Abstract
Emotion regulation is essential for successful social interactions and function, which are important aspects of middle childhood. The current study is one of the first to examine associations between neural correlates of implicit emotion regulation and indices of social behavior and experience during late middle childhood. We examined neural activation during the implicit emotion regulation condition of the Emotional N-back task using data from 8987 9- to 11-year-olds from the Adolescent Brain Cognitive Development℠ study. The brain regions assessed included areas linked to social cognition, social behavior, and emotion recognition, including the amygdala, insula, middle temporal gyrus, and inferior parietal lobe. Greater number of close friends was associated with significantly higher activation of the fusiform gyrus, insula, temporoparietal junction, inferior parietal lobe, and superior temporal gyrus during implicit emotion regulation. Greater reciprocal social impairments were linked to decreased fusiform gyrus activation during implicit emotion regulation. More experiences of discrimination were associated with a significantly lower activation in the middle temporal gyrus during implicit emotion regulation. This study provides evidence that both positive and negative indices of children’s social experiences and behaviors are associated with neural correlates of implicit emotion regulation during late middle childhood. These findings suggest that both positive and negative indices of social behavior and experience, including those within and not within the youth’s control, are associated with generally unique neural correlates during implicit emotion regulation.
Subject terms: Emotion, Human behaviour
Emotion regulation encompasses the processes influencing the experience and expression of emotions [1, 2]. There are two types of emotion regulation: explicit and implicit [3]. Implicit emotion regulation, the focus of the current study, encompasses strategies such as affect labeling and is characterized by a lack of intention and supervision [4]. Successful emotion regulation is associated with an increased ability to engage in socially appropriate emotions and behaviors, which is itself associated with adaptive social interactions and social competence [5–10]. In contrast, disruptions in effective emotion regulation strategies may result from negative social experiences (e.g., experiences of bullying) and lead to difficulties with social behaviors and negative social experiences [6, 11, 12]. Few, if any, previous studies have explicitly explored the relationship between indices of social behavior or experience and the neural correlates of implicit emotion regulation during late middle childhood.
Previous cross-sectional research has examined the neural correlates of social behaviors, social experiences, and emotion regulation separately. Several overlapping regions, including the amygdala and ventromedial prefrontal cortex (PFC), have been implicated in previous research examining either the neural correlates of social behaviors, social experiences, or emotion regulation as assessed by the Emotional N-back (EN-back task) [13–18]. Emotion regulation is generally are associated with activity in frontal (e.g., superior frontal gyrus [SFG], orbitofrontal gyrus, lateral and dorsolateral PFC) regions, temporal (e.g., temporoparietal junction [TPJ]) [17–19], and limbic (e.g., amygdala) regions [20–23]. One study by Sebastian et al. (2010) [24] using an implicit emotion regulation task with a mid-adolescence sample found increased ventrolateral PFC activity when words related to social rejection vs. social acceptance were used. This suggests that there is potentially a link between social experience and implicit emotion regulation, although the current study is the first to our knowledge to explore this link in late middle childhood.
Late middle childhood, defined in our study as ages 9–10, is a critical period for social behaviors and experiences [25–30]. As a result of increased exposure to and development of relationships with peers, friends, and teammates during middle childhood, positive social behaviors, social experiences, and social skills become increasingly important during this stage [27, 31–33]. Social behaviors and experiences in late middle childhood can be distinguished by their valence; both positive and negative indices of social experiences are important as each may have important but varying implications for implicit emotion regulation. Positive social indices can be defined as indices that increase or reflect greater social competence, aid development of positive social behaviors, or reflect positive social experiences. In contrast, negative indices reflect negative social behaviors or experiences that may impede development of positive sociability. It is important to note that indices over which children have control (e.g., their overt behaviors) and those over which children have either no or little control over (e.g., others’ behavior towards them) both influence social behaviors and experiences during middle childhood [27, 32, 34–40]. Given the importance of social interactions in late middle childhood, this study aimed to comprehensively examine social indices varying in both valence and individual control, including engagement in extracurricular activities, number of close friendships, aggression, victimization, experiences of discrimination, and prosocial behavior.
Previous research supports the importance of these aspects of social behaviors and experiences in late middle childhood. Certain positive indices, including those in part under the control of the child (e.g., prosocial behavior) as well as more externally influenced factors (e.g., extracurricular activities engaged in during the past 12 months), are associated with more positive social outcomes, such as social competence [41–44]. Greater number of friends has been associated with increased social competence, in addition to fewer victim experiences [45–53]. Prosocial behavior in school-aged children has been linked to constructive social skills, as well as increased empathy and perspective-taking [54–59]. On the other hand, negative aspects of social behaviors and experiences have been associated with poorer social outcomes. Experiences of discrimination [60] are associated with adjustment difficulties and are inversely related to social connectedness [61], although these experiences are likely influenced by other sequelae of systemic racism. Other forms of victimization are linked to decreased social standing and predict negative perceptions of social abilities [62–64]. Aggression in children has been linked to poor social adjustment plus fewer social goals [53, 54, 62, 65–70].
In terms of the neural correlates of positive social behaviors and experiences, the ability to predict others’ behaviors or to understand another’s mental state is generally associated with activation in a number of regions, including frontal (e.g., ventrolateral, ventromedial, and medial PFC) [71], parietal (e.g., fusiform gyrus) [72], temporal (e.g., temporal pole) [71], and limbic (e.g., insula, amygdala) regions [73–76]. In contrast, the neural correlates of negative social behaviors and experiences, including social exclusion, aggression, and victimization, include disrupted activation of the anterior, medial, and dorsolateral PFC, temporal regions (e.g., superior temporal gyrus [STG]), ACC, frontal regions (e.g., IFG, frontal opercular), amygdala, fusiform gyrus, and insula [77–84].
The current study used data from the Adolescent Brain Cognitive Development℠ (ABCD) study to examine associations between neural correlates of implicit emotion using the EN-back task with social behavior and experience in a large late middle childhood sample. This study chose to investigate social behaviors and experiences both within and outside of a child’s control, as it is important to know if those behaviors and experiences beyond a child’s control can affect the neural correlates of implicit emotion regulation. We selected regions of interest (ROIs) that were both consistent with a review of literature which examined neural correlates of emotion regulation [13–24] and social behaviors or experiences [71–84] and were available in the ABCD dataset. Although analyses were largely exploratory in nature, we did expect to see altered activation in regions previously associated with emotion regulation and social behaviors or experiences, such as the STG, fusiform gyrus, and ACC. Specifically, we predicted positive indices of social behavior and experience would be associated with higher activation during the EN-back task in neural regions related to emotion regulation of positive emotions, such as the TPJ. Conversely, we hypothesized negative indices of social behavior and experience would be associated with altered activation during the EN-back task in neural regions related to emotion regulation of negative emotions, such as the amygdala.
Materials and methods
Participants
A sample of 10,372 children who completed the in-scanner EN-back task was obtained from ABCD Study® Data Release 3.0 (Acknowledgements), a large-scale study tracking 11,875 children ages 9–10 from 21 different research sites across the United States. The study was approved by a central Institutional Review Board at University of California, San Diego. All parents and children provided written informed consent and assent, respectively. Participants were removed from analyses either for having task data that did not pass quality assurance criteria (i.e., did not have at least one run that was complete, passed protocol compliance, and was preceded by field maps within the last two scans; n = 714). In addition, participants with missing relevant imaging data (i.e., imaging variables, scanner information, quality assurance information) were removed from analyses (n = 671). The final sample size was 8,987 individuals with imaging data (Table 1 for demographic characteristics; Supplement for study-wide exclusion criteria).
Table 1.
Demographic Characteristics for samplea.
| Variable | Mean (or %) | SD |
|---|---|---|
| Age (years) | 9.93 | 0.007 |
| Sex (female) | 49.2 | 0.005 |
| Ethnicity (%) | ||
| Caucasian | 54.2 | 0.005 |
| African American | 12.8 | 0.007 |
| Hispanic | 12.8 | 0.013 |
| Asian | 2.1 | 0.006 |
| Other | 10.4 | 0.016 |
| Financial adversity (n = 8891) | 0.43 | 0.011 |
| Average motionb | 0.32 | 0.003 |
| Scanner type | ||
| Siemens | 63.0 | 0.005 |
| GE | 24.6 | 0.005 |
| Philips | 12.1 | 0.003 |
| Positive social behavior and experience indices | ||
| Prosocial behavior (n = 8971) | 1.76 | 0.004 |
| Number of activities in the past 12 months (n = 8986) | 3.58 | 0.025 |
| Number of Close Friends (n = 7167) | 5.96 | 0.07 |
| Negative Social Behavior and Experience Indices | ||
| Experience of Being Bullied (n = 8982) | 1.86 | 0.004 |
| Overt aggression (n = 4832) | 3.29 | 0.013 |
| Overt victimization (n = 4832) | 3.64 | 0.019 |
| Reciprocal Social Impairments (n = 8505) | 14.22 | 0.043 |
| Social problems (n = 8980) | 1.53 | 0.023 |
| Experiences of discrimination (n = 8297) | 1.17 | 0.004 |
SD standard deviation, GE general electric.
aModels were performed with available data; see each index for sample size included in models.
bAverage motion is calculated as average framewise displacement.
