Abstract
β-l-5-Iododioxolane uracil was shown to have potent anti-Epstein-Barr virus (EBV) activity (50% effective concentration = 0.03 μM) with low cytotoxicity (50% cytotoxic concentration = 1,000 μM). It exerts its antiviral activity by suppressing replicative EBV DNA and viral protein synthesis. This compound is phosphorylated in cells where the EBV is replicating but not in cells where the EBV is latent. EBV-specific thymidine kinase could phosphorylate β-l-5-iododioxolane uracil to the monophosphate metabolite. The Km of β-l-5-iododioxolane uracil with EBV thymidine kinase was estimated to be 5.5 μM, which is similar to that obtained with thymidine but about fivefold higher than that obtained with 2′ fluoro-5-methyl-β-l-arabinofuranosyl uracil, the first l-nucleoside analogue discovered to have anti-EBV activity. The relative Vmax is seven times higher than that of thymidine. The anti-EBV activity of β-l-5-iododioxolane uracil and its intracellular phosphorylation could be inhibited by 5′-ethynylthymidine, a potent EBV thymidine kinase inhibitor. The present study suggests that β-l-5-iododioxolane uracil exerts its action after phosphorylation; therefore, EBV thymidine kinase is critical for the antiviral action of this drug.
Epstein-Barr virus (EBV) is an important human pathogen. This virus has been determined to cause infectious mononucleosis, fatal acute infectious mononucleosis–X-linked lymphoproliferative syndrome, and oral hairy leukoplakia (16, 17, 48). EBV also has a close association with several types of malignancies, including Burkitt's lymphoma, nasopharyngeal carcinoma (NPC), Hodgkin's disease, (2, 13, 21), some T-cell lymphomas (1, 26, 32, 35, 40, 58), smooth muscle cell leiomyosarcoma (23), and certain cases of gastric adenocarcinomas (38, 46, 53). EBV infection can enhance human immunodeficiency virus type 1 (HIV-1) replication in T cells (56), and EBV is also related to the development of lymphoma induced in AIDS patients (55). It was also reported that almost all posttransplant lymphomas appeared to be EBV genome positive, irrespective of histological appearance or clonability of the lesion (18, 44, 49, 59). A more complex picture of EBV-associated malignancies is emerging, particularly with regard to virus-positive tumors of non-B-cell origin. It is hoped that a better understanding of EBV persistence and the part played by EBV in the oncogenic process will permit the development of new approaches aimed at the prevention and treatment of EBV-associated tumors. Therefore, it would be ultimately useful to have anti-EBV compounds that do not have serious side effects.
Several compounds have shown anti-EBV activity in cell culture systems, including acyclovir (ACV), ganciclovir (DHPG), 2′-fluoro-5-methyl-β-d-arabinofuranosyl uracil (D-FMAU), and (S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine (cidofivir) (6, 9, 26, 27, 36, 50, 52). However, the clinical application of many of these compounds is restricted by severe side effects (52). Recently, our laboratory found a new anti-EBV l-dioxolane nucleoside analogue, β-l-5-iododioxolane uracil (L-I-OddU) (28). L-I-OddU is thus far the most potent anti-EBV agent, with a 50% effective concentration (EC50) of 0.03 μM against EBV replication in cells (28). Since L-I-OddU has good antiviral inhibition, it could be the most selective compound against EBV replication-associated diseases in the clinical setting. However, the mechanism by which L-I-OddU inhibited EBV DNA replication was not clear.
The antiviral spectrum of L-I-OddU was very different from that of all other l-nucleoside or benzimidizole l-riboside analogues studied (3, 7, 8, 29, 30, 39, 43, 54). L-I-OddU has potent antiviral action specific to EBV, weak antiviral activity in Kaposi's sarcoma-associated herpesvirus–human herpesvirus 8 (HHV8), but poor effect against other viruses (HIV, herpes simplex virus type 1 [HSV-1], HSV-2, cytomegalovirus, or hepatitis B virus). While L-I-OddU at 20 μM has activity that is slightly better than that of DHPG at 20 μM, with an anti-HHV8 activity of 60% compared to 41% (data not shown), it shows no advantage over ACV in our varicella-zoster virus system, with EC50s of 17 μM for L-I-OddU and of 4 μM for ACV (data not shown). Only EBV showed significant increased selectivity, with an L-I-OddU EC50 that was >1,000-fold lower than the EC50 of 50 μM for ACV. Our previous studies indicated that the action(s) responsible for the anti-EBV activity of L-I-OddU could be dependent on EBV-specific proteins (28). In the present study we show that EBV thymidine kinase (TK) was necessary for the activation of L-I-OddU and that L-I-OddU was converted to L-I-OddUMP by EBV TK, which has been shown to have a narrow substrate specificity compared to other herpesviruses (20, 47).
MATERIALS AND METHODS
Reagents.
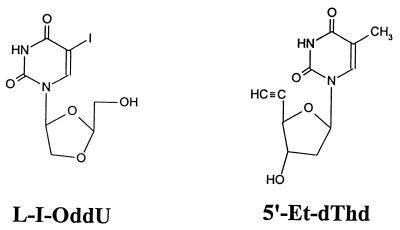
L-I-OddU, β-l-5-bromodioxolane uracil (L-Br-OddU), and L-FMAU were synthesized by C. K. Chu, College of Pharmacy, University of Georgia. An EBV TK inhibitor, 5′-ethynyl-thymidine (5′-Et-dThd), was a gift from M. Bobek, Department of Experimental Therapeutics, Roswell Park Memorial Institute, Buffalo, N.Y. The chemical structures are shown in Fig. 1. [α-32P]dCTP, [γ-32P]ATP, and [14C]thymidine (dThd) were purchased from Amersham, Arlington Heights, Ill. [3H]-L-I-OddU and [3H]-L-FMAU were purchased from Moravek Biochemicals, Inc., Brea, Calif.
FIG. 1.
Chemical structure of L-I-OddU and EBV inhibitor 5′-Et-dThd.
