Abstract
The fifth chapter of the upcoming fifth edition of the 2022 World Health Organization Classification of Tumours of the Head and Neck titled Tumours of the oral cavity and mobile tongue, has had some modifications from the 2017 fourth edition. A new section "Non-neoplastic Lesions", introduces two new entries: necrotizing sialometaplasia and melanoacanthoma. The combined Oral potentially malignant disorders and Oral epithelial dysplasia section in the 2015 WHO has now been separated and submucous fibrosis and HPV-associated dysplasia are also discussed in separate sections. Carcinoma cuniculatum and verrucous carcinoma are described in dedicated sections, reflecting that the oral cavity is the most common location in the head and neck for both these entities which have distinct clinical and histologic features from conventional squamous cell carcinoma. This review summarizes the changes in Chapter 5 with special reference to new additions, deletions, and sections that reflect current clinical, histological, and molecular advances.
Keywords: World Health Organization, Oral cavity, Mobile tongue, Squamous cell, Dysplasia, Proliferative verrucous leukoplakia, Submucous fibrosis, Ectomesenchymal chondromyxoid tumor
Introduction
Periodic updates of the WHO/IARC Classification of Tumours allow for refinements of tumor classifications and addition of new entities based on evolving molecular findings, immunohistochemical advances and clinical behavior. In the 5th edition of The World Health Organization Classification of Tumours of the Head and Neck, the Chapter 5 titled Tumours of the oral cavity and mobile tongue, has had some modifications from the 2017 fourth edition [1].
A Non-neoplastic section is now added to the chapter. Although reactive, necrotizing sialometaplasia has been included as both the clinical and histologic features of this entity can mimic salivary gland tumors and squamous carcinoma. Multifocal epithelial hyperplasia in the past edition was included with the papillomas but in the 5th edition is included in the non-neoplastic section in view of the clinical behavior and spontaneous regression. Another new entry into this section is melanoacanthoma, as the clinical features may be of concern for oral mucosal melanoma.
Tumours of uncertain histogenesis section includes an update on ectomesenchymal chondromyxoid tumor (EMCT) which discusses the recent molecular discovery of a RREB1::MRTFB gene fusion in 90% of cases for this entity. Previously covered in this chapter in the 4th edition, soft tissue and neural tumors, oral mucosal melanoma, salivary gland tumors, and hematolymphoid tumors are now presented in chapters dedicated to those tumor categories.
Unlike past editions where oral carcinoma was discussed first, in the current edition the epithelial tumors are organized by tumor behavior and squamous carcinoma is discussed last. Separate sections have been added on oral potentially malignant disorders, oral epithelial dysplasia, submucous fibrosis, and HPV-associated dysplasia to reflect the advances in our understanding of the clinical, histologic, and molecular findings. Oral epithelial dysplasia grading criteria has been expanded to include several more architectural and cytologic features for the diagnosis of dysplasia with emphasis that architectural features alone may signify dysplasia. Although dysplasia grading remains challenging, the WHO maintains a three-tiered grading system. The section titled Tumours of uncertain histogenesis includes an update on ectomesenchymal myxoid tumor to reflect the molecular findings of a RREB1::MRTFB gene fusion in 90% of cases. Soft tissue and neural tumors, oral mucosal melanoma, salivary gland tumors, and hematolymphoid tumors previously covered in this chapter are now presented in chapters dedicated to those tumor categories.
Non-neoplastic Lesions
A new entry in the 2022 WHO, Non-neoplastic lesions add two new entries. Necrotizing sialometaplasia (NSM) is described, principally to highlight the clinical and histological features of a reactive, self-limiting entity that can mimic squamous cell carcinoma and salivary carcinoma [2–4]. The histologic feature of salivary lobular architecture preservation is emphasized as a desirable diagnostic criterion. Diagnostic challenges can be encountered when lobular preservation is poor or in later stages of NSM when squamous metaplasia of the ducts and acini mimic mucoepidermoid carcinoma (Fig. 1). Pseudoepitheliomatous hyperplasia is another histologic finding in some NSM and may raise the suspicion of squamous cell carcinoma, however the bland cytologic features are helpful criteria [2, 3, 5].
Fig. 1.
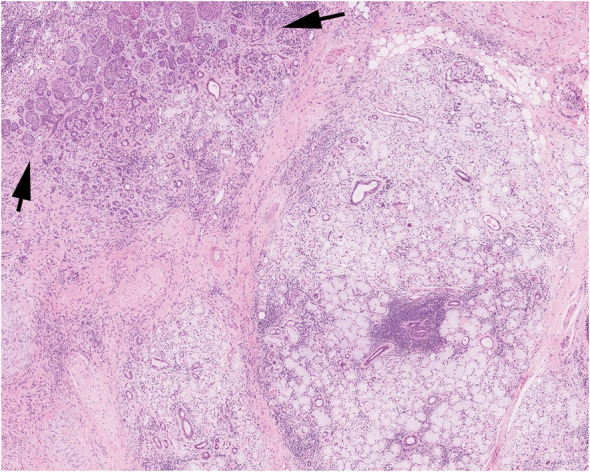
Necrotizing sialometaplasia showing the outline of residual acini with necrosis with preservation of the lobular architecture of the glands and squamous metaplasia of residual ducts (arrows). Courtesy or Dr. K Magliocca
Multifocal epithelial hyperplasia (MFEH) in the 4th edition was grouped with the papilloma section, but it was moved to the Non-neoplastic lesion section for the 5th edition to better reflect the clinical behavior and spontaneous regression of these lesions. When MFEH occurs in children, regression is generally noted at puberty [6]. Low socio-economic status is also associated with MFEH and with improved living conditions the lesions will regress.
