Highlights
-
•
Vitamin D deficiency can not only affect bones but have association with cardiovascular effects.
-
•
The likelihood of unfavorable cardiovascular outcomes, especially major adverse cardiovascular event (MACE), new MI, and all-cause mortality among deficient vitamin D patient is poorly understood.
-
•
This is a first and most comprehensive meta-analysis with the largest sample size thus far comparing vitamin D levels in terms of cardiovascular outcomes.
-
•
High risk patients need to monitor vitamins level and implement vitamins supplementation to avoid adverse outcomes especially elder females.
Keywords: Vitamin D, Hypovitaminosis, Cardiovascular disease, Mortality
Abbreviations: MI, Myocardial Infarction; MACE, Major adverse cardiovascular events; HVD, Hypovitaminosis D; HF, Heart Failure; ACM, All Cause Mortality
Abstract
Background
The relation between blood vitamin D levels and the risk of cardiovascular outcomes is debatable. To our knowledge this is the first comparative meta-analysis of more than 100,000 patients’ data with the aim to inspect the relevance of low vitamin D levels with adverse cardiovascular events.
Methods
Online databases including PubMed, Embase and Cochrane Central were queried to compare the cardiovascular outcomes among hypovitaminosis D (HVD) and control group. The outcomes assessed included differences in major adverse cardiovascular events (MACE), mortality, myocardial infarction, and heart failure. Unadjusted odds ratios (OR) were calculated using a random-effect model with a 95% confidence interval (CI) and P less than 0.05 as a statistical significance.
Results
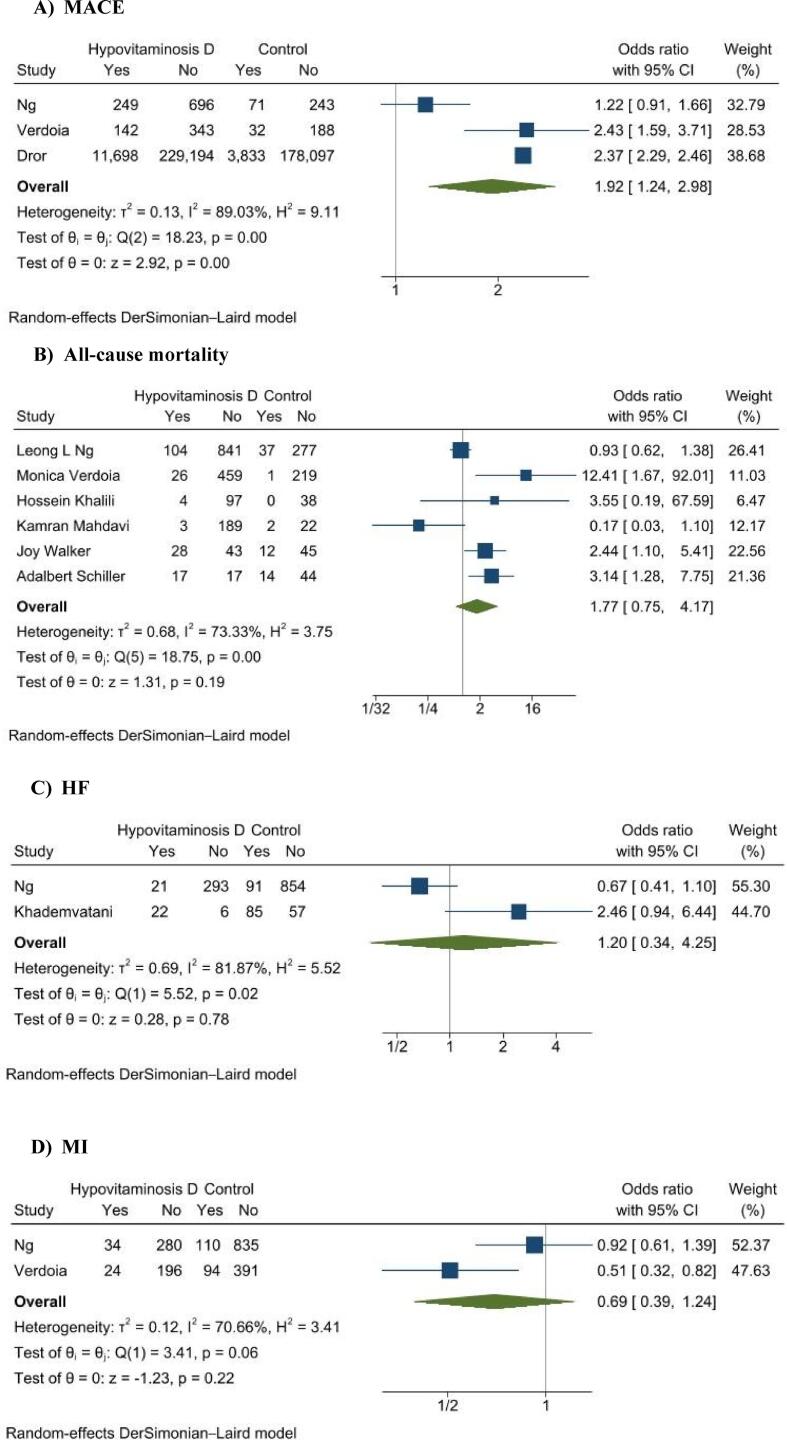
A total of 8 studies including 426,039 patients were included in this analysis. HVD group was associated with a higher incidence of MACE (OR 1.92, 95% CI 1.24 to 2.98, p = 0.003), while there was no significant association of HVD and all-cause mortality (OR 1.77, 95% CI 0.75 to 4.17, p = 0.19), risk of myocardial infarction (OR 0.69, 95% CI 0.39 to 1.24, p = 0.22), and heart failure (OR 1.20, 95% CI 0.34 to 4.25, p = 0.78).
