Abstract
Smooth muscle neoplasms represent an important group of lesions which is rare in the oral cavity. Leiomyoma (LM) is benign smooth muscle/pericytic tumor usually presenting as non-aggressive neoplasm, while leiomyosarcoma (LMS) represents its malignant counterpart. The rarity of these lesions, together with its unspecific clinical presentation and a variable histopathological appearance, lead to a broad list of differential diagnoses, hampering their diagnoses. Therefore, in this study we describe the clinical and microscopic features of a series of oral and maxillofacial LMs and LMSs. A retrospective search from 2000 to 2019 was performed and all cases diagnosed as LM and LMS affecting the oral cavity and gnathic bones were retrieved. Clinical and demographic data were obtained from the patients’ pathology records, while microscopic features and immunohistochemistry were reviewed and completed when necessary to confirm the diagnoses. Twenty-two LMs and five LMSs were obtained. In the LM group, males predominated, with a mean age of 45.7 years. The upper lip was the most affected site, and 18 cases were classified as angioleiomyomas and four as solid LM. In the LMS group, females predominated, with a mean age of 47.6 years. The mandible was the most affected site. Diffuse proliferation of spindle cells, with necrosis and mitotic figures, were frequent microscopic findings. LMs and LMSs were positive for α-smooth muscle actin, HHF-35 and h-caldesmon. In conclusion, oral LM/LMS are uncommon neoplasms with the latter usually presenting as metastatic disease. H&E evaluation may be very suggestive of oral LMs, but h-caldesmon staining is strongly recommended to confirm LMS diagnosis.
Keywords: Angioleiomyoma, Leiomyoma, Leiomyosarcomas, Smooth muscle tumor, Oral cavity, Jaw
Introduction
Soft tissue tumors are relatively uncommon in the head and neck, and sarcomas in this region represent only 5–10% of all cases [1]. Among soft tissue tumors, smooth muscle neoplasms represent an important group of lesions, which mostly affect the endometrium, the gastrointestinal tract, skin and subcutaneous tissues. The occurrence of smooth muscle/pericytic tumors in the oral cavity is very rare, probably due to the scarcity of such components in this anatomic region [2, 3], where these tumors are hypothesized to develop from the muscular walls of larger blood vessels or from the circumvallate papillae of the tongue [4, 5].
Leiomyomas (LMs) are benign smooth muscle/pericytic tumors that may be diagnosed in the oral cavity and lips usually as non-aggressive neoplasms [6, 7]. Although there are several case series describing oral LMs, Silva et al. [8] demonstrated that this benign tumor accounted for only 0.9% of 790 oral soft tissue neoplasms in a Brazilian study. Therefore, the rarity of this lesion, together with its unspecific clinical presentation and a variable histopathological appearance, may lead to a broad list of differential diagnoses [9–11].
Even rarer than oral LM is its malignant counterpart. Leiomyosarcoma (LMS) represents 5 to 10% of all soft tissue sarcomas, more commonly diagnosed in the uterus, retroperitoneum and intra-abdominal structures [2, 12–14]. De Carvalho et al. [15] recently described the distribution of oral sarcomas in a multi-institutional study from Brazil demonstrating that LMS accounted for 6% of the sample, whereas Moreira et al. [16] found only one oral LMS among 69 head and neck sarcomas also in Brazil. Moreover, although some oral and maxillofacial LMSs have been described in literature, many of these reports lack an appropriate diagnostic documentation and the incidence of oral LMSs might be even lower.
Therefore, in this study we aimed to describe the clinical and microscopic characteristics of a series of 22 LMs and five LMSs affecting the oral and maxillofacial region.
Material and Methods
All cases diagnosed as LM and LMS affecting the oral and maxillofacial region were retrieved from six different oral diagnosis services: The Universidade Federal de Minas Gerais (Belo Horizonte/Brazil), the University of Campinas (Piracicaba/Brazil), the Federal University of Rio de Janeiro (Rio de Janeiro/Brazil), the Federal University of Pará (João de Barros Barreto University Hospital) (Belém/Brazil), the Dental Oncology Service of the Instituto do Câncer do Estado de São Paulo (São Paulo/Brazil) and from the School of Dentistry of the National University of Córdoba (Córdoba/Argentina), during a period ranging from January/2000 to December/2019.
The formalin-fixed, paraffin-embedded tissue blocks were obtained and the original or new 3 µm thick, H&E-stained slides were reviewed by an oral pathologist. Demographic data and clinical features of all cases were obtained from the patients’ records and comprised age and sex of patients, lesion size and location, tumor color, time of duration (months),symptomatology, and treatment modality employed. Follow-up data included information regarding recurrences and patients’ status (alive or dead) at last follow-up for those affected by LMS.
Immunohistochemistry was done using the streptavidin–biotin peroxidase complex method to confirm the diagnoses. Briefly, the reactions were done in 3 µm sections obtained from the original formalin-fixed, paraffin-embedded tissue blocks that were de-waxed with xylene and hydrated in an ethanol series. The antigen retrieval was done and the endogenous peroxidase activity was blocked using 10% hydrogen peroxide in five baths, 5 min each. After washing in PBS buffer (pH 7.4), slides were incubated overnight with the following primary antibodies: monoclonal mouse anti-α-SMA diluted 1:200 (Clone 1A4; dilution 1:200; Dako Corp., Carpenteria, CA, USA), muscle actin (clone HHF35; dilution 1:400; Dako Corp., Carpenteria, CA, USA), h-caldesmon (clone h-CALD; dilution 1:100; Medaysis) and Ki67 (clone MIB-1, dilution 1:100; Dako Corp., Carpenteria, CA, USA). Moreover, S100 protein (clone S100; dilution 1:500; Dako Corp., Carpenteria, CA, USA), desmin (clone D33; dilution 1:100; Dako Corp., Carpenteria, CA, USA), CD34 (clone QBEnd/10; dilution 1:100; Cell Marque) and other complementary markers were investigated when necessary. All slides were subsequently exposed to avidin–biotin complex and horseradish peroxidase reagents (LSAB Kit—DakoCytomation, Carpenteria, CA, USA) and diaminobenzidinetetrahydrochloride (DAB, Sigma, St. Louis, MO, USA), and subsequently counterstained with Carazzi hematoxylin. Appropriate positive controls were used for each antibody, while the negative control was obtained by omitting the primary specific antibody.
