Abstract
In light of the need for new antifungal agents, the candidacidal activities of human lactoferrin (hLF) and synthetic peptides representing the first, hLF(1-11), and second, hLF(21-31), cationic domains of its N terminus were compared. The results revealed that hLF(1-11) was more effective in killing fluconazole-resistant Candida albicans than hLF(21-31) and much more effective than lactoferrin, as determined microbiologically and by propidium iodide (PI) staining. By using hLF(1-11) and various derivatives, it was found that the second and third residues of the N terminus of hLF(1-11) were critical for its candidacidal activity. Detailed investigation to elucidate the mechanism of action of hLF(1-11) revealed a dose-dependent release of ATP by Candida upon exposure to hLF(1-11). Our observations that sodium azide reduced the PI uptake and candidacidal activity of hLF(1-11) and that, upon exposure to hLF(1-11), the fluorescent dye rhodamine 123 first accumulated inside the mitochondria and later was released into the cytoplasm indicate that the peptide triggers the energized mitochondrion. Furthermore, oxidized ATP, which interferes with the interaction of ATP with its extracellular receptors, blocked the candidacidal action of hLF(1-11), as measured microbiologically and by PI staining. Addition of ATP (or analogues) was not a sufficient stimulus to kill C. albicans or to act synergistically with suboptimal concentrations of the peptide. The main conclusions are that the first two arginines at the N terminus of hLF are critical in the candidacidal activity of hLF(1-11) and that extracellular ATP is essential but not sufficient for the peptide to exert its candidacidal activity.
Candida albicans is an opportunistic pathogen that causes mucosal and systemic infections in individuals undergoing immunosuppressive therapy for cancer or organ transplantation and in human immunodeficiency virus (HIV)-infected patients. The majority of HIV-infected patients (60 to 80%) develop one or more fungal infections during their illness, the most frequent being oropharyngeal candidiasis (8, 35, 38). Such an infection is so frequently associated with AIDS that it can be considered a criterion for the progression towards AIDS (12). Fungal infections are widely treated with triazole antifungal agents, such as fluconazole. Unfortunately, long-term therapies have led to the emergence of fluconazole-resistant C. albicans strains that are cross resistant not only to other azoles but also to amphotericin B (26). This points to a pressing need for new antifungal agents, e.g., antimicrobial proteins or peptides (20, 21). The 77-kDa antimicrobial protein lactoferrin is part of the innate defense system and is provided to newborns by breast-feeding. It is an iron-binding glycoprotein synthesized by mucosal gland epithelial cells and neutrophils (37) and released by the latter cell type in response to inflammatory stimuli (6, 32). Its role in the innate defense system seems to be related to (i) limitation of the availability of environmental iron (7), (ii) modulation of the innate and specific immune defense systems (37), (iii) neutralization of endotoxin (45), and (iv) release of peptides with microbicidal activity. Indeed, both human lactoferrin (hLF) and bovine lactoferrin, when subjected to pepsinolysis, release the antimicrobial peptides lactoferricin H (residues 1 to 47) and lactoferricin B (residues 17 to 41), respectively (2). Since cationic domains are commonly present in natural antimicrobial peptides (33), it should be noted that lactoferricin H contains two cationic domains (residues 2 to 5 and residues 28 to 31) and lactoferricin B contains one (residues 17 to 41). Recent studies indicated that peptides of bovine lactoferrin origin (23), as well as synthetic peptides that include the first (P. H. Nibbering, E. Ravensbergen, M. M. Welling, L. A. van Berkel, P. H. C. van Berkel, E. K. J. Pauwels, and J. H. Nuijens, Annu. Meet. Dutch Soc. Immunol., abstr. 1b, 1999) or second cationic domain of hLF (9), are more effective in killing bacteria than the native protein. Peptides containing the first cationic domain of hLF are even more effective in killing bacteria than peptides containing the second domain, as shown in in vitro and in vivo experiments (Nibbering et al., Annu. Meet. Dutch Soc. Immunol., 1999). The exact mechanism by which hLF peptides exert their bactericidal activity is not known. A number of antimicrobial peptides have been shown to bind cell wall components of gram-positive and gram-negative bacteria (19) and C. albicans (15), and lactoferrin receptors on a variety of cells have been described (17, 30, 36, 37). Previous studies have shown that the cytotoxic activity of human neutrophil defensins l to 3 against C. albicans (29), bacteria (41), and tumor cells (31) is energy dependent. A similar observation has been reported for the action of the antimicrobial peptide histatin 5 against C. albicans (18, 22, 27). In general, the mechanism of action of antimicrobial peptides is thought to involve an increase in membrane potential and permeability (19, 44) and, recently, the antimicrobial mechanism has been putatively associated with conductive ATP transport (27). Although these data may offer a unifying principle for the mechanism of action of antimicrobial peptides, it cannot be concluded that hLF peptides kill C. albicans by a similar mechanism. Indeed, some peptides, such as bactenecin and indolicidin, do not permeabilize the cytoplasmic membrane, suggesting a different mechanism of action (44).
The present study was undertaken (i) to compare the ability of hLF and synthetic peptides representative of the first and second cationic domains of hLF to kill a fluconazole-resistant C. albicans strain, (ii) to delineate the essential residues in the N-terminal peptide of hLF for candidacidal activity, and (iii) to gain more insight into the role of extracellular ATP (ATPe) and energized mitochondria in the candidacidal action of hLF-related peptides.
MATERIALS AND METHODS
Lactoferrin and synthetic peptides.
