Abstract
Myoepithelial carcinoma (MECA) is a rare salivary gland (SG) neoplasm (0.1–0.45% of all SG tumors) that often presents with bland cytomorphology and can be misclassified as cellular pleomorphic adenoma (PA) or myoepithelioma. This is particularly challenging in MECA ex-PA cases, especially if tumor shows minimal to no capsular invasion. We report a rare case of a 76-year-old female; history of left superficial parotidectomy with diagnosis (outside hospital) of cellular PA, who re-presented 9 months post surgery with enlarging left parotid mass, neck lymphadenopathy and facial nerve deficits. FNAB of parotid and neck lymph node revealed cellular aspirates with loosely cohesive clusters of myoepithelial cells with occasional chondromyxoid stroma. Prior resection slides were reviewed, and diagnosis of MECA ex-PA was made. Patient underwent left radical parotidectomy, selective neck dissection, with facial nerve sacrifice (due to extensive encasing by tumor). Histology showed a multinodular tumor with pushing borders, zonal arrangement comprising of a hypocellular, necrotic/myxoid center, and a peripheral rim of myoepithelial cells, confirmed by positive S100, and p63. Tumor extensively infiltrated peri parotid soft tissues with multiple foci of lymphovascular and perineural invasion; and metastatic neck lymph nodes. Next generation sequencing revealed a novel TERT promoter mutation (c.-124C > T), not usually described in SG neoplasms. Further, PD-L1 immunohistochemistry showed positive expression, making patient eligible for anti-PDL-1 immunotherapy. This case highlights importance of recognizing the subtle malignant features of MECA in distinguishing it from benign mimics like PA. In addition, presence of TERT mutation opens a new arena for future research to explore potential treatment targets.
Keywords: Myoepithelial, Carcinoma ex-Pleomorphic adenoma, PD-L1, TERT
Introduction
Salivary gland neoplasms are histologically diverse, with 20 distinct types recognized in the World Health Organization (WHO) classification of head and neck tumors (4th edition, 2017) [1]. Myoepithelial carcinoma (MECA) accounts for 0.1–0.45% of all salivary gland tumors and generally arises in the parotid gland, although other major and minor salivary glands can also be affected. These tumors typically present in the sixth and seventh decades of life with no sex predilection [2, 3]. MECA can arise de novo or in association with a pre-existing pleomorphic adenoma (PA) aka myoepithelial carcinoma ex-pleomorphic adenoma (MECA ex-PA) [2, 3].
CA ex-PA is an epithelial/ myoepithelial malignancy that arises in a pleomorphic adenoma (primary or recurrent) and usually presents as rapidly growing mass in a pre-existing parotid nodule. CA ex-PA accounts for approximately 3.6% of all salivary gland neoplasms. These tumors can range from < 1 to > 20 cm [4]. MECA has been reported to be the second most common histologic type of CA ex-PA after salivary duct carcinoma [2].
Histologically, MECA is a tumor composed almost entirely of myoepithelial cells and exhibits an invasive growth pattern [1, 4]. Diagnosis of malignancy is straightforward when tumor shows overt cytologic atypia and infiltrative growth. However, MECA often presents with bland cytomorphology, making the diagnosis particularly challenging. It is not uncommon that these tumors get misclassified as a benign salivary gland tumor such as cellular PA or myoepithelioma [2]. This is particularly challenging in MECA ex-PA cases, especially with the in situ and/or minimally invasive ones [2, 3].
MECA ex-PA is an aggressive neoplasm with a high frequency of local recurrence, (37%), and distant metastasis (22%) even when intracapsular or minimally invasive [2]. Therefore, it is important to distinguish MECA from its benign mimics early in the course of disease, to provide adequate management and follow up for the patients. Herein, we present a case of MECA ex-pleomorphic adenoma of the parotid gland in a 76-year-old female, that was initially misinterpreted as benign PA and recurred within a short span after initial surgery, with extensive loco-regional metastasis. In addition, a novel TERT mutation was detected in the tumor by Next Generation sequencing, the significance of which is unknown at this time. Further, literature on this rare entity is reviewed.
Case Presentation
Clinical History
76-year-old female with multiple comorbidities, including chronic kidney disease, left thigh juxta-articular myxoma resected in 2013, and cellular pleomorphic adenoma (PA) s/p left superficial parotidectomy in June 2019 presented for evaluation of an enlarging left parotid mass and left neck lymphadenopathy. The patient noted an increase in size of her left parotid over the previous five months with tenderness to palpation and left frontal facial nerve deficits.
Imaging
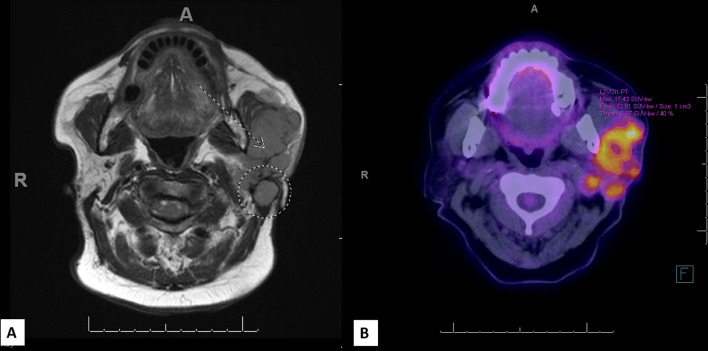
MRI showed a heterogeneous mass centered in the superficial portion of the left parotid gland with irregular enhancement measuring 6.6 × 5.0 × 4.8 cm (Fig. 1A). The mass extended to the stylomastoid notch and showed abnormal thickening and enhancement of the left facial nerve mastoid segment. The mass also abutted and displaced the medial pterygoid and masseter. Pathologic adenopathy involving left level II and V as well as the external jugular chain were identified. Subsequent PET scan showed multiloculated, hypermetabolic, potentially centrally necrotic left parotid disease, essentially contiguous with multifocal left cervical adenopathy (Fig. 1B).