Melanoacanthoma is another new entry in the 2022 WHO, chiefly added due to the alarming clinical presentation of an initially rapidly growing pigmented lesion, raising the concern for oral mucosal melanoma (Fig. 2A). The unique histology of melanoacanthoma is supported by epithelial acanthosis and dendritic melanocytes within the intercellular spaces which can be highlighted by S100 protein, HMB45, or Melan A immunohistochemistry (Fig. 2B) [7–9]. Melanoacanthoma lacks atypia and invasion of melanocytic nests are absent, important features to distinguish from mucosal melanoma.
Fig. 2.
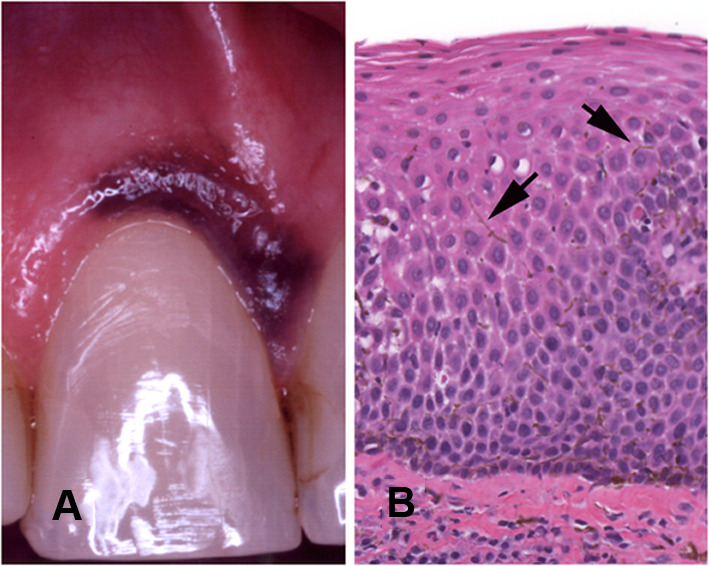
Melanoacanthoma. A Diffuse melanin pigment affecting the attached gingiva of the maxillary central incisor. B Acanthotic epithelium with dendritic melanocytes (arrows) within the intercellular spaces
Epithelial Tumors
In the 5th edition, the epithelial tumor section is organized by tumor behavior with squamous carcinoma discussed last. Papillomas are presented first and are combined with condyloma acuminatum and verruca vulgaris. As previously stated, MFEH is now discussed under Non-neoplastic lesions.
Oral Potentially Malignant Disorders and Oral Epithelial Dysplasia
Oral potentially malignant disorders (OPMD) and oral epithelial dysplasia (OED) sections have been greatly expanded to reflect advances in clinical, histological, and molecular findings. Oral lichenoid lesions have been added as an OPMD [10, 11]. These are specified as lesions that may resemble oral lichen planus but lack the typical clinical or histopathological appearance. An asterisk next to smokeless tobacco keratosis has been added acknowledging that risk varies with tobacco type. Oral lichen planus is still listed as an OPMD although this was a contentious issue. Many publications have recently refined the histologic and clinical features of oral lichenoid lesions to distinguish them from oral lichen planus, but these were thought to be insufficient to remove lichen planus as an OPMD [12, 13]. Oral graft versus host disease is seen in patients receiving allogeneic stem cell transplants for hematologic malignancies, and progression to cancer has been reported [14]. Although lupus erythematosus remains listed as an OPMD it is uncertain whether the risk is solely to the lip vermilion rather than inside the oral cavity [14]. Familial cancer syndromes in addition to dyskeratosis congenita have expanded to include Fanconi anemia, xeroderma pigmentosum, Li Fraumeni syndrome, Blooms syndrome, ataxia telangiectasia and Cowden syndrome. Deletions from the OPMD table include chronic candidiasis, syphilitic glossitis, and actinic keratosis, the latter being associated with UV exposure.
Grading of OED is a contentious issue and reaching a consensus difficult as different regions of the world apply different grading criteria. This edition expands the architectural and cytological features of OED (Table 1; Fig. 3A-D) [15–18]. A distinction between generalized premature keratinization and single cell keratinization was made. Single cell keratinization was moved to cytological features. Generalized premature keratinization refers to cells in the lower spinous layer with prominent eosinophilic cytoplasm (Fig. 3B). A three-tiered OED grading system is maintained, although it is acknowledged that defining dysplasia by thirds oversimplifies the complexity of OED [15]. There are instances when OED may be limited to the basal third and yet may qualify as severe dysplasia due to a number of cytological and architectural features. Like the last edition, a binary grading system was mentioned but this system continues to lack validation against malignant transformation [19, 20]. A lichenoid host response in OED is discussed, which is an important addition to the 2022 edition, due to the confusion this may cause with oral lichen planus. It is emphasized that the presence of any cytological or architectural features of dysplasia in the presence of a lymphohistiocytic band-like infiltrate precludes a diagnosis of oral lichen planus [12, 15]. With absent or minimal cytological features of dysplasia, OED grading is challenging. This is particularly true in papillary and verrucous lesions which is discussed in more detail below. The term carcinoma in situ in the oral cavity is not recommended and is considered synonymous with severe dysplasia.
Table 1.
Modifications to the WHO diagnostic criteria for oral epithelial dysplasia
| Architectural features | Cytological features |
|---|---|
| Irregular epithelial stratification | Abnormal variation in nuclear size |
| Loss of polarity of basal cells | Abnormal variation in nuclear shape |
| Drop-shaped rete ridges | Abnormal variation in cell size |
| Increased number of mitotic figures (moved to cytological features) | Abnormal variation in cell shape |
| Abnormally superficial mitotic figures (now: mitoses high in epithelium) | Increased N:C ratio |
| Premature keratinization in single cells (now: generalized premature keratinization) | Atypical mitotic figures |
| Keratin pearls within rete ridges | Increased number and size of nucleoli |
| Loss of epithelial cell cohesion | Hyperchromasia |
| 2022 | Additions |
|---|---|
| Altered keratin pattern for oral sub-site | Single cell keratinization |
| Verrucous or papillary architecture | Apoptotic mitoses |
| Extension of changes along minor gland ducts | Increased nuclear size |
| Sharply defined margin to changes | |
| Multiple different patterns of dysplasia | |
| Multifocal or skip lesions | |
| Expanded proliferative compartment | |
| Basal cell clustering/nesting |
Fig. 3.
