Abstract
Salivary and mammary glands are both exocrine organs sharing multiple tumorigenic processes. To the best of our knowledge, salivary gland tumors mimicking invasive lobular carcinoma of the breast have not yet been described. Herein, we report a case of a 62-year-old male who presented with progressive facial paralysis. Pathologic examination revealed an ill-defined epithelial neoplasm exhibiting discohesive growth set within an extensively fibrotic stroma. Both perineural and intraneural invasion were present. E-cadherin and p120 immunostaining showed aberrant cytoplasmic expression. Targeted next-generation sequencing detected a frameshift mutation of the CTNNA1 gene as the only known pathogenic variant. The patient was treated with surgical resection, immunotherapy, and chemotherapy. Currently, he is alive with disease twenty months after disease onset.
Keywords: Lobular carcinoma, Breast, Cadherins, Salivary glands, CTNNA1, Salivary duct carcinoma
Introduction
Salivary and mammary glands are both exocrine organs that share multiple tumorigenic processes. Some examples include secretory carcinoma, adenoid cystic carcinoma, acinic cell carcinoma, and mucoepidermoid carcinoma, which share histologic, immunophenotypic and molecular features in both organs. Further, salivary duct carcinoma (SDC) of the salivary gland can mimic high grade in situ and invasive ductal carcinoma of the breast. To the best of our knowledge, a salivary gland tumor mimicking invasive lobular carcinoma of the breast (ILC) has not yet been reported. The closest entity would be salivary duct carcinoma with rhabdoid-like features (SDCRF) first described by Kusafuka et al. in 2014 [1].
Morphologically, SDCRF is characterized by a diffuse proliferation of large rhabdoid cells with eosinophilic cytoplasm, eccentric nuclei, and reduced cell-cell adhesion. By immunohistochemistry, the majority of the cases shows no or aberrant staining of E-cadherin. Genetically, there is some evidence of CDH1 gene alteration [1–3]. Although SDCRF is considered by Kusafuka et al. as a salivary counterpart of pleomorphic lobular carcinoma of the breast, as the name implies, it is listed in the 4th edition of the WHO classification of Head and Neck Tumors as a variant of SDC based on the coexistence of conventional SDC or intraductal carcinoma with high-grade cytologic features.
We herein report a case of salivary gland carcinoma bearing morphologic and genetic resemblance to ILC, supported by exclusive discohesive growth without an accompanying conventional SDC or intraductal carcinoma component, androgen receptor (AR) negativity, aberrant E-cadherin and p120 immunostaining, and pathogenic CTNNA1 mutation.
Case History
A 62-year-old Asian male presented with progressive left facial paralysis for seven months. Imaging performed at an outside hospital at onset was reportedly negative but unavailable for review. The patient was noted to have a remote history of stage III nasopharyngeal carcinoma status post neck radiation and chemotherapy with cisplatin/fluorouracil thirty-five years ago that was complicated by right mandible osteoradionecrosis, and a T3N0M0 conventional high-grade papillary urothelial carcinoma of the renal pelvis status post left nephroureterectomy and adjuvant chemotherapy thirteen years ago. The case of patient’s nasopharyngeal carcinoma was no longer available for review. The slides from his nephroureterectomy specimen were re-reviewed and the diagnosis of conventional papillary urothelial carcinoma was confirmed. Follow-up magnetic resonance imaging (MRI) performed at our institution two years ago was negative for residual or recurrent disease. Other comorbidities included carotid stenosis, hypercholesterolemia, hypertension, hypothyroidism, polio, prediabetes, vitamin D deficiency, elevated urine levels of catecholamines, Barrett’s esophagus with dysplasia, and intestinal metaplasia of gastric mucosa. The patient was a never smoker, did not use alcohol recently, and denied drug use. His serial prostate-specific antigen levels had been stable and within normal range over the past sixteen years, with the most recent value reported being 1.2 ng/mL (reference range ≤ 4.0 ng/mL). Notably, his family history is positive for lung cancer of unspecified type in his brother.
