Abstract
The new WHO classification of head and neck tumors provides a comprehensive overview of lesions by summarizing their clinical, epidemiological, histological, immunohistochemical, molecular and genetic features. The chapters related to the description of oropharyngeal and nasopharyngeal lesions have thus been largely modified.
Keywords: WHO classification, Oropharyngeal carcinoma, Nasopharyngeal carcinoma, HPV, EBV
Introduction
The fifth edition of the WHO classification of head and neck tumors has incorporated changes regarding oropharyngeal (Chapter 6) and nasopharyngeal (Chapter 4) lesions. Tumors in these two anatomical locations are associated with various risk factors, among which viral infections play a central role. This article summarizes the new classification of oropharyngeal and nasopharyngeal lesions, with particular emphasis on the changes between the 4th and 5th editions of the WHO Blue Book.
Oropharynx
Like the previous edition [1], the new version of the WHO Blue Book [2] contains a chapter dedicated to specific diseases of the oropharynx. The oropharynx is the middle part of the pharynx located behind the oral cavity that extends from the soft palate to the level of the hyoid bone (C3). This very particular anatomical location comprises the base of the tongue (posterior third) the lingual and palatine tonsils, the epiglottic vallecula and the soft palate [3].
Compared to the previous edition, this chapter presents many changes. One of them is the increased significance of the distinction between carcinomas that are related to high-risk HPV infection and those that are not. The differences in histologic and clinical manifestations, the epidemiology, and the management of these squamous cell carcinomas (SCC) deserve special attention. It should also be noted that chapters and passages on salivary gland and neuroendocrine tumors and lymphoid malignancies have been removed from the oropharynx chapter and inserted into other chapters to reduce redundancy.
Hamartomatous Polyps
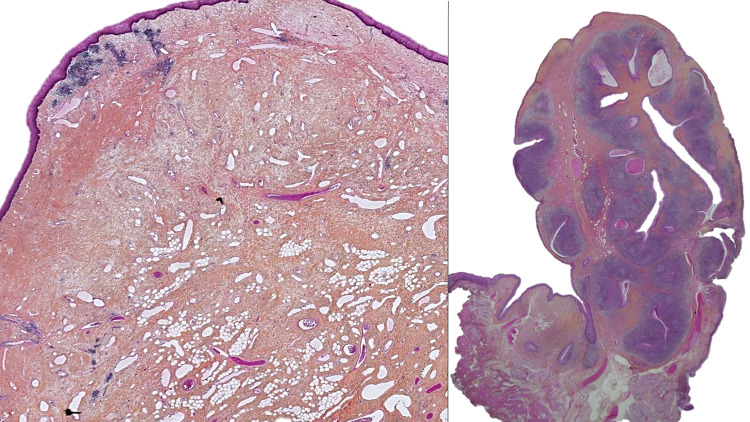
A new item on hamartomatous polyps (Fig. 1) has been added to the oropharynx chapter. It is necessary for pathologists to be able to recognize these rare, benign, and often polypoid lesions of the palatine tonsil to avoid interpreting them as malignant. They are also called lymphangiectatic fibrous polyp, fibrovascular polyp, polypoid lymphangioma, papillary lymphoid polyp, benign hamartomatous polyp or lymphangiectatic fibrolipomatous polyp. Adipocytes, muscle fibers, or a lymphocytic infiltrate may be seen mixed in with this submucosal proliferation of lymphovascular channels that usually contain proteinaceous fluid and lymphocytes [4–6]. There is no recurrence after complete surgical excision [3].
Fig. 1.
Two hamartomatous polyps. The polypoidal tissue show flattened squamous epithelium. For only one hamartomatous polyp (right) presence of residual lymphoid follicles of the tonsil (H&E, 5 × and 10 ×). The stroma of the polyps show multiple dilated lymphatics mixed to a fibrous matrix
Squamous Cell Carcinoma
Head and neck SCC (HNSCC) are a group of malignant tumors located in the oropharynx, larynx, hypopharynx, nasopharynx and oral cavity. In total, they account for approximately 800,000 new cases and 400,000 deaths per year [7]. Oropharyngeal cancers account for approximately 100,000 new cases per year worldwide. Typical risk factors are smoking and alcohol consumption [8], but it is now established that human papillomavirus (HPV) plays a major role in the carcinogenesis of oropharyngeal SCCs (OPSCC) [9, 10]. The role of HPV infection in OPSCC is an important topic and is extensively discussed in the chapter on oropharyngeal pathologies. It should be noted that the role of HPV is unclear in nasopharyngeal SCCs, although some publications suggest that there may be an association between HPV infection and nasopharyngeal carcinomas (NPCs). The latter issue is discussed in the section on NPCs, which are primarily associated with Epstein–Barr virus (EBV) infection [11, 12].
The new “WHO 2022” classification Oropharyngeal Chapter makes a clear distinction between HPV-associated and HPV-independent OPSCCs. The existence of a separate terminology for these two tumor types emphasizes the difference in their carcinogenesis based on the potential involvement of a transcriptionally active high-risk HPV (HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59 and HPV68).
HPV-Associated Squamous Cell Carcinoma
Epidemiology and Etiology
HPV infection is present in approximately 20% to 60% of OPSCCs, depending on geographic location [13]. For example, approximately 10–20% of OPSCCs are HPV-associated in Asia [14, 15] and 60–80% in North America and Northern Europe [9, 16]. As outlined in the new classification, patients with HPV-associated OPSCCs are typically male (male to female ratio of 4:1), white, and of high socioeconomic status. HPV-associated OPSCCs tends to occur more often in nonsmokers, but this is highly country dependent, with the proportion of smokers exceeding 60% in some countries [17]. Another important factor is sexual activity. It appears that the number of oral sex partners is one of the major risk factors for HPV-associated OPSCCs. Partner profile and sexual intensity may also be relevant indicators [18]. Immunosuppression is also a risk factor.
Finally, although many publications have reported that patients with HPV-associated OPSCC tend to belong to a younger age group, this point has been revisited in light of more recent research indicating that HPV-associated carcinomas occur in older patients in certain geographic areas and sociosexual conditions [19].
