Abstract
Portable spirometers has been approved for diagnosing chronic obstructive pulmonary disease (COPD). However, their diagnostic accuracy has not been reviewed. Therefore, the purpose of this study was to systematically evaluate the diagnostic value of portable spirometers in detecting COPD. A comprehensive literature search for relevant studies was conducted in PubMed, Embase, CNKI, Wan Fang, and Web of Science databases. Pooled sensitivity, specificity, summary receiver operating characteristic (SROC), area under the curve (AUC), and other related indices were calculated using the bivariate mixed-effect model. Subgroup analysis was performed to explore the source of heterogeneity. Thirty one studies were included in the meta-analysis. The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic ratio (DOR), SROC, and AUC of the SROC of portable spirometers were 0.85 (0.81–0.88), 0.85 (0.81–0.88), 5.6 (4.4–7.3), 0.18 (0.15–0.22), 31 (21–46) and 0.91 (0.89–0.94), respectively. Among the three commonly used types of portable spirometers, the accuracy of PIKO-6 was higher (0.95) than that of COPD-6 (0.91) and PEF (0.82). Subgroup analysis indicated that the accuracy of a multi-indices portable spirometer was higher than that of a single-index one (P < 0.05). In addition, portable spirometry performed by professional technicians in tertiary hospitals was more accurate than for those conducted by trained technicians in primary care facilities and communities (P < 0.05). Moreover, the accuracy of studies conducted in developing country was superior to developed country (P < 0.05). Portable spirometers have high accuracy in the diagnosis of COPD. Multi-index COPD-6 and PIKO-6 displayed higher accuracy than others. Standardized training of instrument operators should be considered to achieve reliable results.
Subject terms: Physical examination, Chronic obstructive pulmonary disease, Health care economics
Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory condition characterized by persistent and progressive limitation in the airflow1. As one of the leading causes of disability and mortality globally, COPD accounted for nearly 3 million deaths in 20162. Due to an increasing proportion of an aging population, coupled with the high cigarette smoking rate, the prevalence of COPD in China has increased by 67% in the last 10 years. The total number of COPD patients stands close to 100 million3. As a result, COPD has become one of the major public health challenges in China, with attendant heavy economic and social burden.
The 2021 Global Initiative for Chronic Obstructive Lung Disease (GOLD) document categorically points out that pulmonary function test (PFT) is the gold standard for COPD diagnosis. In addition, it states that PFT is the reference basis for grading the severity of COPD and guiding follow-up treatment; thus making it key in the diagnosis, treatment, and management of COPD1. Studies suggest that the prevalence of undiagnosed and underdiagnosed cases of COPD in primary care is substantial4, with most patients only getting diagnosed when they have already lost their lung function5,6. Gao and colleagues summarized the current status of the application of PFTs in China and decry that these tests are under-used in primary care. In fact, they noted, some primary care centers did not even provide these tests7. The low utilization of pulmonary function tests has been cited as the main reason for the failure to diagnose or underdiagnose COPD8. Conducting the traditional laboratory PFTs may not be feasible under primary care settings due prohibitive costs relating to acquisition, storage, and maintenance of the instruments besides the lack of professional technicians capable of operating the machines. However, if all patients suspected to have COPD are referred to tertiary hospitals for PFTs, this will increase their costs of seeking medical care.
Portable spirometers are attractive to use in clinical practice due to their affordability, portability, and easy-to-operate characteristics. Several studies have shown that the measurements obtained with the use of portable spirometers are highly consistent with those of traditional spirometers9,10. Thus, portable spirometers have gained prominence in medical practice and clinical research and can offer a suitable alternative for the early detection of COPD in resource-limited healthcare settings. The purpose of this systematic review and meta-analysis was to quantitatively evaluate the diagnostic accuracy and feasibility of the use of portable spirometers in the diagnosis of COPD.
Methods
Study identification and selection
Two authors searched independently from PubMed, Embase, CNKI, Wan Fang and the Web of Science databases. The search strategy was based on the following keywords and text words: (“COPD” OR “chronic obstructive pulmonary disease” AND (“portable spirometers” OR “handheld spirometry” OR “screening tool”) and related synonym extensions. The search time was from January 2000 to July 2021 with no language restrictions.
Inclusion and exclusion criteria
For inclusion, Studies that designated the target disease as COPD. In addition, the individuals must have completed respiratory examinations using both a portable and traditional spirometer. Although peak flow meters are technically not spirometers, it was found to be used for COPD detection and pulmonary function evaluation in some studies. Therefore, we included peak flow meters in our study for comparing their sensitivity and specificity in COPD detection with other spirometers.
The following were excluded from the meta-analysis: (1) studies which did not report the numbers of true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN) as well as any other relevant data for the construction of two-by-two contingency tables; (2) onference proceedings, expert forums, systematic reviews, translations, and such like articles.
Data extraction
Data were extracted and cross-checked independently by two researchers. In case of any discrepancies, a third researcher was involved to adjudicate over the differences so that a common decision was reached. Information obtained from the studies include: (1) basic information such as author’s name(s), date of publication and sample size; (2) use of portable spirometers (including types, clinical setting, operators); (3) the number of TP, FP, FN, TN, and the threshold for identifying the positive values of the two tests. If more than one set of data (TP, FP, FN, and TN) was found, the set of data with the best diagnostic performance was chosen.
Quality assessment
We divided the risk of bias of included studies into “high risk”, “low risk”, and “unclear risk”. The quality of each article included in this meta-analysis was assessed using the QUADAS-2 checklist as provided in Review Manager, version 5.2 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012)11.
Ethics statement
Procedures and experiment protocols were performed in accordance with the National Institute of Health Guide for Care and were approved by the Ethics Committee of China Medical University in accordance with the Declaration of Helsinki.
Statistical analysis
Forest plots of sensitivity and specificity were constructed using Review Manager, version 5.2. These plots were used to visually explore the diagnostic accuracy of each test. Statistical analyses was conducted using Stata, version 13.1 (Stata-Corp, College Station, Texas, USA). The “midas” command was used to fit the bivariate mixed-effects model to estimate coefficients and the variable-covariate matrix. The same was used to calculate the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with 95% confidence intervals (CI) for each of the included studies. A summary receiver operating characteristic curve (SROC) was drawn, and the area under the curve (AUC) was calculated to describe and compare the accuracy of portable spirometers in the diagnosis of COPD. The accuracy of the diagnostic test was evaluated according to the value of the AUC, which was divided into five parts: non-informative (AUC = 0.5), less accurate (0.5 < AUC < 0.7), moderately accurate (0.7 < AUC < 0.9), highly accurate (0.9 < AUC < 1), and perfect tests (AUC = 1)12.
The I2 test was used to estimate the heterogeneity of the included studies contributing to the pooled estimate. After the influence of the threshold effect was excluded by Meta-Disc version 1.4 (Unit of Clinical Biostatistics Team of the Ramón y Cajal Hospital, Madrid, Spain). Random effect model was used to provide a conservative estimate of statistics, afterwards, potential heterogeneity was explored using subgroup analysis which both were conducted using STATA 13.1 Software. The subgroup analyses included the grouping based on threshold selection method (fixed value or the cutoff value), the type of portable spirometer indicators (multi-index or single index), country (developed countries or developing countries), study setting (hospital or normal population), type of executive place (tertiary hospitals or primary cares and communities), population (non COPD or the whole crowd), and the year of publication (2000–2016 or 2017–2021). The level of significance ɑ was adjusted for multiple comparisons. Sensitivity analyses were performed to determine the reliability of the results, and Deeks’ funnel plot was used to detect publication bias. The results were considered statistically significant when P < 0.05.
Results
Search results
A total of 2578 related articles were obtained in the initial database inspection according to the previously described search strategy (Fig. 1). After removal of duplicate publications, title, and abstract screening, 244 articles were identified as being potentially suitable for inclusion. Subsequently, 188 articles were selected after reading the full text. Finally, 29 articles were included in the meta-analysis after applying the inclusion and exclusion criteria (including 2 in Chinese and 27 in English). One of the articles used two portable spirometers in the same population, and one of the articles conducted one portable spirometer in two different population, for these reason, it was split into two independent studies in the subsequent analysis. Consequently, 31 studies were included in this meta-analysis.