Fig. 1.
A MRI showed a heterogeneous, irregular enhancing mass in left parotid gland measuring 6.6 cm in largest dimension (A, dotted arrow). Pathologic adenopathy involving left level V station was identified (Dotted circle). B PET scan showed a hypermetabolic, multiloculated, centrally necrotic, left parotid mass with multifocal left cervical adenopathy (B)
Fine Needle Aspiration (FNA)
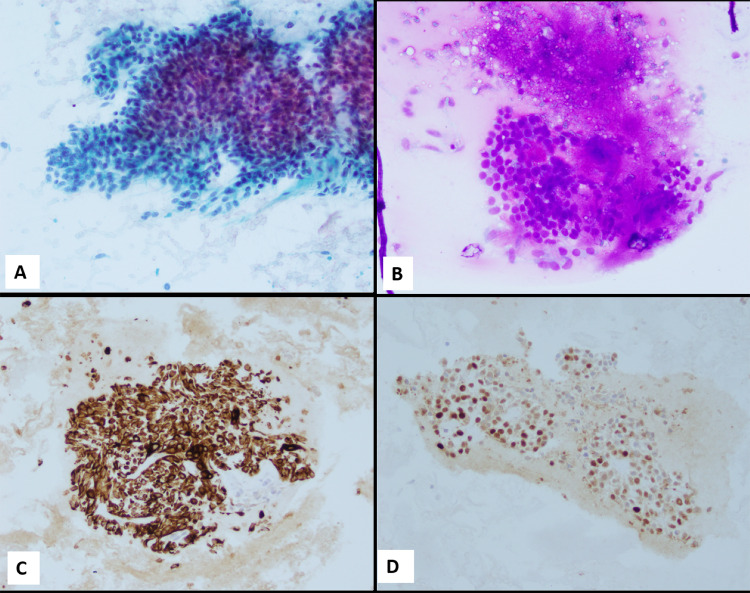
FNA biopsies of the recurrent left parotid gland mass as well as the left neck mass revealed highly cellular aspirates with groups as well as singly scattered bland appearing myoepithelial cells. Background showed focal evidence of necrosis and, chondromyxoid stromal fragments associated with few myoepithelial groups (Fig. 2A and B). Mild anisonucleosis was appreciated, however, marked atypia, increased mitotic figures were absent. Immunocytochemistry confirmed the myoepithelial origin of the tumor cells; positive for CK-pan keratin {AE1/AE3: Cell Marque, Rocklin, CA, USA; CK5: Leica Biosystems, Buffalo Grove, IL, USA; Cam5.2: Becton Dickinson, San Jose, CA, USA; CK8 + 18: Abcam, Cambridge, MA, USA, (Fig. 2C)}, and S100 {Biocare Medical, Pacheco, CA, USA, (Fig. 2D)} while negative for GFAP (Ventana Medical Systems, Oro Valley, AZ, USA). A cytologic diagnosis of “Positive for neoplasm, with myoepithelial features” was rendered.
Fig. 2.
A, B FNAB of recurrent parotid mass and neck lymph node (both) revealed cellular aspirates with loosely cohesive clusters of myoepithelial cells (A, Papanicolaou stain); with occasional chondromyxoid stroma (B, Diff Quik stain). C, D Tumor cells show strong positive expression of Pan-keratin stain and S100 (respectively). Magnification in A–D is 20 ×
Because the patient presented less than one year post surgery with rapid recurrence of her parotid mass, it led us to review the initial outside surgical pathology report from June 2019.
Review of Patient’s Prior Superficial Parotidectomy Specimen (Outside Pathology)
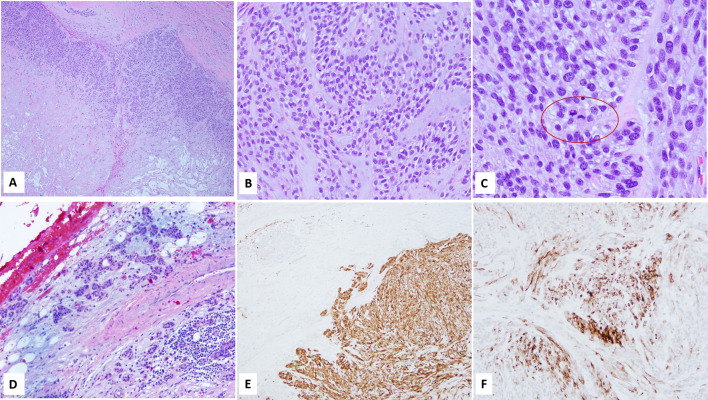
Sections from the specimen showed a cellular, largely encapsulated lesion with an expansile lobulated growth pattern, hypo- and hyper cellular areas (zonal distribution), and a monotonous tumor cell population of myoepithelial cells. Approximately 30% tumor showed central necrosis with few hypocellular areas exhibiting a chondromyxoid stroma like appearance and few other foci of hyalinization. Mitotic figures were readily appreciated, up to 6/10 hpf (Fig. 3A–C). In addition, a single focus suspicious for capsular breech was identified.