Histopathology
The tonsillar crypt epithelium and the surface epithelium have distinct histologic features. The surface epithelium is a stratified, non-keratinizing, morphologically mature and polarized tissue, whereas the tonsillar crypt epithelium is reticular with a discontinuous basement membrane. Tonsils and base of the tongue are part of Waldeyer's lymphatic ring, the epithelium is lymphoepithelial. A recent paper by Roberts et al. and other publications have described strong interactions between HPV, basal keratinocytes and the immune microenvironment [20–23]. In addition, crypt cells overexpress PD-L1, which allows for immunotolerance and promotes cell proliferation [24]. The concept of dysplasia or carcinoma in situ has been and remains highly controversial in HPV-associated OPSCCs. Especially, since many lesions that used to be considered strictly localized in the epithelium are in fact clinically associated with metastatic lymph nodes. Histologically, it is difficult to analyze the tonsillar reticular epithelium, as it most often contains lymphocytes that make interpretation of epithelial lesions or identification of infiltration challenging. Therefore, these non-keratinizing HPV-associated OPSCCs located in the epithelium should be considered a priori as infiltrating, as these carcinomas arise from cryptic epithelium (i.e., lingual, and palatal tonsils) with a porous basement membrane.
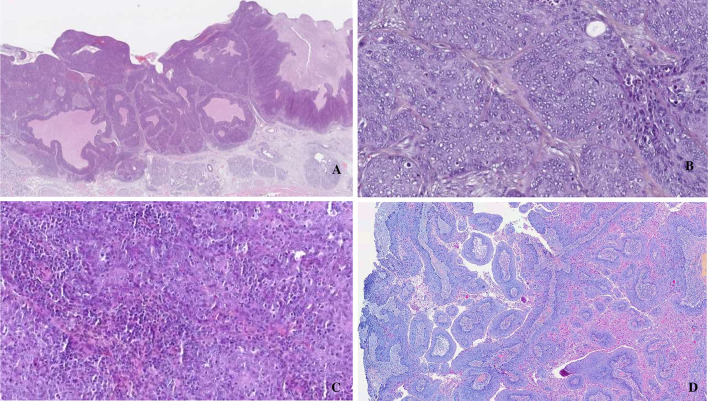
All subtypes of SCCs have been observed and described among HPV-associated OPSCCs, including papillary, adenosquamous, ciliated [25], lymphoepithelial (undifferentiated), sarcomatoid/spindle cell and basaloid carcinomas (Fig. 2). However, the most common histologic description according to new classification for nonkeratinizing SCC is "tumor cells with high nuclear/cytoplasmic ratios, oval or spindle-shaped nuclei, syncytial cytoplasm (indistinct cell borders) without intercellular bridges and usually no significant cytoplasmic keratinization." According to both 4th and 5th edition recommendations, histologic differentiation grading is to be avoided. It may sometimes be necessary for the less keratinizing forms to confirm the epithelial or and squamous phenotype of these carcinomas by immunohistochemical staining using p40, p63, or CK5/6, at least.
Fig. 2.
HPV associated oropharyngeal squamous cell carcinoma. A basaloid (H&E, 2 ×)), B non-keratinizing (H&E, 40 ×)), C lymphoepithelial (H&E, 40 ×)), D papillary (H&E, 10 ×)) subtypes
The new WHO classification highlights the elements of the differential diagnosis of neuroendocrine carcinomas, which are no longer included in this chapter. In addition to specific morphological features, expression of neuroendocrine markers (chromogranin, synaptophysin or INSM1) is mandatory for the diagnosis of neuroendocrine carcinoma. CD56 being unspecific, its use is not recommended. Neuroendocrine carcinomas may locally and heterogenically express p63. P40 expression has not been observed in neuroendocrine carcinomas and is more specific for squamous differentiation. Finally, it should be noted that p16 is often overexpressed in neuroendocrine carcinomas. However, this overexpression might be HPV-unrelated [26]. Nonetheless, authentic HPV-associated large and small cell neuroendocrine carcinomas have been identified in small series and appear to exhibit aggressive clinical behavior compared with HPV-associated OPSCC [27–31]. In the case of these neuroendocrine carcinomas of the oropharynx, p16 immunohistochemical staining is not specific enough and it is necessary to perform HPV tests such as PCR or in situ hybridization in order to identify the possible presence of high risk HPV.
In HPV-associated OPSCCs, lymph node involvement of the upper and middle lymphatic chains (levels II and III) is more frequent [10, 32], and metastatic nodes are more often found at diagnosis than in HPV-independent OPSCCs (75% vs 54%) [33]. The frequently cystic appearance of metastases is a highly specific feature with positive prognostic value [34, 35]. Finally, the prevalence of extranodal extension is higher than in HPV-independent OPSCC (77% vs 56%) and unlike what was initially reported in the literature this corresponds to a negative prognostic factor, albeit not a strongly adverse one [36–38].
HPV Testing and Diagnostic Molecular Pathology
High-risk HPV infection (specifically HPV16 infection, which is present in 90% of cases), was reported for the first time, as one of the main risk factors for OPSCCs, in the 4th edition of the WHO classification published in 2017 [1]. In addition to the epidemiological, clinical, and prognostic differences, it is important to highlight the genetic, epigenetic, and molecular characteristics associated with HPV infection. HPV High-risk genotypes express the E6 and E7 oncoproteins. E6 binds to the tumor suppressor p53 and forms an E6/E6AP/p53 trimeric complex [39], leading to proteolytic degradation of p53 [40]. E7 binds to phosphorylated retinoblastoma protein (pRb), releasing the transcription factor E2F and subsequently promoting cell cycle progression, and consequently overexpression of p16. The p16 protein is a cyclin-dependent kinase (CDK) inhibitor that plays a major role in the cell cycle: it interacts with pRb, and it inhibits the S phase. Overexpression of p16 prevents phosphorylation of Rb family members, resulting in E2F uptake by Rb proteins and thus cell cycle arrest in the G1 phase [41]. The role of other viral oncoproteins such as E5 is less clear and their involvement in the regulation of immune surveillance remains to be clarified. Immunostaining for p16 protein is a cost-effective method for diagnosing high-risk HPV infection in tissues. Overexpression of p16 protein is an indirect sign of E7 protein expression [42, 43]. The sensitivity of p16 immunostaining in the oropharynx is approximately 80–90% [44]. One study compared the performance of p16 immunostaining. It concluded that determination of p16 positivity using a 75% threshold is associated with poor reproducibility, whereas a 50% threshold is more reproducible [45, 46]. The new WHO OPSCC classification sets the threshold at 70% of tumor cells staining positive for p16 in both nuclear and cytoplasmic staining. Immunostaining for p16 is strongly recommended as a first-line diagnostic tool and is considered sufficient to identify high-risk HPV infection in OPSCC and metastases from these carcinomas.