Cell culture.
H1, a high-EBV-producing human B cell, cloned from the P3HR1 cell line, was used in this study (51). H1 has >95% of the EBV DNA in the replicating linear form, while the EBV TK− L5 clone, also derived from P3HR1 cells, is latently infected with EBV and does not produce viral particles. The EBV DNA exists as a supercoiled form in L5 cells. Cells were grown at 37°C in a 5% CO2 humidified incubator. The culture medium was HEPES-buffered RPMI 1640 supplemented with 100 μg of kanamycin per ml and 10% dialyzed fetal bovine serum. Dialyzed serum was used so that small molecules would not interfere with the nucleoside studies performed.
Exposure of H1 cells to compounds.
H1 cells were maintained in a logarithmic phase of growth for 2 days prior to the initiation of treatment. The H1 cells were seeded into 24-well tissue culture plates at a density of 2 × 105 cells per ml in 2 ml of fresh medium with or without the compound to be examined for antiviral activity and were then incubated at 37°C for 5 days. After the period of drug exposure, the cells were pelleted and washed by centrifugation at 2,000 rpm in a tabletop centrifuge. Slot blot analysis of these cell samples was used to determine the inhibitory effect of the compounds on EBV DNA.
EBV DNA detection.
The slot blot assay was performed as described previously (12), with some modifications. A total of 4 × 105 H1 cells were treated with different compounds at various concentrations for the time indicated and were lysed in 400 μl of 10 mM Tris-HCl (pH 7.5) solution by freeze-thawing three times in an ethanol-dry ice bath. The cell lysate was treated with RNase A at a final concentration of 50 μg/ml at 37°C for 30 min. The lysate was then treated with proteinase K at a final concentration of 100 μg/ml at 55°C for 2 h. An equal volume of 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) was added to each sample. After heating for 10 min in a 100°C water bath, the samples were spotted onto a positively charged nylon membrane using a manifold connected to a vacuum system. The samples were denatured on the membrane by washing them with 0.4 N NaOH–10 mM EDTA (pH 8.2). Then, the [α-32P]dCTP-labeled EBV EcoRI C fragment was used as a probe for DNA hybridization. Autoradiographic results were analyzed by Personal Densitometer SI (Molecular Dynamics, Inc., Sunnyvale, Calif.).
Western blotting.
Cell pellets (106 cells) were lysed with 30 μl of lysis buffer (0.05 M Tris-HCl [pH 6.8], 1% sodium dodecyl sulfate [SDS], 0.5% mercaptoethanol, 20% glycerol, 0.1% bromphenol blue) and boiled for 5 min. The samples were electrophoresed on an SDS–7.5% polyacrylamide gel. The proteins were electroblotted onto a nitrocellulose membrane using the Bio-Rad Transblot apparatus method as described by the manufacturer. The membrane was treated with blocking buffer (phosphate-buffered saline [PBS] with 1.5% Triton X-100 and 5% dry milk) for 1 h. The membrane was incubated overnight at 4°C with a monoclonal antibody to EBV TK, a gift from J.-Y. Chen, College of Medicine, National Taiwan University. After three 10-min washes in PBS with 0.15% Triton X-100 at room temperature, the membrane was incubated with anti-mouse immunoglobulin G (IgG) conjugated with peroxidase (Sigma, St. Louis, Mo.) at room temperature for 1 h. The membranes were washed three more times as described above, followed by exposure to a Western blot chemiluminesence reagent for 2 min (NEN Life Science Products, Boston, Mass.). The membranes were placed on X-ray films, which were exposed to the light. After development, the exposed bands were quantified by using a scanning densitometer.
In situ gel lysis method.
For analysis of the EBV linear and supercoiled DNA, we used an in situ gel lysis method. The procedure for in situ gel analysis was as described by Gardella et al. (15). Loads were normalized by counting cells at the end of the drug treatment period and exactly 2 × 106 cells were resuspended in 60 μl of solution that contained 15% Ficoll, 1× Tris-borate-EDTA (TBE), 2 μl of 10-mg/ml RNase A, and 0.25% bromphenol blue solution. These samples were incubated for 30 min at room temperature and then applied to the gel. This discontinuous agarose gel is designed to permit only viral DNA (both circular and linear) to enter the body of the gel (15). The gel was electrophoresed at 15 V for 3 h, and then the voltage was increased to 100 V for 48 h at 4°C. The nucleic acids in the gel were transferred to a nylon membrane by the usual Southern blotting procedure (42).
EBV TK purification.
The cloned EBV TK gene, which was derived from P3HR1 cells, was a gift from J. Y. Chen, College of Medicine, National Taiwan University (22, 33). Approximately 100 ng of PET-TK B1B plasmid was used to transform competent cells, the BL21(DE3)/pLysS expression host, by the standard procedure of Sambrook et al. (42). Once the insert was shown to be present in one of the colonies, a large-scale induction was performed using isopropyl-β-d-thioglucopyranoside with shaking at room temperature for 15 h. The bacterial preparation was centrifuged at 6,000 × g for 30 min at 4°C. The pellet was resuspended in lysis buffer containing 25 mM Tris-HCl (pH 8.0), 50 mM glucose, 10 mM EDTA, and lysozyme at 200 μg/ml. The mixture was kept on ice for 30 min and sonicated using a Branson Sonifier (80% duty cycle; output, 7; four 10-s bursts). The solution was centrifuged for 30 min at 15,000 rpm in a Beckman J21M centrifuge using a JA20 rotor. The pellet was resuspended in lysis buffer containing 50 μM thymidine and then sonicated to resuspend it. The bacterial lysate was brought to 75% ammonium sulfate (AmSO4) by the slow addition of 750 g of solid salt per liter while stirring in the cold room. The suspension was centrifuged at 15,000 rpm for 15 min at 4°C, the supernatant was decanted, and the pellet was redissolved in buffer A′ (Tris-HCl [pH 7.5], 10 mM; dithiothreitol [DTT], 2 mM; glycerol, 10%). The solution was dialyzed against 500 ml of buffer A′ for 2 h, with two changes of buffer at 4°C. The conductivity of the sample was measured, and the ionic strength was adjusted to be lower than that of buffer A. The sample was loaded onto a TK affinity column and eluted as described by Lee and Cheng (25) using various ionic strengths and thymidine concentrations. The EBV TK eluted in buffer E, which contained 400 mM Tris-HCl (pH 7.5), 300 μM thymidine, 2 mM DTT, and 10% glycerol. CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} was added to a final concentration of 0.6 mM to stabilize the enzyme.
