Abstract
The World Health Organization Classification of Head and Neck Tumours recently published the 5th edition. There are new entities, emerging entities, and significant updates to the taxonomy and characterization of tumor and tumor-like lesions, specifically in this article as it relates to nasal cavity, paranasal sinuses and skull base. Importantly, the number of diagnostic entries has been reduced by creating category-specific chapters for soft tissue, hematolymphoid, melanocytic, neuroectodermal, and metastatic tumors. Bone and salivary gland tumors are also not separately reported in the sinonasal tract, but included in the jaw and salivary gland sections, respectively. Repetition of characteristic entities in each anatomic site was also reduced, instead highlighting only the unique features in each anatomic site. Two new entities (SWI/SNF complex-deficient sinonasal carcinomas and HPV-related multiphenotypic sinonasal carcinoma) will be highlighted in this review, with a discussion of several emerging entities. There is a short description of updated information for all 24 diagnostic entities included in this edition to allow the reader a snapshot of current state of knowledge, but to encourage more investigation and further broaden understanding of these diverse and rare entities.
Keywords: Nasal cavity, Paranasal sinuses, Paranasal sinus neoplasms, Skull base, Carcinoma, World Health Organization, SWI/SNF complex, Papillomavirus neoplasms, Immunohistochemistry
Introduction
The 2022 5th edition of the World Health Organization (WHO) Classification of Tumours of the Head and Neck, specifically as it relates to the nasal cavity, paranasal sinuses and skull base (Chapter 1, herein after referred to collectively as sinonasal tract [1]), has undergone a significant classification realignment, in keeping with all of the 5th series WHO classification books.
While several of these changes are stylistic, they allow for a more logical development of a hierarchical classification with successive entities in the system viewed as more significant and progressive towards malignant and then higher grades of malignancy, modified to take tumor incidence into account. As such, sinonasal tract hamartomas are followed by sinonasal papillomas, and then carcinomas and adenocarcinomas. A broad category of other tumors includes several unique sinonasal tract mesenchymal entities (sinonasal tract angiofibroma, glomangiopericytoma, biphenotypic sinonasal sarcoma, and chordoma) or tumor types that are considered within the differential diagnosis for other neoplasms (ameloblastoma, adamantinomatous craniopharyngioma, meningioma, olfactory neuroblastoma).
One of the most significant systematic changes is to aggregate tumors which affect all head and neck sites and move them into their own chapter, recognizing the tumors can affect specific sites, but to avoid unnecessary duplication and redundant repetition of epidemiology, pathogenesis and pathology criteria, these entities are all included in a single chapter. Thus, all head and neck soft tissue tumors are included in a soft tissue tumor chapter, with some site-specific exceptions. Similarly, hematolymphoid proliferations and neoplasms are reported in a chapter devoted to these entities, while melanocytic tumors and metastases to head and neck sites are also each included in their own separate chapters. Bone tumors may develop in the sinonasal tract but are included in the odontogenic and maxillofacial bone tumors chapter. Salivary gland-type neoplasms may arise from the minor mucoserous glands of the sinonasal tract, but again, all salivary gland tumors are classified within their own chapter rather than being repeated in each head and neck anatomic site. A new chapter was introduced for all neuroendocrine neoplasms and paraganglioma, taking into consideration the major emphasis towards nomenclature harmonization across all organ systems, using neuroendocrine tumor (grade 1, 2 and 3) and neuroendocrine carcinoma (small cell, large cell, and Merkel cell), with a separate entry for head and neck paragangliomas. Ectopic/invasive pituitary neuroendocrine tumor (PitNET; formerly pituitary adenoma) is reported in the neuroendocrine neoplasms chapter rather than in the sinonasal tract or nasopharynx chapters. Finally, given the complex interplay between head and neck tumors and various genetic tumor syndromes, a new chapter devoted specifically to genetic tumor syndromes that have head and neck manifestations as their major clinical findings was introduced and includes 15 different syndromes.
Five additional changes deserve specific mention, as they represent major improvements in access and in providing gold standards for pathology. The books are hosted as interactive on-line books. Optimized for both desktop and mobile devices, these online versions of the books allow for anytime, anywhere, on-demand access. All diagnostic entity sections contain at least one virtual whole slide image of the category, which allows the user to personally review an expert-vetted case to help reinforce diagnostic criteria. All references are linked to PubMed identification numbers (PMID), which can be clicked to open a new browser window directly to the PubMed.gov website, permitting the reader access to the source material used in classification development. To further aid in snapshot review, essential and desirable diagnostic criteria are included for each diagnostic entity, features considered indispensable in rendering the pathological diagnosis. Finally, this is the first time that a radiologist and a cytopathologist were included as editorial board members, facilitating the incorporation of pertinent imaging findings into the classification as a multidisciplinary approach to meaningful diagnosis and patient management, while the cytology and fine needle aspiration findings were highlighted were applicable to further aid in diagnostic evaluation and triage. These enhancements to the volume significantly contribute to ease of use and transparency of the process.
For the sinonasal tract, there is a very focused coverage of entities unique to the site (i.e., olfactory neuroblastoma) or those that develop anywhere in the head and neck but account for a significant proportion of disease in the sinonasal tract. Obviously, squamous cell carcinoma (SCC) is covered in each major anatomic site, but specific attention to given to the keratinizing and non-keratinizing types along with related tumors in the sinonasal tract (Table 1). Spindle cell (sarcomatoid) squamous cell carcinoma is now covered in the larynx chapter, where the tumor subtype is more frequent. The new entities in this chapter include SWI/SNF complex-deficient sinonasal carcinoma (provisionally included in the 4th edition) and HPV-related multiphenotypic sinonasal carcinoma (provisionally included as HPV-related carcinoma with adenoid cystic-like features in the 4th edition), and will be the focus of this discussion, along with including selected emerging entities to reflect the current state of understanding for these tumors. Further, a brief snapshot of each diagnostic entity is included to highlight updated information.
Table 1.
2022 5th edition of the World Health Organization (WHO) Classification of Tumours of the nasal cavity, paranasal sinuses, and skull base
| Diagnostic Group | Category | Diagnostic Entity Section |
|---|---|---|
| Hamartomas |
Respiratory epithelial adenomatoid hamartoma Seromucinous hamartoma Nasal chondromesenchymal hamartoma |
|
| Respiratory epithelial lesions | ||
| Sinonasal papillomas |
Sinonasal papilloma, inverted type Sinonasal papilloma, oncocytic type Sinonasal papilloma, exophytic type |
|
| Carcinomas |
Keratinizing squamous cell carcinoma Non-keratinizing squamous cell carcinoma NUT carcinoma SWI/SNF complex-deficient sinonasal carcinoma Sinonasal lymphoepithelial carcinoma Sinonasal undifferentiated carcinoma Teratocarcinosarcoma HPV-related multiphenotypic sinonasal carcinoma |
|
| Adenocarcinoma |
Intestinal-type adenocarcinoma of the sinonasal tract Non-intestinal-type sinonasal adenocarcinoma |
|
| Mesenchymal tumors of sinonasal tract |
Sinonasal tract angiofibroma Sinonasal glomangiopericytoma Biphenotypic sinonasal sarcoma Chordoma |
|
| Other tumors |
Sinonasal ameloblastoma Adamantinomatous craniopharyngioma Meningioma of sinonasal tract Olfactory neuroblastoma |
Hamartomas
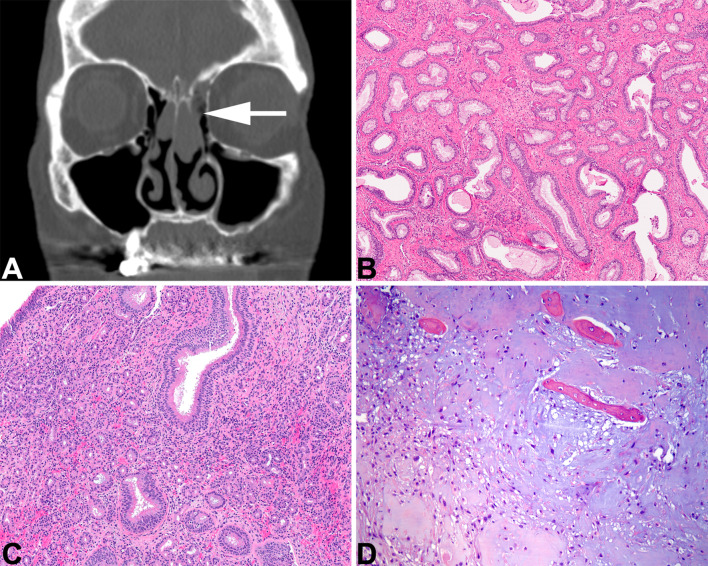
Respiratory epithelial adenomatoid hamartoma (REAH) may represent a neoplasm rather than a hamartoma based on increased fractional allelic loss [2], but clonal studies have not yet documented a clonal expansion [3]. Imaging studies frequently show olfactory cleft expansion without bone erosion [4] (Fig. 1a). Lesions are polypoid benign acquired overgrowths of indigenous glands of the sinonasal tract that arise from the surface epithelium, lacking any ectodermal or mesodermal elements. A serrated hyperplastic, ciliated epithelium is surrounded by a thick, eosinophilic basement membrane which displaces normal elements (Fig. 1b). When cartilaginous or osseous trabecular are admixed, then the lesion is called a chondroosseous and respiratory epithelial (CORE) hamartoma [5]. It is not uncommon to have a combined REAH with seromucinous hamartoma (SH) [6]. However, SH is a benign proliferation of small eosinophilic glands without atypia arising within the sinonasal tract (Fig. 1c). This proliferation resembles microglandular adenosis of the breast, lacking destructive or infiltrative growth, any complex architecture, while also lacking papillae and gland fusion [3, 6]. The cuboidal cells have small nuclei, are noted lining tubules or glands while lacking any significant myoepithelial component [7, 8].
Fig. 1.
Sinonasal hamartomas. A Respiratory epithelial adenomatoid hamartoma. An axial computed tomography shows expanded olfactory cleft (white arrow) with a soft tissue-density mass. B Evenly spaced glandular units have a prominent basement membrane surrounding them. C. Seromucinous hamartoma shows a proliferation of small eosinophilic glands in the stroma, lacking destructive growth. D A chondromesenchymal hamartoma shows cartilaginous nodules with a myxoid stroma and islands of bony tissue (courtesy Dr. D. Baumhoer)
Chondromesenchymal hamartoma remains a distinct lesion, although whether the tumor is a neoplasm is still unresolved. There is a strong association with DICER1 mutations, and as such is considered part of the pleuropulmonary blastoma tumor predisposition syndrome, where pediatric patients comprise the majority of affected individuals [9–11]. A spindled stroma with variably sized nodules of hyaline cartilage (primitive to mature) are often associated with bony trabeculae (Fig. 1d) and mature adipose tissue [10, 12, 13].
