Abstract
BACKGROUND AND OBJECTIVES:
Experimental data suggest that trace elements, such as arsenic (As), cadmium (Cd), and selenium (Se) can influence the bone remodeling process. We evaluated the cross-sectional association between As, Cd, and Se biomarkers with bone mineral density (BMD) measured at the calcaneus, in a representative sample of a general population from Spain. As secondary analyses we evaluated the associations of interest in subgroups defined by well-established BMD determinants, and also conducted prospective analysis of osteoporosis-related incident bone fractures restricted to participants older than 50 years-old.
METHODS:
In N=1365 Hortega Study participants > 20 years-old, urine As and Cd were measured by inductively coupled-plasma mass spectrometry (ICPMS); plasma Se was measured by atomic absorption spectrometry (AAS) with graphite furnace; and BMD at the calcaneus was measured using the Peripheral Instaneuous X-ray Imaging system (PIXI). As levels were corrected for arsenobetaine (Asb) to account for inorganic As exposure.
RESULTS:
The median of total urine As, Asb-corrected urine As, urine Cd, and plasma Se was 61.3, 6.53 and 0.39 μg/g creatinine, and 84.9 μg/L, respectively. In cross-sectional analysis, urine As and Cd were not associated with reduced BMD (T-score < −1 SD). We observed a non-linear dose-response of Se and reduced BMD, showing an inverse association below ~105 μg/L, which became increasingly positive above ~105 μg/L. The evaluated subgroups did not show differential associations. In prospective analysis, while we also observed a U-shape dose-response of selenium with the incidence of osteoporosis-related bone fractures, the positive association above ~105 μg/L was markedly stronger, compared to the cross-sectional analysis.
CONCLUSIONS:
Our results support that Se, but not As and Cd, was associated to BMD-related disease. The association of Se and BMD-related disease was non-linear, including a strong positive association with osteoporosis-related bone fractures risk at the higher Se exposure range. Considering the substantial burden of bone loss in elderly populations, additional large prospective studies are needed to confirm the relevance of our findings to bone loss prevention in the population depending on Se exposure levels.
Keywords: Bone mineral density, arsenic, cadmium, selenium, osteoporosis
INTRODUCTION
Osteoporosis, a systemic skeletal disorder with bone remodeling failure resulting in microarchitecture and bone mass loss [1], is a major Public Health problem due to its high prevalence. In the US, the National Health and Nutrition Examination Survey (NHANES) (2013–2014) estimated that 10–17% of women older than 50 years had prevalent osteporosis [2]. The corresponding prevalence in women older than 70 years old was 39% [3]. In 2010, 22 million women and 5.5 million men were estimated to have osteoporosis in the European Union [4]. Osteoporosis-associated complications such as increased fracture risk, disability and mortality, have a substantial burden of disease in elderly populations [5,6]. While, recent osteoporosis prevalence trends are unclear [7–10], the projections indicated that the cost associated to osteoporosis burden of disease is expected to increase by a 25% in 2025 in the EU [4]. Numerous factors (genetic, nutritional, metabolic and endocrine, including menopause) are associated to osteoporosis [11,12]. Osteoporosis determinants other than age and menopause, however, are not completely understood. The identification of novel factors such as environmental determinants that could provide support for bone fragility prevention and control is needed.
Trace elements, such as As, Cd, and Se could influence the bone remodeling process [13,14]. Chronic exposure to Cd, a well-established nephrotoxicant [15], can alter renal function, including vitamin D activation [16], which has a key role in bone metabolism and mineralization. In addition, Cd decreases intestinal absorption of calcium in the enterocyte which promotes secondary hyperparathyroidism and bone resorption [17], and also stimulates osteoblast (cells related to bone formation) apoptosis and autophagy [18]. As has been associated with a decreased osteoblast proliferation and increased osteoclasts multiplication (cells related to bone resorption) [14], possibly due to the As-induced decrease in transcription factors expression in osteoclasts (RUNX2, BMP2, osteocalcin) and osteoblasts (RANKL expression), resulting in alterations of both cortical and trabecular bone microarchitecture [19,20]. The essential nutrient Se is a key cofactor of antioxidant proteins and reactive oxygen species (ROS) are involved in osteoclasts activation and osteoblasts differentiation [21–23]. Several studies have evaluated the association between these trace elements and bone disease [24–27] with mixed results. However, the non-linearity of the dose-response has rarely been evaluated, specially in the setting of prospective studies.
The Hortega Follow-Up Study investigates novel determinants of selected chronic conditions, especially cardiovascular, but also bone disease, in a population-based sample of a general population from a region of Spain. The study includes measures of bone mineral density (BMD) at the calcaneus bone using the Peripheral Instantaneous X-ray Imaging (PIXI) system. Peripherical densitometry has been associated to fractures risk, similarly to other established measures of osteoporosis such as central BMD determined at the lumbar spine or hip bones [28]. The predictive value of calcaneus BMD for osteoporosis screening has been validated in our study population [29]. We selected urine As and Cd, and plasma Se because they are well established biomarkers of exposure. While urine Cd reflects the long-term accumulated cadmium in the body [30], urine As [31] and plasma Se [32] reflect short-term exposure.
The main objective of this study was to evaluate the cross-sectional association between As, Cd and Se exposures with reduced BMD as determined in the calcaneus in Hortega Study participants. As secondary objectives we conducted subgroup analysis in biologically meaningful subgroups defined by sex, age, renal function, smoking and alcohol drinking status and physical activity, and also, a prospective analysis of time to event data after a 14-year follow-up to evaluate the incidence of osteoporosis-related bone fractures in the subset of participants older than 50 years.
METHODS
Study population
A population-based survey was carried out in public health system beneficiaries older than 20 years included in the Hospital Universitario Rio Hortega’s catchment area in Valladolid (Spain). The multi-stage complex sampling yielded a representative sample of the source population, which is an adult general population from Spain [33]. Among the 1502 baseline participants, we excluded 58 missing BMD values, 58 missing trace element biomarker determination, 2 missing smoking status, 1 missing body mass index and 18 missing physical exercise records, resulting in a study population of 1365 individuals for the cross-sectional analysis. Fractures are the most aparent manifestation of bone fragility, specially in individuals older than 50 years [34]. For prospective analysis of osteoporosis-related bone fractures in the subset of participants older than 50 years (N=702), we further excluded 49 participants who were considered losses to follow-up (i.e. there was only baseline, but not follow-up, information in their health records), and 15 participants with a personal history of osteoporosis-related bone fractures at baseline, resulting in a study population of 638 individuals.
Trace metals determinations
Total plasma metal and metalloid levels were measured in 2012 by atomic absorption spectrometry with graphite furnace on a Varian AAS 240 Zeeman (Varian Inc., US) at Cerba International Laboratories Ltd. 1 ml of urine was dissolved in 5 ml at 5% (v/v) HNO3 control of Ultra Metal Traces and 100ng/ml of Rh aqueous solution was added as an internal standard for the samples and their calibrators. The quality control of the analysis was conducted using the lyophilized Clincheck urine control reference material (RECIPE) Level I and Level II, and Material Standard Reference 2670 for urine toxic metals from the National Institute of Standards and Technology (NIST). The lower detection limit of detection and coefficient of variation (CV) for plasma Se were 29.9 μg/L and 5.6 %, with all individuals showing detectable concentrations.
Urine trace elements biomarkers were measured in the Laboratory of Environmental Chemistry and Bioanalysis of Huelva University (Spain) in 2016. Total urine Cd and As levels were determined by inductively coupled-plasma mass spectrometry (ICPMS) on an Agilent 7500Cex ICPMS (Agilent Technologies) equipped with an octapole collision cell (Agilent Technologies, Japan). The limits of detection (and corresponding CV) were 0.0005 μg/L (5.2%) for Cd and 0.024 μg/L (6.5%) for As. All individuals showed detectable concentrations of urinary Cd and As. Urine As species concentrations, including arsenite (AsIII), arsenate (AsV), methylarsonate (MMA), dimethylarsinate (DMA) and arsenobetaine (Asb), were determined by ICPMS Thermo XSeries2 (Thermo Scientific, Germany) equipped with an octapole reaction cell, coupled to an anion exchange liquid chromatography (IEC-HPLC) system on a Agilent 1100 (Agilent, USA). The As speciation occurred only in a random subsample of 295 individuals of study population.
Asb, an organic As specie, is mostly found in seafood and it is considered non-toxic for human health [35]. In populations with significant seafood intake, as in Spain, it is necessary to account for the contribution of organic As in the interpretation of urinary As concentrations as a biomarker of inorganic As exposure [36]. In a previous report within the Hortega Study [37], we used the As speciation information available in the random subsample of our study participants to generate a distribution of Asb imputed values (for the participants with As speciation missing completely at random [MCAR]) as the 50th percentile of each subject-specific posterior distribution obtained from a Markov Chain Monte Carlo (MCMC) by Gibbs sampling nested linear model. This MCMC model and all the details regarding its development and implementation have been published [37]. In the present analysis, we used the complete dataset including observed and imputed MCAR Asb values to calculate a biomarker for total urine As concentrations not derived from seafood by regressing total urine As concetrations on Asb using a residual-based method [38]. To have levels of As exposure that are meaningful for the population, the marginal mean of total As concentrations among participants with low Asb (defined as inviduals below the second percentile of As distribution [4.78 μg/L]) was added to the residuals.
