Abstract
In the absence of clear pathologic differences, clinical history may differentiate potential primary parotid squamous cell carcinomas (SCC) from metastases. The presence of an ultraviolet (UV) signature can distinguish between tumors of cutaneous and non-cutaneous origin. This study aimed to investigate rates of UV signature mutations in squamous cell carcinomas of the parotid gland as well as differences in clinical features between tumors of cutaneous and non-cutaneous origin. Clinical and pathologic data were collected from 71 patients with SCC involving the parotid gland, of which 48 had cutaneous, 10 had mucosal, and 13 had no history of SCC. In 34 available cases, genomic DNA was isolated from formalin-fixed paraffin-embedded tissue specimens and sequenced using a targeted hybrid capture 1213 gene panel. Tumor mutational burden and COSMIC (Catalogue of Somatic Mutations in Cancer) mutational signatures were calculated. Most (74%) were UV-positive. Patients with UV-positive tumors were significantly older, white, and had higher rates of sun exposure. Patients with UV-negative tumors had a significantly higher mortality rate and shorter time to death: 6 (67%) died of disease with a median time to death of 9 months compared to 5 (20%) UV-positive patients who died of disease with a median time to death of 32 months. Pathologic features did not significantly vary by clinical history or UV status. The presence of a UV-signature combined with clinical history can be used to determine the primary source of SCC involving the parotid gland. UV-positivity may reflect a less aggressive disease course in an older population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12105-021-01349-x.
Keywords: Squamous cell carcinoma, Parotid, Salivary, Ultraviolet signature
Introduction
Squamous cell carcinoma (SCC) of the parotid gland is an uncommon and controversial but potentially aggressive neoplasm that accounts for less than 10% of all salivary gland neoplasms and conveys a 5-year survival rate of approximately 50% [1–11]. Due to its rarity, primary parotid SCC is poorly understood and may even be non-existent [12]. Though prior studies have described the demographic features of affected patients, accurate data on clinical behavior and survival remains limited [8–11].
Due to the gland’s unique position within a complex web of lymphatics, cases of SCC involving the parotid may represent metastatic, not primary, disease. Cutaneous disease of the scalp, cheeks, and auricles drains directly to parotid nodes en route to the upper cervical nodes, while mucosal disease must take a more circuitous route to enter the parotid lymphatics and may instead spread to the parotid via direct invasion through the buccal mucosa [13, 14]. Given significant clinical and pathologic overlap between SCCs originating in various sites, distinguishing potential primary parotid SCC versus metastatic SCC from other sites of the head and neck is a diagnostic challenge. A diagnosis of primary parotid SCC can be considered once a history of SCC of the head and neck is excluded [1–8, 10, 15–19], however, some authors do not differentiate between primary or metastatic disease at all [9, 11]. One study suggested that the origin of the SCC is not clinically relevant as all parotid SCCs share similar pathologic features and clinical outcomes [20]. Estimates of the frequency of presumed primary parotid SCC range from 6.1 to 23% of all SCC found in the parotid [2, 8, 10]. Though there is significant histologic overlap between potential primary parotid SCC and metastatic SCC to the parotid, clinical differences between the two might emerge with a more rigorous toolkit [1].
The presence of an ultraviolet (UV) signature has shown promise as a mechanism by which to distinguish between tumors of cutaneous and non-cutaneous origin [21, 22]. That is, while tumors of cutaneous origin will show evidence of sun exposure in the form of C>T or CC>TT mutations at dipyrimidine sites, primary salivary or mucosal tumors will not contain such UV signature mutations as they arise in sites that have not been exposed to the sun [23–27]. In practice, the presence of a UV signature has been used to identify a head and neck SCC of unknown origin as cutaneous-derived [25]. However, this technique has not yet been applied specifically or systematically to SCCs involving the parotid gland. The aim of this study is therefore to investigate the use of clinical history and UV signature mutations to distinguish between non-cutaneous SCC involving the parotid gland and cutaneous SCC metastatic to the parotid gland. In doing so, we aim to more precisely compare the prognostic factors and outcomes of SCC involving the parotid gland, whether potentially primary, potentially mucosal-derived, or potentially cutaneous-derived.
Materials and Methods
Patient Identification
This retrospective study was performed with Institutional Review Board approval. Patients diagnosed with SCC involving the parotid gland between 2000 and 2018 at the University of Chicago Medical Center were identified. A total of 71 patients were included.
Data Collection
Pathologic, clinical, and follow-up data were collected from the electronic medical record. All surgical pathology diagnoses were reported by University of Chicago pathologists. Patient metadata collected included: age at diagnosis, sex, race/ethnicity, history of sun exposure including diagnosis of actinic keratosis or clinically noted history of exposure, history of smoking, history of prior or concurrent head and neck SCC (mucosal versus cutaneous), presenting symptoms, extent of surgical resection, cervical lymph node dissection, tumor size, extent of local invasion, surgical margin status, pre- or post-operative chemo and/or radiotherapy, recurrence, site of recurrence, and survival. Tumor size was defined by the largest dimension. Treatment was divided into three groups: preoperative chemo and/or radiotherapy, surgery only, and postoperative chemo and/or radiotherapy. Surgical treatment was further categorized into partial or complete parotidectomy and selective or complete neck dissection. Disease outcome after treatment was defined as either alive with no disease, alive with disease, dead of disease, or dead of other causes. If applicable, recurrence time was calculated from time of index surgery to time of recurrence. Survival time was calculated from date of diagnosis to whichever one of the following came first: death, the last date the patient was known to be alive, or April 1, 2020. Patient and pathologic characteristics were stratified and compared by patient history of cutaneous HNSCC, mucosal HNSCC, or no prior HNSCC.