The H&E-stained slides and immunohistochemical results were descriptively evaluated and the final diagnoses followed the guidelines of the latest World Health Organization Classification of Soft Tissue and Bone Tumors [6]. In cases with primary sarcoma of the head and neck, CAP/AJCC protocol for the examination of resection specimens of soft tissue tumors is recommended and was also used in this study [12]. Grading and staging is strongly advised for soft tissue sarcomas; however, because the diagnosis of all LMS cases included in this study were based on incisional biopsy samples, some of them relatively small, we did not render a definitive grading for each case, but microscopic findings were detailed provided.
The ethical committee of the Universidade Federal de Minas Gerais approved this study (72775717.8.0000.5149).
Results
Demographic Data and Clinical Features
The demographic data and clinical features of oral LMs are presented in Table 1. The patients’ age ranged from 28 to 73 years with a mean age of 45.7 ± 13.6 years-old, with the highest frequencies in the fourth and sixth decades of life. LMs were more frequent in men (1.9:1), but the mean age for women (53.4 ± 15.1) was higher than for men (40.1 ± 9.2). The lesions more commonly affected the upper lip (39.1%) and hard palate (26.1%). While over half of all cases in males occurred in the lip (53%), an anatomic predominance was not observed among females.
Table 1.
Demographic data and clinical features of the 22 cases diagnosed as oral leiomyomas in the present series
| Case | Age (years) | Sex | Size (cm) | Site | Color | Symptoms | Duration (month) | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 34 | F | NS | Tongue | NS | NS | NS | Surgical excision |
| 2 | 40 | M | NS | Upper lip | NS | NS | NS | Surgical excision |
| 3 | 53 | M | NS | Upper lip | NS | NS | NS | Surgical excision |
| 4 | 30 | M | NS | Soft palate | NS | NS | NS | Surgical excision |
| 5 | 32 | F | 1.5 | Gingiva | Reddish | Asymptomatic | NS | Surgical excision |
| 6 | 54 | M | 1.2 | Upper lip | NS | NS | 60 | Surgical excision |
| 7 | 69 | F | 0.8 | Buccal mucosa | NS | NS | NS | Surgical excision |
| 8 | 45 | M | 1.5 | Lower lip | Purplish | NS | 4 | Surgical excision |
| 9 | NS | M | NS | Hard palate | Bluish | NS | NS | Surgical excision |
| 10 | NS | M | NS | Lip | NS | NS | NS | Surgical excision |
| 11 | 28 | M | 0.3 | Lower lip | Normal | Symptomatic | NS | Surgical excision |
| 12 | 42 | M | 1.2 | Upper lip | Normal | Asymptomatic | 120 | Surgical excision |
| 13 | 59 | F | 2.0 | Buccal mucosa | Normal | Asymptomatic | 48 | Surgical excision |
| 14 | 52 | F | 1.0 | Hard palate | Reddish | Asymptomatic | NS | Surgical excision |
| 15 | 34 | M | 1.7 | Hard palate | Reddish | Asymptomatic | 180 | Surgical excision |
| 16 | 33 | M | 0.6 | Hard palate | Bluish | Asymptomatic | NS | Surgical excision |
| 17 | 44 | M | 1 | Hard palate | Normal | Asymptomatic | NS | Surgical excision |
| 18 | 34 | M | 0.4 | Lingual frenulum | Normal | Asymptomatic | 2 | Surgical excision |
| 19 | 64 | F | 1 | Buccal space | Red | Asymptomatic | 5 | Surgical excision |
| 20 | 73 | F | 0.9 | Lower lip | Normal | Asymptomatic | 12 | Surgical excision |
| 21 | 56 | F | 1.5 | Tongue | Red | Asymptomatic | 2 | Surgical excision |
| 22 | 31 | M | 3 | Buccal mucosa | NS | Asymptomatic | 12 | Surgical excision |
F Female, M male, NS Not specified
All LMs presented as solitary small nodules and just one case had more than 2 cm in its largest diameter. Most of the cases (91.0%) were asymptomatic, with overlying mucosa ranging from normal-colored to reddish or dark bluish (Fig. 1). The lesions had a mean time of duration of 44.5 months (range 2–180 months). The most common initial clinical diagnoses included mucocele, varix, reactive proliferative process, benign salivary gland neoplasm and benign mesenchymal neoplasm. All lesions were treated by conservative surgical excision, with no recurrences reported.
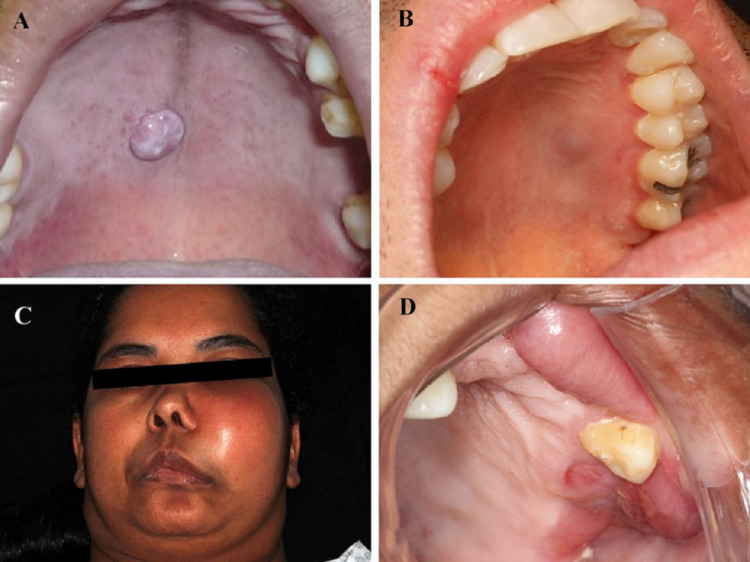
Fig. 1.
