with constipation) | Odds ratio* (95%CI) | Odds ratio** (95%CI) | |
|---|---|---|---|
| CRC with constipation | Comparators with constipation | ||
| 1 254 (7.0%) | 2 468 (6.6%) | 1.05 (0.97–1.13) | 1.04 (0.97–1.13) |
| 736 (9.2%) | 1 511 (8.2%) | 1.04 (0.91–1.18) | 1.03 (0.91–1.18) |
| 518 (5.3%) | 957 (5.0%) | 1.06 (0.92–1.23) | 1.07 (0.92–1.24) |
| 4 (7.7%) | 0 | - | - |
| 5 (1.8%) | 6 (1.1%) | 0.65 (0.06–6.60) | 0.65 (0.06–6.61) |
| 30 (3.1%) | 54 (2.1%) | 1.70 (0.86–3.37) | 1.70 (0.86–3.38) |
| 104 (3.7%) | 240 (3.2%) | 1.15 (0.82–1.60) | 1.17 (0.83–1.64) |
| 417 (6.0%) | 884 (5.8%) | 1.10 (0.93–1.29) | 1.11 (0.94–1.31) |
| 694 (10.3%) | 1 284 (11.1%) | 0.90 (0.80–1.01) | 0.89 (0.79–1.00) |
| 1 239 (7.0%) | 2 448 (6.6%) | 1.04 (0.97–1.13) | 1.04 (0.97–1.12) |
| 15 (9.2%) | 20 (6.3%) | 1.12 (0.49–2.58) | 1.11 (0.48–2.55) |
| 401 (7.2%) | 890 (7.3%) | 0.89 (0.75–1.05) | 0.89 (0.75–1.05) |
| 533 (7.1%) | 1 014 (6.3%) | 1.24 (1.07–1.45) | 1.24 (1.07–1.45) |
| 311 (6.7%) | 543 (6.1%) | 1.11 (0.90–1.36) | 1.11 (0.90–1.36) |
| 221 (3.6%) | 411 (3.1%) | 1.14 (0.95–1.36) | 1.14 (0.95–1.36) |
| 478 (7.8%) | 882 (6.9%) | 1.14 (1.00–1.29) | 1.13 (1.00–1.28) |
| 555 (9.9%) | 1 175 (10.2%) | 0.94 (0.84–1.06) | 0.94 (0.84–1.06) |
| 705 (10.7%) | 1 169 (13.6%) | 0.78 (0.67–0.90) | 0.78 (0.67–0.91) |
| 363 (9.1%) | 518 (13.7%) | 0.70 (0.51–0.95) | 0.70 (0.51–0.95) |
| 222 (11.7%) | 300 (14.4%) | 1.18 (0.80–1.75) | 1.17 (0.79–1.74) |
| 305 (16.1%) | 581 (17.6%) | 0.85 (0.58–1.25) | 0.85 (0.57–1.26) |
| 328 (4.0%) | 867 (3.5%) | 1.13 (0.95–1.34) | 1.10 (0.93–1.31) |
CRC, colorectal cancer.
Conditioned on matching set (family);
Conditioned on matching set and further adjusted for education
Sensitivity analyses
In sensitivity analyses defining chronic constipation as ≥2 visits ever for any constipation in inpatient or non-primary outpatient care starting in 1987, we identified 126,650 cases of biopsy-verified CRC from 1989–2016. After exclusions there remained 111,125 CRC patients who were matched to 547,773 controls (Figure S1).
Overall, the number of patients meeting criteria for chronic constipation was greatly reduced. The crude proportion of chronic constipation in CRC patients was 0.16% compared to 0.18% in matched controls. Conditioning on matching set, and adjusting for education there was no association between chronic constipation and later CRC (OR=0.88, 95% CI=0.75–1.04)(Table S3). Additional sensitivity analyses found no association between CRC and chronic constipation, when the latter was the main diagnosis of their encounters. Further alternative definitions of chronic constipation including II) ≥2 visits for constipation within 12 months between any 2 constipation diagnoses or III) ≥2 visits for constipation within 12 months between any 2 constipation diagnoses with ≥2 prescriptions of laxatives during an 18-month period starting 6 months before the index date (since 2008) similarly demonstrated no increased risk for chronic constipation in CRC: OR=1.01, 95%CI=0.86–1.18 and OR=1.18, 95CI=0.88–1.57, respectively. Varying the time between the two visits for constipation to short (6–12 months), medium (1–5 years), and long (≥5 years) also showed no increased likelihood of chronic constipation in patients with CRC).