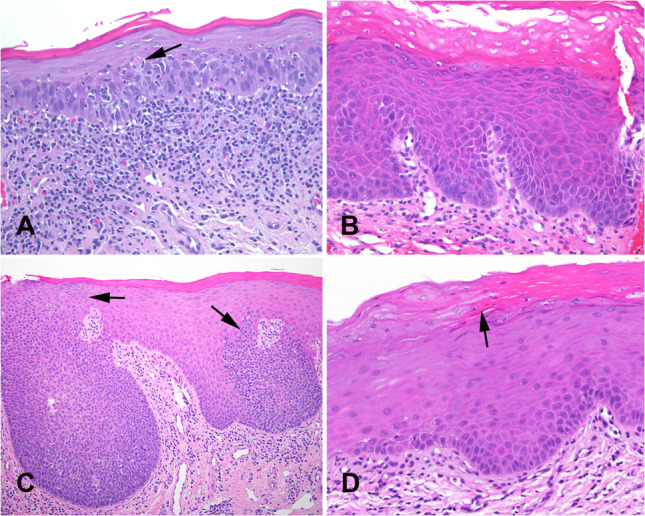
Oral epithelial dysplasia. A Moderate dysplasia with a band-like inflammatory infiltrate at the epithelial-stromal interface imparting a lichenoid appearance but with general preservation of a slightly expanded and hyperchromatic basal cell layer. A dyskeratotic cell (arrow) is present in the spinous layer. B Mild dysplasia showing primarily an architectural or differentiated pattern of dysplasia with marked orthohyperkeratosis, loss of basal cell polarization, budding of rete, increased hyperchromasia, and mitotic figures confined to basal and parabasal layer. C Skip areas of dysplasia (arrows) indicate a clonal pattern which can be present in HPV-associated dysplasia. This case was negative for HPV16 by in situ hybridization. D Sharp demarcation from normal parakeratotic epithelium to orthokeratotic epithelium in mild epithelial dysplasia. The small basal cells give rise to cells with eosinophilic cytoplasm without cytologic atypia. This pattern of dysplasia is primarily an architectural pattern
Proliferative Verrucous Leukoplakia
The section on proliferative verrucous leukoplakia (PVL) has been expanded to reflect the several studies that have been published since the 2017 edition. The early lesions of PVL demonstrate many of the newly added architectural features of OED including premature keratinization, sharp lateral margins, skip keratoses, and increased keratin (Table 1; Fig. 4A, B). In the presence of a band-like lymphohistiocytic infiltrate the early lesions of PVL can be misdiagnosed as oral lichen planus [12, 15, 17, 21]. Unlike lichen planus, intact basal cells at the epithelial-stroma interface and/or dysplasia is seen in PVL. Corrugated hyperortho- or parakeratotic lesions with a verucco-papillary architecture in the absence of or with minimal cytologic atypia are typical in early PVL. This specific architectural feature is not commonly present in other oral lesions and this verrucous morphology without cytologic atypia should be considered mild dysplasia [17, 18, 21]. With disease progression, PVL can have both an exophytic or endophytic growth pattern showing a verrucous, nodular, or bulky architecture with variable dysplasia (Fig. 4C, D) [21].
Fig. 4.
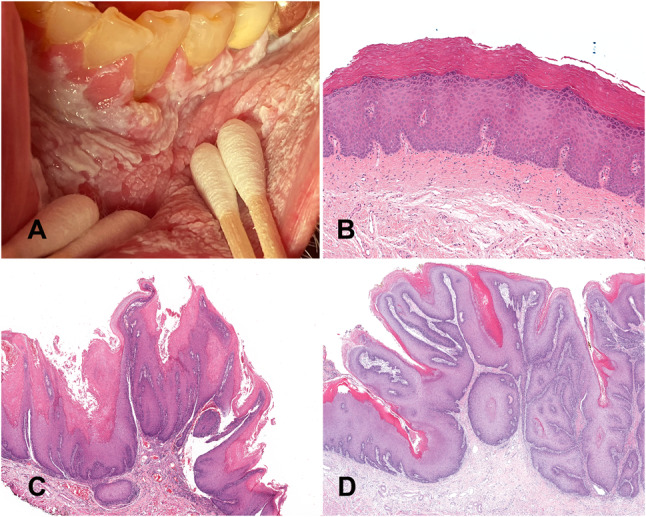
Proliferative verrucous leukoplakia. A An advanced case with a large area of leukoplakia involving the labial mucosa, mandibular vestibule and attached gingiva. B Early lesion exhibiting subtle histology with orthokeratosis, prominent granular layer, and no cytologic atypia. C A late stage of PVL showing acanthosis and a well-developed papillary architecture with a lichenoid immune response. D Bulky hyperkeratotic exophytic and endophytic growth pattern covered by parakeratin with an undulating surface and surface crypts filled with parakeratin
Submucous Fibrosis
Oral submucous fibrosis (OSF) is a chronic, insidious disease characterized by progressive submucosal fibrosis of the oral cavity and the oropharynx with a risk of transformation to squamous cell carcinoma. OSF is added to the WHO classification for the first time allocating a separate section. It was listed in the group of Oral potential malignant disorders in the previous editions.
OSF is mostly prevalent among people in South Asia and among the Pacific Islanders. Cases are also reported in a few Southeast Asian countries and among migrants from the Indian subcontinent to Europe, USA and South and East Africa. The worldwide prevalence of OSF has been reported as 4.96% with a 95% CI = 2.28–8.62 [22, 23]. Areca nut is the main etiological factor for OSF [23]. The clinical features vary according to the stage of the disease. Early cases present with burning sensation to spicy food. With the progression of the disease patients develop depapillation of the tongue, blanching, and leathery mucosa (Fig. 5A). Fibrous bands in the buccal mucosae, lip, or palate leads to progressive limitation of mouth opening [24].