Measures
The current study focuses on positive and negative social behavior and experience indices, as both are important indicators of social experience. All measures were collected at baseline assessment, unless otherwise noted. See Supplemental Table 1 for associations between each of these measures.
Positive social behavior & experience
Prosocial behavior
The Prosocial Behaviors Subscale (PBS) is a 5-item subscale from the Strengths and Difficulties Questionnaire [85] measuring prosocial thoughts and behaviors in a child (e.g., “I try to be nice to other people. I care about their feelings.”), rated from 0=Not true to 2=Certainly true. The current study used an average of the responses to the 5 items, with a higher score indicating more prosocial behavior. We analyzed both youth-report and caregiver-report prosocial behavior, with results remaining consistent.
Number of activities in the past 12 months
The caregiver-reported Sports Activities Involvement questionnaire, based on the Vermont Child Health and Behavior Questionnaire [86], asks caregivers about youth participation in 32 different activities, including both sport (e.g., gymnastics) and non-sport (e.g., music). Caregivers were asked to indicate what type of activities their child participated in and whether they participated in that activity in the past 12 months. The measure used was a summation of the number of activities participated in the past 12 months.
Number of close friends
Caregivers reported on participants’ number of male and female close friends [87], with the current study examining the summation of the number of reported close friendships.
Negative social behavior & experience indices
Experience of being bullied
To measure bullying, the Introduction to KSADS asks caregivers whether the youth was bullied at school or in the neighborhood [87]. The question was rated as either “yes” or “no.”
Overt aggression and overt victimization
Overt Aggression and Overt Victimization are both subscales of the youth-reported Peer Experiences Questionnaire, measured at 2-year-follow-up, each with three items [88, 89]. A higher summed score suggests either more aggression or victimization. The summed scores for these subscales were analyzed as separate predictor variables.
Reciprocal social impairments
Reciprocal social impairments [90] were measured by the Short Social Responsiveness Scale (SSRS) at 1-year-follow-up, which is composed of 12 caregiver-reported questions (e.g., “has difficulty relating to peers”) taken from the Social Responsiveness Scale (SRS) [91]. Although often used to index Autism Spectrum Disorder (ASD) symptoms, the SSRS has been used to study general social impairments in non-ASD populations [92]. A higher summed score suggests greater impairments during reciprocal social interactions.
Social problems
The Child Behavior Checklist (CBCL) is made up of 112 caregiver-reported questions, with a higher score suggesting greater psychopathology symptoms [93]. The current study examined the CBCL social problems subscale (e.g., “doesn’t get along with other kids”).
Experiences of discrimination
The Perceived Discrimination Scale [94], measured at 1-year-follow-up, consists of seven youth-reported questions on being treated unfairly or negatively and feeling unaccepted based on demographic characteristics (e.g., “I feel that others behave in an unfair or negative way toward my ethnic group”), with each experience rated from 1=almost never to 5=very often. A higher average score suggests greater experiences of discrimination. Although this measure was created using children ages 14–19, it has been used previously with children as young as age 9 [95].
EN-back
The EN-back [96] is a variant of the original HCP n-back task [15] measuring implicit emotion regulation and working memory (Supplement for task description). Although both explicit and implicit emotion regulation are important in late middle childhood, the EN-back is the only emotion regulation task in the ABCD study. For imaging analyses, we examined the emotion vs. neutral contrast after collapsing across N-back level. In this contrast, the happy and fearful faces were contrasted with neutral faces (emotion vs. neutral) to examine responses specific to emotionally evocative stimuli as a measure of implicit emotion regulation and reactivity [97, 98].
Imaging procedure
All children were run on a 3 T scanner (either Siemens or General Electric) with a 32-channel head coil. Each child completed T1-weighted and T2-weighted structural scans (1-mm isotropic) before completing tasks, which were counterbalanced across subjects (see Casey et al., 2018 for details [16]). Head motion was monitored in real time using a system called FIRMM (fMRI Integrated Real-time Motion Monitor) [99], which calculates motion values and summary statistics during scan acquisition, providing estimates of movement.
A pre-processing pipeline was created using the Multi-Modal Processing Stream (MMPS), a software package developed by the Center for Multimodal Imaging and Genetics (CMIG). Using this software, head motion was corrected by registering each frame to the first using AFNI’s 3dvolreg [100] and B0 distortions were corrected using a reversing polarity method [101]. Task-related activation strength was then calculated at the individual level using a general linear model (GLM) in AFNI’s 3dDeconvolve [100]. The hemodynamic response function was modeled as a gamma function with temporal derivatives using AFNI. The GLM coefficients and t-statistics were then sampled onto the FreeSurfer-generated cortical surface. Processed task data were mapped to 18 cortical ROIs for each hemisphere based on the Desikan-Killany atlas [102]. Subcortical structure segmentations were based on FreeSurfer (aseg) sub-cortical parcellations [103]. Based on previous research, ROIs focused on the amygdala, nucleus accumbens, insula, medial orbitofrontal cortex (mOFC), IFG, ACC (caudal, rostral, and average), posterior cingulate cortex (PCC), inferior temporal gyrus (ITG), middle temporal gyrus (MTG), SFG, STG, temporal pole, fusiform gyrus, occipitotemporal gyrus (OTG), inferior parietal lobe (IPL), and TPJ [104–114]. The TPJ was defined in the current study as an aggregate of the IPL and STG ROIs as both regions contribute to the TPJ’s response to representational mental states [115]. The average beta weights for the emotion vs. neutral contrast for each of these ROIs were examined (i.e., average across both trial runs).
Statistical analyses
Due to the inclusion of siblings in the ABCD Study dataset, family unit was clustered as a random intercept, as were the 21 research sites. Age, sex, race/ethnicity, financial adversity (an assessment of material hardship or deprivation recommended as a measure of socioeconomic status [116]), average head motion, and scanner type were included as covariates. Every predictor (e.g., amygdala, IPL) and outcome variable (e.g., prosocial behavior, overt aggression) was winsorized to three standard deviations from the mean to minimize the influence of extreme values. All analyses were conducted in R lme4 package [117]. For imaging analyses, hierarchical linear models (HLMs) analyzed associations between each individual outcome (i.e., either positive or negative social behavior or experience index) with the average beta weights for each a priori ROI (e.g., amygdala, IPL) separately by hemisphere for the emotion vs. neutral contrast as the predictor variables, as well as covariates. For example, one HLM examined the association between overt victimization and activity of the left OTG. We ran 36 HLMs for our 9 social indices, totaling 324 models. HLMs were run using the available data for each social experience and behavior index (Table 1 for sample sizes). ROIs were False Discovery Rate corrected (FDR-corrected) for multiple comparisons for each individual social experience or behavior index separately by hemisphere (i.e., 18 FDR-corrected models for left ROIs, 18 FDR-corrected models for right ROIs). Results with FDR-corrected p values < 0.05 were considered significant. This multiple comparison method was utilized to control type 1 error-rate and hemispheres were FDR-corrected separately to account for the strong correlations between left and right ROIs. See the Supplement for follow-up analyses and results, including analyses incorporating EN-task behavioral results, with results reported below remaining consistent with the inclusion of task accuracy as a predictor in the models.