Clinical presentation of smooth muscle tumours of the oral and maxillofacial region. A LM presenting as a well-defined nodular lesion in the central region of the palate. B LM showing a superficial elevated mucosa lesion in the left side of the hard palate with a slight bluish appearance. C LMS demonstrates aggressive clinical behaviour in Case #1 causing a large facial asymmetry in the left side. D In this same case #1, intra-orally, LMS presented as an ulcerated expansive swelling in the maxilla
The clinical features of five oral and maxillofacial LMSs are shown in Table 2. This malignant neoplasm affected 4 women and one man, ranging from 36 to 69 years (mean: 47.6 ± 14.4 years). Two patients presented primary tumors and 3 cases were metastatic diseases occurring in the oral and maxillofacial region from previous retroperitoneal LMS (Cases #2 and #4) and uterine LMS (Case #3). Case #2 also presented other hepatic metastases and the full report of this case is available in Azevedo et al. [17].
Table 2.
Demographic data and clinical features of the five oral and maxillofacial leiomyosarcomas included in the present series
| Case | Sex | Age (years) | Size (cm) | Site | Occurence | Color | Symptoms | Duration (months) | Treatment | Follow-up (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 39 | > 10 | Maxilla | Primary | Swelling and facial asymmetry | NS | NS | Surgical excision | NS | NS |
| 2* | M | 69 | 1 | Mandible | Metastasis | Swelling with ulcerative regions | Numbness in lower lip | 4 | Surgical excision | 12 | Dead |
| 3 | F | 38 | 4 | Mandible | Metastasis | NS | Numbness in lower lip | 3 | NS | NS | NS |
| 4 | F | 56 | 2 | Gingiva | Metastasis | Swelling with ulcerative regions | NS | 2 | NS | NS | NS |
| 5 | F | 36 | 14 | Mandible | Primary | Swelling and bone destruction | Inferior alveolar nerve paresthesia | NS | NS | NS | NS |
M male, F female, NS Not specified
*Previously reported case: [17]
The oral and maxillofacial LMSs presented a tumor size ranging from 1 to 14 cm in their greatest diameter and a history of rapid growth, with duration ranging from 2 to 4 months. Four cases affected the jawbones, 3 the mandible and one the maxilla, while one case affected the gingiva. At extra-oral examination, one case exhibited facial asymmetry with the displacement of the nasal wing and elevation of the inferior eyelid (Case #1). The patients also reported other clinical manifestations like lower lip numbness (Cases #2 and #3) and inferior alveolar nerve paresthesia (Case #5). At intraoral clinical examination, LMSs presented single, well- to ill-defined, non-mobile, sessile, painful swelling with irregular and ulcerated surface ranging in color from normal to erythematous (Fig. 1). Regarding the radiographic findings, intraosseous LMSs appeared as radiolucent/hypodense images with ill-defined margins and bone fracture was observed in one case (Case #3) (Fig. 2).
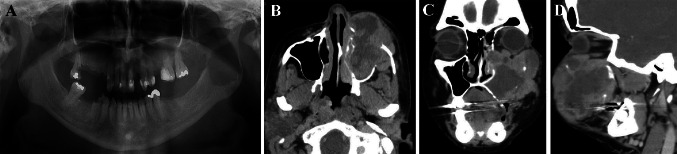
Fig. 2.
Radiographic findings of oral LMS. A A localized, single, well-defined, perforating, rounded, measured 4.0 × 3.0 cm, radiolucent image in the posterior region of the mandible, right side, caused by a metastatic LMS, leading to a pathological bone fracture (Case #3). B Cone bean computed tomography axial image illustrating 6.0 cm in the largest diameter, ill-defined, destructive, expansive, heterogeneous, hypodense lesion, involving the left maxillary sinus and the nasal cavity. There was evidence of bone destruction of the lateral, media, anterior and posterior walls of the sinus of the LMS (Case #1). C Coronal image of Case #1 demonstrating bone destruction all walls and the nasal concha was partially involved. D Sagittal image of Case #1
Data regarding treatment protocols used were available for two patients with oral and maxillofacial LMS (Cases #1 and #2), both treated by surgical resections. Follow-up information was available for only one patient that died of the disease after 12 months of follow-up.
Histopathological Findings
Grossly, oral LM specimens presented as pinkish to dark bluish, fibrous to rubbery, rounded to oval nodules with smooth to irregular surface. The cut surfaces were homogeneous (Fig. 3). On the other hand, oral and maxillofacial LMSs were characterized by irregular-shaped tissues, whitish to brownish with an irregular surface and fibrous consistency.
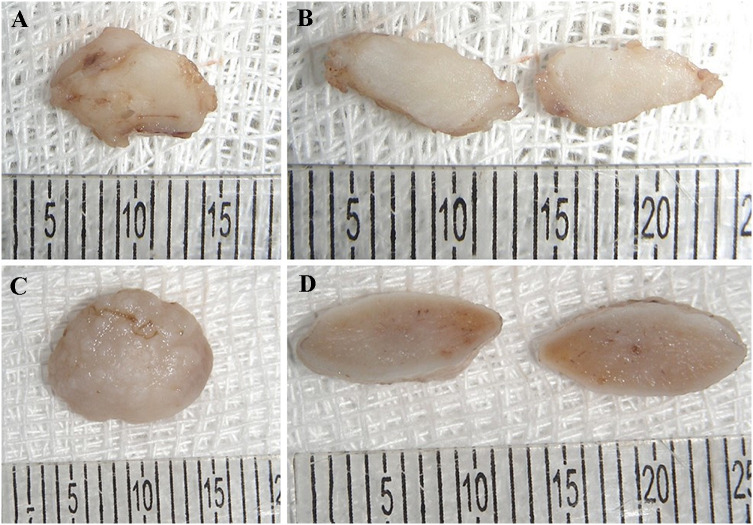
Fig. 3.