DISCUSSION
In this nationwide, population-based study of over 40,000 individuals with CRC—representing virtually all CRC cases in Sweden over a decade—we found only a modest association between chronic constipation and later CRC—that vanished when we compared CRC cases to sibling comparators, thereby taking intrafamilial confounding into account. A sensitivity analysis of 126,000 CRC cases diagnosed 1989–2016 found no association with earlier inpatient or non-primary outpatient care-diagnosed constipation. Varying the definition of chronic constipation did not materially alter our findings even in the setting of prolonged (≥5 years) duration of symptoms, and ORs consistently remained around 1.
Fear of the potential adverse effects of constipation has had a strong and enduring grip on the popular imagination,29 and the potential impact on risk of CRC is no exception. In fact, the literature to date has largely suggested that these fears are at least modestly founded with the largest and most recent study demonstrating an incidence risk ratio of 1.2 (95%CI 1.07–1.35), representing 1,434 CRC cases in a much larger (n=28,854) population of chronic constipation patients.15 Almost 30% of these cases occurred in the first two years after diagnosis of constipation, raising the concern for reverse causation—that is that constipation was a sign of rather than a risk factor for CRC.
While CRC is a common malignancy, the incidence in a population is relatively small over time. By identifying almost all CRC cases in Sweden a priori and then looking at the occurrence of chronic constipation earlier, we were able to identify over 40,000 CRC cases. The next largest study identified 1,207 CRC cases over a 13.3-year period,18 though notably also did not identify an increased risk of CRC with chronic constipation.
The high prevalence of both CRC and chronic constipation implies that any data connecting the two could have significant financial implications. Unlike the United States, most countries in Europe and worldwide do not have colonoscopy-based CRC screening program.30 Any suggestion that individuals with chronic constipation represent a population that may need extra surveillance could generate a significant increase in need for colonoscopy with perceived risk of CRC thought to drive colonoscopy utilization.31 In fact, fear of CRC is higher in both women and those with comorbid anxiety,32, 33 the same population that is disproportionately affected by chronic constipation.1, 2
We did observe effect modification for the association between chronic constipation and risk of later CRC across age, level of education, and year of CRC diagnosis, though the magnitude of the effects would be small. Although there is an increased effect size seen in some of the younger ages, increased education levels, and later years of CRC diagnosis, the effect sizes are virtually identical for those with and without CC.
Our study has several notable strengths. Most importantly, it represents analysis of almost 40 times more CRC cases than ever previously assembled, with strict linkage to histopathology data to confirm diagnosis. Patients who successfully seek care for constipation may be more likely to see specialists and undergo more diagnostic procedures with corresponding increases in CRC detection. By using a population-based cohort with CRC as the starting point, we were able to ensure that any association with constipation was not merely a reflection of diagnostic access bias since all CRC cases in Sweden are included in the analysis. The ability to leverage sibling analyses to further account for residual confounding further strengthens our case that the association between chronic constipation and CRC is most likely null. We were also careful to account for potential reverse causation where constipation reflects a symptom of CRC rather than a risk factor, by using an exclusion period where the first laxative dispensation or constipation diagnosis needed to occur ≥2 years prior to the CRC diagnosis with the second dispensation or diagnosis ≥6 months before the CRC diagnosis. Extensive sensitivity analyses varying the definition of chronic constipation (including one where we extended our study period to 1989–2016) did not significantly alter our results.