Other testing methods that can be suggested to confirm HPV include PCR (detection of HPV DNA), RT-PCR (detection of E6 and E7 mRNA), DNA-targeted in situ hybridization (DNA HSI), and RNA-targeted in situ hybridization (RNA HSI). Table 1 summarizes the advantages and disadvantages of these tests, and it is essential that pathologists be able to interpret their results [41]. These HPV tests may be useful in specific situations (e.g., uncertain immunostaining results, P16 + keratinizing carcinoma, inclusion in a research study) [42, 47]. According to WHO guidelines, all OPSCC samples should be tested for HPV.
Table 1.
Description of the benefits and drawbacks of different types of HPV-detection testing [41] (modified)
| Detection technique | Benefits | Drawbacks |
|---|---|---|
| HPV PCR | High sensitivity | No information about viral transcription |
| HPV genotype information | High risk of contamination (intrinsic and extrinsic) | |
| FFPE manageable | Morphological pre-validation of the sample quality might be needed | |
| Easy-to-use and inexpensive technique | ||
| Routine use with automation | ||
| E6/E7 mRNA RT-PCR | High sensitivity and specificity | Time-consuming |
| Active detection of viral infection | Non FFPE manageable (fresh or frozen tissue only) | |
| RNA fragility | ||
| Technical expertise required | ||
| E6/E7 mRNA in situ hybridization | High specificity and good sensitivity | RNA degradation over time |
| In situ detection of a transcriptionally active HPV infection | Expensive technique | |
| FFPE manageable | Technical expertise required | |
| Automation possible | ||
| HPV DNA in situ hybridization | In situ detection of HPV DNA | Sensitivity reduced by the minimum DNA copy number required |
| High specificity | ||
| FFPE manageable | ||
| Routine use | ||
| P16 immunochemistry | High sensitivity | Moderate specificity |
| Inexpensive technique | Surrogate marker of HPV infection | |
| FFPE manageable | ||
| Easy routine use | ||
| First-intention staining |
As for somatic mutations, a comparable mutational load was observed between the two tumor types. Of note, all HNSCCs have been described as having a high rate of somatic mutations, regardless of the location of tumor growth [48, 49]. Mutations in PIK3CA predominate in HPV-associated tumors, whereas TP53 is the most frequently mutated gene in HPV-independent tumors (41%) [50]. HPV-induced cancers involve the viral oncogenes E6 and E7 that alter the cell cycle via inactivation of p53 [39]. The different pathways could explain the differences. Note that loss of PTEN (Phosphatase and Tensin Homolog) is common, independent of HPV status [50].
Prognosis
HPV-associated OPSCs have a better prognosis than HPV-independent OPSCs, with better sensitivity to radiation and better overall survival [10]. More generally, HPV-associated OPSCs have a better prognosis regardless of treatment modality [51, 52]. Approximately 20% of patients do not experience cancer recurrence after treatment, with 3- and 5-year overall survival rates of 86% and 80%, respectively, which is very different from the survival rates seen in patients with HPV-independent OPSCs [32]. Because of these significant biological and clinical differences, HPV-associated OPSCCs have their own classification, dependent on p16 labeling; in the eighth edition of the International Union of Cancer Control (UICC) TNM classification or in the American Joint Committee on Cancer (AJCC) staging systems [53, 54]. In addition, several trials are currently evaluating the performance of alternative treatment modalities such as radiation therapy dose de-escalation and immunotherapy (instead of using chemotherapy) according to HPV status in OPSCCs [55]. In this context, determination of HPV status in all OPSCCs is mandatory.
HPV-Independent Squamous Cell Carcinoma
HPV-independent OPSCCs are distinguished from those described above by the absence of high-risk HPV infection. Most commonly, these OPSCCs are located in the tonsillar region, but they can also be detected in non-lymphoepithelial sites, such as the soft palate. The main risk factors are smoking and chronic alcohol abuse. Nitrosamines (tobacco-specific nitrosamines or TNSAs) and polycyclic aromatic hydrocarbons (PAHs) are the main substances involved in smoking-related carcinogenesis [56]. Alcohol consumption is another important risk factor for cancers of the upper aerodigestive tract, including oropharyngeal cancers [57, 58]. Recent studies suggest that the role of alcohol alone (without tobacco) in oropharyngeal cancers may even be underestimated [59]. The relative risk of developing oropharyngeal cancer is 1.21 for patients consuming ≤ 1 drink/day, and 5.24 for those consuming ≥ 4 drinks/day [59, 60]. As with oropharyngeal cancers, carcinogenesis is synergistically triggered by tobacco and alcohol.
These SCCs have specific biological features (mutated TP53, hypoxia signature) or a characteristic immune microenvironment [61]. The OPSCCs microenvironment is being studied to determine the potential impact of immunomodulatory therapies and to understand the differences between carcinomas according to viral and environmental risk factors [62].
Histopathology
The most common form is keratinizing SCC, but basaloid, papillary, spindle cell/sarcomatoid, adenosquamous, or lymphoepithelial forms have also been described. As with all OPSCCs, immunohistochemical staining for p40 and p63 or cytokeratin 5/6 can indicate the squamous epithelial origin of these cancers [63]. It is important to note that, unlike HPV-associated carcinomas, the use of a three-level classification (well-, moderately-, and poorly differentiated) is recommended in HPV-independent forms. Table 2 provides a comprehensive comparison between the two types of OPSCC.
Table 2.