Macroscopic features of oral LM. A Irregular shape and surface, fibrous tissue showing a whitish to brownish colour. B A homogeneous whitish cut surface of the lesion. C A rounded shape and rugged surface, fibrous tissue with a whitish to brownish colour (Case #21) was removed and the overlying mucosa can be seen. D The homogeneous cut surface can also be found in this case
Oral LMs were usually well-circumscribed often growing in a nodular fashion, although some cases were poorly delimited (Cases #1 and #21). Dilated blood vascular spaces of varying sizes commonly filled by red blood cells were observed in 19 cases (angioleiomyoma). Occasionally, the tumor cells were arranged in concentric rings around the thick muscular walls of the vessels, with vascular spaces that looked compressed and merged into tumor stroma. In contrast, a solid pattern exhibiting small, closely compacted or slit-like blood vascular spaces and larger amounts of spindle tumor cells was observed in 4 cases (Cases #1, #5, #18and #21). LMs were characterized by bundles or fascicles of tumor cells with differentiation towards smooth muscle exhibiting relatively abundant eosinophilic cytoplasm and round to elongated spindle nuclei, often with blunt ends (cigar shaped). The tumor cells were arranged in short to large fascicles perpendicularly to haphazardly or in clusters within a variable fibrous connective tissue stroma. Less common histologic findings included mature lipomatous component (Cases #2, #14and #16), hemangiopericytoma-like foci (Cases #4 and #13) and myxohyaline degeneration (Case #19) (Fig. 4). Cellular and nuclear pleomorphism, as well as atypical mitotic figures were absent.
Fig. 4.
Histopathology and immunohistochemical features of oral LM. A An angioleiomyoma showing the presence of irregular blood vessels and demonstrating the proliferation of smooth muscle cells originating from the vascular structures (H&E, ×50 magnification). B Solid LM demonstrating hypercellular areas with a fascicular growth pattern (H&E, ×50 magnification). C Neoplastic spindle cells with a bland aspect and the so-called cigar-shaped nucleus (H&E, × 200 magnification). D Lipomatous component was found in some cases (H&E, × 100 magnification. E Tumour cells were positive for h-caldesmon (DAB, ×200 magnification) and F Ki67 nuclear staining showed a low proliferative index of tumour cells (DAB, ×200 magnification)
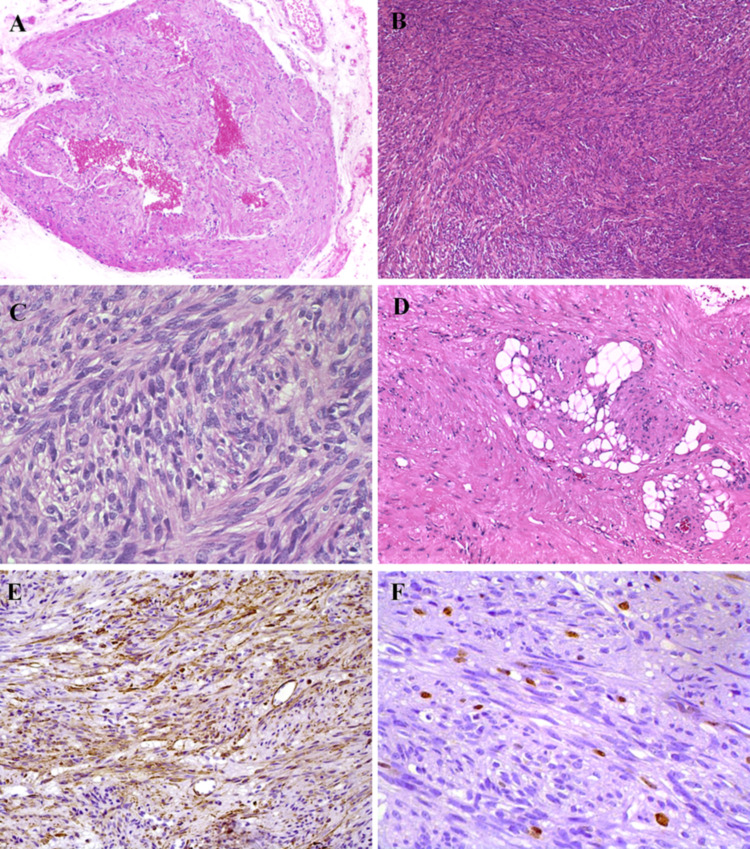
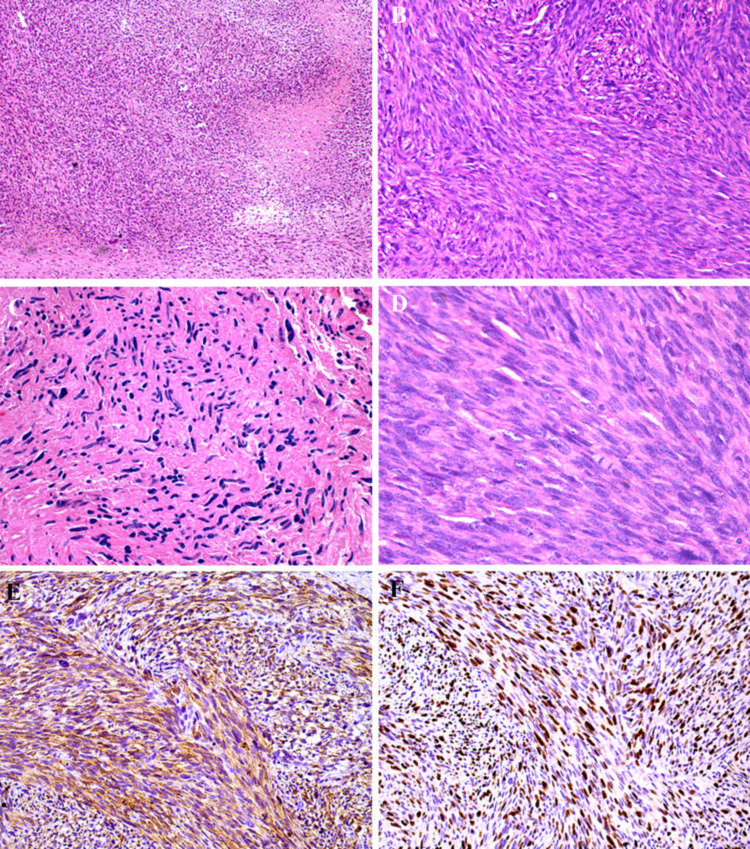
Histopathologically, all five oral LMSs demonstrated an infiltrative or ill-demarcated fascicular growth with focal to extensive necrotic areas, and moderate to poorly differentiated regions. The malignant tumor cells were arranged in fascicles of varying size intersecting perpendicularly or randomly, consisting of spindle cells with well to poorly defined eosinophilic cytoplasm and oval to elongated nuclei, also with the blunt-ended (cigar) shape. Mitotic figures were scattered present in four cases and more frequent in one case, and occasional cells showed perinuclear vacuoles (Fig. 5).
