Abstract
Endocrine mucin-producing sweat gland carcinoma (EMPSCG) is a rare, low-grade cutaneous adnexal neoplasm with evidence of neuroendocrine differentiation, predominantly involving the eyelids of elderly. It has a striking resemblance to solid papillary carcinoma of breast which similarly displays neuroendocrine features. EMPSGC is considered a precursor of cutaneous mucinous carcinoma, and the term “mucinous carcinoma” is also recommended for hybrid lesions which reveal an invasive mucinous component associated with EMPSGC. While local recurrences are well- documented in EMPSGC, metastases had not been encountered until very recently; two reports in the past year have described metastases from eyelid EMPSGC to the parotid gland after a prolonged interval from the primary presentation. We report the case of a 78-year-old male with eyelid EMPSGC metastatic to the parotid gland nine years after excision of the primary tumor, which had initially been diagnosed as a poorly differentiated carcinoma. Development of metastasis after a prolonged interval is similar to both the previously described cases, and emphasizes the need to reevaluate the stated indolent nature of this neoplasm. It also aims to draw attention of pathologists to this uncommon tumor of the eyelid which is often misdiagnosed on primary presentation.
Keywords: Eyelid, Eccrine, Parotid, Metastasis, Mucinous carcinoma
Introduction
Endocrine mucin-producing sweat gland carcinoma (EMPSCG) is a rare, low grade skin adnexal tumor most frequently involving the eyelid and periorbital skin in elderly patients, with a female preponderance [1]. It has a striking histomorphological and immunohistochemical resemblance to solid papillary carcinoma of breast. EMPSGC and solid papillary carcinoma of breast both display neuroendocrine features, and are considered precursors of mucinous carcinoma of the skin and breast, respectively [2, 3].
Since its first description in 1997 as a cutaneous analogue to endocrine ductal carcinoma in situ of breast, less than 200 cases of EMPSGC have been reported in literature, most of which have had an unremarkable clinical course following excision [1–3]. Local recurrences are well documented in a proportion of cases; however, there have been no reports of metastases of EMPSGC until very recently, with only two case reports describing metastases from eyelid EMPSGC [1, 3–5]. Herein, we report a case of eyelid EMPSGC metastatic to the parotid gland nine years after excision of the primary tumor. Development of metastasis after a prolonged interval is similar to both the previously described cases, and questions the long opined indolent nature of this neoplasm.
Case Description
This 78 year-old male patient presented with a swelling in the left preauricular region for one and a half years. He also complained of facial pain and facial asymmetry for the last three months. The patient gave history of a tumor in left upper eyelid nine years prior, which had been treated with wide local excision. On histopathology, the tumor had been reported as a poorly differentiated carcinoma; the surgical resection margins were free.
On examination of the current preauricular swelling, a 3 × 2 cm firm and tender mass with limited mobility was noted, extending from the zygoma till the insertion of the lobule inferiorly. He had Grade 5 House Brackman facial nerve function with obvious facial asymmetry at rest. Contrast-enhanced MRI (Fig. 1A, B) revealed an enhancing predominantly solid lesion with cystic components and irregular margins, which was T1-isointense and T2-hyperintense, in the superficial lobe of left parotid gland. The lesion was infiltrating into the masseter and abutting the mandibular ramus. FDG PET-CT showed a metabolically active soft tissue mass in the preauricular region with sub-centimetric cervical lymph nodes at levels I, II and V; no other foci of uptake were noted elsewhere in the body.
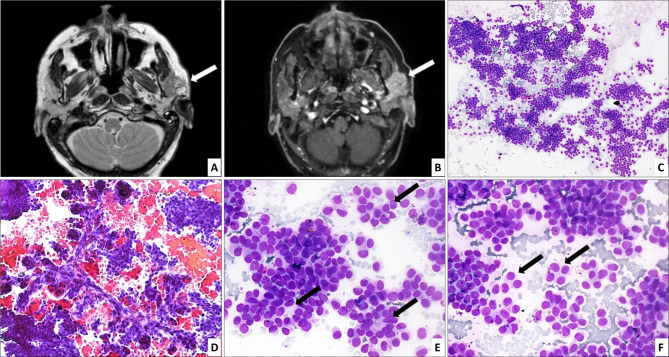
Fig. 1.
