Abstract
Kimura disease (KD) is a rare inflammatory disorder which involves the head and neck. Due to its rarity and various findings, definitive diagnosis can be difficult to ascertain. Kimura disease is distinguished from other conditions, including angiolymphoid hyperplasia, by histopathological features including follicular hyperplasia, reactive germinal centers, abundant eosinophilia, eosinophilic microabscesses, preserved nodal architecture, Warthin-Finkeldy polykaryocytes, and capsular fibrosis. Herein, we describe the clinical presentation, pathology, and diagnosis of a single case of a 39-year-old treated at an academic center in Texas.
Keywords: Kimura, Lymphadenopathy, Kimura disease, Angiolymphoid hyperplasia with eosinophilia, Neck mass, Lymphoid disorders, Facial mass
Introduction
Kimura disease (KD) is an extremely rare chronic inflammatory disease with approximately 200 individual cases reported in the literature worldwide [1]. It clinically presents as multifocal subcutaneous masses of the head and neck involving soft tissue, cervical lymph nodes, and salivary gland tissue. Its pathogenesis is largely unknown; however, some believe there is a concordance with atopy due to the presence of peripheral eosinophilia, eosinophilic infiltrate within the masses, and elevated IL-5/IgE [2]. Histopathologic evaluation demonstrates multiple lymphoid follicles with germinal centers and eosinophilic infiltration with occasional eosinophilic microabscess formation. KD is often confused with other lymphoid disorders, especially angiolymphoid hyperplasia (ALHE). Key differences outlined by Rosai et al. differentiated these two lesions based upon histopathology, but recent work provides some evidence that the two clinical entities could be on a spectrum of disease [3–5].
Kimura disease almost exclusively affects individuals of Southeast Asian descent with 3:1 male predilection and peak incidence of 20–40 years old [6, 7]. No standard of care has been established given its rarity, but immunomodulating agents such as corticosteroids and cyclosporine are frequently implemented as first line therapy. Upon medical failure, radiation and surgery are employed [8, 9]. KD has a high rate of local recurrence with reported rates of up to 62% following surgery [10]. Herein, we describe an uncommon presentation of Kimura disease with shared histopathology representative of ALHE in a Hispanic male.
Case Report
A 39 year-old male presented to the University of Texas Medical Branch Otolaryngology clinic with facial pain and cosmetic deformity caused by a slowly growing right facial mass. The mass first appeared in 2015, but the patient did not seek care until 2017. At that time, a community plastic surgeon performed a surgical resection. The lesion recurred shortly thereafter and the patient was then treated with systemic corticosteroids without improvement. Upon presentation to our clinic, physical exam revealed a large right facial mass extending from the supraorbital rim superiorly to below the angle of the mandible inferiorly without overlying skin change. CT and MRI demonstrated a subcutaneous heterogeneously enhancing mass involving the right parotid as well as infra-temporal and supraorbital soft tissues without extension into the orbit (Figs. 1 and 2). Additionally, multiple pathologic lymph nodes were seen in the region surrounding the right parotid as well as right levels II-IV.
Fig. 1.
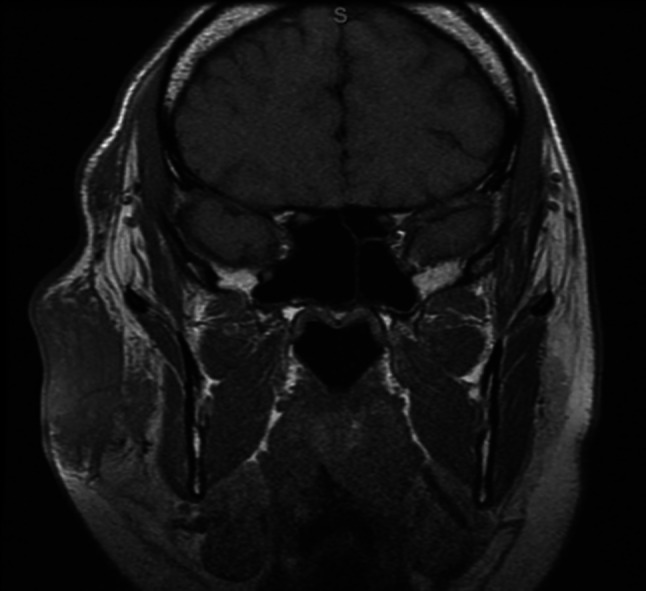
MRI orbits without contrast T1 sequence showing large right sided preauricular lymphadenopathy
Fig. 2.