Fig. 1. Studies selection for meta-analysis.
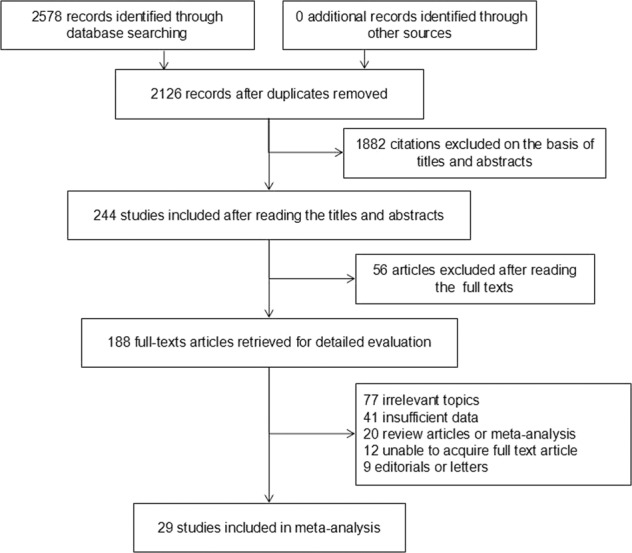
COPD chronic obstructive pulmonary disease.
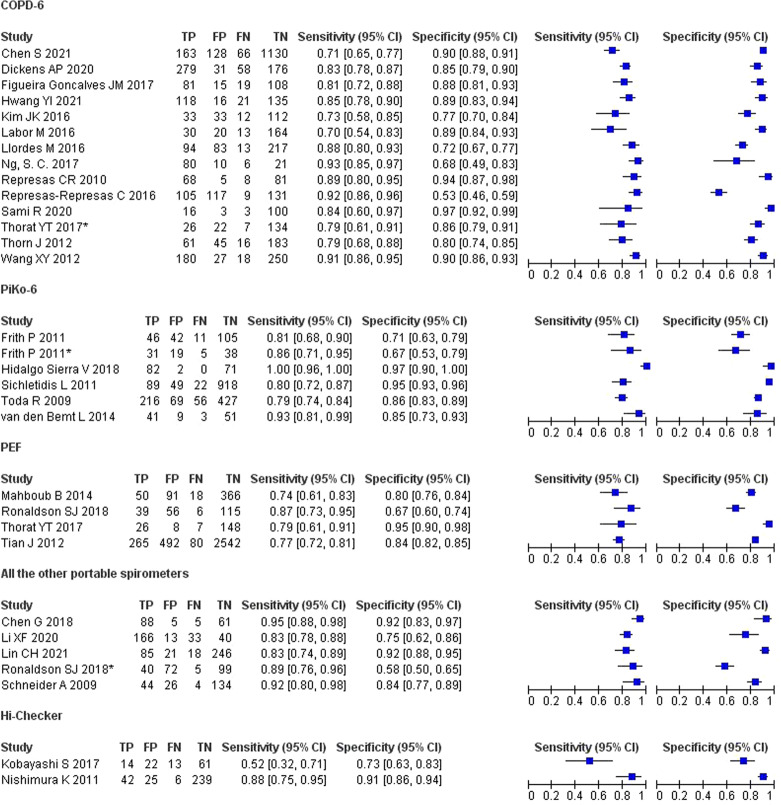
The studies included in this meta-analysis were conducted in 15 countries (6 in China, 5 in Spain, 3 in the UK, 3 in Japan, 2 in Australia, 2 in Korea, 2 in India, and 1 in the United Arab Emirates, Germany, Netherlands, Croatia, Sweden, Iran, Malaysia, and Greece, respectively), in tertiary hospitals (10 articles), primary care units or community settings (21 pieces), and utilized nine types of portable spirometers. These devices were COPD-6 (n = 14), PIKO-6 (n = 6), PEF (n = 4), Hi-Checker (n = 2), and IQ-Spiro (n = 1), Medikro SpiroStar (n = 1), MS01 Micro spirometer (n = 1), SP10BT (n = 1), Spirobank Smart (n = 1) (Table 1).
Table 1.
Characteristics of studies included in the meta-analysis.
| Author and year | Country | Setting | Study designs | Portable spirometer | Test threshold of portable spirometers | Definition of airflow obstruction | Portable spirometers operator | Inclusion and exclusion criteria | Sample size (male) | Age (years, mean ±SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen G 201810 | China | 1 tertiary hospital | randomized | IQ-spiro |
Cut-off value unclear-FEV1/FEV6<0.74 |
post-FEV1/FVC<0.70 | Professional technician | Subjects who visited a tertiary hospital | 159 (-) | 55±14.7 |
| Chen S 202137 | China | 8 primary cares | cross-sectional study | COPD-6 |
Cut-off value unclear-FEV1/FVC<0.77 |
post-FEV1/FVC<0.70 | Trained physician | Aged ≧ 40 years | 1487 (-) | - |
| Dickens AP 202038 | UK | 71 general practices | case-control | COPD-6 |
Cut-off value Pre-FEV1/FEV6<0.78 |
post-FEV1/FVC<LLN | Trained research assistants | aged≧40, who either had previously clinically diagnosed COPD or had reported chronic respiratory symptoms. | 544 (349) | 69.6±9.1 |
| Figueira Goncalves JM 201739 | Spain | 1 tertiary hospital | cross-sectional observational study | COPD-6 |
Cut-off value Pre-FEV1/FEV6<0.75 |
pre-FEV1/FVC<0.70 | Professional technician | patients referred to laboratory for respiratory functional tests | 233 (133) | 59±15 |
| Frith P 201140 | Australia | 4 primary care practices | prospective, multicenter | PiKo-6 |
Cut-off value preFEV1/FEV6<0.75 |
post-FEV1/FVC<0.70 | Trained nurse or general practitioner (GP) | current or former smokers, aged > 50 years, no previous diagnosis of obstructive lung disease, and no treatment for obstructive lung disease in the past year. | 204 (69) | 61±8 |
| Frith P 2011*40 | Australia | 4 primary care practices | prospective, multicenter | PiKo-6 |
Cut-off value Pre-FEV1/FEV6<0.75 |
post-FEV1/FVC<0.70 | Trained nurse or general practitioner (GP) | current and former smokers aged >50 years, a previous diagnosis of or treatment for asthma, and no previous diagnosis of COPD. | 93 (54) | 62±8.8 |
| Hidalgo Sierra V 201841 | Spain | 2 primary care centers* | - | PiKo-6 |
Cut-off value unclear-FEV1/FEV6≦0.70 |
post-FEV1/FVC<0.70 | Professional technician* | aged ≧40 years, a pack-year index (PYI)≧10, and typical symptoms, such as cough, expectoration and dyspnea, and with no previous diagnosis of COPD | 155 (111) | 63±14 |
| Hwang YI 202142 | Korea | 5 tertiary hospitals | - | COPD-6 |
Cut-off value pre-FEV1/FEV6<0.73 |
post-FEV1/FVC<0.70 | Professional technician |
Aged ≧ 40 years; respiratory symptoms and PYI≧10 pack-years. Subjects who had a history of disease such as tuberculous sequalae, bronchiectasis, asthma, and lung cancer that might influence pulmonary function tests were excluded. |
290 (-) | 63.1 ± 11.0 |
| Kim JK 201643 | Korea | 9 primary clinics | prospective cohort study | COPD-6 |
Cut-off value preFEV1/FEV6≦0.77 |
post-FEV1/FVC<0.70 | primary care physicians |
Subjects who visited a primary clinic complaining of respiratory symptoms and aged ≧40 years, PYI≧10 irrespective of their current smoking state and had no previous diagnosis of COPD. Patients with a history of disease that might have influenced spirometry results, such as tuberculosis-destroyed lungs, bronchiectasis, asthma, or lung cancer were excluded. |
190 (-) | 60.3±10.6 |
| Kobayashi S 201744 | Japan | 16 primary care clinics and 4 hospitals | prospective multi-center, observational study | Hi-Checker | Fixed value unclear-FEV1/FEV6≦0.75 | post-FEV1/FVC<0.70 | primary care physicians | Patients > 40 years of age who received outpatient care for chronic disease at primary care clinics Patients with known chronic respiratory diseases, including asthma and COPD, and patients suffering from acute respiratory symptoms were excluded. | 110 (91) | 68.5±0.8 |
| Labor M 20169 | Croatia | 26 general practitioners’ office | prospective cohort study | COPD-6 |
Cut-off value unclear-FEV1/FEV6≦0.78 |
post-FEV1/FVC<0.70 | Trained GPs |
written consent. aged 40–65 years with a smoking history of PYI≧2; with no previous diagnosis of COPD. |
227 (112) | 52.5±6.8 |
| Li XF 202045 | China | 1 district hospitals and ten primary care centers. | - | SP10BT | Cut-off value post-FEV1/FVC<0.7 and FVC>80%pred | post-FEV1/FVC<0.70 and FVC<80%pred | 2 trained primary care physicians | aged > 40 years, typical symptoms such as chronic cough expectoration, or asthma, and risk factor exposure history | 252 (182) | 65.7±10.1 |
| Lin CH 202146 | Taiwan, China | 26 outpatient clinics | prospective multi-center, | Spirobank Smart | Cut-off value pre-FEV1/FVC<0.74 | post-FEV1/FVC<0.70 | trained nurses and physicians | Aged ≧ 40 years, PYI≧10 pack-years., with chronic respiratory disease and no previous diagnosis of COPD. | 370 (349) | 60.9±9.7 |
| Llordes M 201747 | Spain | 8 primary care centers | - | COPD-6 | Cut-off value unclear-FEV1/FEV6≦0.78 | post-FEV1/FVC<0.70 | Trained primary care physician, a nurse or a technician | Aged ≧ 40 years with a smoking history of PYI≧1; with no previous diagnosis of COPD and attended the primary care centers for any reason | 407 (265) | 57.4±8.9 |