Human lactoferrin (molecular mass, 77 kDa) was purified from fresh milk of a single donor as previously described (40). hLF, which was free of contamination with endotoxin or human lysozyme (40) and found to be pure by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, was dialyzed against saline and stored at −70°C at a concentration of approximately 20 mg/ml. The synthetic peptides used were as follows: peptides corresponding to residues 1 to 11 of hLF (GRRRRSVQWCA; molecular mass, 1,494 Da), representative of the first cationic domain and further referred to as hLF(1-11), and fragments thereof lacking, respectively, the first, the first two, and the first three N-terminal residues [hLF(2-11), hLF(3-11), and hLF(4-11)]; hLF(1-11) peptides comprised of alanines instead of arginines at positions 2 and/or 3 [hLF(1-11)2A, hLF(1-11)3A, and hLF(1-11)2A/3A]; and peptides corresponding to residues 21 to 31 (FQWQRNMRKVR; molecular mass, 1,567 Da), representative of the second cationic domain. These peptides were prepared and purified as described previously (13; Nibbering et al., Annu. Meet. Dutch Soc. Immunol, 1999). The purity of the various peptides usually exceeded 88%, as determined by reverse-phase high-performance liquid chromatography. Stocks of the synthetic peptides at a concentration of 1 mg/ml of 0.01% acetic acid (HAc; pH 3.7) were stored at −20°C and, immediately before use, dried in a Speed-Vac (Savant Instruments Inc., Farmingdale, N.Y.). Synthetically prepared protegrin-1 (RGGRLCYCRRRFCVCVGR; molecular mass, 2,161 Da) and peptide 4 (RPVVSTQLLLNGSLAEEEVV; molecular mass, 2,171 Da; part of gp120 from HIV type 1) were used as the positive and negative controls, respectively.
Materials.
Sodium azide, benzoyl-benzoyl ATP (BzATP), periodate-oxidized ATP (oATP), and adenosine 5′-O-(3-thiotriphosphate) (ATPγS) were purchased from Sigma Chemical Co. (St. Louis, Mo.). ATP was purchased from Molecular Probes (Eugene, Oreg.). Stocks of BzATP, oATP, ATPγS, and ATP at a concentration of 100 mM were prepared in phosphate-buffered saline and stored at −20°C until use.
Source of C. albicans strain.
Fluconazole-resistant C. albicans strain Y01-19 was purchased from Pfizer (Groton, Conn.). The yeast was identified using Candiselect (Sanofi Pasteur, Paris, France) and confirmed by demonstration of a typical C. albicans pattern of sugar utilization (API ID 32C; bioMerieux, Marcy I'Etoile, France). Fluconazole resistance (MIC > 256 μg/ml) was evaluated using the E-test (Oxoid Unipath Ltd., Basingstoke, United Kingdom). Yeasts were cultured overnight in Sabouraud broth (Oxoid) at 37°C and subcultured for 2.5 h on a rotary wheel at 37°C.
Assay for candidacidal activities of hLF and related peptides.
An in vitro assay was used to assess the candidacidal activities of hLF and related peptides. Briefly, yeast cells were harvested in mid-log phase by centrifugation at 1,500 × g for 10 min, washed twice in 10 mM sodium phosphate buffer (NaPB; pH 7.4), and diluted to a concentration of 106 cells/ml of NaPB supplemented with 2% Sabouraud broth. Equal volumes of this suspension and various concentrations of hLF or related peptides were mixed. Where indicated, the mixture was incubated in the presence of sodium azide (5 mM), BzATP (from 100 μM to l0 mM), ATPγS (5 mM), or ATP (from 1 μM to 1 mM) or preincubated for 30 min at 37°C with 300 μM oATP. The optimal concentrations were determined in preliminary experiments to avoid toxic effects of these compounds on C. albicans. After incubation for 2 h at 37°C with hLF peptides or for 24 h with the native protein, the number of viable blastoconidia was determined by plating serial dilutions of each sample on Sabouraud agar. Results are expressed as CFU per milliliter.
Assay for membrane permeability.
Changes in the membrane permeability of blastoconidia upon exposure to hLF or related peptides were monitored using the DNA-binding fluorescent probe propidium iodide (PI; Sigma) and fluorescence-activated cell sorter (FACS) analysis, as described previously (9, 34). A stock solution of 1 mg of PI/ml of deionized water was prepared. Blastoconidia were grown to mid-log phase and diluted to 2 × 106 cells/ml of NaPB. Equal volumes of this suspension and various concentrations of hLF or related peptides were mixed. After a 2-h (for peptide) or 24-h (for hLF) incubation at 37°C, Candida cells were reincubated with 1 μg of PI/ml (final concentration) for 5 min at room temperature before analysis on a FACScan (Becton Dickinson, San Jose, Calif.) equipped with an argon laser at 488 nm. The photomultiplier voltage was set at 465 V for PI fluorescence intensity in the second channel. Data acquisition and analysis were controlled using the Lysis II software. Results are expressed as the percent PI-positive cells.
Assay for mitochondrial permeabilization.
The fluorescent probe rhodamine 123 (Molecular Probes) (10) was used to investigate mitochondrial permeabilization of C. albicans. Rhodamine 123 is a positively charged probe believed to enter cells by diffusion (4). In mammalian systems, it has been shown to accumulate in mitochondria (25). Its accumulation depends on the mitochondrial transmembrane potential. Briefly, C. albicans cells in mid-log phase were resuspended in potassium phosphate buffer (PPB; 1 mM [pH 7.0]) and preincubated for 10 min at 37°C with 10 μM rhodamine 123 in PPB. After washes with PPB, Candida cells were treated for 10 min at 37°C with 17 μM hLF(1-11) and then prepared for microscopic inspection of the distribution of rhodamine 123 using a fluorescent microscope (Axiolab; Zeiss, Werttenberg, Germany).