Fig. 3.
A Superficial parotidectomy showed a relatively well circumscribed, nodular mass with zonal (hypocellular and hypercellular) growth pattern and central necrosis; B, C High power view showed monomorphic growth of myoepithelial cells with clear cytoplasm, intervening chondromyxoid stroma, and numerous mitotic figures (C); D Residual duct like formations, reminiscent of pleomorphic adenoma were identified in and around the capsule; E, F. Immunohistochemistry confirmed myoepithelial origin of the tumor cells, S100 (E) and Myosin (F). Magnification in A is 4 ×, B, D and F is 20 ×, C is 40 ×, and E is 10 ×
No duct like structures (usually seen in a PA), were identified within the encapsulated lesion. However, few ductal components reminiscent of PA were present in and outside the capsule (Fig. 3D), a finding not so infrequently observed in benign PAs.
Immunohistochemical workup was performed on the histology material from outside case and showed diffuse expression of S100 (Biocare Medical, Pacheco, CA, USA), and patchy myosin (Cell Marque, Rocklin, CA, USA) in the myoepithelial cells (Figs. 3E, F). Myb (Abcam, Cambridge, MA, USA), performed to rule out the possibility of solid variant of adenoid cystic carcinoma, was negative.
Given the monotonous myoepithelial cell proliferation (based on morphology and diffuse S100 positivity), expansile growth pattern, numerous mitotic figures, and extensive necrosis along with a focus suspicious for capsular breach, this lesion was re-characterized as a “myoepithelial carcinoma, ex PA, at least in situ” with focus suspicious for minimal (~ 1 mm) capsular invasion.
Considering the revisited diagnosis, the cytopathologic findings from the current parotid and neck masses were suspicious for recurrence of the patient’s left parotid primary neoplasm with possible metastasis from the patient’s known parotid primary. Excision of both the left parotid and neck mass were suggested to confirm the diagnostic impression and resolve this diagnostic conundrum.
Surgery
A left radical parotidectomy with facial nerve sacrifice (due to extensive encasing by the tumor), left selective neck dissection (levels IB, IIA, and IIB, and VA), with left facial preauricular skin excision and submental flap reconstruction was performed.
Pathology
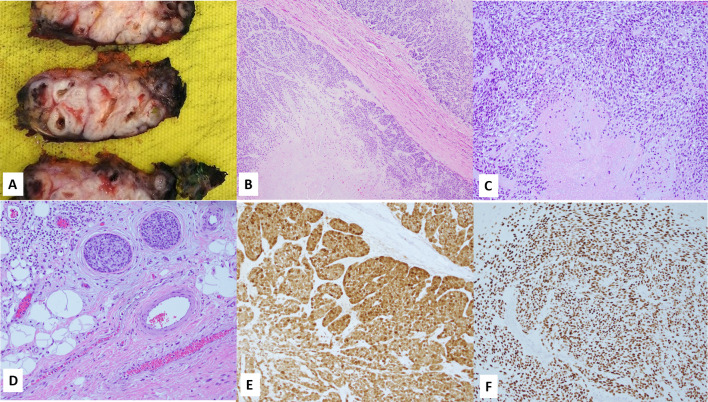
Grossly, tumor measured 6.9 (anterior–posterior) × 6.7 (superior-inferior) × 3.7 (superficial-deep) cm and sectioning showed a multinodular, tan, white tumor with pushing borders and multiple cysts like foci filled with translucent to brownish material (Fig. 4A). The accompanying neck dissection specimen showed several matted, grossly positive lymph nodes from level V.
Fig. 4.
A Gross exam showed a white tan lesion with multinodular configuration and multiple cystic foci filled with translucent to brownish material; B Histology of the radical parotidectomy specimen showed similar morphology to the prior specimen (Fig. 3) in the form of a multinodular myoepithelial cell tumor with pushing borders, zonal arrangement, and necrotic center (C); D Lymphovascular invasion; E, F Immunohistochemistry for S100 and p63 re confirmed the myoepithelial origin of tumor cells. Magnification in B is 4 ×, C, E and F are 10 ×, while D is 20 ×
Histology revealed nodules of tumor tissue extensively infiltrating the parotid gland as well as peri-parotid connective tissue and skeletal muscle of the neck. The tumor displayed a multinodular architecture with lobulated borders and zonal cellular arrangement with a hypercellular peripheral rim surrounding a hypocellular, necrotic center and areas of hyalinization {similar morphology to that seen in prior resection specimen without evidence of histologic progression, Fig. 4B, C)}. Perineural and lymphovascular invasion (Fig. 4D) were readily appreciated. Multiple lymph nodes (levels IIb and V) were positive for metastatic disease with the largest metastatic deposit measuring 2 cm along with extra-nodal invasion. Immunohistochemistry (select stains) were reperformed on the resection specimen and showed strong, diffuse S100 and p63 expression (Fig. 4E and F). Tumor was staged as pT3N3b.
Immunohistochemistry for PD-L1 (Dako, 22C3 pharmDx™) showed high positive expression with a combined positive score of 10, making patient eligible for immunotherapy (if needed).
Next generation sequencing {(NGS), NeoType™} detected a novel TERT promoter mutation (c.-124C > T) in the tumor. In addition, tumor cells expressed pan-TRK, however reflex NTRK NGS fusion studies were negative for mutation involving the NTRK genes.
Post-surgery, patient received 4 cycles of carboplatin along with radiation therapy. Three months post completion of chemo-radiation therapy, patient is doing well with minimal complaints of fatigue and congestion.