Bone mineral density-related endpoints.
Peripheral BMD.
A densitometry of the calcaneus was performed on the right calcaneus using the Peripheral Instataneous X-ray Imaging System (PIXI). Calibration and quality assurance testing of scanners were performed daily, both of which were always within the limits set by the software. The results were expressed in grams per square centimeters. We then calculated the T-score for each sex based on a reference Spanish population provided by the manufacturers. Reduced BMD was defined as a T- score lower than one standard deviation from the mean (−1 SD), which yielded the highest sensitivity to identify osteoporosis in our study population compared to the gold standard (central DEXA as measured in the hip bone) [29].
Incidence of osteoporosis-related bone fractures.
In the Hortega Study we have available information on osteoporotic incident fractures (mainly, hip, humerus, vertebral and Colles) and associated time to event based on the date of the first pathological imaging technique (including X-ray, CT scan or NMR) from review of clinical records for a 14-year follow-up [33]. Follow-up time was calculated in years from the date of baseline visit to the date of pathological imaging for patients with osteoporosis-related bone fracture during the follow-up (i.e cases) and, for those without bone fracture (i.e non-cases), to the date of death, if death happened during the follow-up, or the date of the administrative censoring (Novermber 30, 2015).
Other relevant variables
Sociodemographics data, such as sex, age, education (< high school, > or equal to high school) and lifestyle habits including physical activity, diet, drinking and smoking intake were collected by questionaires administered by trained staff in an in-person interview and physical examination. Physical activity questions included information on the type and frequency of walking and physically active hobbies, sports, or exercises, including jogging or running, riding a bicycle or an exercise bicycle, swimming, aerobic dancing, other dancing, calisthenics or floor exercises, gardening or yard work, and weight lifting. Open-ended questions assessed information on physical activities not previously listed. Physical activity was estimated in metabolic equivalents (METs) per minute/week based on standardized intensity scores [39]. For descriptive and interaction pourposes we categorized physical activity below and above 3000 METs minute/week, since it has been shown that lower risk for diabetes, stroke and other outcomes occurred specially above 3000 METs minute/week [40]. Smoking and alcohol consumption status were classified as former, current and never. Urine cotinine was measured by enzyme-linked immunosorbent assay (ELISA) (“Análisis DRI® Cotinine” Kit, Ref. 0395 Microgenics laboratories). Concentrations below the limit (34 ng/mL) were detected in 77% of the participants. Body mass index (BMI) was calculated using measured weight (kilograms) by height (meters) squared. Urine and serum creatinine were measured by the modified kinetic Jaffé method by isotope dilution mass spectrometry on a Hitachi 917 analyzer (Rocher, Boheringer, Germany). The glomerular filtration rate was estimated based on serum creatinine determinations (eGFR) by the abbreviated CKD-EPI equation [41]. Chronic kidney disease (CKD) was defined as glomerular filtration rate lower than 60 ml/min/1.73m2.
The research protocol was approved by ethical committee of the Hospital Universitario Rio Hortega of Valladolid.
Statistical Analysis
Descriptive analysis.
All analyses were weighted to reflect the distribution of participant characteristics in the underlying source population. Urine biomarkers in μg/L were divided by urine creatinine in g/L to account for the dilution of urine. Trace elements levels were log-transformed. We described the median and interquartile range of each trace element distribution across participant characteristics. Age and sex are strong, non-modifiable, determinants of BMD. Thus, to describe other BMD determinants independently of age and sex, we estimated summary statistics (i. e. marginal proportions and means) of participant characteristics overall and by BMD categories using age and sex-adjusted generalized linear models. Cutoffs for trace metals and BMD percentiles were based on the weighted distribution in the study sample.
Association and subgroup analysis.
We evaluated the association of log-transformed trace elements levels (as independent variables in separate models for Asb-corrected As, Cd and Se) with reduced BMD (T-score < −1 SD) by using logistic regression models. Trace element concentrations were introduced in the models as tertiles comparing each of the 2 highest tertiles of trace elements with the lowest tertile, or as a log-transformed (continuous) variable for an interquantile range comparison. We conducted two progressively adjusted statistical models. Model 1 was adjusted for age, sex, education (>high school, <high school) and body mass index (BMI). Model 2 was additionally adjusted for physical activity (METs min/week), eGFR, alcohol intake (never, former, current), cotitine (<34, 34–500, ≥500 ng/mL), cumulative smoking (packs-years) and smoking status (never, former, current). In preliminary analysis, we also adjusted for meat and fish consumption with no substantial change in the observed estimates. These variables had a number of missing values and were, thus, not included in subsequent analysis. We evaluated differential associations in subgroups defined by biologically relevant covariates, including sex (men and women), age (< 50 and ≥ 50 years), smoking status (never and ever smoking), alcohol intake (never and ever drinking), eGFR (>60 and ≤ 60 ml/min/1.23 m2) and physical activity (<3000 and ≥3000 METs min/week).
Prospective analysis.
We repeated the cross-sectional associations for reduced BMD and also evaluated osteoporosis-related fractures risk associated to Se among individuals older than 50 years. The reason to restrict the prospective analysis to older participants was that the number of osteoporosis-related fractures in individuals below 50 years is low. The prospective analysis was performed using multi-adjusted cox proportional regression models with urine Asb-corrected As, urine Cd and plasma Se as independent variables in separate models, with age introduced in the survival models as the time scale.
All statistical analyses were conducted with the “survey” package in R software (R Studio, version 1.1) to account for the complex sampling design.
RESULTS
Descriptive analysis.
The median of urine As, Asb-corrected As and Cd and plasma Se was 61.3, 6.53, and 0.39 μg/g creatinine and 84.9 μg/L, respectively (Table 1). In this study, participants with higher levels of Asb-corrected As and Cd were somewhat older, males, with lower BMI and less physically active. Former and current smokers had higher urine cadmium levels. Among never smokers, median levels of cadmium were 0.3 μg/g creatinine for men and 0.4 μg/g creatinine for women. Se levels were lower in women compared to men. The average calcaneus BMD was 0.47 g/cm2 in women and 0.59 g/cm2 in men. The weighted % of participants with reduced BMD was 28.4 overall, and 41.9% among participants older than 50 years (data not shown). Participants with higher BMD in the calcaneus tended to be younger, mostly males, with higher BMI, higher education, with decreased cumulative smoking and more physically active (Table 2).
Table 1.
Median (interquartile range) of urine As, Asb-corrected As and Cd (μg/g), and plasma Se (μg/l) levels by participants’ characteristics (n=1365).