| Mahboub B 201422 | United Arab Emirates | 5 primary cares, 2 large shopping malls and 1 industrial city | cross sectional study | PEF |
Fixed value Pre-PEF<2.2L(s*m2) |
pre-FEV1/FVC<0.70 | Trained primary care physicians or nurses | Aged ≧ 40 years | 525 (358) | 49.6±4.1 |
| Ng, S. C. 201748 | Malaysia | Medical Outpatient Department and health care clinics | cross-sectional study | COPD-6 | Cut-off value post-FEV1/FEV6<0.75 | post-FEV1/FVC<0.70 | Trained stuff | Aged ≧50 years; history of dyspnoea; history of chronic cough or chronic sputum production; history of exposure to risk factors; and any smoker even in the absence of above symptoms. | 117(101) | 67.38±11.58 |
| Nishimura K 201149 | Japan | 1 tertiary hospital | - | Hi-Checker | Cut-off value unclear-FEV1/FEV6<0.746 | post-FEV1/FVC<0.70 | Professional technician | industrial workers who underwent annual health checks | 312 (312) | 55±9.4 |
| Represas CR 201050 | Spain | 1 tertiary hospital | prospective, descriptive transversal study | COPD-6 | Cut-off value unclear-FEV1/FEV6<0.77 | unclear-FEV1/FVC<0.70 | Professional technician | those who attended pulmonary function laboratory for functional respiratory tests | 162 (95) | 56±16 |
| Represas-Represas C 201651 | Spain | 8 primary care centers, 15 community pharmacies and 4 emergency services | prospective, multi-cohort study | COPD-6 | Cut-off value preFEV1/FEV6<0.80 | post-FEV1/FVC<0.70 | Trained primary care physicians or nurses |
Aged ≧40 years, with a smoking history of PYI≧10, and symptoms suggestive of COPD. Individuals who had already been diagnosed with a respiratory disease were excluded. |
362 (224) | 55.4±9.9 |
| Ronaldson SJ 201823 | UK | general practices | prospective case-finding stud | PEF |
Fixed value Pre-PEF<80%pred |
post-FEV1/FVC<0.70 and FEV1%<80%pred or FEV1%>80%pred with at least 1 symptom | Trained nurses in primary care | aged≧35; current smokers, including those who had comorbidities, such as COPD or asthma | 216 (109) | 53.4±11.0 |
| Ronaldson SJ 2018*23 | UK | general practice | prospective case-finding stud | MS01 Micro spirometer |
Fixed value FEV1/FVC<0.7, FEV1<80%pred, or FVC<80%pred |
post-FEV1/FVC<0.70 and FEV1%<80%pred or FEV1%>80%pred with at least 1 symptom | Trained nurses in primary care | aged≧35; current smokers, including those who had comorbidities, such as COPD or asthma | 216 (109) | 53.4±11.0 |
| Sami R 202052 | Iran | 1 tertiary hospital | cross-sectional descriptive study | COPD-6 | Cut-off value post-FEV1/FVC<0.72 | post-FEV1/FVC<0.70 | Professional technician |
Aged ≧ 40 years; PYI≧10 pack-years; symptoms suggestive of COPD. Patients with previously diagnosed respiratory diseases were excluded. |
122 (-) | 53.2±9.0 |
| Schneider A 200953 | Germany | 10 primary care centers | cross-sectional study | Medikro SpiroStar | Fixed value post-FEV1/FVC<0.70and FVC>80%pred | post-FEV1/FVC≦0.70 and/or FEV1<80%pred | Trained primary care physicians and practice assistants | Complaints suggestive of obstructive airway disease (OAD), had not been diagnosed previously for OAD and had not received any previous anti-obstructive medicine. | 219 (92) | 43.8±15.6 |
| Sichletidis L 201154 | Greece | Two Primary cares Centers | - | PiKo-6 | Fixed value post-FEV1/FEV6<0.70 and/or FEV1<80% | post-FEV1/FVC<0.70 | Trained primary care general practitioners |
Aged >40years. Medically confirmed diagnosis of an obstructive lung disease (e.g. COPD, asthma, bronchiectasis) and any other pulmonary disease (e.g. tuberculosis, interstitial lung disease, lung cancer), thoracic surgery in the previous 6 months or acute respiratory infection were excluded. |
1078 (616) | 65.3±11.4 |
| Thorat YT 201724 | India | Specialized Hospital | cross-sectional study | PEF | Cut-off value pre-PEF<80%pred | post-FEV1/FVC<0.70 | Professional technician |
Adult patients with respiratory complains. Patients with history of pulmonary tuberculosis, and those with contra-indications for spirometry, and also pregnant and nursing mothers were excluded. |
189 (111) | 51±17 |
| Thorat YT 2017*24 | India | Specialized Hospital | cross-sectional study | COPD-6 | Cut-off value preFEV1/FEV6<0.75 | post-FEV1/FVC<0.70 | Professional technician |
Adult patients with respiratory complains. Patients with history of pulmonary tuberculosis, and those with contra-indications for spirometry, and also pregnant and nursing mothers were excluded. |
189 (111) | 51±17 |
| Thorn J 201255 | Sweden | Twenty-one urban and rural Primary cares Centers | - | COPD-6 | Cut-off value preFEV1/FEV6<0.73 | post-FEV1/FVC<0.70 | Trained nurse | Aged 45–85 years; with a smoking history of at least 15 pack-years | 305 (132) | 61.2±8.4 |
| Tian J 201225 | China | Community settings | cluster Sampling; Prospective study | PEF | Fixed value pre-PEF<80%pred | post-FEV1/FVC<0.70 | Trained primary care physicians or nurses | Aged ≧40years | 3379 (1400) | - |
| Toda R 200956 | Japan | 1 tertiary hospital | - | PiKo-6 | Cut-off value pre-FEV1/FEV6<0.749 | pre-FEV1/FVC<0.70 | Professional technician | subjects including non-smokers, smokers, COPD, bronchial asthma, and interstitial lung diseases. | 768 (402) | 57.8±0.6 |
| van den Bemt L201457 | Nether lands. | several sites for general practitioners | randomized cross-sectional diagnostic study | PiKo-6 | Fixed value pre-FEV1/FEV6<0.73 | post-FEV1/FVC<0.70 | Trained primary care physicians | Aged ≧50 years; current or former smokers (⩾1 pack year) with no previous diagnosis of COPD | 104 (62) | - |
| Wang XY 201858 | China | 1 community | - | COPD-6 | Fixed value pre-FEV1/FEV6<0.70 | post-FEV1/FVC<0.70 | Trained technician | Aged 40-75 years | 475 (134) | 57.78±9.18 |
*In this study, both portable spirometers and traditional laboratory PFT were conducted by the high-resource medical institutions.