Axial T2 weighted (A) and post gadolinium fat saturated T1 weighted (B) images of contrast-enhanced MRI show an irregular solid lesion with cystic components in left parotid gland (arrow) infiltrating into the masseter and abutting the mandibular ramus, showing hyperintense signal on T2 weighted images and intense contrast enhancement. FNAC shows a tumor with predominantly trabecular (C; HE, 100× ) and focal pseudopapillary architecture (D; HE, 200× ). The cells form rosette-like structures (arrows) at places (E; HE, 400× ). They appear monotonous, have scant granular cytoplasm and round to ovoid nuclei, some of which are eccentrically placed, imparting a plasmacytoid (arrows) appearance (F; HE, 400× )
Fine needle aspiration cytology performed from the lesion at another centre was submitted to us for review (Fig. 1C–F); smears showed a tumor with predominantly trabecular and focal pseudopapillary architecture. The cells were monotonous, had scant granular cytoplasm, and round to ovoid nuclei, and formed rosette-like structures at places. Some of the tumor cells had eccentrically placed nuclei imparting a plasmacytoid appearance. The aspirate was reported as a malignant neoplasm, Milan category VI. The patient underwent left radical parotidectomy with comprehensive neck dissection (level I-V lymph nodes). Intraoperatively, a firm tumor was seen encasing the facial trunk and pes anserinus, and adherent to the masseter.
Gross examination showed a grey-white tumor measuring 3.5 × 2 × 1.7 cm in the superficial lobe of parotid. Sections examined showed a tumor (Fig. 2A–E) composed of monomorphic cells arranged in well- demarcated, expansile nodules having solid (Fig. 2A), cribriform, trabecular (Fig. 2B), and papillary architecture, within the parotid parenchyma. Tumor cells were small to intermediate sized, polygonal, with scant amphophilic cytoplasm and monomorphic round to oval nuclei with fine chromatin and inconspicuous nucleoli. Rosette- like structures were identified (Fig. 2D). Mitoses were infrequent. Multiple foci of perineural invasion were present around small nerves (Fig. 2E); however, lymphovascular emboli were absent. At places, extracellular mucin was identified between the tumor nodules, accounting for approximately 5% of the tumor area (Fig. 2C). These mucin-containing foci were limited to the central and internodular regions of the tumor, while the periphery was comprised of the expansile solid nodules. Floating tumor cells, and infiltrating glands or tumor cell nests were absent.
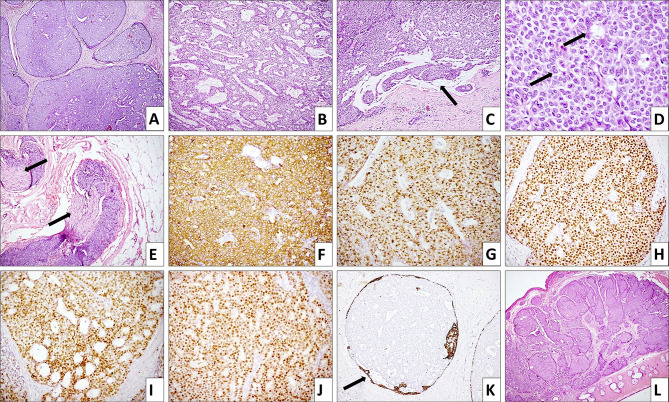
Fig. 2.
Microscopic examination of total parotidectomy specimen shows a tumor composed of well- demarcated, expansile nodules having solid, cribriform (A; HE, 40× ) and trabecular (B; HE, 100× ) architecture. At places, extracellular mucin (arrow) is identified between the tumor nodules (C; HE, 100× ). Tumor cells focally form rosette- like structures (arrow) and are small to intermediate sized, polygonal, with scant amphophilic cytoplasm and monomorphic round to oval nuclei with fine chromatin and inconspicuous nucleoli (D; HE, 400× ); foci of perineural invasion are noted (E; HE, 100× ). Tumor cells are positive for synaptophysin (F; IHC, 200× ), chromogranin (G; IHC, 200× ), estrogen receptor (H; IHC, 200× ), progesterone receptor (I; IHC, 200× ) and androgen receptor (J; IHC, 200× ). An intact myoepithelial layer at the periphery is highlighted by CK5/6 (K; IHC, 200× ) in some of the smaller nodules. Archival sections of the excised primary eyelid lesion show a tumor with similar histomorphology (L; HE, 20× )
On immunohistochemistry (Fig. 2F–K), diffuse positivity was noted with CK7, chromogranin, synaptophysin, estrogen receptor, progesterone receptor, androgen receptor, mammaglobin and GCDFP-15. Ki-67 proliferation index was around 5%. Some of the smaller nodules showed an intact myoepithelial layer at the periphery highlighted by CK5/6 and p40. Immunohistochemistry for SOX10, p40, CD117, smooth muscle actin (SMA), NUT protein, CD99 and NKX2.2 was negative. Residual lymphoid tissue or lymph nodes were not identified in any of the sections from the parotid gland; however, the possibility of metastasis to intra-parotid lymph nodes with spill over into the parotid could not be excluded. Eleven cervical lymph nodes dissected were free of tumor.