Fig. 5.
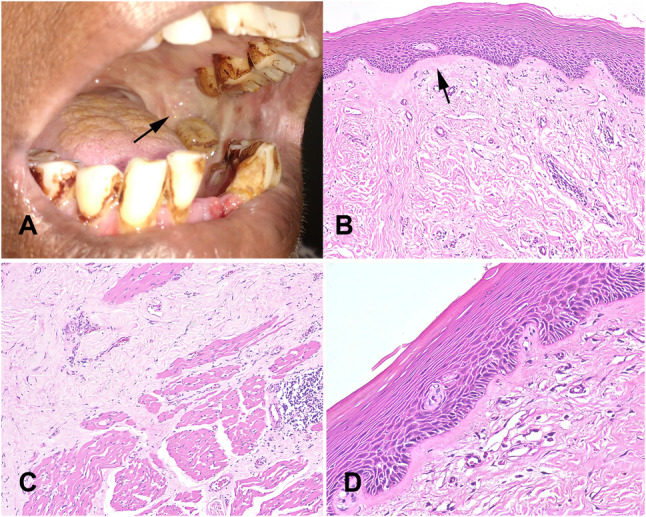
Submucous fibrosis A. Leathery mucosa, blanching and trismus (arrow). Teeth show betel quid staining. B Atrophic epithelium and dense fibrosis of the corium with juxta-epithelial hyalinization (arrow). C Progressive replacement of muscle by fibrous tissue. D Epithelial dysplasia starts to appear with budding rete morphology in the atrophic epithelium
Microscopically, initial lesions show mildly hyperplastic epithelium and subsequently progresses to marked atrophy with loss of rete ridges. The corium shows minimal changes at early stage including slightly increased vascularity, inflammatory infiltrate, and increased fibrillar collagen. With the progression of the disease, collagen becomes homogeneous, starting superficially with juxta-epithelial hyalinization (Fig. 5B) [25–27]. Advanced cases show loss of vascularity with dense fibrosis extending to underlying muscle and complete replacement of muscle by fibrous tissue (Fig. 5C). Epithelial dysplasia starts to appear with progression of the disease with characteristic budding rete morphology in severely atrophic epithelium (Fig. 5D). This feature is unique to OSF. Prevalence of epithelial dysplasia varies across different studies. [28, 29]
Significant number of oral cancers in South Asia arise in the background of OSF. The malignant transformation rate is approximately 4.2% (CI 2.7–5.6%). It has been reported that cases with oral epithelial dysplasia have a higher potential for malignant transformation compared to cases without dysplasia [30]. Mechanisms of malignant transformation have been described in many studies including both genetic and epigenetic pathways. There are no established molecular predictive markers. LOH in genes within the 13 q14-q33, hypoxia, and ROS may have some predictive value for transformation [30, 31].
HPV-Associated Dysplasia
Since the last edition there has been greater characterization of HPV-associated dysplasia (HPVOED) and due to its distinction from conventional OEDs has been classified as a separate entity. HPVOED has a strong male predilection (M:F = 6:1), with a wide age range of presentation, showing a peak in the 6th decade [32–35]. The ventral/lateral tongue and floor of mouth are the most common sites, although the buccal mucosa, palate, labial mucosa, and gingiva are affected [32, 34, 36]. Presenting generally as a flat and well-demarcated white to red patch indistinguishable from other oral leukoplakias. The etiology is high risk HPV subtypes, usually HPV 16, but the pathogenesis is unclear but presumed to be comparable to HPV associated cervical dysplasia [32, 34, 36]. The histologic features of HPVOED are distinct from conventional OED. A brightly eosinophilic layer of keratin (usually parakeratin) is usually present overlying epithelial cells with a basaloid morphology with a high nuclear to cytoplasmic ratio (Fig. 6A). Koilocytes may be seen in the superficial epithelium [32, 33, 36, 37]. Karyorrhectic cells exhibiting coarse chromatin that resemble cells in mitosis (mitosoid bodies), with a peri-cellular halo are characteristic for HPVOED. Apoptotic cells with dense eosinophilic cytoplasm are also associated with HPVOED. Although HPVOED shows strong and diffuse p16 immunoexpression, confirmation with high-risk HPV by DNA or RNA in situ hybridization is still recommended as there is insufficient validation to recommend p16 as a surrogate marker (Fig. 6B, C). Invasive squamous cell carcinoma occurs in 5% to 15% of cases [34]. No grading criteria for HPVOED has been established and it is recommended to currently grade similar to conventional OED pending further studies.
Fig. 6.
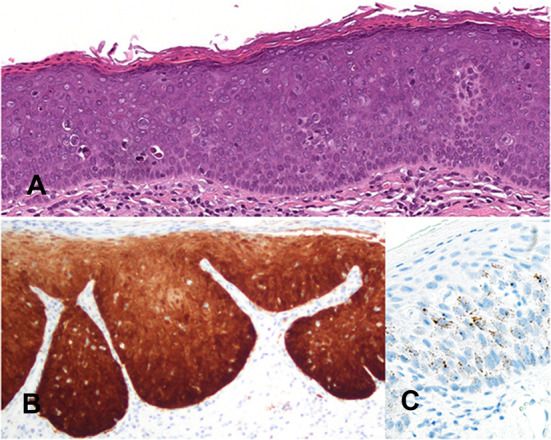
HPV-associated dysplasia. A Epithelium with a brightly eosinophilic keratin surface and basaloid morphology showing marked architectural and cytological changes of dysplasia with karyorrhectic and apoptotic cells. B Full thickness diffuse and intense cytoplasmic and nuclear immunoreactivity for p16. C RNA in situ hybridization for high-risk HPV shows punctate dot-like nuclear and cytoplasmic staining
Squamous Cell Carcinoma
The 2022 WHO section on oral squamous cell carcinoma (OSCC) has been updated to reflect current epidemiology, pathogenesis, and histological prognostic factors. The listed subtypes of OSCC are unchanged but both carcinoma cuniculatum and verrucous carcinoma are described in dedicated sections, reflecting that the oral cavity is the most common location in the head and neck for both these entities which have distinct clinical and histologic features from conventional squamous cell carcinoma.