COPD, chronic obstructive pulmonary disease; FEV1,forced expiratory volume in 1 s; FEV6, forced expiratory volume in 6 s; FVC, forced vital capacity; GP, general practitioner; PYI, pack year index; SD, Standard Deviation.
Literature bias risk assessment and publication bias
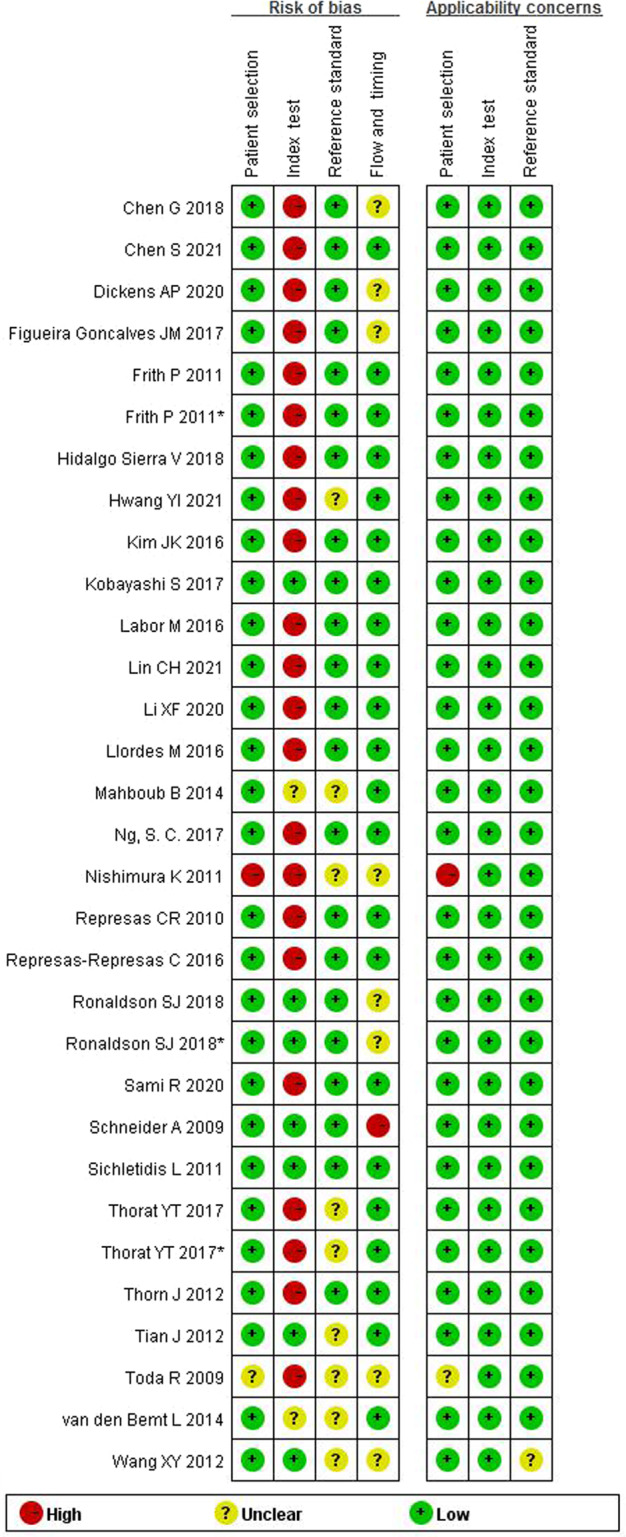
Generally, the quality of the included studies ranged between medium to high. Assessment using QUADAS-2 tools found that “patient selection” and “flow and timing” parts of tools were clear for most studies. The risk of bias mainly arose from the selection method of the threshold of the index test and the lack of a strict blinding method between the index test and the reference test (Figs. 2 and 3).
Fig. 2. Summary map of risk of bias across domains of the included studied.
Using QUADAS-2 tool. Key domains: patient selection; index test; reference standard; study flow and timing. The risk of bias is indicated by three colors, red for high risk of bias, yellow for unclear risk of bias, and green for low risk of bias.
Fig. 3. Risk of bias and corresponding applicability concerns across included studies.
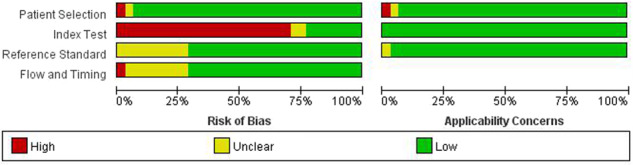
Using QUADAS-2 tool. Key domains: patient selection; index test; reference standard; flow and timing. The risk of bias is indicated by three colors, red for high risk of bias, yellow for unclear risk of bias, and green for low risk of bias.
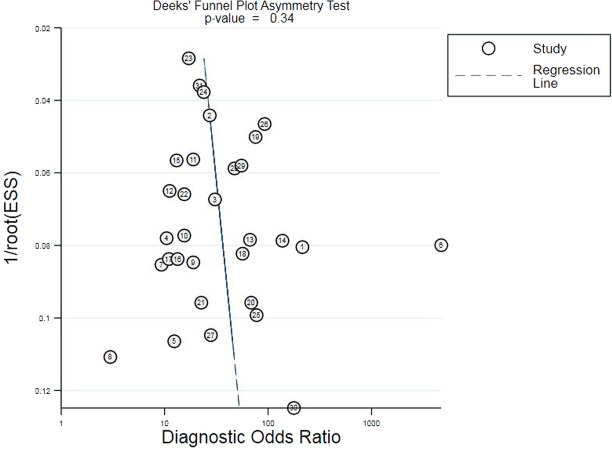
There was no significant publication bias as determined by the Deeks’ funnel chart, which showed that the angle between the regression line and the horizontal axis was close to 90° (P = 0.34) (Fig. 4).
Fig. 4. Deeks’ funnel plot asymmetry test for evaluation of publication bias.
The closer the angle between the regression line of the Deeks’ funnel plot and the horizontal axis (x) is to 90, the less likely it is to suggest that there is publication bias.
Diagnostic accuracy of spirometry
The results of TP, FP, FN, and TN in the diagnosis of COPD in each study are shown in (Fig. 5). The Spearman correlation coefficient between the logit of sensitivity and logit of 1-specificity was 0.011 (P = 0.955), indicating that there was no threshold effect in the study. However, the I2 values were high (I2 = 99%, P < 0.01). We chose the random-effects model to conservatively estimate the summary statistics. The results show that the pooled sensitivity, specificity, PLR, NLR, and DOR with 95% CI are 0.85 (0.81–0.88), 0.85 (0.81–0.88), 5.6 (4.4–7.3), 0.18 (0.15–0.22), and 31 (21–46), respectively. The area under the SROC (AUC) was 0.91 (0.89–0.94) showing that the accuracy of the portable spirometer is 91% and is very close to the reference test (Fig. 6).
Fig. 5. Forest plot of sensitivity and specificity of each screening test.