Fig. 5.
Histopathology and immunohistochemical features of oral and maxillofacial LMS. A The presence of tissue necrosis was a common finding in the oral and maxillofacial LMS cases evaluated (H&E, ×50 magnification). B Under higher magnification it is possible to illustrate tumour cells showing a fascicular growth pattern (H&E, ×100 magnification). C In one of the metastatic LMS it was observed frequent pleomorphic cells with hyperchromatic nuclei (H&E, ×100 magnification). D Mitotic figures were found in hypercellular regions (H&E, ×200 magnification). E) h-caldesmon was strongly positive in all LMS cases (DAB, ×100 magnification) and F that also presented a high proliferative index measured by Ki67 expression (DAB, ×100 magnification)
Immunohistochemical Findings
Regarding the immunohistochemical findings, LMs exhibited diffuse and strong cytoplasmic positivity for αSMA, calponin, HHF35 and h-caldesmon, while desmin (Fig. 4) was weaker and diffuse. Tumor cells were negative for S100 and CD34. Regarding LMSs, immunohistochemistry showed positivity for αSMA, h-caldesmon and HHF35 in all five cases. Desmin was also focally observed. Additionally, tumor cells were negative for CD34, S100, MyoD1and cytokeratin. The proliferative index determined by Ki67 expression was very low for LMs with only scattered positive nuclei, whereas LMSs showed very high proliferative indexes (Fig. 5).
Discussion
Smooth muscle tumors affecting the oral cavity and the jaws are uncommon, more frequently representing benign lesions. Oral and maxillofacial LMSs, although very rare, have been described in the literature, but given the lack of appropriate and well-documented immunohistochemical investigations in many reports, available data are incomplete. Therefore, in this study we described the clinicopathological features of 22 LMs sand five LMSs following strict diagnostic criteria. LMs presented as benign nodules with different histopathological architectural patterns, whereas LMSs were frequently metastatic diseases with very aggressive clinical behavior and that demanded rigorous histological evaluation and immunohistochemical investigation including h-caldesmon stain for correct diagnosis.
LMs most commonly arise in the soft tissue of female genital tract, skin and gastrointestinal tract [18] only rarely affecting the oral cavity [18–20]. In the mouth there is a slight male predominance, usually affecting adult patients [7, 10, 21, 22]. However, Kim et al. [23] reported a possible congenital LM in the posterior tongue of a 2-months-old infant. In agreement with the literature, our LM series showed a mean age of 45.8 years-old and a similar sex distribution.
Oral LMs usually present as solitary small nodules, which rarely exceed more than 2 cm in the largest diameter, with slow-growing and normal-colored to reddish/bluish surface, which demands from oral diagnosticians that LMs are frequently included in the list of differential diagnoses of many oral benign soft tissue tumors [3, 18, 24]. This indolent behavior was demonstrated by Gueiros et al. [21] and Kim et al. [23] that reported long-standing cases with over 20 years of duration. Moreover, virtually all cases are asymptomatic, although pain and swallowing impairment may be exceptionally reported [3, 18]. The lips, tongue, cheek mucosa and palate are the most affected locations [18, 22], but cases in the jaws may rarely occur mostly in the mandible [10, 25]. In accordance with these findings, all cases in our series were asymptomatic and the upper lip and the palate were the most affected locations. Given this benign nature, oral LMs are treated conservatively by surgical procedures and recurrences are extremely rare [10, 18, 21, 22].
LMs are characterized by bundles of eosinophilic, blunt-ended (cigar-shaped) spindle cells perpendicularly oriented. Focal degenerative changes, such as fibrosis, calcification, myxoid change and fatty differentiation may occur. Unlike LMS, mitotic figures are rare or absent [4, 11, 26]. As shown in our series, LMs usually present as angioleiomyomas, which are believed to derive from pericytic cells, showing many vascular spaces surrounded by thick muscular walls [2, 6, 10]. However, the solid variant is also observed and less common microscopic findings may be found in the oral cavity, including extensive areas of calcification [27, 28], presence of granular cells [29] and clear cells [30]. It is important to differentiate oral LMs from other benign spindle cell tumors, such as myofibromas, solitary fibrous tumors, benign fibrous histiocytomas, neurofibromas and schwannomas, some of which may carry an important clinical significance [9–11].
Oral and maxillofacial LMSs are very rare, representing less than 1% of oral sarcomas [2, 3, 13, 31]. However, in a multicenter study developed in Brazil, de Carvalho et al. [15] found that LMSs comprised 6% of the sarcomas in the oral cavity. Recently, Ko [13] reviewed the clinicopathological features of 29 primary oral LMSs, demonstrating that females were more affected and patients’ mean age at diagnosis was 36.7 years, although other authors [15, 32, 33] observed a higher mean age (44–45 years), as also demonstrated in our series (47.6 years-old). In LMS pain is a frequent finding, which was reported by our patients that also complained of chin and lower lip paresthesia. Moreover, LMS affecting the sinonasal tract is also very rare, and in these cases, facial swelling may be found [15, 32–36].