Labeling procedure.
hLF and related peptides were labeled with technetium-99m (99mTc), as previously described (42). In short, 10 μl of a peptide solution (1 mM peptide in 0.01 M HAc; pH 3.7) was added to 2 μl of an aseptic 0.5-mg/ml solution of stannous pyrophosphate (Department of Clinical Pharmacy and Toxicology, Leiden University Medical Center). Immediately thereafter, 4 μl of a solution containing 10 mg of KBH4 (crystalline; Sigma) per ml of 0.1 M NaOH was added. After addition of 0.1 ml of 99mTc-sodium pertechnetate solution (200 MBq/ml) obtained from a 99mTc generator (Ultratechnekow; Mallinckrodt Medical, Petten, The Netherlands), the mixture (pH between 5 and 6) was gently stirred at room temperature for 1 h and then used. The final preparation was a solution containing the peptides labeled with 99mTc. The yield of labeling of the peptides was determined by instant thin-layer chromatography, using saline as the eluent. By this method, only 99mTc in the form of pertechnetate is eluted; other radioactive species remain at the site of origin. In addition, the composition of the samples was analyzed by high-performance liquid chromatography cation-exchange analysis.
In vitro binding of hLF and related peptides to blastoconidia.
Binding of 99mTc-labeled peptides to cells was assessed at 4°C unless indicated otherwise (42). In short, 0.1 ml of a 10-fold dilution of 99mTc-labeled peptides in NaPB was transferred to an Eppendorf vial. Next, 0.8 ml of a 50% (vol/vol) dilution of 0.01 M HAc in NaPB (containing 0.01% [vol/vol] Tween 80) and 0.1 ml of NaPB containing 2 × 107 C. albicans cells in mid-log phase were mixed. The mixture, with a final pH of 5, was incubated for 1 h and then the vials were centrifuged at 2,000 × g for 5 min. The supernatant was removed and the pellet was gently resuspended in 1 ml of NaPB and centrifuged again. This supernatant was also removed and the radioactivity in this pellet was determined in a dose calibrator (VDC 101; Veenstra Instruments, Joure, The Netherlands). The radioactivity associated with blastoconidia was expressed as the percentage of added 99mTc activity bound to 2 × 107 yeasts.
ATP bioluminescence assay.
ATP levels in cultures of C. albicans were measured as described previously (1, 11). Briefly, yeast cells were harvested in mid-log phase, washed twice as described above, and then diluted to a concentration of 108 cells/ml of NaPB. Equal volumes of this suspension and of various concentrations of hLF peptides were mixed, and after an incubation at 37°C for various intervals, samples were centrifuged at 10,000 × g at 4°C. Supernatants were collected, and the cells were then resuspended in an equal volume of phosphate-buffered saline (pH 7.4). The cell suspensions were boiled for an additional 3 min. Extracellular and intracellular ATP levels were measured by luminometry using an ATP determination kit (Molecular Probes) according to the manufacturer's instructions. A luciferin-luciferase assay mixture (180 μl) was added to 20 μl of cell lysates or extracellular medium, 150 μl of each sample was transferred into a 96-well microtiter plate, and light emission was monitored using a 1420 Multilabel Counter Victor2 luminometer (EG&G Wallac, Turku, Finland). Results were measured as bioluminescence relative light units, and ATP concentrations were calculated using a standard curve constructed with various concentrations of ATP.
Statistical analysis.
Differences between the values for hLF(1-11) and fragments thereof were analyzed using the Mann-Whitney U test. The level of significance was set at a P value of <0.05. Correlation between the percent PI-positive cells and ATPe was analyzed using the Spearman rank test.
RESULTS
Candidacidal activities of hLF and related peptides.
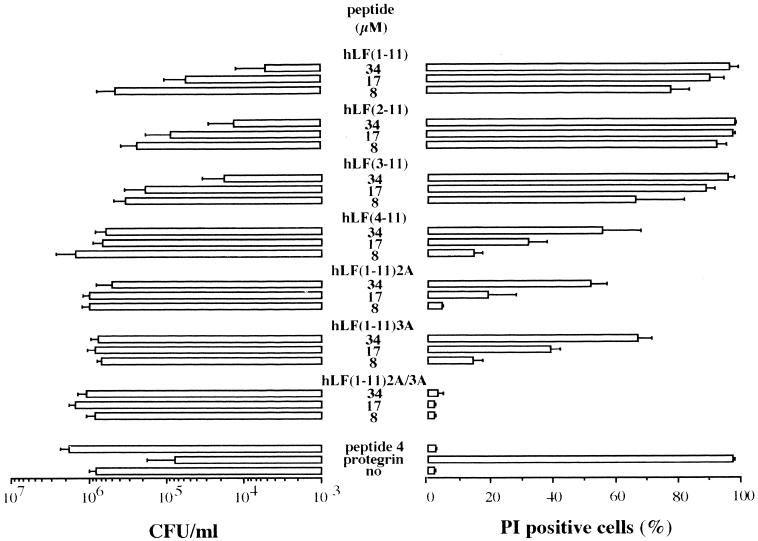
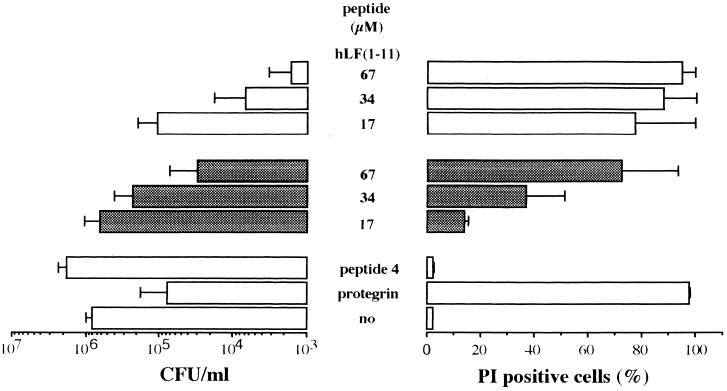
For comparison we first determined the candidacidal activity of native lactoferrin using killing assays. The results revealed that the candidacidal activity of the intact protein, even at concentrations of up to 40 μM for a prolonged incubation time (24 h), was rather poor, reaching only a 40% reduction in the number of viable C. albicans cells. Although the native protein is not very active in killing C. albicans, hLF-derived peptides are believed to be more effective (3). The candidacidal activities of peptides representative of the first and second cationic domains of hLF were determined. The results of the dose-effect study revealed that hLF(1-11) is more efficient (P < 0.05) than hLF(21-31) in killing C. albicans, since it was necessary to use 10 times more hLF(21-31) than hLF(1-11) for a similar level of candidacidal activity. Next, to find out which N-terminal amino acids of hLF are essential in the candidacidal activity of the peptide comprising the first cationic domain, we compared the candidacidal activities of hLF(1-11) and fragments thereof. The results revealed that hLF(1-11), hLF(2-11), and hLF(3-11) each display candidacidal activity in a dose-dependent manner, without significant differences among these peptides (Fig. 1). However, hLF(4-11) displayed a significantly (P < 0.05) reduced killing activity compared to these other hLF peptides. In addition, hLF(1-11)2A and hLF(1-11)3A showed a significantly (P < 0.05) reduced killing activity compared to hLF(1-11), and hLF(1-11)2A/3A was completely inactive (Fig. 1).
