To the Editor:
Given the considerable overlap between endothelial dysfunction caused by the coronavirus disease 2019 (COVID-19)-associated endotheliitis and the one observed after allogeneic HSCT such as veno-occlusive disease (VOD), transplant associated-thrombotic microangiopathy (TA-TMA), idiopathic pneumonia syndrome (IPS) and graft-versus-host disease (GvHD) we analyzed long- term outcomes of patients who received allogeneic-HSCT with recovered COVID-19 [1, 2]. Favorable short-term outcomes after HSCT were previously reported by our group [3].
A total of 14 patients with a history of resolved COVID-19 were transplanted at the University Medical Center Hamburg Eppendorf UKE (n = 9) and Department of Medicine of Goethe University Frankfurt (n = 5) and provided informed consent for data collection and analysis. Patients were 57.1% female and 42.9% male with a median age of 56.5 (range 33–69) years (Table 1). COVID-19 was diagnosed between February 2020 and June 2021 by polymerase chain reaction (PCR) a median of 26 (−99 - 134) days before or after induction chemotherapy for advanced or high-risk acute myeloid (AML), lymphoblastic leukemia (ALL) and blast crisis of CML. During COVID-19, 11 patients (79%) developed lung infiltrates, six patients (43%) required ICU admission and two were treated with casirivimab/imdevimab (H8) or bamlanivimab (Fr2, Table 1). All patients recovered after median 47.5 (11–70) days after diagnosis of COVID-19 and were transplanted a median of 94 (35–136) days after COVID-19 resolution in CR1 (n = 8), PR or CR3 (n = 2) of high risk AML, high risk ALL (n = 3) or second chronic phase after CML blast crisis (n = 1). Donors were matched related (n = 3) or unrelated (n = 4), haploidentical related (n = 4) or mismatched unrelated (n = 3). Conditioning was myeloablative (MAC; n = 6), of reduced intensity (RIC; n = 7) and of reduced toxicity (RTC; n = 1). All patients received fludarabine in combination with fractionated total body irradiation (TBI; 8 or 12 Gy, n = 7), thiotepa/busulfan (n = 3), melphalan (n = 3) or treosulfan (n = 1). GvHD prophylaxis was performed with anti-thymocyte globulin (ATG) followed by cylosporine and mycofenolate mofetil (MMF) in 10 patients and with post-transplant cyclophosphamide (PT-CY) and tacrolimus/MMF in the haploidentical (4) and in one mismatched unrelated HSCT. Ursodiol as prophylaxis was given to all patients transplanted in Frankfurt/Main. TA-TMA was defined as previously described [4] and VOD diagnosed according to Seattle criteria [5]. Differences between groups were analyzed with the Fisher’s exact test.
Table 1.
Characteristics of patients recovered pretransplant from COVID-19 and diagnosis of endothelial complications.
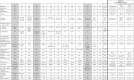 |
H Hamburg, Fr Frankfurt/Main, M male, F female, AML acute myeloid leukemia, t therapy related, ALL acute lymphoblastic leukemia, CML BC blast crisis CML, chr.Ph.CML chronic phase CML, n.a. not available, ven/aza venetoclax/azacytidine, 7 + 3 according to [9]; mido midostaurin, GO gentuzumab ozogamicin, HAM high-dose cytosine arabinoside (HDAraC) and mitoxantrone, mito-FLAG fludarabine AraC mitoxantrone granulocyte colony-stimulating factor, GMALL German ALL protocol, blina binatumumab, OSHO East German Study Group protocol, EWALL European Working Group on Adult ALL, CT computed tomography, pneum pneumonia, pneum§§ bilateral pneumonia, peri pericarditis, RI respiratory insufficiency, ARF acute renal failure, CRBSI catheter-related blood stream infection, DVT deep venous thrombosis, O2 oxygen, ICU intensive care unit.
*Intubated; HSCT hematopoietic stem cell transplantation.