Histologic and Molecular Analysis
H&E slides and FFPE (Formalin-Fixed Paraffin-Embedded) tissue blocks were retrieved from the Department of Pathology Archives. All available H&E slides were reviewed (S.F. and N.A.C) to confirm sites of involvement (skin overlying parotid, intra- and/or peri-parotid lymph nodes, parotid glandular tissue). Cases originating in the skin overlying the parotid gland (and subsequently invading the superficial parotid) were assumed to be cutaneous primaries and were excluded from molecular analysis. For all remaining available cases, tumor-rich blocks were identified. Patients excluded from testing were reviewed for clinical histories that have been reported to confer a higher likelihood of non-UV-related cutaneous SCC, including: the presence of immunosuppression on azathioprine, underlying genetic diseases (xeroderma pigmentosa or recessive dystrophic epidermolysis bullosa), presence of SCC on a non-sun-exposed site, and/or presence of SCC on the nose in which origination on the mucosal versus skin side was difficult to determine [28, 29]. Immunosuppressed patients not receiving azathioprine remain at risk for UV-related SCC [29].
Sequencing was performed using the targeted hybrid capture 1213 gene UCM-OncoPlus panel, as previously described [30]. In brief, genomic DNA was isolated from macrodissected tumor-enriched formalin-fixed, paraffin-embedded tissue using the QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. Following extraction, DNA was quantified using the Qubit fluorometric assay (Thermo Fisher Scientific, Foster City, CA) and further assessed for quantity and quality using a quantitative polymerase chain reaction assay (hgDNA Quantitation and QC kit; KAPA Biosystems, Wilmington, MA). Library preparation and sequencing were performed as previously described for the UCM-OncoPlus Assay [30]. Briefly, ∼100 ng DNA was fragmented using the Covaris S2 (Covaris, Woburn, MA). The fragmented DNA was amplified using the KAPA HTP Library Preparation Kit (Kapa Biosystems) along with a set of patient-specific indexes (Roche, Indianapolis, IN). The pooled library was captured using a custom SeqCap EZ capture panel (Roche) featuring a collection xGen LockdownProbes (IDT, Coralville, IA) for 1213 genes. The pooled captured library was sequenced on the Illumina Hi-Seq. 2500 system (Illumina, San Diego, CA) in rapid run mode (2 × 101 bp paired-end sequencing). Somatic mutation calling was performed across all 1213 genes using a custom in-house bioinformatics pipeline previously described [30].
Mutations from coding regions of OncoPlus genes were used to calculate tumor mutational burden (TMB). Individual basecalls with instrument quality scores greater than 30 were included (quality score of 30 indicates approximate chance of error of 1/1000). For a given base, DP30 is defined as is the depth of basecalls at a quality of 30 or above. Therefore, mutations from coding regions of OncoPlus genes having > 50 × DP30, variant allele frequency (VAF) > 10%, and filtered for variants within the 1000 Genomes or ExAC population databases were used to calculate TMB (variants with > 10 entries in COSMIC (Catalogue of Somatic Mutations in Cancer) and ExAC frequency of < 0.001 were rescued from filtering). TMB is defined as the number of these mutants per Mb of genome interrogated for variants. Mutational signatures and percentage match scores were determined as well from this set using the SigProfiler single sample tool [31]. Percentage match represents similarity of individual sample single base substitution (SBS) profiles to COSMIC SBS profiles [30]. COSMIC profiles 7a–d have been strongly associated with UV exposure [31], and samples having a sum percentage of > 60% for signatures 7a–d with TMB > 20 were determined to be UV signature positive. Correspondingly, samples with sum percentage sig7 < 60% and TMB < 20 were determined to be UV signature negative. UV signature and TMB cutoffs were chosen in accordance with those described in the literature [24, 32].
Statistical Analysis
Microsoft Excel, MedCalc statistical software, and VassarStats were used for statistical analysis using two-tailed Fisher Exact Probability Test, F-statistic, p-value, and χ2 statistic. A p-value of < 0.05 was considered significant.
Results
Patient Population
The study population included 71 patients. The average age was 72 years (range 47–96 years). The majority of patients were white (92%) and male (82%). Most patients had a known history of alcohol (60%) and tobacco (59%) use. A significant sun exposure history was present in 48%. Of all 71 patients, 48 (68%) had a known history of cutaneous SCC of the head and neck on the ipsilateral side of the parotid tumor. Ten patients (14%) had a known history of head and neck mucosal SCC (including oral tongue, base of tongue, tonsil, gingiva, buccal mucosa, or nasal cavity) on the ipsilateral side of the parotid tumor. Thirteen patients (18%) had no known history of SCC of the head and neck.
Molecular Analysis
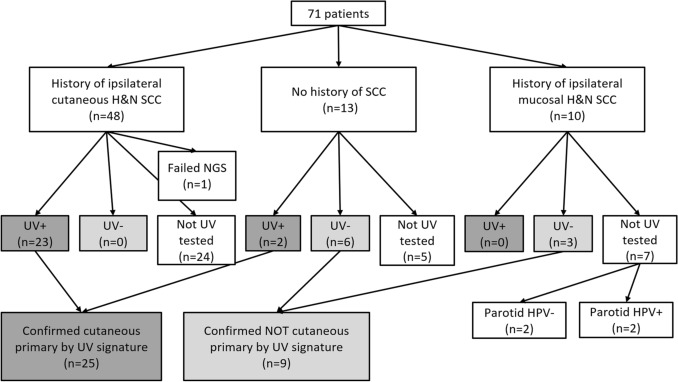
In total, specimens from 35 patients (49%) underwent molecular analysis with one failing sequencing (Fig. 1). Nine were excluded from sequencing based on direct extension from cutaneous primary and the remaining 27 did not have parotid tumor available for testing. Of the 34 successfully sequenced patients, 25 (74%) were found to be UV signature positive and 9 (26%) were UV signature negative (Figs. 2, 3). Specifically, cases ranged from having 0 to 100% signature 7 and 3.17 to 122.98 tumor mutational burden (see Supplemental Digital Content; Supplemental Table 1). Cases determined to be UV-negative had 0–40% signature 7 (median 0%, mean 8%) and 3.17–12.97 tumor mutational burden (median 5.25, mean 7.28). Cases determined to be UV-positive had 60–100% signature 7 (median 88%, mean 87%) and 22.28–122.98 tumor mutational burden (median 62.77, mean 65.77).
Fig. 1.