| N | U. As | U. As (Asb-corrected) | U. Cd | P. Se | |
|---|---|---|---|---|---|
| Overall | 1365 | 61.3 (23.4, 176.9) | 6.53 (4.05, 11.38) | 0.39 (0.23, 0.65) | 84.9 (72.3, 100.1) |
| Age, years | |||||
| <50 | 663 | 49.5 (20.3, 132.7) | 6.07 (3.92, 10.87) | 0.37 (0.22, 0.64) | 84.2 (72.5, 101) |
| 50–65 | 212 | 81.3 (32.6, 223) | 7.33 (4.60, 12.32) | 0.43 (0.26, 0.70) | 88.4 (72.6, 99.4) |
| ≥65 | 490 | 104.6 (34.2, 287.4) | 6.74 (4.04, 12.09) | 0.39 (0.23, 0.66) | 83.9 (71.9, 98.5) |
| Sex | |||||
| Male | 684 | 69.1 (25.3, 189.6) | 6.57 (4.26, 11.39) | 0.42 (0.25, 0.68) | 87.1 (73.7, 103.0) |
| Women | 681 | 54.0 (21.6, 162.1) | 6.31 (3.75, 11.21) | 0.37 (0.20, 0.61) | 83.2 (71.5, 97.6) |
| BMI, kg/m2 | |||||
| <30 | 1115 | 61.7 (23.4, 178.8) | 6.62 (4.15, 11.82) | 0.39 (0.23, 0.66) | 84.8 (72.7, 100.3) |
| ≥30 | 250 | 58.6 (23.8, 157.7) | 5.89 (3.57, 9.92) | 0.38 (0.21, 0.65) | 85.0 (70.8, 98.6) |
| Smoking status | |||||
| Never | 640 | 57.1 (22.9, 188.4) | 6.31 (3.86, 10.83) | 0.35 (0.2, 0.59) | 84.3 (72.1, 98.7) |
| Former | 407 | 80.1 (27.1, 205.2) | 6.69 (4.32, 12.43) | 0.41 (0.25, 0.70) | 85.5 (71.9, 100.5) |
| Current | 318 | 53.4 (22.2, 135.9) | 6.31 (4.13, 11.86) | 0.45 (0.26, 0.69) | 85.0 (72.9, 100.6) |
| Cumulative smoking, pack-year | |||||
| 0 | 654 | 57.2 (22.3, 189) | 6.31 (3.89, 10.89) | 0.35 (0.2, 0.59) | 84.1 (71.9, 98.6) |
| 0–12 | 361 | 52.4 (20.4, 157.9) | 6.36 (4.09, 11.81) | 0.37 (0.23, 0.63) | 84.6 (70.8, 100.6) |
| ≥12 | 350 | 75.7 (32, 185.9) | 6.86 (4.28, 12.02) | 0.52 (0.3, 0.80) | 86.8 (74.2, 100.9) |
| Urine cotinine mg/dl | |||||
| <34 | 1048 | 64 (23.7, 200.7) | 6.55 (4.02, 11.14) | 0.37 (0.22, 0.62) | 84.3 (71.9, 99.6) |
| 34–500 | 72 | 50.5 (18.8, 126.3) | 4.99 (2.65, 9.80) | 0.31 (0.19, 0.63) | 91.3 (76.7, 101.6) |
| ≥500 | 245 | 56.7 (22.6, 135.5) | 6.62 (4.49, 12.07) | 0.48 (0.27, 0.78) | 84.3 (71.3, 100.1) |
| Alcohol intake | |||||
| Never | 560 | 57.2 (22.8, 165.4) | 6.46 (3.78, 11.70) | 0.40 (0.23, 0.63) | 84.1 (71.9, 99.3) |
| Former | 113 | 54.5 (17.6, 222.2) | 6.38 (4.25, 10.45) | 0.37 (0.23, 0.66) | 84.3 (72.9, 99.5) |
| Current | 692 | 63.3 (24.3, 178.7) | 6.54 (4.17, 11.29) | 0.38 (0.23, 0.66) | 85.1 (72.3, 100.5) |
| eGFR, ml/min/1.73m2 | |||||
| ≥60 | 1227 | 61.0 (23.4, 176.7) | 6.61 (4.15, 11.58) | 0.40 (0.23, 0.66) | 84.8 (72.3, 100.3) |
| <60 | 138 | 66.1 (22.9, 187.0) | 4.92 (3.04, 9.18) | 0.33 (0.19, 0.59) | 85.0 (71.5, 96.0) |
| High education | |||||
| No | 382 | 72.2 (25.7, 236.6) | 6.55 (3.77, 11.23) | 0.40 (0.23, 0.64) | 85.1 (71.2, 98.8) |
| Yes | 983 | 59.2 (22.5, 163.4) | 6.48 (4.08, 11.44) | 0.39 (0.23, 0.65) | 84.7 (72.8, 100.5) |
| Physical activity, METs min/week | |||||
| <3000 | 844 | 62.5 (25.1, 183.2) | 6.56 (4.16, 11.20) | 0.40 (0.23, 0.67) | 84.3 (72.3, 98.7) |
| ≥3000 | 521 | 58.8 (20.9, 164.9) | 6.34 (3.86, 11.41) | 0.37 (0.23, 0.63) | 85.4 (72.1, 101.8) |
Abbreviations: U. urine, P. plasma; Asb, arsenobetaine; eGFR, estimated glomerular filtration rate
Table 2.
Age and gender-adjusted* baseline characteristics of participants by BMD (n=1365).
| BMD in the calcaneus (g/cm2) | |||||
|---|---|---|---|---|---|
| Overall | ≤0.33 | ≥0.33–0.66 | >0.66 | P-value | |
| N=1365 | N=445 | N=465 | N=445 | ||
| Age, years; mean (SE) | 48.69 (0.30) | 56.15 (0.73) | 47.24 (0.82) | 43.96 (1.30) | <0.001 |
| Women; % (SE) | 51.0 (1.6) | 82.4 (5.2) | 53.4 (5.4) | 21.0 (4.7) | <0.001 |
| BMI, kg/m2; mean (SE) | 26.15 (0.30) | 24.36 (0.50) | 25.94 (0.46) | 27.64 (0.67) | <0.001 |
| Smoking status | |||||
| Never; % (SE) | 44.7 (4.1) | 36.3 (7.6) | 41.5 (6.6) | 42.3 (7.7) | 0.27 |
| Former; % (SE) | 28.4 (3.4) | 28.3 (6.5) | 33.1 (5.6) | 31.1 (6.2) | 0.50 |
| Current; % (SE) | 27.0 (3.8) | 35.4 (7.5) | 25.4 (6.0) | 26.6 (7.2) | 0.07 |
| Cumulative smoking, pack-year; mean (SE) | 8.46 (0.88) | 10.85 (1.43) | 9.58 (1.47) | 9.23 (1.80) | 0.33 |
| Urine cotinine > 500 mg/dl; % (SE) | 1.48 (0.07) | 1.60 (0.13) | 1.47 (0.11) | 1.48 (0.14) | 0.10 |
| Alcohol intake status | |||||
| Never; % (SE) | 37.9 (3.8) | 38.5 (7.5) | 37.8 (6.2) | 35.0 (7.1) | 0.40 |
| Former; % (SE) | 8.7 (2.4) | 8.1 (4.3) | 9.4 (4.0) | 9.1 (4.6) | 0.78 |
| Current; % (SE) | 53.5 (4.0) | 53.5 (7.6) | 52.8 (6.5) | 55.9 (7.5) | 0.53 |
| eGFR < 60 ml/min/1.73m2; % (SE) | 7.0 (1.7) | 8.4 (3.5) | 6.0 (2.7) | 6.5 (3.1) | 0.61 |
| High education; % (SE) | 77.6 (2.2) | 75.6 (4.0) | 78.6 (3.8) | 81.0 (3.9) | 0.05 |
| Physical activity, METs min/week; mean (SE) | 3262 (275) | 2947 (366) | 3036 (393) | 3653 (676) | 0.02 |
| Urine As levels (μg/g); geometric mean (95% CI) | 66.25 (52.29, 83.95) | 58.50 (36.82, 92.96) | 74.00 (51.55, 106.21) | 72.31 (45.77, 114.24) | 0.11 |
| Urine Asb (μg/g); geometric mean (95% CI)** | 50.44 (30.74, 82.78) | 51.73 (21.82, 122.62) | 51.30 (24.41, 107.80) | 69.38 (26.17, 183.89) | 0.62 |
| Asb-corrected urine As (μg/g); geometric mean (95% CI) | 6.71 (5.80, 7.77) | 6.28 (4.75, 8.31) | 6.98 (5.51, 8.84) | 6.77 (5.13, 8.92) | 0.51 |
| Urine Cd (μg/g); geometric mean (95% CI) | 0.38 (0.33, 0.45) | 0.41 (0.30, 0.54) | 0.38 (0.30, 0.49) | 0.39 (0.30, 0.52) | 0.97 |
| Plasma Se (μg/l); geometric mean (95% CI) | 84.7 (81.2, 88.3) | 82.8 (76.6, 89.4) | 84.7 (79.8, 90.0) | 85.7 (78.7, 93.3) | 0.29 |
Age and sex are very strong, non modifiable, determinants of BMD. We, thus, accounted for sex and age because we wanted to descriptively assess other determinants independently than age and sex.
Subset of 284 participants with measured urine As species.
Abbreviatons: SE, standard error; eGFR, estimated glomerular filtration rate; CI, confidence interval
Association and subgroup analyses.
In cross-sectional analysis, urine As and Cd were not associated with reduced BMD. The fully adjusted odds ratio (OR [95% CI]) comparing the highest to the lowest tertiles of Asb-corrected As and Cd distributions were, respectively, 0.80 (0.58, 1.10) and 1.06 (0.77, 1.45) (Table 3). We observed a non-linear association between plasma Se levels and reduced BMD (p-value of nonlinearity = 0.01) (Table 3). Supplemental Figure 1 shows the flexible dose-response relationship for the evaluated trace elements with reduced BMD. We observed that the association for Se was inverse at plasma concentrations below ~105 μg/L and became increasingly positive at plasma Se concentrations above ~105 μg/L. The odds ratios (95% CI) of reduced BMD comparing the 80th to the 20th percentiles of plasma Se in non-linear models was 0.63 (0.44, 0.90). In subgroup analysis, we mostly observed no differencial associations by the subgroups evaluated (Supplemental Table 1).
Table 3.
Odds ratio (95% confidence interval) of reduced BMD, by Asb-corrected As, Cd and Se levels (n=1365).
| Cases/No cases | Model 1 | Model 2 | |
|---|---|---|---|
| Urine Asb-corrected As, μg/g | |||
| Tertil 1 | 149/304 | 1.00 (Reference) | 1.00 (Reference) |
| Tertil 2 | 152/303 | 0.78 (0.57, 1.08) | 0.78 (0.56, 1.07) |
| Tertil 3 | 147/310 | 0.82 (0.59, 1.14) | 0.80 (0.58, 1.10) |
| 80th vs 20th | 448/917 | 0.91 (0.74, 1.12) | 0.89 (0.72, 1.10) |
| p-value | 0.37 | 0.27 | |
| Urine Cd, μg/g | |||
| Tertil 1 | 143/308 | 1.00 (Reference) | 1.00 (Reference) |
| Tertil 2 | 134/320 | 0.78 (0.56, 1.09) | 0.78 (0.56, 1.09) |
| Tertil 3 | 171/289 | 1.09 (0.80, 1.49) | 1.06 (0.77, 1.45) |
| 80th vs 20th | 488/917 | 1.01 (0.83, 1.24) | 0.99 (0.81, 1.22) |
| p-value | 0.91 | 0.96 | |
| Plasma Se, μg/L | |||
| Tertil 1 | 163/297 | 1.00 (Reference) | 1.00 (Reference) |
| Tertil 2 | 142/315 | 0.81 (0.59, 1.11) | 0.80 (0.58, 1.11) |
| Tertil 3 | 143/305 | 0.83 (0.60, 1.14) | 0.79 (0.57, 1.09) |
| 80th vs 20th | 488/917 | 0.66 (0.46, 0.94)* | 0.63 (0.44, 0.90)* |
| p-value | 0.03* | 0.01* |
Model 1 adjusted for age (years), sex (men, women), BMI (kg/m2) and high education (no, yes).