OSCC is the 16th most common cancer worldwide accounting for more than 377,000 per year with males accounting for 70% of cases [38]. There is marked variation in incidence throughout the world with the highest estimated incidence in Melanesia and South-Central Asia [39]. The incidence of tongue cancer in persons < 45 years-of age has increased worldwide, although the etiology is unclear [40]. The etiology of OSCC is primarily tobacco consumption although areca nut use is the primary carcinogen in some geographic regions [41, 42]. To date, high risk HPV while important for oropharyngeal SCC, has not been shown to be a significant etiologic factor in OSCC [43]. Most OSCC arise from pre-existing OED, however malignant transformation of OED remains poorly understood. OSCC has high mutational load and gross chromosomal changes [15, 17, 44]. Somatic mutations in several genes in OSCC have been identified including NOTCH1, KMT2D, CASP8, AJUBA, NSD1, HLA-A, and TGFBR2. With increasing chromosomal instability, the development of OSCC becomes more likely.
Most OSCC are well-differentiated, conventional keratinizing type. Tumor grade alone does not correlate well with prognosis. Adverse histological prognostic factors in OSCC include depth of invasion > 5 mm, tumor budding, perineural invasion, lymphovascular invasion, bone invasion, worst pattern of invasion 5, and stroma-tumor ratio > 50% in a deeply invasive tumor [45–50]. Positive lymph nodes, particularly those lymph nodes with extracapsular extension are also considered adverse prognostic factors.
Verrucous Carcinoma
In past edition verrucous carcinoma (VC) was covered in the Hypopharynx, larynx, trachea, and parapharyngeal space chapter. As the presentation of VC differs in the oral cavity, and accounts for 50–75% of all VC cases in the head and neck, a section devoted to VC was added to Chapter 5 in the new edition. The buccal mucosa, gingiva, and tongue are the most frequent oral cavity sites, presenting as a slow growing exophytic tumor that can erode and destroy bone if left untreated. VC accounts for 2–16% of all OSCC and shows a male predominance presenting in the 6th decade or later [51]. The characteristic histologic features of VC are unchanged and will not be described. VC is associated with a much better prognosis than conventional SCC with an overall 5-year survival rate of 77–86% [51, 52]. In 20% of cases VC may be a precursor to conventional OSCC. The presence of focal dysplasia in VC or invasion ≤ 2 mm in depth do not adversely affect outcome and should be treated as VC [53].
Carcinoma Cuniculatum
Carcinoma cuniculatum (CC), a rare well-differentiated locally destructive SCC with a unique burrowing invasive pattern, now has a dedicated section in Chapter 5. CC is a non-metastasizing SCC, and most are located on the gingiva-alveolar mandible followed by the maxilla and rarely in other oral cavity sites [54]. CC has no gender predilection and most common in the 7th to 8th decades [55]. The histology of CC is characteristic, with a primarily endophytic growth pattern, but a minor exophytic component may be seen [56, 57]. The tumor consists of burrowing interconnecting keratin-filled crypts of well-differentiated SCC with no more than mild cytologic atypia (Fig. 7). Keratin microabscesses and intraepithelial and stromal neutrophils are typically present. Bone sequestrum are indicative of bone invasion [58]. Treatment of CC is complete excision and local recurrence is uncommon.
Fig. 7.
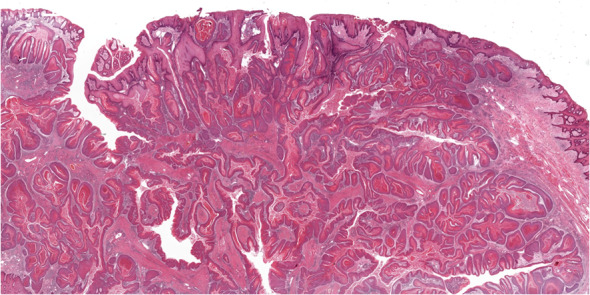
Carcinoma cuniculatum showing the characteristic infiltrative labyrinthine network of keratin-filled crypts. Courtesy of Dr. Amrita Jay
Tumors of Uncertain Histogenesis
This section includes congenital granular cell epulis, granular cell tumor (GCT), ectomesenchymal chondromyxoid tumor (EMCT), and melanotic neuroectodermal tumor of infancy (MNTI).
In the new 2022 WHO edition the pathogenesis of GCT has been updated to reflect the molecular findings associated with the LEOPARD, Noonan syndrome and PTEN hamartoma tumor syndrome which present with multiple GCT [59, 60]. PTPN11 and PTEN mutations are not associated with sporadic GCT [61]. Overexpression of transcription factor E3 (TFE3) is observed in skin and subcutaneous lesions, but not in oral GCTs [62]. ATP6AP1, ATP6AP2 and ATP6V1A, mutations, components of the vacuolar APTase (V-ATPase) complex have been reported in GCT affecting different tissue types [63]. The histogenesis of GCT is still uncertain but a Schwann cell origin is favored. The oral cavity accounts for 70% of GCTs in the head and neck with the tongue being the most common location. The microscopic features are unchanged from the prior edition.