Conclusions
This meta-analysis suggested that low blood levels of vitamin D are associated with MACE, but no such difference in all-cause mortality, myocardial infarction or heart failure was observed. Appropriate supplementation of vitamin D in selected populations might be cardioprotective in nature and warrants extensive trials.
1. Introduction
A fat-soluble vitamin, Vitamin D is a predominant modulator of calcium and bone homeostasis but has an equally important regulatory function on 2000 to 8000 gene transcription factors [1]. Vitamin D downregulates the renin-angiotensin system, protects against angiogenesis, augments insulin secretion and sensitivity, and regulates inflammatory processes [2]. Recently, it has been shown that knocking out vitamin D receptors present in vascular endothelium and cardiomyocytes may give rise to left ventricular hypertrophy, change in the extracellular matrix, and activate plasma renin [3]. Hypovitaminosis D (HVD) is present in over 1 billion people worldwide across all ages, ethnicities, and comorbidities [4], [5]. Although HVD has primarily been thought to cause pathologies of muscles and bones, it has also been linked to cardiovascular, and various inflammatory, neoplastic, and infectious diseases [1], [3].
As cardiovascular events are the leading cause of death worldwide and HVD is a burgeoning problem with people leading a more sedentary lifestyle, it is very worthwhile to study a link between these two and help mitigate this preventable cause of cardiovascular events [6]. While there are certain definite non-modifiable risk factors, according to the INTERHEART study, 9 out of 10 myocardial infarctions are related to 9 easily modifiable factors [7]. Lack of physical activity is one such prominent risk factor. This subgroup is more prone to lower vitamin D levels, apart from other causes of HVD. Hence, if HVD is proven as a cardiovascular risk factor, we can substantially reduce the cardiovascular disease burden with vitamin D supplementation.
In the previously published reviews on vitamin D and its association with possible cardiovascular outcomes, none have reported or analyzed the optimal threshold range in which vitamin D can serve as a cardioprotective agent [6], [8], [9], [10], [11]. Thus, in this paper we conducted a comparative systematic review and meta-analysis with more than 100,000 study participants from the available published data to see if there is an association between different vitamin D levels and cardiovascular adverse events.
2. Methods
This study was carried out in compliance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines and performed according to established methods, as described previously [12]. A prespecified study protocol has been registered on Prospero: CRD42022306943.
2.1. Definition and outcomes
At present, the optimal 25(OH)-vitamin D levels for benefits beyond skeletal health are yet to be determined. Thus, we adopted the recommendations by the National Academy of Medicine which approximates 25(OH)-vitamin D levels of more than 20 ng/ml as adequate for skeletal benefits and considered levels below 20 ng/ml as hypovitaminosis D [13]. Our primary outcomes were the prevalence of major adverse cardiovascular events (MACE) between the two groups, and our secondary outcomes were all-cause mortality, myocardial infarction (MI) and heart failure (HF).