Respiratory Epithelial Lesions
Sinonasal Papillomas
Updated information for the inverted, oncocytic, and exophytic types of sinonasal papilloma (SNP, formerly Schneiderian papilloma family) includes imaging findings, epidemiology, etiology, and pathogenesis. An inverted SNP can be suggested when imaging shows lateral nasal cavity origin, associated osteitis, a lobulated shape, with a cerebriform (columnar) pattern on T2 weighted and post-contrast T1 weighted magnetic resonance imaging (MRI). The lack of bone erosion helps to exclude a malignant tumor [10, 14].
Updated RNA in situ hybridization (ISH) shows a consistent lack of high-risk HPV E6/E7 transcripts in inverted SNP, though low-risk HPV transcripts can be seen in a subset of cases [15, 16]. Further work on EGFR profiling shows consistent somatic mutations in about 90% of inverted SNP, and about 80% of carcinomas developing from these tumors [16–18], mutually exclusive from the occasional low-risk HPV infection that can be an alternative oncogenic driver [16, 19]. Malignant progression is associated with TP53 and/or CDKN2A alterations [20].
Oncocytic sinonasal papilloma (OSP) frequently display a high signal on T1 weighted MR, multiple mucinous cystic foci, and generally lack focal osteitis [14]. Importantly, HPV infection is not an etiologic factor but instead hotspot mutations in KRAS have been consistently identified [21, 22], with malignant progression also associated with TP53 and/or CDKN2A alterations [20].
Exophytic SNP frequently harbor low-risk HPV (types 6 and 11) [23, 24], without a well-developed pathogenesis identified yet. While the majority arise on the lower anterior nasal septum [25], malignant transformation is exceptionally rare, although recurrences are common due to incomplete excision [26, 27].
Carcinomas
Keratinizing Squamous Cell Carcinoma
Keratinizing squamous cell carcinoma (KSCC) is histologically identical to any other affected site, arranged in sheets, nests, islands, and single cells, showing a variable degree of keratinization, and separated into well, moderately and poorly differentiated categories. This category is only rarely associated with transcriptionally active high-risk human papillomavirus (HR HPV) [28, 29]. In some cases, KSCC may be associated with a precursor SNP [30].
Non-keratinizing Squamous Cell Carcinoma
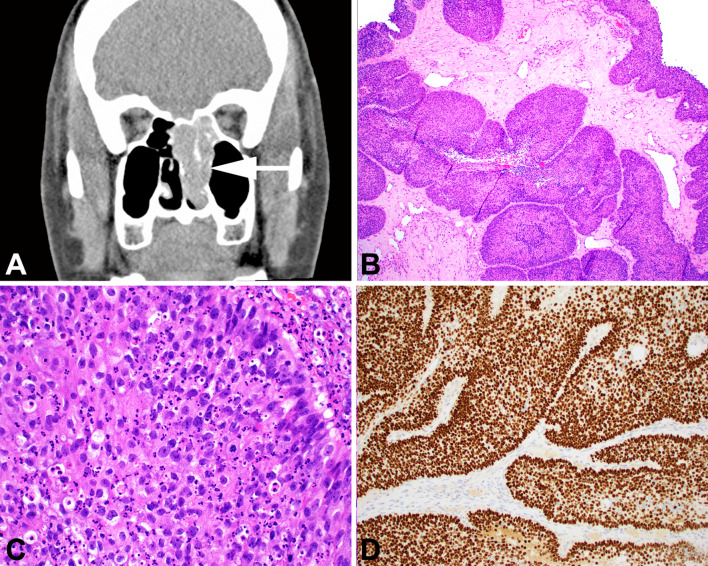
Non-keratinizing squamous cell carcinoma (NKSCC) is a distinctive sinonasal tumor. By imaging, the tumor is a soft tissue density that often erodes bone (Fig. 2a). It is histologically characterized by minimal to no keratinization combined with invasion in the form of expansile nests, lobules, or ribbons with a smooth stromal interface and minimal associated desmoplastic response (Fig. 2b). Papillary architecture, both exophytic and inverted, is also common. At the cellular level these tumors are often more monotonous than pleomorphic, with hyperchromatic round nuclei often with prominent nucleoli (Fig. 2c). NKSCC is diffusely positive for squamous markers such as p40 (Fig. 2d) and CK5/6, an important feature that separates it from morphologic mimics like sinonasal undifferentiated carcinoma (SNUC) and neuroendocrine carcinoma (NEC) which are negative or at most focal for these stains. NKSCC can also closely resemble NUT carcinoma and adamantinoma-like Ewing sarcoma; negative results for NUT, CD99, and NKX2.2 help rule out these possibilities. [31–33].
Fig. 2.
Nonkeratinizing SCC. A A computed tomography scan demonstrates a soft tissue mass involving the nasal cavity and maxillary sinus, with bone erosion. B Tumor invasion is as smooth-edged lobules and ribbons, reminiscent of inverted papilloma. C This case harbored DEK::AFF2. Cases with this fusion typically have nuclear monotony and in infiltrate of neutrophils. D Nonkeratinizing SCC is characteristically positive for p40 in a diffuse pattern
In contrast to KSCC, NKSCC commonly harbors transcriptionally active HR HPV in up to 60% of cases [29, 34–39]. In addition, up to half of NKSCC harbor DEK::AFF2. DEK::AFF2 carcinomas may represent an emerging, distinctive tumor type analogous to NUT carcinoma and others. Carcinomas with this fusion tend to be rich with inflammatory cells, especially neutrophils, and are often deceptively bland; these features, when combined with similar growth patterns, often result in misdiagnoses as inverted SNP [40–42]. Despite its sometimes bland features, DEK::AFF2 carcinoma often behaves in an aggressive manner.
NUT Carcinoma
With improved testing by increased availability of commercial antibodies, NUT carcinoma has been recognized in up to 18% of upper aerodigestive tract poorly differentiated carcinomas [43, 44]. Further case evaluation has identified that NUTM1 gene (chromosome 15q14) is either translocated or fused to an expanded number of partner genes, although BRD4 is identified in the majority of cases, while BRD3, NSD3, ZNF532, ZNF592, and unidentified genes make up the remainder [45–47]. These NUT-fusion oncoproteins act as the single drivers of carcinoma by blocking differentiation and maintaining proliferation [45, 48]. The neoplasm is composed of monotonous evenly spaced sheets of evenly-sized nuclei with vesicular chromatin and prominent nucleoli. Abrupt keratinization is classic, but only seen in about a third of cases [49], while a rich inflammatory infiltrate is common. The monoclonal NUT antibody yielding a nuclear speckled pattern of reactivity is highly specific [33, 50, 51], recognizing epithelial markers (CK-pan, p40, p63) are seen in the majority of cases.
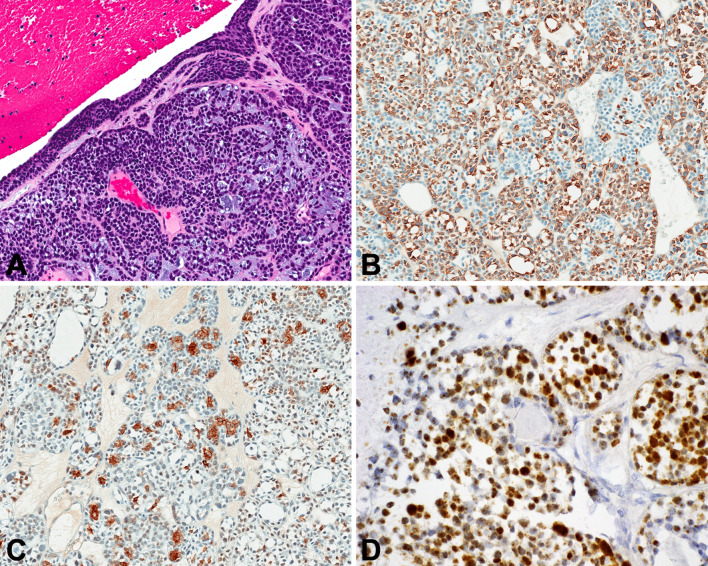
SWI/SNF Complex-Deficient Sinonasal Carcinoma
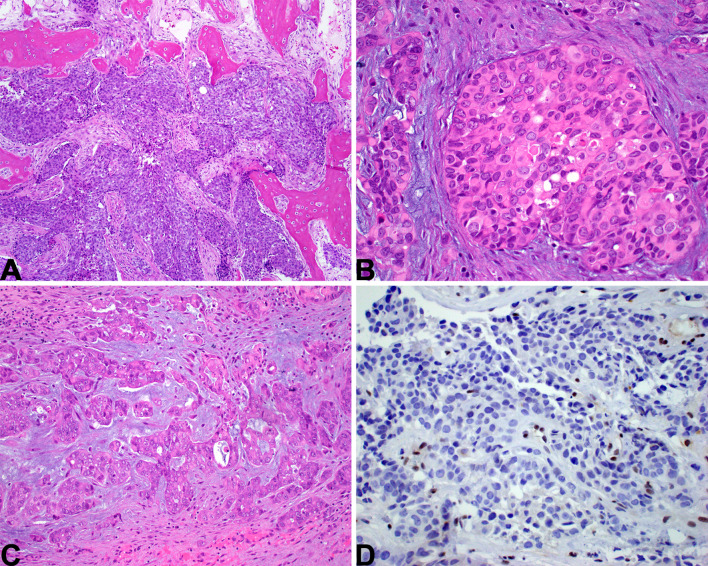
This tumor group is defined by inactivation of one of the SWI/SNF complex genes. By far the most common subtype is SMARCB1-deficient sinonasal carcinoma. Most cases have a basaloid, undifferentiated appearance (Fig. 3a), but about a third are more eosinophilic, with tumor cells showing plasmacytoid cytomorphology (Fig. 3b) [52–54]. While SMARCB1-deficient sinonasal carcinoma usually has an undifferentiated appearance, occasionally they can be overtly gland-forming (Fig. 3c) or even yolk sac-like [55]. By immunohistochemistry, SMARCB1-deficient sinonasal carcinoma is cytokeratin positive and completely SMARCB1 negative (Fig. 3d), but is otherwise quite variable. Gland-forming SMARCB1-deficient sinonasal adenocarcinomas frequently express yolk sac markers (e.g., SALL4, AFP, CDX2). [55].
Fig. 3.