Discussion
Myoepithelial carcinoma is an extremely rare (< 2% of malignant salivary gland neoplasms), histologically diverse entity which is often misdiagnosed owing to its diverse array of morphologic features. MECA was first described by Stromeyer et al. [5] in 1975 and has been included in the World Health Organization classification of salivary gland tumors since 1991 [6]. MECA is defined as a malignant tumor composed exclusively of myoepithelial cells and showing evidence of invasion into the surrounding parenchyma [7, 8]. The importance of distinguishing MECA from its benign counterparts, specifically pleomorphic adenoma, lies in the considerable difference in clinical outcomes [3].
Clinically, MECA presents as a rapidly enlarging, painless mass, often associated with a previous benign tumor such as myoepithelioma or pleomorphic adenoma (MECA ex-PA). In fact, MECA has been reported as the second most common histologic type of CA ex-PA after salivary duct carcinoma [3]. The tumor mostly occurs in parotid gland but can arise in other major and minor salivary glands [2, 8]. Patients are usually in the 6th decade and there is no sex predilection; however, a study reported slight female predominance for this malignancy [9].
Histologic diagnosis of MECA can be challenging due to the following reasons: 1. Bland cytomorphology (absence of cytologic atypia), 2. Presence of focal tubular differentiation. The presence of former feature makes it particularly challenging in cases of MECA ex-PA with minimal to no capsular invasion [2]. The diagnostic problem is further confounded by the presence of pushing borders in majority of MECAs rather than infiltrative borders, latter being the traditional histologic criteria for diagnosing malignancy.
Kong et al. and later Xu et al. [2, 3] described two distinctive features of MECA that assist in differentiating it from benign neoplasms such as myoepithelioma and pleomorphic adenoma. The first is a multinodular, lobulated pattern of growth with pushing borders and tumor composed of a uniform population of myoepithelial cells [2, 3]. Xu et al. described this growth as an “expression of invasive pattern even when confined to the capsule of the tumor” [2]. Sternlicht et al. [10] demonstrated that neoplastic myoepithelial cells secrete abundant extracellular matrix as well as large amounts of proteinase inhibitors; a possible explanation for nodular growth pattern in MECA. In addition, it has been proposed that the monotony of the myoepithelial cells exemplifies their clonal growth and malignant transformation [2].
The second feature is a zonal cellular arrangement comprised of a hypocellular, occasionally necrotic or myxoid center with a hypercellular peripheral rim of myoepithelial cells [2, 3, 11]. Sampling of these hypocellular areas on FNAB can show chondromyxoid stromal fragments like those seen in a PA [12] and can mislead the pathologist to render a benign diagnosis, resulting in improper clinical management of these aggressive neoplasms. Our case showed all the above mentioned cytologic and histologic features, with the FNB showing metachromatic stroma closely associated with neoplastic myoepithelial cells (Fig. 2B), which led to the misdiagnosis of PA prior to the first resection.
Additional histologic features described in literature include presence of more than one morphologic cell types such as epithelioid, spindle cell, plasmacytoid and clear cells, numerous mitotic figures, and varying degree of intra-tumoral hyalinization [7, 9, 11, 13]. Histologic evidence of a preexisting PA can be difficult in cases of MECA arising in a PA. McCluggage et al. suggested histologic examination of the entire lesion to help identify a small residual PA component in a MECA ex-PA. In the absence of histological evidence, history of sudden increase in the size of a mass which has been present for some time, is suggestive of Ca ex-PA [14]. In the absence of this histological evidence, a history of sudden rapid increase in the size of a mass which has been present for some time is suggestive of an origin in a benign PA. Our case (both previous misinterpreted resection and the recurrent tumor) showed similar histology in the form of expansile, lobulated multinodular tumor with a zonal cellular pattern consisting of monotonous, bland myoepithelial cells and a hypocellular center with mixed foci of hyaline and chondromyxoid stroma and, necrosis. Evidence of PA was found in the first resection specimen in the form of bilayered ducts, within and immediately adjacent to the capsule (a finding commonly seen in conventional PAs).
Review of the English literature using the keywords “myoepithelial carcinoma, carcinoma ex-pleomorphic adenoma, myoepithelial carcinoma ex pleomorphic adenoma, myoepithelial carcinoma parotid gland” was performed. A total of 276 cases of MECA were found. Various case series reported cases in the range of 7–51 [2, 3, 7, 9, 11, 15–18]. In addition, 6 isolated case reports were included in this review [4, 12–14, 19, 20]. The two largest studies, [Skalova et al., 2015, and Kane et al., 2010] reported 72 and 51 cases of MECA, respectively. Skalova et al. [16] tested a variety of salivary gland carcinomas with clear cell features by fluorescence in situ hybridization (FISH) for EWSR1 rearrangement. Of the 94 cases they studied, 51 cases were clear cell MECA denovo, while 21 were clear cell MECA ex-PA. Authors found EWSR1 rearrangement in 25 MECA cases (20 denovo and 5 MECA ex-PA), all of which shared common histology showing large polyhedral tumor cells arranged in nodules, separated by varying degree of hyalinization. Majority affected the parotid gland; 19/25 cases showed pushing borders while 6 showed infiltration into the surrounding parotid parenchyma.
Kane et al. [7] on the contrary, reported higher incidence of MECAs in minor salivary glands (71%) compared to major salivary glands (29%). This retrospective review study found diffuse sheetlike growth pattern with cord like infiltration as the predominant pattern in MECA. The authors reported wide cytomorphologic variation in the 51 tumors they reviewed, with large, atypical epithelioid type tumor cells forming the majority.