2.2. Search strategy and study selection
We conducted a systematic search in PubMed, Embase, and Scopus for articles from inception until January 10, 2022. The following MeSH terms were used: “hypovitaminosis D” OR “vitamin D deficiency” AND “acute coronary syndrome” OR “cardiovascular disease” AND “outcomes”. Eligible reports were assessed for methodological quality. Two authors (VJ and MV) reviewed the abstract and title of the articles for screening the eligibility of those articles in the analysis. The senior author resolved any inclusion related discrepancy. Only full-text articles were included. Studies were included if they fulfilled the following criteria: 1) patients with vitamin D deficiency compared with patients with normal vitamin D levels, 2) studies with patients > 18 years of age, 3) studies with data on baseline characteristics and outcomes measured, 4) all multicenter, case control, observational studies, and randomized clinical trials were included. We excluded literature or systematic reviews, studies with undesirable data, letters, commentaries, animal studies, and studies with patients less than 18 years of age. We did not use any language restrictions. References of previously published articles and conferences abstract were checked for additional studies.
2.3. Data extraction and statistical analysis
Data of the eligible selected studies such as demographic, comorbidities, risk factors, and outcomes of both groups were extracted in the spreadsheet by two authors (VJ and NS). Baseline continuous variables were summarized in mean (SD), whereas dichotomous variables were described in frequencies or percentages. We performed a conventional meta-analysis for primary and secondary outcome and adopted the Dersimonian and Laird random-effects model for the study variations [14]. We considered a two-tailed p value of less than 0.05 to be statistically significant. In addition, we assessed the between-study heterogeneity using the Q and I2 test and considered the results to be substantially heterogeneous if the I2 test is more than 50% [15]. Subgroup analyses, where possible, were conducted using follow-up duration, cardiovascular (CV) risk or CKD and non-CKD population. Publication bias was performed for outcome with at least 6 studies and was assessed through funnel plot and quantified using Egger’s test. All statistical work including analyses and graphical illustrations were conducted using STATA (version 17.0, StataCorp) [16].
2.4. Quality assessment
AI independently assessed the quality of the included studies using the Newcastle-Ottawa Scale for cohort studies and cross-sectional studies [17]. In case of disagreement, senior author consensus (VJ) was involved. The details of quality assessment are presented in Supplementary Tables 1 and 2.
3. Results
3.1. Study selection
Preliminary database search using keywords stated above yielded 7812 articles, of which 852 studies were excluded after removal of duplicates. 7343 studies were further excluded after initial title and abstract screening based on our inclusion and exclusion criteria and comparison arm between lower vitamin D levels vs normal levels (control group). Full-text review was conducted for the remaining 80 articles identified. 65 full-text articles were retrieved for screening. 57 studies were excluded as they either had the wrong target population, were not a primary research article, or lacked a comparison arm. Hence, a total of 8 studies that met the eligibility criteria were included in our meta-analysis. The PRISMA flow diagram is shown in Supplementary Fig. 1.
3.2. Patient and study demographics:
8 studies with a total of 426,039 patients; 182,669 patients in control group (Vitamin D levels > 20 ng/ml) and 242,862 patients with HVD (Vitamin D levels < 20 ng/ml) were included in the meta-analysis. There was a worldwide distribution of the studies; 4 from Asia [18], [19], [20], [21], 3 from Europe [1], [3], [22], and 1 from North America [23]. The baseline demographics of the study participants are summarized in Table 1. Mean age of the HVD and control group were 63 and 66 years, respectively. The majority (68.7%) of participants were female (71% in the HVD group and 66% in the control group). The patients presented with various comorbidities. Average BMI was increased in both groups; 26.6 kg/m2 in the HVD group and 25.9 kg/m2 in the control group. The participants suffered from various comorbidities. The two most common comorbidities were type 2 diabetes (49% in the HVD group and 42% in the control group) and hypertension (14.9% in the HVD group and 12.1% in the control group). History of MI, stroke and hyperlipidemia were reported in less than 1% of participants of both groups. 26% participants of both the comparator group and control were smokers.
Table 1.
Baseline demographics, comorbidities and study characteristics of studies included in the meta-analysis.