Results
Positive social behaviors and experiences
Number of close friends
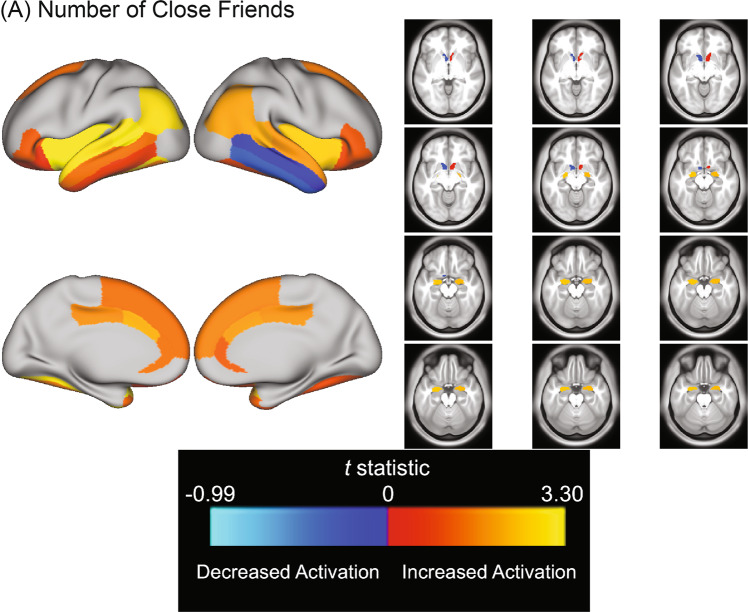
For the emotion vs. neutral contrast, greater number of close friends was associated with higher left fusiform gyrus and left insula activation (Table 2, Fig. 1). Greater number of close friends was also associated with both higher left IPL activation and higher left STG activation. Lastly, greater number of close friends was associated with higher left TPJ activation.
Table 2.
Positive social behavior and experience and emotion faces versus neutral faces: significant resultsa.
| ROI | Number of close friends | ||||||
|---|---|---|---|---|---|---|---|
| Beta | SE | T-statistic | P value | FDR corrected P value | R2 | ||
| ACC | Left | 0.016 | 0.179 | 1.470 | 0.14 | 0.29 | |
| Right | 0.014 | 0.182 | 1.342 | 0.18 | 0.62 | ||
| Amg | Left | 0.025 | 0.147 | 2.373 | 0.018* | 0.05 | |
| Right | 0.023 | 0.158 | 2.173 | 0.030* | 0.62 | ||
| cACC | Left | 0.020 | 0.188 | 1.926 | 0.05 | 0.14 | |
| Right | 0.018 | 0.186 | 1.673 | 0.09 | 0.62 | ||
| FuG | Left | 0.030 | 0.156 | 2.765 | 0.006** | 0.03* | 0.009 |
| Right | 0.008 | 0.152 | 0.747 | 0.45 | 0.62 | ||
| IFG | Left | 0.010 | 0.094 | 0.925 | 0.36 | 0.41 | |
| Right | 0.009 | 0.099 | 0.892 | 0.37 | 0.62 | ||
| ITG | Left | 0.006 | 0.121 | 0.521 | 0.60 | 0.64 | |
| Right | −0.010 | 0.115 | −0.984 | 0.32 | 0.62 | ||
| Ins | Left | 0.035 | 0.234 | 3.292 | 0.001*** | 0.018* | 0.009 |
| Right | 0.024 | 0.221 | 2.309 | 0.021* | 0.62 | ||
| IPL | Left | 0.028 | 0.214 | 2.648 | 0.008** | 0.029* | 0.009 |
| Right | 0.019 | 0.212 | 1.780 | 0.08 | 0.62 | ||
| mOFC | Left | 0.004 | 0.064 | 0.358 | 0.72 | 0.72 | |
| Right | 0.007 | 0.062 | 0.696 | 0.49 | 0.62 | ||
| MTG | Left | 0.012 | 0.179 | 1.173 | 0.24 | 0.35 | |
| Right | −0.003 | 0.183 | −0.274 | 0.78 | 0.84 | ||
| NAcc | Left | −0.010 | 0.140 | −0.902 | 0.37 | 0.41 | |
| Right | 0.002 | 0.141 | 0.205 | 0.84 | 0.84 | ||
| OTG | Left | 0.015 | 0.126 | 1.410 | 0.16 | 0.29 | |
| Right | 0.008 | 0.121 | 0.735 | 0.46 | 0.62 | ||
| PCC | Left | 0.016 | 0.209 | 1.461 | 0.14 | 0.29 | |
| Right | 0.016 | 0.208 | 1.526 | 0.13 | 0.62 | ||
| rACC | Left | 0.013 | 0.142 | 1.190 | 0.23 | 0.35 | |
| Right | 0.010 | 0.150 | 0.952 | 0.34 | 0.62 | ||
| SFG | Left | 0.012 | 0.227 | 1.148 | 0.25 | 0.35 | |
| Right | 0.014 | 0.228 | 1.332 | 0.18 | 0.62 | ||
| STG | Left | 0.028 | 0.250 | 2.668 | 0.008** | 0.029* | 0.009 |
| Right | 0.017 | 0.241 | 1.602 | 0.11 | 0.62 | ||
| TmP | Left | 0.011 | 0.083 | 1.051 | 0.29 | 0.38 | |
| Right | 0.011 | 0.079 | 1.013 | 0.31 | 0.62 | ||
| TPJ | Left | 0.031 | 0.251 | 2.890 | 0.004** | 0.029* | 0.008 |
| Right | 0.020 | 0.248 | 1.873 | 0.06 | 0.62 | ||
SE standard error, FDR false discovery rate, R2 R-squared, ROI region of interest, Amg amygdala, NAcc nucleus accumbens, Ins insula, mOFC medial orbitofrontal cortex, IFG inferior frontal gyrus, cACC caudal anterior cingulate cortex, rACC rostral anterior cingulate cortex, ACC anterior cingulate cortex, OTG occipitotemporal gyrus, IPL inferior parietal lobe, TPJ temporoparietal junction, ITG inferior temporal gyrus, MTG middle temporal gyrus, PCC posterior cingulate cortex, SFG superior frontal gyrus, STG superior temporal gyrus, TmP temporal pole, FuG fusiform gyrus.
Significance codes: *=<0.05; **=<0.01; ***=<0.001.
aNote, FDR-corrections were performed by hemisphere (i.e., 18 FDR-corrections for left ROIs; 18 FDR-corrections for right ROIs.).
Fig. 1. Association between positive social behavior and experience indices and activity in a priori ROIs during Emotion Faces versus Neutral Faces contrast.
Color bar depicts t-statistic range. Warm colors indicate increased activation, cool colors indicate decreased activation relative to baseline. A t-statistics from all models examining associations between a priori ROIs and number of close friends, whether or not they passed FDR correction.
No other positive social behavior or experience indices (i.e., prosocial behavior, number of activities in the past 12 months) were significantly associated with activation in any of the examined regions (Supplemental Table 2–4 for model results).
Negative social behaviors and experiences
Reciprocal social impairments
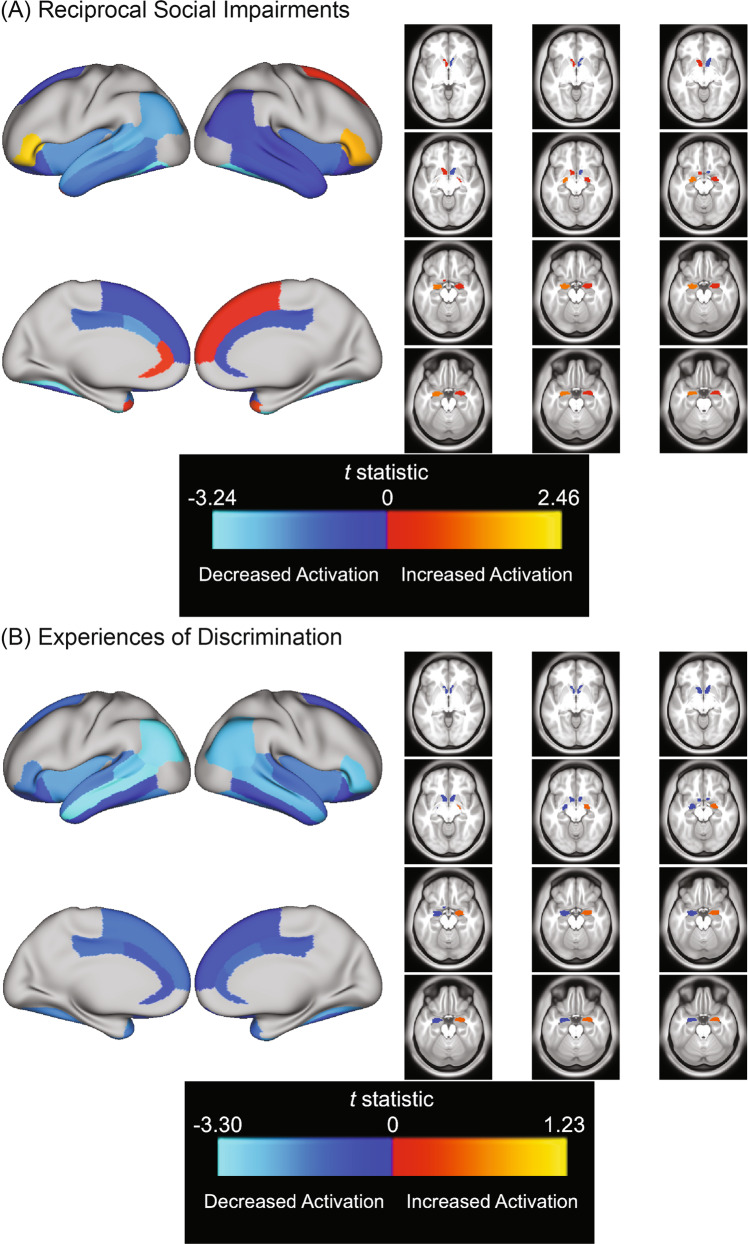
There was an association between greater reciprocal social impairments and lower left fusiform gyrus activation for the emotion vs. neutral contrast (Table 3; Fig. 2).