SMARCB1-deficient sinonasal carcinoma. A Most cases have a basaloid low-power appearance. Bone invasion is common. B A minority of cases have a “pink” cell appearance, where the tumor cells are somewhat plasmacytoid, with hyaline-appearing cytoplasm. C Rare cases show glandular or even yolk sac-like differentiation. D A complete loss of SMARCB1 expression by immunohistochemistry defines this tumor. Note the retained staining in lymphocytes, a helpful internal control
SMARCA4-deficient sinonasal carcinoma is much less common. While cases have a similar appearance to SMARCB1-deficient carcinomas, most SMARCA4-deficient sinonasal carcinomas actually more closely resemble high-grade neuroendocrine carcinomas with trabecular and nested architecture and abortive rosettes. SMARCA4 expression is lost by definition, while SMARCB1 is retained. Many cases express neuroendocrine markers focally. Because teratocarcinosarcoma also exhibits neuroendocrine differentiation and may show SMARCA4 loss, some cases may be difficult to categorize into either group, especially on a small biopsy.
SWI/SNF complex-deficient sinonasal carcinomas are highly aggressive neoplasms, with more than 50% of patients dying within 2 years of diagnosis. Mortality may be higher for SMARCA4-deficient cases compared to SMARCB1-deficient carcinomas [56, 57].
Sinonasal Lymphoepithelial Carcinoma
The histologic features of sinonasal lymphoepithelial carcinoma (LEC) are identical to nasopharyngeal carcinoma (NPC), and it is only the sinonasal tract location that aids in separation. Just like NPC, sinonasal LEC are strongly associated with Epstein-Barr virus (EBV) infection, even more so when identified in endemic area patients [58, 59]. The syncytium of large neoplastic cells with vesicular chromatin, associated with a heavy lymphoplasmacytic infiltrate and reactive with EBV-encoded small RNA (EBER) by ISH will help to confirm the diagnosis in the vast majority of cases [60, 61].
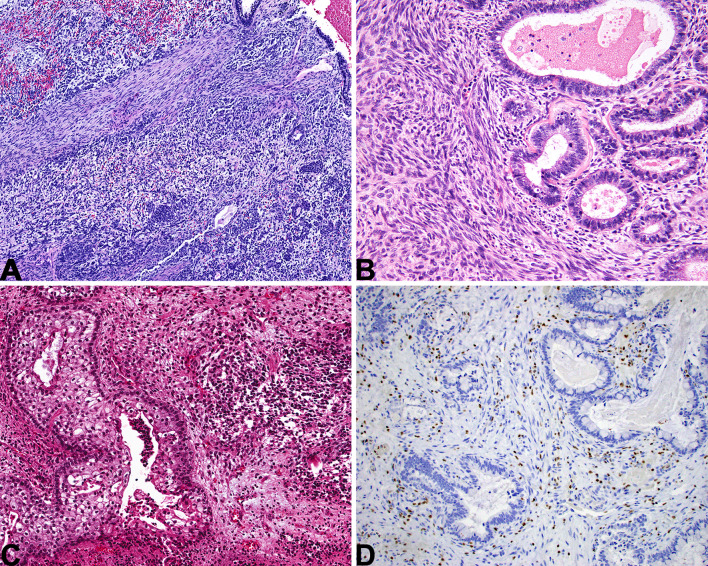
Sinonasal Undifferentiated Carcinoma
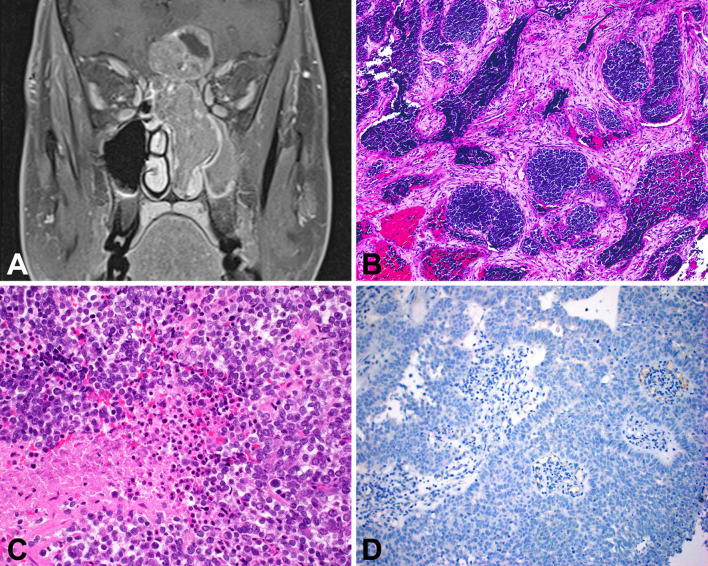
Sinonasal undifferentiated carcinoma is a diagnosis of exclusion, consisting of a high-grade carcinoma that lacks any significant squamous, glandular, or neuroendocrine differentiation by histology and/or immunohistochemistry. Tumors are typically large and locally destructive (Fig. 4a) [62, 63]. Its histologic appearance is nonspecific, consisting of lobules or sheets of basaloid tumor cells with high-grade features but no evidence of differentiation (Fig. 4b–c). SNUC is negative or, at most, focally positive with squamous markers (e.g., p40, CK5/6) (Fig. 4d) and neuroendocrine markers (e.g., synaptophysin, INSM1).
Fig. 4.
Sinonasal undifferentiated carcinoma (SNUC). A SNUC is an aggressive tumor, commonly showing invasion of local structures like the orbit or brain. B A nonspecific, basaloid, nested appearance is typical. C The tumor cells are monotonous with necrosis and a high mitotic rate. D SNUC is usually completely negative for squamous markers like p40
As tumor classification has been refined, this diagnosis is becoming less common, with newly defined entities (e.g., SWI/SNF complex-deficient sinonasal carcinomas) being removed from this group. Recent genetic studies have shown that a significant subset of what remains in the sinonasal undifferentiated carcinoma group harbors IDH2 hotspot mutations, and these tumors appear to have an improved prognosis [64–67]. While these tumors are not easily distinguished from IDH2-wild type sinonasal undifferentiated carcinomas by routine histology or immunohistochemistry, IDH2 mutated sinonasal carcinoma may nevertheless be regarded as a separate tumor entity in future editions.
Teratocarcinosarcoma
A high-grade mixed epithelial, mesenchymal, and primitive neuroectodermal malignancy, teratocarcinosarcoma (TCS) has recently been shown to have recurrent molecular alterations, specifically as biallelic inactivation of SMARCA4 and activating CTNNB1 mutations [68, 69]. The multitude of elements in this tumor result in significant difficulties rendering a diagnosis, especially in limited or crushed/electrocauterized material. It is important to recognize epithelial (squamous and/or glandular) components, whether benign or malignant and sometimes with clear cell change, combined with hypercellular mesenchymal elements, while primitive neuroepithelial cells are seen in sheets and nests (Fig. 5). Many immunohistochemical markers are reactive, each highlighting a specific constituent, potentially resulting in a complex immunoprofile. However, germ cell markers are negative, while a majority of cases will show some degree of SMARCA4 (BRG1) loss [69], with aberrant nuclear ß-catenin in a few cases [68].
Fig. 5.
Teratocarcinosarcoma. A) There is a blend of spindled cells, epithelial elements and primitive cells. B) The mesenchymal spindled cell component is juxtaposed with malignant glandular elements. C) An area of squamoid differentiation shows clear cell change, while the primitive neuroectodermal component has a high nuclear to cytoplasmic ratio. D SMARCA4 is lost as reflected by a negative BRG1 stain. Note retained expression in lymphocytes, a helpful internal control
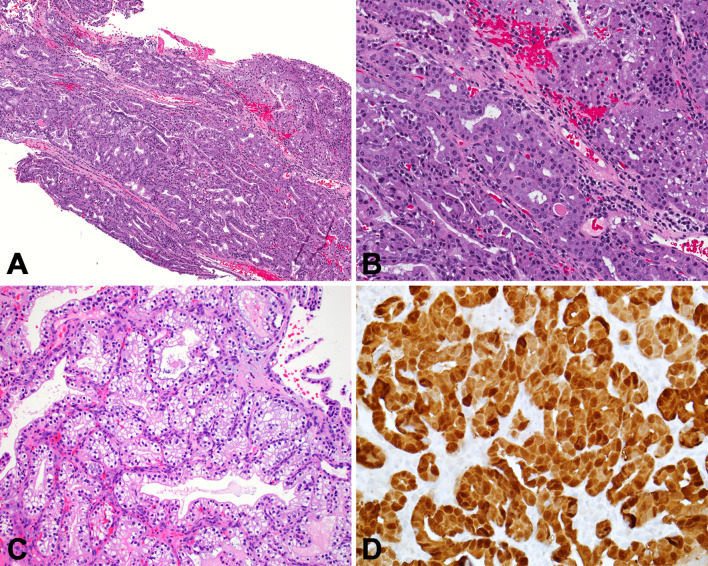
HPV-Related Multiphenotypic Sinonasal Carcinoma
HPV-related multiphenotypic sinonasal carcinoma (HMSC) is a very unique neoplasm that is seemingly restricted to the sinonasal area. Previously known as HPV-related carcinoma with adenoid cystic-like features, HMSC has histologic and immunophenotypic features of both a salivary-type carcinoma (biphasic ductal and myoepithelial differentiation, often in a cribriform arrangement resembling adenoid cystic carcinoma) and a squamous cell carcinoma (frequent squamous cell carcinoma in situ, occasional squamous differentiation within the invasive tumor) [70–72] (Fig. 6). HMSC harbors high-risk HPV, with the rare type 33 being the most common type isolated. HMSC has a favorable prognosis despite often-aggressive appearing histologic features (e.g., necrosis, high mitotic rates). [70, 72].
Fig. 6.
HPV-related multiphenotypic sinonasal carcinoma. A The tumor infiltrates as basaloid nests and strands within a myxoid stroma. Surface epithelial dysplasia is seen overlying the tumor. B Myoepithelial cell markers like SMA are positive in an abluminal pattern. C CD117 highlights tumor ducts which are often subtle on routine microscopy. D The presence of high-risk HPV required for a diagnosis of HPV-related multiphenotypic sinonasal carcinoma. In this case, it was demonstrated by RNA in situ hybridization
Because of the prognotic significance to making an HMSC diagnosis, a sensitive threshold is needed. The main diagnostic considerations are squamous cell carcinoma (which lacks myoepithelial differentiation) and a true salivary-type carcinoma (which does not harbor high-risk HPV). Notably, basaloid squamous cell carcinoma is often positive for SOX10 and occasionally positive for S100 protein; these markers, by themselves, do not reliably establish a salivary-type phenotype [73]. Moreover, while p16 immunohistochemistry is also always positive in HMSC, this marker is not sufficiently specific by itself to make the diagnosis as it is also positive in adenoid cystic carcinoma and other tumors [29, 74].