Primary oral LMSs more commonly affect buccal mucosa, tongue, palate and floor of the mouth, while mandibular involvement is unusual, and the lesion size usually ranges from one to 10 cm, as shown in our series [13–15, 32, 33]. Although our two primary LMSs affected the mandible and the maxilla, after such diagnosis a complete systemic evaluation is mandatory, since metastatic diseases may occur [37, 38]. Ko [13] showed that 10.3% of primary oral LMSs may develop metastases, while Saluja et al. [31] demonstrated that metastatic head and neck LMSs have a poor prognosis and most of these cases mainly originate from the uterus and retroperitoneum, which is confirmed by our three metastatic diseases that disseminated from these regions.
Histopathologically, LMS consists of interlacing fascicles of spindle cells with eosinophilic to pale cytoplasm and oval to elongated hyperchromatic nuclei with a blunt-ended feature. Although LMs and LMSs are usually easily differentiated in the oral and maxillofacial region, in deep soft tissues this distinction can be very difficult. Cytologic atypia, cellularity, pleomorphism, and presence of tumor necrosis are consistent with malignancy, but mitotic activity may be very low [39]. In our five cases, the presence of histopathological findings consistent with malignancy was rapidly observed and facilitated the recognition of the tumor nature, which was further supported by clinical aspects. Nonetheless, other spindle-cell sarcomas must also be considered as differential diagnoses for LMS, including fibrosarcoma, rhabdomyosarcoma, malignant peripheral nerve sheath tumor, spindle cell carcinoma, melanoma and others [13, 32, 33].
According to the CAP/AJCC guidelines [12] genetic analyses for tumor-specific molecular translocations can be used to help pathologists to classify soft tissue tumors, and LMS is characterized by complex events with frequent deletion of chromosome 1p. Moreover, microscopic grading of LMS is also very important and the French Federation of Cancer Centers Sarcoma Group (FNCLCC) system is one of the most recommended, which combines three microscopic parameters: differentiation, mitotic activity, and necrosis. Although we have described these findings in our series, because we used incisional biopsies to perform the histological investigation of our LMSs, we decided not to render a definitive grading for the cases included, but rather descriptively provide the histological findings.
Although the smooth muscle differentiation should be confirmed with the expression of at least two myogenic markers [11], the most appropriate panel of antibodies remains controversial and many authors have provided incomplete data. In various reports, diagnoses were rendered based on histopathology and positivity for αSMA only, whereas some authors also described positivity for other smooth muscle proteins like desmin, muscle-specific actin (HHF-35) and calponin, combined with negativity to other mesenchymal markers like S100 [13, 32]. However, all these smooth muscle markers are also variably expressed in myofibrosarcomas [13, 40]. Therefore, because the expression pattern of the above-mentioned myogenic markers are usually not well described or documented and the lack of specificity of these proteins for smooth muscle differentiation, it is possible that some of the previously reported oral LMSs do not show true myogenic differentiation, but rather myofibroblastic.
On the other hand, Ceballos et al. [40] demonstrated that h-caldesmon would be a reliable marker for differentiating smooth muscle tumors from myofibroblastic neoplasms, which was further supported by subsequent studies that investigated tumors from different anatomic locations [41–43], including in the oral cavity [44]. However, Yu et al. [45] more recently found h-caldesmon expression in gastrointestinal stromal tumors (GIST), slightly decreasing its specificity. Therefore, we understand that the use of h-caldesmon is very important for confirming the diagnosis of oral LMSs and for difficult cases of LMs.
Extensive surgical approaches followed or not by chemotherapy and/or radiotherapy are usually applied for LMSs, but no standard therapeutic scheme is recognized [14, 32, 33]. Local recurrences occur in approximately 23% of the cases, while metastases are observed in 34% [35]. The overall survival rate of patients affected by oral LMSs ranges from 55 to 61.87% [32, 33], but the literature review of Ko [13] observed a much higher value (94.1%). The presence of metastatic tumors might significantly impact this rate.
Conclusion
Oral LMs are uncommon lesions with innocuous clinical behavior that are usually well suspected under histopathological investigation, while LMSs are aggressive neoplasms whose diagnosis demands the use of an immunohistochemical panel containing h-caldesmon and other myogenic markers which expression pattern must also be considered during pathological interpretation. Moreover, oral LMS diagnosis necessarily requires a systemic evaluation of the patient to rule out a possible metastatic disease.
Funding
This study was supported by grants from the São Paulo State Research Foundation (FAPESP 2020/03818–8), the Minas Gerais State Research Foundation (FAPEMIG) and the Coordination for the Improvement of Higher Education Personel (CAPES) (Financial code 001). FPF, RSG, RAM, JSN, PAV and ARSS are research fellows of the National Council for Scientific and Technological Development (CNPq) (Brazil).
Declarations
Conflict of interest
No conflict of interest to disclose.