Immunohistochemistry is often required to confirm the myoepithelial differentiation of tumor cells, especially in cases where morphology is suggestive but not definitive of MECA [3]. Various studies have reported variable staining patterns in these tumors, and it is suggested to use a panel of myoepithelial markers, including S100, p63, GFAP, calponin, myosin, and SMA as well as at least two–three different keratin stains to confirm the diagnosis of MECA [4, 7]. It has been reported [7, 11] that S100 and vimentin are extremely sensitive (not specific) markers of neoplastic myoepithelial cells, while SMA, calponin, and myosin might not be that helpful due to alteration in the neoplastic myoepithelial cells’ smooth muscle phenotype [7, 21].
Xu et al. and Savera et al. reported a 100% rate of expression for S100 in their studies, making it a must have myoepithelial marker in the work up of suspected myoepithelial carcinomas [2, 11]. In Savera et al.’s study, calponin and SMA were positive in 75% and 50% cases respectively (weak and limited expression of SMA than calponin), while MSA and GFAP were positive in 31% cases each [11]. Findings in our study agree with those reported in literature, in terms of strong and diffuse positive S100 expression, negative calponin, and weak patchy myosin expression. In addition, we found strong diffuse nuclear expression of p63 which corroborates with Wang et al. [9] and Xu et al.’s [2] findings (percentage of cases showing positive expression in these studies: 91% and 89% respectively).
At the molecular level, PLAG1 and HMGA2 rearrangements are the most common genetic aberrations in CA ex-PA including MECA type [2, 22]. Dalin et al. reported TGFBR3-PLAG1 fusion in 15% of studied MECAs while none of the benign neoplasms showed the same [23]. Most of the literature reports PLAG1 aberrations in Ca ex-PA while Dalin et al. found fusions of the same gene in de novo cases as well [23]. EWSR1 is a highly promiscuous gene and has been identified as a translocation partner in a wide variety of histomorphologically diverse tumors, such as the Ewing family of tumors, desmoplastic round cell tumor, myoepithelial tumors of skin and soft tissue and many other soft tissue tumors [16]. Skalova et al. studied 94 clear cell salivary gland tumors and found EWSR1 rearrangement in 25 MECA cases {20 de novo and 5 Ca ex-PA from major and minor salivary gland origin, [16]}.
Next generation sequencing of our patient’s tumor revealed a novel TERT promoter (c.-124C > T) mutation, not previously described in MECA. Isolated studies have investigated role of TERT mutations in salivary gland tumors, however; substantial evidence of its possible role in carcinogenesis of salivary gland malignancies is not elucidated. To the best of our knowledge, this is the first case report of a TERT promoter mutation in Myoepithelial carcinoma ex-PA of salivary gland. This finding raises the possibility of an alternate mechanism of carcinogenesis in these tumors and opens a new arena for future research into the same.
Telomeres are guanine-rich nucleotide repeats and serve as binding sites for telomere binding proteins [24]. The primary role of telomeres is to cap the chromosome ends and prevent chromosomal instability. Majority of human cells (except stem cells) lose telomerase activity after birth due to down regulation of the active protein component (called TERT). Activation of telomerase is the critical step for the immortalization of > 90% of all human tumors. Recent studies have found non-coding mutations in the promoter of the catalytic subunit of telomerase (TERT) as one of the most prevalent mutations in human cancer. TERT promoter mutations involved in carcinogenesis generate novel binding sites for the ETS (E26 transformation-specific) family of transcription factors and are located close to the translational start site of TERT (e.g., − 57A/C, − 124C/T, and − 146C/T) [25].
A study from Japanese researchers investigated telomerase activity and expression of telomerase components (hTERT, hTEP1, p23, Hsp90 and dyskerin) in a variety of salivary gland tumors (malignant tumors included mucoepidermoid carcinoma (MEC), acinic cell carcinoma, and ADCC), and found that both telomerase activity and hTERT mRNA expression are useful markers for the detection of malignant cells in salivary gland carcinomas [24].
A recent study by Alen et al. [26] performed an integrated genomic analysis of 1045 cases of advanced/ metastatic adenoid cystic carcinomas (ADCC) and found TERT promoter mutations in 13.1% of recurrent/ metastatic cases of the same. The authors found that TERT mutation were mutually exclusive with the other mutations found in ADCC such as NOTCH1 mutations and MYB/MYBL1 fusions, thereby raising the possibility of a discrete, alternative mechanism of tumorigenesis in ADCC.
Programmed cell death 1 (PD-1) is a transmembrane glycoprotein expressed on NK cells and T and B lymphocytes which on activation (on binding to its ligands PD-L1 or PD-L2), can inhibit cytotoxic T-Cell immune response leading to immune tolerance by tumor cells [27].
Anti-PD-1 therapy in the form of Pembrolizumab and atezolizumab has been approved by US Food and Drug administration for metastatic non-small cell lung carcinoma and bladder cancer, respectively. Few recent studies have investigated prognostic role of PD-L1 expression by IHC in salivary gland cancers and have found high PD-L1 expression in aggressive salivary gland carcinoma including salivary duct carcinoma and squamous cell carcinoma [28–30]. Harada et al. [30] studied 47 malignant salivary gland tumors (MEC, adenocarcinoma, and ADCC) and found PD-L1 expression in 51.1% malignancies with significant association to tumor stage, recurrence/metastasis after surgery and overall survival (OS). In addition, PD-L2 expression has been found in several tumor types, including adenoid cystic carcinoma [29].