Comparison and specificity of HPV-associated and HPV-independent OPSCC
| HPV-associated OPSCC | HPV-independent OPSCC | |
|---|---|---|
| Gender | Mainly men | Mainly men |
| Primary tumour | Small | Large (main symptom) |
| Neck nodes | Large (main symptom) | Small |
| Secondary upper aero digestive tract cancer | Exceptional | 10–15% of cases |
| Age | Younger cohorts | Older cohorts |
| Smoking | No or moderate | Significant ± alcohol |
| Medical condition | Good | Numerous comorbidities |
| Association with sexual practices | Yes | No |
| Socio-economic level | Medium to high | Low to medium |
| Location | Tonsil, tongue base | All oropharyngeal anatomical subsites |
| Crypt epithelium | Surface epithelium | |
| Histology | Mainly Non Keratinizing SCC | Mainly Keratinizing SCC |
| Precancerous lesion | Not applicable | Squamous dysplasia |
| Grading | Not applicable | Applicable |
| HPV testing | p16 + | p16 - |
| p16 immunostaining | ||
| Survival | Better | Worse |
Nasopharynx
Introduction
The chapter on the nasopharynx in the 5th edition of the WHO classification of head and neck tumors, as with the chapter on the oropharynx, covers only those elements specific to this region. To avoid overloading this chapter, salivary gland tumors (with the exception of salivary gland anlage tumor), soft tissue tumors, hematolymphoid neoplasms, melanocytic tumors, and neuroendocrine neoplasms are covered in respective chapters. It is important to note that nasopharyngeal lymphomas account for 15% of all head and neck lymphomas. Diffuse large B-cell lymphomas are the most common type in this category [1]. NK/T cell lymphomas are mainly found in the nasopharynx with almost certain infection of the lymphoma cells by EBV, especially in Asia [64]. The "WHO 2022" classification [2] mentions that: "ectopic pituitary adenoma is covered in the chapter on neuroendocrine neoplasms and paraganglioma, under the designation "ectopic or invasive PitNET/adenoma". Craniopharyngioma is described in the chapter on nasal cavity, paranasal sinuses, and skull base, under the designation "adamantinomatous craniopharyngioma." Chordoma, which typically occurs in the clivus (base of the skull), is also discussed in the same chapter. Nasopharyngeal angiofibroma is covered in the chapter on the nasal cavity, paranasal sinuses, and skull base, under the designation "angiofibroma of the sinonasal tract."
Therefore, this new chapter only covers features specific to the nasopharynx. They can be either benign or borderline (hairy polyp and salivary gland anlage tumor) or malignant (low-grade papillary nasopharyngeal adenocarcinoma and NPC).
Benign and Borderline Lesions
Hairy Polyp
This rare tumor has already been discussed in the previous edition. It is a benign polypoid lesion arising from the lateral wall of the nasopharynx. It contains both ectodermal and mesodermal elements suggesting that it is a form of teratoma [65, 66].
Salivary Gland Anlage Tumor
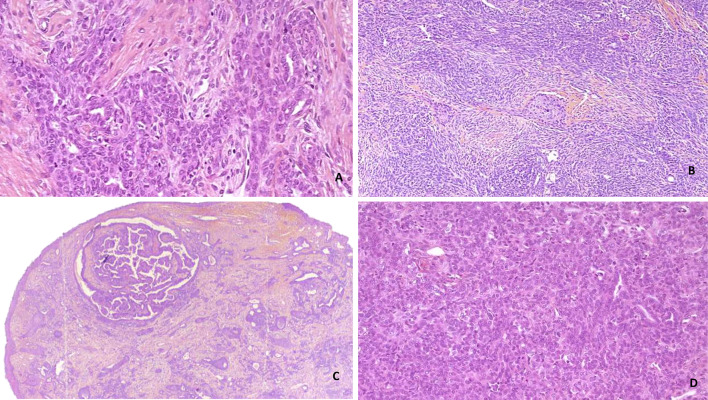
The salivary gland anlage tumor was also discussed in the previous edition, specifically in the chapter on salivary glands of the nasopharynx. This is an extremely rare form of tumor that usually occurs in a pediatric population. It involves squamous and myoepithelial epithelial components that are continuous with the surface epithelium and resemble a developing salivary gland (Fig. 3). Whole exome sequencing studies propose to classify this entity as a hamartomatous (non-neoplastic) process [67, 68].
Fig. 3.
Salivary gland anlage tumor. A, D Complex network of tubules and ducts (H&E, 40 ×): B proliferation showing squamous cells arranged in fascicles or nodules (H&E, 40 ×), C polypoid mass comprising spindle cells, tubes or nodules cribriform under a thin squamous epithelium (H&E, 5 ×)
Carcinoma
Low-Grade Papillary Nasopharyngeal Adenocarcinoma
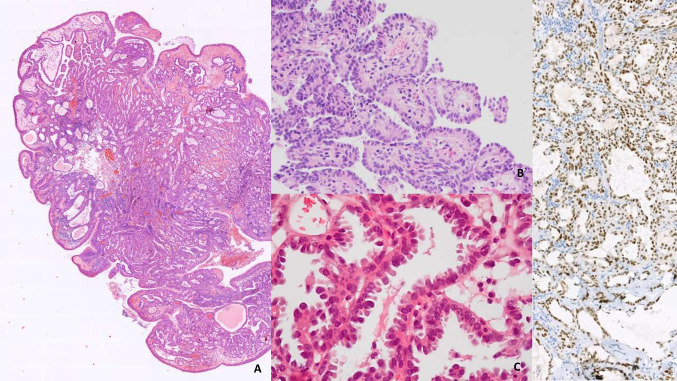
The most notable change in “WHO 2022” regarding this chapter is that nasopharyngeal papillary adenocarcinoma has been renamed "low-grade nasopharyngeal papillary adenocarcinoma" to emphasize the bland morphology as well as the lack of aggressive behavior [2]. These carcinomas are surface-derived malignant glandular neoplasms; therefore, it is recommended to look for transition zones between the surface and the carcinoma. These carcinomas have a predominantly papillary structure with scarce cytonuclear atypia. They are classified as low-grade adenocarcinomas because of their indolent behavior (Fig. 4). No specific etiologic agent or association with EBV or HPV has been identified for these rare carcinomas. Finally, it is important to note that they are positive for thyroid transcription factor (TTF1) immunostaining, and that thyroglobulin and PAX8 staining should also be performed, the latter two being negative [69–71].