| Variable | Ng et al, 2013[3] | Dror et al, 2013[18] | Verdoia et al., 2021[1] | Khalili et al., 2012[19] | Khademvatani et al., 2017[20] | Madhavi et al., 2013[21] | Walker et al., 2014[23] | Schiller et al., 2015[22] |
|---|---|---|---|---|---|---|---|---|
| Sample (n) Hypovitaminosis D/ Control |
945/314 | 240892/181930 | 485/220 | 101/38 | 142/28 | 192/24 | 71/57 | 34/58 |
| Age, y(Mean) | 65.5/66.2 | NA | 67.2/67.5 | 59.6/63.1 | 57.71/68.1 | 65.7/60.89 | 64.4/69.7 | NA |
| Male, % | 70.3/77.3 | 28.7/34.1 | 78.3/75.4 | 84.1/ 77.1 |
71.8/57.1 | 40.1/45.8 | 95.8/98.2 | NA |
| BMI, kg/m2 (mean) | NA | NA | 27.8/26.2 | NA | NA | 25.71/25.36 | NA | 26.4/26.37 |
| Comorbidities | ||||||||
| HTN, % | 52.4/42.6 | 14.5/12 | 75.5/72.7 | 60.3/36.8 | 49.3/50 | 66.1/58.3 | 95.8/100 | NA |
| HLD, % | NA | NA | 61.6/59 | 40.6/42.1 | NA | 44.8/50 | 67.6/80.7 | NA |
| DM, % | 26.4/13.7 | 49/42 | 43.7/31.8 | 25.7/31.6 | 80.2/89.2 | 66.7/50 | 59.1/63.1 | 100/100 |
| Smoker, % | 43.8/45.9 | 25.8/26 | 55.2/51.4 | NA | 50/42.9 | 34.8/12.5 | 21.2/15.8 | NA |
| Previous PCI, % | NA | NA | 59.1/60 | 28.7/21 | NA | NA | NA | NA |
| Previous MI, % | 24.2/13.4 | 4.7/5.1 | 24.5/19.5 | NA | 10.6/17.9 | NA | 47.9/52.6 | NA |
| Previous Stroke, % | NA | NA | NA | NA | NA | NA | 21.1/7 | NA |
HTN – Hypertension; HLD – Hyperlipidemia; DM – Diabetes Mellitus (type 2); PCI – Percutaneous Coronary Intervention; MI – Myocardial Infarction.
3.3. Meta-analysis of outcomes
3.3.1. Primary outcome - MACE
3 studies reported results on the primary outcome, MACE, with a total of 242,786 patients (242,322 in the HVD group and 182,464 patients in the control group). The meta-analysis showed that patients with suboptimal levels of vitamin D were at a 1.92 (95% CI 1.24 to 2.98, p = 0.003; I2 = 90%) increased odds of MACE (Fig. 1A).
Fig. 1.
Forest plots of outcomes: (A) MACE, major adverse cardiovascular events; (B) all-cause mortality; (C) HF (heart failure) and (D) MI (myocardial infarction).
3.3.2. Secondary outcomes - all cause Mortality, MI, and HF
6 studies reported results on all-cause mortality. As shown in Fig. 1B results of our analysis showed that there was no significant association between vitamin D levels and all-cause mortality (OR 1.77, 95% CI 0.75 to 4.17, p = 0.19, I2 = 73%). 2 studies reported on incidence of MI and heart failure. There was also no significant association between vitamin D levels and risk of MI event (OR 0.69, 95% CI 0.39 to 1.24, p = 0.22, I2 = 71%) or incidence of heart failure (OR 1.20, 95% CI 0.34 to 4.25, p = 0.78, I2 = 82%) as shown in Fig. 1C and Fig. 1D respectively.
3.3.3. Subgroup analyses
Subgroup analyses were conducted for the outcomes MACE and all-cause mortality using either CV risk, follow-up period or population with CKD. For MACE, subgroup analysis was done by CV risk and showed that patients with suboptimal vitamin D did not have increased odds of MACE among the subgroup with high CV risk (OR 1.70, 95% CI 0.87 to 3.32, p = 0.12) (supplementary Fig. 2). For all-cause mortality, we found that CKD populations with suboptimal vitamin D level had higher odds of mortality (OR 2.73, 95% CI 1.50 to 4.95, p = 0.001) compared to those with adequate vitamin D level while there was no difference among the non-CKD populations (p = 0.67) (supplementary Fig. 3A). Follow-up period using 1 year as cut-off period for all-cause mortality did not result in a significant difference between the two groups (supplementary Fig. 3B).
3.3.4. Publication bias
There was no evidence of publication bias on studies with all-cause mortality by visual inspection of funnel plot (supplementary Fig. 4) or with the Egger’s regression test (p = 0.45).
4. Discussion
This systematic review includes 8 observational studies in diverse populations with a total of 428,328 participants. The meta-analysis of the included studies showed HVD to significantly increase odds of suffering from MACE. However, we found no significant association between HVD with all-cause mortality, risk of MI or heart failure.
Previous reviews including observational studies demonstrated similar results as ours where HVD was associated with increased risk of cardiovascular (CV) events [2], [6], [8], [24]. One of the studies further detailed the linear, inverse relation between circulating 25(OH)-vitamin D levels between 8 and 24 ng/mL and the risk of CV events [2]. In another meta-analysis of prospective observational studies by Zhang et al., higher level of vitamin D was found to have a protective effect on total cardiovascular events (pooled RR per 10 ng/mL increment in vitamin D level 0.90, 95% CI 0.86, 0.94) [11]. However, the protective effect was evident when serum 25(OH)D was 25 ng/mL, contrary to our cutoff value (20 ng/mL) to define HVD. A non-linear Mendelian Randomization (MR) study suggested that deficiency of vitamin D could increase the risk of CV events and high blood pressure [25]. An L-shaped association of genetically predicted serum 25(OH)D was observed with both cardiovascular events and systolic/diastolic blood pressures [25]. The results are suggestive of the beneficial cardiovascular effects of vitamin D supplementation to people with low concentrations.