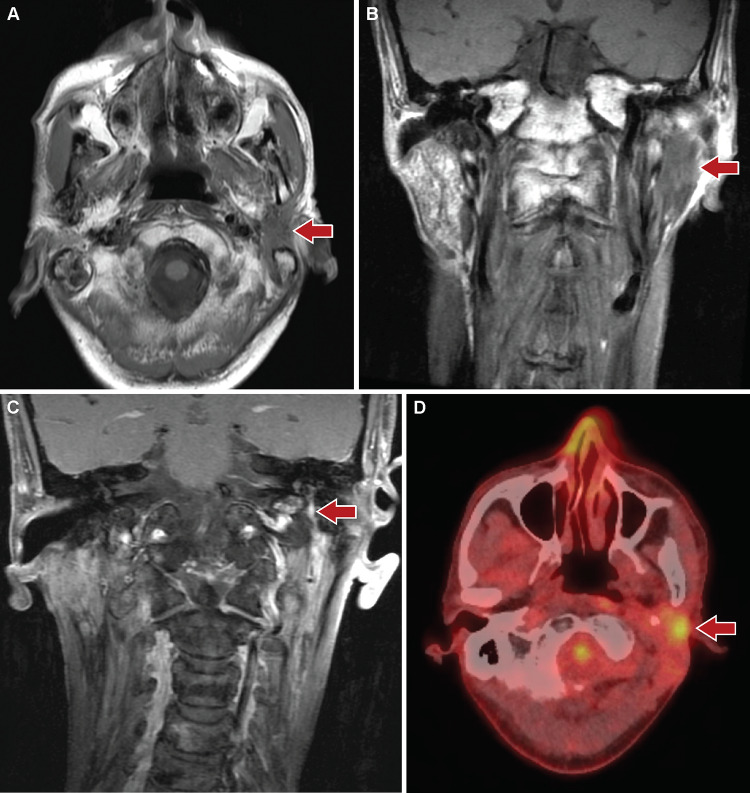
MRI of the head and neck at presentation to our hospital revealed enhancing infiltrative soft tissue thickening involving the deep and superficial lobes of the left parotid gland and extending to the left stylomastoid foramen and facial nerve (Fig. 1a-c). The findings were considered indeterminate, as the features may be seen with delayed post-radiation changes, infectious/ inflammatory changes, or malignancy. Further evaluation by positron emission tomography/ computed tomography (PET/CT) showed the left parotid lesion to be hypermetabolic (Fig. 1d). The lesion measured approximately 2.6 × 1.8 × 3.6 cm in size (anteroposterior by transverse by craniocaudal). Subsequent fine needle aspiration specimen from the left parotid was suspicious for a basaloid neoplasm; however, interpretation was limited by hypocellularity of the sample.
Fig. 1.
Radiologic findings of the parotid tumor
Repeat MRI three months later demonstrated a similar appearance of an infiltrative soft tissue abnormality in the left parotid bed with even more prominent enhancement of the left facial nerve through the stylomastoid foramen and into the temporal bone. These findings were highly suspicious for retrograde perineural spread of tumor. A parotidectomy was therefore performed. Intraoperatively, a dense fibrotic mass was identified intimately associated with the facial nerve and engulfing the greater auricular nerve.
Pathologic Findings
Gross examination of the parotidectomy specimen revealed a white-tan firm mass with irregular borders, measuring 3.5 cm in greatest dimension. Histologic sections demonstrated an ill-defined neoplasm (Fig. 2a) comprised of discontinuous small clusters as well as single-file or individual epithelioid cells embedded within an extensively fibrotic stroma (Fig. 2b). Rounded nests of tumor cells were present, suggestive of an in-situ process (Fig. 2c). There was frequent perineural and intraneural invasion (Fig. 2d). The largest contiguous focus of tumor, 0.4 cm in size, was located within a lymph node (Fig. 2e). The tumor cells exhibited variable amounts of clear, vacuolated or eosinophilic cytoplasm, large round nuclei, and small nucleoli (Fig. 2f). The tumor was entirely submitted and showed no evidence of conventional SDC or pleomorphic adenoma. Entrapped residual salivary gland tissue was noted.