Fig. 4.
Nasopharyngeal papillary adenocarcinoma: A polypoid formation covered by respiratory epithelium. Submucosal tumor proliferation corresponding to papillary adenocarcinoma (H&E, 5 ×). B, C Papillae with hyalinized fibrovascular centers. Tumor cells ovoid in shape, ground-glass in appearance, (H&E, 40 ×). D Immunohistochemical positivity for thyroid transcription factor-1 (TTF1 20 ×)
Nasopharyngeal Carcinoma
By definition, NPC is a malignant mucosal epithelial neoplasm usually with evidence of squamous differentiation [72].
Established etiologic factors for NPC include genetic, viral, and environmental elements: genetically predisposed patients are likely to develop NPC when exposed to a viral and/or environmental factor. NPC is considered rare, and its overall incidence is declining. However, because of its association with EBV infection, incidence rates are significantly higher in some endemic regions. These areas include Southwest Asia (Canton and Hong Kong), the Mediterranean region, North Africa, and Greenland and Alaska (Inuit population) [73, 74]. The average age of affected patients is 45–55 years, but pediatric cases have also been described. The male/female incidence ratio is 2.75 [75]. Many other risk factors, dietary (nitrosamines), genetic (HLA), or environmental (dust, smoke) were described in the previous edition and have been maintained in the new edition. It should be noted that the role of HPV infections has gained importance among the established risk factors for these cancers. A study in southern China, where EBV is endemic, found that among 1328 NPCs, 91.3% were EBV + /HPV-, 0.6% were EBV + /HPV + , 7.1% were EBV-/HPV + and 1.0% were EBV-/HPV- [76]. It should also be mentioned that depending on the country and the level of EBV endemicity, the importance of the role of HPV infection in the development of these cancers remains highly variable, exceeding 35% according to some studies [11, 77, 78]. In non-endemic countries, the frequency of HPV infection seems to be higher in keratinizing cancers. The association with prognosis is not as clear as for the oropharynx.
Histopathology
There are no significant differences between the two editions of the Blue Book in terms of histological description and classification of these carcinomas. Three subtypes are described: (i) non-keratinizing SCC (NK-NPC), (ii) keratinizing SCC (K-NPC) and (iii) basaloid SCC. Therefore, this article provides only a brief overview of the histologic subtypes that are described in detail in the WHO classification (Fig. 5).
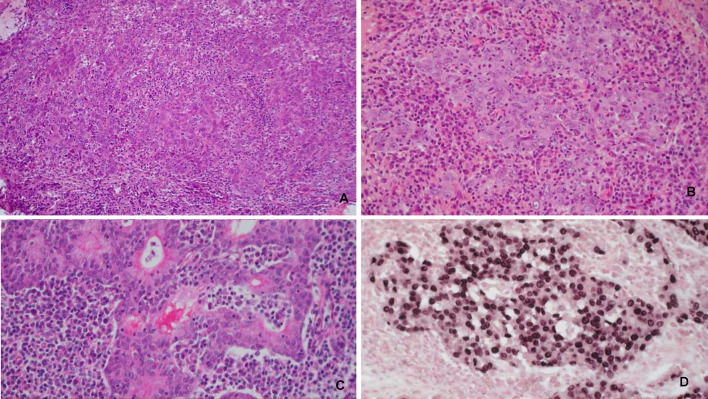
Fig. 5.
Nasopharyngeal carcinoma. A, B Nasopharyngeal nonkeratinizing carcinoma: sheets of tumour with a squamous cell differentiation separated by a dense infiltrate of lymphocytes and plasma cells. C Subtype with glandular differentiation. D In situ hybridization with EBER probe
Nonkeratinizing SCC (NK-NPC) is the most common histologic subtype that may have some cytologic variations or lymphoepithelial features. Most of these tumors are related to EBV infection. Keratinizing SCC (K-NPC) does not differ from other keratinizing HNSCC in terms of histologic aspects. Basaloid SCC does not differ in histologic appearance from other locations.
Immunohistochemistry and In Situ Hybridization
These carcinomas show positive staining for pan-cytokeratin, which shows their epithelial origin, while differentiation of squamous cells can be demonstrated by positivity of p40 and p63. The use of latent membrane protein (LMP1) immunohistochemical staining is not recommended because of its low sensitivity and difficulty in determining EBV infection. Indeed, LMP1 is an oncogenic factor in EBV-infected cells, but its presence cannot be demonstrated in half of the NPC biopsies. EBV in situ hybridization for viral RNA is recommended. It is positive in most cases of NK-NPC but gives variable results in K-NPC and basaloid subtypes. In summary, there are no major changes in this chapter regarding histologic and immunohistologic analysis or EBV testing. However, the new edition covers in detail the major advances in our understanding of the molecular genetics of nasopharyngeal carcinoma. The process of carcinogenesis from normal mucosa to invasive NPC involves intermediate low- and high-grade dysplastic lesions, and it is accompanied by a cascade of genetic abnormalities. In the early stages of carcinogenesis, these abnormalities are rather rare. The new "WHO 2022" classification points out that "genomic sequencing reveals distinct mutational signatures associated with high APOBEC3 activity, DNA mismatch repair and homologous recombination repair deficiencies, evidencing viral driven genomic instability during pathogenesis”. The deregulation of the cell cycle is triggered by multiple factors, and it plays a major role in the development of NPCs. It is worth noting that p53 mutations appear rather late in the carcinogenic process, which could explain why these tumors respond well to chemo- and radiation therapy in the early stages.
Prognosis Prediction and Staging
The use of the 8th edition of the UICC/AJCC TNM staging system is recommended for NPCs, as it includes the most important prognostic factors [79–81]. The efficacy of staging can be enhanced using other prognostic factors such as the inclusion of histological and virological data or the analysis of the immune microenvironment [79]. Further studies are needed to prove the validity of these hypotheses. Thus, for example, the expression of the epithelial growth factor EGFR is not correlated with the T and N stages and with the occurrence of metastases, but an expression higher than 25% in immunohistochemistry could be a poor prognostic factor [82].