Archival sections of the excised eyelid tumor were retrieved and showed a tumor with similar histomorphology (Fig. 2L). Lymphovascular emboli were not seen in these sections. Immunostaining performed for synaptophysin and estrogen receptor was positive; CK5/6 showed an intact myoepithelial layer in a few nodules, while the rest lacked a myoepithelial layer, indicative of pushing invasion. A final diagnosis of EMPSGC of the left eyelid with metastasis to the ipsilateral parotid gland was made. There was no prior history of breast carcinoma, and breast clinical examination was unremarkable.
Discussion
Delayed encapsulation of the parotid gland during development leads to entrapment of lymphoid tissue within its parenchyma, due to which the parotid contains up to 20 intraglandular and periglandular lymph nodes which drain the skin of part of the face, the eyelids, conjunctiva, lacrimal gland, anterior ear and the external auditory canal [6]. Consequent to this, primary malignancies from these sites can metastasize to the nodes within the parotid. Metastases to parotid gland account for 6–8% of all parotid neoplasms, and up to 70% of all parotid malignancies, being higher in countries with high sun exposure [7, 8]. Most metastases to the parotid have primaries in the head and neck region, with the most frequently described being cutaneous and mucosal squamous cell carcinoma and melanoma [7, 8]. Tumors of the eye and ocular adnexa have a fair share in this list, and there are reports of metastases from eyelid sebaceous carcinoma, conjunctival melanoma, and retinoblastoma [8–10]. We have previously documented a case of ocular medulloepithelioma metastatic to ipsilateral parotid gland [11].
EMPSGC shares notable similarities with solid papillary carcinoma of the breast. Both the entities have been proposed as precursors of mucinous carcinomas of their respective organs of origin, namely primary cutaneous mucinous carcinoma, and type B mucinous carcinoma of breast, both of which display neuroendocrine features [2, 3]. Consequently, a morphological spectrum of tumors exists, including in-situ only lesions, hybrid lesions with both in-situ and invasive mucinous components, and invasive carcinomas, and the term “EMPSGC” was previously being used for all of them. However, the recent WHO Classification of Skin tumors (2018) recommends the use of the term mucinous carcinoma for the hybrid lesions, while solid tumors with a minimal mucinous component and expression of neuroendocrine markers are to be diagnosed as EMPSGC [12].
Histomorphologically, EMPSGC has been described as a well-circumscribed nodular lesion with variable cystic, solid, papillary and cribriform architecture. The tumor is composed of bland, eccrine-appearing, round to oval cells displaying stippled or “salt and pepper” chromatin. Rosettes-like structures may be seen. Foci of intra- and extracellular mucin are noted. Small clefts of mucin have also been described. Myoepithelial cells are preserved at the rim of some of the neoplastic nodules akin to ductal carcinoma in-situ of breast, indicating progression from an in-situ lesion. Progression to neuroendocrine mucinous adenocarcinoma is characterized by pools of extracellular stromal mucin and/or infiltrating glands and nests of tumor cells with loss of myoepithelial rim. However, a tumor with “pushing invasion” characterized by loss of myoepithelial cells at the border of large solid expansile nodules is diagnosed as EMPSGC as it lacks a definitive invasive mucinous component [1, 3].
Tumor cells express markers of epithelial differentiation (pancytokeratin, epithelial membrane antigen, CK7), neuroendocrine differentiation (synaptophysin, chromogranin, INSM1, neuron specific enolase), hormone receptors (estrogen receptor, progesterone receptor, androgen receptor), and others such as GCDFP-15, GATA3 and mammaglobin. At primary sites, myoepithelial cells at the circumference of the nodules are highlighted by p63, p40, smooth muscle actin, calponin and CK5/6 [1]. The presence of a focally intact myoepithelial layer at a metastatic site is undescribed, and possibly represents colonization and expansion of native ductal structures within the parotid by the tumor cells, rather than being indicative of an in-situ lesion.