Cohen et al. analyzed the safety and efficacy of Pembrolizumab in advanced salivary gland cancers and found it’s promising antitumor activity and a manageable safety profile in these patients [31]. Our findings in this case resonate the findings of high PD-L1 expression in recurrent and metastatic salivary gland carcinoma;MECA-ex-PA in this case, making patient eligible for immunotherapy with Pembrolizumab (if required). Our patient has not received any immunotherapy as of current and is disease free after a three month follow up period post completion of platinum-based chemotherapy and radiation therapy.
The differential diagnosis of MECA is broad and includes both benign and malignant neoplasms. Depending on the predominant cell type, differentials include epithelioid neoplasms like ADCC, adenocarcinoma NOS, basaloid squamous carcinoma in case of epithelioid cells; plasmacytoma, lymphoma, and malignant melanoma in case of plasmacytoid cells; hyalinizing clear cell carcinoma, epithelial myoepithelial carcinoma, metastatic renal cell carcinoma in case of clear cells; schwannoma, spindle cell squamous carcinoma, malignant peripheral nerve sheath tumor (MPNST), and fibrosarcoma in spindle cell morphology [7, 11]. It can be difficult to differentiate myoepithelial carcinoma showing epithelioid morphologic characteristics from other salivary gland neoplasms showing myoepithelial differentiation, especially ADCC and polymorphous adenocarcinoma. Evidence of luminal differentiation by EMA and CEA favors diagnosis of ADCC.
MECA is often diagnosed as a benign entity, specifically PA. Pleomorphic adenomas consist of ducts, myoepithelial cells, and stroma [2, 3]. The varied arrangement of these three components within the adenoma and the absence of myoepithelial cell expansion favor the benign process [2]. Additionally, pleomorphic adenomas typically do not display nuclear pleomorphism, atypia, significant mitotic activity, or tumor necrosis [2]. Of note, the malignant nodular architecture with pushing borders seen in MECA should not be mistaken for the benign protrusions (satellite nodules) typically observed in hypocellular myxoid PA.
As the overall prognosis of MECA is poor with high rate of recurrence [9], radical surgery with negative margins, with or without cervical lymph node dissection remains the treatment of choice. Wang et al. found lymph nodes to be the most common metastatic site in their study of MECA. 3/7 cases with metastatic cervical lymph nodes had a submandibular primary: thereby authors emphasized the need for supraomohyoid lymph node dissection in submandibular MECAs [9].
Chemotherapy is reserved for palliation or advanced disease while role of radiation therapy is still controversial [7, 9, 11]. In our case, the initial pleomorphic adenoma was treated as recommended in literature, by superficial parotidectomy. Upon recurrence and a revised diagnosis of MECA ex PA, subsequent management involved radical parotidectomy with attempted facial nerve preservation and neck dissection. However, during operation it was found that tumor was grossly involving the facial nerve, hence, it had to be sacrificed. Post-surgery, patient completed 4 cycles of chemoradiation therapy and is currently free of disease with a 3 month follow up period.
Clinically, MECA seems to be relatively aggressive and is associated with high risk of recurrence (cumulative recurrence rate of 43%), lymph node metastasis (cumulative rate of 21%), and distant metastasis (cumulative rate of 22%) [2]. Xu et al. highlighted that in contrast to MECA, its closest histologic mimic, cellular PA, did not show any distant metastasis and all patients previously studied were alive without evidence of disease. Moreover, in this study, 12 out of 14 MECA patients who developed recurrences had an initial benign diagnosis (7 PA and 3 myoepitheliomas). Although local recurrence alone is not indicative of malignant behavior, the high frequency of local recurrence in MECAs (33%) compared with the local recurrence rate (6%) in cellular PA suggests that MECA may correlate with more aggressive behavior [2]. Given the apparent diagnostic difficulty and the drastically different clinical outcomes between MECA and PA, it is crucial to recognize the pathologic features of MECA to distinguish this tumor from benign neoplasms and offer proper management to these patients.
Previous studies have lacked agreement regarding differences in clinical outcomes and prognosis based on the presence or absence of a PA component in MECA. Kong et al. and Xu et al. reported that MECA ex-PA correlated with significantly worse clinical behavior compared to de novo MECA and that MECA ex-PA can recur and cause death even when it is intracapsular, minimally invasive, or displays cytologically bland features with low mitotic activity [2, 3]. They also found that the majority of recurrent MECAs (71%) were MECA ex-PA and mostly (80%) intracapsular or minimally invasive tumors. In contrast, Di Palma and Guzzo [15] consider MECA ex-PA to be low-grade while de novo MECA to be high grade.
Similarly, no clear correlation was found between different histologic features of MECA and its clinical behavior [3, 7]. Kane et al. and Kong et al. did not find any correlation between nuclear atypia, mitosis, and disease-free survival (DFS) [3, 7]. Kong et al. suggested tumor necrosis as the defining feature of high grade MECA [3]. This suggestion was based on their finding of zero recurrence in all 23 patients lacking tumor necrosis with a median follow up of 4 years. Defining high-grade MECA on basis of tumor necrosis would make grading more feasible and reproducible. They also found that CA ex-PA and tumor necrosis may have independent effects [3], leading to an even poorer prognosis if both features are present. Our case has both features, and its aggressive clinical behavior is quite evident from its presentation and extensive disease recurrence.
The current case highlights the many challenges of this diagnostic entity, including a preexisting benign lesion, cytomorphologic heterogeneity, and bland overall cytology which resulted in the misdiagnosis of this aggressive malignancy as a benign neoplasm.