FIG. 1.
Dose-dependent killing of fluconazole-resistant C. albicans by N-terminal human lactoferrin-derived peptides. The peptides are hLF(1-11), which comprises the first cationic domain of hLF, fragments thereof [hLF(2-11), hLF(3-11), and hLF(4-11)], and peptides in which the first and/or second arginine is replaced by alanine, [hLF(1-11)2A, hLF(1-11)3A, and hLF(1-11)2A/3A]. Protegrin and peptide 4 are positive and negative controls, respectively. “no” means no peptide. Results are means plus standard deviations of at least three independent experiments. In addition, the effect of these hLF-related peptides on membrane permeability of C. albicans was assessed using FACS analysis. The percentage of PI-positive C. albicans cells was measured. The results are means plus standard deviations of at least three independent experiments.
Effect of hLF and related peptides on membrane permeability of C. albicans.
To further confirm their candidacidal activity, the effect of hLF and related peptides on membrane integrity was monitored by determining the percentages of PI-positive C. albicans cells. Again, hLF(1-11) induced a higher percentage (P < 0.05) of PI-positive cells than did hLF(21-31). The percentage of PI-positive cells increased dose dependently after addition of the various peptides except for hLF(1-11)2A/3A (Fig. 1) and intact hLF (data not shown). It should be noted that even at the highest concentration, 34 μM, hLF(4-11), hLF(1-11)2A, and hLF(1-11)3A did not reach maximum values (Fig. 1).
Binding of 99mTc-labeled hLF and related peptides to C. albicans.
To find out whether candidacidal activity could be correlated to binding of hLF and related peptides to C. albicans, we employed a binding assay using 99mTc-labeled peptides. To reduce the number of synthetic peptides evaluated in our further experiments, we decided to focus on hLF(1-11) and shorter fragments thereof, since such truncated forms of hLF occur in nature (24, 40). The results revealed that binding of hLF(1-11) and hLF(2-11) to C. albicans was, respectively, fourfold (P < 0.05) and twofold higher than that of hLF(3-11) and hLF(4-11) (Table 1). No difference in binding was found between hLF(1-11) and hLF(21-31) (Table 1). The binding of hLF(1-11) to Candida was twofold higher than that of intact hLF. The Spearman rank test revealed no correlation between binding and candidacidal activity.
TABLE 1.
Binding of 99mTc-labeled hLF peptides to C. albicans
| Peptidea | Binding (at 4°C)b |
|---|---|
| hLF(1-692) | 18 ± 3 |
| hLF(1-11) | 31 ± 4 |
| hLF(2-11) | 17 ± 2 |
| hLF(3-11) | 8 ± 9 |
| hLF(4-11) | 8 ± 8 |
| hLF(21-31) | 38 ± 2 |
hLF(1-692), native lactoferrin; hLF(1-11), peptide comprising first cationic domain of hLF; hLF(2-11), hLF(3-11), and hLF(4-11) are fragments of hLF(1-11); hLF(21-31), peptide comprising second cationic domain of hLF.
Radioactivity associated with blastoconidia, expressed as a percentage of added 99mTc activity bound to 2 × 107 yeasts (mean ± standard deviation) (three experiments).
Effect of hLF peptides on ATP levels.