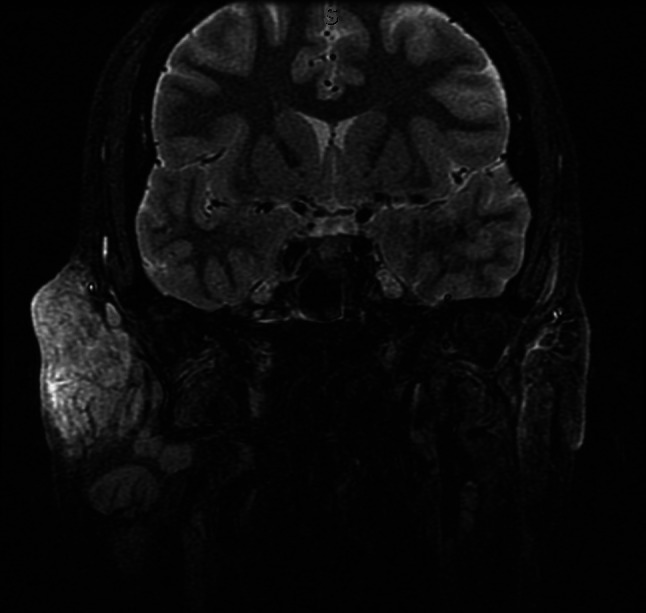
Short TI Inversion Recovery sequence (fat suppression) showing enhancement of right sided preauricular lymphadenopathy
A fine-needle aspirate was taken of the mass and cervical lymph nodes, which demonstrated atypical T-cells and prominent eosinophilia. A subsequent incisional biopsy revealed extensive lymphoid and eosinophilic infiltrate, follicular hyperplasia with germinal centers, extensive fibrosis, and neoangiogensis consisting of mostly thin-walled vessels, but with occasional thick-walled vessels (Figures 3, 4, 5 and 6). In addition, Warthin-Finkeldy polykaryocytes were identified.
Fig. 3 .

Incisional biopsy at low power magnification revealing extensive fibrosis and follicular hyperplasia (H&E, ×40)
Fig. 4 .
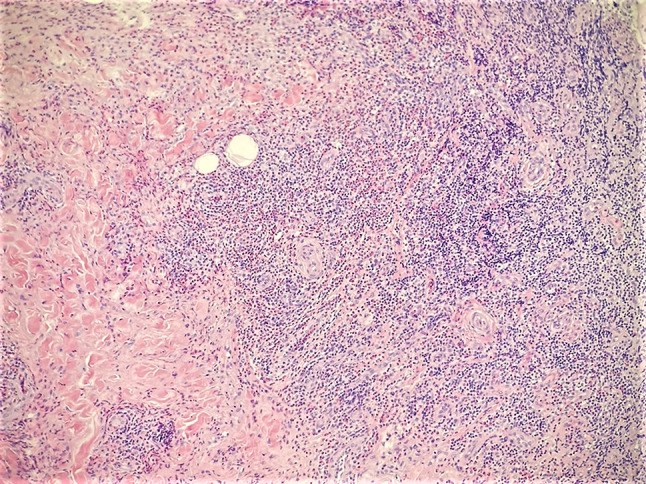
Incisional biopsy with features of Kimura disease including sclerosis, neoangiogenesis, and extensive eosinophilic inflammation (H&E, ×100)
Fig. 5 .

Incisional biopsy revealing neoangiogenesis and extensive lymphoid and eosinophilic infiltration (H&E, ×200)
Fig. 6 .
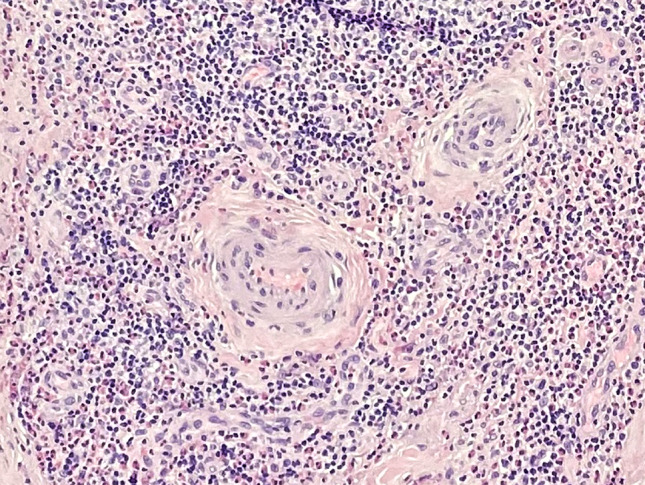
Incisional biopsy with proliferation of thick-walled vessels—a feature concerning for ALHE (H&E, ×400)
Immunohistochemistry demonstrated an increased ratio of CD4 to CD8 T-cells (5:1). The involved CD4 T-cells demonstrated expression of CD2, CD3, CD5, and down-regulation of CD7. Corresponding flow cytometry and T-cell receptor (TCR) gene rearrangement studies did not detect monoclonal B or T-cell populations. Additional peripheral blood testing revealed a serum IgE of 28,000 kU/L and peripheral eosinophilia of 591 cells/µL. The patient was presented at the multidisciplinary head and neck tumor board with recommendation for external beam radiation due to the failure of medical and surgical therapy.