Cellular kinase purification.
The TK and mitochondrial deoxypyrimidine kinase (dPydK) enzymes were affinity purified from human chronic lymphocytic leukemia cells by methods described previously (34) and on the same column as described above (25, 34).
TK assay.
The TK activity was determined essentially as described by Cheng and Ostrander (4) and Lee and Cheng (25). The TK assay mixture contained 0.12 M Tris-HCl (pH 7.5), 1.8 mM ATP-Mg2, 20 μM dThd, 6.8 mM sodium fluoride, 71 U of creatine phosphokinase, 8.8 mM creatine phosphate, 0.07% bovine serum albumin, and 0.1 μCi of [14C]dThd (55 mCi/mmol) and purified TK in a final volume of 100 μl. The reaction mixture was incubated at 37°C, and then 50 μl of reaction mixture was spotted onto Whatman 2.3-cm anion-exchange DE81 disks to stop the reaction. The disks were then immediately dropped into 95% ethanol (5 ml/disk) and washed three times for 5 min each time. The disks were dried and inserted into scintillation vials, and 5 ml of Safe Scint (American Bioanalytical, Natic, Mass.) was added to each vial. The amount of dThd converted to dTMP was quantitated by radiospectrometry using a Beckman LS 100C or LS 5000TD apparatus. [3H]-L-I-OddU was used in a similar manner as dThd for kinase assays except that the disks were washed in 1 mM ammonium formate. To improve the counting efficiency of the tritiated analogue, the disks were dried and placed in scintillation vials containing 1 ml of 2 mM NaCl and 0.2 N HCl, which eluted the isotope from the ion-exchange paper, and 10 ml of scintillation fluid was added.
Determination of Km and Vmax.
To determine the Km and relative Vmax for the EBV TK with dThd and the l-nucleoside analogues, we used reaction conditions that were similar to those for the standard TK assay. The substrate concentrations varied from 6.25 to 100 μM for dThd, 0.5 to 4 μM for L-FMAU, and 6.25 to 100 μM for L-I-OddU. Lineweaver-Burk plots were used to determine the Km and Vmax values.
HPLC analysis of phosphorylate metabolites.
Separation of cellular metabolites was performed by high-pressure liquid chromatography (HPLC) using a Perkin-Elmer system with a Whatman Particil SAX column (3). A gradient of potassium phosphate buffer from 0.03 to 300 mM (pH 6.6) at a flow rate of 1 ml/min was employed. When isotopic l-nucleosides were used, an in-line Packard 150TR Radiation Detector with National Diagnostics Monoflow 5 scintillation fluid was mixed at a rate of 4 ml/min. The amount of radiation under the metabolite peak was used to determine the picomoles of phosphorylated metabolites generated.
RESULTS
L-I-OddU inhibits EBV replication.
In EBV lytic replicating cells, such as H1 cells, the majority of EBV DNA exists in the linear form, whereas in EBV latent cells, such as L5 cells, the supercoiled form of EBV DNA is the major form detected (Fig. 2A). After treatment with 1 μM L-I-OddU or L-Br-OddU, only the linear form of EBV DNA was greatly decreased in H1 cells, with no apparent effect on the supercoiled form of EBV DNA in these cells (Fig. 2A, lanes 3 and 4).
FIG. 2.
(A) Detection of linear and circular EBV DNA in cells with or without L-I-OddU and L-Br-OddU. Lanes: 1, H1 cells with no treatment; 2, H1 cells plus dimethyl sulfoxide (DMSO); 3, H1 cells plus 1 μM L-I-OddU; 4, H1 cells plus 1 μM L-Br-OddU; 5, L5 cells with no treatment; 6, L5 cells plus DMSO; 7, L5 cells plus 1 μM L-I-OddU; 8, L5 cells plus 1 μM L-Br-OddU; 9, CEM cells with no treatment (CEM cells are human T cells which do not contain any EBV genome). (B) Western blotting by anti-EBV TK antibody. Lanes: 1, H1 cells with no treatment; 2, H1 cells plus DMSO; 3, H1 cells plus 1 μM L-I-OddU; 4, H1 cells plus 1 μM L-Br-OddU; 5, L5 cells with no treatment; 6, L5 cells plus DMSO; 7, L5 cells plus 1 μM L-I-OddU; 8, L5 cells plus 1 μM L-Br-OddU; 9, CEM cells with no treatment. The TK protein is marked at 69 kDa and a human protein (HP) that has bound nonspecifically to this antibody serves as a load control.
To examine the effect of 1 μM L-I-OddU and L-Br-OddU on the replication of EBV, cell extracts of treated and untreated H1 and L5 cells were electrophoresed and blotted onto nitrocellulose membranes. Using a monoclonal antibody, EBV TK was detected in H1 cells but was below detection in drug-treated H1 or latently infected L5 cells or in the negative control CEM cells (Fig. 2B).
Formation of L-I-OddU metabolites in cells.
The metabolism of L-I-OddU in EBV replicating cells (H1 cells) and EBV latently infected cells (L5) was examined. [3H]-L-I-OddU was incubated with these cell lines at 1 and 2 μM for 24 h. The acid-soluble fractions were extracted, and the L-I-OddU metabolites were separated by HPLC. The major metabolite of [3H]-L-I-OddU was found to be L-I-OddUMP in H1 cells, along with some putative L-I-OddUDP and L-I-OddUTP metabolites which need to be verified. However, when 2 μM [3H]-L-I-OddU was added to the cells, no metabolites were detected in L5 cells under the same conditions. The amount of the mono- and diphosphate metabolites formed in H1 cells increased in a dose-dependent manner; however, the amount of L-I-OddUTP formed was not proportional to the amount of drug added (Table 1).