Table 3.
Negative social behavior and experience and emotion faces versus neutral faces: significant resultsa.
| ROI | Experiences of discrimination | Reciprocal social impairments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | T-statistic | P value | FDR corrected P value | R2 | Beta | SE | T-statistic | P value | FDR corrected P value | R2 | ||
| ACC | Left | −0.006 | 0.009 | −0.523 | 0.60 | 0.64 | −0.007 | 0.111 | −0.624 | 0.53 | 0.77 | ||
| Right | −0.010 | 0.010 | −0.917 | 0.36 | 0.56 | −0.005 | 0.113 | −0.481 | 0.63 | 0.92 | |||
| Amg | Left | −0.003 | 0.008 | −0.329 | 0.74 | 0.74 | 0.016 | 0.092 | 1.467 | 0.14 | 0.32 | ||
| Right | 0.013 | 0.008 | 1.225 | 0.22 | 0.53 | 0.003 | 0.098 | 0.302 | 0.76 | 0.92 | |||
| cACC | Left | −0.007 | 0.010 | −0.696 | 0.49 | 0.64 | −0.017 | 0.117 | −1.620 | 0.11 | 0.32 | ||
| Right | −0.011 | 0.010 | −1.064 | 0.29 | 0.53 | −0.006 | 0.116 | −0.576 | 0.56 | 0.92 | |||
| FuG | Left | −0.016 | 0.008 | −1.488 | 0.14 | 0.39 | −0.035 | 0.096 | −3.240 | 0.001*** | 0.022* | 0.041 | |
| Right | −0.020 | 0.008 | −1.826 | 0.07 | 0.24 | −0.031 | 0.094 | −2.848 | 0.004** | 0.08 | |||
| IFG | Left | −0.015 | 0.005 | −1.435 | 0.15 | 0.39 | 0.026 | 0.058 | 2.454 | 0.014* | 0.13 | ||
| Right | −0.025 | 0.005 | −2.369 | 0.02 | 0.22 | 0.021 | 0.061 | 1.955 | 0.05 | 0.46 | |||
| ITG | Left | −0.006 | 0.006 | −0.536 | 0.59 | 0.64 | −0.012 | 0.075 | −1.143 | 0.25 | 0.46 | ||
| Right | −0.005 | 0.006 | −0.510 | 0.61 | 0.73 | −0.007 | 0.071 | −0.641 | 0.52 | 0.92 | |||
| Ins | Left | −0.018 | 0.012 | −1.718 | 0.09 | 0.39 | −0.016 | 0.145 | −1.496 | 0.13 | 0.32 | ||
| Right | −0.014 | 0.012 | −1.309 | 0.19 | 0.53 | −0.011 | 0.137 | −1.068 | 0.29 | 0.92 | |||
| IPL | Left | −0.030 | 0.011 | −2.763 | 0.006** | 0.05 | −0.020 | 0.132 | −1.891 | 0.06 | 0.26 | ||
| Right | −0.024 | 0.011 | −2.250 | 0.024* | 0.22 | −0.003 | 0.131 | −0.285 | 0.78 | 0.92 | |||
| mOFC | Left | −0.007 | 0.003 | −0.694 | 0.49 | 0.64 | −0.006 | 0.040 | −0.536 | 0.59 | 0.77 | ||
| Right | −0.004 | 0.003 | −0.398 | 0.69 | 0.73 | −0.001 | 0.039 | −0.111 | 0.91 | 0.96 | |||
| MTG | Left | −0.035 | 0.009 | −3.297 | 0.001*** | 0.018* | 0.062 | −0.014 | 0.111 | −1.316 | 0.19 | 0.38 | |
| Right | −0.022 | 0.010 | −2.096 | 0.036* | 0.22 | −0.004 | 0.113 | −0.415 | 0.68 | 0.92 | |||
| NAcc | Left | −0.006 | 0.007 | −0.557 | 0.58 | 0.64 | 0.004 | 0.087 | 0.350 | 0.73 | 0.77 | ||
| Right | −0.004 | 0.007 | −0.350 | 0.73 | 0.73 | −0.008 | 0.087 | −0.752 | 0.45 | 0.92 | |||
| OTG | Left | −0.016 | 0.007 | −1.503 | 0.13 | 0.39 | −0.005 | 0.078 | −0.426 | 0.67 | 0.77 | ||
| Right | −0.010 | 0.006 | −0.894 | 0.37 | 0.56 | −0.011 | 0.075 | −1.052 | 0.29 | 0.92 | |||
| PCC | Left | −0.011 | 0.011 | −1.066 | 0.29 | 0.47 | −0.011 | 0.129 | −0.989 | 0.32 | 0.53 | ||
| Right | −0.008 | 0.011 | −0.727 | 0.47 | 0.60 | −0.007 | 0.128 | −0.674 | 0.50 | 0.92 | |||
| rACC | Left | −0.006 | 0.008 | −0.523 | 0.60 | 0.64 | 0.004 | 0.088 | 0.346 | 0.73 | 0.77 | ||
| Right | −0.009 | 0.008 | −0.805 | 0.42 | 0.58 | −0.004 | 0.093 | −0.409 | 0.68 | 0.92 | |||
| SFG | Left | −0.012 | 0.012 | −1.121 | 0.26 | 0.47 | −0.005 | 0.140 | −0.440 | 0.66 | 0.77 | ||
| Right | −0.005 | 0.012 | −0.459 | 0.65 | 0.73 | 0.001 | 0.140 | 0.054 | 0.96 | 0.96 | |||
| STG | Left | −0.014 | 0.013 | −1.279 | 0.20 | 0.40 | −0.019 | 0.154 | −1.727 | 0.08 | 0.30 | ||
| Right | −0.011 | 0.013 | −1.044 | 0.30 | 0.53 | −0.003 | 0.148 | −0.236 | 0.81 | 0.92 | |||
| TmP | Left | −0.014 | 0.004 | −1.363 | 0.17 | 0.39 | 0.001 | 0.052 | 0.108 | 0.91 | 0.91 | ||
| Right | −0.012 | 0.004 | −1.123 | 0.26 | 0.53 | 0.006 | 0.049 | 0.564 | 0.57 | 0.92 | |||
| TPJ | Left | −0.025 | 0.013 | −2.304 | 0.021* | 0.13 | −0.021 | 0.155 | −1.977 | 0.048* | 0.26 | ||
| Right | −0.020 | 0.013 | −1.839 | 0.07 | 0.24 | −0.004 | 0.153 | −0.406 | 0.68 | 0.92 | |||
SE standard error, FDR false discovery rate, R2 R-squared, ROI region of interest, Amg amygdala, NAcc nucleus accumbens, Ins insula, mOFC medial orbitofrontal cortex, IFG inferior frontal gyrus, cACC caudal anterior cingulate cortex, rACC rostral anterior cingulate cortex, ACC anterior cingulate cortex, OTG occipitotemporal gyrus, IPL inferior parietal lobe, TPJ temporoparietal junction, ITG inferior temporal gyrus, MTG middle temporal gyrus, PCC posterior cingulate cortex, SFG superior frontal gyrus, STG superior temporal gyrus, TmP temporal pole, FuG fusiform gyrus.
Significance codes: *=<0.05; **=<0.01; ***=<0.001.
aNote, FDR-corrections were performed by hemisphere (i.e., 18 FDR-corrections for left ROIs; 18 FDR-corrections for right ROIs.).
Fig. 2. Association between negative social behavior and experience indices and activity in a priori ROIs during Emotion Faces versus Neutral Faces contrast.
Color bar depicts t-statistic range. Warm colors indicate increased activation, cool colors indicate decreased activation relative to baseline. A t-statistics from all models examining associations between a priori ROIs and reciprocal social impairments, whether or not they passed FDR correction. B t-statistics from all models examining associations between a priori ROIs and experiences of discrimination, whether or not they passed FDR correction.
Experiences of discrimination
Greater experiences of discrimination were associated with lower left MTG activation for the emotion vs. neutral contrast (Table 3; Fig. 2).