While this would support population-wide vitamin D supplementation to eradicate vitamin D deficiency, results from recent large randomized controlled trials (RCTs) are not supportive. VITAL trial, ViDA study and FIND trial found supplemental vitamin D not to be beneficial to reduce major cardiovascular events [26], [27], [28]. This was most probably due to an inadequate number of participants with very low vitamin D levels which would not permit detection of beneficial effects for relatively rare disease endpoints such as cardiovascular events. In another recent study, when vitamin D-replete individuals (baseline serum 25(OH)D greater than 20 ng/mL) were supplemented with vitamin D, it did not provide demonstrable health benefits [29]. Future trials with an adequate number of participants with very low vitamin D levels might be necessary while addressing practical issues and ethical dilemmas.
We found no significant association of HVD with all-cause mortality in our study. However, previous meta-analyses dealing mostly with women over 70 years have revealed a 6–11% reduction in all-cause mortality [24], [30]. After addition of new RCTs which recruited younger, mostly vitamin-D replete individuals [26], [27], [31] the beneficial effect has been eliminated [32]. Thus, it is possible that supplemental vitamin D is only beneficial in subjects with poor vitamin D status and the elderly. This might explain the results of our analysis as well, since the mean age of participants in our analysis is around 65 years. However, the recently published results of the D-Health Trial which included only the participants older than 60 years, is not supportive of the beneficial role of increased age during vitamin D supplementation [33]. The mean age of treatment and control groups is 69.3 years with participants between 60 and 70 years and over 70 years being almost equal. On further study, we found the levels of 25(OH)D in both groups well above 30 ng/mL. This might suggest that for vitamin D to benefit an individual, one must have the simultaneous presence of two factors: old age and vitamin D deficient state.
It is worth investigating the possible association between low vitamin D levels and increased all-cause mortality and cardiovascular mortality. A recent large MR study involving more than half a million subjects reported an increase in all-cause mortality risk for 25(OH)D concentrations below 10 ng/mL [34]. The study showed an inverse association between genetically predicted 25(OH)D concentrations and all-cause mortality up to 16 ng/mL [34]. In another 2017 review, pooling of prospective observational studies revealed the beneficial effect of higher levels of vitamin D in cardiovascular mortality (RR 0.88 per 10 ng/mL increase in vitamin D, 95% CI 0.80, 0.96) [11].
Individuals having HVD with CKD had increased odds of all-cause mortality on subgroup analysis. Majority of patients in both the included studies had diabetes and other comorbidities. In diabetic patients with mild or moderately reduced kidney function, HVD is associated with increased mortality [35], [36]. Jayedi et al. in a meta-analysis of cohort studies demonstrated the significant risk of mortality in CKD patients ranging from 1.22 to 1.63 from vitamin D deficiency and concluded that higher levels of 25(OH)D is associated with lower risk of all-cause mortality until 35 ng/mL [37]. For every 10 ng/mL increment in serum 25(OH)D, there was a 21% reduction in risk of overall mortality (RR, 0.79; 95% CI 0.70, 0.87) [37]. A brief functional overview of vitamin D would further clarify the above associations. Vitamin D has immunomodulatory and anti-inflammatory effects [5] which explains the association of HVD with increased risk of viral and bacterial infections, cancer, autoimmune disorders, excessive systemic inflammation that causes atherosclerosis and endothelial dysfunction [38], [39], [40], [41]. Uremia in CKD results in disturbed biochemical state leading to inflammatory and oxidative stress as well as further vitamin D deficiency [42].
Regarding the role of vitamins in the risk of MI, we found no association. However, Huang et al. in a similarly designed review as ours found that vitamin D is significantly lower in MI patients and sufficient blood vitamin D levels might be protective against MI [43]. On the contrary, other meta-analyses haven't found the beneficial effect of supplemental vitamin D against MI [9], [32], [44], [45]. In a most recent meta-analysis of RCTs, vitamin D supplementation resulted in a non-significant decrease in the risk of MI (RR 0.96, 95% CI 0.85, 1.09) [32].