Discussion
Kimura disease remains a difficult disease to diagnose and treat. The differential is large and may include dermatopathic lymphadenopathy, parasitic lymphadenitis, eosinophilic granuloma, epithelioid hemangioma, Hodgkin’s disease, cutaneous T-cell lymphomas, angioimmunoblastic lymphoma, and angiolymphoid hyperplasia with eosinophilia (ALHE) [3]. KD and ALHE have similar clinical and histopathological findings, rendering a definitive diagnosis difficult.
Both diseases present with slowly growing subcutaneous and nodal masses of the head and neck, particularly in the pre-auricular region. However, several clinical and epidemiologic differences do exist between the two entities. KD predominantly affects men of Southeast Asian descent with few cases described in other ethnic groups [8]. The disease is characterized by deep subcutaneous masses (typically without overlying skin changes), cervical adenopathy, peripheral eosinophilia, and elevated serum IgE. In contrast, ALHE is a benign vascular proliferative disorder with a female predilection that effects a diversity of ethnic groups and presents with superficial dermal papules and nodules that are frequently erythematous, pruritic, and friable [11]. Peripheral blood eosinophilia is uncommon in ALHE.
Histologic differences also exist between the two. In addition to the follicular hyperplasia, reactive germinal centers, abundant eosinophilia, and eosinophilic microabscesses characteristic of Kimura, there is commonly sclerosis and proliferation of thin-walled vessels. Nodal architecture is typically preserved, but capsular fibrosis may be seen. The inflammatory response often extends into the soft tissue surrounding the lymph node. Eosinophilic infiltrates are seen in the interfollicular areas, sinusoids, and perinodal soft tissues [12]. ALHE, on the other hand, demonstrates a more diffuse pattern of lymphocyte infiltrate with less prominent sclerosis. Most notably, it exhibits a unique thick-walled vascular proliferation with hypertrophic endothelial cells that protrude into the vascular lumen with a hobnail appearance.
Though these two diseases are now largely recognized as separate entities, recent evidence suggests that they may be subsets of the same disease process. Chong et al. described five cases of Kimura disease evaluated for clonal T-cell rearrangement in which one case demonstrated a T-cell population with clonal rearrangement of the TCRδ gene [4]. Liu et al. described a case of a single Chinese male that presented with lesions consistent with both disease processes [13]. Likewise, Kempf et al. reviewed seven cases of ALHE and found that five demonstrated clonal rearrangements of the TCRγ gene [5]. In this study the proliferative lymphocytes were CD4+ T-cells, suggesting that ALHE may be a lymphoproliferative disorder of CD4+ T-cell origin rather than a vascular lesion with reactive inflammation. Given the presence of clonal T-cells with TCR gene rearrangements in cases of both ALHE and Kimura disease as well as documentation of the coexistence of Kimura and ALHE in single patients [13], we must consider the possibility that the two conditions may both be part of the same disease process.
Our patient’s biopsy demonstrated many of the features of Kimura Disease, namely, a lymphocytic infiltrate with prominent eosinophilia, follicular hyperplasia with germinal centers, abundant fibrosis, and Warthin-Finkeldy polykaryocytes. Though small-vessel vascular proliferation was predominant, occasional thick-walled vessels with hobnail endothelial cells raised suspicion for ALHE. Though it is less common, ALHE can involve reactive follicles and present with peripheral eosinophilia. The preponderance of features was, however, most consistent with KD.
Further complicating this case was the immunohistochemistry profile of the lymphocytes which was suggestive of cutaneous T-cell lymphoma or angioimmunoblastic lymphoma. Both of these entities may show similar histopathology to KD. However, further characterization of the cells with flow cytometry, PCR, and chromosomal analysis did not illuminate a diagnosis of lymphoma.