No other negative social behavior or experience indices (i.e., experience of being bullied, overt aggression, overt victimization, or social problems) were significantly associated with any of the examined ROIs (Supplemental Tables 5–8 for model results).
Additional analyses
Further statistical and behavioral analyses and their results can be found in the Supplement and Supplemental Tables 9–14.
Discussion
This paper investigates the relationship between implicit emotion regulation and aspects of positive and negative social behavior and experiences. For positive social behavior and experiences, more close friends were associated with higher activation in several regions previously linked to emotion regulation and mentalization. For negative social behaviors and experiences, greater reciprocal social impairments were associated with lower fusiform gyrus activation and greater experiences of discrimination were associated with lower MTG activation, regions which have been associated with emotion regulation in previous studies [110, 112, 113]. Overall, the current study found both indices inside (i.e., number of close friends) and outside (i.e., experiences of discrimination and reciprocal social impairments) of the child’s control were significantly associated with implicit emotion regulation neural correlates. Our study shows both social indices within and beyond a child’s control relate to neural activation associated with implicit emotion regulation, indicating it is possible a social behavior or experience may have an impact even if it does not appear explicitly influential to social relationships or development. The results of the current study improve our understanding of the associations between neural correlates of implicit emotion regulation and social behaviors and experiences, finding both positive and negative social behavior and experience indices were associated with generally distinct regions of activation during implicit emotional regulation.
The current paper was the first to our knowledge to investigate neural correlates of implicit emotion regulation with quantity of close friendships, a positive social behavior and experience index that is at least partially within the child’s control. The results showed higher activation of the STG, IPL, TPJ, fusiform gyrus, and insula during implicit emotion regulation when associated with greater number of close friends. Consistent with our findings, higher left insula activation has been shown to be associated with implicit emotion processing and parasympathetic activation, which has in turn been linked to affiliative emotions [118, 119]. Research has also shown greater left insula compared to right insula activation in various functional imaging studies of unconditional love [120], which is arguably present in close friendship [121]. Thus, the present study found greater close friendships are associated with greater activation in a region related to affiliative emotions and love during implicit emotion regulation.
Potentially consistent with our finding that higher TPJ activation, as well as the IPL and STG which comprise the TPJ [115], were all associated with greater number of close friends, the TPJ has been previously linked to predicting others’ behaviors [73], reasoning about other people [73], emotion regulation [17, 18], and increased up- and down-regulation of interpersonal emotions [19]. In the context of the current study, perhaps greater implicit emotion regulation abilities, possibly reflected by higher TPJ activation during the EN-back task, may be associated with better social skills and therefore greater quantity of friendships. This interpretation is consistent with previous research linking increased mentalizing skills to increased number of a close friends [122]. Overall, these results also showed that increased number of close friends, a social behavior and experience index partly within the youth’s control, is correlated to higher activation in regions implicated in mentalizing abilities.
The link we found between activation in the left fusiform gyrus during implicit emotion regulation and increased number of close friends is indirectly consistent with research showing the fusiform face area is linked to face perception, and better facial recognition is associated with better social skills and more social activity [123, 124]. Another study found that those with more social ties and a greater in-degree network size also had a larger regional fusiform gyrus volume [125]. Thus, the current results indicate that a larger network of close friends, perhaps a proxy of social skills, is associated with a region critically implicated in face perception. One possible interpretation is that greater emotion regulation abilities are associated with greater facial processing, leading to more close friends, or vice versus (i.e., more close friends result in better face processing and implicit emotion regulation).
The results of our study also found that increased reciprocal social impairments—a negative social behavior not under the child’s control—were associated with lower left fusiform gyrus activation during implicit emotion regulation. Social impairments, including those present in populations clinically diagnosed with ASD, have been linked to abnormal face perception and hypoactive fusiform gyri [82, 83]. Additionally, individuals with 22q11.2 deletion syndrome, a chromosomal mutation that results in a condition linked to social withdrawal and poor emotion processing, exhibit decreased fusiform gyrus activation during face processing [126]. Our findings show greater reciprocal social impairments are linked to decreased activation in a region implicated in facial perception during implicit emotion regulation.
Our study also found lower activation in the MTG during implicit emotion regulation was associated with greater experiences of discrimination. The MTG has been previously linked to emotion regulation and social perception [127, 128]. One study racially primed Black participants with a White confederate interaction prior to a social exclusion event and found greater MTG activation when participants attributed exclusion to racial discrimination, potentially suggesting the use of emotion regulation to cope with negative emotions during racial discrimination [78]. In contrast, the current study found lower MTG activation during discrimination. Thus, although the MTG appears to be critically implicated in the experience of discrimination and exclusion, perhaps contextual factors (e.g., temporal distance from the experience, severity, etc.) result in a modulation of the direction of the effect. In this speculative explanation, while the ongoing experience of social exclusion results in higher MTG activation [78], perhaps prior experiences of discrimination as measured in the current study may have later downstream effects, including lower MTG activation in the context of implicit emotion regulation. Future research should disentangle the influence of other correlates of systemic racism on neural correlates of implicit emotion regulation.
The current study had several limitations. First, the analyses found small effect sizes, as well as a modest number of findings. We implemented strict control of false positives in our analyses, which limited the number of findings, both in terms of indices of social behavior and experience as well as in terms of neural correlates. Furthermore, the generally small effect sizes (βs < 0.055) are consistent with previous research [129, 130]. Small effects are expected given the ABCD Study is an epidemiologically informed study with a demographically diverse sample [131] and therefore effects are being examined in the context of a complex set of contextual variables. In addition, the current study utilized pre-defined ROIs for a priori brain regions in our hypotheses. While a whole-brain or voxel-wise analyses would provide much more comprehensive results, due to computational challenges of such analyses in datasets of this size, we are not currently able to conduct these analyses. ROIs used in the current study were derived from the Desikan atlas [102], with ROIs varying in size. It is possible that utilizing a different atlas or more fine-grained analyses may have yielded different results. Lastly, although most social experiences and behaviors were measured at the baseline assessment wave, some indices were measured at a later assessment wave and therefore were not completed at the same time as the EN-back fMRI task.
The current study found that both positive and negative social behavior and experience indices, including those both inside and outside of the youth’s agency, were associated with generally unique regions of activation during implicit emotion regulation. This indicates a social behavior or experience may possibly have an impact on social relationships or development even if it does not appear explicitly influential. Indices of positive social behavior and experience were associated with higher activation in several regions previously implicated in adaptive emotion regulation and social processing, such as the insula, IPL, STG, fusiform gyrus, and TPJ. Negative social function behavior and experience indices were associated with lower activation in regions previously associated with emotion regulation and social impairments, including the fusiform gyrus and MTG. The potential implications of these findings are especially important because the study’s late middle childhood sample. First, these findings suggest the possibility of potential emotion regulation benefits with positive social behavior and experience interventions, such as interventions to increase the development of friendships. Second, this paper shows that even at a young age, negative social experiences, specifically experiencing discrimination and greater reciprocal social impairments, are associated with neural correlates during implicit emotion. These externally controlled experiences were linked to neural activity, indicating those social experiences that the child may not even directly influence have the potential to be associated with neural correlates of implicit emotion regulation. This further points to the necessity of amplifying attempts in schools to prevent or work to ameliorate the effects of these types of experiences.
Supplementary information
Acknowledgements
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/Consortium_Members.pdf. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from DOI 10.15154/1519007.
Author contributions
Concept and design: KCG, NRK, Acquisition, analysis, or interpretation of data: KCG, NRK Drafting of the manuscript: KCG, NRK, DMB Critical revision of the manuscript for important intellectual content: KCG, NRK, DMB Statistical analysis: KCG, NRK Supervision: KCG, NRK Final approval: KCG, NRK, DMB.
Funding
This work was supported by National Institute on Drug Abuse (U01 DA041120 to DMB); National Institute of Mental Health (K23 MH121792-01 and L30 MH120574-01 to NRK). KCG received no support for this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01286-5.