Fig. 2.
Histologic findings of the parotid tumor
Ancillary studies were performed to characterize the tumor. Periodic acid–Schiff (PAS) and PAS with diastase stains demonstrated intracytoplasmic glycogen (Fig. 2g-h). A mucicarmine stain was negative for intracytoplasmic mucin (Fig. 2i). Immunohistochemical stains showed that the tumor was positive for CK7, S100, SOX10 (Fig. 3a), GATA-3 (Fig. 3b), and ER (weak to moderate). The tumor cells were negative for p40, p63 (Fig. 3c), AR (Fig. 3d), GCDFP15, mammaglobin, calponin, smooth muscle actin, DOG-1, CD117, NKX3.1, PSA, and synaptophysin. Myoepithelial markers such as p40, p63, calponin and smooth muscle actin highlighted in situ lesions (Fig. 3c). E-cadherin (Fig. 3e) and p120 (Fig. 3f) showed diffuse cytoplasmic and Golgi staining despite focal membranous and cytoplasmic staining in the in-situ component. PR labeled rare tumor cells. HER2 immunohistochemistry was equivocal. In situ hybridization for EBER was negative.
Fig. 3.
Immunoprofile of the parotid tumor
Using the formalin-fixed paraffin-embedded tissue, next-generation sequencing performed at Foundation One detected a frameshift mutation in CTNNA1 (NM_001903:c.2049_2053del; p.K683fs*17); also detected were thirteen variants of uncertain significance, including ALK D1529K, BCOR P1283L, C17ORF39 P72S, CCND2 R22G, CTNNB1 R124H, CTNNB1 R661Q, DAXX R230C, MLL2 E1072K, NBN amplification, NOTCH1 G1360S, RAD21 amplification, SETD2 A2350T, SUFU P482L, and TSC1 Q654E. The tumor was microsatellite stable, with an estimated tumor mutation burden of 3 mutations per megabase. There was no evidence of ERBB2 gene amplification or EWSR1 gene rearrangement. The salivary gland next-generation sequencing fusion panel performed at NeoGenomics on the same archival tissue showed no evidence of translocations involving the following genes: ARID1A, ATF1, CRTC1, CRTC3, DDX3X, ETV6, EWSR1, HMGA2, MAML1, MAML2, MYB, MYBL1, MYH9, NCOA4, NFIB, NTRK1, NTRK2, NTRK3, PLAG1, PRKD1, PRKD2, PRKD3, RET, and USP6.
Follow up
Five months post-surgery, the patient presented with multiple subcutaneous nodules at the left neck level II/III/IV/Va as well as with mucosal bumps at left retromolar trigone/buccal surface. Punch biopsy of the left neck lesions revealed tumor cells arranged in minute nests or singly dispersed amongst a background of fibrosis, which was morphologically similar to the parotid tumor (Fig. 4a). The scattered tumor cells were highlighted by immunostaining for CK7 (Fig. 4b). The extent of the disease was beyond the prior procedural site, a finding likely related to tumor aggressiveness.
Fig. 4.
Histologic and immunophenotypic findings of the metastatic disease
Subsequent PET/CT and physical examination detected suspicious, non-specific right axillary lymph nodes as well as multiple dermal/subcutaneous nodules of the bilateral occipital regions. Fine needle aspiration of the right axillary lymph node showed metastatic carcinoma consistent with salivary gland origin. The patient was started on pembrolizumab for three cycles with suboptimal response. Chemotherapy consisting of carboplatin/fluorouracil was subsequently added with clinical evidence of tumor shrinkage. Currently, he is alive with disease twenty months after disease onset.