Conclusion
The new WHO 2022 classification includes numerous modifications in the chapters covering the oropharynx and the nasopharynx. Several items have been extracted and reinserted into specific chapters (neuroendocrine neoplasms, salivary gland tumors, soft tissue tumors or hematolymphoid proliferations and neoplasia). This has restricted the breadth of the chapters to lesions that are specific to the oro- and nasopharyngeal regions.
Acknowledgements
Viktoria Nagy, Sophie Outh-Gauer, Michel Wassef, Aurore Coulomb-Lhermine, Valérie Costes.
Author Contributions
Conceptualization, literature search, data analysis, writing, review and editing by CB.
Funding
Not applicable.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
The author declares that there is no conflict of interest relative to tis research project.
Ethical Approval
This review article did not involve the use of human or animal subjects and has complied with the ethical standards as outlined by the publisher.
Consent to Participate
Not applicable.
Consent for Publication
The author has read and agreed to the published version of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. 4. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 2.WHO Classification of Tumours Editorial Board. WHO classification of tumours series. Head and neck tumours. 5th ed., vol. 9. Lyon: International Agency for Research on Cancer; 2022.
- 3.Fossum CC, Chintakuntlawar AV, Price DL, Garcia JJ. Characterization of the oropharynx: anatomy, histology, immunology, squamous cell carcinoma and surgical resection. Histopathology. 2017;70:1021–1029. doi: 10.1111/his.13140. [DOI] [PubMed] [Google Scholar]
- 4.Kardon DE, Wenig BM, Heffner DK, Thompson LDR. Tonsillar lymphangiomatous polyps: a clinicopathologic series of 26 cases. Mod Pathol. 2000;13:1128–1133. doi: 10.1038/modpathol.3880208. [DOI] [PubMed] [Google Scholar]
- 5.Barreto I, Costa AF, Martins MT, Furuse C, de Araújo VC, Altemani A. Immunohistochemical study of stromal and vascular components of tonsillar polyps: high endothelial venules as participants of the polyp’s lymphoid tissue. Virchows Arch. 2011;459:65–71. doi: 10.1007/s00428-011-1088-8. [DOI] [PubMed] [Google Scholar]
- 6.Min HJ, Kim KS. Lymphangiomatous polyp arising from the palatine tonsil. Ear Nose Throat J. 2021;100:NP154–NP155. doi: 10.1177/0145561319863367. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer . WHO Global Cancer Observatory. Lyon: International Agency for Research on Cancer; 2020. [Google Scholar]
- 8.Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67:51–64. doi: 10.3322/caac.21384. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wotman M, Oh EJ, Ahn S, Kraus D, Costantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40:705–710. doi: 10.1016/j.amjoto.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Verma V, Simone CB, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40:696–706. doi: 10.1002/hed.24978. [DOI] [PubMed] [Google Scholar]
- 13.Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108:div403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 14.Lam EWH, Chan JYW, Chan ABW, Ng CS, Lo STH, Lam VSC, et al. Prevalence, clinicopathological characteristics, and outcome of human papillomavirus-associated oropharyngeal cancer in Southern Chinese Patients. Cancer Epidemiol Biomark Prev. 2016;25:165–173. doi: 10.1158/1055-9965.EPI-15-0869. [DOI] [PubMed] [Google Scholar]
- 15.Shaikh MH, Khan AI, Sadat A, Chowdhury AH, Jinnah SA, Gopalan V, et al. Prevalence and types of high-risk human papillomaviruses in head and neck cancers from Bangladesh. BMC Cancer. 2017;17:792. doi: 10.1186/s12885-017-3789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim MY, Dahlstrom KR, Sturgis EM, Li G. Human papillomavirus integration pattern and demographic, clinical, and survival characteristics of patients with oropharyngeal squamous cell carcinoma. Head Neck. 2016;38:1139–1144. doi: 10.1002/hed.24429. [DOI] [PubMed] [Google Scholar]
- 17.Augustin J, Mandavit M, Outh-Gauer S, Grard O, Gasne C, Lépine C, et al. HPV RNA CISH score identifies two prognostic groups in a p16 positive oropharyngeal squamous cell carcinoma population. Mod Pathol. 2018;31:1645–1652. doi: 10.1038/s41379-018-0090-y. [DOI] [PubMed] [Google Scholar]
- 18.Drake VE, Fakhry C, Windon MJ, Stewart CM, Akst L, Hillel A, et al. Timing, number, and type of sexual partners associated with risk of oropharyngeal cancer. Cancer. 2021;127:1029–1038. doi: 10.1002/cncr.33346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakhry C, Waterboer T, Westra WH, Rooper LM, Windon M, Troy T, et al. Distinct biomarker and behavioral profiles of human papillomavirus-related oropharynx cancer patients by age. Oral Oncol. 2020;101:104522. doi: 10.1016/j.oraloncology.2019.104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts S, Evans D, Mehanna H, Parish JL. Modelling human papillomavirus biology in oropharyngeal keratinocytes. Philos Trans R Soc B Biol Sci. 2019;374:20180289. doi: 10.1098/rstb.2018.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westra WH. The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6:48–54. doi: 10.1007/s12105-012-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry ME. The specialised structure of crypt epithelium in the human palatine tonsil and its functional significance. J Anat. 1994;185(Pt 1):111–127. [PMC free article] [PubMed] [Google Scholar]
- 23.Kranjec C, Holleywood C, Libert D, Griffin H, Mahmood R, Isaacson E, et al. Modulation of basal cell fate during productive and transforming HPV-16 infection is mediated by progressive E6-driven depletion of Notch. J Pathol. 2017;242:448–462. doi: 10.1002/path.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop JA, Westra WH. Ciliated HPV-related carcinoma: a well-differentiated form of head and neck carcinoma that can be mistaken for a benign cyst. Am J Surg Pathol. 2015;39:1591–1595. doi: 10.1097/PAS.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alos L, Hakim S, Larque A-B, de la Oliva J, Rodriguez-Carunchio L, Caballero M, et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 2016;469:277–284. doi: 10.1007/s00428-016-1982-1. [DOI] [PubMed] [Google Scholar]