References
- 1.Gross JJ. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. 1998;2:271–99. doi: 10.1037/1089-2680.2.3.271. [DOI] [Google Scholar]
- 2.McRae K, Gross JJ. Emotion regulation. Emotion. 2020;20:1–9. doi: 10.1037/emo0000703. [DOI] [PubMed] [Google Scholar]
- 3.Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cognition Emot. 2011;25:400–12. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre JB, Lieberman MD. Putting feelings into words: affect labeling as implicit emotion regulation. Emot Rev. 2018;10:116–24. doi: 10.1177/1754073917742706. [DOI] [Google Scholar]
- 5.Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: a longitudinal study. Child Dev. 1995;66:1360. doi: 10.2307/1131652. [DOI] [PubMed] [Google Scholar]
- 6.Rubin KH, Coplan RJ, Fox NA, Calkins SD. Emotionality, emotion regulation, and preschoolers’ social adaptation. Dev Psychopathol. 1995;7:49–62. doi: 10.1017/S0954579400006337. [DOI] [Google Scholar]
- 7.Gross JJ. Sharpening the focus: emotion regulation, arousal, and social competence. Psychological Inq. 1998;9:287–90. doi: 10.1207/s15327965pli0904_8. [DOI] [Google Scholar]
- 8.Raver CC, Blackburn EK, Bancroft M, Torp N. Relations between effective emotional self-regulation, attentional control, and low-income preschoolers’ social competence with peers. Early Educ Dev. 1999;10:333–50. doi: 10.1207/s15566935eed1003_6. [DOI] [Google Scholar]
- 9.Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: their role in predicting quality of social functioning. J Personal Soc Psychol. 2000;78:136–57. doi: 10.1037/0022-3514.78.1.136. [DOI] [PubMed] [Google Scholar]
- 10.Dollar JM, Stifter CA. Temperamental surgency and emotion regulation as predictors of childhood social competence. J Exp Child Psychol. 2012;112:178–94. doi: 10.1016/j.jecp.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calkins SD, Gill KL, Johnson MC, Smith CL. Emotional reactivity and emotional regulation strategies as predictors of social behavior with peers during toddlerhood. Soc Dev. 2001;8:310–34. doi: 10.1111/1467-9507.00098. [DOI] [Google Scholar]
- 12.Eisenberg N, Fabes RA, Guthrie IK, Murphy BC, Maszk P, Holmgren R, et al. The relations of regulation and emotionality to problem behavior in elementary school children. Dev Psychopathol. 1996;8:141–62. doi: 10.1017/S095457940000701X. [DOI] [PubMed] [Google Scholar]
- 13.Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, et al. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–89. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzig MM, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cogn Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen AO, Conley MI, Dellarco DV, Casey BJ The impact of emotional cues on short-term and long-term memory during adolescence. Proceedings of the Society for Neuroscience: San Diego, CA; 2016b.
- 16.Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- 17.Koush Y, Pichon S, Eickhoff SB, Van De Ville D, Vuilleumier P, Scharnowski F. Brain networks for engaging oneself in positive-social emotion regulation. NeuroImage. 2019;189:106–15. doi: 10.1016/j.neuroimage.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 18.Bukowski H, Lamm C Temporoparietal junction. In: Zeigler-Hill V, Shackelford TK, editors. Encyclopedia of personality and individual differences. Cham: Springer; 2020.
- 19.Grecucci A, Giorgetta C, Bonini N, Sanfey AG. Reappraising social emotions: The role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Front Hum Neurosci. 2013;7:1–12. doi: 10.3389/fnhum.2013.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak AK, Hu Z-G, Zhang JX, Xiao Z-W, Lee TM. Neural correlates of regulation of positive and negative emotions: an fMRI study. Neurosci Lett. 2009;457:101–6. doi: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- 22.Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, et al. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 2014;45:202–11. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed SP, Bittencourt-Hewitt A, Sebastian CL. Neurocognitive bases of emotion regulation development in adolescence. Developmental Cogn Neurosci. 2015;15:11–25. doi: 10.1016/j.dcn.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sebastian CL, Roiser JP, Tan GCY, Viding E, Wood NW, Blakemore S-J. Effects of age and MAOA genotype on the neural processing of social rejection. Genes, Brain Behav. 2010;9:628–37. doi: 10.1111/j.1601-183X.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- 25.Alkire D, Levitas D, Warnell KR, Redcay E. Social interaction recruits mentalizing and reward systems in middle childhood. Hum Brain Mapp. 2018;39:3928–42. doi: 10.1002/hbm.24221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erikson EH Childhood and society. 3rd ed. W. W. Norton & Company: New York, NY; 1993.
- 27.McHale SM, Dariotis JK, Kauh TJ Social development and social relationships in middle childhood. In Lerner RM, Easterbrooks MA, Mistry J, Weiner IB, editors. Handbook of Psychology: Developmental Psychology. 6th ed. Hoboken, NJ: John Wiley & Sons; 2003. . 241-65.
- 28.Sullivan HS, editor. The interpersonal theory of psychiatry. Routledge: Abingdon, OX; 2013.
- 29.Kopp CB. Regulation of distress and negative emotions: a developmental view. Developmental Psychol. 1989;25:343–54. doi: 10.1037/0012-1649.25.3.343. [DOI] [Google Scholar]
- 30.Warnell KR, Sadikova E, Redcay E. Let’s chat: developmental neural bases of social motivation during real-time peer interaction. Developmental Sci. 2017;21:1–14. doi: 10.1111/desc.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parke RD, Simpkins SD, McDowell DJ, Kim M, Killian C, Dennis J, et al. Relative contributions of families and peers to children’s social development. In: Smith PK, Hart CH, editors. Blackwell Handbook of Childhood Social Development. Oxford, UK: Blackwell Publishing; 2002. p. 156–77.
- 32.Vandell DL. Parents, peer groups, and other socializing influences. Dev Psychol. 2000;36:699–710. doi: 10.1037/0012-1649.36.6.699. [DOI] [PubMed] [Google Scholar]
- 33.Krappman L The development of manifold social relationships among children. In Awhagen AE, Von Salisch M, editors. Interpersonal relationships. Goettingen: Hagrete; 1992. p. 37–58.
- 34.Cairns RB, Cairns BD Lifelines and risks: pathways of youth in our time. New York, NY: Cambridge University Press; 1994.
- 35.Lerner RM. Children and adolescents as producers of their own development. Dev Rev. 1982;2:342–70. doi: 10.1016/0273-2297(82)90018-1. [DOI] [Google Scholar]
- 36.Cairns RB. Multiple metaphors for a singular idea. Dev Psychol. 1991;27:23–26. doi: 10.1037/0012-1649.27.1.23. [DOI] [Google Scholar]
- 37.Rogoff B, Baker‐Sennett J, Lacasa P, Goldsmith D. Development through participation in sociocultural activity. New Dir Child Dev. 1995;1995:45–65. doi: 10.1002/cd.23219956707. [DOI] [PubMed] [Google Scholar]
- 38.Smith PK, Hart CH, editors. Blackwell handbook of childhood social development. Oxford, UK: Blackwell Publishing; 2002.
- 39.Harkness S Culture and social development: Explanations and evidence. In Smith PK, Hart CH, editors. Blackwell Handbook of Childhood Social Development. Oxford, UK: Blackwell Publishing; 2002. p. 60–77.