Discussion
The current case demonstrated a peculiar morphology and mutational profile that does not fit into any of the entities recognized by the 4th edition of the WHO classification of Head and Neck tumors. Single-file growth and significant perineural invasion are features of polymorphous adenocarcinoma, low-grade variant. In contrast to the current case, it almost always involves minor salivary glands and demonstrates diverse histologic patterns including the characteristic “eye of the storm” or whorled appearance, focal slate blue-gray stroma, p63 positivity, and an indolent clinical course. Its higher-grade counterpart, cribriform variant, also affects minor salivary glands, exhibits cribriform to solid architecture, and harbors PRKD fusions. These features are absent in the current case. Clear cell carcinoma of the salivary gland often shows hyalinized stroma and glycogen-rich clear cytoplasm. However, the discohesion and pleomorphism seen in this case are unusual for clear cell carcinoma. In addition, the tumor lacks a squamoid immunophenotype and EWSR1-ATF1 gene rearrangement characteristic of clear cell carcinoma. SDC with abundant desmoplastic stromal reaction may have areas resembling the current case, but the lack of more conventional areas of SDC such as Roman-bridge or cribriform architecture, comedonecrosis, and granularly eosinophilic cytoplasm argues against this possibility. Moreover, SDC is typically AR and HER2 positive. Comprehensive molecular characterization by whole-exome sequencing and RNA sequencing showed the most frequent genetic alterations of SDC to be mutations involving TP53, HRAS, and PIK3CA genes, with additional ERBB2 amplification seen in the setting of carcinoma ex pleomorphic adenoma [4, 5]. None of these molecular alterations were present in this case. Furthermore, the salivary gland next-generation sequencing fusion panel performed at NeoGenomics showed no evidence of known characteristic fusions encountered in a variety of salivary gland tumors.
The discohesive growth, in particular single-file pattern, seen in the current case is reminiscent of ILC. The morphologic impression is further supported by aberrant cytoplasmic staining for E-cadherin and p120 catenin. Although rare for a male patient, metastatic lobular carcinoma of mammary origin is certainly a consideration [6], particularly in the presence of axillary lymph node metastasis. However, there is no clinical or radiographic evidence of a breast lesion in our patient. As a breast lineage marker, strong diffuse SOX10 expression is typically seen in triple-negative breast tumors rather than luminal-type tumors and argues against a breast primary when estrogen receptor is positive. Another possibility is a cutaneous tumor with features of ILC, i.e. cutaneous signet-ring cell/histiocytoid carcinoma, involving the parotid gland. However, these reported cases typically involve the eyelid or axilla and exhibit apocrine differentiation as evidenced by GCDFP15 and AR positivity [7]; these features are absent in the current case. Moreover, our patient presented with facial paralysis but no skin lesions on physical examination except for a 2mm actinic keratosis of the right superior helix. Normal serum prostate-specific antigen level and PSA/NKX3.1 negativity by immunohistochemistry provide no support for a metastatic prostatic adenocarcinoma with plasmacytoid features. Lastly, the presence of in situ lesions, as confirmed by immunostaining for myoepithelial markers, further supports the parotid gland as the primary site. Axillary nodal metastasis from cancers of head and neck, including the parotid, is uncommon but can occur [8–10]. Most of these patients had been treated with surgery and/or chemoradiation. Consequent regional fibrosis may alter usual lymphatic drainage [9].
Overall, the clinical course and the pathologic findings of our case are most consistent with a primary salivary gland tumor, which bears morphologic and genetic resemblances to ILC. To the best of our knowledge, similar cases have not been described in the literature. This case is distinct from SDCRF described by Kusafuka et al. in multiple ways. In addition to the aforementioned conventional SDC component, AR expression and rhabdoid features (i.e., eosinophilic cytoplasm and eccentric nuclei), SDCRF is also characterized by intracytoplasmic mucin, frequent preexisting/coexistent pleomorphic adenoma, and worse prognosis than conventional SDC [2, 11]. In contrast, the current case lacks conventional SDC areas and AR expression. The tumor cells contained glycogen-rich clear cytoplasm and had non-eccentric nuclei; a mucicarmine stain was negative for intracytoplasmic mucin. There is no clinical, radiographic or histologic evidence of a preexisting/coexistent pleomorphic adenoma in our patient. PLAG1 or HMGA2 rearrangements, molecular changes suggestive of preexisting pleomorphic adenoma, were not identified. In the case series reported by Kusafuka et al., six of eight SDCRF patients with follow-up data died of disease within 16 days to 30 months [2]. Although follow-up is short in our case, the local recurrence and additional nodal metastases also portend an aggressive clinical course.