- 27.Bates T, McQueen A, Iqbal MS, Kelly C, Robinson M. Small cell neuroendocrine carcinoma of the oropharynx harbouring oncogenic HPV-infection. Head Neck Pathol. 2014;8:127–131. doi: 10.1007/s12105-013-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hojilla CV, Yu ES, Perez-Ordonez B. Human papillomavirus-associated poorly differentiated (small cell) neuroendocrine carcinoma of the oropharynx. Diagnos Histopathol. 2013;19:20–24. [Google Scholar]
- 29.Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35:1679–1684. doi: 10.1097/PAS.0b013e3182299cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraft S, Faquin WC, Krane JF. HPV-associated Neuroendocrine carcinoma of the oropharynx. Am J Surg Pathol. 2012;36:321–330. doi: 10.1097/PAS.0b013e31823f2f17. [DOI] [PubMed] [Google Scholar]
- 31.Thompson ED, Stelow EB, Mills SE, Westra WH, Bishop JA. Large cell neuroendocrine carcinoma of the head and neck. Am J Surg Pathol. 2016;40:471–478. doi: 10.1097/PAS.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/nejmoa065497. [DOI] [PubMed] [Google Scholar]
- 33.Würdemann N, Wagner S, Sharma SJ, Prigge E-S, Reuschenbach M, Gattenlöhner S, et al. Prognostic impact of AJCC/UICC 8th editon new staging rules in oropharyngeal squamous cell carcinoma. Front Oncol. 2017 doi: 10.3389/fonc.2017.00129/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 35.Yasui T, Morii E, Yamamoto Y, Yoshii T, Takenaka Y, Nakahara S, et al. Human papillomavirus and cystic node metastasis in oropharyngeal cancer and cancer of unknown primary origin. PLoS ONE. 2014;9:e95364. doi: 10.1371/journal.pone.0095364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morani AC, Eisbruch A, Carey TE, Hauff SJ, Walline HM, Mukherji SK. Intranodal cystic changes. J Comput Assist Tomogr. 2013;37:343–345. doi: 10.1097/RCT.0b013e318282d7c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An Y, Park HS, Kelly JR, Stahl JM, Yarbrough WG, Burtness BA, et al. The prognostic value of extranodal extension in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer. 2017;123:2762–2772. doi: 10.1002/cncr.30598. [DOI] [PubMed] [Google Scholar]
- 38.Day AT, Yang AM, Tanamal P, Blackwell J-M, Wang E, Sumer BD, et al. Extracapsular extension, pathologic node status, and adjuvant treatment in primary surgery patients with human papillomavirus-mediated oropharyngeal cancer: National hospital-based retrospective cohort analysis. Head Neck. 2021;43:3345–3363. doi: 10.1002/hed.26825. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Zapien D, Ruiz FX, Poirson J, Mitschler A, Ramirez J, Forster A, et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature. 2016;529:541–545. doi: 10.1038/nature16481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018;26:158–168. doi: 10.1016/j.tim.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Augustin JG, Lepine C, Morini A, Brunet A, Veyer D, Brochard C, et al. HPV detection in head and neck squamous cell carcinomas: what is the issue? Front Oncol. 2020 doi: 10.3389/fonc.2020.01751/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis JS, Beadle B, Bishop JA, Chernock RD, Colasacco C, Lacchetti C, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the college of american pathologists. Arch Pathol Lab Med. 2018;142:559–597. doi: 10.5858/arpa.2017-0286-CP. [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin-Drubin ME, Crum CP, Münger K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc Natl Acad Sci USA. 2011;108:2130–2135. doi: 10.1073/pnas.1009933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nauta IH, Rietbergen MM, van Bokhoven AAJD, Bloemena E, Lissenberg-Witte BI, Heideman DAM, et al. Evaluation of the eighth TNM classification on p16-positive oropharyngeal squamous cell carcinomas in the Netherlands and the importance of additional HPV DNA testing. Ann Oncol. 2018;29:1273–1279. doi: 10.1093/annonc/mdy060. [DOI] [PubMed] [Google Scholar]
- 45.Mehanna H, Evans M, Beasley M, Chatterjee S, Dilkes M, Homer J, et al. Oropharyngeal cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S90–S96. doi: 10.1017/S0022215116000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fakhry C, Lacchetti C, Perez-Ordonez B. Human papillomavirus testing in head and neck carcinomas: ASCO clinical practice guideline endorsement summary of the CAP guideline. J Oncol Pract. 2018;14:613–617. doi: 10.1200/JOP.18.00433. [DOI] [PubMed] [Google Scholar]
- 47.El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459–461. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–641. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feldman R, Gatalica Z, Knezetic J, Reddy S, Nathan C-A, Javadi N, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1625–1638. doi: 10.1002/hed.24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Misiukiewicz K, Gupta V, Miles BA, Bakst R, Genden E, Selkridge I, et al. Standard of care vs reduced-dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients: the Quarterback trial. Oral Oncol. 2019;95:170–177. doi: 10.1016/j.oraloncology.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 52.Wang MB, Liu IY, Gornbein JA, Nguyen CT. HPV-positive oropharyngeal carcinoma. Otolaryngol Neck Surg. 2015;153:758–769. doi: 10.1177/0194599815592157. [DOI] [PubMed] [Google Scholar]
- 53.Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 54.Lewis JS, Adelstein DJ, Agaimy A, Carlson DL, Faquin WC, Helliwell T, et al. Data set for the reporting of carcinomas of the nasopharynx and oropharynx: explanations and recommendations of the guidelines from the international collaboration on cancer reporting. Arch Pathol Lab Med. 2019;143:447–451. doi: 10.5858/arpa.2018-0405-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howard J, Dwivedi RC, Masterson L, Kothari P, Quon H, Holsinger FC. De-intensified adjuvant (chemo)radiotherapy versus standard adjuvant chemoradiotherapy post transoral minimally invasive surgery for resectable HPV-positive oropharyngeal carcinoma. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD012939.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khariwala SS, Hatsukami D, Hecht SS. Tobacco carcinogen metabolites and DNA adducts as biomarkers in Head and Neck cancer: potential screening tools and prognostic indicators. Head Neck. 2012;34:441–447. doi: 10.1002/hed.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006 doi: 10.1515/BC.2006.047/html. [DOI] [PubMed] [Google Scholar]