In a previous analysis too, vitamin D supplementation was not found to have any beneficial effect in the treatment of chronic heart failure. However, it did increase quality of life and exercise tolerance while decreasing the levels of inflammatory mediators in chronic heart failure patients [2], [46]. It is important to note here that both included studies investigated the association of vitamin D status with heart failure in patients with acute coronary syndromes [3], [20]. Thus, the result could not be generalized to other populations.
Our study has several limitations. We have included only the observational studies in our analysis; the results of which might have been influenced by confounding variables thus affecting the overall pooled results as well. In addition, significant heterogeneity was observed across studies included in the pooled analysis of MACE events. Besides, not all included studies mentioned all the predefined primary and secondary outcomes along with different levels of Vitamin D for comparison. Thus, only a few studies could be included in the final analyses of different outcomes. While the findings from our study could be well generalized since the included studies are from different continents, some studies [3], [19], [20], [21], [22], [23] included only cardiovascular disease patients which would decrease the generalizability of our findings to the general population.
5. Conclusion
In conclusion, HVD was found to be significantly associated with MACE. However, due to the observational nature of included studies, the evidence is not yet strong enough to suggest increased screening for HVD or vitamin supplementation for cardiovascular risk reduction. Future randomized controlled trials with enough power and a large sample of vitamin D deficient participants are required to determine whether there is causal association between HVD and cardiovascular events, and to further explore the beneficial effect of vitamin D supplementation in CVD events.
Source of funding
None.
CRediT authorship contribution statement
Vikash Jaiswal: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition. Angela Ishak: Writing – original draft, Writing – review & editing, Funding acquisition. Song Peng Ang: Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Funding acquisition. Nishan Babu Pokhrel: Writing – original draft, Writing – review & editing, Funding acquisition. Nishat Shama: Writing – original draft, Writing – review & editing, Funding acquisition. Kriti Lnu: Conceptualization, Writing – original draft, Writing – review & editing. Jeffy Susan Varghese: Funding acquisition. Tatyana Storozhenko: Funding acquisition. Jia Ee Chia: Funding acquisition. Sidra Naz: Writing – review & editing. Prachi Sharma: Writing – review & editing, Funding acquisition. Akash Jaiswal: Conceptualization, Validation, Supervision, Review and edit.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We want to thank Gazala Hitawala for helping in graphical illustrations, Gaurav Nepal, and Dr Ruchika all for their immense help in the manuscript. This research has not been funded by any authors or third party source.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2022.101019.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Verdoia M., Nardin M., Rolla R., Negro F., Gioscia R., Afifeh A.M.S., Viglione F., Suryapranata H., Marcolongo M., De Luca G. Prognostic impact of Vitamin D deficiency in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur. J. Internal Med. 2021;83:62–67. doi: 10.1016/j.ejim.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Wang L.u., Song Y., Manson J.E., Pilz S., März W., Michaëlsson K., Lundqvist A., Jassal S.K., Barrett-Connor E., Zhang C., Eaton C.B., May H.T., Anderson J.L., Sesso H.D. Circulating 25-Hydroxy-Vitamin D and Risk of Cardiovascular Disease: A Meta-Analysis of Prospective Studies. Circ: Cardiovasc. Quality Outcomes. 2012;5(6):819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng L.L., Sandhu J.K., Squire I.B., Davies J.E., Jones D.J.L. Vitamin D and prognosis in acute myocardial infarction. Int. J. Cardiol. 2013;168(3):2341–2346. doi: 10.1016/j.ijcard.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Palacios C., Gonzalez L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014;144:138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick M.F., Vitamin D. Deficiency. N Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Grandi N.C., Breitling L.P., Brenner H. Vitamin D and cardiovascular disease: Systematic review and meta-analysis of prospective studies. Prev. Med. 2010;51(3-4):228–233. doi: 10.1016/j.ypmed.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S., Hawken S., Ôunpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 8.Sokol S.I., Tsang P., Aggarwal V., Melamed M.L., Srinivas V.S. Vitamin D Status and Risk of Cardiovascular Events: Lessons Learned via Systematic Review and Meta-Analysis. Cardiol. Review. 2011;19(4):192–201. doi: 10.1097/CRD.0b013e31821da9a5. [DOI] [PubMed] [Google Scholar]
- 9.Barbarawi M., Kheiri B., Zayed Y., et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019;4:765. doi: 10.1001/jamacardio.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elamin M.B., Abu Elnour N.O., Elamin K.B., Fatourechi M.M., Alkatib A.A., Almandoz J.P., Liu H., Lane M.A., Mullan R.J., Hazem A., Erwin P.J., Hensrud D.D., Murad M.H., Montori V.M. Vitamin D and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2011;96(7):1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R., Li B., Gao X., Tian R., Pan Y., Jiang Y., Gu H., Wang Y., Wang Y., Liu G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017;105(4):810–819. doi: 10.3945/ajcn.116.140392. [DOI] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;2021:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giustina A., Adler R.A., Binkley N., Bouillon R., Ebeling P.R., Lazaretti-Castro M., Marcocci C., Rizzoli R., Sempos C.T., Bilezikian J.P. Controversies in Vitamin D: Summary Statement From an International Conference. J. Clin. Endocrinol. Metab. 2019;104(2):234–240. doi: 10.1210/jc.2018-01414. [DOI] [PubMed] [Google Scholar]
- 14.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Method. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 15.J.P.T. Higgins, J. Thomas, J. Chandler, et al. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions. 1st ed. Wiley, 2019.