TABLE 1.
Phosphorylated metabolites of L-I-OddU after 48 h of treatment
| Cell line | L-I-OddU concn (μM) | L-I-OddU
phosphorylated metabolites (pmol/106 cells ±
SD)a
|
||
|---|---|---|---|---|
| L-I-OddUMP | L-I-OddUDPb | L-I-OddUTPb | ||
| H1 | 0.25 | 0.42 ± 1.0 | 0.06 ± 0.02 | 0.02 ± 0.01 |
| 0.50 | 1.03 ± 0.14 | 0.10 ± 0.01 | 0.03 ± 0.01 | |
| 1.00 | 1.64 ± 0.17 | 0.14 ± 0.03 | 0.02 ± 0.01 | |
| L5 | 1.00 | NDc | ND | ND |
| 2.00 | ND | ND | ND | |
These numbers represent an average of three experiments.
These are putative L-I-OddUDP and L-I-OddUTP values since authentic markers were not available.
ND, not detected (i.e., below the detection limit).
L-I-OddU is a selective substrate for EBV TK.
The preferential phosphorylation of L-I-OddU in H1 cells indicated that the L-I-OddU may act as a selective substrate for EBV TK. The phosphorylation of [3H]-L-I-OddU and [14C]dThd by EBV TK and the human thymidine phosphorylating kinases was examined (Table 2). L-I-OddU was phosphorylated at a 2.5-fold-higher rate than was dThd by EBV TK at 10 μM. However, L-I-OddU was not phosphorylated by human mitochondrial dPydK or cytoplasmic TK. This indicates that L-I-OddU is a specific substrate of EBV TK but not a favorable substrate for cellular kinases.
TABLE 2.
Phosphorylation of L-I-OddU and dThd by various kinases
| Enzyme and compounda | Phosphorylation rate (% ± SD) to dThd |
|---|---|
| EBV TK | |
| L-I-OddU | 251.5 ± 35.0 |
| dThd | 100.0 |
| Human mitochondrial dPydk | |
| L-I-OddU | NDb |
| dThd | 100.0 |
| Human cytosolic TK | |
| L-I-OddU | ND |
| dThd | 100.0 |
The concentrations of L-I-OddU and dThd were each 10 μM. The enzyme activities used were 4, 1.7, and 0.1 nmol/min for EBV TK, cytosolic TK, and mitochondrial dPydK, respectively. The enzyme sources were as described in Materials and Methods. EBV was from a bacterial vector, and the human enzymes were from chronic lymphocytic leukemic cells. Enzyme protein levels from the affinity column were too low to be measured with the Bio-Rad protein assay.
ND, not detected.
The Km values and relative Vmaxs of L-I-OddU and L-FMAU, another anti-EBV l-nucleoside, toward EBV TK were determined (Table 3). L-I-OddU had a Km about seven times higher than that for L-FMAU and about the same as that for dThd. L-I-OddU showed a relative Vmax 1.3 times higher than that for L-FMAU and 3.9 times higher than that for dThd.
TABLE 3.
The Km and Vmax values of compounds to EBV TKa
| Compound | Mean Km (μM) ± SD | Mean relative Vmax ± SD |
|---|---|---|
| L-I-OddU | 5.5 ± 0.08 | 3.9 ± 0.25 |
| L-FMAU | 0.8 ± 1.2 | 3.0 ± 0.51 |
| dThd | 4.5 ± 0.9 | 1.00 |
The enzyme used in these experiments was the EBV affinity-purified enzyme from the PET-TK B1B plasmid described in Materials and Methods. These numbers represent the average of five experiments.
5′-Et-dThd as an EBV TK inhibitor.
5′-Et-dThd (Fig. 1), which is a selective HSV TK inhibitor (37), was able to inhibit EBV TK but not human cytoplasmic TK or mitochondrial dPydK in the concentration range studied (Fig. 3A). These results indicated that 5′-Et-dThd is also a selective inhibitor of EBV TK. Detailed kinetic studies were performed using multiple concentrations of [3H]-L-I-OddU and 5′-Et-dThd. 5′-Et-dThd was shown to exert its action as a competitive inhibitor with respect to L-I-OddU with a KI value of 4 μM (Fig. 3B).
FIG. 3.
(A) Effect of 5′-Et-dThd on thymidine phosphorylation by EBV ( ), human cytosolic TK (○), and mitochondrial dPydK (▴). The concentration of cold dThd in the reaction mix was 20 μM. (B) Lineweaver-Burk plots of various L-I-OddU concentrations with or without 5′-Et-dThd (large figure) and the replot (left-side inset). The Ki value of 5′-Et-dThd obtained was 4 μM. The 5′-Et-dThd concentrations tested were 0 μM (●), 7.5 μM (▿), 15 μM (+), 30 μM (▴), and 60 μM (▪).
Inhibition of metabolism and anti-EBV activity of L-I-OddU by 5′-Et-dThd.
The effect of 5′-Et-dThd on the amount of L-I-OddU metabolites formed in H1 cells exposed to 2 μM [3H]-L-I-OddU was examined. When H1 cells were exposed to L-I-OddU for 24 h, 6.5 pmol of L-I-OddUMP, 1 pmol of the putative L-I-OddUDP, and 0.1 pmol of the putative L-I-OddUTP were formed per 106 cells. In the presence of 5 μM 5′-Et-dThd, the amounts of L-I-OddUMP, L-I-OddUDP, and L-I-OddUTP were reduced to 3 pmol, 0.2 pmol, and below the detection limit, respectively. When the effect of 5′-Et-dThd on the anti-EBV action of L-I-OddU was examined, the antiviral activity was reduced in a dose-dependent manner as measured by the amount of EBV DNA formed (Fig. 4). However, 5′-Et-dThd, up to a concentration of 100 μM alone, could not inhibit EBV lytic replication (data not shown).