Adenocarcinoma
Intestinal-Type Adenocarcinomas of Sinonasal Tract
Intestinal-type adenocarcinoma (ITAC) is morphologically and immunophenotypically nearly identical to primary intestinal type adenocarcinomas. Distinction from other glandular-type neoplasms is important as there is a strong etiologic relationship with occupational exposures to wood and leather dusts and because of well recognized aggressive biologic behavior [75–77]. Arranged in various patterns (papillary, tubular, solid), the cells show a cuboidal to columnar appearance, often with nuclear stratification, while a signet-ring pattern is uncommon [78]. Neoplastic cells are typically positive for markers of intestinal differentiation, including cytokeratin 20, CDX2, MUC2 and villin [79, 80]. Because ITAC is essentially identical to intestinal adenocarcinomas, the distinction from a distant metastasis to the sinonasal tract must be made on clinical and radiographic grounds.
The genetic alterations in ITAC are similar to those observed in colorectal adenocarcinoma [81]. TP53 is most frequently mutated (40–50%) [82–84], while APC, KRAS, and BRAF mutations are found in a subset [81, 85–87].
Non-intestinal-Type sinonasal Adenocarcinoma
Non-intestinal-type adenocarcinoma (non-ITAC) is a heterogenous group of tumors that demonstrate glandular differentiation but are otherwise a diagnosis of exclusion. They not only lack intestinal differentiation but also cannot be better classified as any specific salivary gland-type tumor. Non-ITAC is dichotomous, subdivided into low-grade and high-grade tumors. Low-grade non-ITAC is very indolent and defined by seromucinous-type gland with architectural atypia (fused glands, cribriforming, and papillary formations) in spite of very bland cellular features [88–90] (Fig. 7a, b). Renal cell carcinoma-like adenocarcinoma is a low-grade subtype with optically clear cytoplasm resembling renal cell carcinoma [91, 92] (Fig. 7c). In contrast, high-grade non-ITAC is an aggressive tumor that is histologically poorly differentiated, sometimes showing only focal evidence of glandular differentiation [93]. Low-grade non-ITAC tends to show seromucinous gland-like differentiation, with frequent staining with S100 protein (Fig. 7d), SOX10, and DOG1, while high-grade non-ITAC is variable by immunohistochemistry [94].
Fig. 7.
Low-grade non-intestinal-type sinonasal adenocarcinoma. A At low-power, there is a markedly increased number of seromucinous glands within the nasal submucosa. B The tumor glands have minimal cellular atypia, but demonstrate architectural atypia in the form of fusion and cribriforming with no intervening stroma. C A rare variant known as renal cell carcinoma-like adenocarcinoma has water-clear cytoplasm and prominent cell membranes. D S100 protein is usually positive, supporting seromucinous differentiation
Emerging molecular studies suggest that low-grade non-ITAC is heterogeneous with some cases harboring distinctive mutations (e.g., CTNNB1) or fusions (e.g., ETV6::NTRK3) [95, 96]. The molecular underpinnings of high-grade non-ITAC are not well studied.
Mesenchymal Tumors of the Sinonasal Tract
Sinonasal Tract Angiofibroma
The specific site of origin is known to include the posterolateral wall and roof of the nasal cavity along with lateral nasopharynx, and so the tumor name has been more correctly designated as sinonasal tract angiofibroma, moved out of the nasopharynx chapter in this edition. The pathogenesis is defined by somatic mutations in CTNNB1, the ß-catenin encoding gene in the majority of tumors, while nuclear ß-catenin localization is seen immunophenotypically in nearly all cases [97–99]. The tumor is characterized by numerous vessel types and sizes, with variable smooth muscle wall content, stellate stromal fibroblasts and a variably collagenized stroma. Androgen receptor immunoreactivity in the stromal cells is common [98–100].
Sinonasal Glomangiopericytoma
The unique sinonasal tract origin of this tumor has permitted this soft tissue tumor to remain in the sinonasal tract chapter. The tumor shows a perivascular myoid phenotype, with recurrent missense mutations within CTNNB1 exon 3, leading to aberrant nuclear translocation and accumulation of ß-catenin, detected immunohistochemically [101–103]. The tumor is composed of an ovoid to spindled syncytium of myoid-type cells set within a richly vascularized stroma, showing a peritheliomatous hyalinization, extravasated erythrocytes, eosinophils, and mast cells [104]. In addition to SMA or MSA reactivity, CD34 reactivity has been showed to be clone dependent, with absent staining with clone My10 [104, 105].
Biphenotypic Sinonasal Sarcoma
Biphenotypic sinonasal sarcoma (BSNS) was originally described as low-grade sinonasal sarcoma with neural and myogenic features. BSNS characteristically arises in the nasal cavity or ethmoid sinus of middle-aged women (Fig. 8a). It is an infiltrative tumor that often entraps invaginations of surface epithelium and is made up of uniform spindled cells arranged as fascicles, often in a herringbone pattern (Fig. 8b, c). Dilated, hemangiopericytoma-like vessels are common. Tumor nuclei are elongated, wavy, and hypochromatic, with few mitotic figures. Rhabdomyoblasts can occasionally be encountered (Fig. 8d).
Fig. 8.
Biphenotypic sinonasal sarcoma. A The right-sided tumor involves the nasal cavity with bone erosion. B The tumor is infiltrative and often entraps downward invaginations of surface respiratory-type epithelium. C The tumor grows as fascicles of uniform spindle cells with minimal mitotic activity or atypia. Slit-like vessels are common. D In some cases the tumor can show overt rhabdomyoblastic differentiation in the form of strap cells
By immunohistochemistry, BSNS expresses S100 and SMA, although the extent and intensity of the staining varies. EMA, CK-pan, and desmin are variable, and myogenin/MyoD1 highlights rhabdomyoblasts, when present. SOX10 is always negative. Most cases show focal nuclear beta-catenin, and pan-TRK is also often positive. Most cases of BSNS harbor a fusion involving PAX3, most often PAX3::MAML3. PAX3 immunohistochemistry has been reported to be positive in BSNS, but it can be technically challenging to optimize.
Chordoma
This malignant bone tumor recapitulating notochordal differentiation is a neoplasm that affects the skull base and upper mobile spine, and as such was included in the sinonasal tract chapter rather than the nasopharynx chapter, recognizing it may present in either location. The tumors are usually large and destructive, lytic midline masses. The aberrant expression of TBXT is recognized in the pathogenesis of chordomas, with associated brachyury expression by immunohistochemistry. PBRM1 and SETD2 alterations, members of the SWI/SNF complex are frequently identified [106, 107], while homozygous deletion of SMARCB1 with loss of protein expression is seen in poorly differentiated tumors [108]. Lobules of large epithelioid cells with bubbly cytoplasm are suspended in a myxoid or chondroid matrix, separated by fibrous septa. The co-expression of pancytokeratin, EMA, and S100 protein, along with brachyury (TBXT) are considered characteristic [109, 110].
Other Sinonasal Tumors
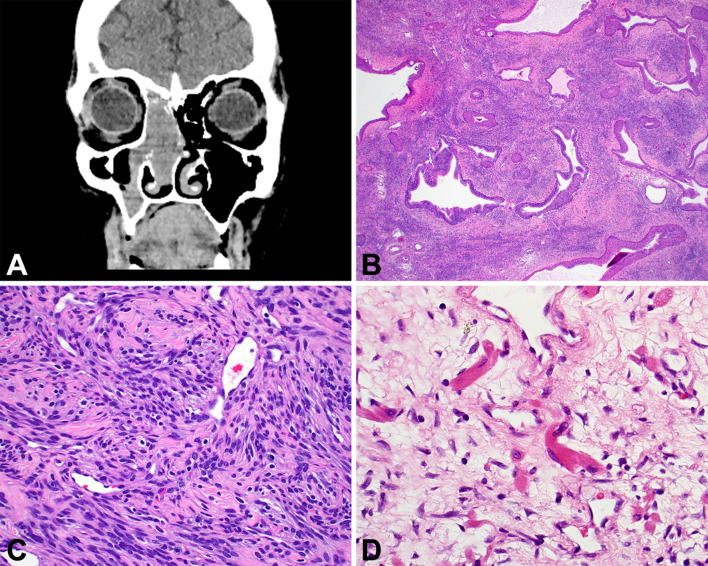
Sinonasal Ameloblastoma
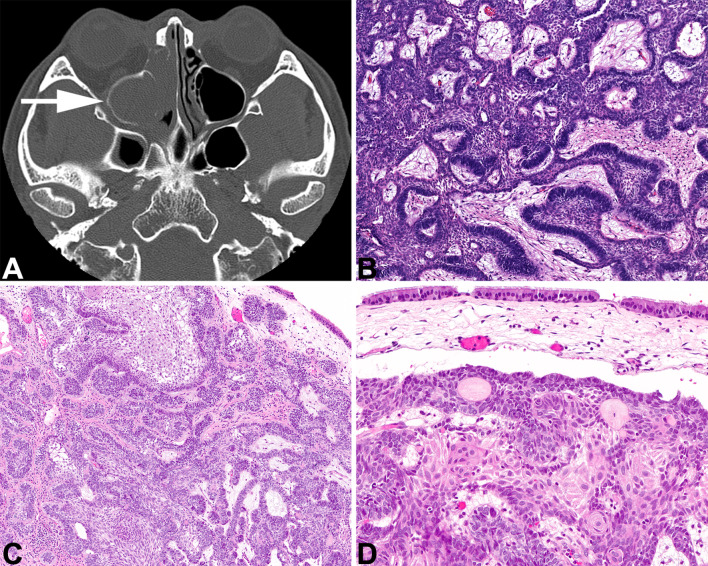
Morphologically indistinguishable from gnathic counterparts, sinonasal tract ameloblastoma must be centered in the sinonasal tract, with imaging showing a solid soft tissue-density mass associated with bone remodeling or destruction of the sinus bony walls (Fig. 9). Tumors develop in older patients than jaw counterparts. BRAF or RAS mutations are detected in jaw lesions along with co-occurring SMO mutations, and are extrapolated to be similar in sinonasal tract tumors [111–113]. A central stellate reticulum is surrounded by a basaloid, reverse polarized columnar ameloblast-like component, showing well developed subnuclear vacuoles (Fig. 9).
Fig. 9.