- 16.StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC, 2021.
- 17.G. Wells, B. Shea, D. O’Connell, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- 18.Dror Y., Giveon S.M., Hoshen M., Feldhamer I., Balicer R.D., Feldman B.S. Vitamin D Levels for Preventing Acute Coronary Syndrome and Mortality: Evidence of a Nonlinear Association. J. Clin. Endocrinol. Metab. 2013;98(5):2160–2167. doi: 10.1210/jc.2013-1185. [DOI] [PubMed] [Google Scholar]
- 19.Khalili H., Talasaz A.H., Salarifar M. Serum vitamin D concentration status and its correlation with early biomarkers of remodeling following acute myocardial infarction. Clin. Res. Cardiol. 2012;101(5):321–327. doi: 10.1007/s00392-011-0394-0. [DOI] [PubMed] [Google Scholar]
- 20.Khademvatani K., Mohammadzad M.S., Yekta Z., et al. The association of serum vitamin D concentration and ventricular dysfunction among patients with acute coronary syndrome. TCRM. 2017;13:1455–1461. doi: 10.2147/TCRM.S144437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahdavi K., Amirajam Z., Yazdankhah S., Majidi S., Adel M.H., Omidvar B., Alasti M. The Prevalence and Prognostic Role of Vitamin D Deficiency in Patients with Acute Coronary Syndrome: A Single Centre Study in South-West of Iran. Heart Lung Circulation. 2013;22(5):346–351. doi: 10.1016/j.hlc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Schiller A., Gadalean F., Schiller O., Timar R., Bob F., Munteanu M., Stoian D., Mihaescu A., Timar B., Seguro A.C. Vitamin D Deficiency—Prognostic Marker or Mortality Risk Factor in End Stage Renal Disease Patients with Diabetes Mellitus Treated with Hemodialysis—A Prospective Multicenter Study. PLoS ONE. 2015;10(5):e0126586. doi: 10.1371/journal.pone.0126586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker J.P., Hiramoto J.S., Gasper W.J., Auyang P., Conte M.S., Rapp J.H., Lovett D.H., Owens C.D. Vitamin D deficiency is associated with mortality and adverse vascular access outcomes in patients with end-stage renal disease. J. Vasc. Surg. 2014;60(1):176–183. doi: 10.1016/j.jvs.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdhury R., Kunutsor S., Vitezova A., Oliver-Williams C., Chowdhury S., Kiefte-de-Jong J.C., Khan H., Baena C.P., Prabhakaran D., Hoshen M.B., Feldman B.S., Pan A., Johnson L., Crowe F., Hu F.B., Franco O.H. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348(apr01 2) doi: 10.1136/bmj.g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou A., Selvanayagam J.B., Hyppönen E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2021 doi: 10.1093/eurheartj/ehab809. [DOI] [PubMed] [Google Scholar]
- 26.Scragg R., Stewart A.W., Waayer D., Lawes C.M.M., Toop L., Sluyter J., Murphy J., Khaw K.-T., Camargo C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017;2(6):608. doi: 10.1001/jamacardio.2017.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manson J.E., Bassuk S.S., Buring J.E. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J. Steroid Biochem. Mol. Biol. 2020;198:105522. doi: 10.1016/j.jsbmb.2019.105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virtanen J.K., Nurmi T., Aro A., Bertone-Johnson E.R., Hyppönen E., Kröger H., Lamberg-Allardt C., Manson J.E., Mursu J., Mäntyselkä P., Suominen S., Uusitupa M., Voutilainen A., Tuomainen T.-P., Hantunen S. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: a randomized controlled trial. Am. J. Clin. Nutrit. 2022 doi: 10.1093/ajcn/nqab419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouillon R., Manousaki D., Rosen C., Trajanoska K., Rivadeneira F., Richards J.B. The health effects of vitamin D supplementation: evidence from human studies. Nat. Rev. Endocrinol. 2022;18(2):96–110. doi: 10.1038/s41574-021-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.G. Bjelakovic, L.L. Gluud, D. Nikolova, et al., Vitamin D supplementation for prevention of mortality in adults. Cochrane Metabolic and Endocrine Disorders Group (Ed.). Cochrane Database of Systematic Reviews 2014, DOI: 10.1002/14651858.CD007470.pub3. [DOI] [PMC free article] [PubMed]