TP true positive, FP false positive, FN false negative, TN true negative.
Fig. 6. Bivariate summary estimates of sensitivity and specificity, with corresponding 95% confidence ellipse around the mean values, for all studies.
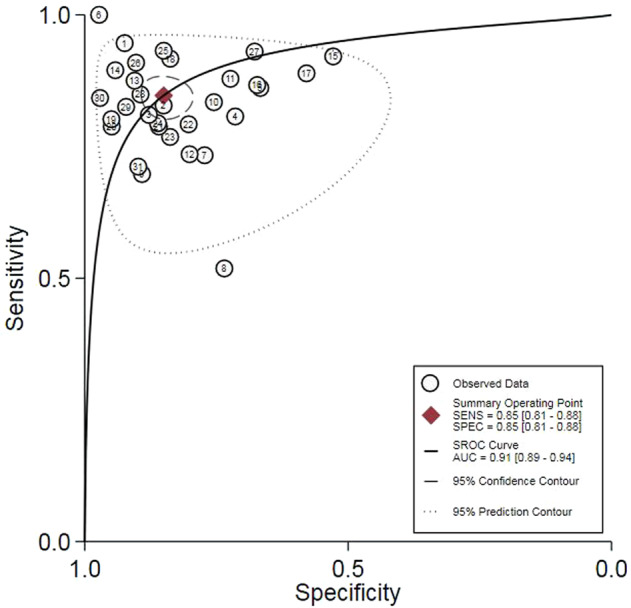
SENS sensitivity, SPEC specificity, SROC summary receiver operating characteristic, AUC area under the curve.
Subgroup analysis
The outcomes of the subgroup analyses are summarized in Table 2. PIKO-6 had the highest diagnostic accuracy with the area under the SROC (AUC) of 0.95 (0.92–0.96). AUC value for COPD-6 was 0.91 (0.88–0.93), and for PEF it was 0.82 (0.78–0.85). There were statistically significant differences in the diagnostic accuracy indices among COPD-6, PIKO-6, and PEF (P all < 0.0167) after adjusting the level of significance ɑ due to multiple comparisons (Fig. 7). According to the classification of detection indicators, portable spirometers with FEV1/FEV6 showed the area under the SROC (AUC) was 0.92 (0.90–0.94). There were statistically significant differences in the diagnostic accuracy indices between PEF and FEV1/FEV6 (P < 0.001), and between FEV1/FEV6 and FEV1/FVC (P = 0.007) after adjusting the level of significance ɑ due to multiple comparisons.
Table 2.
Subgroup analysis of all included studies.
| Factor | Studies | Sensitivity (95% CI) | I2 | Model used | Specificity (95% CI) | I2 | Model used | SROC (95% CI) |
|---|---|---|---|---|---|---|---|---|
| All studies | 31 | 0.85 (0.81–0.88) | 77.87% | Random | 0.85 (0.81–0.88) | 95.48% | Random | 0.91 (0.89–0.94) |
| Type of the device | ||||||||
| COPD-6 | 14 | 0.84 (0.80–0.88) | 79.22% | Random | 0.85 (0.79–0.90) | 95.85% | Random | 0.91 (0.88–0.93) |
| Piko-6 | 6 | 0.89 (0.76–0.96) | 84.17% | Random | 0.88 (0.75–0.94) | 96.11% | Random | 0.95 (0.92–0.96) |
| PEF | 4 | 0.77 (0.73–0.81) | 1.13% | Fixed | 0.83 (0.71–0.91) | 94.04% | Random | 0.82 (0.78–0.85) |
| Hi-checker | 2 | 0.75 (0.63–0.84) | 91.20% | Random | 0.86 (0.82–0.90) | 92.80% | Random | – |
| IQ-spiro | 1 | 0.94 | – | – | 0.92 | – | – | – |
| SP10BT | 1 | 0.83 | – | – | 0.75 | – | – | – |
| Medikro SpiroStar | 1 | 0.92 | – | – | 0.84 | – | – | – |
| MS01 Micro spirometer | 1 | 0.89 | – | – | 0.58 | – | – | – |
| Spirobank Smart | 1 | 0.83 | – | – | 0.92 | – | – | – |
| Detection indicators | ||||||||
| PEF | 4 | 0.77 (0.73–0.81) | 1.13% | Fixed | 0.83 (0.71–0.91) | 94.04% | Random | 0.82 (0.78–0.85) |
| FEV1/FVC | 4 | 0.85 (0.81–0.88) | 6.47% | Fixed | 0.80 (0.65–0.90) | 96.14% | Random | 0.87 (0.84–0.90) |
| FEV1/FEV6 | 23 | 0.85 (0.81–0.89) | 82.09% | Random | 0.86 (0.81–0.90) | 95.54% | Random | 0.92 (0.90–0.94) |
| Threshold selection method | ||||||||
| Fixed | 4 | 0.83 (0.65–0.92) | 90.39% | Random | 0.89 (0.79–0.94) | 94.40% | Random | 0.93 (0.90–0.95) |
| Cutoff | 19 | 0.86 (0.81–0.89) | 80.62% | Random | 0.85 (0.80–0.90) | 95.43% | Random | 0.92 (0.89–0.94) |
| P | 0.673 | 0.385 | 0.581 | |||||
| Type of indicators | ||||||||
| Multi-index | 27 | 0.85 (0.82–0.88) | 79.21% | Random | 0.85 (0.81–0.89) | 95.60% | Random | 0.92 (0.89–0.94) |
| Single index | 4 | 0.77 (0.73–0.81) | 1.13% | Fixed | 0.83 (0.71–0.91) | 94.04% | Random | 0.82 (0.78–0.85) |
| P | <0.001 | 0.716 | <0.001 | |||||
| Country | ||||||||
| Developed | 23 | 0.85 (0.81–0.88) | 73.60% | Random | 0.83 (0.78–0.87) | 95.52% | Random | 0.90 (0.88–0.93) |
| Developing | 8 | 0.84 (0.77–0.89) | 88.47% | Random | 0.90 (0.85–0.93) | 96.57% | Random | 0.94 (0.91–0.95) |
| P | 0.778 | 0.023 | 0.015 | |||||
| Executive place | ||||||||
| Tertiary hospital | 10 | 0.89 (0.83–0.93) | 81.27% | Random | 0.92 (0.89–0.95) | 82.86% | Random | 0.96 (0.94–0.97) |
| Primary care/community | 21 | 0.83 (0.79–0.87) | 76.98% | Random | 0.80 (0.75–0.85) | 95.97% | Random | 0.89 (0.86–0.91) |
| P | 0.066 | <0.001 | <0.001 | |||||
| Study setting | ||||||||
| Hospital-based | 27 | 0.85 (0.81–0.88) | 77.64% | Random | 0.85 (0.80–0.89) | 95.81% | Random | 0.91 (0.89–0.93) |
| Population-based | 4 | 0.85 (0.78–0.90) | 86.53% | Random | 0.87 (0.84–0.90) | 94.79% | Random | 0.93 (0.90–0.95) |
| P | 0.999 | 0.469 | 0.222 | |||||
| Population | ||||||||
| No COPD | 13 | 0.86 (0.78–0.91) | 80.71% | Random | 0.87 (0.81–0.92) | 94.67% | Random | 0.93 (0.90–0.95) |
| The whole crowd | 18 | 0.84 (0.81–0.87) | 76.22% | Random | 0.83 (0.78–0.87) | 95.81% | Random | 0.90 (0.87–0.93) |
| P | 0.584 | 0.271 | 0.133 | |||||
| Publication year | ||||||||
| 2000–2016 | 15 | 0.83 (0.79–0.86) | 66.10% | Random | 0.83 (0.77–0.87) | 95.94% | Random | 0.89 (0.86–0.92) |
| 2017–2021 | 16 | 0.86 (0.80–0.90) | 83.90% | Random | 0.87 (0.81–0.91) | 95.02% | Random | 0.92 (0.89–0.95) |
| P | 0.336 | 0.267 | 0.178 | |||||
The study divides countries into developed and developing countries according to HDI. When HDI > 0.80, it is classified as a developed country and if otherwise, developing country59.