The diagnosis of Kimura disease remains challenging due to its clinical and histopathologic similarity to multiple lymphoid disorders, most specifically angiolymphoid hyperplasia. Recent molecular studies have blurred the lines that previously delineated these conditions. Significant work remains to help differentiate this rare disease from clinically and pathologically similar conditions [14].
Funding
No funding obtained.
Data Availability
No datasets were used in this paper.
Declarations
Conflict of interest
None of the authors have any conflicts of interest to report.
Ethical Approval
This paper received an IRB waiver from the University of Texas Medical Branch Institutional Review Board.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Othman SK, Daud KM, Othman NH. Kimura’s disease: a rare cause of nephrotic syndrome with lymphadenopathy. Malays J Med Sci MJMS. 2011;18(4):88–90. [PMC free article] [PubMed] [Google Scholar]
- 2.Rascovan N, Monteil Bouchard S, Grob J-J, et al. Human polyomavirus-6 infecting lymph nodes of a patient with an angiolymphoid hyperplasia with eosinophilia or Kimura disease. Clin Infect Dis. 2016;62(11):1419–1421. doi: 10.1093/cid/ciw135. [DOI] [PubMed] [Google Scholar]
- 3.Rosai J, Gold J, Landy R. The histiocytoid hemangiomas. A unifying concept embracing several previously described entities of skin, soft tissue, large vessels, bone, and heart. Hum Pathol. 1979;10(6):707–730. doi: 10.1016/S0046-8177(79)80114-8. [DOI] [PubMed] [Google Scholar]
- 4.Chong W-S, Thomas A, Goh C-L. Kimura’s disease and angiolymphoid hyperplasia with eosinophilia: two disease entities in the same patient. Case report and review of the literature. Int J Dermatol. 2006;45(2):139–145. doi: 10.1111/j.1365-4632.2004.02361.x. [DOI] [PubMed] [Google Scholar]
- 5.Kempf W, Haeffner AC, Zepter K, et al. Angiolymphoid hyperplasia with eosinophilia: evidence for a T-cell lymphoproliferative origin. Hum Pathol. 2002;33(10):1023–1029. doi: 10.1053/hupa.2002.128247. [DOI] [PubMed] [Google Scholar]
- 6.Tseng C-F, Lin H-C, Huang S-C, Su C-Y. Kimura’s disease presenting as bilateral parotid masses. Eur Arch Oto-Rhino-Laryngol Head Neck. 2005;262(1):8–10. doi: 10.1007/s00405-003-0677-9. [DOI] [PubMed] [Google Scholar]
- 7.Fouda MA, Gheith O, Refaie A, et al. Kimura disease: a case report and review of the literature with a new management protocol. Int J Nephrol. 2010;2010(2010):4. doi: 10.4061/2010/673908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nonaka M, Sakitani E, Yoshihara T. Anti-IgE therapy to Kimura’s disease: a pilot study. Auris Nasus Larynx. 2014;41(4):384–388. doi: 10.1016/j.anl.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Kottler D, Barète S, Quéreux G, et al. Retrospective multicentric study of 25 Kimura disease patients: emphasis on therapeutics and shared features with cutaneous IgG4-related disease. Dermatology (Basel) 2015;231(4):367–377. doi: 10.1159/000439346. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka N, Minato T, Yoshida S, et al. Kimura’s disease with unusual eosinophilic epithelioid granulomatous reaction: a finding possibly related to eosinophil apoptosis. Hum Pathol. 2002;33(5):561–564. doi: 10.1053/hupa.2002.124037. [DOI] [PubMed] [Google Scholar]
- 11.Lin P, Medeiros LJ. Hematopoietic lesions. In: Gnepp DR, editor. Diagnostic surgical pathology of the head and neck. 2. Philadelphia: W.B. Saunders; 2009. pp. 933–974. [Google Scholar]
- 12.Chen DR, Thompson SI, Aguilera L, Abbondanzo L. Kimura disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2004;28(4):505–513. doi: 10.1097/00000478-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Liu XK, Ren J, Wang XH, Li XS, Zhang HP, Zeng K. Angiolymphoid hyperplasia with eosinophilia and Kimura's disease coexisting in the same patient: evidence for a spectrum of disease. Australas J Dermatol. 2012;53(3):e47–50. doi: 10.1111/j.1440-0960.2011.00744.x. [DOI] [PubMed] [Google Scholar]
- 14.Sia KJ, Kong CK, Tan TY, Tang IP. Kimura's disease: diagnostic challenge and treatment modalities. Med J Malays. 2014;69(6):281–283. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were used in this paper.