Sequencing detected a frameshift variant in CTNNA1 gene, K683fs*17. Although this particular variant has not been functionally characterized in the literature, CTNNA1 K683fs*40 has been reported in a patient with breast cancer [12]; loss of protein expression was confirmed in cases of diffuse-type gastric adenocarcinoma carrying CTNNA1 R27fs*17 or R451*, truncating mutations similar to the one detected in the current case [13, 14]. The absence of other pathogenic alterations supports CTNNA1 K683fs*17 to be a driver mutation in this tumor.
CTNNA1 encodes α catenin, which is indispensable for tethering β/γ catenin-cadherin complex to the cytoskeleton. Loss of function genetic alterations involving the E-cadherin complex result in loss of cell adhesion and consequent discohesive growth, characteristic features of a biologic family of tumors including ILC, gastric signet ring cell carcinoma, and plasmacytoid urothelial carcinoma. Tumors of similar morphology are occasionally seen in other organs, such as skin and prostate [7, 15]. In terms of underlying genetic causes, inactivating alterations of CDH1 gene, which encodes E-cadherin, appear to be the most common. CTNNA1 inactivation, the second most common, has been reported in ILC and gastric signet ring cell carcinoma [13, 14, 16, 17]. For as yet unidentified reasons, the risk of breast cancer associated with CTNNA1 inactivation is several-fold higher than the risk of gastric cancer. In a large cohort study of germline CTNNA1 loss-of-function variants, among 33 carriers with detailed history available, 4 (12 %) individuals had diffuse-type gastric cancer and 22 (67 %) had breast cancer while 83 % of carriers had either a personal history of or a first-degree relative with breast cancer [14]. In a case of cutaneous signet-ring cell/histiocytoid carcinoma as well as a case of plasmacytoid acinar adenocarcinoma of the prostate, normal E-cadherin expression was lost by immunohistochemistry; Sequencing revealed no genomic alterations in CDH1 gene for both cases, but it is unclear whether CTNNA1 was covered by the panels [7, 15]. Kusafuka et al. sequenced CDH1 gene in their SDCRF cases and reported seven of eleven cases examined carried single nucleotide variants. However, except for R74* and W20fs*14 which are predicted to be pathogenic, all other alterations are variants of uncertain significance [3]. It would be interesting to know the status of CTNNA1 in these cases as well.
Although not routinely covered by targeted sequencing panels, CTNNA1 alterations appear to be infrequent in salivary gland carcinomas. Ross et al. analyzed 623 consecutive cases of salivary gland carcinoma sequenced by Foundation Medicine and found CTNNA1 variants in approximately 2–3 % of salivary duct carcinomas [18]. Relatively more common than CTNNA1 variants, CDH1 variants were detected in 5–10 % of cases of carcinoma ex pleomorphic adenoma and adenocarcinoma not otherwise specified, and in less than 5 % of mucoepidermoid carcinomas, adenoid cystic carcinomas and carcinomas not otherwise specified [18]. Unfortunately, no details are available regarding the nature of these genetic alterations, the mutational profiles or the morphologic features of the unclassified cases. However, it is possible that some of these unclassified cases may represent unrecognized examples of mammary lobular carcinoma-like carcinoma of the salivary gland.
Sasaki et al. reported a case of secretory carcinoma of salivary gland with dominant macrocystic architecture and CTNNA1-ALK fusion [19]. The impact of this fusion on α catenin function has not been studied. It is uncertain whether CTNNA1 gene rearrangement plays a role in the dominant macrocystic and less solid growth pattern. Prognostic data on CTNNA1 or α-catenin alterations in salivary gland tumors are currently lacking. However, in urothelial carcinoma, a highly significant correlation was observed between the loss of expression of E-cadherin/α-catenin/γ-catenin, but not β-catenin, and increased TNM stage [20].