SROC summary receiver operating characteristic curve.
aOwing to the complexity of the bivariate model and the limited number of studies, the groups with n ≥ 4 were pooled using a bivariate model. The remaining data were pooled by univariate random-effects logistic regression model. We also tested for the difference in the sensitivity or specificity between the two groups using the bivariate model. The AUC difference in the area under the SROC between the groups was obtained by Z test.
Fig. 7. The SROC of portable spirometers classified by type.
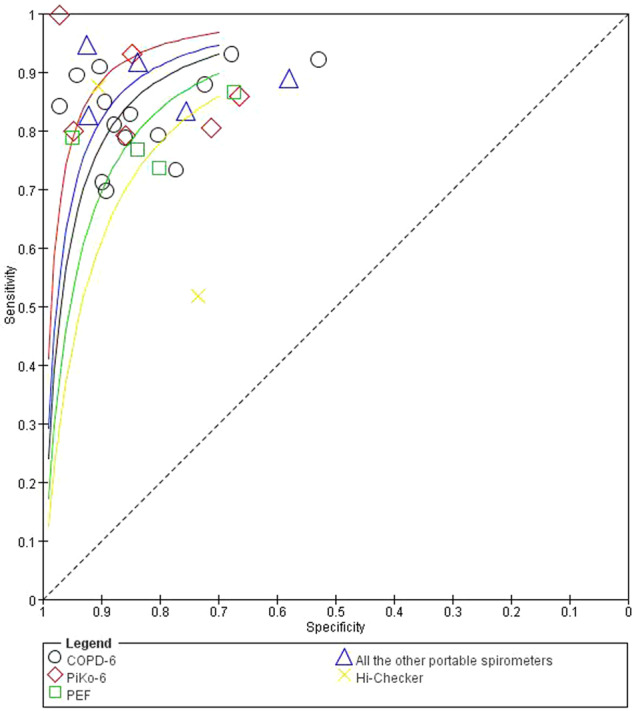
All the other portable spirometers including IQ-spiro, SP10BT, Medikro SpiroStar, MS01 Micro spirometer and Spirobank Smart.
Based on the subgroup analyses, sources of heterogeneity could not be traced with regard to the threshold selection method, study setting, population, and year of publication. However, the source of heterogeneity can be attributed to the place of execution, the type of portable spirometer classified by indicators and the country sorted by Human Development Index (HDI). When classified by indicator type, the area under the SROC (AUC) was 0.92 (0.89–0.94) for the multi-indices group and 0.82 (0.78–0.85) for the single-index group. This difference was statistically significant both in sensitivity and AUC (P all < 0.001). When we grouped studies by the clinical setting in which the spirometry was conducted, the area under the SROC (AUC) of the tertiary hospital group was 0.96 (0.94–0.97), and 0.89 (0.86–0.91) for the primary care and community group. Statistically significant differences in AUC and specificity were noted between these groups (P all < 0.001). When we grouped studies by country, the area under the SROC (AUC) of the developed country was 0.90 (0.88–0.93), and 0.94 (0.91–0.95) for the developing country statistically significant differences in AUC (P = 0.015) and specificity (P = 0.023) were noted between these groups.
Discussion
COPD has been widely underdiagnosed so far. PFT has been recommended as the gold standard for COPD diagnosis and monitoring1. However, such tests are not readily available or applied to all patients in need, leading to the absence of standard diagnosis and treatment, and subsequently the deterioration of COPD13. A decision-analytic model conducted by Qu S et al. showed that portable spirometer is likely the optimal option in the early screening and follow-up of patients in China14. In addition, multiple screening questionnaires have been developed as active case-finding tools to identify potential COPD patients in primary care15. Haroon16 compared the diagnostic accuracy of screening tests in primary care in 2015, finding that portable spirometers had a sensitivity of 79.9% (74.2–84.7%) and a specificity of 84.4% (68.9–93.0%). He concluded that portable spirometers demonstrated higher test accuracy than questionnaires for COPD screening in primary care. However, There were only three relevant references in Haroon’s study concerning portable spirometers, which was too small to further analyze the clinical application effects and influencing factors of portable spirometers. To address this gap, we performed a more detailed and comprehensive meta-analysis in this field by including 31 studies for systematic evaluation and quantitative analysis. Across the studies, nine types of portable spirometers were used under three different kinds of medical environments. We excluded the influence of threshold effects and used random-effects models to pool the data. The results show that the area under the SROC (AUC) of 0.91 indicate that the portable spirometer has high accuracy and can be used as an alternative for traditional pulmonary function tests in COPD screening, primary diagnosis and subsequent monitoring. COPD-6, PIKO-6, and PEF are three commonly used portable spirometers in clinical practice. From a diagnostic accuracy perspective, PIKO-6 has the highest diagnostic accuracy rate (95%), followed by COPD-6 (91%) and PEF (82%) with statistically significant difference among them (P < 0.05).
The heterogeneity in these studies was explored by subgroup analyses. According to the GOLD guideline, post-bronchodilator FEV1/FVC < 0.70 is the criterion for diagnosing COPD1. However, a qualified FVC measurement based on the ATS guideline has high requirements for the subject and the operator. A growing number of studies indicated that the FEV1/FEV6 could be served as an alternative choice for FEV1/FVC17–19. FEV6 is more accessible to measure than FVC and reduces the probability of spirometry complications. Several portable spirometers, such as COPD-6, PIKO-6, were designed to measure FEV6 instead of the original FVC. As mentioned above, some studies in this meta-analysis defined the fixed FEV1/FEV6 < 0.70 as airflow obstruction. However, considering that the reference formula for FEV6 was originated from the lung function database of the Third National Health and Nutrition Examination Survey(NHANES III) conducted in American20. There is still a debate on whether its application to the population of other countries and regions will make a difference. Some studies have been modified its ratio from the fixed value to an optimal cutoff value based on the national population. Therefore, we compared the diagnostic accuracy of portable spirometer using the fixed value with the cutoff value. Our study found that differences in the diagnostic accuracy of portable spirometers have nothing to do with the threshold selection method. Although the cutoff value can get the best diagnostic effect, a fixed value can also be acceptable if applied to primary diagnosis or community screening. Besides, we also investigated the diagnostic accuracy of all spirometers using FEV1/FEV6 ratio. Our pooled estimates showed a diagnostic accuracy of 92% with FEV1/FEV6, compared with the gold standard using FEV1/FVC. This study showed that the FEV1/FEV6 could be served as an alternative choice for FEV1/FVC for the diagnosis of COPD. Still, Soares et al. compared the sensitivity of FEV1/FEV6 with that of FEV1/FVC and concluded that although FEV1/FEV6 showed a good sensitivity of 85.6–95%, when it comes to mild airway obstruction, the sensitivity will be decreased21.
We found that the heterogeneity in test accuracy between studies was likely to arise from differences in the type of portable spirometer index, executive place, and country, but not in the threshold selection method, study setting, population, or year of publication.
Portable spirometers, for example, PIKO-6 and COPD-6 can give multiple respiratory function indicators, such as FEV1, FEV6, and FVC. These indicators can help in the diagnosis of patients with COPD, as well as in estimating the severity of the disease, and then guiding the choice of an appropriate treatment plan. However, there are still some studies22–25 that applied PEF for screening and diagnosis of COPD. As single-index spirometry, PEF has been widely used to diagnose and monitor asthma patients26,27. Liu YN28 and Jackson et al.29 concluded that PEF has extremely high sensitivity (98.5–100%) in screening moderate to severe COPD patients. To assess whether the type of indicators impacts the diagnostic accuracy. This meta showed that the diagnostic accuracy of multi-index spirometry (92%) was higher than that of single-index (82%) (P < 0.001) and also showed an advantage in sensitivity, 85% in multi-index and 77% in single-index. Therefore, from a clinical implementation perspective, PEF is inexpensive, easy to operate, and the patient can use it easily. Still, its diagnostic accuracy rate renders it unsuitable for diagnosing COPD. It is appropriate to be used to follow-up patients with stable COPD. Previous studies have shown that PEF could be regarded as a prediction tool for the prognosis of the disease30,31. Large variability in daily PEF indicated instability in the condition and was susceptible to acute exacerbations.