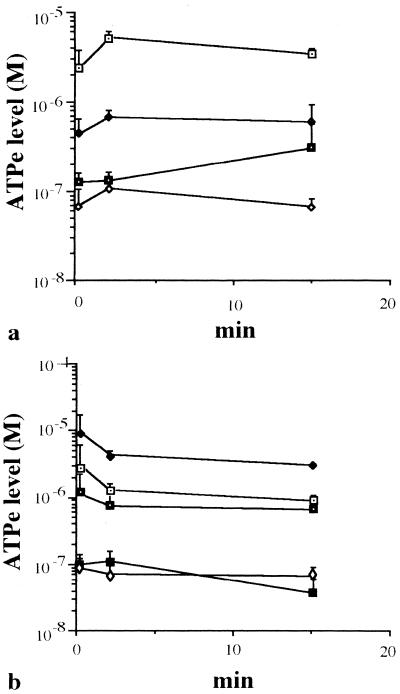
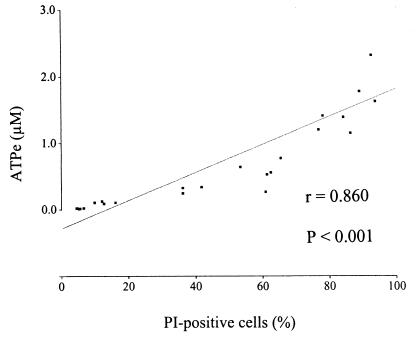
In order to obtain some understanding of the role of cellular metabolism in the candidacidal activity of hLF peptides, changes in the intracellular and extracellular ATP levels of Candida were quantified at different intervals up to 2 h after addition of hLF peptides. Intact hLF was not included in these experiments because it binds ATP (39); hLF(21-31) was not included because of its poor candidacidal activity. Time-response curves indicated that the major increase in ATPe occurred within the first few minutes, with the ATPe level reaching a steady state after about 15 min (Fig. 2). For this reason the effect of hLF(1-11) and fragments thereof on extracellular and intracellular ATP levels was monitored at 0, 2, and 15 min after addition of the peptide. The results revealed that hLF(1-11) induced a dose-dependent increase (P < 0.05) in the levels of ATPe (Fig. 2a), and maximum values were reached within 2 min. Similar results were obtained for hLF(2-11) and hLF(3-11) but not for hLF(4-11) (Fig. 2b). Interestingly, the intracellular ATP concentration, 0.5 ± 0.1 nM, did not change significantly after exposure to the various peptides. Furthermore, a significant (P < 0.001) correlation between the percentage of PI-positive cells and the level of ATPe induced after addition of hLF(1-11) was found (Fig. 3).
FIG. 2.
(a) Effect of different doses of hLF(1-11) on ATPe levels. In short, approximately, 108 C. albicans cells/ml were incubated with peptide concentrations of 16 μM (open squares with black dot), 8 μM (closed diamonds), and 4 μM (closed squares with white dot) or with no peptide (open diamonds). (b) Effect of various peptides on ATPe levels. In short, approximately, 108 C. albicans cells/ml were incubated with 8 μM of hLF(1-11) (open squares with black dot), hLF(2-11) (closed diamonds), hLF(3-11) (closed squares with white dot), and hLF(4-11) (open diamonds) or with no peptides (closed squares). ATPe levels were measured at various intervals using an ATP determination kit. The results are expressed as means plus standard deviations of at least three independent experiments.
FIG. 3.
Correlation between the percentage of PI-positive C. albicans cells and the level of ATPe induced after addition of various concentrations of hLF(1–11) (four experiments).
Effect of sodium azide on candidacidal activity of hLF(1-11).
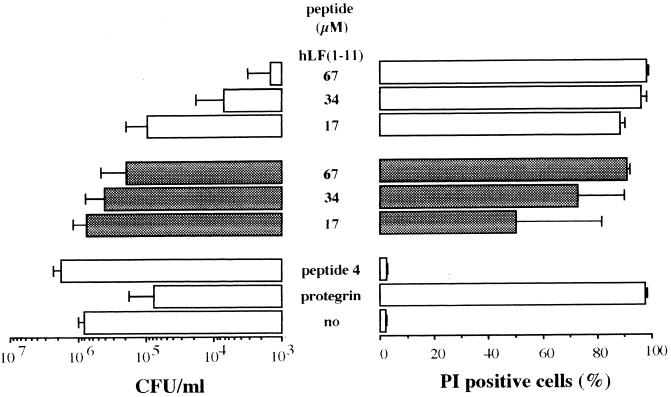
To determine whether the candidacidal activity of hLF(1-11) is dependent on the metabolic activity of Candida, killing assays were performed in the presence of sodium azide, which blocks mitochondrial respiration. The results revealed that azide rescued (P < 0.05) the cells from the candidacidal activity of hLF(1-11), as assessed by microbiological assays and FACS analysis (Fig. 4), thus indicating that the killing activity of hLF(1-11) is dependent on cellular oxidative metabolism. Of course, the hLF(1-11)-induced increase of ATPe was completely blocked (P < 0.05) in the presence of azide. Furthermore, azide induced a twofold reduction (P < 0.05) of hLF(1-11) associated with C. albicans at 37°C, decreasing from 24% ± 4% to 13% ± 1% (three experiments).
FIG. 4.
Dose-dependent candidacidal activity of hLF(1-11) in the presence (hatched bars) or absence (open bars) of azide. For each concentration of the peptide, the difference between azide-treated and nontreated Candida is statistically significant (P < 0.05). Protegrin and peptide 4 served as positive and negative controls, respectively. “no” means no peptide. Results are means and standard deviations of at least three independent experiments. In addition, the effect of hLF(1-11), in the presence or absence of azide, on the membrane permeability of C. albicans was assessed using FACS analysis. The percentage of PI-positive C. albicans cells was measured. Results are means plus standard deviations of at least three independent experiments.
Effect of oATP on candidacidal activity of hLF(1-11).
To assess the role of ATPe in the candidacidal activity of hLF(1-11), the effect of oATP, which irreversibly blocks the interaction between ATPe and extracellular receptors (28), was investigated microbiologically and by PI staining. Preincubation with 300 μM oATP conferred significant (P < 0.05) protection on C. albicans against hLF(1-11) in killing assays (Fig. 5). In addition, FACS analysis demonstrated that the percentage of hLF(1-11)-stimulated PI-positive cells was considerably decreased (P < 0.05) in C. albicans cells preincubated with oATP, indicating that pore formation is mediated by ATPe.
FIG. 5.