Flow diagram of H&N SCC cases, based on clinical history and next-generation sequencing results. Of those tested, 25 had UV signature (confirming cutaneous primary) and 9 did not. Additionally, 2 cases were HPV positive, confirming oropharyngeal mucosal primary. H&N head and neck, SCC squamous cell carcinoma, NGS next generation sequencing, UV ultraviolet, HPV human papillomavirus
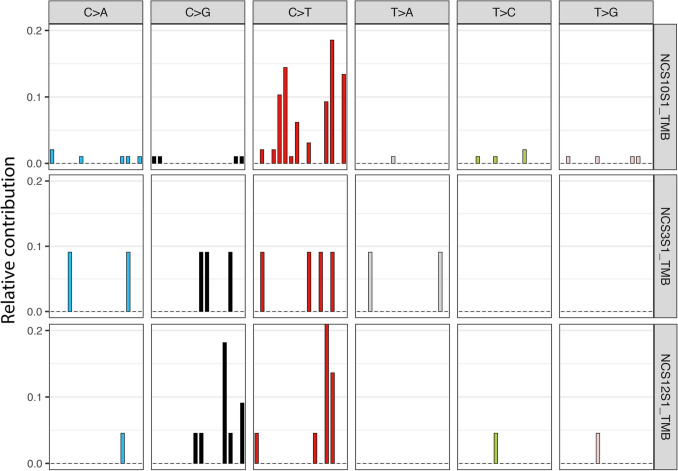
Fig. 2.
Single base substitution signatures of three select cases. NCS10 (top row) is from an 89-year-old white male with a history of cutaneous head and neck SCC, whose ipsilateral parotid tumor contained 79.38% subset 7 and high tumor mutational burden (34.44), consistent with UV signature mutation. NCS3 (middle row) is from an 82-year-old while male with a history of tongue SCC, whose ipsilateral parotid tumor contained 0% subset 7 and low tumor mutational burden (4.58), therefore lacking UV signature. NCS12 (bottom row) is from a 47-year-old black male with no history of SCC, whose parotid tumor contained 31.82% subset 7 and low tumor mutational burden (8.85), therefore lacking UV signature
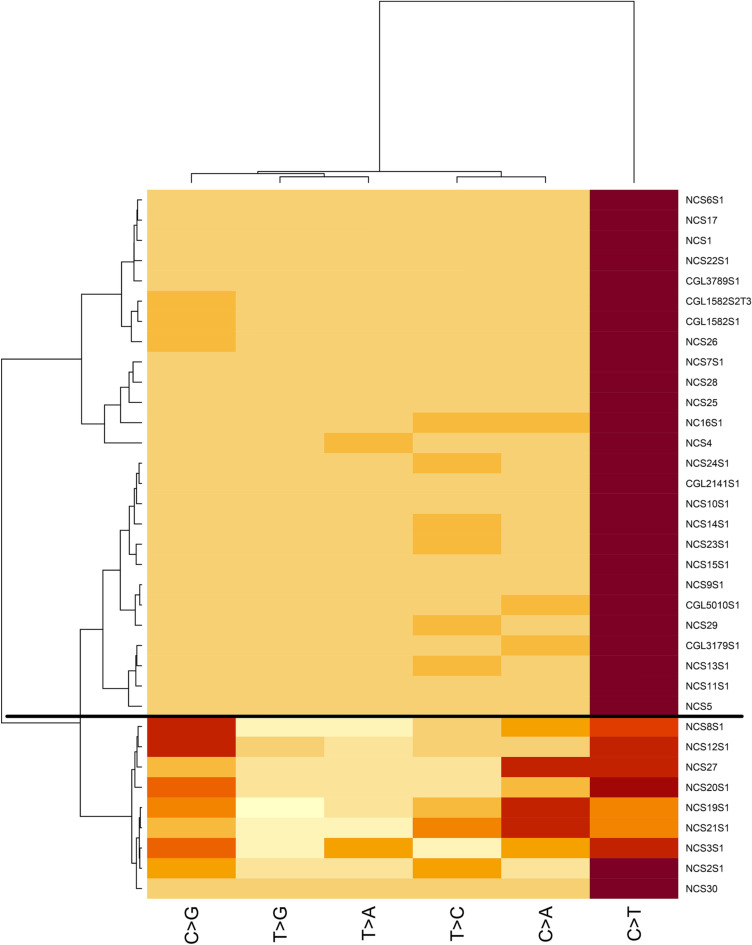
Fig. 3.
Heatmap of tested squamous cell carcinoma cases (35 cases from 34 patients). Cases above the horizontal black line are compatible with UV signature; cases below are UV signature negative. X-axis represents nucleotide change; Y-axis represents case tested
The UV-positive patients represented 23 patients with a known history of cutaneous head and neck SCC and 2 patients with no known history of SCC. These 25 patients were considered to have a confirmed cutaneous primary tumor on the basis of positive UV signature. The UV-negative patients represented 6 patients with no known history of SCC and 3 patients with a history of mucosal SCC. These 9 patients were considered to NOT have a cutaneous primary tumor on the basis of negative UV signature. Of tested patients with histories of cutaneous head and neck SCC, none were UV-negative.
In the review of patients excluded from molecular analysis, only one had possible risk for non-UV-related cutaneous SCC, specifically related to renal transplant. At the time of parotid involvement, he had concurrent SCC of the ipsilateral ear. He was immunosuppressed with azathioprine 1 year prior to parotid disease. However, he also had a longstanding history of actinic keratosis for years prior to receipt of azathioprine, suggesting he also has risk for UV-related SCC. The remaining untested patients were considered not to have significant risk for non-UV-related cutaneous SCC.
Clinical Characteristics
Demographic features were compared based on clinical history (Table 1) and UV status (see Supplemental Digital Content; Supplemental Table 2). Patients with a history of cutaneous SCC were older: median age of 76 years compared to median age of 67 years in those with no history of SCC and 56 years in those with a history of mucosal SCC (p = 0.000056). Race also differed significantly by clinical history (p = 0.014), as those with cutaneous SCC were more likely to be white: 47 (98%) compared to 10 (77%) of those with no history of SCC, and 8 (80%) of those with a history of mucosal SCC. Patients with a history of cutaneous SCC were also significantly more likely to have had sun exposure: 31 (65%) compared to 1 (8%) among those with no history of SCC and 2 (20%) among those with a history of mucosal SCC (p = 0.00021).
Table 1.