In our study, the PFTs in tertiary hospitals were all conducted by professional technicians while in primary care centers and communities, these were completed by trained doctors, nurses, or physician assistants. The area under the SROC obtained by the portable spirometer in tertiary hospitals was much higher (0.96) as compared to that in primary care centers or communities (0.89). The results show that PFTs performed by trained general practitioners, nurses, or laboratory assistants in primary care centers and communities can effectively identify persistent airflow limitation, however, compared with professional technicians, there is still room for improvement in diagnostic accuracy measurement. Previous studies have demonstrated that at least 90% of subjects can get acceptable and reproducible results under the operation of experienced professional technicians32 but this rate is much lower (58.5–71%) for primary care institutions33–35. Taken together, the observations suggest the need to strengthen the supervision of the normative diagnosis and treatment of COPD in resource-limited settings. The professional knowledge, reproducibility, and accuracy of PFTs can be significantly improved for practitioners in medical institutions who have undergone standardized training33.
The prevalence of COPD differed across countries and regions. In this study, the diagnostic accuracy of portable spirometers conducted in developing countries was superior to developed countries. We examined the composition of two groups and found that the difference may be that the executive place of the portable spirometer in developed countries was a larger proportion of primary care or community, 73.91% (17 articles) in developed countries, and 50% (4 articles) in developing countries. As mentioned above, there was a difference in the quality of PDTs between trained general practitioners in primary cares and professional technicians in tertiary hospitals. Generally speaking, the accuracy of spirometers conducted by professional technicians in tertiary hospitals could meet an acceptable quality, whereas temporary trained operators in primary cares or communities could not meet for so far. Therefore, regardless of countries or regions, we need to strengthen regular training on spirometer operators to turn this situation around, especially operators in primary care.
Although providing useful insights, there are some limitations to the present study. First, although we used subgroup analyses to explore the sources of heterogeneity, the subgroup variables could not offer complete explanations. This suggests that there may be other confounding variables as sources of heterogeneity. Second, only three included studies were randomly assigned the test order. A portable spirometry test usually precedes the traditional one, which may cause a bias in the test order, that is, the learning effect36. Our results show that portable spirometers exhibit high accuracy even in the presence of learning effect. Third, in some studies, both PFTs were performed by the same operator so that blinding was not strictly achieved. Finally, the accuracy of the instrument itself, the choice of the target population, the differences in research design, and the operating procedures may also affect the accuracy of results achieved.
In conclusion, portable spirometers have high accuracy in the diagnosis of COPD. Multi-index Spirometer, such as COPD-6 and PIKO-6, shows superior accuracy over single-indicator. Compared with FEV1/FVC, FEV1/FEV6 can be regarded as a viable surrogate indicator for diagnosing COPD. It is worth noting that although portable spirometers are easier to manoeuvre than laboratory spirometries, they also need to be performed under strict quality control. Standardized training for operators should be strengthened to ensure reliable and reproducible measurements. Portable spirometers are characterized by high accuracy, user-friendly, patient-friendly, inexpensive, and portable, making them suitable for primary care use and providing a feasible pathway for early diagnosis of COPD.
Acknowledgements
This study was funded by the National Key R&D Program of China (Grant #2018YFC1311600), and from the Distinguished professor of LiaoNing Province (issued by the Education Department of Liaoning Province [2015] No. 153).
Author contributions
J.Z. took part in drafting the article, analyzing, and interpreting data; X.L. participated in the acquisition of data; N.Y. and X.W. took part in manuscript revision. W.W. and N.Y. made substantial contributions to study design and data interpretation.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Na Yu, Wei Wang.
Contributor Information
Na Yu, Email: yunacmu@163.com.
Wei Wang, Email: wwbycmu@126.com.
References
- 1.GOLD Science Committee. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2021 report[OL]. https://goldcopd.org/gold-reports/ (2021).
- 2.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 4.Diab N, et al. Underdiagnosis and Overdiagnosis of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018;198(Nov):1130–1139. doi: 10.1164/rccm.201804-0621CI. [DOI] [PubMed] [Google Scholar]
- 5.Calverley PM. COPD: early detection and intervention. Chest. 2000;117:365–71S. doi: 10.1378/chest.117.5_suppl_2.365S. [DOI] [PubMed] [Google Scholar]
- 6.Exarchos KP, et al. Validation of the portable Bluetooth® Air Next spirometer in patients with different respiratory diseases. Respir. Res. 2020;21:79. doi: 10.1186/s12931-020-01341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Y, Zheng JP. Carrying out standardized training on pulmonary function test to help comprehensive prevention and control of chronic respiratory diseases. Chin. J. Pr. Int Med. 2019;39:481–484. [Google Scholar]
- 8.Ho T, et al. Under- and over-diagnosis of COPD: a global perspective. Breathe (Sheff.) 2019;15:24–35. doi: 10.1183/20734735.0346-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labor M, et al. Diagnostic accuracy of a pocket screening spirometer in diagnosing chronic obstructive pulmonary disease in general practice: a cross sectional validation study using tertiary care as a reference. BMC Fam. Pract. 2016;17:112. doi: 10.1186/s12875-016-0518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, et al. The accuracy of a handheld “disposable pneumotachograph device” in the spirometric diagnosis of airway obstruction in a Chinese population. Int J. Chron. Obstruct Pulmon Dis. 2018;13:2351–2360. doi: 10.2147/COPD.S168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiting PF, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 12.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 2000;45:23–41. doi: 10.1016/S0167-5877(00)00115-X. [DOI] [PubMed] [Google Scholar]
- 13.Jain VV, et al. Impact of an integrated disease management program in reducing exacerbations in patients with severe asthma and COPD. Respir. Med. 2014;108:1794–1800. doi: 10.1016/j.rmed.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Qu S, et al. Cost-effectiveness analysis of COPD screening programs in primary care for high-risk patients in China. NPJ Prim. Care Respir. Med. 2021;31:28. doi: 10.1038/s41533-021-00233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spyratos D, et al. Comparison of Three Screening Questionnaires for Chronic Obstructive Pulmonary Disease in the Primary Care. Respiration. 2017;93:83–89. doi: 10.1159/000453586. [DOI] [PubMed] [Google Scholar]
- 16.Haroon S, et al. Diagnostic accuracy of screening tests for COPD: a systematic review and meta-analysis. BMJ Open. 2015;5:e008133. doi: 10.1136/bmjopen-2015-008133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing JY, et al. Should FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A meta analysis. Chest. 2009;135:991–998. doi: 10.1378/chest.08-0723. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Gong W, Tian Y, Zhou J. FEV1/FEV6 in Primary Care Is a Reliable and Easy Method for the Diagnosis of COPD. Respir. Care. 2016;61:349–353. doi: 10.4187/respcare.04348. [DOI] [PubMed] [Google Scholar]
- 19.Venkatachalam P, Dwivedi DP, Govindraj V. FEV1/FEV6 is effective as a surrogate for FEV1/FVC in the diagnosis of chronic obstructive pulmonary disease. Indian J. Tuberc. 2021;68:230–235. doi: 10.1016/j.ijtb.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 21.Soares AL, Rodrigues SC, Pereira CA. Airflow limitation in Brazilian Caucasians: FEV1/FEV6 vs. FEV1/FVC. J. Bras. Pneumol. 2008;34:468–472. doi: 10.1590/S1806-37132008000700006. [DOI] [PubMed] [Google Scholar]