Sinonasal ameloblastoma. A An axial CT shows an ethmoid sinus mass expanding and filling the sinus with a soft tissue-density mass. B A central stellate reticulum with ameloblastic palisade. C The surface respiratory epithelium overlies the proliferation of ameloblastoma. D An intact, ciliated respiratory-type epithelium is seen overlying a proliferation of columnar, basaloid cells associated with a well-developed central stellate reticulum
Adamantinomatous Craniopharyngioma
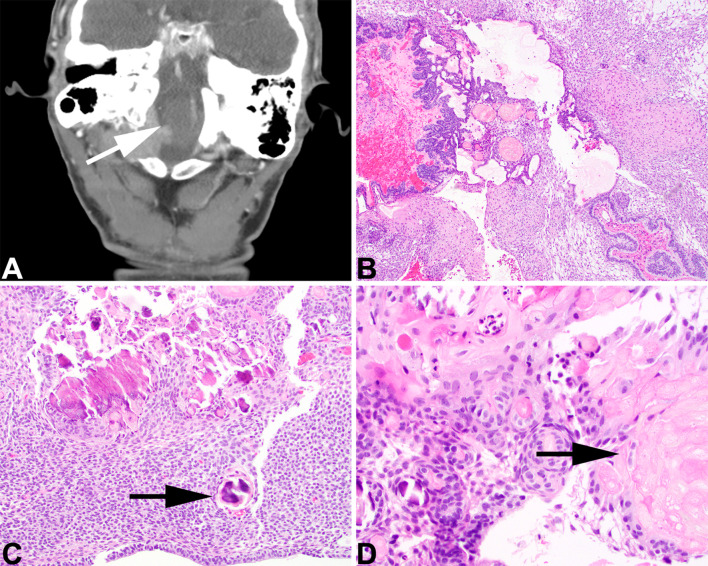
Craniopharyngioma is separated into adamantinomatous and papillary, with the former documented to occasionally exclusively present in the sinonasal tract or nasopharynx. For taxonomic clarity, the tumor was included in the sinonasal tract rather than nasopharynx chapter (previously in 4th edition), since Rathke cleft origin is considered part of the nasopharynx embryologic development and this is an ectopic presentation in the sinonasal tract. The tumor shows a mixed solid and cystic benign squamous epithelium associated with a stellate reticulum and anucleated ghost-like remnants of squamous cells (referred to as “wet keratin”). There is a blending of these elements (Fig. 10), frequently showing calcification and with secondary changes to ruptured cyst content quite common. Tumor cells express p63, CK5/6, CK903, CK7 and SOX9 [114–116]. Nuclear ß-catenin expression is usually spatially restricted to small epithelial whorls, and may be detected even when CTNNB1 mutations are not identified [117–119], although activating CTNNB1 mutations are clonal drivers of most tumors. There are histologic and molecular parallels with odontogenic tumors, hence occasionally teeth will be seen in adamantinomatous craniopharyngioma [120].
Fig. 10.
Adamantinomatous craniopharyngioma. A A destructive skull base mass extends into the nasopharynx (white arrow). B A solid and cystic proliferation of stellate reticulum and anucleated squamous epithelium. C A cellular stellate reticulum with numerous calcifications (black arrow). D Wet keratin is identified as anucleated squamous cell adjacent to the epithelium
Meningioma of the Sinonasal Tract
Imaging studies are crucial in documenting the exact extent, location, and presence or absence of intracranial involvement, as meningiomas may secondarily involve the sinonasal tract much more commonly than arising ectopically [121–123]. Further, as many meningiomas will express somatostatin receptor 2a (SSTR2a) [124–126], imaging studies based on somatostatin receptors (such as 68 Ga-DOTATATE PET-CT) may further aid in tumor detection and potentially provide alternative treatment options [127, 128]. If atypical features are detected (increased mitoses, tumor necrosis, sheet-like growth, pleomorphism), detection of TERT promoter mutations may impact management as there is an associated lower overall survival [129, 130].
Olfactory Neuroblastoma
Given the unique anatomic predilection of this tumor, even though there is neuroendocrine differentiation, olfactory neuroblastoma (ONB) was retained in the sinonasal tract chapter. The derivation from the specialized sensory olfactory neuroepithelium in part dictates involvement of the cribriform plate and/or adjacent structures. As a neuroendocrine tumor, somatostatin receptor expression is seen in most ONBs, which allows for 68Ga-DOTATATE PET-CT to potential aid in documenting disease, recurrence, and/or metastasis [131–134], in addition to radionuclide therapy options. The histologic features of uniform cells with a salt-and-pepper nuclear chromatin arranged in sharply demarcated lobules and nests help to make low grade tumors easily recognizable; but with greater nuclear pleomorphism, lack of neuropil, tumor necrosis, and increased mitoses, high grade tumors become more difficult to recognize. Tumor grading using the Hyams grading system is advocated as there are prognostic outcome differences [135–137]. Rare divergent differentiation (melanin, ganglion cells, rhabdomyoblasts, true glands, clear cells) may hamper recognition of the tumor type. Use of a selected panel of immunohistochemistry studies helps to support the diagnosis and to exclude tumors in the differential diagnosis [134, 138–141].
Conclusions
With two major new entities included in this edition, along with several emerging entities discussed, it is important to appreciate that for the most part, sinonasal tract tumors are still defined and recognized by their histological features, with the addition of selected ancillary tests to narrow the diagnosis for possible differences in treatment and prognostication. Further, it should be realized that classification based on tumor type (soft tissue, hematolymphoid, melanocytic, neuroendocrine) has been introduced to supplement anatomic site for tumors which are known to affect more than one site. It is hoped that this summary spurs the reader to tackle the knowledge gaps and to report new findings such that the next classification update in 5 years will continue to better the understanding of tumor biology and thereby improve treatment of patients because of it.
Funding
No external funding was obtained for this study.
Declarations
Conflict of interest
All authors have contributed to this work and both authors declare they have no conflict of interest as it relates to this study.
Ethical Approval
All evaluations performed in this analysis do not involve any individual patient’s data, but was still performed in accordance with the ethical standards of the institutional review board (IRB #5968) of Southern California Permanente Medical Group. The opinions or assertions contained herein are the private views of the authors.
Consent Statement
No personally identifiable information is included and thus informed consent is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lester D. R. Thompson, Email: Lester.D.Thompson@gmail.com
Justin A. Bishop, Email: Justin.Bishop@UTSouthwestern.edu
References
- 1.Bishop JA, Loney EL, Thompson LDR. Nasal cavity, paranasal sinuses, and skull base tumours. In: Board WCTE, editor. Head and neck tumours. 5. Lyon: International Agency for Research on Cancer; 2022. [Google Scholar]
- 2.Ozolek JA, Hunt JL. Tumor suppressor gene alterations in respiratory epithelial adenomatoid hamartoma (REAH): comparison to sinonasal adenocarcinoma and inflamed sinonasal mucosa. Am J Surg Pathol. 2006;30:1576–1580. doi: 10.1097/01.pas.0000213344.55605.77. [DOI] [PubMed] [Google Scholar]
- 3.Baneckova M, Michal M, Laco J, et al. Immunohistochemical and genetic analysis of respiratory epithelial adenomatoid hamartomas and seromucinous hamartomas: are they precursor lesions to sinonasal low-grade tubulopapillary adenocarcinomas? Hum Pathol. 2020;97:94–102. doi: 10.1016/j.humpath.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Lee JT, Garg R, Brunworth J, Keschner DB, Thompson LD. Sinonasal respiratory epithelial adenomatoid hamartomas: series of 51 cases and literature review. Am J Rhinol Allergy. 2013;27:322–328. doi: 10.2500/ajra.2013.27.3905. [DOI] [PubMed] [Google Scholar]
- 5.Daniel A, Wong E, Ho J, Singh N. Chondro-osseous respiratory epithelial adenomatoid hamartoma (COREAH): case report and literature review. Case Rep Otolaryngol. 2019;2019:5247091. doi: 10.1155/2019/5247091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan RA, Chernock RD, Lewis JS., Jr Seromucinous hamartoma of the nasal cavity: a report of two cases and review of the literature. Head Neck Pathol. 2011;5:241–247. doi: 10.1007/s12105-011-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinreb I, Gnepp DR, Laver NM, et al. Seromucinous hamartomas: a clinicopathological study of a sinonasal glandular lesion lacking myoepithelial cells. Histopathology. 2009;54:205–213. doi: 10.1111/j.1365-2559.2008.03198.x. [DOI] [PubMed] [Google Scholar]
- 8.Fleming KE, Perez-Ordonez B, Nasser JG, Psooy B, Bullock MJ. Sinonasal seromucinous hamartoma: a review of the literature and a case report with focal myoepithelial cells. Head Neck Pathol. 2012;6:395–399. doi: 10.1007/s12105-012-0339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz KA, Yang J, Doros L, et al. DICER1-pleuropulmonary blastoma familial tumor predisposition syndrome: a unique constellation of neoplastic conditions. Pathol Case Rev. 2014;19:90–100. doi: 10.1097/PCR.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason KA, Navaratnam A, Theodorakopoulou E, Chokkalingam PG. Nasal Chondromesenchymal Hamartoma (NCMH): a systematic review of the literature with a new case report. J Otolaryngol Head Neck Surg. 2015;44:28. doi: 10.1186/s40463-015-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz KAP, Williams GM, Kamihara J, et al. DICER1 and associated conditions: identification of at-risk individuals and recommended surveillance strategies. Clin Cancer Res. 2018;24:2251–2261. doi: 10.1158/1078-0432.CCR-17-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozolek JA, Carrau R, Barnes EL, Hunt JL. Nasal chondromesenchymal hamartoma in older children and adults: series and immunohistochemical analysis. Arch Pathol Lab Med. 2005;129:1444–1450. doi: 10.5858/2005-129-1444-NCHIOC. [DOI] [PubMed] [Google Scholar]
- 13.Priest JR, Williams GM, Mize WA, Dehner LP, McDermott MB. Nasal chondromesenchymal hamartoma in children with pleuropulmonary blastoma: a report from the International Pleuropulmonary Blastoma Registry registry. Int J Pediatr Otorhinolaryngol. 2010;74:1240–1244. doi: 10.1016/j.ijporl.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Yang B, Li J, Dong J. MR imaging and CT features of oncocytic papilloma of the sinonasal tract with comparison to inverted papilloma. Br J Radiol. 2018;91:20170957. doi: 10.1259/bjr.20170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooper LM, Bishop JA, Westra WH. Transcriptionally active high-risk human papillomavirus is not a common etiologic agent in the malignant transformation of inverted schneiderian papillomas. Head Neck Pathol. 2017;11:346. doi: 10.1007/s12105-017-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehrad M, Stelow EB, Bishop JA, et al. Transcriptionally active HPV and targetable EGFR mutations in sinonasal inverted papilloma: an association between low-risk HPV, condylomatous morphology, and cancer risk? Am J Surg Pathol. 2020;44:340–346. doi: 10.1097/PAS.0000000000001411. [DOI] [PubMed] [Google Scholar]