FIG. 4.
Effect of 5′-Et-dThd on the anti-EBV activity of L-I-OddU. The virus amount produced without L-I-OddU treatment was used as the 100% level. The 5′-Et-dThd concentrations tested were 0 μM (▿), 0.8 μM (▴), 4 μM (○), and 20 μM ( ).
DISCUSSION
Most biologically active l-nucleosides are monophosphorylated by cytosolic deoxycytidine kinase(dCydK) (14, 19, 34, 57), while L-FMAU can be phosphorylated by cytosolic TK and mitochondrial dPydK in addition to dCydK. L-I-OddU is the first potent l-nucleoside that requires a viral protein to convert the drug to its active antiviral metabolite in a way that is similar to that of ACV, based on viral TK and viral polymerase.
To show that the activation of L-I-OddU was related not only to a viral protein but also to one involving virion replication, Western blot analysis of protein extracts of H1 and L5 cell lines was used to visualize viral TK. When cells were treated with L-I-OddU or L-Br-OddU, the viral TK band of H1 became similar to that of L5. This suggested that the replicating virus DNA synthesis apparatus may be the target of action of L-I-OddU or L-Br-OddU. Indeed, L-I-OddU inhibited the replicating linear form of EBV DNA present in H1 cells but had no effect on the supercoiled form of EBV DNA associated with latent infection present in L5 and H1 cells. Although the patterns of viral DNA and protein in H1 drug-treated cells changed to resemble that of L5 cells, the EBV is not truly latent. When the drugs were removed for 25 days, the linear viral DNA reappeared, suggesting the presence of a small amount of replicating virus (28). The virus-specific TK protein is demonstrable in H1 EBV replicating cells but not demonstrable in L5 cells (31, 45). The metabolism of tritiated L-I-OddU in H1 and L5 cells showed no phosphorylated metabolites in L5 but showed the formation of monophosphate as well as very small amounts of the putative di- and triphosphate forms in H1. Based on this study the EBV TK was suspected to be the enzyme responsible for the activation of L-I-OddU. All the other l-nucleosides that we have studied had diphosphate and triphosphate metabolite pools that were severalfold higher than the amount of monophosphate metabolite. These pools also increased in amount in direct proportion to the concentration of compound used. This is not the case with L-I-OddU, where the amount of triphosphate is low and not proportional to the drug concentration. Therefore, it is not clear which metabolite of L-I-OddU is the active form, but the formation of phosphorylated metabolites is essential for its antiviral action. A monoclonal antibody to EBV TK was used in a Western blot analysis of H1 and L5 cells treated with L-I-OddU or L-Br-OddU. There was a decrease in the amount of EBV TK compared to the untreated control (Fig. 2B).
When affinity column-purified EBV TK was examined for its ability to utilize L-I-OddU as a substrate, it was phosphorylated at a rate 2.5-fold higher than that for dThd. Since purified cytoplasmic TK and mitochondrial dPydK cannot phosphorylate L-I-OddU and since EBV TK is an enzyme associated with virus replication, its selective antiviral activity should be targeted on cells with replicating EBV.
To demonstrate the importance of EBV TK for the activation of L-I-OddU, another dThd analog, 5′-Et-dThd, was utilized. We have shown previously that 5′-Et-dThd is a competitive inhibitor of human HSV TK but has no effect on cytoplasmic TK or mitochondrial dPydK. While 5′-Et-dThd had no anti-EBV effect alone at 100 μM in H1 cells, the higher the concentration of 5′-Et-dThd, coincubated with L-I-OddU, the less the effect of the L-I-OddU on the formation of viral linear DNA. When purified EBV TK was used to study the impact of 5′-Et-dThd on L-I-OddU, the Lineweaver-Burk plot (Fig. 3B) showed that the Kmapp value of L-I-OddU increased with increasing concentrations of 5′-Et-dThd, whereas the Vmax remained unchanged. This observation suggests that 5′-Et-dThd is a competitive inhibitor of L-I-OddU. The marked decrease of L-I-OddU metabolites in H1 cells that were coincubated with 5′-Et-dThd demonstrated that EBV TK is necessary to activate L-I-OddU.
Unlike L-FMAU, which was the first l-nucleoside with potent anti-EBV activity, L-I-OddU is phosphorylated preferentially by EBV TK. These results suggest that L-I-OddU could serve as a “selective alternate substrate” as described in our previous study (5) and can explain the good selective therapeutic index observed with this compound. While EBV TK appears to be responsible for the formation of L-I-OddUMP, further phosphorylation may occur by human dTMP kinase and NDP kinase to form L-I-OddUDP and L-I-OddUTP, respectively. The L-I-OddUTP formed may then act either as an alternative substrate of dTTP or as a competitive inhibitor of EBV DNA polymerase. It is also possible that L-I-OddU may function like L-FMAU; a recent report from this laboratory demonstrated that L-FMAUTP was not a substrate for EBV DNA polymerase. However, L-FMAUTP could potentially prevent EBV replication by binding to the EBV polymerase at a site different than the deoxynucleoside triphosphates, causing inhibition of chain elongation (“allosteric regulation”) and exonuclease activity (24). However, it is still unclear whether the active form of L-I-OddU is the mono-, di-, or triphosphate metabolite because the amounts of di- and triphosphate are quite low compared to the monophosphorylated form.
In summary, it has been suggested that anti-EBV therapy could be useful in the clinic for the prevention or therapy of EBV-associated diseases, including posttransplant lymphoma and hairy leukoplakia lesions of AIDS patients (10, 11, 17, 41). Since L-I-OddU is the most effective anti-EBV l-nucleoside studied to date, its potential should be examined. While its metabolism and mechanisms of action are still under investigation, L-I-OddU, based on its low toxicity and potent antiviral effect, warrants further investigation as a specific anti-EBV chemotherapeutic agent.