**Diagnosis COVID-HSCT days; L lopinavir, RIT ritonavir, P prednison, R remdesivir, dexa dexamethason, casi casirivimab, serum convalenscence serum, imde imdevimab, bamla bamlanivimab, CR complete remission, MAC myeloablative conditioning, RIC reduced intensity conditioning, RTC reduced toxicity conditioning, Gy Gray, TBF thiotepa busulfan fludarabine, flu fludarabine, mel melphalan, TBI total body irradiation, treo treosulfan, ATG antithymocyte globulin, PT-CY post-transplant cyclophosphamide; MRD matched related donor, MUD matched unrelated donor, MMUD mismatched unrelated donor, haplo haploidentical related donor, TA-TMA transplant associated thrombotic microangiopathy, VOD venoocclusive disease, defib defibrotide, ecu eculizumab, n.ap. not applicable, ple pleural effusion, peri pericardial effusion, asc ascites.
After a median follow-up of 221 (69–492) days, 11 (79%) of the 14 patients are alive. One patient died on day +146 from complications following AML relapse, one from cardio-pulmonary insufficiency following fungal infection (d +208) and one from not further specified liver failure (d +179). Three patients developed VOD, TA-TMA or both, all of them associated with polyserositis, at a median of day +67.5 (9–242) and are still alive a median of 451 (221–492) days after HSCT. Additional four patients had serositis without clinical signs of TA-TMA or VOD.
Patients with TA-TMA/VOD were exclusively female transplanted from a male mismatched unrelated or family donor (p = 0.01) in comparison to patients without TA-TMA/VOD. In addition, seven out of the 14 patients developed pleural, pericardial effusion and ascites after HSCT. In contrast, no differences in age, interval diagnosis leukemia to COVID-19, COVID-19 duration, interval from COVID-19 diagnosis to HSCT, conditioning including TBI, conditioning intensity and GvHD prophylaxis were detected between the non- and TA-TMA/VOD patients (Table 1; p = n.s.). In a subgroup of patients (n = 9) median peak levels of acute phase proteins such as ferritin (3440 vs 3817 µg/l), IL-6 (323.45 vs 767.8 ng/l), procalcitonin (PCT; 2.48 vs 1.87 µg/l) and C-reactive protein (CRP; 185 vs 256 mg/l) during COVID-19 were found to be not significantly different in patients with as compared to those without endothelial damage.
One 69 years old female patient (H1) with AML achieved CR1 after induction with azacytidine and venetoclax and developed on the day of leukemia diagnosis pulmonary COVID-19 requiring mechanical ventilation. Peak ferritin reached 2875 µg/l, IL-6 538 ng/l, PCT 5 µg/l and CRP 361 mg/l. She was tested PCR negative after 12 days. Haploidentical HSCT was performed 106 days after COVID-19 following a conditioning regimen with thiotepa, busulfan, fludarabine and ATG. Tacrolimus/MMF in combination with PT-CY were administered for GvHD prophylaxis. Cytomegalovirus (CMV) status was negative in both donor and recipient. After engraftment on day +17, she developed histologically (renal biopsy) confirmed TA-TMA eight months after HSCT. CMV, urogenital and clostridium difficile infections may have triggered TA-TMA. The patient recovered from TA-TMA after steroids and antiviral therapy and deteriorated again in a subsequent CMV reactivation. The newly diagnosed polyserositis was treated with prednisone. The patient is alive 492 days after HSCT.
One patient (H4) with ALL was treated according to GMALL (German ALL) protocol and three courses of blinatumomab and recovered from COVID-19 after treatment with convalescent serum with peak levels of ferritin 4006 µg/l, IL-6 109 ng/l, PCT 0 µg/l and CRP of 85 mg/l. Duration of infection was 56 days. After conditioning regimen of TBI and fludarabine a haplo-identical HSCT from a male, CMV positive donor (recipient positive) was performed. For GvHD prophylaxis, immunosuppression with tacrolimus/MMF and PT-CY was given. Engraftment was observed on day +21 and on day +59, CMV reactivation, BK cystitis, polyserositis and ascites were diagnosed. Pathological liver enzymes and liver histology confirmed VOD, which was treated successfully with defibrotide. The patient is alive 451 days post-HSCT.