- 17.Udager AM, Rolland DCM, McHugh JB, et al. High-frequency targetable EGFR mutations in sinonasal squamous cell carcinomas arising from inverted sinonasal papilloma. Cancer Res. 2015;75:2600–2606. doi: 10.1158/0008-5472.CAN-15-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Li H, Hu L, et al. EGFR and KRAS mutations in Chinese patients with sinonasal inverted papilloma and oncocytic papilloma. Histopathology. 2019;75:274–281. doi: 10.1111/his.13868. [DOI] [PubMed] [Google Scholar]
- 19.Udager AM, McHugh JB, Goudsmit CM, et al. Human papillomavirus (HPV) and somatic EGFR mutations are essential, mutually exclusive oncogenic mechanisms for inverted sinonasal papillomas and associated sinonasal squamous cell carcinomas. Ann Oncol. 2018;29:466–471. doi: 10.1093/annonc/mdx736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown NA, Plouffe KR, Yilmaz O, et al. TP53 mutations and CDKN2A mutations/deletions are highly recurrent molecular alterations in the malignant progression of sinonasal papillomas. Mod Pathol. 2021;34:1133–1142. doi: 10.1038/s41379-020-00716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajalingam K, Schreck R, Rapp UR, Albert S. Ras oncogenes and their downstream targets. Biochim Biophys Acta. 2007;1773:1177–1195. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Maisch S, Mueller SK, Traxdorf M, et al. Sinonasal papillomas: a single centre experience on 137 cases with emphasis on malignant transformation and EGFR/KRAS status in "carcinoma ex papilloma". Ann Diagn Pathol. 2020;46:151504. doi: 10.1016/j.anndiagpath.2020.151504. [DOI] [PubMed] [Google Scholar]
- 23.Buchwald C, Franzmann MB, Jacobsen GK, Lindeberg H. Human papillomavirus (HPV) in sinonasal papillomas: a study of 78 cases using in situ hybridization and polymerase chain reaction. Laryngoscope. 1995;105:66–71. doi: 10.1288/00005537-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Syrjanen K, Syrjanen S. Detection of human papillomavirus in sinonasal papillomas: systematic review and meta-analysis. Laryngoscope. 2013;123:181–192. doi: 10.1002/lary.23688. [DOI] [PubMed] [Google Scholar]
- 25.Glatre R, De Kermadec H, Alsamad IA, et al. Exophytic sinonasal papillomas and nasal florid papillomatosis: a retrospective study. Head Neck. 2018;40:740–746. doi: 10.1002/hed.25042. [DOI] [PubMed] [Google Scholar]
- 26.Mafee MF, Peyman GA, Grisolano JE, et al. Malignant uveal melanoma and simulating lesions: MR imaging evaluation. Radiology. 1986;160:773–780. doi: 10.1148/radiology.160.3.3737917. [DOI] [PubMed] [Google Scholar]
- 27.Blumberg JM, Escobar-Stein J, Vining EM, Prasad ML. Low-grade, nonintestinal nonsalivary sinonasal adenocarcinoma associated with an exophytic schneiderian papilloma: a case report. Int J Surg Pathol. 2015;23:662–666. doi: 10.1177/1066896915599060. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JS, Jr, Westra WH, Thompson LD, et al. The sinonasal tract: another potential "Hot Spot" for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol. 2013;8:241. doi: 10.1007/s12105-013-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8:269–286. doi: 10.1007/s12105-014-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCuiston A, Bishop JA. Usefulness of NKX2.2 immunohistochemistry for distinguishing Ewing sarcoma from other sinonasal small round blue cell tumors. Head Neck Pathol. 2017;12:89–91. doi: 10.1007/s12105-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop JA, Alaggio R, Zhang L, Seethala RR, Antonescu CR. Adamantinoma-like Ewing family tumors of the head and neck: a pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol. 2015;39:1267–1274. doi: 10.1097/PAS.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–991. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larque AB, Hakim S, Ordi J, et al. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod Pathol. 2014;27:343–351. doi: 10.1038/modpathol.2013.155. [DOI] [PubMed] [Google Scholar]
- 35.Laco J, Sieglova K, Vosmikova H, et al. The presence of high-risk human papillomavirus (HPV) E6/E7 mRNA transcripts in a subset of sinonasal carcinomas is evidence of involvement of HPV in its etiopathogenesis. Virchows Arch. 2015;467:405–415. doi: 10.1007/s00428-015-1812-x. [DOI] [PubMed] [Google Scholar]
- 36.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 37.Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115:2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury N, Alvi S, Kimura K, et al. Outcomes of HPV-related nasal squamous cell carcinoma. Laryngoscope. 2017;127:1600–1603. doi: 10.1002/lary.26477. [DOI] [PubMed] [Google Scholar]
- 39.Jiromaru R, Yamamoto H, Yasumatsu R, et al. HPV-related sinonasal carcinoma: clinicopathologic features, diagnostic utility of p16 and Rb immunohistochemistry, and EGFR copy number alteration. Am J Surg Pathol. 2020;44:305–315. doi: 10.1097/PAS.0000000000001410. [DOI] [PubMed] [Google Scholar]
- 40.Bishop JA, Gagan J, Paterson C, McLellan D, Sandison A. Nonkeratinizing squamous cell carcinoma of the sinonasal tract with DEK-AFF2: further solidifying an emerging entity. Am J Surg Pathol. 2021;45:718–720. doi: 10.1097/PAS.0000000000001596. [DOI] [PubMed] [Google Scholar]
- 41.Rooper LM, Agaimy A, Dickson BC, et al. DEK-AFF2 carcinoma of the sinonasal region and skull base: detailed clinicopathologic characterization of a distinctive entity. Am J Surg Pathol. 2021;45:1682–1693. doi: 10.1097/PAS.0000000000001741. [DOI] [PubMed] [Google Scholar]
- 42.Kuo YJ, Lewis JS, Jr, Zhai C, et al. DEK-AFF2 fusion-associated papillary squamous cell carcinoma of the sinonasal tract: clinicopathologic characterization of seven cases with deceptively bland morphology. Mod Pathol. 2021;34:1820–1830. doi: 10.1038/s41379-021-00846-2. [DOI] [PubMed] [Google Scholar]
- 43.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 44.Lee T, Cho J, Baek CH, et al. Prevalence of NUT carcinoma in head and neck: analysis of 362 cases with literature review. Head Neck. 2020;42:924–938. doi: 10.1002/hed.26067. [DOI] [PubMed] [Google Scholar]
- 45.French CA, Rahman S, Walsh EM, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov. 2014;4:928–941. doi: 10.1158/2159-8290.CD-14-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alekseyenko AA, Walsh EM, Zee BM, et al. Ectopic protein interactions within BRD4-chromatin complexes drive oncogenic megadomain formation in NUT midline carcinoma. Proc Natl Acad Sci USA. 2017;114:E4184–E4192. doi: 10.1073/pnas.1702086114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiota H, Elya JE, Alekseyenko AA, et al. "Z4" complex member fusions in NUT carcinoma: implications for a novel oncogenic mechanism. Mol Cancer Res. 2018;16:1826–1833. doi: 10.1158/1541-7786.MCR-18-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang R, Liu W, Helfer CM, et al. Activation of SOX2 expression by BRD4-NUT oncogenic fusion drives neoplastic transformation in NUT midline carcinoma. Cancer Res. 2014;74:3332–3343. doi: 10.1158/0008-5472.CAN-13-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chau NG, Ma C, Danga K, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr. 2020;4:94. doi: 10.1093/jncics/pkz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tilson MP, Bishop JA. Utility of p40 in the differential diagnosis of small round blue cell tumors of the sinonasal tract. Head Neck Pathol. 2014;8:141–145. doi: 10.1007/s12105-013-0496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuda K, Kashima J, Yatabe Y. The isoform matters in NUT carcinoma: a diagnostic pitfall of p40 immunohistochemistry. J Thorac Oncol. 2020;15:e176–e178. doi: 10.1016/j.jtho.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38:1282–1289. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agaimy A, Weichert W. SMARCA4-deficient sinonasal carcinoma. Head Neck Pathol. 2017;11:541–545. doi: 10.1007/s12105-017-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agaimy A, Koch M, Lell M, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol. 2014;38:1274–1281. doi: 10.1097/PAS.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah AA, Jain D, Ababneh E, et al. SMARCB1 (INI-1)-deficient adenocarcinoma of the sinonasal tract: a potentially under-recognized form of sinonasal adenocarcinoma with occasional yolk sac tumor-like features. Head Neck Pathol. 2019;14:465. doi: 10.1007/s12105-019-01065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agaimy A, Hartmann A, Antonescu CR, et al. SMARCB1 (INI-1)-deficient sinonasal carcinoma: a series of 39 cases expanding the morphologic and clinicopathologic spectrum of a recently described entity. Am J Surg Pathol. 2017;41:458–471. doi: 10.1097/PAS.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agaimy A, Jain D, Uddin N, Rooper LM, Bishop JA. SMARCA4-deficient sinonasal carcinoma: a series of 10 cases expanding the genetic spectrum of SWI/SNF-driven sinonasal malignancies. Am J Surg Pathol. 2020;44:703–710. doi: 10.1097/PAS.0000000000001428. [DOI] [PubMed] [Google Scholar]
- 58.Zong Y, Liu K, Zhong B, Chen G, Wu W. Epstein-Barr virus infection of sinonasal lymphoepithelial carcinoma in Guangzhou. Chin Med J (Engl) 2001;114:132–136. [PubMed] [Google Scholar]
- 59.Jeng YM, Sung MT, Fang CL, et al. Sinonasal undifferentiated carcinoma and nasopharyngeal-type undifferentiated carcinoma: two clinically, biologically, and histopathologically distinct entities. Am J Surg Pathol. 2002;26:371–376. doi: 10.1097/00000478-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 60.Wenig BM. Lymphoepithelial-like carcinomas of the head and neck. Semin Diagn Pathol. 2015;32:74–86. doi: 10.1053/j.semdp.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Rytkonen AE, Hirvikoski PP, Salo TA. Lymphoepithelial carcinoma: two case reports and a systematic review of oral and sinonasal cases. Head Neck Pathol. 2011;5:327–334. doi: 10.1007/s12105-011-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiersen DA, Pahilan ME, Devaiah AK. Meta-analysis of treatment outcomes for sinonasal undifferentiated carcinoma. Otolaryngol Head Neck Surg. 2012;147:7–14. doi: 10.1177/0194599812440932. [DOI] [PubMed] [Google Scholar]