The 2017 WHO introduced EMCT with about 60 cases reported at that time. To date fewer than 100 cases have been reported and the anterior dorsal tongue remains the predominant site of occurrence [64, 65]. Rare occurrences include the palate and mandible. EMCT occurs over a wide age range (7 to 78 years-of age) but is most common in young adults. No gender predilection is seen. The histogenesis of EMCT is unknown but an uncommitted ectomesenchymal progenitor cell is favored. Recent molecular studies show that about 90% of EMCT have RREB1::MKL2 fusions while a minority of EMCT have other molecular drivers, including EWSR1 [66]. This RREB1::MKL2 fusion is not specific to EMCT which has been reported in biphenotypic sinonasal sarcoma, mediastinal mesenchymal neoplasms, and oropharyngeal sarcoma [67]. The histology of EMCT shows diverse morphology of sheets, cords and/or reticular arrangement of spindle-stellate to polygonal cells. The intervening stroma varies including myxoid, hyaline or collagenous features. Most tumors are diffusely GFAP positive. Other immunostains show variable patchy or weak expression in EMCT including S100, actin, desmin, smooth muscle actin, myogenin CD56, CD57, keratin, and EMA [64–66].
MNTI in the 2017 WHO edition was in Chapter 8, Odontogenic and maxillofacial bone tumours. MNTI is not an exclusive tumor of bone, also presenting in the brain, neck, the testis, and epididymis, among other locations. Therefore, MNTI is now in Chapter 5 under Tumours of uncertain histogenesis. The pathogenesis of MNTI is still unknown but germline mutations in CDKN2A and BRAF p.V600E mutations are reported [68, 69]. Of note is that MNTI shares DNA methylation profile with high grade medulloblastoma (MB G3) but does not share in its clinical behavior [70]. MNTI, although rapidly growing and locally destructive has low grade behavior, although about 2% of MNTI behave in a malignant fashion with metastases [71].
Other Changes
The previous edition included various neoplasms in this chapter that are now included elsewhere. Rhabdomyoma, lymphangioma, hemangioma, neural tumors and Kaposi sarcoma are now in the Soft Tissue Tumour chapter. Oral mucosal melanoma now appears in the Melanocytic Tumour chapter. Salivary gland lesions are now included in the Salivary gland chapter and CD30-positive T-cell lymphoproliferative disorder, plasmablastic lymphoma, Langerhans cell histiocytosis, and extramedullary myeloid sarcoma are in the Haematolymphoid proliferations and neoplasia chapter.
Conclusion
Pathology is a dynamic and rapidly evolving field and the fifth chapter of the upcoming fifth edition of the 2022 World Health Organization Classification of Tumours of the Head and Neck titled Tumours of the oral cavity and mobile tongue reflects this. With future discoveries, further WHO blue book updates, modifications, and reclassifications will be required. Stay tuned!
Author Contributions
Both authors contributed to the writing and editing of the submission.
Funding
Not Applicable.
Data Availability
Not Applicable.
Code Availability
Not Applicable.
Declarations
Conflict of interest
Neither author has any conflicts of interest to disclose.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Not Applicable.
Consent for Publication
Not Applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Classification of Tumours Editorial Board. Head and neck tumours (WHO classification of tumours series, 5th ed. vol. 9). Lyon (France): International Agency for Research on Cancer 2022.
- 2.Carlson DL. Necrotizing sialometaplasia: a practical approach to the diagnosis. Arch Pathol Lab Med. 2009;133(5):692–698. doi: 10.5858/133.5.692. [DOI] [PubMed] [Google Scholar]
- 3.Abrams AM, Melrose RJ, Howell FV. Necrotizing sialometaplasia. A disease simulating malignancy. Cancer. 1973;32(1):130–135. doi: 10.1002/1097-0142(197307)32:1<130::aid-cncr2820320118>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Fowler CB, Brannon RB. Subacute necrotizing sialadenitis: report of 7 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(5):600–609. doi: 10.1067/moe.2000.105943. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan I, et al. The clinical, histologic, and treatment spectrum in necrotizing sialometaplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5):577–585. doi: 10.1016/j.oooo.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, et al. An update on Heck's disease-a systematic review. J Public Health (Oxf) 2021. [DOI] [PubMed]
- 7.Fornatora ML, et al. Oral melanoacanthoma: a report of 10 cases, review of the literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol. 2003;25(1):12–15. doi: 10.1097/00000372-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Albuquerque DM, et al. Oral pigmented lesions: a retrospective analysis from Brazil. Med Oral Patol Oral Cir Bucal. 2021;26(3):e284–e291. doi: 10.4317/medoral.24168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantudo-Sanagustín E, et al. Pathogenesis and clinicohistopathological caractheristics of melanoacanthoma: a systematic review. J Clin Exp Dent. 2016;8(3):e327–e336. doi: 10.4317/jced.52860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145(1):45–56. doi: 10.14219/jada.2013.10. [DOI] [PubMed] [Google Scholar]
- 11.Ramos-García P, González-Moles MÁ, Warnakulasuriya S. Oral cancer development in lichen planus and related conditions-3.0 evidence level: a systematic review of systematic reviews. Oral Dis. 2021;27(8):1919–1935. [DOI] [PubMed]
- 12.Muller S. Oral lichenoid lesions: distinguishing the benign from the deadly. Mod Pathol. 2017;30(s1):S54–s67. doi: 10.1038/modpathol.2016.121. [DOI] [PubMed] [Google Scholar]
- 13.Aguirre-Urizar JM, Lafuente-Ibáñez de Mendoza I, Warnakulasuriya S. Malignant transformation of oral leukoplakia: systematic review and meta-analysis of the last 5 years. Oral Dis 2021;27(8):1881–1895. [DOI] [PubMed]
- 14.Warnakulasuriya S, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021;27(8):1862–1880. doi: 10.1111/odi.13704. [DOI] [PubMed] [Google Scholar]
- 15.Odell E, et al. Oral epithelial dysplasia: recognition, grading and clinical significance. Oral Dis. 2021;27(8):1947–1976. doi: 10.1111/odi.13993. [DOI] [PubMed] [Google Scholar]