FNAC findings of EMPSGC are not well established, with two case reports till date [4, 5]. The parotid FNAC in our patient showed cellular fragments with trabecular and focal acinar architecture. The cells were monotonous and a fair number showed plasmacytoid appearance. They had scant granular cytoplasm and round to ovoid nuclei, closely recapitulating the histomorphology of the tumor. Mucin was not identified in the background.
Differential diagnosis of EMPSGC metastases to parotid includes primary parotid carcinomas composed of small basaloid cells with solid, cribriform and trabecular architecture, such as adenoid cystic carcinoma, and basal cell adenocarcinoma, rarer translocation-associated neoplasms seen in the head and neck region viz. NUT carcinoma and adamantinoma-like Ewing sarcoma (ALES), and metastases from basal cell carcinoma (BCC), the salient diagnostic features of which are summarized in Table 1 [13–15]. Lastly, although rare, primary breast carcinoma metastatic to the parotid needs to be excluded. In the few such reports, these metastases are mostly from invasive ductal carcinomas that are morphologically distinct from EMPSGC. Breast solid papillary carcinoma which is morphologically and immunohistochemically identical to EMPSGC has indolent behavior with rare metastases to axillary lymph nodes, and metastasis to parotid has not been described. Nevertheless, detailed clinical history and physical examination are a requisite to exclude metastatic breast cancer, particularly in female patients, in whom EMPSGC is almost twice as common [1].
Table 1.
Differential diagnosis of Endocrine mucin producing sweat gland carcinoma (EMPSGC)
| Differential diagnosis | Morphological features | IHC | Molecular alterations |
|---|---|---|---|
| EMPSGC | Variably cystic tumor with cribriform, solid, papillary architecture; small cells with uniform nuclei, “salt and pepper” chromatin; rosettes; variable intracellular, extracellular mucin | Positive for CK, CK7, ER, PR, AR, neuroendocrine markers; myoepithelial markers (p63, p40, SMA) partly retained at periphery of tumor nodules | No diagnostic molecular alteration |
| Adenoid cystic carcinoma, solid type | Solid sheets of basaloid cells with scant cytoplasm, angulated hyperchromatic nuclei; scarce ductal cells | SOX 10 positive; CK7, CD117 in ductal cells; p40, SMA in myoepithelial cells; Myb protein overexpression may be present | MYB, MYBL1 gene rearrangements |
| Basal cell adenocarcinoma | Biphasic tumor with jigsaw puzzle- like solid nests; dark and pale cells; peripheral palisading; hyaline basal lamina material may be present around tumor islands | CK, myoepithelial markers (p40, p63, SMA) positive; nuclear β-catenin may be present; negative for neuroendocrine markers | No diagnostic molecular alteration |
| NUT carcinoma | Monomorphic basaloid cells with scant cytoplasm, prominent nucleoli; abrupt squamous differentiation; necrosis, frequent mitoses | Positive for NUT protein (speckled staining), p63, p40; neuroendocrine markers are negative or focal faint positive | NUTM1 gene rearrangements |
| Adamantinoma-like Ewing sarcoma | Sheets, nests, lobules of basaloid cells; variably myxoid to hyalinized stroma; keratinization, squamous pearls; rosettes, peripheral palisading of nuclei, basement membrane material; necrosis, frequent mitoses | Diffuse CD99, NKX2.2, CK, p63 and p40 positivity; neuroendocrine markers may be positive; negative for hormone receptors | EWSR1-FLI1 gene fusion |
| Metastases from basal cell carcinoma | Basaloid cells with peripheral palisading; retraction spaces around tumor islands; frequent apoptosis | BER-EP4 positive; negative for neuroendocrine markers, hormone receptors | No diagnostic molecular alteration |
AR androgen receptor, CK cytokeratin, ER estrogen receptor, PR progesterone receptor, SMA smooth muscle actin
EMPSGC at primary sites is managed by complete surgical excision. The largest series of EMPSGCs with 63 cases, including hybrid lesions better classified as primary cutaneous mucinous carcinoma, documented an overall local recurrence rate of 14.3%. Among these, EMPSGC with an in-situ component or with pushing invasion had a recurrence rate of 9.5%, while the recurrence rate in EMPSGC associated with mucinous carcinoma was 23.8%. Distant metastases were not identified over a follow-up period ranging from 1 to 67 months [3]. More recently, Au et al. reviewed 190 cases of EMPSGC in literature, of which 35.7% were associated with mucinous carcinoma. Overall recurrence rate was found to be 8.4%, that for EMPSGC with mucinous carcinoma was 12.3%, while that for EMPSGC without an invasive component was 6.8% [1]. This data highlights the differences in outcome between EMPSGC and EMPSGC associated with mucinous carcinoma, and mandates a meticulous search for foci of mucinous carcinoma in all cases of EMPSGC.