Ethical Approval
The questionnaire and methodology for this study was approved by the Human Research Ethics committee of the Universidade Federal de Minas Gerais (72775717.8.0000.5149).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
The participant has consented to the submission of the case report to the journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fletcher CDM. Distinctive soft tissue tumors of the head and neck. Mod Pathol. 2002;15:324–330. doi: 10.1038/modpathol.3880526. [DOI] [PubMed] [Google Scholar]
- 2.Solar AA, Jordan RCK. Soft tissue tumors and common metastases of the oral cavity. Periodontol. 2000;2011(57):177–197. doi: 10.1111/j.1600-0757.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- 3.Wertheimer-Hatch L, Hatch GF, III, Davis GB, Blanchard DK, Foster RS, Jr, Skandalakis JE. Tumors of the oral cavity and pharynx. World Journal Surg. 2000;24(4):395–400. doi: 10.1007/s002689910064. [DOI] [PubMed] [Google Scholar]
- 4.Stout AP. Solitary cutaneous and subcutaneous leiomyoma. Am J Cancer. 1937;29:435. [Google Scholar]
- 5.Castaldi A, Arcuri T, Carta M, Quilici P, Derchi LE. Primary leiomyosarcoma of the oral tongue: magnetic resonance and ultrasonography findings with histopathologic correlation. Acta Radiol. 2006;47:514–517. doi: 10.1080/02841850600647124. [DOI] [PubMed] [Google Scholar]
- 6.The WHO Classification of Tumours Editorial Board . WHO Classification of tumours soft tissue and bone tumours, 5. Lyon: IARC Press; 2020. [Google Scholar]
- 7.Ide F, Mishima K, Yamada H, Saito I, Horie N, Shimoyama T, Kusama K. Perivascular myoid tumors of the oral region: a clinicopathologic re-evaluation of 35 cases. J Oral Pathol Med. 2008;37:43–49. doi: 10.1111/j.1600-0714.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 8.Silva LAB, Monroy EAC, Serpa MS, Souza LB. Oral benign neoplasms: A retrospective study of 790 patients over a 14-year period. Acta Otorrinolaringol Esp. 2018;70:158–164. doi: 10.1016/j.otorri.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Luaces-Rey R, Lorenzo-Franco F, Gómez-Oliveira G, Patiño-Seijas B, Guitián D, López-Cedrún-Cembranos JL. Oral leiomyoma in retromolar trigone. A case report. Med Oral Patol Oral Cir Bucal. 2007;12:42–44. [PubMed] [Google Scholar]
- 10.Cepeda LAG, Rivera DQ, Rocha FT, Huerta ERL, Sánchez ERM. Vascular leiomyoma of the oral cavity. Clinical, histopathological and immunohistochemical characteristics. Presentation of five cases and review of the literature. Med Oral Patol Oral Cir Bucal. 2008;13:483–488. [PubMed] [Google Scholar]
- 11.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. World Health Organization classification of head and neck tumours. 4th ed. Lyon; 2017
- 12.College of American Pathologists. AJCC protocol for the examination of resection specimens from patients with soft tissue tumors. 8th ed.; 2019
- 13.Ko E. Primary oral leiomyosarcoma: A systematic review and update. J Oral Pathol Med. 2019;48:780–787. doi: 10.1111/jop.12858. [DOI] [PubMed] [Google Scholar]
- 14.Yan B, Li Y, Pan J, Xia H, Li L-J. Primary oral leiomyosarcoma: A retrospective clinical analysis of 20 cases. Oral Dis. 2010;16:198–203. doi: 10.1111/j.1601-0825.2009.01635.x. [DOI] [PubMed] [Google Scholar]
- 15.de Carvalho WRS, de Souza LL, Pontes FSC, Uchôa DCC, Corrêa DL, de Cáceres CVBL, et al. A multicenter study of oral sarcomas in Brazil. Oral Dis. 2019;26:43–52. doi: 10.1111/odi.13211. [DOI] [PubMed] [Google Scholar]
- 16.Moreira DGL, Silva LP, Morais EF, Queiroz SIML, Santos EM, Souza LB, et al. The occurrence and pattern of head and neck sarcomas: a comprehensive cancer center experience. Eur Arch Otorhinolaryngol. 2020;277:1473–1480. doi: 10.1007/s00405-020-05834-x. [DOI] [PubMed] [Google Scholar]
- 17.Azevedo RS, Pires FR, Gouvêa AF, Lopes MA, Jorge J. Leiomyosarcomas of the oral cavity: report of a radiation-associated and a metastatic case. Oral Maxillofac Surg. 2012;16(2):227–232. doi: 10.1007/s10006-011-0294-5. [DOI] [PubMed] [Google Scholar]
- 18.Veeresh M, Sudhakara M, Girish G, Naik C. Leiomyoma: a rare tumor in the head and neck and oral cavity: report of 3 cases with review. J Oral Maxillofac Pathol. 2013;17:281–287. doi: 10.4103/0973-029X.119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baden E, Doyle JL, Lederman DA. Leiomyoma of the oral cavity: a light microscopic and immunohistochemical study with review of the literature from 1884 to 1992. Eur J Cancer Part B: Oral Oncol. 1994;30:1–7. doi: 10.1016/0964-1955(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 20.Ranjan S, Singh KT. Gingival angioleiomyoma-infrequent lesion of oral cavity at a rare site. J Oral Maxillofac Pathol. 2014;18:107–110. doi: 10.4103/0973-029X.131928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gueiros LA, Romañach MJ, Pires-Soubhia AM, Pires FR, Paes-de-Almeida O, Vargas PA. Angioleiomyoma affecting the lips: report of 3 cases and review of the literature. Med Oral Patol Oral Cir Bucal. 2011;16:482–487. doi: 10.4317/medoral.16.e482. [DOI] [PubMed] [Google Scholar]
- 22.Aitken-Saavedra J, da Silva KD, Gomes APN, Vasconcelos ACU, Etges A, Nóbrega TG, et al. Clinicopathologic and immunohistochemical characterization of 14 cases of angioleiomyomas in oral cavity. Med Oral Patol Oral Cir Bucal. 2018;23:564–568. doi: 10.4317/medoral.22649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YH, Jang YW, Pai H, Kim SG. Congenital angiomyoma of the tongue: case report. Dentomaxillofac Radiol. 2010;39:446–448. doi: 10.1259/dmfr/32524441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherrick HM, Dunlap CL, King OH. Leiomyomas of the oral cavity Review of the literature and clinicopathologic study of seven new cases. Oral Surg Oral Med Oral Pathol. 1973;35:54–66. doi: 10.1016/0030-4220(73)90094-7. [DOI] [PubMed] [Google Scholar]