- 16.Barnes L, Eveson JW, Reichart P, Sidransky D. Pathology and genetics of head and neck tumours. In: Kleihues P, Sobin LH, editors. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 17.Woo SB. Oral epithelial dysplasia and premalignancy. Head Neck Pathol. 2019;13(3):423–439. doi: 10.1007/s12105-019-01020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller S. Oral epithelial dysplasia, atypical verrucous lesions and oral potentially malignant disorders: focus on histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(6):591–602. doi: 10.1016/j.oooo.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Warnakulasuriya S, et al. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008;37(3):127–133. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 20.Nankivell P, et al. The binary oral dysplasia grading system: validity testing and suggested improvement. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(1):87–94. doi: 10.1016/j.oooo.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Thompson LDR, et al. Proliferative verrucous leukoplakia: an expert consensus guideline for standardized assessment and reporting. Head Neck Pathol. 2021;15(2):572–587. doi: 10.1007/s12105-020-01262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mello FW, et al. Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. J Oral Pathol Med. 2018;47(7):633–640. doi: 10.1111/jop.12726. [DOI] [PubMed] [Google Scholar]
- 23.Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr Eval Carcinog Risks Hum 2004;85:1–334. [PMC free article] [PubMed]
- 24.Rao NR, et al. Oral submucous fibrosis: a contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management. J Otolaryngol Head Neck Surg. 2020;49(1):3. doi: 10.1186/s40463-020-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt P, et al. Assessment and correlation between functional and histological staging of oral submucous fibrosis: a clinicohistopathologic study. Natl J Maxillofac Surg. 2019;10(1):27–32. doi: 10.4103/njms.NJMS_15_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Q, et al. Oral submucous fibrosis in Asian countries. J Oral Pathol Med. 2020;49(4):294–304. doi: 10.1111/jop.12924. [DOI] [PubMed] [Google Scholar]
- 27.Shen YW, et al. Oral submucous fibrosis: a review on biomarkers, pathogenic mechanisms, and treatments. Int J Mol Sci 2020;21(19). [DOI] [PMC free article] [PubMed]
- 28.Tilakaratne WM, et al. Oral submucous fibrosis: review on aetiology and pathogenesis. Oral Oncol. 2006;42(6):561–568. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Utsunomiya H, et al. Extracellular matrix remodeling in oral submucous fibrosis: its stage-specific modes revealed by immunohistochemistry and in situ hybridization. J Oral Pathol Med. 2005;34(8):498–507. doi: 10.1111/j.1600-0714.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 30.Ekanayaka RP, Tilakaratne WM. Oral submucous fibrosis: review on mechanisms of malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(2):192–199. doi: 10.1016/j.oooo.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Kujan O, Mello FW, Warnakulasuriya S. Malignant transformation of oral submucous fibrosis: a systematic review and meta-analysis. Oral Dis. 2021;27(8):1936–1946. doi: 10.1111/odi.13727. [DOI] [PubMed] [Google Scholar]
- 32.Lerman MA, et al. HPV-16 in a distinct subset of oral epithelial dysplasia. Mod Pathol. 2017;30(12):1646–1654. doi: 10.1038/modpathol.2017.71. [DOI] [PubMed] [Google Scholar]
- 33.McCord C, et al. Association of high-risk human papillomavirus infection with oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(4):541–549. doi: 10.1016/j.oooo.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Hendawi N, et al. Human papillomavirus (HPV) can establish productive infection in dysplastic oral mucosa, but HPV status is poorly predicted by histological features and p16 expression. Histopathology. 2020;76(4):592–602. doi: 10.1111/his.14019. [DOI] [PubMed] [Google Scholar]
- 35.Alsabbagh A, et al. Surrogate markers for high-risk human papillomavirus infection in oral epithelial dysplasia: a comparison of p16, Ki-67, and ProExC. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;129(3):246–259.e1. doi: 10.1016/j.oooo.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 36.de la Cour CD, et al. Prevalence of human papillomavirus in oral epithelial dysplasia: systematic review and meta-analysis. Head Neck. 2020;42(10):2975–2984. doi: 10.1002/hed.26330. [DOI] [PubMed] [Google Scholar]
- 37.Khanal S, et al. Histologic variation in high grade oral epithelial dysplasia when associated with high-risk human papillomavirus. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(5):566–585. doi: 10.1016/j.oooo.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 39.Kuruvilla J, Nayar KR. Distribution pattern and its correlation for oral cancer rate and human development rank for countries: an ecological approach. Contemp Clin Dent. 2021;12(1):9–13. doi: 10.4103/ccd.ccd_1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng JH, et al. Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck. 2017;39(2):297–304. doi: 10.1002/hed.24589. [DOI] [PubMed] [Google Scholar]
- 41.Asthana S, et al. Association of smokeless tobacco use and oral cancer: a systematic global review and meta-analysis. Nicotine Tob Res. 2019;21(9):1162–1171. doi: 10.1093/ntr/nty074. [DOI] [PubMed] [Google Scholar]
- 42.Gupta B, Johnson NW. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS ONE. 2014;9(11):e113385. doi: 10.1371/journal.pone.0113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zafereo ME, et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol. 2016;56:47–53. doi: 10.1016/j.oraloncology.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Freitas Silva BS, et al. Binary and WHO dysplasia grading systems for the prediction of malignant transformation of oral leukoplakia and erythroplakia: a systematic review and meta-analysis. Clin Oral Investig. 2021;25(7):4329–4340. doi: 10.1007/s00784-021-04008-1. [DOI] [PubMed] [Google Scholar]