ACKNOWLEDGMENTS
This work was supported by NIH grants CA-63477, AI-38204, and AI-33655.
T.K. and S.P.G. contributed equally to this work.
REFERENCES
- 1.Ambinder R F, Mann R B. Detection and characterization of Epstein-Barr virus in clinical specimens. Am J Pathol. 1994;145:239–252. [PMC free article] [PubMed] [Google Scholar]
- 2.Ambinder R F, Robertson K D, Moore S M, Yang J. Epstein-Barr virus as a therapeutic target in Hodgkin's disease and nasopharyngeal carcinoma. Semin Cancer Biol. 1996;7:217–226. doi: 10.1006/scbi.1996.0029. [DOI] [PubMed] [Google Scholar]
- 3.Chang C-N, Doong S-L, Zhou J H, Beach J W, Jeong L S, Chu C K, Tsai C-H, Cheng Y-C. Deoxycytidine deaminase-resistant stereoisomer is the active form of (±)-2′,3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992;267:13938–13942. [PubMed] [Google Scholar]
- 4.Cheng Y-C, Ostrander M. Deoxythymidine kinase induced in HeLa TK−cells by herpes simplex virus type I and type II. Purification and characterization. J BiolChem. 1976;251:2605–2610. [PubMed] [Google Scholar]
- 5.Cheng Y-C, Domin B A, Sharma R A, Bobek M. Antiviral action and cellular toxicity of four thymidine analogues: 5-ethyl-, 5-vinyl-, 5-propyl-, and 5-allyl-2′-deoxyuridine. Antimicrob Agents Chemother. 1976;10:119–122. doi: 10.1128/aac.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Y-C, Huang E-S, Lin J-C, Mar E-C, Pagano J S, Dutschman G E, Grill S P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy) methyl]-guanine against herpesvirus in vitroand its mode of action against herpes simplex virus type I. Proc Natl Acad Sci USA. 1983;80:2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu C-K, Ma T, Shanmuganathan K, Wang C, Xiang Y, Pai S B, Yao G-Q, Sommadossi J-P, Cheng Y-C. Use of 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil as a novel antiviral agent for hepatitis B virus and Epstein-Barr virus. Antimicrob Agents Chemother. 1995;39:979–981. doi: 10.1128/aac.39.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates J A V, Cammack N, Jenkinson H J, Mutton I M, Pearson B A, Storer R, Cameron J M, Penn C R. The separated enantiomers of 2′-deoxy-3′-thiacytidine (BCH-189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob Agents Chemother. 1992;36:202–205. doi: 10.1128/aac.36.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colby B M, Shaw J E, Elion G B, Pagano J S. Effect of acyclovir [9-(2-hydroxyethoxymethyl)guanine] on Epstein-Barr virus DNA replication. J Virol. 1980;34:560–568. doi: 10.1128/jvi.34.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellemijn P L I, Brandenburg A, Niesters H G M, van den Bent M J, Rothbarth P H H, Vlasveld L T. Successful treatment with ganciclovir of presumed Epstein-Barr meningo-encephalitis following bone marrow transplant. Bone Marrow Transplant. 1995;16:311–312. [PubMed] [Google Scholar]
- 11.Derenkov I A, Marcarelli M A, Basadonna G P, Friedman A L, Lorber K M, Howe J G, Corouch J, Bia M J, Kliger A S, Lorber M I. Reduced incidence of Epstein-Barr virus-associated posttransplant lymphoproliferative disorder using preemptive antiviral therapy. Transplantation. 1997;65:848–852. doi: 10.1097/00007890-199709270-00010. [DOI] [PubMed] [Google Scholar]
- 12.Doong S-L, Tsai C-H, Schinazi R F, Liotta D C, Cheng Y-C. Inhibition of the replication of hepatitis B virus in vitroby 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc Natl Acad Sci USA. 1991;88:8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drouet E, Brousset P, Fares F, Icart J, Verniol C, Meggetto F, Schlaifer D, Desmorat-Coat H, Rigal-Huguet F, Niveleau A, Delsol G. High Epstein-Barr virus serum load and elevated titers of anti-ZEBRA antibodies in patients with EBV-harboring tumor cells of Hodgkin's disease. J Med Virol. 1999;57:383–389. doi: 10.1002/(sici)1096-9071(199904)57:4<383::aid-jmv10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Dutschman G E, Bridges E G, Liu S-H, Gullen E, Guo X, Kukhanova M, Cheng Y-C. Metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine and its activity in combination with clinically approved anti-human immunodeficiency virus β-d(+) nucleoside analogs in vitro. Antimicrob Agents Chemother. 1998;42:1799–1804. doi: 10.1128/aac.42.7.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardella T, Medveczky P, Sairenji T, Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984;50:248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenspan J S, Greenspan D, Lennette E T, Abrams D I, Conant M A, Petersen V, Freese U K. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985;313:1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan D, De Souza Y G, Conant M A, Hollander H, Chapman S K, Lennette E T, Petersen V, Greenspan J S. Efficacy of desciclovir in the treatment of Epstein-Barr virus infection in oral hairy leukoplakia. J Acquir Immune Defic Syndr. 1990;3:571–578. [PubMed] [Google Scholar]
- 18.Gross T G, Steinbuch M, DeFor T, Shapiro R S, McGlave P, Ramsay N K C, Wagner J E, Filipovich A H. B cell lymphoproliferative disorders following hematopoietic stem cell transplantation: risk factors, treatment and outcome. Bone Marrow Transplant. 1999;23:251–258. doi: 10.1038/sj.bmt.1701554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grove K L, Guo X, Liu S-H, Gao Z, Chu C K, Cheng Y-C. Anticancer activity of beta-l-dioxolane-cytidine, a novel nucleoside analogue with the unnatural lconfiguration. Cancer Res. 1995;55:3008–3011. [PubMed] [Google Scholar]