Model 2 is model 1 further adjusted for physical activity (METs min/week), urine cotinine categories (<34, 34–500, >=500), glomerular filtration rate (ml/min/1.73m2), cumulative-tobacco smoking (pack-years), smoking status and alcohol intake status (never, former, current).
The tertiles cutoffs were 4.7 and 9.2 μg/g for Asb-corrected urine As, 0.27 and 0.54 μg/g for urine Cd, and 76.7 and 94.3 μg/L for plasma Se.
The 80th and 20th percentiles of urine biomarker distributions were 13.6 and 3.5 μg/g for Asb-corrected As and 0.76 and 0.20 μg/g for Cd. The corresponding percentiles of plasma Se distribution were 104.8 and 69.4 μg/L.
Association obtained from a regression models with plasma Se modelled as restricted quadratic splines with knots at the 10th, 50th and 90th percentiles. The p-value of non-linearity was obtained from a wald-test of the spline terms. Other p-values in the table were obtained from a wald test of the regression coefficient for log-transformed urine arsenic and cadmium.
Prospective analysis.
In analysis restricted to the 638 individuals older than 50 years, the number of newly diagnosed osteoporosis-related fractures and accumulated follow up was 66 and 7006.9 person-year, respectively. The dose-response relation between plasma Se and BMD -related endpoints was consistently non-linear in both the cross-sectional and prospective analysis, with a stronger positive association of selenium with the incidence of osteoporosis-related bone fractures observed above ~100 μg/L (Table 4 and Figure 1). The hazard ratio (95% CI) for incident fractures comparing the 80th to the 20th percentiles of plasma Se in non-linear models was 2.25 (1.13, 4.49) (p-value of non-linearity=0.01) (Table 4). We conducted a sensitivity analysis by excluding participants with reduced BMD at baseline (resulting in 20 cases and 329 non-cases), with consistent results (corresponding HR [95%CI] and p-value were 1.85 [1.08, 3.18] and 0.03, respectively).
Table 4.
Odds ratio (95% confidence interval) of reduced BMD and Hazard ratio (95% confidence interval) of fractures incidence, by urine Asb-corrected As, Cd and plasma Se levels in the subsample of individuals older than 50 years.
| OR (95% CI) of reduced BMD (N=702) | HR (95% CI) of incident osteoporosis-related fractures (N=638) | |||||
|---|---|---|---|---|---|---|
| Cases/No cases | Model 1a | Model 2b | Cases/No cases | Model 1 | Model 2 | |
| Urine Asb-corrected As | ||||||
| Tertil 1 | 100/108 | 1.00 (Reference) | 1.00 (Reference) | 25/183 | 1.00 (Reference) | 1.00 (Reference) |
| Tertil 2 | 119/130 | 0.91 (0.60, 1.38) | 0.89 (0.58, 1.35) | 19/204 | 0.90 (0.45, 1.79) | 0.80 (0.39, 1.65) |
| Tertil 3 | 105/140 | 0.75 (0.49, 1.15) | 0.72 (0.47, 1.10) | 22/185 | 1.26 (0.66, 2.4) | 1.27 (0.66, 2.45) |
| 80th vs 20th | 324/378 | 0.79 (0.60, 1.05) | 0.78 (0.59, 1.03) | 66/572 | 1.07 (0.71, 1.62) | 1.09 (0.71, 1.66) |
| p-value | 0.10 | 0.08 | 0.76 | 0.70 | ||
| Urine Cd | ||||||
| Tertil 1 | 99/118 | 1.00 (Reference) | 1.00 (Reference) | 26/171 | 1.00 (Reference) | 1.00 (Reference) |
| Tertil 2 | 97/137 | 0.77 (0.50, 1.20) | 0.75 (0.48, 1.17) | 22/195 | 0.97 (0.51, 1.83) | 0.99 (0.53, 1.85) |
| Tertil 3 | 128/123 | 1.14 (0.76, 1.72) | 1.14 (0.75, 1.73) | 18/206 | 0.75 (0.38, 1.46) | 0.75 (0.39, 1.46) |
| 80th vs 20th | 324/378 | 1.14 (0.86, 1.50) | 1.13 (0.85, 1.50) | 66/572 | 1.13 (0.81, 1.58) | 1.09 (0.80, 1.50) |
| p-value | 0.37 | 0.40 | 0.477 | 0.586 | ||
| Plasma Se | ||||||
| Tertil 1 | 122/117 | 1.00 (Reference) | 1.00 (Reference) | 20/196 | 1.00 (Reference) | 1.00 (Reference) |
| Tertil 2 | 102/133 | 0.70 (0.47, 1.06) | 0.68 (0.45, 1.03) | 18/191 | 1.03 (0.52, 2.01) | 1.09 (0.55, 2.16) |
| Tertil 3 | 100/128 | 0.75 (0.50, 1.13) | 0.70 (0.46, 1.06) | 28/185 | 1.44 (0.77, 2.7) | 1.67 (0.91, 3.04) |
| 80th vs 20th* | 324/378 | 0.61 (0.38, 0.97) | 0.57 (0.36, 0.91) | 66/572 | 1.96 (0.95, 4.06) | 2.25 (1.13, 4.49) |
| p-value* | 0.006 | 0.003 | 0.02 | 0.01 | ||
Model 1 adjusted by age (years), sex (men, women), BMI (kg/m2) and high education (no, yes).
Model 2 is model 1 further adjusted for physical activity (METs min/week), urine cotinine categories (<34, 34–500, ≥500), glomerular filtration rate (ml/min/1.73m2), cumulative-tobacco smoking (pack-years), smoking status, and alcohol intake status (never, former, current).
Urine As was adjusted for urine Asb. The 80th and 20th percentiles of urine biomarker distributions were 13.5 and 3.5 μg/g for Asb-corrected As and 0.76 and 0.20 μg/g for Cd. The corresponding percentiles of plasma Se distribution were 103.7 and 68.9 μg/L.
Association obtained from regression models with plasma Se modelled as restricted quadratic splines with knots at the 10th, 50th and 90th percentiles. The p-value of non-linearity was obtained from a wald-test of the spline terms. Other p-values in the table were obtained from a wald test of the regression coefficient for log-transformed urine arsenic and cadmium.
Figure 1. Odds ratio (95% CI) of reduced Bone Mineral Density and Hazard Ratio (95% CI) of Osteoporosis-Related Bone Fractures by plasma Se concentrations (μg/L) in the subsample of individuals older than 50 years (n= 702 and 638, for the cross-sectional and prospective analysis, respectively).