Demographics by clinical group
| History of cutaneous SCC (n = 48) | No history of SCC (n = 13) | History of mucosal SCC (n = 10) | F-statistic or χ2 statistic | p value | ||
|---|---|---|---|---|---|---|
| Age | Range | 55–96 | 47–83 | 47–82 | ||
| Median | 76 | 67 | 56 | F(2, 68) = 11.35 | 0.000056 | |
| Sex | Male | 41 (85%) | 9 (69%) | 8 (80%) | 0.33 | |
| Female | 7 (15%) | 4 (31%) | 2 (20%) | |||
| Race | White | 47 (98%) | 10 (77%) | 8 (80%) | 0.014 | |
| Non-white | 1 (2%) | 3 (23%) | 2 (20%) | |||
| Sun exposure | Exposed | 31 (65%) | 1 (8%) | 2 (20%) | χ2 = 16.89 | 0.00021 |
| Non-exposed | 17 (35%) | 12 (92%) | 8 (80%) | |||
| Smoking | Exposed | 27 (56%) | 6 (46%) | 9 (90%) | χ2 = 5.01 | 0.081 |
| Non-exposed | 21 (44%) | 7 (54%) | 1 (10%) | |||
| Alcohol | Exposed | 27 (56%) | 9 (69%) | 7 (70%) | χ2 = 1.16 | 0.56 |
| Non-exposed | 21 (44%) | 4 (31%) | 3 (30%) |
Bold indicates statistical significance (p-value < 0.05)
Age also differed by UV signature status (see Supplemental Digital Content; Supplemental Table 2): the median age of UV-positive patients was 78 years compared with 66 years in the UV-negative patients (p = 0.0096). UV-positive tumors also contained a higher proportion of white patients (100%) compared to UV-negative tumors (67%) (p = 0.014). The UV-positive patients were significantly more likely to have had sun exposure (p = 0.017). All 25 patients with UV-positive tumors were white and 18 (72%) had a known history of sun exposure. Conversely, 6 (67%) patients with UV-negative tumors were white and only 2 (20%) had a known history of sun exposure.
Pathologic Features
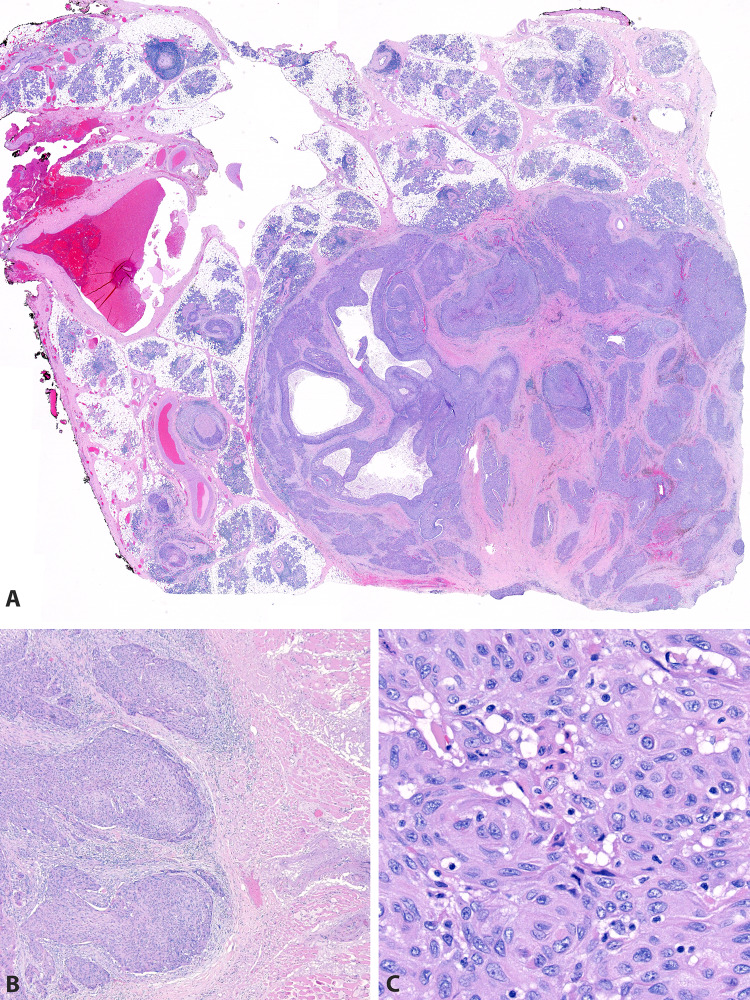
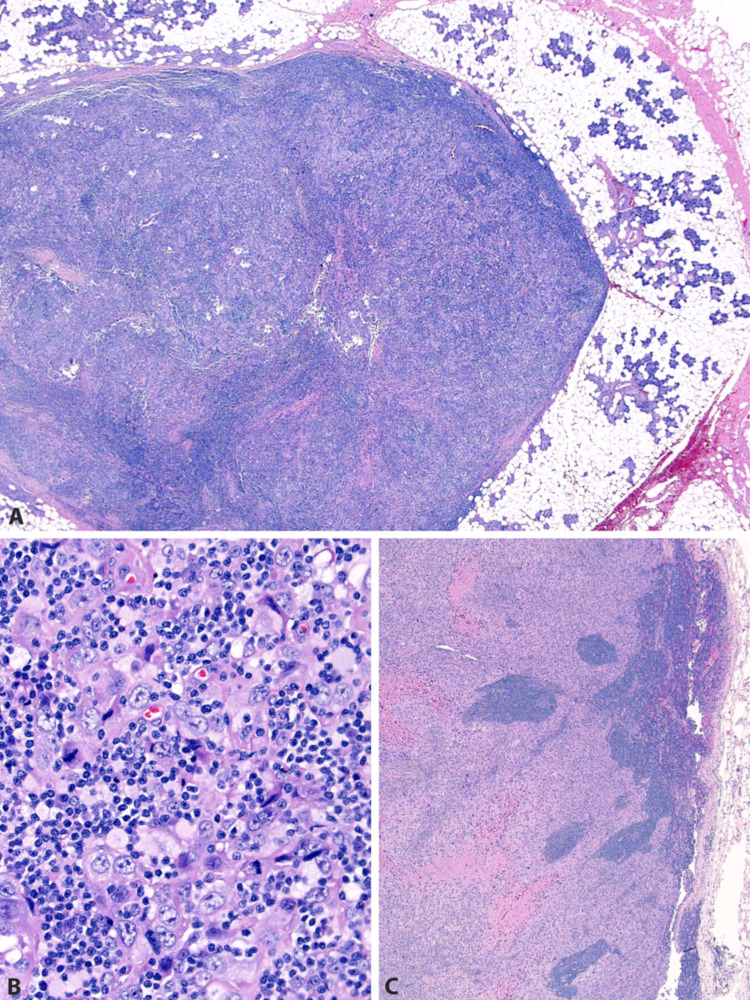
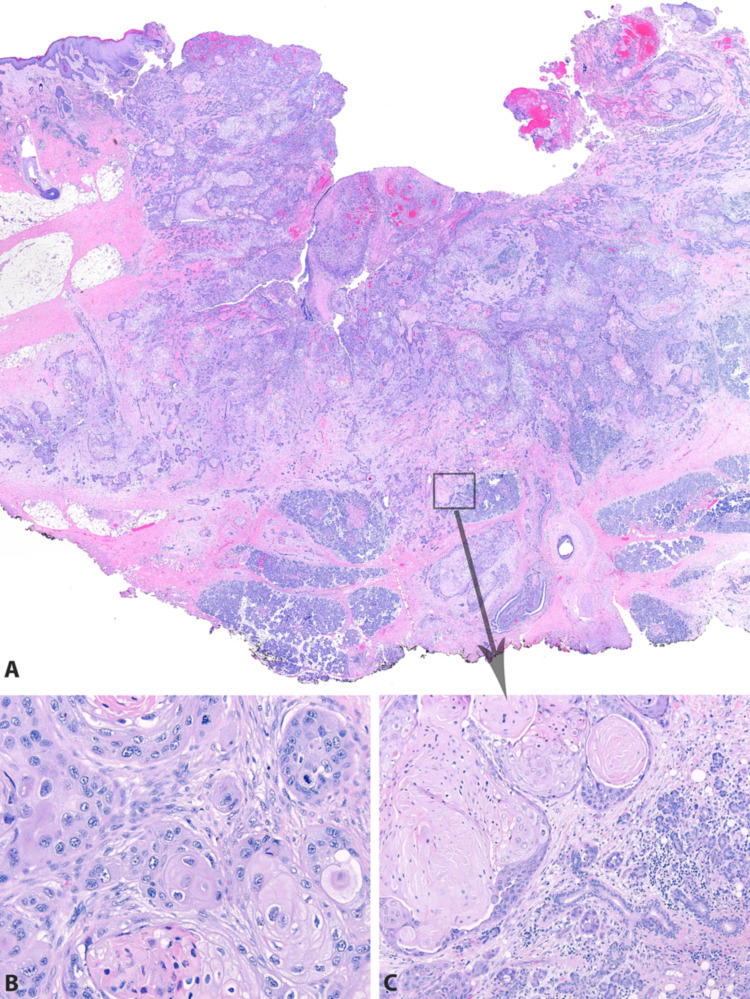