- 40.Guralnick MJ. Family and child influences on the peer-related social competence of young children with developmental delays. Ment Retardation Developmental Disabilities Res Rev. 1999;5:21–29. doi: 10.1002/(SICI)1098-2779(1999)5:1<21::AID-MRDD3>3.0.CO;2-O. [DOI] [Google Scholar]
- 41.Simpkins SD, Ripke M, Huston AC, Eccles JS. Predicting participation and outcomes in out-of-school activities: similarities and differences across social ecologies. New Dir Youth Dev. 2005;105:51–69. doi: 10.1002/yd.107. [DOI] [PubMed] [Google Scholar]
- 42.Balyer A, Gunduz Y. Effects of structured extracurricular facilities on students’ academic and social development. Procedia—Soc Behav Sci. 2012;46:4803–7. doi: 10.1016/j.sbspro.2012.06.338. [DOI] [Google Scholar]
- 43.Brooks BA, Floyd F, Robins DL, Chan WY. Extracurricular activities and the development of social skills in children with intellectual and specific learning disabilities. J Intellect Disabil Res. 2015;59:678–87. doi: 10.1111/jir.12171. [DOI] [PubMed] [Google Scholar]
- 44.Fleming CB, Catalano RF, Mazza JJ, Brown EC, Haggerty KP, Harachi TW. After-school activities, misbehavior in school, and delinquency from the end of elementary school through the beginning of high school. J Early Adolescence. 2008;28:277–303. doi: 10.1177/0272431607313589. [DOI] [Google Scholar]
- 45.Hoza B, Bukowski WM, Beery S. Assessing peer network and dyadic loneliness. J Clin Child Psychol. 2000;29:119–28. doi: 10.1207/S15374424jccp2901_12. [DOI] [PubMed] [Google Scholar]
- 46.Bowker JCW, Rubin KH, Burgess KB, Booth-Laforce C, Rose-Krasnor L. Behavioral characteristics associated with stable and fluid best friendship patterns in middle childhood. Merrill-Palmer Q. 2006;52:671–93. doi: 10.1353/mpq.2006.0000. [DOI] [Google Scholar]
- 47.Parker JG, Seal J. Forming, losing, renewing, and replacing friendships: applying temporal parameters to the assessment of children’s friendship experiences. Child Dev. 1996;67:2248–68. doi: 10.2307/1131621. [DOI] [Google Scholar]
- 48.Clark ML, Drewry DL. Similarity and reciprocity in the friendships of elementary school children. Child Stduy J. 1985;15:251–64. [Google Scholar]
- 49.Newcomb AF, Bagwell CL. Children’s friendship relations: a meta-analytic review. Psychological Bull. 1995;117:306–47. doi: 10.1037/0033-2909.117.2.306. [DOI] [Google Scholar]
- 50.Berndt TJ. Friendship quality and social development. Curr Dir Psychol Sci. 2002;11:7–10. doi: 10.1111/1467-8721.00157. [DOI] [Google Scholar]
- 51.Rubin KH, Bukowski WM, Parker JG. Peer interactions, relationships, and groups. Handb Child Psychol. 2007;3:1–180. [Google Scholar]
- 52.Parker JG, Asher SR. Friendship and friendship quality in middle childhood: Links with peer group acceptance and feelings of loneliness and social dissatisfaction. Dev Psychol. 1993;29:611–21. doi: 10.1037/0012-1649.29.4.611. [DOI] [Google Scholar]
- 53.Ojanen T, Grönroos M, Salmivalli C. An interpersonal circumplex model of children’s social goals: links with peer-reported behavior and sociometric status. Dev Psychol. 2005;41:699–710. doi: 10.1037/0012-1649.41.5.699. [DOI] [PubMed] [Google Scholar]
- 54.Rodkin PC, Ryan AM, Jamison R, Wilson T. Social goals, social behavior, and social status in middle childhood. Dev Psychol. 2013;49:1139–50. doi: 10.1037/a0029389. [DOI] [PubMed] [Google Scholar]
- 55.Eisenberg N, Fabes RA, Karbon M, Murphy BC, Wosinski M, Polazzi L, et al. The relations of children’s dispositional prosocial behavior to emotionality, regulation, and social functioning. Child Dev. 1996;67:974–92. doi: 10.2307/1131874. [DOI] [PubMed] [Google Scholar]
- 56.Gülay H. Assessment of the prosocial behaviors of young children with regard to social development, social skills, parental acceptance-rejection and peer relationships. J Instructional Psychol. 2011;38:164–72.
- 57.Bengtsson H, Johnson L. Perspective taking, empathy, and prosocial behavior in late childhood. Child Study J. 1992;22:11–22. [Google Scholar]
- 58.Garner PW. The relations of emotional role taking, affective/moral attributions, and emotional display rule knowledge to low-income school-age children’s social competence. J Appl Dev Psychol. 1996;17:19–36. doi: 10.1016/S0193-3973(96)90003-9. [DOI] [Google Scholar]
- 59.Litvack-Miller W, McDougall D, Romney DM. The structure of empathy during middle childhood and its relationship to prosocial behavior. Genet Soc Gen Psychol Monogr. 1997;123:303–25. [PubMed] [Google Scholar]
- 60.Major B, Quinton WJ, Mccoy SK. Antecedents and consequences of attributions to discrimination: Theoretical and empirical advances. Adv Exp Soc Psychol. 2002;34:251–330. doi: 10.1016/S0065-2601(02)80007-7. [DOI] [Google Scholar]
- 61.Duru E, Poyrazli S. Perceived discrimination, social connectedness, and other predictors of adjustment difficulties among Turkish international students. Int J Psychol. 2011;46:446–54. doi: 10.1080/00207594.2011.585158. [DOI] [PubMed] [Google Scholar]
- 62.Archer J, Coyne SM. An integrated review of indirect, relational, and social aggression. Personal Soc Psychol Rev. 2005;9:212–30. doi: 10.1207/s15327957pspr0903_2. [DOI] [PubMed] [Google Scholar]
- 63.Paquette JA, Underwood MK. Young adolescents’ experiences of peer victimization: Gender differences in accounts of social and physical aggression. Merrill-Palmer Q. 1999;45:233–58. [Google Scholar]
- 64.Crick NR, Bigbee MA. Relational and overt forms of peer victimization: a multiinformant approach. J consulting Clin Psychol. 1998;66:337. doi: 10.1037/0022-006X.66.2.337. [DOI] [PubMed] [Google Scholar]
- 65.Coie JD, Dodge K, Kupersmidt JB Peer group behavior and social status. In Asher SR, Coie JD, editors. Peer rejection in childhood. New York, NY: Cambridge University Press; 1990. p. 17–59.
- 66.Coie JD, Lochman JE, Terry R, Hyman C. (1992). Predicting early adolescent disorder from childhood aggression and peer rejection. J Consulting Clin Psychol. 1992;60:783–92. doi: 10.1037/0022-006X.60.5.783. [DOI] [PubMed] [Google Scholar]
- 67.Panak WF, Garber J. Role of aggression, rejection, and attributions in the prediction of depression in children. Dev Psychopathol. 1992;4:145–65. doi: 10.1017/S0954579400005617. [DOI] [Google Scholar]
- 68.Crick NR. The role of overt aggression, relational aggression, and prosocial behavior in the prediction of children’s future social adjustment. Child Dev. 1996;67:2317–27. doi: 10.2307/1131625. [DOI] [PubMed] [Google Scholar]
- 69.Xie H, Cairns RB, Cairns BD. The development of social aggression and physical aggression: A narrative analysis of interpersonal conflicts. Aggressive Behav. 2002;28:341–55. doi: 10.1002/ab.80008. [DOI] [Google Scholar]
- 70.Crick NR, Grotpeter JK. Relational aggression, gender, and social-psychological adjustment. Child Dev. 1995;66:710–22. doi: 10.2307/1131945. [DOI] [PubMed] [Google Scholar]
- 71.Krämer UM, Mohammadi B, Doñamayor N, Samii A, Münte TF. Emotional and cognitive aspects of empathy and their relation to social cognition—an fMRI-study. Brain Res. 2010;1311:110–20. doi: 10.1016/j.brainres.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 72.Schultz RT, Grelotti DJ, Klin A, Kleinman J, Gaag CVD, Marois R, et al. The role of the fusiform face area in social cognition: Implications for the pathobiology of autism. Philos Trans R Soc Lond Ser B: Biol Sci. 2003;358:415–27. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soto-Icaza P, Aboitiz F, Billeke P Development of social skills in children: Neural and behavioral evidence for the elaboration of cognitive models. Front Neurosci. 2015;9. [DOI] [PMC free article] [PubMed]
- 74.Rogers CR A way of being. Houghton Mifflin: Boston, MA; 1995.