Dose-dependent candidacidal activity of hLF(1-11) in the presence of oATP, which inhibits the interaction between extracellular ATP and its receptors (hatched bars), and without oATP (open bars). For each concentration of the peptide, the difference between oATP-treated and nontreated Candida is statistically significant (P < 0.05). Protegrin and peptide 4 served as positive and negative controls, respectively. “no” means no peptide. Results are means and standard deviations of at least three independent experiments. In addition, the effect of hLF(1-11) in C. albicans, preincubated or not with oATP, on membrane permeability was assessed using FACS analysis. The percentage of PI-positive C. albicans cells was measured. Results are means plus standard deviations of at least three independent experiments.
Candidacidal activity of ATP and ATP analogues.
First, ATP and ATP analogues, such as BzATP and ATPγS, were used to evaluate whether ATPe alone was sufficient for inducing pores and killing of Candida. Second, the possible synergistic effects between ATP analogues and intact hLF or suboptimal concentrations of hLF(1-11) were investigated. Third, we evaluated whether ATP analogues were able to restore the candidacidal activity of hLF(1-11) for azide-incubated Candida. The results revealed no effects of ATP or ATP analogues in these experiments.
Effect of hLF(1-11) on mitochondrial membrane integrity.
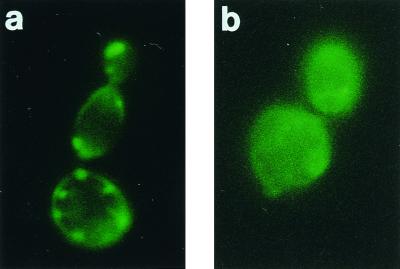
To investigate the effect of hLF(1-11) on mitochondria of C. albicans, the mitochondrial fluorescent probe rhodamine 123 was used. Microscopic analysis revealed that cells preloaded with rhodamine 123 showed immediately after addition of hLF(1-11) the typical granular appearance indicative of a mitochondrial localization (Fig. 6a). Upon a 10-min incubation with hLF(1-11), rhodamine 123-treated C. albicans cells stained diffusely and homogeneously throughout the cytoplasm (Fig. 6b).
FIG. 6.
Fluorescence microscopy results of hLF(1-11) treatment of rhodamine 123-labeled C. albicans. In short, cells preloaded with rhodamine 123 for 10 min at 37°C were washed and treated with hLF(1-11). Pictures were made immediately after addition (a) or after a 10-min incubation with hLF(1-11) at 37°C (b).
DISCUSSION
The main conclusion from the present results is that the candidacidal activity of hLF(1-11) depends on mitochondrial respiration and the subsequent rise in ATPe, with the latter event being essential but not sufficient to kill C. albicans. This conclusion is based on the following findings. First, ATP was released extracellularly in a dose-dependent fashion after the addition of hLF(1-11), and a statistically significant correlation (P < 0.001) was found between peptide-stimulated PI-positive cells and the level of ATPe, suggesting that ATPe plays a role in pore formation. Analysis of ATPe induced by hLF(1-11) and fragments thereof confirmed this observation. hLF(4-11), which did not induce candidacidal activity or increase the percentage of PI-stained cells, indeed did not cause a release of ATP from Candida. Moreover, sodium azide, which is a specific inhibitor of cytochrome oxidase (43) that reduces oxygen consumption, inhibited the uptake of the peptide at 37°C and blocked the release of ATP in the medium, thus providing protection to cells against downstream events, such as the candidacidal activity of hLF(1-11) and pore formation, as evidenced by PI staining. These data strongly suggest that hLF(1-11) requires an energized mitochondrion for its candidacidal activity. Indeed, under anaerobic conditions the candidacidal activity of hLF(1-11) was considerably decreased (data not shown). To unequivocally demonstrate that hLF(1-11) targets the mitochondrion, rhodamine 123 was used. This fluorescent dye was accumulated by energized mitochondria immediately after addition of hLF(1-11) and was released into the cytoplasm during a 10-min incubation with hLF(1-11). Second, oATP was used to inhibit the interaction between ATPe and its receptors on Candida. The results revealed that preincubation with oATP conferred significant (P < 0.05) protection to C. albicans after addition of hLF(1-11) in killing assays. The direct involvement of ATPe in pore formation was demonstrated by the significant (P < 0.05) reduction in the percentage of PI-positive cells after preincubation of C. albicans with oATP. This demonstrates that oATP blocks the candidacidal effect of hLF(1-11) by inhibiting the interaction of ATPe with ATP binding sites. The main question that remains is whether these sites are purinergic receptors or other molecules present on C. albicans that are also affected by this ligand. An attractive possibility is P2X7 receptor-like molecules (14, 16), as suggested by Koshlukova et al. (27). Unfortunately, the National Center for Biotechnology Information database did not indicate the existence of a protein homologous to human P2X7 in Candida spp. or Saccharomyces cerevisiae. Third, analysis of the activity of ATP or ATP analogues revealed that (i) they did not show candidacidal activity, in agreement with observations by others (W. van 't Hof, Academic Centre for Dentistry, Department of Oral Biochemistry, Vrije Universiteit, Amsterdam, The Netherlands, personal communication) and in contrast with data recently shown (27); (ii) they had no synergistic effect with hLF or suboptimal concentrations of hLF(1-11); and (iii) they were not able to restore the candidacidal activity that hLF(1-11) lost when in the presence of azide. These data suggest that ATPe is not sufficient for the induction of cell death.