Of all 71 cases, 49 (69%) were available for histologic review. Of these, 16 (34%) involved only parotid glandular tissue (Figs. 4, 5). Nine (18%) involved only intraparotid lymph nodes (Fig. 6). Nine (18%) involved both parotid glandular tissue and intraparotid lymph nodes. Nine (18%) involved the parotid by direct extension from overlying skin (Figs. 4, 7). Six (12%) involved the parotid by direct extension from surrounding soft tissue. None of the patients had histologic features of a differentiated salivary-type carcinoma (such as salivary duct or mucoepidermoid) that might otherwise suggest primary salivary carcinoma with squamous differentiation.
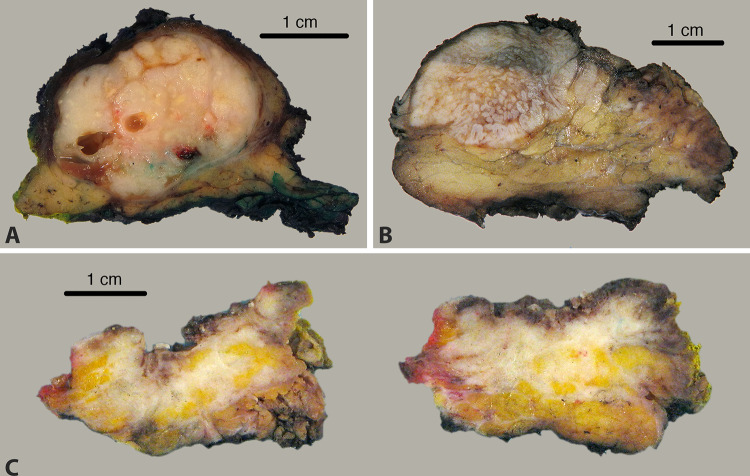
Fig.4.
A 3 cm tumor involving the right parotid gland in a 96 year old white male with a history of cutaneous squamous cell carcinoma of the right ear. UV signature was identified, confirming cutaneous metastasis. A A 3.8 cm tumor involving the right parotid gland in a 59 year old black male with no history of prior squamous cell carcinoma. UV signature was not identified, suggesting that this tumor may represent a parotid or occult mucosal primary. B A 1.8 cm tumor arising from the skin of an 87 year old white male with deep infiltration into the superficial parotid gland. UV testing was not performed, as the tumor was clinically and histologically a cutaneous primary with direct extension into the parotid gland (C)
Fig. 5.
H&E images from a 3.8 cm tumor in the right parotid gland of a 59 year old black male (see Fig. 4B) with no history of prior squamous cell carcinoma and negative UV signature. The mass is part-cystic/part-solid with perineural invasion (A), extraparenchymal extension into skeletal muscle (B), and keratinizing morphology (C)
Fig. 6.
A 93 year old white male with a history of squamous cell carcinoma of the left pinna. Recurrent/metastatic carcinoma involves an intraparotid lymph node (A), manifesting as infiltrative cords within lymph node parenchyma (B). Metastases were also present in the left lateral neck (C). UV mutation signature was identified, confirming cutaneous metastasis
Fig. 7.