- 22.Mahboub B, et al. Case-finding of chronic obstructive pulmonary disease with questionnaire, peak flow measurements and spirometry: a cross-sectional study. BMC Res. Notes. 2014;7:241. doi: 10.1186/1756-0500-7-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronaldson SJ, et al. Determining the optimal approach to identifying individuals with chronic obstructive pulmonary disease: The DOC study. J. Eval. Clin. Pract. 2018;24:487–495. doi: 10.1111/jep.12896. [DOI] [PubMed] [Google Scholar]
- 24.Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim. Care Respir. Med. 2017;27:32. doi: 10.1038/s41533-017-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian J, et al. Peak expiratory flow as a screening tool to detect airflow obstruction in a primary health care setting. Int J. Tuberc. Lung Dis. 2012;16:674–680. doi: 10.5588/ijtld.11.0429. [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. (NICE, 2021). [PubMed]
- 27.Cloutier MM, et al. Managing Asthma in Adolescents and Adults: 2020 Asthma Guideline Update From the National Asthma Education and Prevention Program. JAMA. 2020;324:2301–2317. doi: 10.1001/jama.2020.21974. [DOI] [PubMed] [Google Scholar]
- 28.Liu YN, et al. Exploring the effectiveness of peak expiratory flow rate detection by peak flow meter in the screening of chronic obstructive pulmonary disease. Chin. J. Respir. Crit. Care Med. 2015;14:250–254. [Google Scholar]
- 29.Jackson H, Hubbard R. Detecting chronic obstructive pulmonary disease using peak flow rate: cross sectional survey. BMJ. 2003;327:653–654. doi: 10.1136/bmj.327.7416.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cen J, et al. Monitoring peak expiratory flow could predict COPD exacerbations: A prospective observational study. Respir. Med. 2019;148:43–48. doi: 10.1016/j.rmed.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 31.So JY, et al. Daily Peak Expiratory Flow Rate and Disease Instability in Chronic Obstructive Pulmonary Disease. Chronic Obstruct. Pulmon. Dis. 2015;3:398–405. doi: 10.15326/jcopdf.3.1.2015.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yawn BP, et al. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest. 2007;132:1162–1168. doi: 10.1378/chest.06-2722. [DOI] [PubMed] [Google Scholar]
- 33.Giraud V, et al. Feasibility of spirometry in primary care to screen for COPD: a pilot study. Int. J. Chron. Obstruct. Pulmon. Dis. 2016;11:335–340. doi: 10.2147/COPD.S96385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegewald MJ, Gallo HM, Wilson EL. Accuracy and Quality of Spirometry in Primary Care Offices. Ann. Am. Thorac. Soc. 2016;13:2119–2124. doi: 10.1513/AnnalsATS.201605-418OC. [DOI] [PubMed] [Google Scholar]
- 35.Weiss G, et al. Detection of chronic obstructive pulmonary disease in primary care in Salzburg, Austria: findings from the real world. Respiration. 2014;87:136–143. doi: 10.1159/000354796. [DOI] [PubMed] [Google Scholar]
- 36.Koyama H, et al. A comparison of different methods of spirometric measurement selection. Respir. Med. 1998;92:498–504. doi: 10.1016/S0954-6111(98)90298-0. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, et al. Validity of the Handheld Expiratory Flowmeter for COPD Screening in the Primary Care Setting of China. Int J. Chron. Obstruct Pulmon Dis. 2021;16:2039–2047. doi: 10.2147/COPD.S312190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickens AP, et al. Accuracy of Vitalograph lung monitor as a screening test for COPD in primary care. NPJ Prim. Care Respir. Med. 2020;30:2. doi: 10.1038/s41533-019-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueira Gonçalves JM, et al. Impact of body mass index on the predictive capacity of the COPD-6 device in the detection of airflow obstruction. Med. Clin. (Barc.) 2017;149:483–487. doi: 10.1016/j.medcli.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 40.Frith P, et al. Simplified COPD screening: validation of the PiKo-6® in primary care. Prim. Care Respir. J. 2011;20:190–198. doi: 10.4104/pcrj.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hidalgo Sierra V, et al. Usefulness of The Piko-6 Portable Device for Early COPD Detection in Primary Care. Arch. Bronconeumol. (Engl. Ed.) 2018;54:460–466. doi: 10.1016/j.arbr.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Hwang YI, et al. Cut-off value of FEV1/FEV6 to determine airflow limitation using handheld spirometry in subjects with risk of chronic obstructive pulmonary disease. Korean J. Intern. Med. 2021;36:629–635. doi: 10.3904/kjim.2019.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JK, et al. Active case finding strategy for chronic obstructive pulmonary disease with handheld spirometry. Medicine. 2016;95:e5683. doi: 10.1097/MD.0000000000005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi S, et al. Early Detection of Chronic Obstructive Pulmonary Disease in Primary Care. Intern. Med. 2017;56:3153–3158. doi: 10.2169/internalmedicine.8717-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XF, et al. The feasibility exploration of portable spirometer in COPD community screening. J. Clin. Pulmon. Med. 2020;25:834–838. [Google Scholar]
- 46.Lin CH, et al. Novel App-Based Portable Spirometer for the Early Detection of COPD. Diagnostics (Basel) 2021;11:785. doi: 10.3390/diagnostics11050785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Llordés M, et al. Which is the Best Screening Strategy for COPD among Smokers in Primary Care? COPD. 2017;14:43–51. doi: 10.1080/15412555.2016.1239703. [DOI] [PubMed] [Google Scholar]
- 48.Ng SC, et al. “Comparison between FEV1/FEV6 and FEV1/FVC as screening of chronic obstructive pulmonary disease.”. Med. J. Malays. 2017;72:286–290. [PubMed] [Google Scholar]
- 49.Nishimura K, et al. Case identification of subjects with airflow limitations using the handheld spirometer “Hi-Checker™”: comparison against an electronic desktop spirometer. COPD. 2011;8:450–455. doi: 10.3109/15412555.2011.626817. [DOI] [PubMed] [Google Scholar]
- 50.Represas CR, et al. Assessment of the portable COPD-6 device for detecting obstructive airway diseases. Arch. Bronconeumol. 2010;46:426–432. doi: 10.1016/j.arbres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Represas-Represas C, et al. Screening for chronic obstructive pulmonary disease: validity and reliability of a portable device in non-specialized healthcare settings. PLoS One. 2016;11:e0145571. doi: 10.1371/journal.pone.0145571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sami R, Omidi A, Sadegh R. Validity and Reliability of COPD-6 Device for Detecting Chronic Obstructive Pulmonary Disease in High-Risk Individuals. Tanaffos. 2020;19:201–207. [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider A, et al. Diagnostic accuracy of spirometry in primary care. BMC Pulm. Med. 2009;9:31. doi: 10.1186/1471-2466-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sichletidis L, et al. A combination of the IPAG questionnaire and PiKo-6® flow meter is a valuable screening tool for COPD in the primary care setting. Prim. Care Respir. J. 2011;20:184–189. doi: 10.4104/pcrj.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorn J, et al. Improved prediction of COPD in at-risk patients using lung function pre-screening in primary care: a real-life study and cost-effectiveness analysis. Prim. Care Respir. J. 2012;21:159–166. doi: 10.4104/pcrj.2011.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toda R, et al. Validation of “lung age” measured by spirometry and handy electronic FEV1/FEV6 meter in pulmonary diseases. Intern Med. 2009;48:513–521. doi: 10.2169/internalmedicine.48.1781. [DOI] [PubMed] [Google Scholar]
- 57.van den Bemt L, et al. Diagnostic accuracy of pre-bronchodilator FEV1/FEV6 from microspirometry to detect airflow obstruction in primary care: a randomised cross-sectional study. NPJ Prim. Care Respir. Med. 2014;24:14033. doi: 10.1038/npjpcrm.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang XY, et al. The application value of using a portable spirometer to screen COPD among tobacco-exposed populations. Int J. Respir. 2018;38:1381–1385. [Google Scholar]
- 59.The United Nations Development Programme (UNDP). Human Development Report 2020 | UNDP HDR.http://report.hdr.undp.org/ (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.