In any observational study, there is a risk of residual confounding. Although data were collected on comorbidities as per Table 1, we did not adjust for medical comorbidities in our final analysis. Matching comorbidities at the time of CRC diagnosis would introduce an additional source of bias, as we would be accounting for comorbidities were potentially diagnosed after the onset of constipation. Our use of sibling comparisons allowed us to examine the influence of intrafamilial confounding associated with shared genetic and early environmental factors on chronic constipation and CRC. In the absence of a chronic constipation diagnostic code, there may be some misclassification of patients using the laxative prescription surrogate, and some patients may obtain laxatives over-the-counter without a prescription. However, we conducted several sensitivity analyses using diagnostic codes alone or in combination with laxative dispensations to examine the effects of more rigorous definitions of chronic constipation with similar results. Most importantly, the prevalence of chronic constipation in our cohort (8.8–9.5%) compares favorably with the prevalence of chronic constipation in Sweden using the most recent Rome IV definitions derived a self-completed questionnaire (10.3%, 95%CI 9.0—11.6), respectively).34 Finally, Sweden represents a single country with a homogenous population such that our findings may have more limited generalizability. However, during the study period, Sweden’s colon cancer screening methodology was similar to most countries in Europe and elsewhere and has not used a colonoscopy-based program.
In summary, in this large population-based cohort of patients with CRC diagnosed over a period of 9 years, previous chronic constipation was no more common in CRC patients than in matched controls. Our findings will hopefully offer clarity to providers struggling to balance previously discordant data on this topic with real patient concerns about risk of cancer.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background:
Prolonged colon transit times may increase contact time between potential carcinogens in the stool and the colonic mucosa. Previous studies have yielded conflicting results connecting chronic constipation with risk of colorectal cancer.
Findings:
In a large, nationwide case-control study accounting for 41,299 colorectal cancer cases, we found no association between chronic constipation and later colorectal cancer.
Implications for Clinical Care:
These findings may alleviate patient fears and result in cost savings by avoiding unnecessary testing in chronic constipation patients likely to undergo colonoscopy as part of their workup for symptoms.
Financial Support:
KS is supported by NIH K23DK120945.
Disclosures:
KS has received research support from AstraZeneca, Ironwood, and Urovant, has served as a speaker for Shire, and has served as a consultant to Arena, Gelesis, GI Supply, Synergy, and Shire. JFL coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). This study has received funding from Janssen. OO has been PI for projects (unrelated to the current paper) at KI partly financed by investigator-initiated grants from Janssen and Pfizer.
Abbreviations:
- CI
confidence interval.
- GI
Gastrointestinal.
- OR
Odds ratio.
- CRC
colorectal cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Transparency Statement: Other researchers can apply for our data through the Swedish National Board of Health and Welfare, and through the individual Swedish histopathology departments.
REFERENCES
- 1.Bharucha AE, Lacy BE. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology. 2020;158(5):1232–49 e3. Epub 2020/01/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilleri M, Ford AC, Mawe GM, et al. Chronic constipation. Nat Rev Dis Primers. 2017;3:17095. Epub 2017/12/15. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. Epub 2021/02/05. [DOI] [PubMed] [Google Scholar]
- 4.Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer. 2012;118(14):3636–44. Epub 2012/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings JH, Bingham SA, Heaton KW, et al. Fecal weight, colon cancer risk, and dietary intake of nonstarch polysaccharides (dietary fiber). Gastroenterology. 1992;103(6):1783–9. Epub 1992/12/01. [DOI] [PubMed] [Google Scholar]