There are only two recently described reports of eyelid EMPSGC with metastasis (Table 2). In both these cases, metastasis developed after a prolonged interval of 9–10 years after primary diagnosis, and a diagnosis of EMPSGC was made on examination of the metastases accompanied by review of the primary tumor in one case, as well as in ours [4, 5]. Eyelid carcinomas are known to have a propensity for delayed metastasis to regional lymph nodes. Nodal metastases have been reported in 8 to 23% of eyelid carcinomas [16]. The risk of metastasis increases with size greater than 20 mm, i.e. beyond stage T2. Hashimoto et al. in a series of 268 eyelid carcinomas, documented parotid metastasis in 21 cases, the majority being sebaceous carcinomas [17]. Seventeen of these (81%) developed parotid lesions subsequent to treatment of the primary, with a median interval of 20 months (range: 5–133 months) [17]. Our case, coupled with the two previous ones, highlights similar clinical behavior of EMPSGC.
Table 2.
Cases of Endocrine mucin-producing sweat gland carcinoma (EMPSGC) with metastases
| Author, year | Present case | Froehlich et al. (2020) [5] | Hadi et al. (2021) [4] | Shah et al. (2021) [18] |
|---|---|---|---|---|
| Age (years)/ sex | 78/Male | 71/Female | 66/Male | 60/Male |
| Primary site | Upper eyelid | Upper eyelid | Upper eyelid | Scrotum |
| Histopathology diagnosis of primary tumor | Poorly differentiated carcinoma | Unclear | Hidradenoma with atypia | EMPSGC |
| Treatment | Excision with negative margins | Excision | Excision with negative margins | Excision biopsy, radiotherapy and palliative chemotherapy |
| Time interval to local recurrences | No local recurrence | 10 years |
First: 7 years Second: 9 years |
Not applicable |
| Time interval to metastasis from primary diagnosis | 9 years | 11 years | 9 years | Synchronous |
| Sites of metastasis | Ipsilateral parotid | Ipsilateral parotid | Ipsilateral parotid, 8th rib | Lymph nodes, pancreas, bone on PET scan |
| Specimen on which EMPSGC was diagnosed | Parotidectomy, with review of histology of primary tumor | Incision biopsy of recurrence | Parotidectomy, with review of histology of primary tumor | Excision biopsy of primary |
The only other reported EMPSGC with metastasis, also documented recently, occurred in the penoscrotal skin [18]. However, unlike the eyelid EMPSGCs, regional nodal and systemic metastases were seen at initial presentation in this patient, suggesting that while eyelid EMPSGC is a relatively indolent neoplasm, with risk of local recurrence and delayed metastases, EMPSGC at other sites may behave more aggressively with widespread metastases at presentation.
Conclusions
The present case demonstrates an extremely rare event of EMPSGC of eyelid metastasizing to ipsilateral parotid gland nine years after excision of the primary tumor. With two prior similar reports, these cases call attention to the possible metastatic potential of this apparently indolent tumor. In both reported cases, the diagnosis of EMPSGC was clinched on histological examination of the recurrent/ metastatic tumor. This case highlights the express need to consider a metastatic tumor to the parotid in the differential diagnosis of any parotid neoplasm that lacks the classical histomorphology of a primary salivary gland carcinoma. Documentation and further analysis of these tumors is warranted to explore the histomorphological, immunohistochemical and molecular clues to their metastatic potential.
Author Contributions
AK diagnosed the case, conceptualized and supervised the manuscript; JS and KK assisted with the diagnosis and drafted the manuscript; AKo, KS are the treating clinicians; SS contributed the archival material for the primary eyelid tumor; all authors approved the final version of the manuscript.
Funding
No funding has been received.
Data Availability
No associated data as this is a case report.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Ethics approval was waived as it is a single case report.