- 63.Xu CC, Dziegielewski PT, McGaw WT, Seikaly H. Sinonasal undifferentiated carcinoma (SNUC): the Alberta experience and literature review. J Otolaryngol Head Neck Surg. 2013;42:2. doi: 10.1186/1916-0216-42-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dogan S, Chute DJ, Xu B, et al. Frequent IDH2 R172 mutations in undifferentiated and poorly-differentiated sinonasal carcinomas. J Pathol. 2017;242:400–408. doi: 10.1002/path.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jo VY, Chau NG, Hornick JL, Krane JF, Sholl LM. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol. 2017;30:650–659. doi: 10.1038/modpathol.2016.239. [DOI] [PubMed] [Google Scholar]
- 66.Mito JK, Bishop JA, Sadow PM, et al. Immunohistochemical detection and molecular characterization of IDH-mutant sinonasal undifferentiated carcinomas. Am J Surg Pathol. 2018;42:1067–1075. doi: 10.1097/PAS.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 67.Riobello C, Lopez-Hernandez A, Cabal VN, et al. IDH2 mutation analysis in undifferentiated and poorly differentiated sinonasal carcinomas for diagnosis and clinical management. Am J Surg Pathol. 2020;44:396–405. doi: 10.1097/PAS.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 68.Birkeland AC, Burgin SJ, Yanik M, et al. Pathogenetic analysis of sinonasal teratocarcinosarcomas reveal actionable beta-catenin overexpression and a beta-catenin mutation. J Neurol Surg B Skull Base. 2017;78:346–352. doi: 10.1055/s-0037-1601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rooper LM, Uddin N, Gagan J, et al. Recurrent loss of SMARCA4 in sinonasal teratocarcinosarcoma. Am J Surg Pathol. 2020;44:1331–1339. doi: 10.1097/PAS.0000000000001508. [DOI] [PubMed] [Google Scholar]
- 70.Bishop JA, Andreasen S, Hang JF, et al. HPV-related multiphenotypic sinonasal carcinoma: an expanded series of 49 cases of the tumor formerly known as HPV-related carcinoma with adenoid cystic carcinoma-like features. Am J Surg Pathol. 2017;41:1690–1701. doi: 10.1097/PAS.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bishop JA, Ogawa T, Stelow EB, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37:836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bishop JA, Westra WH. Human papillomavirus-related multiphenotypic sinonasal carcinoma: an emerging tumor type with a unique microscopic appearance and a paradoxical clinical behaviour. Oral Oncol. 2018;87:17–20. doi: 10.1016/j.oraloncology.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Rooper LM, McCuiston AM, Westra WH, Bishop JA. SOX10 immunoexpression in basaloid squamous cell carcinomas: a diagnostic pitfall for ruling out salivary differentiation. Head Neck Pathol. 2018;13:543–547. doi: 10.1007/s12105-018-0990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antony VM, Kakkar A, Sikka K, et al. p16 Immunoexpression in sinonasal and nasopharyngeal adenoid cystic carcinomas: a potential pitfall in ruling out HPV-related multiphenotypic sinonasal carcinoma. Histopathology. 2020;77:989–993. doi: 10.1111/his.14212. [DOI] [PubMed] [Google Scholar]
- 75.Choussy O, Ferron C, Vedrine PO, et al. Adenocarcinoma of ethmoid: a GETTEC retrospective multicenter study of 418 cases. Laryngoscope. 2008;118:437–443. doi: 10.1097/MLG.0b013e31815b48e3. [DOI] [PubMed] [Google Scholar]
- 76.Donhuijsen K, Kollecker I, Petersen P, Gassler N, Wolf J, Schroeder HG. Clinical and morphological aspects of adenocarcinomas of the intestinal type in the inner nose: a retrospective multicenter analysis. Eur Arch Otorhinolaryngol. 2016;273:3207–3213. doi: 10.1007/s00405-016-3987-4. [DOI] [PubMed] [Google Scholar]
- 77.Fiaux-Camous D, Chevret S, Oker N, et al. Prognostic value of the seventh AJCC/UICC TNM classification of intestinal-type ethmoid adenocarcinoma: systematic review and risk prediction model. Head Neck. 2017;39:668–678. doi: 10.1002/hed.24663. [DOI] [PubMed] [Google Scholar]
- 78.Barnes L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1986;10:192–202. doi: 10.1097/00000478-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Franchi A, Massi D, Palomba A, Biancalani M, Santucci M. CDX-2, cytokeratin 7 and cytokeratin 20 immunohistochemical expression in the differential diagnosis of primary adenocarcinomas of the sinonasal tract. Virchows Arch. 2004;445:63–67. doi: 10.1007/s00428-004-1030-4. [DOI] [PubMed] [Google Scholar]
- 80.Kennedy MT, Jordan RC, Berean KW, Perez-Ordonez B. Expression pattern of CK7, CK20, CDX-2, and villin in intestinal-type sinonasal adenocarcinoma. J Clin Pathol. 2004;57:932–937. doi: 10.1136/jcp.2004.016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sjostedt S, Schmidt AY, Vieira FG, et al. Major driver mutations are shared between sinonasal intestinal-type adenocarcinoma and the morphologically identical colorectal adenocarcinoma. J Cancer Res Clin Oncol. 2021;147:1019–1027. doi: 10.1007/s00432-020-03421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perrone F, Oggionni M, Birindelli S, et al. TP53, p14ARF, p16INK4a and H-ras gene molecular analysis in intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Int J Cancer. 2003;105:196–203. doi: 10.1002/ijc.11062. [DOI] [PubMed] [Google Scholar]
- 83.Holmila R, Bornholdt J, Heikkila P, et al. Mutations in TP53 tumor suppressor gene in wood dust-related sinonasal cancer. Int J Cancer. 2010;127:578–588. doi: 10.1002/ijc.25064. [DOI] [PubMed] [Google Scholar]
- 84.Perez-Escuredo J, Martinez JG, Vivanco B, et al. Wood dust-related mutational profile of TP53 in intestinal-type sinonasal adenocarcinoma. Hum Pathol. 2012;43:1894–1901. doi: 10.1016/j.humpath.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 85.Garcia-Inclan C, Lopez F, Perez-Escuredo J, et al. EGFR status and KRAS/BRAF mutations in intestinal-type sinonasal adenocarcinomas. Cell Oncol (Dordr) 2012;35:443–450. doi: 10.1007/s13402-012-0103-7. [DOI] [PubMed] [Google Scholar]
- 86.Lopez F, Garcia Inclan C, Perez-Escuredo J, et al. KRAS and BRAF mutations in sinonasal cancer. Oral Oncol. 2012;48:692–697. doi: 10.1016/j.oraloncology.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 87.Franchi A, Innocenti DR, Palomba A, et al. Low prevalence of K-RAS, EGF-R and BRAF mutations in sinonasal adenocarcinomas. Implications for anti-EGFR treatments. Pathol Oncol Res. 2014;20:571–579. doi: 10.1007/s12253-013-9730-1. [DOI] [PubMed] [Google Scholar]
- 88.Heffner DK, Hyams VJ, Hauck KW, Lingeman C. Low-grade adenocarcinoma of the nasal cavity and paranasal sinuses. Cancer. 1982;50:312–322. doi: 10.1002/1097-0142(19820715)50:2<312::aid-cncr2820500225>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 89.Neto AG, Pineda-Daboin K, Luna MA. Sinonasal tract seromucous adenocarcinomas: a report of 12 cases. Ann Diagn Pathol. 2003;7:154–159. doi: 10.1016/s1092-9134(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 90.Skalova A, Cardesa A, Leivo I, et al. Sinonasal tubulopapillary low-grade adenocarcinoma. Histopathological, immunohistochemical and ultrastructural features of poorly recognised entity. Virchows Arch. 2003;443:152–158. doi: 10.1007/s00428-003-0844-9. [DOI] [PubMed] [Google Scholar]
- 91.Storck K, Hadi UM, Simpson R, Ramer M, Brandwein-Gensler M. Sinonasal renal cell-like adenocarcinoma: a report on four patients. Head Neck Pathol. 2008;2:75–80. doi: 10.1007/s12105-008-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen T, Shi Q, Velosa C, et al. Sinonasal renal cell-like adenocarcinomas: robust carbonic anhydrase expression. Hum Pathol. 2015;46:1598–1606. doi: 10.1016/j.humpath.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 93.Stelow EB, Jo VY, Mills SE, Carlson DL. A histologic and immunohistochemical study describing the diversity of tumors classified as sinonasal high-grade nonintestinal adenocarcinomas. Am J Surg Pathol. 2011;35:971–980. doi: 10.1097/PAS.0b013e31821cbd72. [DOI] [PubMed] [Google Scholar]
- 94.Purgina B, Bastaki JM, Duvvuri U, Seethala RR. A subset of sinonasal non-intestinal type adenocarcinomas are truly seromucinous adenocarcinomas: a morphologic and immunophenotypic assessment and description of a novel pitfall. Head Neck Pathol. 2015;9:436–446. doi: 10.1007/s12105-015-0615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Andreasen S, Skalova A, Agaimy A, et al. ETV6 gene rearrangements characterize a morphologically distinct subset of sinonasal low-grade non-intestinal-type adenocarcinoma: a novel translocation-associated carcinoma restricted to the sinonasal tract. Am J Surg Pathol. 2017;41:1552–1560. doi: 10.1097/PAS.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 96.Villatoro TM, Mardekian SK. Two cases of sinonasal non-intestinal-type adenocarcinoma with squamoid morules expressing nuclear beta-catenin and CDX2: a curious morphologic finding supported by molecular analysis. Case Rep Pathol. 2018;2018:8741017. doi: 10.1155/2018/8741017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abraham SC, Montgomery EA, Giardiello FM, Wu TT. Frequent beta-catenin mutations in juvenile nasopharyngeal angiofibromas. Am J Pathol. 2001;158:1073–1078. doi: 10.1016/s0002-9440(10)64054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sanchez-Romero C, Carlos R, Diaz Molina JP, Thompson LDR, de Almeida OP, Rumayor PA. Nasopharyngeal angiofibroma: a clinical, histopathological and immunohistochemical study of 42 cases with emphasis on stromal features. Head Neck Pathol. 2018;12:52–61. doi: 10.1007/s12105-017-0824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carlos R, Thompson LD, Netto AC, et al. Epstein-Barr virus and human herpes virus-8 are not associated with juvenile nasopharyngeal angiofibroma. Head Neck Pathol. 2008;2:145–149. doi: 10.1007/s12105-008-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang HC, Mills SE, Patterson K, Gown AM. Expression of androgen receptors in nasopharyngeal angiofibroma: an immunohistochemical study of 24 cases. Mod Pathol. 1998;11:1122–1126. [PubMed] [Google Scholar]