Conclusion
Salivary gland neoplasms provide a diagnostic challenge due to their rarity and diverse histomorphology. The current case represents a rare case of MECA ex-PA which was misdiagnosed as benign neoplasm on initial resection, and that recurred within a span of 9 months with extensive local disease and lymph node metastasis.
MECA is a challenging entity and may be easily overlooked and misclassified as a benign salivary gland neoplasm, especially when it is histologically bland and arises in association with a previous PA (MECA ex-PA). Cellular uniform myoepithelial growth with an expansile lobulated nodular pattern, and zonal cellular distribution are common histologic features of MECA and can help distinguish it from benign mimics. MECAs arising within a preexisting benign tumor should be suspected if there is a long history of benign parotid tumor with history of rapid growth and/or multiple recurrences in a preexisting PA with or without lymph node metastasis [8].
Such distinction is important as MECA can have adverse outcomes with a significant risk of distant and local recurrence even when it is intracapsular or minimally invasive MECA ex-PA [2] as was seen in our patient.
This case highlights the diagnostic dilemma of these heterogeneous malignancies and emphasizes the importance of recognizing these aggressive neoplasms and differentiating them from benign entities with overlapping morphology like PA and myoepithelioma. Thorough assessment must include diligent attention to clinical presentation, intraoperative findings, and histopathologic features to ensure that the proper diagnosis is established.
In addition, (to the best of our knowledge) this is the first case report of MECA ex-PA with TERT promotor mutation, the clinical significance of which is currently unknown in salivary gland carcinomas. Our finding hereby opens a new area for investigation of significance of TERT mutation in MECA and/ or Ca ex-PA and elucidate a possible alternate pathway of tumorigenesis in these rare and aggressive neoplasms.
Author Contributions
CC performed the literature search and wrote the first draft of the manuscript. SA had the idea for the article, was the primary diagnostician in this case, and critically revised the manuscript.
Declarations
Ethical Approval
This is a case report; hence, ethics approval is not applicable.
Consent for Publication
No patient’s personal data/ identifiers are disclosed; hence, no consent was taken.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brandwein-Gensler M, Bell D, Inagaki H, Katabi N, Leivo I, Seethala R, Triantafyllou A. Malignant tumours of the salivary gland. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. World Health Organization classification of Head and Neck Tumors. Lyon: IARC; 2005. pp. 160–184. [Google Scholar]
- 2.Xu B, Mneimneh W, Torrence DE, et al. Misinterpreted myoepithelial carcinoma of salivary gland: a challenging and potentially significant pitfall. Am J Surg Pathol. 2019;43(5):601–609. doi: 10.1097/PAS.0000000000001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong M, Drill EN, Morris L, et al. Prognostic factors in myoepithelial carcinoma of salivary glands: a clinicopathologic study of 48 cases. Am J Surg Pathol. 2015;39(7):931–938. doi: 10.1097/PAS.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sload RL, Carbone P, Johnson C, Johnson T. Carcinoma ex pleomorphic adenoma of the parotid gland. Acta Oto-Laryngologica Case Reports. 2016;1(1):67–70. doi: 10.1080/23772484.2016.1226140. [DOI] [Google Scholar]
- 5.Stromeyer FW, Haggitt RC, Nelson JF, Hardman JM. Myoepithelioma of minor salivary gland origin: light and electron microscopical study. Arch Pathol. 1975;99(5):242–245. [PubMed] [Google Scholar]
- 6.World Health Organization. Seifert G, Sobin LH. World Health Organization international histological classification of tumors. 2. Berlin: Springer; 1991. [PMC free article] [PubMed] [Google Scholar]
- 7.Kane SV, Bagwan IN. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 51 cases in a tertiary cancer center. Arch Otolaryngol Head Neck Surg. 2010;136(7):702–712. doi: 10.1001/archoto.2010.104. [DOI] [PubMed] [Google Scholar]
- 8.Thompson LDR, Wenig B. Myoepithelial carcinoma. In: Pathology D, editor. Head and neck. Salt Lake City: Elsevier; 2017. pp. 582–585. [Google Scholar]
- 9.Wang C, Zhang Z, Ge Y. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 29 patients. J Oral Maxillofac Surg. 2015;73:1938–1945. doi: 10.1016/j.joms.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Sternlicht MD, Safarians S, Rivera SP, Barsky SH. Characterizations of the extracellular matrix and proteinase inhibitor content of human myoepithelial tumors. Lab Invest. 1996;74(4):781–796. [PubMed] [Google Scholar]
- 11.Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24(6):761–774. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Romano A, Dell’Aversana Orabona G, Pansini A, Salzano G, Cozzolino I, Cieri M, et al. Clear cell myoepithelial carcinoma ex pleomorphic adeno-ma of parotid gland: case report and review of literature. Oral Maxillofac Surg Cases. 2018;4(1):12–16. doi: 10.1016/j.omsc.2017.12.002. [DOI] [Google Scholar]