- 20.Gustafson E A, Chillemi A C, Sage D R, Fingeroth J D. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob Agents Chemother. 1998;42:2923–2931. doi: 10.1128/aac.42.11.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbst H. Epstein-Barr virus in Hodgkin's disease. Semin Cancer Biol. 1996;7:183–189. doi: 10.1006/scbi.1996.0025. [DOI] [PubMed] [Google Scholar]
- 22.Hsu T-Y, Pai C-Y, Shieh S-M, Cho S-M, Liu M-Y, Chen J-Y, Yang C-S. Use of antigen expressed in bacteria for detection of EBV-specific thymidine kinase antibodies in sera from the patients with nasopharyngeal carcinoma. J Med Virol. 1992;38:214–219. doi: 10.1002/jmv.1890380311. [DOI] [PubMed] [Google Scholar]
- 23.Jenson H B, Montalvo E A, McClain K L, Ench Y, Heard P, Christy B A, Dewalt-Hagan P J, Moyer M P. Characterization of natural Epstein-Barr virus infection and replication in smooth muscle cells from a leiomyosarcoma. J Med Virol. 1999;57:36–46. [PubMed] [Google Scholar]
- 24.Kukhanova M, Lin Z-Y, Yas'co M, Cheng Y-C. Unique inhibitory effect of l-(2′-deoxy-2′-fluoro-β-l-arabinofuranosyl)-5-methyluracil 5′-triphosphate on Epstein-Barr virus and human DNA polymerases. Biochem Pharmacol. 1998;55:1181–1187. doi: 10.1016/s0006-2952(97)00598-4. [DOI] [PubMed] [Google Scholar]
- 25.Lee L-S, Cheng Y-C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozyme derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976;251:2600–2604. [PubMed] [Google Scholar]
- 26.Lin J-C, Pagano J S. Cellular transformation by the herpesviruses and antiviral drugs. Pharmacol Ther. 1985;28:135–161. doi: 10.1016/0163-7258(85)90010-5. [DOI] [PubMed] [Google Scholar]
- 27.Lin J-C, DeClercq E, Pagano J S. Inhibitory effects of acyclic nucleoside phosphonate analogs, including (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine on Epstein-Barr virus replication. Antimicrob Agents Chemother. 1991;35:2440–2443. doi: 10.1128/aac.35.11.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J-S, Kira T, Gullen E, Qu F, Choi Y, Chu C K, Cheng Y-C. Structure activity relationship of l-dioxolane uracil analogues against EBV replication. J Med Chem. 1999;42:2212–2217. doi: 10.1021/jm9806749. [DOI] [PubMed] [Google Scholar]
- 29.Lin T-S, Luo M-Z, Liu M-C, Pai S B, Dutschman G E, Cheng Y-C. Synthesis and biological evaluation of 2′,3′-dideoxy-l-pyrimidine nucleosides as potential antiviral agents against human immunodeficiency virus (HIV) and hepatitis B virus (HBV) J Med Chem. 1994;37:798–803. doi: 10.1021/jm00032a013. [DOI] [PubMed] [Google Scholar]
- 30.Lin T-S, Luo M-Z, Liu M-C, Zhu Y-L, Gullen E, Dutschman G E, Cheng Y-C. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-β-l-5-cytidine (β-L-d4C), and 2′,3′-dideoxy-2′,3′-didehydro-β-l-5-fluorocytidine (β-L-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent inhibitors of human immunodeficiency virus (HIV) in vitro. J Med Chem. 1996;39:1757–1759. doi: 10.1021/jm950836q. [DOI] [PubMed] [Google Scholar]
- 31.Litter E, Zeuther J, Mcbride A A, Sørensen E T, Powell K L, Walsh-Arrand J E, Arrand J R. Identification of an Epstein-Barr virus-encoded thymidine kinase. EMBO J. 1986;5:1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litter E, Baylis S A, Zeng Y, Conway M J, Mackett M, Arrand J R. Diagnosis of nasopharyngeal carcinoma by means of recombinant Epstein-Barr virus proteins. Lancet. 1991;337:685–689. doi: 10.1016/0140-6736(91)90275-t. [DOI] [PubMed] [Google Scholar]
- 33.Liu M-Y, Hsu T-Y, Pai C-Y, Shieh S-M, Liu J-Y, Chen J-Y, Yang C-S. Cloning and expression of a cDNA encoding the Epstein-Barr virus thymidine kinase gene. J Virol Methods. 1992;40:107–118. doi: 10.1016/0166-0934(92)90012-3. [DOI] [PubMed] [Google Scholar]
- 34.Liu S-H, Grove K L, Cheng Y-C. Unique metabolism of a novel antiviral l-nucleoside analog, 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil: a substrate for both thymidine kinase and deoxycytidine kinase. Antimicrob Agents Chemother. 1998;42:833–839. doi: 10.1128/aac.42.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meijer C J L M, Jiwa N M, Dukers D F, Oudejans J J, de Bruin P C, Walboomers J M M, van den Brule A J C. Epstein-Barr virus and human T-cell lymphomas. Semin Cancer Biol. 1996;7:191–196. doi: 10.1006/scbi.1996.0026. [DOI] [PubMed] [Google Scholar]
- 36.Neyts J, Sadler R, De Clercq E, Raab-Traub N, Pagano J S. The antiviral agent cidofovir [(S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl) cytosine] has pronounced activity against nasopharyngeal carcinoma grown in nude mice. Cancer Res. 1998;58:384–388. [PubMed] [Google Scholar]
- 37.Nutter L M, Grill S P, Dutschman G E, Sharma R A, Bobek M, Cheng Y-C. Demonstration of viral thymidine kinase inhibitor and its effect on deoxynucleotide metabolism in cells infected with herpes simplex virus. Antimicrob Agents Chemother. 1987;31:368–374. doi: 10.1128/aac.31.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osato T, Imai S. Epstein-Barr virus and gastric carcinoma. Semin Cancer Biol. 1996;7:175–182. doi: 10.1006/scbi.1996.0024. [DOI] [PubMed] [Google Scholar]