H&E images from a 1.8 cm tumor arising from the skin of an 87 year old white male with deep infiltration into the superficial parotid gland (see Fig. 4C). The carcinoma arises from the facial skin (A) and has a keratinizing morphology (B). It invades deeply through the subcutis into parotid parenchyma (C)
Pathologic features were compared based on clinical history (Table 2) and UV status (see Supplemental Digital Content; Supplemental Table 3). Of the 49 cases available for review, 18 (37%) involved intraparotid lymph nodes, which was not different among groups. The median tumor size by largest dimension was 2.9 cm. Seven (14%) demonstrated lymphovascular invasion. Eighteen (37%) demonstrated perineural invasion. Twenty-three (47%) demonstrated extraparenchymal extension. These characteristics did not vary significantly by clinical history or UV status.
Table 2.
Pathologic features by clinical group
| History of cutaneous SCC (n = 34) | No history of SCC (n = 9) | History of mucosal SCC (n = 6) | F-statistic | p value | |
|---|---|---|---|---|---|
| Intraparotid node involvement | 15 (44%) | 1 (11%) | 2 (33%) | 0.20 | |
| Median size (cm) | 2.5 | 2.5 | 3.1 | F(2, 43) = 0.19 | 0.82 |
| Lymphovascular invasion | 5 (15%) | 1 (11%) | 1 (17%) | 1.0 | |
| Perineural invasion | 12 (35%) | 5 (55%) | 1 (16%) | 0.37 | |
| Extraparenchymal extension | 17 (50%) | 5 (55%) | 1 (16%) | 0.33 |
Treatment
Sixty-eight patients (96%) underwent surgical intervention with 52 of these (76%) undergoing both parotidectomy and neck dissection and the remaining 16 (24%) undergoing only parotidectomy. All but 9 patients received postoperative radiation, chemotherapy, or chemoradiation. Treatments were largely consistent across groups with 23 (92%) UV-positive patients and 7 (78%) UV-negative patients undergoing parotidectomy and neck dissection (χ2 = 1.28, p = 0.26). Twenty-one (84%) UV-positive patients and 5 (56%) UV-negative patients underwent subsequent radiation and/or chemotherapy (χ2 = 2.97, p = 0.08).
Clinical Outcomes
Average follow-up from date of surgery was 26.5 months (SD = 24). Rates of recurrence did not vary by clinical history (Table 3) or UV status (see Supplemental Digital Content; Supplemental Table 4). In total, 40 (56%) patients had a known recurrence of SCC. Most had locoregional recurrence (80%), though 5 patients had distant metastasis to the lungs.
Table 3.
Outcomes by clinical group
| History of cutaneous SCC (n = 48) | No history of SCC (n = 13) | History of mucosal SCC (n = 10) | F-statistic | p value | ||
|---|---|---|---|---|---|---|
| Recurrence | Disease recurred | 28 (58%) | 5 (38%) | 7 (70%) | 0.24 | |
| No recurrence | 12 (25%) | 6 (46%) | 2 (20%) | |||
| Mortality | Alive | 21 (44%) | 6 (46%) | 2 (20%) | 0.014 | |
| Dead of disease | 9 (19%) | 7 (54%) | 6 (60%) | |||
| Dead of something else | 13 (27%) | 0 (0%) | 2 (20%) | |||
| Average time to death (months) | 34 | 21 | 20 | F(2, 38) = 1.20 | 0.31 |
Bold indicates statistical significance (p-value < 0.05)
Patients without cutaneous SCC and patients with UV-negative tumors did have a significantly higher mortality rate: 9 (19%) patients with cutaneous SCC died of disease compared to 7 (54%) patients with no SCC and 6 (60%) patients with mucosal SCC (p = 0.014) (Table 3). Similarly, only 5 (20%) UV-positive patients died of disease compared to 6 (67%) UV-negative patients (p = 0.047) (see Supplemental Digital Content; Supplemental Table 4). The average time to death from date of presentation with parotid involvement also differed by UV status. The median time to death of UV-negative patients was 9 months compared to 32 months among UV-positive patients (p = 0.038).
Determination of Final Status
Of the 48 patients with prior/concurrent ipsilateral skin head and neck SCC, 23 (48%) underwent sequencing and all had positive UV signature. One failed attempted sequencing and the remaining 24 were untested. The 25 patients with a known history of cutaneous SCC on the ipsilateral side of the parotid tumor who did not undergo molecular analysis were presumed, but not confirmed, to have a skin primary based on clinical history. Only one patient whose tissue was not available for testing had both longstanding acinic keratoses and recent immunosuppression with azathioprine, placing him at risk for both UV-related and non-UV-related SCC. Of the 13 patients with no history of SCC, 2 (15%) were UV positive, rendering them cutaneous metastases. One had a documented history of sun exposure. The remaining 11 (85%) with no history of SCC (6 UV negative and 5 not tested) could be considered unknown or potentially parotid primary. Of the 10 patients with a history of mucosal SCC, 3 (30%) were UV negative and 7 were not tested: 9 were determined to have parotid involvement by mucosal SCC based on clinical history and/or HPV testing; 1 could be considered unknown or potentially parotid primary (see Supplemental Digital Content; Supplemental Text Addendum “Detailed analysis of mucosal primary patients”).
Discussion
This study investigates rates of UV signature mutations in squamous cell carcinomas involving the parotid gland as well as differences in clinical features between tumors of cutaneous origin and tumors of non-cutaneous (parotid or mucosal) origin. This is the first study of UV signature mutations as a mechanism to determine the primary source of a parotid SCC. These tumors are rare and difficult to distinguish from one another using traditional histology and immunohistochemistry [1–11]. The presence of UV signature mutations may not only provide a solution to this diagnostic dilemma but may also have important ramifications for prognosis and treatment, and therefore, clinical teams may elect to perform routine testing of these tumors.
To date, the true incidence of primary parotid SCC has been difficult to ascertain. The patient’s clinical history has traditionally been used in order to support a diagnosis of metastasis, and the clinician must rely on the patient to provide accurate historical data [1–8]. Primary parotid SCC has therefore been a diagnosis of exclusion, and it is likely that, in the absence of relevant clinical history, some tumors may have been misclassified.