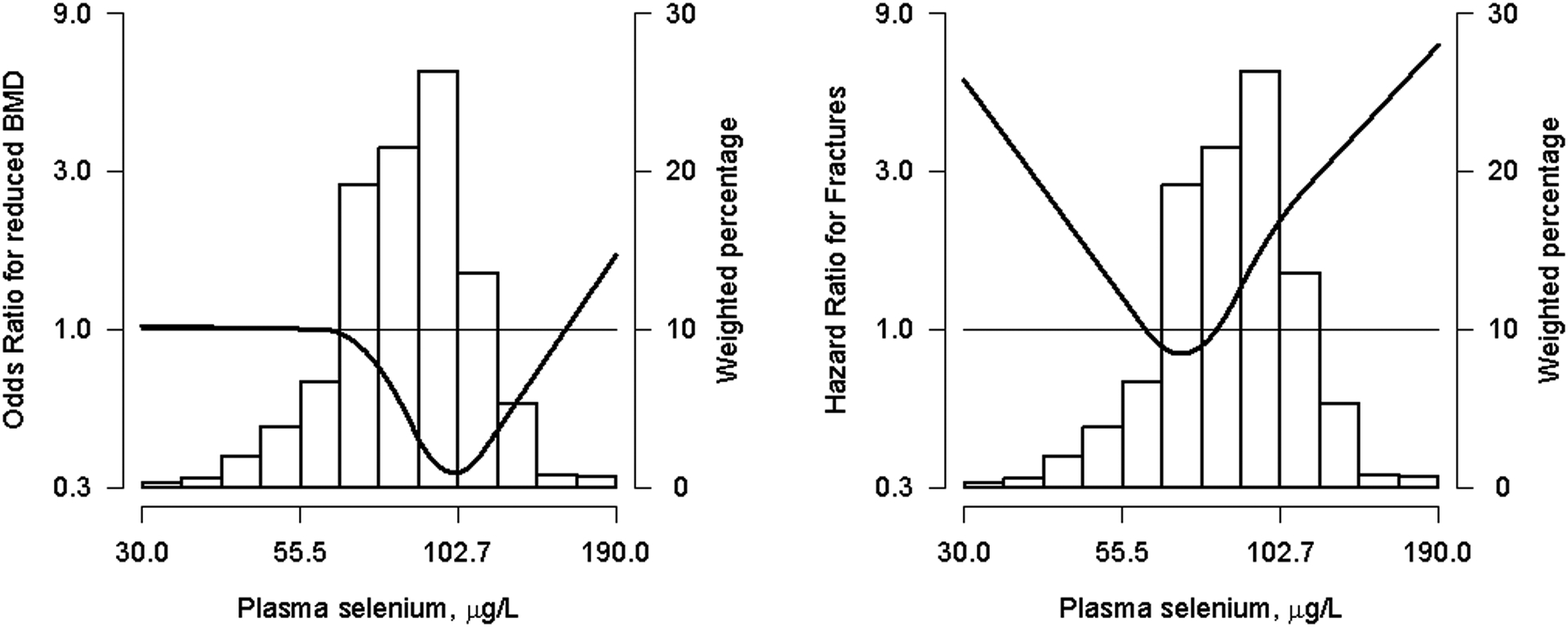
The curve (gray shades) represent the odds ratio (95% confidence interval) of reduced BMD levels (BMD below −1.0 SD) and hazard ratio (95% confidence interval) of osteoporosis-related fractures based on restricted quadratics splines with knots at the 10th, 50th and 90th percentiles of plasma Se distribution (29.92, 84.46 and 115 μg/L, respectively). The reference value was set at the 10th of plasma Se distribution (29.9 μg/L). Odds ratio and Hazard ratio were estimated with logistic and cox proportional hazards models, for the cross-sectional and prospective analysis, respectively. Models were adjusted for age, sex, education, body mass index, smoking status (never, former and current), cumultavie smoking (pack-years), urine cotinine levels (34, 34–500 y >500 ng/ml), alcohol intake (never, former and curent), glomerular filtration rate (ml/min/1.73m2) and physical activity (METs min/week). The histogram represents the frequency distribution of plasma Se in the study sample.
DISCUSSION
As and Cd exposure were not associated to bone disease in our study population. Our data suggest, however, a non-linear association of Se exposure with BMD-related endpoints. Below ~105 μg/L, the association of plasma Se with reduced BMD was inverse. Above 105 μg/L, the corresponding association became positive, being particularly strong for the risk of osteoporosis-related bone fractures among participants older than 50 years. There was no supportive evidence in favor of a differencial association of Se exposure with reduced BMD by the evaluated subgroups.
In 2001, a general population study used central densitometry by dual-energy X-ray absorptiometry (DEXA) to estimate that in Spain two million women and eight hundred thousand men have osteoporosis (the overall osteoporosis prevalence was 12.7% )[42]. We could not estimate a central-BMD based prevalence of osteoporosis in the present study, as we did not have available lumbar spine or femoral neck BMD measures in the complete study population. In a previous study, however, peripheral DEXA allowed to identify individuals with prevalent osteoporosis as diagnosed by central DEXA in our study population with consistent findings [29]. Other studies have also concluded that peripheral DEXA is a useful tool for osteoporosis identification [43,44]. Moreover, well-known determinants of BMD including age, sex, physical activity, smoke status and alcohol intake, are consistently associated with calcaneus BMD in our study population, which add robustness to our data.
Some human studies have evaluated the association of Se biomarkers and outcomes related to BMD and have found mixed results [24,25,45–50], possibly due to the small samples sizes, heterogeneous adjustment for confounders and different underlying selenium distributions. Importantly, while our data suggest that association of selenium and bone outcomes is non-linear, the potential non-linearity of selenium associations was not explored in previous studies. A case-control study from Iran (mean Se was 57.58 μg/L in 90 osteoporotic cases and 81.09 μg/L in 90 controls) showed moderate-to-strong positive correlations between high serum Se levels and high lumbar spine (r=0.63; p<0.001), and femoral neck (r= 0.69; p<0.001) BMD [24]. A cross-sectional in Dutch men (N=387, median plasma Se=91.9 μg/L) also found a positive significant association of Se with central BMD as measured in the hip bone [45]. Similarly, another study on 1144 postmenopausal European women (mean Se = 94.3 μg/L) observed a positive cross-sectional, but not prospective, association of Se with hip BMD [46]. In this study, lumbar spine BMD was not associated with plasma Se cross-sectionally nor prospectively, and no relationship with risk fracture was identified. Moreover, two case-control studies from China (N=91, mean plasma Se was ~134.8 μg/L) and from Turkey (N=107, mean plasma Se ~66.7 μg/L for each group), found no association between plasma Se and central BMD [25,47]. Other studies have evaluated the association between dietary Se intake and risk of osteoporotic fractures[48–50]. A large cross-sectional study in China (N= 6267) observed a stadistically significant association between dietary Se intake (mean ~39.1μg/day) and peripheral BMD, OR [95%CI]=0.47 [0.31,0.73], p<0.001, comparing the highest to the lowest quartiles)[48]. A case-control study in participants from the Utah Study of Nutrition and Bone Health (N=2564) (mean Se ~110 μg/day), found that increased Se intake levels were associated with lower risk of osteoporotic fractures, especially in ever smokers (OR [95% CI]=0.27 [0.12, 0.58], p<0.001) [49]. Consistently, another case-control study from China (N=1452, Se intake ~45 μg/day) reported an inverse association of Se with the risk of hip fracture (OR [95% CI]=0.43 [0.26, 0.70], p=0.005, comparing the highest to the lowest Se quartiles) [50]. In these studies, Se exposure was assessed based on self-reported dietary intake, which can undergo exposure miss-classification.
Additional dose-response studies are needed to reproduce our findings. Our results, however, are consistent with some experimental studies in rats showing that Se deficit decreased tibia [51] and cortical femur [52] bone density. Se inhibited the receptor activador of nuclear factor κ-B ligand (RANKL) production, which, essential for osteoclasts differentiation [21], and has been related to a beneficial effect on bone in rats [53]. Interestingly, an experimental study in rats with anatrozole treatment (an aromatase inhibitor that decreases estrogen production, which has been associated with a decrease in mineral bone) showed that supplemental Se nanoparticles decreased bone reduction using biomarkers such as TRAP, which is a specific marker for osteoclast cells function [54]. Additional animal studies evaluating Se excess, and not only deficiency, on bone disease, are needed.
Other studies point to redox mechanisms as biological link for Se-related associations with bone endpoints [22,23,55,56]. Specifically, selenoproteins play a main role in oxidative stress regulation and bone homeostasis maintenance [21,57]. Glutathione peroxidase and thioredoxin reductase (TrxR) are involved in redox mechanism, through peroxidase reduction, decrease ROS generation, which in experimental studies inhibited osteoclast differentiation and activity [21,55,57,58] Consistently, an experimental study in mice observed lower levels in GPx1 and percentage of femoral trabecular bone in the control group compared to Se supplementation group [56]. In rats, GPX1 expression enhanced the type I collagen and alkaline phosphatase expression, and the deposition of calcium in bone marrow stromal cells [21,57]. The TrxR gene has been found to be the responsive to the 1-α,25(OH)2-vitamin D3, which stimulates bone cell growth and differentiation [21]. Alternatively, selenoprotein P (SePP), another antioxidant selenoprotein, is involved in Se transport and storage [57]. Plasma SePP concentrations were positively associated to hip and lumbar spine BMD in the OPUS study [46], supporting that selenium transport into the bone marrow is important for bone homeostasis. While there is limited evidence assessing whether other selenoproteins are directly involved on bone metabolism, they likely have a role through redox balance regulation.
In selenium-replete populations, selenium intake above 55 μg/day level does not increase selenoprotein synthesis or activity [59–64]. In our study population, plasma Se levels below 110 μg/L were inversely associated with oxidative stress biomarkers including GSSG/GSH and MDA [65]. Above 110 μg/L of plasma Se, the association with GSSG/GSH reached a plateau, and was positive for 8-oxo-DG [65]. Indeed, GPx1, reaches the saturation point at ~110 μ/L [66], which, interestingly, approximates the inflection point in our dose response curves for selenium and reduced BMD. Thus, our data support the hypothesis that selenium above selenoproteins saturation levels may increase non-specific incorporation of selenomethionine into proteins [59], and induce alterations in osteoblast proliferation and differentiation, and, in osteoclast activity [21].