- 58.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 59.Gormley M, Dudding T, Sanderson E, Martin RM, Thomas S, Tyrrell J, et al. A multivariable Mendelian randomization analysis investigating smoking and alcohol consumption in oral and oropharyngeal cancer. Nat Commun. 2020;11:6071. doi: 10.1038/s41467-020-19822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tramacere I, Negri E, Bagnardi V, Garavello W, Rota M, Scotti L, et al. A meta-analysis of alcohol drinking and oral and pharyngeal cancers. Part 1: overall results and dose-risk relation. Oral Oncol. 2010;46:497–503. doi: 10.1016/j.oraloncology.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 61.Kim HAJ, Zeng PYF, Shaikh MH, Mundi N, Ghasemi F, Di Gravio E, et al. All HPV-negative head and neck cancers are not the same: analysis of the TCGA dataset reveals that anatomical sites have distinct mutation, transcriptome, hypoxia, and tumor microenvironment profiles. Oral Oncol. 2021;116:105260. doi: 10.1016/j.oraloncology.2021.105260. [DOI] [PubMed] [Google Scholar]
- 62.Outh-Gauer S, Morini A, Tartour E, Lépine C, Jung AC, Badoual C. The microenvironment of head and neck cancers: papillomavirus involvement and potential impact of immunomodulatory treatments. Head Neck Pathol. 2020;14:330–340. doi: 10.1007/s12105-020-01147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bishop JA, Montgomery EA, Westra WH. Use of p40 and p63 immunohistochemistry and human papillomavirus testing as ancillary tools for the recognition of head and neck sarcomatoid carcinoma and its distinction from benign and malignant mesenchymal processes. Am J Surg Pathol. 2014;38:257–264. doi: 10.1097/PAS.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tse E, Kwong Y-L. NK/T-cell lymphomas. Best Pract Res Clin Haematol. 2019;32:253–261. doi: 10.1016/j.beha.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Cone BM, Taweevisit M, Shenoda S, Sobol S, Schemankewitz E, Shehata BM. Pharyngeal hairy polyps: five new cases and review of the literature. Fetal Pediatr Pathol. 2012;31:184–189. doi: 10.3109/15513815.2011.648722. [DOI] [PubMed] [Google Scholar]
- 66.Melzer JM, Morgan A, Darrow D. Congenital choristoma (Hairy Polyp) of the Eustachian tube: surgical management of a rare clinical entity. Ear Nose Throat J. 2016;95:E43–E45. doi: 10.1177/014556131609500106. [DOI] [PubMed] [Google Scholar]
- 67.Herrmann BW, Dehner LP, Lieu JEC. Congenital salivary gland anlage tumor: a case series and review of the literature. Int J Pediatr Otorhinolaryngol. 2005;69:149–156. doi: 10.1016/j.ijporl.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 68.Peters SM, Turk AT. Salivary gland anlage tumor: molecular profiling sheds light on a morphologic question. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127:e108–e113. doi: 10.1016/j.oooo.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Lai Y, Li W, Zhai C, Song X, Yang J, Sun X, et al. Low-grade nasopharyngeal papillary adenocarcinoma: a review of 28 patients in a Single Institution. Cancer Manage Res. 2021;13:1271–1278. doi: 10.2147/CMAR.S288007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pineda-Daboin K, Neto A, Ochoa-Perez V, Luna MA. Nasopharyngeal adenocarcinomas: a clinicopathologic study of 44 cases including immunohistochemical features of 18 papillary phenotypes. Ann Diagn Pathol. 2006;10:215–221. doi: 10.1016/j.anndiagpath.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Thompson LDR. Nasopharyngeal papillary adenocarcinoma. Ear Nose Throat J. 2017;96:456–457. doi: 10.1177/014556131709601203. [DOI] [PubMed] [Google Scholar]
- 72.Yu MC, Yuan J-M. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–429. doi: 10.1016/s1044579x02000858. [DOI] [PubMed] [Google Scholar]
- 73.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today. Lyon: International Agency for Research on Cancer; 2020. [Google Scholar]
- 74.Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, et al. Cancer incidence in Five Continents. Lyon: International Lyon; 2017. [Google Scholar]
- 75.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 76.Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124:530–536. doi: 10.1002/cncr.31031. [DOI] [PubMed] [Google Scholar]
- 77.Augustin J, Outh-Gauer S, Mandavit M, Gasne C, Grard O, Denize T, et al. Evaluation of the efficacy of the 4 tests (p16 immunochemistry, polymerase chain reaction, DNA, and RNA in situ hybridization) to evaluate a human papillomavirus infection in head and neck cancers: a cohort of 348 French squamous cell carcinomas. Hum Pathol. 2018;78:63–71. doi: 10.1016/j.humpath.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 78.Lin Z, Khong B, Kwok S, Cao H, West RB, Le Q-T, et al. Human papillomavirus 16 detected in nasopharyngeal carcinomas in white Americans but not in endemic Southern Chinese patients. Head Neck. 2014;36:709–714. doi: 10.1002/hed.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Sullivan B, Brierley JD, D’Cruz A, Fey M, Pollock RE, Vermorken J, et al. Nasopharynx. UICC Manual of Clinical Oncology. 9th ed. 2015. p. 512–23.
- 80.Pan JJ, Ng WT, Zong JF, Chan LLK, O’Sullivan B, Lin SJ, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122:546–558. doi: 10.1002/cncr.29795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang X-L, Wang Y, Liang S-B, He S-S, Chen D-M, Chen H-Y, et al. Comparison of the seventh and eighth editions of the UICC/AJCC staging system for nasopharyngeal carcinoma: analysis of 1317 patients treated with intensity-modulated radiotherapy at two centers. BMC Cancer. 2018;18:606. doi: 10.1186/s12885-018-4419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chua DTT, Nicholls JM, Sham JST, Au GKH. Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:11–20. doi: 10.1016/j.ijrobp.2003.10.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.