Besides discohesive growth, another peculiar feature of the current case is extensive stromal fibrosis. Although this might be related to the patient’s remote history of radiation and chemotherapy to some extent, intriguingly, cardiospecific Ctnna1 knockout mice exhibited dysregulation of fatty acid metabolism, perivascular fibrosis, and replacement fibrosis [21]. More in-depth investigations are needed to understand this potential association. It is still unclear what extensive fibrosis means in terms of prognosis. As for plasmacytoid urothelial carcinoma, the desmoplastic subtype has been reported to be associated with high stage and worst overall survival compared to the classic or pleomorphic subtypes [22].
Conclusions
In summary, we report a rare case of parotid gland carcinoma closely mimicking invasive lobular carcinoma of the breast. The tumor is characterized by discohesive growth, aberrant E-cadherin and p120 staining, and pathogenic CTNNA1 mutation.
Acknowledgements
The authors would like to thank Norman L Cyr for his expertise in figure illustration.
Author Contributions
All authors contributed to the study conception. Material preparation, pathology data collection and interpretation were performed by Li Lei. Radiology data collection and interpretation were performed by Eric Van Staalduinen and Michael Zeineh. The first draft of the manuscript was written by Li Lei and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding obtained.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest, either financial or non-financial.
Ethical Approval
This study was approved by Stanford University Institutional Review Board.
Informed Consent
All data have been de-identified.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Li Lei, Email: lilei@stanford.edu.
Eric Van Staalduinen, Email: ericvs@stanford.edu.
Megan Troxell, Email: megant@stanford.edu.
Michael G. Ozawa, Email: mgozawa@stanford.edu
Michael Zeineh, Email: mzeineh@stanford.edu.
Gerald Berry, Email: gerald.berry@stanford.edu.
References
- 1.Kusafuka K, Onitsuka T, Muramatsu K, Miki T, Murai C, Suda T, et al. Salivary duct carcinoma with rhabdoid features: report of 2 cases with immunohistochemical and ultrastructural analyses. Head Neck. 2014;36:E28–35. doi: 10.1002/hed.23466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusafuka K, Kawasaki T, Maeda M, Yamanegi K, Baba S, Ito Y, et al. Salivary duct carcinoma with rhabdoid features: a salivary counterpart of pleomorphic lobular carcinoma of the breast. Histopathology. 2017;70:164–73. doi: 10.1111/his.12987. [DOI] [PubMed] [Google Scholar]
- 3.Kusafuka K, Yamada H, Ishino K, Maeda M, Yamanegi K, Baba S, et al. Salivary duct carcinoma with rhabdoid features-No or aberrant expression of E-cadherin and genetic changes in CDH1: Immunohistochemical and genetic analyses of 17 cases. Am J Surg Pathol. 2021;45:439–49. doi: 10.1097/PAS.0000000000001672. [DOI] [PubMed] [Google Scholar]
- 4.Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee KW, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22:4623–33. doi: 10.1158/1078-0432.CCR-16-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiosea SI, Thompson LD, Weinreb I, Bauman JE, Mahaffey AM, Miller C, et al. Subsets of salivary duct carcinoma defined by morphologic evidence of pleomorphic adenoma, PLAG1 or HMGA2 rearrangements, and common genetic alterations. Cancer. 2016;122:3136–44. doi: 10.1002/cncr.30179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murzabdillaeva A, Ali Y, Aakash N, Armstrong J, Ghorbani R, Zhang S. Pleomorphic invasive lobular carcinoma of the male breast: two case reports with opposite hormone receptor status. Ann Clin Lab Sci. 2019;49:534–8. [PubMed] [Google Scholar]