While a number of experimental studies provide biological support for As and Cd-related bone effects, we did not find statistically significant associations in our population-based study. It is known that at very high exposure levels Cd induces bone disease including osteopenia and osteomalacia [67]. At lower exposure levels, multiple studies in humans, including a meta-analysis of observational studies, have investigated the relation between Cd and osteoporosis-related endpoints with heterogeneous results [26,27,68,69]. Interestingly, the systematic review and meta-analysis [69] estimated that the pooled risk ratio of bone fracture was 1.30 (95% IC [1.13, 1.49]) when comparing the highest to the lowest Cd exposure categories. Nevertheless, these results are to be taken cautiously since the number of meta-analized studies was low (N=8), and there was heterogeneous adjustment for confounders. In addition, different biomarkers were used to assess Cd exposure (one study measured Cd in erythrocytes [70], three considered dietary Cd [71–73] and four used urine Cd [74–77]. Three of the studies using urinary biomarkers showed higher Cd levels than in our data (mean urinary cadmium levels were 11.18 μg/g and 3.55 μg/g in high and medium exposures areas in China [77], 0.74 μg/g in Swedish individuals [76] and 16.9 μg/day in a Belgian population [75]). Nonetheless, all the individual studies in the meta-analyses showed a statistically significant association between Cd exposure and fracture risk, including some studies conducted in postmenopausal women and participants older than 50 years. In post-hoc analysis, we observed a trend towards a positive association of cadmium with reduced BMD above 105 μg/L of selenium (OR= 0.93 [0.62,1.38] and 1.14 [0.50, 2.63] in participants with plasma selenium <105μg/L and ≥105μg/L, respectively). However, the p-interaction clearly was not statistically significant (p interaction=0. 94) and the confidence intervals are wide. These results, thus, do not support that the association between cadmium levels and bone mineral density is differential by selenium status in our study population.
For As, bone disease has not been described as a typical manifestation of Arsenicosis in areas with disproportionate exposure [78]. At lower exposure levels, two case-control studies from Turkey (N=336, median hair As=1.01 μg/g) [79], and Korea (n=985, mean urinary As ~ 8.9 μg/g) [80] reported no statistically significant associations of As with central and peripheral BMD, respectively. No other epidemiological studies have evaluated the association between As and BMD-related outcomes at the lower exposure range.
Our study is not exempt of limitations. For instance, total plasma Se levels do not provide information of individual Se species. More detailed analyses of specific selenoproteins levels and activity would allow to better understand the relation of Se with bone disease. Another limitation is the lack of information about central BMD measure for the whole study population. However, a calcaneus T-score <−1.0 showed a sensitivity of 85% and a negative predictive value of 79% for the identification of participants with osteoporosis in a small subset of study population [29]. The T-score cut-off associated with the highest specificity in our study population was −2.5 SD. However, only 57 participants in our study population showed calcaneus BMD levels below this cut-off [29]. Finally, we cannot discard residual confounding in our results as in other observational studies. For instance, the menopause status of women in our study population was unknown. Nonetheless, our data does not support a differential association of Se and reduced BMD in women or individuals younger than 50 years old. Conversely, an important strength of our data is that the study population is a representative sample of a general population. Another strength is the availability of both prevalent and incident BMD-related endpoints, and well-established trace elements biomarkers of exposure, which have been obtained by standardised protocols including a rigorous quality control.
CONCLUSIONS
In conclusion, Cd and As exposure were not associated with BMD loss. For Se, however, we observed a non-linear association with BMD endpoints, including a markedly strong positive association with fractures risk at the higher Se exposure range among individuals older than 50 years. Our findigs are compatible with a role of redox unbalance in explaining Se-associated bone disease. Given the substantial impact of bone mass loss in the elderly population, additional large prospective studies are needed to confirm the relevance of our findings to bone loss prevention in the population depending on Se exposure levels.
Supplementary Material
Funding
This work was supported by the Strategic Action for Research in Health sciences [CP12/03080, PI15/00071, PI10/0082, PI13/01848, PI14/00874, PI16/01402 and PI11/00726], the State Agency for Research (PID2019-108973RB-C21 and C22), the Valencia Government (GRUPOS 03/101; PROMETEO/2009/029 and ACOMP/2013/039), the Castilla-Leon Government (GRS/279/A/08) and European Network of Excellence Ingenious Hypercare (EPSS- 037093) from the European Commission; CIBER Fisiopatología Obesidad y Nutrición (CIBEROBN) (CIBER-02-08-2009, CB06/03 and CB12/03/30016). The Strategic Action for Research in Health sciences, CIBEROBN are initiatives from Carlos III Health Institute Madrid and co-funded with European Funds for Regional Development (FEDER). The State Agency for Research and Carlos III Health Institue belong to the Spanish Ministry of Science and Innovation. The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. MGP received the support of a fellowship from “la Caixa” Foundation (ID 100010434, fellowship code LCF/BQ/IN18/11660001).
Abbreviations
- BMD
bone mineral density
- ROS
reactive oxygen species
- As
arsenic
- Cd
cadmium
- Se
selenium
- Asb
arsenobetaine
- NMR
nuclear magnetic resonance
- DEXA
dual-energy x-ray absorptiometry
- ICPMS
inductively coupled-plasma mass spectrometry
- AAS
atomic absorption spectrometry
- PIXI
peripheral instaneuous x-ray imaging system
- NIST
National Institute of Standards and Technology
- As III
arsenite
- AsV
arsenate
- MMA
methylarsonate
- DMA
dimethylarsinate
Footnotes
Declaration of interest None to be declared
REFERENCES
- [1].Klibanski A, Adams-Campbell L, Bassford T, Blair SN, Boden SD, Dickersin K, Gifford DR, Glasse L, Goldring SR, Hruska K, Johnson SR, McCauley LK, Russell WE, Osteoporosis prevention, diagnosis, and therapy, J. Am. Med. Assoc 285 (2001) 785–795. 10.1001/jama.285.6.785. [DOI] [Google Scholar]
- [2].Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA, Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014, Osteoporos. Int 28 (2017) 1979–1988. 10.1007/s00198-017-3996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duan W, Meng X, Sun Y, Jia C, Association between polycyclic aromatic hydrocarbons and osteoporosis: data from NHANES, 2005–2014, Arch. Osteoporos 13 (2018) 112. 10.1007/s11657-018-0527-4. [DOI] [PubMed] [Google Scholar]
- [4].Svedbom A, McCloskey EV, Ivergård M, Kanis JA, Stenmark J, Hernlund E, Jönsson B, Cooper C, Compston J, Osteoporosis in the European Union: medical management, epidemiology and economic burden, Arch. Osteoporos 8 (2013). 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johnell O, Kanis J, Epidemiology of osteoporotic fractures, Osteoporos. Int 16 (2005) S3–S7. 10.1002/9781119266594.ch50. [DOI] [PubMed] [Google Scholar]
- [6].Sànchez-Riera L, Carnahan E, Vos T, Veerman L, Norman R, Lim SS, Hoy D, Smith E, Wilson N, Nolla JM, Chen JS, Macara M, Kamalaraj N, Li Y, Kok C, Santos-Hernańdez C, March L, The global burden attributable to low bone mineral density, Ann. Rheum. Dis 73 (2014) 1635–1645. 10.1136/annrheumdis-2013-204320. [DOI] [PubMed] [Google Scholar]
- [7].Xu Y, Wu Q, Decreasing trend of bone mineral density in US multiethnic population: analysis of continuous NHANES 2005–2014, Osteoporos. Int 29 (2018) 2437–2446. 10.1007/s00198-018-4648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tarantino U, Piscitelli P, Feola M, Neglia C, Rao C, Gimigliano F, Iolascon G, Decreasing trend of hip fractures incidence in Italy between 2007 and 2014: epidemiological changes due to population aging, Arch. Osteoporos 13 (2018) 23. 10.1007/s11657-018-0423-y. [DOI] [PubMed] [Google Scholar]
- [9].Trajanoska K, Schoufour JD, de Jonge EAL, Kieboom BCT, Mulder M, Stricker BH, Voortman T, Uitterlinden AG, Oei EHG, Arfan Ikram M, Carola Zillikens M, Rivadeneira F, Oei L, Fracture incidence and secular trends between 1989 and 2013 in a population based cohort: The Rotterdam Study, Bone. 114 (2018) 116–124. 10.1016/j.bone.2018.06.004. [DOI] [PubMed] [Google Scholar]