Despite this diagnostic challenge, other studies have confirmed generally lower rates of primary parotid SCC compared to metastases to the parotid. In a prospective review of 232 parotidectomies, Bron et al. reported that 77% represented metastatic disease and 23% likely represented primary salivary gland disease [8]. In a retrospective review of 40 parotid SCCs, Flynn et al. reported 80% represented metastatic disease [2]. Finally, in a review of tumor registry data, Ying et al. found that 62% of patients with parotid SCC had metastatic disease from another known primary [6]. Similarly, the current study found that 73% of tumors that underwent molecular analysis showed evidence of a UV signature, confirming metastasis from a cutaneous source. Unlike the current study, prior studies have found no difference in survival between patients with potential primary parotid SCC compared to metastatic SCC [6, 8, 11]. This discrepancy suggests one of two possibilities: either that there is no difference in clinical outcomes between these tumor types, or that some of the reported tumors may have been misclassified, obscuring a possible clinical difference.
Indeed, this study finds key differences between cutaneous metastatic SCC and non-cutaneous SCC involving the parotid gland, supported by mutational analysis. Patients with confirmed UV-negative tumors experienced significantly higher rates of mortality, with 67% dying of disease compared to 20% patients with UV-positive tumors dying of disease (p = 0.016). Patients with UV-negative tumors also had a more rapid disease course, with an average time to death of 9 months from diagnosis compared to patients whose tumors showed UV positivity, who had an average time to death from diagnosis of 32 months (p = 0.038). Furthermore, patients with UV-negative tumors were significantly younger and more racially diverse. Together, these findings suggest that UV-positivity, and therefore metastasis from a cutaneous source, reflects a less aggressive disease course in an older population.
Based on the present findings, a combination of clinical history and molecular analysis can be used to determine the primary source of SCC involving the parotid gland. In the current study, two patients without a known history of cutaneous squamous cell carcinoma were determined to be UV-positive. Therefore, UV signature analysis might identify additional patients without clear histories, thereby suggesting a better prognosis for these patients. Clinicians could consider more aggressive treatment or more frequent follow-up in patients with confirmed UV-negative tumors.
One remaining challenge is the identification of metastatic tumors from a mucosal site. Though confirmed UV-negativity can rule out metastasis from a sun-exposed site, the tumor may still represent a primary parotid carcinoma or metastasis/direct extension from a primary mucosal site. In such cases, clinical history is essential to most accurately determine the primary site. When a prior mucosal site is available for comparative testing, HPV status can be a useful marker to determine the primary site [33]. In the current study, two tumors were confirmed to be HPV-positive, supporting metastasis from known oropharyngeal primary. Additionally, the possibility that UV-negative SCCs could represent salivary-type carcinomas with diffuse or extensive squamous differentiation obscuring underlying morphology should be considered. However, prognosis and treatment will likely be driven by the high grade nature, regardless of classification as squamous cell carcinoma or salivary type carcinoma mimicking squamous cell carcinoma.
Limitations to this study include: this is a single-institution study with a moderate number of patients. Some samples were not available for histologic and molecular analysis, as some contained scant tissue insufficient for testing and some were referral cases in which H&E slides and tissue blocks were not available. Despite these limitations, the study results are consistent with rates of metastasis found in other studies.
In conclusion, parotid SCC most frequently represents metastasis/direct extension from a cutaneous site but may also potentially represent a primary tumor of the parotid or metastasis/extension from a mucosal site. Patients with cutaneous primaries (UV-positive parotid tumors) tend be older, white males. Outcomes in these patients are better than in those with non-cutaneous primaries (UV-negative parotid tumors). UV signature mutations can be a useful indicator of cutaneous origin, and sequencing should be considered in patients with tumors of unclear origin in order to guide treatment and prognostication.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the University of Chicago Pritzker School of Medicine and the Human Tissue Resource Center at the University of Chicago Medicine (Terri Li, Technical Director; Dr. Mark Lingen; Faculty Director).
Funding
No relevant grant funding to report.
Data Availability
Not applicable.
Code Availability (Software Application or Custom Code)
Not applicable.
Consent for Publication
All authors have given consent for publication.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was waived based on approval by Institutional Review Board.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shira Fishbach, Email: Shira.Fishbach@uchospitals.edu.
George Steinhardt, Email: gsteinhardt@bsd.uchicago.edu.
Chao Jie Zhen, Email: ChaoJie.Zhen@uchospitals.edu.
Rutika Puranik, Email: rpuranik@luriechildrens.org.