Another conclusion to be drawn from the present results is that the first two arginines together play a key role in the candidacidal activity of peptides, e.g., hLF(1-11) derived from the N terminus of hLF, in agreement with our earlier findings for the antibacterial activity of hLF and related peptides (Nibbering et al., Annu. Meet. Duch Soc. Immunol., 1999). This conclusion is based on the following results. Comparison of the candidacidal activities of hLF(1-11) and fragments lacking one or more of the first three N-terminal residues revealed that hLF(1-11), hLF(2-11), and hLF(3-11) showed comparable candidacidal activities and PI staining, whereas a significantly lower activity was found for hLF(4-11). Moreover, a significant reduction in the activity of hLF(1-11) was observed when the second and/or third N-terminal residues were replaced by alanine. The first and second residues seem to play a role in binding to Candida. The amounts of hLF(1-11) and hLF(2-11) bound to this microorganism were, respectively, fourfold and twofold greater than the values for hLF(3-11) and hLF(4-11). Although binding to C. albicans is required for the peptide to exert a candidacidal effect, no correlation was found between the candidacidal activity of hLF or related peptides and their binding to this microorganism. Indeed, little hLF(3-11) bound to Candida and it still had a good candidacidal activity, while hLF(1-11) and hLF(21-31) had similar binding but significantly different candidacidal activities. Another conclusion from the present results is that hLF-related peptides are much more effective than native hLF in killing fluconazole-resistant C. albicans. It should be realized that, even at higher concentrations and for longer incubation times, the candidacidal activity of hLF was rather poor. In addition, the peptide comprising the first cationic domain showed higher killing activity than the peptide comprising the second cationic domain, again in agreement with findings on the antibacterial activity (Nibbering et al., Annu. Meet. Dutch Soc. Immunol., 1999).
All these data taken together indicate that (i) hLF-derived peptides are more active than the native protein in killing C. albicans, and the peptide comprising the first cationic domain is more active than the peptide comprising the second cationic domain of hLF; (ii) the second and third N-terminal residues are essential in the activity of hLF(1-11); and (iii) the mechanism of action of peptides derived from the N terminus of hLF seems to involve a particular sequence of events. The peptide interacts with structural elements of the plasma membrane of blastoconidia and is taken up in an energy-dependent way; it triggers the energized mitochondrion to synthesize and secrete ATP; and it is released extracellularly, where it interacts with surface ATP binding sites, resulting in pore formation. These events, combined with the effects of hLF(1-11), induce progression towards cell death, possibly involving mitochondria (5).
ACKNOWLEDGMENT
This study was financially supported by Pharming (Leiden, The Netherlands).
REFERENCES
- 1.Ånséhn S, Nilsson L. Direct membrane-damaging effect of ketoconazole and tioconazole on Candida albicans demonstrated by bioluminescent assay of ATP. Antimicrob Agents Chemother. 1984;26:22–25. doi: 10.1128/aac.26.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy W, Wakabayashi H, Takase M, Kawase K, Shimamura S, Tomita M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med Microbiol Immunol. 1993;182:97–105. doi: 10.1007/BF00189377. [DOI] [PubMed] [Google Scholar]
- 4.Bernal S D, Lampidis T J, Summerhayes I C, Chen L B. Rhodamine-123 selectively reduces clonal growth of carcinoma cells in vitro. Science. 1982;218:1117–1119. doi: 10.1126/science.7146897. [DOI] [PubMed] [Google Scholar]
- 5.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 6.Brock J. Lactoferrin: a multifunctional immunoregulatory protein? Immunol Today. 1995;16:417–419. doi: 10.1016/0167-5699(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 7.Bullen J J. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 8.Chandler F W. Epidemiology of AIDS and its opportunistic infections. In: Vanden Bossche H, et al., editors. Mycoses in AIDS patients. New York, N.Y: Plenum Press; 1990. pp. 3–12. [Google Scholar]
- 9.Chapple D S, Mason D J, Joannou C L, Odell E W, Gant V, Evans R W. Structure-function relationship of antibacterial synthetic peptides homologous to a helical surface region on human lactoferrin against Escherichia coli serotype O111. Infect Immun. 1998;66:2434–2440. doi: 10.1128/iai.66.6.2434-2440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark F S, Parkinson T, Hitchcock C A, Gow N A R. Correlation between rhodamine 123 accumulation and azole sensitivity in Candida species: possible role for drug efflux in drug resistance. Antimicrob Agents Chemother. 1996;40:419–425. doi: 10.1128/aac.40.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cockayne A, Odds F C. Interactions of Candida albicans yeast cells, germ tubes and hyphae with human polymorphonuclear leucocytes in vitro. J Gen Microbiol. 1984;130:465–471. doi: 10.1099/00221287-130-3-465. [DOI] [PubMed] [Google Scholar]
- 12.Coleman D C, Bennett D E, Sullivan D J, Gallagher P J, Henman M C, Shanley D B, Russell R J. Oral Candida in HIV infection and AIDS: new perspectives/new approaches. Crit Rev Microbiol. 1993;19:61–82. doi: 10.3109/10408419309113523. [DOI] [PubMed] [Google Scholar]
- 13.de Koster H S, Amons R, Benckhuijsen W E, Feijlbrief M, Schellekens G A, Drijfhout J W. The use of dedicated peptide libraries permits the discovery of high affinity binding peptides. J Immunol Methods. 1995;187:179–188. doi: 10.1016/0022-1759(95)00182-a. [DOI] [PubMed] [Google Scholar]