- 7.Ito Y, Ishida M, Ohe C, Miyasaka C, Tsuta K. Signet-ring cell/histiocytoid carcinoma in the axilla: A case report with genetic analysis using next-generation sequencing. J Cutan Pathol. 2021;48:102–5. doi: 10.1111/cup.13838. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy J, Krishnamurty D, Baker J, Zhen W, Lydiatt D, Ganti A. Salivary duct carcinoma responding to trastuzumab-based therapy: case report and review of the literature. Head Neck. 2013;35:E372–5. doi: 10.1002/hed.23307. [DOI] [PubMed] [Google Scholar]
- 9.Oo A, Yamaguchi S, Iwaki H, Amagasa T. Axillary nodal metastasis from oral and maxillofacial cancers: a report of 3 cases. J Oral Maxillofac Surg. 2004;62:1019–24. doi: 10.1016/j.joms.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Hafez M, Refky B, Elwahab K, Arafa M, Abdou I, Elnahas W. Axillary lymph nodes metastasis in a patient with recurrent papillary thyroid cancer: a case report. J Med Case Rep. 2015;9:181. doi: 10.1186/s13256-015-0668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akaki M, Ishihara A, Nagai K, Naono H, Taguchi K, Yamamoto H, et al. Signet ring cell differentiation in salivary duct carcinoma with rhabdoid features: report of three cases and literature review. Head Neck Pathol. 2020 doi: 10.1007/s12105-020-01186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson TR, Udyavar AR, Chang CW, Spoerke JM, Aimi J, Savage HM, et al. Genomic alterations associated with recurrence and TNBC subtype in high-risk early breast cancers. Mol Cancer Res. 2019;17:97–108. [DOI] [PubMed]
- 13.Majewski IJ, Kluijt I, Cats A, et al. An alpha-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J Pathol. 2013;229:621–9. doi: 10.1002/path.4152. [DOI] [PubMed] [Google Scholar]
- 14.Clark DF, Michalski ST, Tondon R, Nehoray B, Ebrahimzadeh J, Hughes SK, et al. Loss-of-function variants in CTNNA1 detected on multigene panel testing in individuals with gastric or breast cancer. Genet Med. 2020;22:840–6. doi: 10.1038/s41436-020-0753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Hussain T, Haffner MC, Altaweel WM, Epstein JI. Plasmacytoid acinar adenocarcinoma of the prostate: a newly described variant of prostate cancer. Hum Pathol. 2019;94:86–91. doi: 10.1016/j.humpath.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 16.de Groot JS, Ratze MA, van Amersfoort M, Eisemann T, Vlug EJ, Niklaas MT, et al. alphaE-catenin is a candidate tumor suppressor for the development of E-cadherin-expressing lobular-type breast cancer. J Pathol. 2018;245:456–67. doi: 10.1002/path.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blair VR, McLeod M, Carneiro F, Coit DG, D’Addario JL, van Dieren JM, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol. 2020;21:e386–e397 [DOI] [PMC free article] [PubMed]
- 18.Ross JS, Gay LM, Wang K, Vergilio JA, Suh J, Ramkissoon S, et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann Oncol. 2017;28:2539–46. doi: 10.1093/annonc/mdx399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki E, Masago K, Fujita S, Suzuki H, Hanai N, Hosoda W. Salivary secretory carcinoma harboring a novel ALK fusion: expanding the molecular characterization of carcinomas beyond the ETV6 gene. Am J Surg Pathol. 2020;44:962–9. doi: 10.1097/PAS.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 20.Syrigos K, Harrington K, Waxman J, Krausz T, Pignatelli M. Altered gamma-catenin expression correlates with poor survival in patients with bladder cancer. J Urol. 1998;160:1889–93. doi: 10.1016/S0022-5347(01)62438-8. [DOI] [PubMed] [Google Scholar]
- 21.Balatskyi VV, Macewicz LL, Gan AM, Goncharov SV, Pawelec P, Portnichenko GV, et al. Cardiospecific deletion of alphaE-catenin leads to heart failure and lethality in mice. Pflugers Arch. 2018;470:1485–99. doi: 10.1007/s00424-018-2168-2. [DOI] [PubMed] [Google Scholar]
- 22.Perrino CM, Eble J, Kao CS, Whaley RD, Cheng L, Idrees M, et al. Plasmacytoid/diffuse urothelial carcinoma: a single-institution immunohistochemical and molecular study of 69 patients. Hum Pathol. 2019;90:27–36. doi: 10.1016/j.humpath.2019.04.012. [DOI] [PubMed] [Google Scholar]