- [10].Mazzucchelli Esteban R, Pérez-Fernández E, Crespí-Villarías N, García-Vadillo A, Rodriguez-Caravaca G, Gil de Miguel A, Carmona L, Trends in osteoporotic hip fracture epidemiology over a 17-year period in a Spanish population: Alcorcón 1999–2015., Arch. Osteoporos 12 (2017) 84. 10.1007/s11657-017-0376-6. [DOI] [PubMed] [Google Scholar]
- [11].Yedavally-Yellayi S, Ho AM, Patalinghug EM, Update on Osteoporosis, Prim. Care 46 (2019) 175–190. 10.1016/j.pop.2018.10.014. [DOI] [PubMed] [Google Scholar]
- [12].Compston JE, McClung MR, Leslie WD, Osteoporosis, Lancet. 393 (2019) 364–376. 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- [13].Rodríguez J, Mandalunis PM, A Review of Metal Exposure and Its Effects on Bone Health, J. Toxicol (2018) 1–11. 10.1155/2018/4854152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gaffney-Stomberg E, The Impact of trace minerals on bone metabolism, Biol. Trace Elem. Res 188 (2019) 26–34. 10.1007/s12011-018-1583-8. [DOI] [PubMed] [Google Scholar]
- [15].Nordberg GF, Fowler BA, Nordberg M, Friberg LT, Handbook on the Toxicology of Metals, 3rd ed., 2007. [Google Scholar]
- [16].Rodríguez J, Mandalunis PM, Effect of cadmium on bone tissue in growing animals., Exp. Toxicol. Pathol 68 (2016) 391–7. 10.1016/j.etp.2016.06.001. [DOI] [PubMed] [Google Scholar]
- [17].Ibrahim KS, Beshir S, Shahy EM, Shaheen WA, Effect of occupational cadmium exposure on parathyroid gland, Maced. J. Med. Sci 4 (2016) 302–306. 10.3889/oamjms.2016.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bhattacharyya MH, Cadmium osteotoxicity in experimental animals: mechanisms and relationship to human exposures., Toxicol. Appl. Pharmacol 238 (2009) 258–65. 10.1016/j.taap.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu C-T, Lu T-Y, Chan D-C, Tsai K-S, Yang R-S, Liu S-H, Effects of Arsenic on Osteoblast Differentiation in Vitro and on Bone Mineral Density and Microstructure in Rats, Environ. Health Perspect 122 (2014) 559–565. 10.1289/ehp.1307832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hu Y-C, Cheng H-L, Hsieh B-S, Huang L-W, Huang T-C, Chang K-L, Arsenic trioxide affects bone remodeling by effects on osteoblast differentiation and function., Bone. 50 (2012) 1406–15. 10.1016/j.bone.2012.03.012. [DOI] [PubMed] [Google Scholar]
- [21].Zeng H, Cao JJ, Combs GF, Selenium in bone health: Roles in antioxidant protection and cell proliferation, Nutrients. 5 (2013) 97–110. 10.3390/nu5010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R, Selenium in human health and disease, Antioxid. Redox Signal 14 (2011) 1337–1383. 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- [23].Zofkova I, Davis M, Blahos J, Trace elements have beneficial , as well as detrimental effects on bone homeostasis, 66 (2017) 391–402. 10.33549/physiolres.933454. [DOI] [PubMed] [Google Scholar]
- [24].Al-E-Ahmad A, Parsian H, Fathi M, Faghihzadeh S, Hosseini SR, Nooreddini HG, Mosapour A, ALOX12 gene polymorphisms and serum selenium status in elderly osteoporotic patients, Adv. Clin. Exp. Med 27 (2018) 1717–1722. 10.17219/acem/75689. [DOI] [PubMed] [Google Scholar]
- [25].Wang L, Yu H, Yang G, Zhang Y, Wang W, Su T, Ma W, Yang F, Chen L, He L, Ma Y, Zhang Y, Correlation between bone mineral density and serum trace element contents of elderly males in Beijing urban area., Int. J. Clin. Exp. Med 8 (2015) 19250–7. [PMC free article] [PubMed] [Google Scholar]
- [26].Wallin M, Barregard L, Sallsten G, Lundh T, Karlsson MK, Lorentzon M, Ohlsson C, Mellström D, Low-Level Cadmium Exposure Is Associated With Decreased Bone Mineral Density and Increased Risk of Incident Fractures in Elderly Men: The MrOS Sweden Study, J. Bone Miner. Res 31 (2016) 732–741. 10.1002/jbmr.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Puerto-Parejo LM, Aliaga I, Canal-Macias ML, Leal-Hernandez O, Roncero-Martín R, Rico-Martín S, Moran JM, Evaluation of the dietary intake of cadmium,lead and mercury and Its relationship with bone health among postmenopausal women in Spain., Int. J. Environ. Res. Public Health 14 (2017) 564. 10.3390/ijerph14060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kanis JA, Cooper C, Rizzoli R, Reginster JY, European guidance for the diagnosis and management of osteoporosis in postmenopausal women, Osteoporos. Int 30 (2019) 3–44. 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pérez-Castrillón JL, Martín-Escudero JC, del Pino-Montes J, Blanco FS, Martín FJM, Pérez Paredes MG, Fernández FP, Arés TA, Prevalence of osteoporosis using DXA bone mineral density measurements at the calcaneus: cut-off points of diagnosis and exclusion of osteoporosis, J. Clin. Densitom 8 (2005) 404–408. 10.1385/jcd:8:4:404. [DOI] [PubMed] [Google Scholar]
- [30].Nordberg GF, Nogawa K, Nordberg M, Friberg LT, Cadmium, in: Nordberg GF, Fowler BA, Nordberg M, Friberg LT (Eds.), Handb. Toxicol. Met, 3rd ed., 2007: pp. 445–486. [Google Scholar]
- [31].Fowler BA, Chou C-HSJ, Jones RL, Chen C-J, Arsenic, in: Nordberg GF, Fowler BA, Nordberg M, Friberg LT (Eds.), Handb. Toxicol. Met, Third, 2007: pp. 367–406. [Google Scholar]
- [32].Thomson C, SELENIUM/Physiology, in: Encycl. Food Sci. Nutr, 2nd ed., 2003: pp. 5117–5124. [Google Scholar]
- [33].Tellez-Plaza M, Briongos-Figuero L, Pichler G, Dominguez-Lucas A, Simal-Blanco F, Mena-Martin FJ, Bellido-Casado J, Arzua-Mouronte D, Chaves FJ, Redon J, Martin-Escudero JC, Cohort profile: the Hortega Study for the evaluation of non-traditional risk factors of cardiometabolic and other chronic diseases in a general population from Spain, BMJ Open. 9 (2019) e024073. 10.1136/bmjopen-2018-024073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lorentzon M, Cummings SR, Osteoporosis: The evolution of a diagnosis, J. Intern. Med 277 (2015) 650–661. 10.1111/joim.12369. [DOI] [PubMed] [Google Scholar]
- [35].Mozaffarian D, Rimm EB, Fish intake, contaminants, and human health evaluating the risks and the benefits, J. Am. Med. Assoc 296 (2006) 1885–1899. 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- [36].Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E, Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population, Environ. Res 111 (2011) 110–118. 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Grau-Perez M, Navas-Acien A, Galan-Chilet I, Briongos-Figuero LS, Morchon-Simon D, Bermudez JD, Crainiceanu CM, de Marco G, Rentero-Garrido P, Garcia-Barrera T, Gomez-Ariza JL, Casasnovas JA, Martin-Escudero JC, Redon J, Chaves FJ, Tellez-Plaza M, Arsenic exposure, diabetes-related genes and diabetes prevalence in a general population from Spain, Environ. Pollut 235 (2018) 948–955. 10.1016/j.envpol.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jones MR, Tellez-Plaza M, Vaidya D, Grau M, Francesconi KA, Goessler W, Guallar E, Post WS, Kaufman JD, Navas-Acien A, Estimation of inorganic arsenic exposure in populations with frequent seafood intake: evidence from MESA and NHANES, 184 (2016) 590–602. 10.1093/aje/kww097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS, 2011 compendium of physical activities: A second update of codes and MET values, Med. Sci. Sports Exerc 43 (2011) 1575–1581. 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- [40].Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, Cercy K, Vos T, Murray CJL, Forouzanfar MH, Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: Systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013, BMJ. 354 (2016). 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, A new equation to estimate glomerular filtration rate, Ann. Intern. Med 150 (2009) 604–612. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Diaz Curiel M, Garcia J, Carrasco JL, Honorato J, Perez Cano R, Rapado A, Álvarez C, Prevalencia de osteoporosis determinada por densitometría en la población femenina española, Med. Clin. (Barc) 116(3) (2001) 86–88. [DOI] [PubMed] [Google Scholar]
- [43].Fordham JN, Chinn DJ, Kumar N, Identification of women with reduced bone density at the lumbar spine and femoral neck using BMD at the os calcis, Osteoporos. Int 11 (2000) 797–802. 10.1007/s001980070059. [DOI] [PubMed] [Google Scholar]
- [44].Pacheco EMB, Harrison EJ, Ward KA, Lunt M, Adams JE, Detection of osteoporosis by dual energy X-ray absorptiometry (DXA) of the calcaneus: Is the WHO criterion applicable?, Calcif. Tissue Int 70 (2002) 475–482. 10.1007/s002230020030. [DOI] [PubMed] [Google Scholar]