- 14.Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 15.Edgerton M, Koshlukova S E, Lo T E, Chrzan B G, Straubinger R M, Raj P A. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J Biol Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari D, Los M, Bauer M K A, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999;447:71–75. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 17.Fillebeen C, Descamps L, Dehouck M-P, Fenart L, Benaïssa M, Spik G, Cecchelli R, Pierce A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J Biol Chem. 1999;274:7011–7017. doi: 10.1074/jbc.274.11.7011. [DOI] [PubMed] [Google Scholar]
- 18.Gyurko C, Lendenmann U, Troxler R F, Oppenheim F G. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob Agents Chemother. 2000;44:348–354. doi: 10.1128/aac.44.2.348-354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock R E W. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 20.Hancock R E W. Host defence (cationic) peptides. What is their future clinical potential? Drugs. 1999;57:469–473. doi: 10.2165/00003495-199957040-00002. [DOI] [PubMed] [Google Scholar]
- 21.Hancock R E W, Chapple D S. Minireview: peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helmerhorst E J, Breeuwer P, van 't Hof W, Walgreen-Weterings E, Oomen L C J M, Veerman E C I, Nieuw Amerongen A V, Abee T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem. 1999;274:7286–7291. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 23.Hoek K S, Milne J M, Grieve P A, Dionysius D A, Smith R. Antibacterial activity in bovine lactoferrin-derived peptides. Antimicrob Agents Chemother. 1997;41:54–59. doi: 10.1128/aac.41.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchens T W, Henry J F, Yip T T. Structurally intact (78-kDa) form of maternal lactoferrin purified from urine of preterm infants fed human milk: identification of a trypsin-like proteolytic cleavage event in vivo that does not result in fragment dissociation. Proc Natl Acad Sci USA. 1991;15:2994–2998. doi: 10.1073/pnas.88.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson L V, Walsh M L, Chen L B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci USA. 1980;77:990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly S L, Lamb D C, Kelly D E, Loeffler J, Einsele H. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet. 1996;348:1523–1524. doi: 10.1016/S0140-6736(05)65949-1. [DOI] [PubMed] [Google Scholar]
- 27.Koshlukova S E, Lloyd T L, Araujo M W B, Edgerton M. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J Biol Chem. 1999;274:18872–18879. doi: 10.1074/jbc.274.27.18872. [DOI] [PubMed] [Google Scholar]
- 28.Lammas D A, Stober C, Harvey C J, Kendrick N, Panchalingam S, Kumararatne D S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 29.Lehrer R I, Ganz T, Szklarek D, Selsted M E. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Investig. 1988;81:1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leveugle B, Mazurier J, Legrand D, Mazurier C, Montreuil J, Spik G. Lactotransferrin binding to its platelet receptor inhibits platelet aggregation. Eur J Biochem. 1993;213:1205–1211. doi: 10.1111/j.1432-1033.1993.tb17871.x. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenstein A K, Ganz T, Nguyen T-M, Selsted M E, Lehrer R I. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J Immunol. 1988;140:2686–2694. [PubMed] [Google Scholar]
- 32.Lonnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 33.Martin E, Ganz T, Lehrer R I. Defensins and other endogenous peptide antibiotics of vertebrates. J Leukoc Biol. 1995;58:128–136. doi: 10.1002/jlb.58.2.128. [DOI] [PubMed] [Google Scholar]
- 34.Mason D J, Dybowski R, Larrick J W, Gant V A. Antimicrobial action of rabbit leukocyte CAP18106–137. Antimicrob Agents Chemother. 1997;41:624–629. doi: 10.1128/aac.41.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musial C E, Cockerill III F R, Roberts G D. Fungal infections of the immunocompromised host: clinical and laboratory aspects. Clin Microbiol Rev. 1988;1:349–364. doi: 10.1128/cmr.1.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nillesse N, Pierce A, Lecocq M, Benaïssa M, Spik G. Expression of the lactotransferrin receptor during the differentiation process of the megakaryocyte Dami cell line. Biol Cell. 1994;82:149–159. doi: 10.1016/s0248-4900(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 37.Nuijens J H, van Berkel P H C, Schanbacher F L. Structure and biological actions of lactoferrin. J Mammary Gland Biol Neoplasia. 1996;1:285–295. doi: 10.1007/BF02018081. [DOI] [PubMed] [Google Scholar]
- 38.Odds F C, Schmid J, Soll D R. Epidemiology of Candida infections in AIDS. In: Vanden Bossche H, et al., editors. Mycoses in AIDS patients. New York, N.Y: Plenum Press; 1990. pp. 67–74. [Google Scholar]
- 39.Semenov D V, Kanyshkova T G, Buneva V N, Nevinsky G A. Human milk lactoferrin binds ATP and dissociates into monomers. Biochem Mol Biol Int. 1999;47:177–184. doi: 10.1080/15216549900201183. [DOI] [PubMed] [Google Scholar]
- 40.van Berkel P H, Geerts M E, van Veen H A, Mericskay M, de Boer H A, Nuijens J H. N-terminal stretch Arg2, Arg3, Arg4, and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem J. 1997;328:145–151. doi: 10.1042/bj3280145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walton E, Gladstone G P. Factors affecting the susceptibility of staphylococci to killing by the cationic proteins from rabbit polymorphonuclear leucocytes: the effect of alteration of cellular energetics and of various iron compounds. Br J Exp Pathol. 1976;57:560–570. [PMC free article] [PubMed] [Google Scholar]
- 42.Welling M M, Paulusma-Annema A, Balter H S, Pauwels E K J, Nibbering P H. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med. 2000;27:292–301. doi: 10.1007/s002590050036. [DOI] [PubMed] [Google Scholar]
- 43.Wilson D F, Chance B. Azide inhibition of mitochondrial electron transport. I. The aerobic steady state of succinate oxidation. Biochim Biophys Acta. 1967;131:421–430. doi: 10.1016/0005-2728(67)90002-3. [DOI] [PubMed] [Google Scholar]
- 44.Wu M, Maier E, Benz R, Hancock R E W. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 45.Zhang G H, Mann D M, Tsai C M. Neutralization of endotoxin in vitro and in vivo by a human lactoferrin-derived peptide. Infect Immun. 1999;67:1353–1358. doi: 10.1128/iai.67.3.1353-1358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]