- 6.American Gastroenterological A, Bharucha AE, Dorn SD, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144(1):211–7. Epub 2012/12/25. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Pemberton JH, Locke GR 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144(1):218–38. Epub 2012/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller-Lissner SA, Kamm MA, Scarpignato C, et al. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100(1):232–42. Epub 2005/01/19. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg A, Muller AD. Constipation and cathartics as risk factors of colorectal cancer: a meta-analysis. Pharmacology. 1993;47 Suppl 1:224–33. Epub 1993/10/01. [DOI] [PubMed] [Google Scholar]
- 10.Le Marchand L, Wilkens LR, Kolonel LN, et al. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;57(21):4787–94. Epub 1997/11/14. [PubMed] [Google Scholar]
- 11.Jacobs EJ, White E. Constipation, laxative use, and colon cancer among middle-aged adults. Epidemiology. 1998;9(4):385–91. Epub 1998/07/02. [PubMed] [Google Scholar]
- 12.Roberts MC, Millikan RC, Galanko JA, et al. Constipation, laxative use, and colon cancer in a North Carolina population. Am J Gastroenterol. 2003;98(4):857–64. Epub 2003/05/10. [DOI] [PubMed] [Google Scholar]
- 13.Kojima M, Wakai K, Tokudome S, et al. Bowel movement frequency and risk of colorectal cancer in a large cohort study of Japanese men and women. Br J Cancer. 2004;90(7):1397–401. Epub 2004/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe T, Nakaya N, Kurashima K, et al. Constipation, laxative use and risk of colorectal cancer: The Miyagi Cohort Study. Eur J Cancer. 2004;40(14):2109–15. Epub 2004/09/03. [DOI] [PubMed] [Google Scholar]
- 15.Guerin A, Mody R, Fok B, et al. Risk of developing colorectal cancer and benign colorectal neoplasm in patients with chronic constipation. Aliment Pharmacol Ther. 2014;40(1):83–92. Epub 2014/05/17. [DOI] [PubMed] [Google Scholar]
- 16.Kune GA, Kune S, Field B, et al. The role of chronic constipation, diarrhea, and laxative use in the etiology of large-bowel cancer. Data from the Melbourne Colorectal Cancer Study. Dis Colon Rectum. 1988;31(7):507–12. Epub 1988/07/01. [DOI] [PubMed] [Google Scholar]
- 17.Dukas L, Willett WC, Colditz GA, et al. Prospective study of bowel movement, laxative use, and risk of colorectal cancer among women. Am J Epidemiol. 2000;151(10):958–64. Epub 2000/06/15. [DOI] [PubMed] [Google Scholar]
- 18.Simons CC, Schouten LJ, Weijenberg MP, et al. Bowel movement and constipation frequencies and the risk of colorectal cancer among men in the Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2010;172(12):1404–14. Epub 2010/10/29. [DOI] [PubMed] [Google Scholar]
- 19.Power AM, Talley NJ, Ford AC. Association between constipation and colorectal cancer: systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2013;108(6):894–903; quiz 4. Epub 2013/03/14. [DOI] [PubMed] [Google Scholar]
- 20.Vobecky J, Caro J, Devroede G. A case-control study of risk factors for large bowel carcinoma. Cancer. 1983;51(10):1958–63. Epub 1983/05/15. [DOI] [PubMed] [Google Scholar]
- 21.Bruce Wirta S, Hodgkins P, Joseph A. Economic burden associated with chronic constipation in Sweden: a retrospective cohort study. Clinicoecon Outcomes Res. 2014;6:369–79. Epub 2014/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–36. [DOI] [PubMed] [Google Scholar]
- 23.Ludvigsson JF, Svedberg P, Olen O, et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. [DOI] [PubMed] [Google Scholar]
- 26.Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol. 2019;11:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology. 2016;150(6):1393–407.e5. [DOI] [PubMed] [Google Scholar]
- 28.Ludvigsson JF, Haberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whorton J Civilisation and the colon: constipation as the “disease of diseases”. BMJ. 2000;321(7276):1586–9. Epub 2000/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senore C, Basu P, Anttila A, et al. Performance of colorectal cancer screening in the European Union Member States: data from the second European screening report. Gut. 2019;68(7):1232–44. Epub 2018/12/12. [DOI] [PubMed] [Google Scholar]
- 31.Macrae FA, Hill DJ, St John DJ, et al. Predicting colon cancer screening behavior from health beliefs. Prev Med. 1984;13(1):115–26. Epub 1984/01/01. [DOI] [PubMed] [Google Scholar]
- 32.Robb KA, Miles A, Wardle J. Demographic and psychosocial factors associated with perceived risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(3):366–72. Epub 2004/03/10. [PubMed] [Google Scholar]
- 33.Vernon S, McQueen A, Meissner H, et al. , editors. Sex differences in the prevalence and correlates of colorectal cancer testing. 2002–2003 Health Informaton National Trends Survey. Health Information National Trends Survey Data Users Conference; 2005. January 20. [Google Scholar]
- 34.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160(1):99–114 e3. Epub 2020/04/16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