- [45].Beukhof CM, Medici M, van den Beld AW, Hollenbach B, Hoeg A, Visser WE, de Herder WW, Visser TJ, Schomburg L, Peeters RP, Selenium status is positively associated with bone mineral density in healthy aging European men., PLoS One. 11 (2016) e0152748. 10.1371/journal.pone.0152748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hoeg A, Gogakos A, Murphy E, Mueller S, Köhrle J, Reid DM, Glüer CC, Felsenberg D, Roux C, Eastell R, Schomburg L, Williams GR, Bone turnover and bone mineral density are independently related to selenium status in healthy euthyroid postmenopausal women, J.Clin. Endocrinol. Metab 97 (2012) 4061–4070. 10.1210/jc.2012-2121. [DOI] [PubMed] [Google Scholar]
- [47].Arikan DC, Coskun A, Ozer A, Kilinc M, Atalay F, Arikan T, Plasma selenium, zinc, copper and lipid levels in postmenopausal Turkish women and their relation with osteoporosis, Biol. Trace Elem. Res 144 (2011) 407–417. 10.1007/s12011-011-9109-7. [DOI] [PubMed] [Google Scholar]
- [48].Wang Y, Xie D, Li J, Long H, Wu J, Wu Z, He H, Wang H, Yang T, Wang Y, Association between dietary selenium intake and the prevalence of osteoporosis: A cross-sectional study, BMC Musculoskelet. Disord 20 (2019) 585. 10.1186/s12891-019-2958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wengreen HJ, Munger RG, West NA, Cutler DR, Corcoran CD, Zhang J, Sassano NE, Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah., J. Bone Miner. Res 19 (2004) 537–45. 10.1359/JBMR.040208. [DOI] [PubMed] [Google Scholar]
- [50].Sun L, Li B, Xie H, Fan F, Yu W, Wu B, Xue W, Chen Y, Associations between the dietary intake of antioxidant nutrients and the risk of hip fracture in elderly Chinese: a case–control study, Br. J. Nutr 112 (2014) 1706–1714. 10.1017/S0007114514002773. [DOI] [PubMed] [Google Scholar]
- [51].Moreno-Reyes R, Egrise D, Nève J, Pasteels JL, Schoutens A, Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia, J. Bone Miner. Res 16 (2001) 1556–1563. 10.1359/jbmr.2001.16.8.1556. [DOI] [PubMed] [Google Scholar]
- [52].Martiniaková M, Boboňová I, Omelka R, Grosskopf B, Chovancová H, Španková J, Toman R, Simultaneous subchronic exposure to selenium and diazinon as possible risk factor for osteoporosis in adult male rats, Acta Vet. Scand 55 (2013) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Martiniaková M, Boboňová I, Omelka R, Grosskopf B, Stawarz R, Toman R, Structural changes in femoral bone tissue of rats after subchronic peroral exposure to selenium., Acta Vet. Scand 55 (2013) 8. 10.1186/1751-0147-55-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vekariya KK, Kaur J, Tikoo K, Alleviating anastrozole induced bone toxicity by selenium nanoparticles in SD rats, Toxicol. Appl. Pharmacol 268 (2013) 212–220. 10.1016/j.taap.2013.01.028. [DOI] [PubMed] [Google Scholar]
- [55].Liu H, Bian W, Liu S, Huang K, Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway, Biol. Trace Elem. Res 150 (2012) 441–450. 10.1007/s12011-012-9488-4. [DOI] [PubMed] [Google Scholar]
- [56].Cao JJ, Gregoire BR, Zeng H, Selenium Deficiency Decreases Antioxidative Capacity and Is Detrimental to Bone Microarchitecture in Mice, J. Nutr 142 (2012) 1526–1531. 10.3945/jn.111.157040. [DOI] [PubMed] [Google Scholar]
- [57].Zhang Z, Zhang J, Xiao J, Selenoproteins and selenium status in bone physiology and pathology, Biochim. Biophys. Acta - Gen. Subj 1840 (2014) 3246–3256. 10.1016/j.bbagen.2014.08.001. [DOI] [PubMed] [Google Scholar]
- [58].Zoidis E, Seremelis I, Kontopoulos N, Danezis GP, Selenium-dependent antioxidant enzymes: Actions and properties of selenoproteins, Antioxidants. 7 (2018). 10.3390/antiox7050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Monsen ER, Dietary Reference Intakes for The Antioxidant Nutrients, J. Am. Diet. Assoc 100 (2000) 637–640. 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- [60].Burk RF, Selenium, an antioxidant nutrient., Nutr. Clin. Care 5 (2002) 75–79. 10.1046/j.1523-5408.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- [61].Rayman MP, The importance of selenium to human health, Lancet. 356 (2000) 233–241. 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- [62].Kudva AK, Shay AE, Prabhu KS, Selenium and inflammatory bowel disease, Am. J. Physiol. - Gastrointest. Liver Physiol 309 (2015) G71–G77. 10.1152/ajpgi.00379.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Papp LV, Lu J, Holmgren A, Khanna KK, From selenium to selenoproteins: Synthesis, identity, and their role in human health, Antioxidants Redox Signal. 9 (2007) 775–806. 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- [64].Rayman MP, Food-chain selenium and human health: Emphasis on intake, Br. J. Nutr 100 (2008) 254–268. 10.1017/S0007114508939830. [DOI] [PubMed] [Google Scholar]
- [65].Galan-Chilet I, Tellez-Plaza M, Guallar E, De Marco G, Lopez-Izquierdo R, Gonzalez-Manzano I, Carmen Tormos M, Martin-Nuñez GM, Rojo-Martinez G, Saez GT, Martín-Escudero JC, Redon J, Javier Chaves F, Plasma selenium levels and oxidative stress biomarkers: A gene-environment interaction population-based study, Free Radic. Biol. Med 74 (2014) 229–236. 10.1016/j.freeradbiomed.2014.07.005. [DOI] [PubMed] [Google Scholar]
- [66].Nève J, Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity, J. Trace Elem. Med. Biol 9 (1995) 65–73. 10.1016/S0946-672X(11)80013-1. [DOI] [PubMed] [Google Scholar]
- [67].Nishijo M, Nakagawa H, Suwazono Y, Nogawa K, Kido T, Causes of death in patients with Itai-itai disease suffering from severe chronic cadmium poisoning: A nested case-control analysis of a follow-up study in Japan, BMJ Open. 7 (2017) e015694. 10.1136/bmjopen-2016-015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lavado-García JM, Puerto-Parejo LM, Roncero-Martín R, Moran JM, Pedrera-Zamorano JD, Aliaga IJ, Leal-Hernández O, Canal-Macias ML, Dietary Intake of cadmium, lead and mercury and its association with bone health in healthy premenopausal women., Int. J. Environ. Res. Public Health 14 (2017) 1437. 10.3390/ijerph14121437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cheng X, Niu Y, Ding Q, Yin X, Huang G, Peng J, Song J, Cadmium Exposure and Risk of Any Fracture, Medicine (Baltimore). 95 (2016) e2932. 10.1097/md.0000000000002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sommar JN, Pettersson-Kymmer U, Lundh T, Svensson O, Hallmans G, Bergdahl IA, Hip fracture risk and cadmium in erythrocytes: A nested case-control study with prospectively collected samples, Calcif. Tissue Int 94 (2014) 183–190. 10.1007/s00223-013-9796-5. [DOI] [PubMed] [Google Scholar]
- [71].Thomas LDK, Michaëlsson K, Julin B, Wolk A, Åkesson A, Dietary cadmium exposure and fracture incidence among men: a population-based prospective cohort study., J. Bone Miner. Res 26 (2011) 1601–8. 10.1002/jbmr.386. [DOI] [PubMed] [Google Scholar]
- [72].Dahl C, Søgaard AJ, Tell GS, Flaten TP, Hongve D, Omsland TK, Holvik K, Meyer HE, Aamodt G, Do cadmium, lead, and aluminum in drinking water increase the risk of hip fractures? A NOREPOS study, Biol. Trace Elem. Res 157 (2014) 14–23. 10.1007/s12011-013-9862-x. [DOI] [PubMed] [Google Scholar]
- [73].Engström A, Michaëlsson K, Vahter M, Julin B, Wolk A, Åkesson A, Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women, Bone. 50 (2012) 1372–1378. 10.1016/j.bone.2012.03.018. [DOI] [PubMed] [Google Scholar]
- [74].Engström A, Michaëlsson K, Suwazono Y, Wolk A, Vahter M, Akesson A, Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women., J. Bone Miner. Res 26 (2011) 486–95. 10.1002/jbmr.224. [DOI] [PubMed] [Google Scholar]
- [75].Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R, Environmental exposure to cadmium, forearm bone density, and risk of fractures: Prospective population study, Lancet. 353 (1999) 1140–1144. 10.1016/S0140-6736(98)09356-8. [DOI] [PubMed] [Google Scholar]
- [76].Alfvén T, Elinder CG, Hellström L, Lagarde F, Järup L, Cadmium exposure and distal forearm fractures, J. Bone Miner. Res 19 (2004) 900–905. 10.1359/JBMR.040202. [DOI] [PubMed] [Google Scholar]
- [77].Zhu G, Wang H, Shi Y, Weng S, Jin T, Kong Q, Nordberg GF, Environmental cadmium exposure and forearm bone density, in: BioMetals, Biometals, 2004: pp. 499–503. 10.1023/B:BIOM.0000045728.80518.d9. [DOI] [PubMed] [Google Scholar]
- [78].Hensawang S, Chanpiwat P, Health impact assessment of arsenic and cadmium intake via rice consumption in Bangkok, Thailand, Environ. Monit. Assess 189 (2017). 10.1007/s10661-017-6321-8. [DOI] [PubMed] [Google Scholar]
- [79].Akbal A, Yılmaz H, Tutkun E, Arsenic exposure associated with decreased bone mineralization in male, Aging Male. 17 (2014) 256–258. 10.3109/13685538.2013.819326. [DOI] [PubMed] [Google Scholar]
- [80].Kim YD, Eom SY, Yim DH, Kim IS, Won HK, Park CH, Kim GB, Do Yu S, Choi BS, Park JD, Kim H, Environmental exposure to arsenic, lead, and cadmium in people living near Janghang Copper smelter in Korea, J. Korean Med. Sci 31 (2016) 489–496. 10.3346/jkms.2016.31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