- 45.Subramaniam N, et al. Adverse pathologic features in early oral squamous cell carcinoma and the role of postoperative radiotherapy-a review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(1):24–31. doi: 10.1016/j.oooo.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Almangush A, et al. Clinical significance of tumor-stroma ratio in head and neck cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):480. doi: 10.1186/s12885-021-08222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu B, et al. The prognostic role of histologic grade, worst pattern of invasion, and tumor budding in early oral tongue squamous cell carcinoma: a comparative study. Virchows Arch. 2021;479(3):597–606. doi: 10.1007/s00428-021-03063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almangush A, et al. Staging and grading of oral squamous cell carcinoma: an update. Oral Oncol. 2020;107:104799. doi: 10.1016/j.oraloncology.2020.104799. [DOI] [PubMed] [Google Scholar]
- 49.Caldeira PC, et al. Tumor depth of invasion and prognosis of early-stage oral squamous cell carcinoma: a meta-analysis. Oral Dis. 2020;26(7):1357–1365. doi: 10.1111/odi.13194. [DOI] [PubMed] [Google Scholar]
- 50.Almangush A, et al. Tumour budding in oral squamous cell carcinoma: a meta-analysis. Br J Cancer. 2018;118(4):577–586. doi: 10.1038/bjc.2017.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alonso JE, et al. A population-based analysis of verrucous carcinoma of the oral cavity. Laryngoscope. 2018;128(2):393–397. doi: 10.1002/lary.26745. [DOI] [PubMed] [Google Scholar]
- 52.Koch BB, et al. National survey of head and neck verrucous carcinoma: patterns of presentation, care, and outcome. Cancer. 2001;92(1):110–120. doi: 10.1002/1097-0142(20010701)92:1<110::aid-cncr1298>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 53.Patel KR, et al. Verrucous carcinoma with dysplasia or minimal invasion: a variant of verrucous carcinoma with extremely favorable prognosis. Head Neck Pathol. 2015;9(1):65–73. doi: 10.1007/s12105-014-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farag AF, Abou-Alnour DA, Abu-Taleb NS. Oral carcinoma cuniculatum, an unacquainted variant of oral squamous cell carcinoma: a systematic review. Imaging Sci Dent. 2018;48(4):233–244. doi: 10.5624/isd.2018.48.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Datar UV, Kale A, Mane D. Oral carcinoma cuniculatum: a new entity in the clinicopathological spectrum of oral squamous cell carcinoma. J Clin Diagn Res 2017;11(1):ZD37-ZD39. [DOI] [PMC free article] [PubMed]
- 56.Padilla RJ, Murrah VA. Carcinoma cuniculatum of the oral mucosa: a potentially underdiagnosed entity in the absence of clinical correlation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(6):684–693. doi: 10.1016/j.oooo.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Fonseca FP, et al. Oral carcinoma cuniculatum: two cases illustrative of a diagnostic challenge. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(4):457–463. doi: 10.1016/j.oooo.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 58.Zhang C, et al. Oral carcinoma cuniculatum presenting with moth-eaten destruction of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(4):e86–e93. doi: 10.1016/j.oooo.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Schrader KA, et al. Multiple granular cell tumors are an associated feature of LEOPARD syndrome caused by mutation in PTPN11. Clin Genet. 2009;75(2):185–189. doi: 10.1111/j.1399-0004.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 60.Ramaswamy PV, et al. Multiple granular cell tumors in a child with Noonan syndrome. Pediatr Dermatol. 2010;27(2):209–211. doi: 10.1111/j.1525-1470.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- 61.França JA, et al. Sporadic granular cell tumours lack recurrent mutations in PTPN11, PTEN and other cancer-related genes. J Clin Pathol. 2018;71(1):93–94. doi: 10.1136/jclinpath-2017-204849. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, et al. Granular cell tumors overexpress TFE3 without gene rearrangement: evaluation of immunohistochemistry and break-apart FISH in 45 cases. Oncol Lett. 2019;18(6):6355–6360. doi: 10.3892/ol.2019.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pareja F, et al. Loss-of-function mutations in ATP6AP1 and ATP6AP2 in granular cell tumors. Nat Commun. 2018;9(1):3533. doi: 10.1038/s41467-018-05886-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kato MG, et al. Clinical features of ectomesenchymal chondromyxoid tumors: a systematic review of the literature. Oral Oncol. 2017;67:192–197. doi: 10.1016/j.oraloncology.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 65.Truschnegg A, et al. Ectomesenchymal chondromyxoid tumor: a comprehensive updated review of the literature and case report. Int J Oral Sci. 2018;10(1):4. doi: 10.1038/s41368-017-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dickson BC, et al. Ectomesenchymal chondromyxoid tumor: a neoplasm characterized by recurrent RREB1-MKL2 fusions. Am J Surg Pathol. 2018;42(10):1297–1305. doi: 10.1097/PAS.0000000000001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Makise N, et al. Mesenchymal tumors with RREB1-MRTFB fusion involving the mediastinum: extra-glossal ectomesenchymal chondromyxoid tumors? Histopathology 2020. [DOI] [PubMed]
- 68.Gomes CC, et al. BRAFV600E mutation in melanotic neuroectodermal tumor of infancy: toward personalized medicine? Pediatrics. 2015;136(1):e267–e269. doi: 10.1542/peds.2014-3331. [DOI] [PubMed] [Google Scholar]
- 69.Barnes DJ, et al. A germline mutation of CDKN2A and a novel RPLP1-C19MC fusion detected in a rare melanotic neuroectodermal tumor of infancy: a case report. BMC Cancer. 2016;16:629. doi: 10.1186/s12885-016-2669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopez-Nunez O, et al. Melanotic Neuroectodermal Tumor of Infancy (MNTI) and pineal anlage tumor (PAT) harbor a medulloblastoma signature by DNA methylation profiling. Cancers (Basel) 2021;13(4). [DOI] [PMC free article] [PubMed]
- 71.Chrcanovic BR, Gomez RS. Melanotic neuroectodermal tumour of infancy of the jaws: an analysis of diagnostic features and treatment. Int J Oral Maxillofac Surg. 2019;48(1):1–8. doi: 10.1016/j.ijom.2018.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.
Not Applicable.