Informed Consent
Informed consent was obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021 doi: 10.1111/cup.13983. [DOI] [PubMed] [Google Scholar]
- 2.Flieder A, Koerner FC, Pilch BZ, Maluf HM. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501–1506. doi: 10.1097/00000478-199712000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Agni M, Raven ML, Bowen RC, Laver NV, Chevez-Barrios P, Milman T, et al. An update on endocrine mucin-producing sweat gland carcinoma: clinicopathologic study of 63 cases and comparative analysis. Am J Surg Pathol. 2020;44:1005–1016. doi: 10.1097/PAS.0000000000001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadi R, Xu H, Barber BR, Shinohara MM, Moshiri AS. A case of endocrine mucin-producing sweat gland carcinoma with distant metastasis. J Cutan Pathol. 2021 doi: 10.1111/cup.13999. [DOI] [PubMed] [Google Scholar]
- 5.Froehlich M, Cook J, Bruner E, Stalcup S, Patel K, Day T. Endocrine mucin-producing sweat gland carcinoma of the eyelid with locoregional metastasis to the parotid gland. Dermatol Surg. 2020;46:1116–1118. doi: 10.1097/DSS.0000000000002322. [DOI] [PubMed] [Google Scholar]
- 6.Nuyens M, Schüpbach J, Stauffer E, Zbären P. Metastatic disease to the parotid gland. Otolaryngol Head Neck Surg. 2006;135:844–848. doi: 10.1016/j.otohns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Pastore A, Ciorba A, Soliani M, Di Laora A, Valpiani G, Bianchini C, et al. Secondary malignant tumors of the parotid gland: not a secondary problem. J BUON. 2017;22:513–518. [PubMed] [Google Scholar]
- 8.Bron LP, Traynor SJ, McNeil EB, O'Brien CJ. Primary and metastatic cancer of the parotid: comparison of clinical behavior in 232 cases. Laryngoscope. 2003;113:1070–1075. doi: 10.1097/00005537-200306000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Esmaeli B, Wang X, Youssef A, Gershenwald JE. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101–2105. doi: 10.1016/S0161-6420(01)00782-5. [DOI] [PubMed] [Google Scholar]
- 10.Purkayastha A, Sharma N, Pathak A, Kapur BN, Dutta V. An extremely rare case of metastatic retinoblastoma of parotids presenting as a massive swelling in a child. Transl Pediatr. 2016;5:90–94. doi: 10.21037/tp.2016.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarangi J, Kakkar A, Roy D, Thakur R, Singh CA, Sharma MC. Ocular non-teratoid medulloepithelioma with teratoid metastases in ipsilateral intraparotid lymph nodes. Eur J Ophthalmol. 2019 doi: 10.1177/1120672119870079. [DOI] [PubMed] [Google Scholar]
- 12.Zembowicz A, Argenyi ZB, Brenn T, Calonje E, Mehregan DA, Mehregan DR, et al. Endocrine mucin-producing sweat gland carcinoma. In: Elder DE, Massi D, Scolyer RA, Willemze R, et al., editors. WHO classification of skin tumours. 4. Lyon: IARC Press; 2018. pp. 168–169. [Google Scholar]
- 13.Rooper LM, Jo VY, Antonescu CR, Nose V, Westra WH, Seethala RR, Bishop JA. Adamantinoma-like ewing sarcoma of the salivary glands. Am J Surg Pathol. 2019;43:187–194. doi: 10.1097/PAS.0000000000001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agaimy A, Fonseca I, Martins C, Thway K, Barrette R, Harrington KJ, et al. NUT carcinoma of the salivary glands. Clinicopathologic and molecular analysis of three cases and a survey of NUT expression in salivary gland carcinomas. Am J Surg Pathol. 2018;42:877. doi: 10.1097/PAS.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurian RR, Di Palma S, Barrett AW. Basal cell carcinoma metastatic to parotid gland. Head Neck Pathol. 2014;8:349–353. doi: 10.1007/s12105-013-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sa HS, Rubin ML, Xu S, Ning J, Tetzlaff M, Sagiv O, et al. Prognostic factors for local recurrence, metastasis and survival for sebaceous carcinoma of the eyelid: observations in 100 patients. Br J Ophthalmol. 2019;103:980–984. doi: 10.1136/bjophthalmol-2018-312635. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto K, Yasumatsu R, Toh S, Shiratsuchi H, Yoshida T, Nishiyama K, et al. Patterns of lymphatic spread and the management of eyelid carcinomas. Auris Nasus Larynx. 2016;43:666–671. doi: 10.1016/j.anl.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Shah M, Aman A, Srinivaas K, Gudipati A, Chavali P. Endocrine mucin-producing sweat gland carcinoma of the peno-scrotum with systemic metastases: a rare case report. Ind J Pathol Microbiol. 2021;64:180–182. doi: 10.4103/IJPM.IJPM_804_20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No associated data as this is a case report.