- 13.Said S, Campana J. Myoepithelial carcinoma ex pleomorphic adenoma of salivary glands: a problematic diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:196–201. doi: 10.1016/j.tripleo.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 14.McCluggage WG, Primrose WJ, Toner PG. Myoepithelial carcinoma (malignant myoepithelioma) of the parotid gland arising in a pleomorphic adenoma. J Clin Pathol. 1998;51(7):552–556. doi: 10.1136/jcp.51.7.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Palma S, Guzzo M. Malignant myoepithelioma of salivary glands: clinocopathological features of ten cases. Virchows Archiv A Pathol Anat. 1993;423:389–396. doi: 10.1007/BF01607152. [DOI] [PubMed] [Google Scholar]
- 16.Skálová A, Weinreb I, Hyrcza M, Simpson RH, Laco J, Agaimy A, Vazmitel M, Majewska H, Vanecek T, Talarčik P, Manajlovic S, Losito SN, Šteiner P, Klimkova A, Michal M. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: molecular analysis of 94 salivary gland carcinomas with prominent clear cell component. Am J Surg Pathol. 2015;39(3):338–348. doi: 10.1097/PAS.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Li L, Zeng M, Zhu X, Zhang J, Chen X. Myoepithelial carcinoma of intraoral minor salivary glands: a clinicopathological study of 7 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(1):85–93. doi: 10.1016/j.tripleo.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Nagao T, Sugano I, Ishida Y, Tajima Y, Matsuzaki O, Konno A, Kondo Y, Nagao K. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998;83(7):1292–1299. doi: 10.1002/(SICI)1097-0142(19981001)83:7<1292::AID-CNCR4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Di Palmi S, Guzzo M. Myoepithelial carcinoma with predominance of plasmacytoid cells arising in a pleomorphic adenoma of the parotid gland (let) Histopathology. 1998;33:485. doi: 10.1046/j.1365-2559.1998.0491b.x. [DOI] [PubMed] [Google Scholar]
- 20.Parwani AV, Lujan G, Ali SZ. Myoepithelial carcinoma arising in a pleomorphoc adenoma of the parotid gland: report of a case with cytopathologic findings. Acta Cytol. 2006;50:93–96. doi: 10.1159/000325902. [DOI] [PubMed] [Google Scholar]
- 21.Alos L, Cardesa A, Bombi JA, Mallofre C, Cuchi A, Traserra J. Myoepithelial tumors of salivary glands: a clinicopathologic, immunohistochemical, ultrastructural, and flow-cytometric study. Semin Diagn Pathol. 1996;13(2):138–147. [PubMed] [Google Scholar]
- 22.Bahrami A, Dalton JD, Shivakumar B, et al. PLAG1 alteration in carcinoma ex pleomorphic adenoma: immunohistochemical and fluorescence in situ hybridization studies of 22 cases. Head Neck Pathol. 2012;6:328–335. doi: 10.1007/s12105-012-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalin MG, Katabi N, Persson M, et al. Multi-dimensional genomic analysis of myoepithelial carcinoma identifies prevalent oncogenic gene fusions. Nat Commun. 2017;8:1197. doi: 10.1038/s41467-017-01178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shigeishi H, Sugiyama M, Tahara H, Ono S, Kumar Bhawal U, Okura M, Kogo M, Shinohara M, Shindoh M, Shintani S, Hamakawa H, Takata T, Kamata N. Increased telomerase activity and hTERT expression in human salivary gland carcinomas. Oncol Lett. 2011;2(5):845–850. doi: 10.3892/ol.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiba K, Johnson JZ, Vogan JV, Wagner T, Boyle JM, Hockemeyer D. Cancer-associated TERT promoter mutations abrogate telomerase silencing. Elife. 2015;4:e07918. doi: 10.7554/eLife.07918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho AS, Ochoa A, Jayakumaran G, Zehir A, Valero Mayor C, Tepe J, Makarov V, Dalin MG, He J, Bailey M, Montesion M, Ross JS, Miller VA, Chan L, Ganly I, Dogan S, Katabi N, Tsipouras P, Ha P, Agrawal N, Solit DB, Futreal PA, El Naggar AK, Reis-Filho JS, Weigelt B, Ho AL, Schultz N, Chan TA, Morris LG. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J Clin Invest. 2019;129(10):4276–4289. doi: 10.1172/JCI128227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torrez M, Braunberger RC, Yilmaz E, Agarwal S. Primary squamous cell carcinoma of thyroid with a novel BRAF mutation and High PDL-1 expression: a case report with treatment implications and review of literature. Pathol Res Pract. 2020;216(10):153146. doi: 10.1016/j.prp.2020.153146. [DOI] [PubMed] [Google Scholar]
- 28.Mukaigawa T, Hayashi R, Hashimoto K, et al. Programmed death ligand-1 expression is associated with poor disease-free survival in salivary gland carcinomas. J Surg Oncol. 2016;114:36–43. doi: 10.1002/jso.24266. [DOI] [PubMed] [Google Scholar]
- 29.Sridharan V, Gjini E, Liao X, et al. Immune profiling of adenoid cystic carcinoma: PD-L2 expression and associations with tumor infiltrating lymphocytes. Cancer Immunol Res. 2016;4:679–687. doi: 10.1158/2326-6066.CIR-16-0031. [DOI] [PubMed] [Google Scholar]
- 30.Harada K, Ferdous T, Ueyama Y. PD-L1 expression in malignant salivary gland tumors. BMC Cancer. 2018;18(1):156. doi: 10.1186/s12885-018-4069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen RB, Delord JP, Doi T, Piha-Paul SA, Liu SV, Gilbert J, Algazi AP, Damian S, Hong RL, Le Tourneau C, Day D, Varga A, Elez E, Wallmark J, Saraf S, Thanigaimani P, Cheng J, Keam B. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the Phase 1b KEYNOTE-028 study. Am J Clin Oncol. 2018;41(11):1083–1088. doi: 10.1097/COC.0000000000000429. [DOI] [PMC free article] [PubMed] [Google Scholar]