- 75.Elliott R, Bohart AC, Watson JC, Greenberg LS. Empathy. Psychotherapy. 2011;48:43–49. doi: 10.1037/a0022187. [DOI] [PubMed] [Google Scholar]
- 76.Schnell K, Bluschke S, Konradt B, Walter H. Functional relations of empathy and mentalizing: An fMRI study on the neural basis of cognitive empathy. NeuroImage. 2011;54:1743–54. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 77.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 78.Masten CL, Telzer EH, Eisenberger NI. An fMRI investigation of attributing negative social treatment to racial discrimination. J Cogn Neurosci. 2011;23:1042–51. doi: 10.1162/jocn.2010.21520. [DOI] [PubMed] [Google Scholar]
- 79.Fanning JR, Keedy S, Berman ME, Lee R, Coccaro EF. Neural correlates of aggressive behavior in real time: A review of fMRI studies of laboratory reactive aggression. Curr Behav Neurosci Rep. 2017;4:138–50. doi: 10.1007/s40473-017-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Achterberg M, Duijvenvoorde ACKV, Meulen MVD, Bakermans-Kranenburg MJ, Crone EA. Heritability of aggression following social evaluation in middle childhood: an fMRI study. Hum Brain Mapp. 2018;39:2828–41. doi: 10.1002/hbm.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McIver TA, Bosma RL, Sandre A, Goegan S, Klassen JA, Chiarella J, et al. Peer victimization is associated with neural response to social exclusion. Merrill-Palmer Q. 2018;64:135–61. doi: 10.13110/merrpalmquar1982.64.1.0135. [DOI] [Google Scholar]
- 82.Van Kooten IA, Palmen SJ, Von Cappeln P, Steinbusch HW, Korr H, Heinsen H, et al. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131:987–99. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- 83.Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, et al. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43:373–85. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 84.Will G-J, van Lier PAC, Crone EA, Güroğlu B. Chronic childhood peer rejection is associated with heightened neural responses to social exclusion during adolescence. J Abnorm Child Psychol. 2016;44:43–55. doi: 10.1007/s10802-015-9983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodman R, Meltzer H, Bailey V. The strengths and difficulties questionnaire: A pilot study on the validity of the self-report version. Eur Child Adolesc Psychiatry. 1998;7:125–30. doi: 10.1007/s007870050057. [DOI] [PubMed] [Google Scholar]
- 86.Sibold J, Edwards E, Murray-Close D, Hudziak JJ. Physical activity, sadness, and suicidality in bullied US adolescents. J Am Acad Child Adolesc Psychiatry. 2015;54:808–15. doi: 10.1016/j.jaac.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 87.Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, et al. Demographic, physical and mental health assessments in the Adolescent Brain and Cognitive Development study: Rationale and description. Developmental Cogn Neurosci. 2018;32:55–66. doi: 10.1016/j.dcn.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Los Reyes A, Prinstein MJ. Applying depression-distortion hypotheses to the assessment of peer victimization in adolescents. J Clin Child Adolesc Psychol. 2004;33:325–35. doi: 10.1207/s15374424jccp3302_14. [DOI] [PubMed] [Google Scholar]
- 89.Prinstein MJ, Boergers J, Vernberg EM. Overt and relational aggression in adolescents: social-psychological adjustment of aggressors and victims. J Clin Child Adolesc Psychol. 2001;30:479–91. doi: 10.1207/S15374424JCCP3004_05. [DOI] [PubMed] [Google Scholar]
- 90.Wigham S, McConachie H, Tandos J, Le, Couteur AS. The reliability and validity of the social responsiveness scale in a UK general child population. Res Dev Disabilities. 2012;33:944–50. doi: 10.1016/j.ridd.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 91.Reiersen AM, Constantino JN, Grimmer M, Martin NG, Todd RD. Evidence for shared genetic influences on self-reported ADHD and Autistic symptoms in young adult Australian twins. Twin Res Hum Genet. 2008;11:579–85. doi: 10.1375/twin.11.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Korzeniewski SJ, Joseph RM, Kim SH, Allred EN, O’Shea TM, Leviton A, et al. Social responsiveness scale assessment of the preterm behavioral phenotype in 10-year-olds born extremely preterm. J Developmental Behav Pediatrics. 2017;38:697–705. doi: 10.1097/DBP.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Achenbach TM Achenbach system of empirically based assessment (ASEBA): Development, findings, theory, and applications. University of Vermont, Research Center of Children, Youth & Families: Burlington, VT; 2009
- 94.Phinney JS, Madden T, Santos LJ. Psychological variables as predictors of perceived ethnic discrimination among minority and immigrant adolescents. J Appl Soc Psychol. 1998;28:937–53. doi: 10.1111/j.1559-1816.1998.tb01661.x. [DOI] [Google Scholar]
- 95.Chen L, Su S, Li X, Tam CC, Lin D. Perceived discrimination, schooling arrangements and psychological adjustments of rural-to-urban migrant children in Beijing, China. Health Psychol Behav Med. 2014;2:713–22. doi: 10.1080/21642850.2014.919865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson K, et al. When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychological Sci. 2016;27:549–62. doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- 97.Hare TA, Tottenham N, Galván A, Voss HU, Glove GH, Casey B. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–34. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dreyfuss M, Caudle K, Drysdale AT, Johnston NE, Cohen AO, Somerville LH, et al. Teens impulsively react rather than retreat from threat. Developmental Neurosci. 2014;36:220–7. doi: 10.1159/000357755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dosenbach NU, Koller JM, Earl EA, Miranda-Dominguez O, Klein RL, Van AN, et al. Real-time motion analytics during brain MRI improve data quality and reduce costs. NeuroImage. 2017;161:80–93. doi: 10.1016/j.neuroimage.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 101.Hagler DJ, Jr, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage. 2019;202:1–17. doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 103.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 104.Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–77. doi: 10.1016/S0959-4388(02)00301-X. [DOI] [PubMed] [Google Scholar]
- 105.George MS. Brain regions involved in recognizing facial emotion or identity: an oxygen-15 PET study. J Neuropsychiatry Clin Neurosci. 1993;5:384–94. doi: 10.1176/jnp.5.4.384. [DOI] [PubMed] [Google Scholar]
- 106.Hsieh S, Hornberger M, Piguet O, Hodges J. Brain correlates of musical and facial emotion recognition: Evidence from the dementias. Neuropsychologia. 2012;50:1814–22. doi: 10.1016/j.neuropsychologia.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 107.Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ, Troiani V, et al. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia. 2013;51:2062–9. doi: 10.1016/j.neuropsychologia.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Soc Cogn Affect Neurosci. 2010;7:106–14. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Overwalle FV. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, Williams SC, et al. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Res Neuroimaging. 1998;83:127–38. doi: 10.1016/S0925-4927(98)00036-5. [DOI] [PubMed] [Google Scholar]
- 111.Preston SD, de Waal FBM. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/S0140525X02000018. [DOI] [PubMed] [Google Scholar]
- 112.Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc R Soc Lond Ser B Biol Sci. 1998;265:1927–31. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tso IF, Rutherford S, Fang Y, Angstadt M, Taylor SF. The “social brain” is highly sensitive to the mere presence of social information: An automated meta-analysis and an independent study. Plos One. 2018;13:1–13. doi: 10.1371/journal.pone.0196503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Von Der Heide R, Vyas G, Olson IR. The social network-network: size is predicted by brain structure and function in the amygdala and paralimbic regions. Soc Cogn Affect Neurosci. 2014;9:1962–72. doi: 10.1093/scan/nsu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zaitchik D, Walker C, Miller S, LaViolette P, Feczko E, Dickerson BC. Mental state attribution and the temporoparietal junction: an fMRI study comparing belief, emotion, and perception. Neuropsychologia. 2010;48:2528–36. doi: 10.1016/j.neuropsychologia.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Diemer MA, Mistry RS, Wadsworth ME, López I, Reimers F. Best practices in conceptualizing and measuring social class in psychological research. Analyses Soc Issues Public Policy. 2012;13:77–113. doi: 10.1111/asap.12001. [DOI] [Google Scholar]
- 117.Bates D, Mächler M, Bolker BM, Walker SC Fitting linear mixed-effects models using lme4. J Stat Softw. 2014. http://arxiv.org/abs/1406.5823
- 118.Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–12. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- 119.Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 120.Ortigue S, Bianchi-Demicheli F, Patel N, Frum C, Lewis JW. Neuroimaging of love: fMRI meta-analysis evidence toward new perspectives in sexual medicine. J Sex Med. 2010;7:3541–52. doi: 10.1111/j.1743-6109.2010.01999.x. [DOI] [PubMed] [Google Scholar]
- 121.Sprecher S, Fehr B. Compassionate love for close others and humanity. J Soc Personal Relatsh. 2005;22:629–51. doi: 10.1177/0265407505056439. [DOI] [Google Scholar]
- 122.Stiller J, Dunbar R. Perspective-taking and memory capacity predict social network size. Soc Netw. 2007;29:93–104. doi: 10.1016/j.socnet.2006.04.001. [DOI] [Google Scholar]
- 123.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J, Tian M, Fang H, Xu M, Li H, Liu J. Extraversion predicts individual differences in face recognition. Commun Integr Biol. 2010;3:295–8. doi: 10.4161/cib.3.4.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kwak S, Joo W, Youm Y, Chey J. Social brain volume is associated with in-degree social network size among older adults. Proc R Soc B: Biol Sci. 2018;285:1–10. doi: 10.1098/rspb.2017.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Glaser B, Schaer M, Berney S, Debbane M, Vuilleumier P, Eliez S. Structural changes to the fusiform gyrus: a cerebral marker for social impairments in 22q11.2 deletion syndrome? Schizophrenia Res. 2007;96:82–86. doi: 10.1016/j.schres.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 127.Allison T, Puce A, Mccarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–78. doi: 10.1016/S1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 128.Goldin PR, Mcrae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karcher NR, O’Brien KJ, Kandala S, Barch DM. Resting-state functional connectivity and psychotic-like experiences in childhood: results from the Adolescent Brain Cognitive Development study. Biol Psychiatry. 2019;86:7–15. doi: 10.1016/j.biopsych.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.O’Brien KJ, Barch DM, Kandala S, Karcher NR. Examining specificity of neural correlates of childhood psychotic-like experiences during an emotional n-back task. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:580–90. doi: 10.1016/j.bpsc.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garavan H, Bartsch H, Conway K, Decastro A, Goldstein R, Heeringa S, et al. Recruiting the ABCD sample: design considerations and procedures. Developmental Cogn Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.