- 25.Liang H, Frederiksen NL, Binnie WH, Cheng YS. Intraosseous oral leiomyoma: systematic review and report of one case. Dentomaxillofac Radiol. 2003;32:285–290. doi: 10.1259/dmfr/22632903. [DOI] [PubMed] [Google Scholar]
- 26.Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss's soft tissue tumors. In: Goldblum JR, Weiss SW, Folpe AL, editors. Leiomyosarcoma. 6. Amsterdam: Elsevier; 2008. pp. 517–543. [Google Scholar]
- 27.Nonaka CFWP, Pereira KMA, Costa-Miguel MC. Oral vascular leiomyoma with extensive calcification areas. Braz J Otorhinolaryngol. 2010;76:539. doi: 10.1590/S1808-86942010000400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montague LJ, Fitzpatrick SG, Islam NM, Cohen DM, Bhattacharyya I. Extensively ossifying oral leiomyoma: a rare histologic finding. Head Neck Pathol. 2014;8:311–316. doi: 10.1007/s12105-013-0497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya I, Summerlin DJ, Cohen DM, Ellis GL, Bavitz JB, Gillham LL. Granular cell leiomyoma of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2006;102:353–359. doi: 10.1016/j.tripleo.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Darling MR, Wehrli B, Zeligman E, Smillie J, Daley T. Unusual benign smooth muscle lesions of the tongue: review and report of two cases. Head Neck Pathol. 2012;6:121–124. doi: 10.1007/s12105-010-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saluja TS, Iyer JS, Satyendra K. Leiomyosarcoma: Prognostic outline of a rare head and neck malignancy. Oral Oncol. 2019;95:100–105. doi: 10.1016/j.oraloncology.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Ethunandan M, Stokes C, Higgins B, Spedding A, Way C, Brennan P. Primary oral leiomyosarcoma: a clinic pathologic study and analysis of prognostic factors. Int J Oral Maxillofac Surg. 2007;36:409–416. doi: 10.1016/j.ijom.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Vilos GA, Rapidis AD, Lagogiannis GD, Apostolidis C. Leiomyosarcoma of the oral tissues: clinicopathologic analysis of 50 cases. Int J Oral Maxillofac Surg. 2005;63:1461–1477. doi: 10.1016/j.joms.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Mizutani H, Tohnai I, Yambe M, Ueda M. Leiomyosarcoma of the maxillary gingiva: a case report. Nagoya J Med Sci. 1995;58:165–170. [PubMed] [Google Scholar]
- 35.Dry SM, Jorgensen JL, Fletcher CDM. Leiomyosarcomas of the oral cavity: an unusual topographic subset easily mistaken for non-mesenchymal tumours. Histopathology. 2000;36:210–220. doi: 10.1046/j.1365-2559.2000.00814.x. [DOI] [PubMed] [Google Scholar]
- 36.Chew YK, Noorizan Y, Khir A, Brito-Mutunayagam S. Leiomyosarcoma of the maxillary sinus. Med J Malaysia. 2009;64(2):174–175. [PubMed] [Google Scholar]
- 37.Nusrath MA, Kendall CH, Avery CM. Metastatic uterine leiomyosarcoma masquerading as a primary lesion of the masseter muscle. Int J Oral Maxillofac Surg. 2006;35:466–468. doi: 10.1016/j.ijom.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Schütz A, Smeets R, Driemel O, Hakim SG, Kosmehl H, Hanken H, et al. Primary and secondary leiomyosarcoma of the oral and perioral region-clinicopathological and immunohistochemical analysis of a rare entity with a review of the literature. J Oral Maxillofac Surg. 2013;71:1132–1142. doi: 10.1016/j.joms.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Brooks JK, Nikitakis NG, Goodman NJ, Levy BA. Clinicopathologic characterization of oral angioleiomyomas. Oral Surg Oral Med Oral Pathol Oral Radiol. 2002;94:221–227. doi: 10.1067/moe.2002.125276. [DOI] [PubMed] [Google Scholar]
- 40.Ceballos KM, Nielsen GP, Selig MK, O’Connell JX. Is anti h-caldesmon useful for distinguishing smooth muscle and myofibroblastic tumors? An immunohistochemical study. Am J Clin Pathol. 2000;114:746–753. doi: 10.1309/K5JP-A9EN-UWN7-B5GG. [DOI] [PubMed] [Google Scholar]
- 41.Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, Suster S. Differential expression of smooth muscle myosin, smooth muscle actin, H-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006;28:105–111. doi: 10.1097/01.dad.0000200009.02939.cc. [DOI] [PubMed] [Google Scholar]
- 42.Robin YM, Penel N, Pérot G, Neuville A, Vélasco V, Ranchère-Vince D, et al. Transgelin is a novel marker of smooth muscle differentiation that improves diagnostic accuracy of leiomyosarcomas: a comparative immunohistochemical reappraisal of myogenic markers in 900 soft tissue tumors. Mod Pathol. 2013;26:502–510. doi: 10.1038/modpathol.2012.192. [DOI] [PubMed] [Google Scholar]
- 43.Beck EM, Bauman TM, Rosman IS. A tale of two clones: caldesmon staining in the differentiation of cutaneous spindle cell neoplasms. J Cutan Pathol. 2018;45:581–587. doi: 10.1111/cup.13259. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira DH, da Silveira ÉJ, de Souza LB, Caro-Sanchez CH, Dominguez-Malagon H, Mosqueda-Taylor A, Queiroz LM. Myofibroblastic lesions in the oral cavity: immunohistochemical and ultrastructural analysis. Oral Dis. 2019;1:174–181. doi: 10.1111/odi.12972. [DOI] [PubMed] [Google Scholar]
- 45.Yu G, Xu J, Jiang L, Cai L, Zohar Y, Wu S, et al. Expression and clinical significance of H-caldesmon in gastrointestinal stromal tumor: is it a specific marker for myogenic differentiation. Int J Clin Exp Pathol. 2019;12:2566–2571. [PMC free article] [PubMed] [Google Scholar]