- 101.Lasota J, Felisiak-Golabek A, Aly FZ, Wang ZF, Thompson LD, Miettinen M. Nuclear expression and gain-of-function beta-catenin mutation in glomangiopericytoma (sinonasal-type hemangiopericytoma): insight into pathogenesis and a diagnostic marker. Mod Pathol. 2015;28:715–720. doi: 10.1038/modpathol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haller F, Bieg M, Moskalev EA, et al. Recurrent mutations within the amino-terminal region of beta-catenin are probable key molecular driver events in sinonasal hemangiopericytoma. Am J Pathol. 2015;185:563–571. doi: 10.1016/j.ajpath.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki Y, Ichihara S, Kawasaki T, et al. beta-catenin (CTNNB1) mutation and LEF1 expression in sinonasal glomangiopericytoma (sinonasal-type hemangiopericytoma) Virchows Arch. 2018;473:235–239. doi: 10.1007/s00428-018-2370-9. [DOI] [PubMed] [Google Scholar]
- 104.Thompson LD, Miettinen M, Wenig BM. Sinonasal-type hemangiopericytoma: a clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Am J Surg Pathol. 2003;27:737–749. doi: 10.1097/00000478-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 105.Sangoi AR, Bishop JA. Variability of CD34 expression in sinonasal glomangiopericytoma: a potential diagnostic pitfall. Head Neck Pathol. 2020;14:459–464. doi: 10.1007/s12105-019-01063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cottone L, Eden N, Usher I, et al. Frequent alterations in p16/CDKN2A identified by immunohistochemistry and FISH in chordoma. J Pathol Clin Res. 2020;6:113–123. doi: 10.1002/cjp2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bai J, Shi J, Li C, et al. Whole genome sequencing of skull-base chordoma reveals genomic alterations associated with recurrence and chordoma-specific survival. Nat Commun. 2021;12:757. doi: 10.1038/s41467-021-21026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hasselblatt M, Thomas C, Hovestadt V, et al. Poorly differentiated chordoma with SMARCB1/INI1 loss: a distinct molecular entity with dismal prognosis. Acta Neuropathol. 2016;132:149–151. doi: 10.1007/s00401-016-1574-9. [DOI] [PubMed] [Google Scholar]
- 109.Vujovic S, Henderson S, Presneau N, et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209:157–165. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- 110.Jo VY, Hornick JL, Qian X. Utility of brachyury in distinction of chordoma from cytomorphologic mimics in fine-needle aspiration and core needle biopsy. Diagn Cytopathol. 2014;42:647–652. doi: 10.1002/dc.23100. [DOI] [PubMed] [Google Scholar]
- 111.Schafer DR, Thompson LD, Smith BC, Wenig BM. Primary ameloblastoma of the sinonasal tract: a clinicopathologic study of 24 cases. Cancer. 1998;82:667–674. doi: 10.1002/(sici)1097-0142(19980215)82:4<667::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 112.Kurppa KJ, Caton J, Morgan PR, et al. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol. 2014;232:492–498. doi: 10.1002/path.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brown NA, Rolland D, McHugh JB, et al. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014;20:5517–5526. doi: 10.1158/1078-0432.CCR-14-1069. [DOI] [PubMed] [Google Scholar]
- 114.Momota H, Ichimiya S, Ikeda T, et al. Immunohistochemical analysis of the p53 family members in human craniopharyngiomas. Brain Tumor Pathol. 2003;20:73–77. doi: 10.1007/BF02483450. [DOI] [PubMed] [Google Scholar]
- 115.Xin W, Rubin MA, McKeever PE. Differential expression of cytokeratins 8 and 20 distinguishes craniopharyngioma from rathke cleft cyst. Arch Pathol Lab Med. 2002;126:1174–1178. doi: 10.5858/2002-126-1174-DEOCAD. [DOI] [PubMed] [Google Scholar]
- 116.Thimsen V, John N, Buchfelder M, et al. Expression of SRY-related HMG Box Transcription Factors (Sox) 2 and 9 in craniopharyngioma subtypes and surrounding brain tissue. Sci Rep. 2017;7:15856. doi: 10.1038/s41598-017-15977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buslei R, Holsken A, Hofmann B, et al. Nuclear beta-catenin accumulation associates with epithelial morphogenesis in craniopharyngiomas. Acta Neuropathol. 2007;113:585–590. doi: 10.1007/s00401-006-0184-3. [DOI] [PubMed] [Google Scholar]
- 118.Goschzik T, Gessi M, Dreschmann V, et al. Genomic alterations of adamantinomatous and papillary craniopharyngioma. J Neuropathol Exp Neurol. 2017;76:126–134. doi: 10.1093/jnen/nlw116. [DOI] [PubMed] [Google Scholar]
- 119.Apps JR, Stache C, Gonzalez-Meljem JM, et al. CTNNB1 mutations are clonal in adamantinomatous craniopharyngioma. Neuropathol Appl Neurobiol. 2020;46:510–514. doi: 10.1111/nan.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beaty NB, Ahn E. Images in clinical medicine. Adamantinomatous craniopharyngioma containing teeth. N Engl J Med. 2014;370:860. doi: 10.1056/NEJMicm1308260. [DOI] [PubMed] [Google Scholar]
- 121.Thompson LD, Gyure KA. Extracranial sinonasal tract meningiomas: a clinicopathologic study of 30 cases with a review of the literature. Am J Surg Pathol. 2000;24:640–650. doi: 10.1097/00000478-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 122.Rushing EJ, Bouffard JP, McCall S, et al. Primary extracranial meningiomas: an analysis of 146 cases. Head Neck Pathol. 2009;3:116–130. doi: 10.1007/s12105-009-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Y, Wang H, Shao H, Wang C. Primary extradural meningiomas in head: a report of 19 cases and review of literature. Int J Clin Exp Pathol. 2015;8:5624–5632. [PMC free article] [PubMed] [Google Scholar]
- 124.Menke JR, Raleigh DR, Gown AM, Thomas S, Perry A, Tihan T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. 2015;130:441–443. doi: 10.1007/s00401-015-1459-3. [DOI] [PubMed] [Google Scholar]
- 125.Silva CB, Ongaratti BR, Trott G, et al. Expression of somatostatin receptors (SSTR1-SSTR5) in meningiomas and its clinicopathological significance. Int J Clin Exp Pathol. 2015;8:13185–13192. [PMC free article] [PubMed] [Google Scholar]
- 126.Wu W, Zhou Y, Wang Y, et al. Clinical significance of somatostatin receptor (SSTR) 2 in meningioma. Front Oncol. 2020;10:1633. doi: 10.3389/fonc.2020.01633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cleary JO, Yeung J, McMeekin H, Wilhelm T, Wagner T. The significance of incidental brain uptake on 68Ga-DOTATATE PET-CT in neuroendocrine tumour patients. Nucl Med Commun. 2016;37:1197–1205. doi: 10.1097/MNM.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 128.Parghane RV, Talole S, Basu S. Prevalence of hitherto unknown brain meningioma detected on (68)Ga-DOTATATE positron-emission tomography/computed tomography in patients with metastatic neuroendocrine tumor and exploring potential of (177)Lu-DOTATATE peptide receptor radionuclide therapy as single-shot treatment approach targeting both tumors. World J Nucl Med. 2019;18:160–170. doi: 10.4103/wjnm.WJNM_39_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Juratli TA, Thiede C, Koerner MVA, et al. Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas. Oncotarget. 2017;8:109228–109237. doi: 10.18632/oncotarget.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mirian C, Duun-Henriksen AK, Juratli T, et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: an individual patient data meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91:378–387. doi: 10.1136/jnnp-2019-322257. [DOI] [PubMed] [Google Scholar]
- 131.Rostomily RC, Elias M, Deng M, et al. Clinical utility of somatostatin receptor scintigraphic imaging (octreoscan) in esthesioneuroblastoma: a case study and survey of somatostatin receptor subtype expression. Head Neck. 2006;28:305–312. doi: 10.1002/hed.20356. [DOI] [PubMed] [Google Scholar]
- 132.Hasan OK, Ravi Kumar AS, Kong G, et al. Efficacy of peptide receptor radionuclide therapy for esthesioneuroblastoma. J Nucl Med. 2020;61:1326–1330. doi: 10.2967/jnumed.119.237990. [DOI] [PubMed] [Google Scholar]
- 133.Gains JE, Aldridge MD, Mattoli MV, et al. 68Ga-DOTATATE and 123I-mIBG as imaging biomarkers of disease localisation in metastatic neuroblastoma: implications for molecular radiotherapy. Nucl Med Commun. 2020;41:1169–1177. doi: 10.1097/MNM.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 134.Cracolici V, Wang EW, Gardner PA, et al. SSTR2 expression in olfactory neuroblastoma: Clin. Head Neck Pathol. 2021;15:1185–1191. doi: 10.1007/s12105-021-01329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gallagher KK, Spector ME, Pepper JP, McKean EL, Marentette LJ, McHugh JB. Esthesioneuroblastoma: updating histologic grading as it relates to prognosis. Ann Otol Rhinol Laryngol. 2014;123:353–358. doi: 10.1177/0003489414526368. [DOI] [PubMed] [Google Scholar]
- 136.Tajudeen BA, Arshi A, Suh JD, St John M, Wang MB. Importance of tumor grade in esthesioneuroblastoma survival: a population-based analysis. JAMA Otolaryngol Head Neck Surg. 2014;140:1124–1129. doi: 10.1001/jamaoto.2014.2541. [DOI] [PubMed] [Google Scholar]
- 137.Saade RE, Hanna EY, Bell D. Prognosis and biology in esthesioneuroblastoma: the emerging role of Hyams grading system. Curr Oncol Rep. 2015;17:423. doi: 10.1007/s11912-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 138.Thompson LD. Olfactory neuroblastoma. Head Neck Pathol. 2009;3:252–259. doi: 10.1007/s12105-009-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121:1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bishop JA, Thompson LD, Cardesa A, et al. Rhabdomyoblastic differentiation in head and neck malignancies other than rhabdomyosarcoma. Head Neck Pathol. 2015;9:507–518. doi: 10.1007/s12105-015-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thompson LD. Small round blue cell tumors of the sinonasal tract: a differential diagnosis approach. Mod Pathol. 2017;30:S1–S26. doi: 10.1038/modpathol.2016.119. [DOI] [PubMed] [Google Scholar]