Jeremy P. Segal, Email: jsegal5@bsd.uchicago.edu
Nicole A. Cipriani, Email: Nicole.Cipriani@uchospitals.edu
References
- 1.Fang Q, Wu J, Liu F. Oncologic outcome and potential prognostic factors in primary squamous cell carcinoma of the parotid gland. BMC Cancer. 2019;19(1):752. doi: 10.1186/s12885-019-5969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn MB, Maguire S, Martinez S, et al. Primary squamous cell carcinoma of the parotid gland: the importance of correct histological diagnosis. Ann Surg Oncol. 1999;6(8):768–770. doi: 10.1007/s10434-999-0768-y. [DOI] [PubMed] [Google Scholar]
- 3.Gaughan RK, Olsen KD, Lewis JE. Primary squamous cell carcinoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 1992;118(8):798–801. doi: 10.1001/archotol.1992.01880080020006. [DOI] [PubMed] [Google Scholar]
- 4.Lee SW, Kim GE, Park CS, et al. Primary squamous cell carcinoma of the parotid gland. Am J Otolaryngol. 2001;22(6):400–406. doi: 10.1053/ajot.2001.28068. [DOI] [PubMed] [Google Scholar]
- 5.Sterman BM, Kraus DH, Sebek BA, et al. Primary squamous cell carcinoma of the parotid gland. Laryngoscope. 1990;100(2):146–148. doi: 10.1288/00005537-199002000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Ying YL, Johnson JT, Myers EN. Squamous cell carcinoma of the parotid gland. Head Neck J Sci Spec Head Neck. 2006;28(7):626–632. doi: 10.1002/hed.20360. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar K, Ray PS, Sherwani R, et al. Primary squamous cell carcinoma of the parotid gland: a rare entity. Case Rep. 2013 doi: 10.1136/bcr-2013-009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bron LP, Traynor SJ, McNeil EB, et al. Primary and metastatic cancer of the parotid: comparison of clinical behavior in 232 cases. Laryngoscope. 2003;113(6):1070–1075. doi: 10.1097/00005537-200306000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Chen MM, Roman SA, Sosa JA, et al. Prognostic factors for squamous cell cancer of the parotid gland: an analysis of 2104 patients. Head Neck. 2015;37(1):1–7. doi: 10.1002/hed.23566. [DOI] [PubMed] [Google Scholar]
- 10.Franzen A, Lieder A, Guenzel T, et al. The heterogenicity of parotid gland squamous cell carcinoma: a study of 49 patients. In Vivo. 2019;33(6):2001–2006. doi: 10.21873/invivo.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfisterer MJ, Vazquez A, Mady LJ, et al. Squamous cell carcinoma of the parotid gland: a population-based analysis of 2545 cases. Am J Otolaryngol. 2014;35(4):469–475. doi: 10.1016/j.amjoto.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Thompson L. Parotid gland squamous cell carcinoma: direct cutaneous invasion versus metastatic disease: a clinicopathologic study of 95 cases (abs 661). Abstracts head and neck pathology. Mod Pathol. 2021;34(Suppl 2):791–792. [Google Scholar]
- 13.Hinerman RW, Indelicato DJ, Amdur RJ, et al. Cutaneous squamous cell carcinoma metastatic to parotid-area lymph nodes. Laryngoscope. 2008;118(11):1989–1996. doi: 10.1097/MLG.0b013e318180642b. [DOI] [PubMed] [Google Scholar]
- 14.Badlani J, Gupta R, Smith J, et al. Metastases to the parotid gland—a review of the clinicopathological evolution, molecular mechanisms and management. Surg Oncol. 2018;27(1):44–53. doi: 10.1016/j.suronc.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Audet N, Palme CE, Gullane PJ, et al. Cutaneous metastatic squamous cell carcinoma to the parotid gland: analysis and outcome. Head Neck J Sci Spec Head Neck. 2004;26(8):727–732. doi: 10.1002/hed.20048. [DOI] [PubMed] [Google Scholar]
- 16.Bumpous J. Metastatic cutaneous squamous cell carcinoma to the parotid and cervical lymph nodes: treatment and outcomes. Curr Opin Otolaryngol Head Neck Surg. 2009;17(2):122–125. doi: 10.1097/MOO.0b013e32832924e0. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien CJ, McNeil EB, McMahon JD, et al. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck J Sci Spec Head Neck. 2002;24(5):417–422. doi: 10.1002/hed.10063. [DOI] [PubMed] [Google Scholar]
- 18.Khurana VG, Mentis DH, O'Brien CJ, et al. Parotid and neck metastases from cutaneous squamous cell carcinoma of the head and neck. Am J Surg. 1995;170(5):446–450. doi: 10.1016/S0002-9610(99)80326-2. [DOI] [PubMed] [Google Scholar]
- 19.Veness MJ, Porceddu S, Palme CE, et al. Cutaneous head and neck squamous cell carcinoma metastatic to parotid and cervical lymph nodes. Head Neck J Sci Spec Head Neck. 2007;29(7):621–631. doi: 10.1002/hed.20576. [DOI] [PubMed] [Google Scholar]
- 20.Taxy JB. Squamous carcinoma in a major salivary gland: a review of the diagnostic considerations. Arch Pathol Lab Med. 2001;125(6):740–745. doi: 10.5858/2001-125-0740-SCIAMS. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Cliften PF, Duncavage EJ, et al. UV signature mutations reclassify salivary high-grade neuroendocrine carcinomas as occult metastatic cutaneous Merkel cell carcinomas. Am J Surg Pathol. 2019;43(5):682–687. doi: 10.1097/PAS.0000000000001231. [DOI] [PubMed] [Google Scholar]
- 22.Yang C, Sanchez-Vega F, Chang JC, et al. Lung-only melanoma: UV mutational signature supports origin from occult cutaneous primaries and argues against the concept of primary pulmonary melanoma. Mod Pathol. 2020;33(11):2244–2255. doi: 10.1038/s41379-020-0594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brash DE. UV signature mutations. Photochem Photobiol. 2015;91(1):15–26. doi: 10.1111/php.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan JW, Yeh I, El-Sayed IH, et al. Ultraviolet light-related DNA damage mutation signature distinguishes cutaneous from mucosal or other origin for head and neck squamous cell carcinoma of unknown primary site. Head Neck. 2019;41(6):E82–E85. doi: 10.1002/hed.25613. [DOI] [PubMed] [Google Scholar]
- 26.Ikehata H, Ono T. The mechanisms of UV mutagenesis. J Radiat Res. 2011;52(2):115–125. doi: 10.1269/jrr.10175. [DOI] [PubMed] [Google Scholar]
- 27.Pfeifer GP, You YH, Besaratinia A. Mutations induced by ultraviolet light. Mutat Res/Fundam Mol Mech Mutagen. 2005;571(1–2):19–31. doi: 10.1016/j.mrfmmm.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 28.Pickering CR, Zhou JH, Lee JJ, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20(24):6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang D, Shain AH. The landscape of driver mutations in cutaneous squamous cell carcinoma. bioRxiv. 1 Jan 2020. [DOI] [PMC free article] [PubMed]
- 30.Kadri S, Long BC, Mujacic I, et al. Clinical validation of a next-generation sequencing genomic oncology panel via cross-platform benchmarking against established amplicon sequencing assays. J Mol Diagn. 2017;19(1):43–56. doi: 10.1016/j.jmoldx.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Bergstrom EN, Huang MN, Mahto U, et al. SigProfilerMatrixGenerator: a tool for visualizing and exploring patterns of small mutational events. BMC Genomics. 2019;20(1):1–2. doi: 10.1186/s12864-019-6041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):1–4. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Mofty SK, Zhang MQ, Davila RM. Histologic identification of human papillomavirus (HPV)-related squamous cell carcinoma in cervical lymph nodes: a reliable predictor of the site of an occult head and neck primary carcinoma. Head Neck Pathol. 2008;2(3):163–168. doi: 10.1007/s12105-008-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.
Consent for Publication
All authors have given consent for publication.