- 39.Pai S B, Liu S-H, Zhu Y-L, Cheng Y-C. Inhibition of hepatitis B virus by a novel l-nucleoside, 2′-fluoro-5-methyl-β-l-arabinofuranosyl uracil. Antimicrob Agents Chemother. 1996;40:380–386. doi: 10.1128/aac.40.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pallesen G, Hamilton-Dutoit S J, Zhou X. The association of Epstein-Barr virus (EBV) with T cell lymphoproliferation and Hodgkin's disease: two new developments in the EBV field. Adv Cancer Res. 1993;62:179–239. doi: 10.1016/s0065-230x(08)60319-x. [DOI] [PubMed] [Google Scholar]
- 41.Pirsch J D, Stratta R J, Sollinger H W, Hafez G R, D'Alessandro A M, Kalayoglu M, Belzer F O. Treatment of severe Epstein-Barr virus-induced lymphoproliferative syndrome with ganciclovir: two cases after solid organ transplantation. Am J Med. 1989;86:241–244. doi: 10.1016/0002-9343(89)90279-9. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Schinazi R F, McMillan A, Cannon D, Mathis R, Lloyd R M, Peck A, Sommadossi J-P, St. Clair M, Wilson J, Furman P A, Painter G, Choi W-B, Liotta D C. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2423–2431. doi: 10.1128/aac.36.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro R S, McClain K, Frizzera G, Gajl-Peczalska K J, Kersey J H, Blazar B R, Arthur D C, Patton D F, Greenberg J S, Burke B, Ramsay N K C, McGlave P, Filipovich A H. Epstein-Barr virus-associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood. 1988;71:1234–1243. [PubMed] [Google Scholar]
- 45.Stinchcombe T, Clough W. Epstein-Barr virus induces a unique pyrimidine deoxynucleoside kinase activity in superinfected and virus-producer B cell line. Biochemistry. 1985;24:2027–2033. doi: 10.1021/bi00329a034. [DOI] [PubMed] [Google Scholar]
- 46.Takasaka N, Tajima M, Okinaga K, Hoshikawa Y, Katsumoto T, Kurata T, Sirenji T. Productive infection of Epstein-Barr virus (EBV) in EBV-genome-positive epithelial cell line (GT38 and GT39) derived from gastric tissues. Virology. 1998;247:152–159. doi: 10.1006/viro.1998.9231. [DOI] [PubMed] [Google Scholar]
- 47.Tung P P, Summers W C. Substrate specificity of Epstein-Barr virus thymidine kinase. Antimicrob Agents Chemother. 1994;38:2175–2179. doi: 10.1128/aac.38.9.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webster-Cyriaque J, Raab-Traub N. Transcription of Epstein-Barr virus latent cycle genes in oral hairy leukoplakia. Virology. 1998;248:53–65. doi: 10.1006/viro.1998.9268. [DOI] [PubMed] [Google Scholar]
- 49.Weiss L M, Movahed L A. In situdemonstration of Epstein-Barr viral genomes in viral associated B cell lymphoproliferations. Am J Pathol. 1989;134:651–659. [PMC free article] [PubMed] [Google Scholar]
- 50.Yao G-Q, Grill S, Egan W, Cheng Y-C. Potent inhibition of Epstein-Barr virus by phosphorothioate oligodeoxynucleotides without sequence specification. Antimicrob Agents Chemother. 1993;37:1420–1425. doi: 10.1128/aac.37.7.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao G-Q, Tsai C-H, Cheng Y-C. Characterization of sublines of Epstein-Barr virus producing HR-1 cells and its implication in virus propagation in culture. Virus Genes. 1995;9:247–255. doi: 10.1007/BF01702880. [DOI] [PubMed] [Google Scholar]
- 52.Yao G-Q, Liu S-H, Chou E, Kukhanova M, Chu C K, Cheng Y-C. Inhibition of Epstein-Barr virus replication by a novel l-nucleoside, 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil. Biochem Pharmacol. 1996;51:941–947. doi: 10.1016/0006-2952(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 53.Young L S. Introduction: Epstein-Barr virus and non-B cell tumours. Semin Cancer Biol. 1996;7:163–164. doi: 10.1006/scbi.1996.0022. [DOI] [PubMed] [Google Scholar]
- 54.Zancy V L, Gershburg E, Davis M G, Biron K K, Pagano J S. Inhibition of Epstein-Barr virus replication by a benzimidazole l-riboside: novel antiviral mechanism of 5,6-dichloro-2-(isopropylamino)-1-β-l-ribofuranosyl-1H-benzimidazole. J Virol. 1999;73:7271–7277. doi: 10.1128/jvi.73.9.7271-7277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng Y, Middeldorp J, Madjar J-J, Ooka T. A major DNA binding protein encoded by BALF2 open reading frame of Epstein-Barr virus (EBV) forms a complex with other EBV DNA-binding proteins: DNAase, EA-D, and DNA polymerase. Virology. 1997;239:285–295. doi: 10.1006/viro.1997.8891. [DOI] [PubMed] [Google Scholar]
- 56.Zhang R D, Guan M, Park Y, Tawadros R, Yang J Y, Gold B, Wu B, Henderson E E. Synergy between human immunodeficiency virus type 1 and Epstein-Barr virus in T lymphoblastoid cell lines. AIDS Res Hum Retrovir. 1997;13:161–171. doi: 10.1089/aid.1997.13.161. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y L, Dutschman G E, Liu S-H, Bridges E G, Cheng Y-C. Anti-hepatitis B virus activity and metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine. Antimicrob Agents Chemother. 1998;42:1805–1810. doi: 10.1128/aac.42.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zur Hausen H, Schulte-Holthauser H, Klein G, Henle W, Henle G, Clifford P, Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228:1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]
- 59.Zutter M M, Martin P J, Sale G E, Shulman H M, Fisher L, Thomas E D, Durnam D M. Epstein-Barr virus lymphoproliferation after bone marrow transplantation. Blood. 1988;72:520–529. [PubMed] [Google Scholar]