One patient (Fr2) with AML had disease persistence following 7 + 3+midostaurin and obtained CR1 after high dose cytarabine and mitoxantrone (HAM) followed by gilteritinib. COVID-19 was detected by PCR 104 days after diagnosis and persisted for 70 days despite convalescent serum, dexamethasone, remdesivir and bamlanivimab. HSCT from a HLA-C antigen mismatched unrelated male and CMV concordant positive donor was performed 240 days after AML diagnosis and 136 days after COVID-19. In the immediate post-transplantation period, bacteremia with Enterococcus faecium was treated successfully with antibiotic combination. The patient was diagnosed with VOD (day +9) and treated successfully with defibrotide. On day +40, Epstein Barr Virus reactivation and human poliomavirus 1 - cystitis occurred. On day +64, the patient stabilized and gilteritinib maintenance was initiated. When she developed TA-TMA on day +76, she was switched from cyclosporine to everolimus. On day +81, acute GvHD of the gut was suspected which responded to steroids. Progressive kidney injury developed despite 6 doses of eculizumab starting on day +87, by ruxolitinib or daily plasma exchange starting on day +132. The patient remained on renal replacement therapy 221 days after HSCT.
The association of female patients transplanted from a mismatched male donor with endothelial damage and polyserositis was statistically significant despite the small sample size and needs further confirmation. Gender has shown to have an important role in immune response against COVID-19 and may provide a rationale for this observation [6]. Associations were found in addition with the presence of viral and bacterial infections. No association were found with older age, longer interval diagnosis—COVID-19, treatment of COVID-19, especially with convalescent serum and monoclonal antibodies (Table 1). In five of the six patients pleural effusions may be caused by endothelial dysfunction in the context of idiopathic pneumonia syndrome [7].
The small sample size, the lack of a control group and underdiagnosing endothelial damage may represent limitations of the study. Therefore, incidence of TA-TMA or VOD cannot be compared to that of patients transplanted without recovered COVID-19 [1, 8].
In summary, complications of endothelial damage was not associated with mortality, but with significant morbidity. Prophylaxis with endothelial-protective agents may represent a promising and rationale therapeutic strategy in female patients with mismatched or haploidentical HSCT from male donors and pretransplant COVID-19 history, especially if inflammation triggered by viral and bacterial infection is present. Complement inhibition for treatment of endothelial damage may be another approach to investigate.
Future research is needed in a larger group of patients to confirm our findings, identify new associations possibly missed in this small sample size and investigate prophylactic and treatment interventions.
Author contributions
CN (CMWRESAIV); BW (CMWREIV); GB, NK (CMESA); CN, BW, MR, NG, SA, VS, ZZ, FL, DJ, CW, FA, GB, NK, (RI); All authors read and approved the final manuscript. Conceptualization (C), Methodology (M), Software Writing (W), Reviewing (R) and Editing (E), Supervision (S), Project administration (A), Patient acquisition and collection of clinical information (I) Writing Original Draft Validation (V).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
No conflict of interest, except GB: Honoraria from Jazz Pharmaceuticals.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tiwari NR, Phatak S, Sharma VR, Agarwal SK. COVID-19 and thrombotic microangiopathies. Thromb Res. 2021;202:191–8. doi: 10.1016/j.thromres.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calabretta E, Moraleda JM, Iacobelli M, Jara R, Vlodavsky I, O’Gorman P, et al. COVID-19-induced endotheliitis: emerging evidence and possible therapeutic strategies. Br J Haematol. 2021;193:43–51. doi: 10.1111/bjh.17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christopeit M, Reichard M, Niederwieser C, Massoud R, Klyuchnikov E, Haase N, et al. Allogeneic stem cell transplantation in acute leukemia patients after COVID-19 infection. Bone Marrow Transplant. 2021;56:1478–81. doi: 10.1038/s41409-021-01225-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dandoy CE, Rotz S, Alonso PB, Klunk A, Desmond C, Huber J, et al. A pragmatic multi-institutional approach to understanding transplant-associated thrombotic microangiopathy after stem cell transplant. Blood Adv. 2021;5:1–11. doi: 10.1182/bloodadvances.2020003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116–22. doi: 10.1002/hep.1840040121. [DOI] [PubMed] [Google Scholar]
- 6.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20:442–7. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi D, Jang H, Kim S, Deol A, Ayash L, Bhutani D, et al. Incidence, etiology, and outcome of pleural effusions in allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2016;91:E341–7. doi: 10.1002/ajh.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagliuca S, Michonneau D, Sicre de Fontbrune F, Del Sutra Galy A, Xhaard A, Robin M, et al. Allogeneic reactivity-mediated endothelial cell complications after HSCT: a plea for consensual definitions. Blood Adv. 2019;3:2424–35. doi: 10.1182/bloodadvances.2019000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