- 31.Zittermann A., Ernst J.B., Prokop S., et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur. Heart J. 2017;38:2279–2286. doi: 10.1093/eurheartj/ehx235. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Fang F., Tang J., et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019:l4673. doi: 10.1136/bmj.l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neale R.E., Baxter C., Romero B.D., McLeod D.S.A., English D.R., Armstrong B.K., Ebeling P.R., Hartel G., Kimlin M.G., O'Connell R., van der Pols J.C., Venn A.J., Webb P.M., Whiteman D.C., Waterhouse M. The D-Health Trial: a randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. 2022;10(2):120–128. doi: 10.1016/S2213-8587(21)00345-4. [DOI] [PubMed] [Google Scholar]
- 34.Sofianopoulou E., Kaptoge S.K., Afzal S., et al. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021;9:837–846. doi: 10.1016/S2213-8587(21)00263-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Joergensen C., Gall M.-A., Schmedes A., Tarnow L., Parving H.-H., Rossing P. Vitamin D Levels and Mortality in Type 2 Diabetes. Diabetes Care. 2010;33(10):2238–2243. doi: 10.2337/dc10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joergensen C., Hovind P., Schmedes A., Parving H.-H., Rossing P. Vitamin D Levels, Microvascular Complications, and Mortality in Type 1 Diabetes. Diabetes Care. 2011;34(5):1081–1085. doi: 10.2337/dc10-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayedi A., Soltani S., Shab-Bidar S. Vitamin D Status and All-Cause Mortality in Patients With Chronic Kidney Disease: A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Endocrinol. Metab. 2017;102:2136–2145. doi: 10.1210/jc.2017-00105. [DOI] [PubMed] [Google Scholar]
- 38.London G.M., Guérin A.P., Verbeke F.H., Pannier B., Boutouyrie P., Marchais S.J., Mëtivier F. Mineral Metabolism and Arterial Functions in End-Stage Renal Disease: Potential Role of 25-Hydroxyvitamin D Deficiency. JASN. 2007;18(2):613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 39.Chonchol M., Greene T., Zhang Y., Hoofnagle A.N., Cheung A.K. Low Vitamin D and High Fibroblast Growth Factor 23 Serum Levels Associate with Infectious and Cardiac Deaths in the HEMO Study. JASN. 2016;27(1):227–237. doi: 10.1681/ASN.2014101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krause R., Schober-Halstenberg H.-J., Edenharter G., et al. Vitamin D status and mortality of German hemodialysis patients. Anticancer Res. 2012;32:391–395. [PubMed] [Google Scholar]
- 41.Ginde A.A., Mansbach J.M., Camargo C.A. Association Between Serum 25-Hydroxyvitamin D Level and Upper Respiratory Tract Infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009;169(4):384. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bucharles S., Barberato S.H., Stinghen A.E.M., Gruber B., Piekala L., Dambiski A.C., Custodio M.R., Pecoits-Filho R. Impact of Cholecalciferol Treatment on Biomarkers of Inflammation and Myocardial Structure in Hemodialysis Patients Without Hyperparathyroidism. J. Renal Nutrit. 2012;22(2):284–291. doi: 10.1053/j.jrn.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Huang J., Wang Z., Hu Z., Jiang W., Li B. Association between blood vitamin D and myocardial infarction: A meta-analysis including observational studies. Clin. Chim. Acta. 2017;471:270–275. doi: 10.1016/j.cca.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Bolland M.J., Grey A., Gamble G.D., Reid I.R. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(4):307–320. doi: 10.1016/S2213-8587(13)70212-2. [DOI] [PubMed] [Google Scholar]
- 45.Ford J.A., MacLennan G.S., Avenell A., Bolland M., Grey A., Witham M. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am. J. Clin. Nutrit. 2014;100(3):746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 46.Jiang W.-L., Gu H.-B., Zhang Y.-F., Xia Q.-Q., Qi J., Chen J.-C. Vitamin D Supplementation in the Treatment of Chronic Heart Failure: A Meta-analysis of Randomized Controlled Trials: Vitamin D in treatment of CHF. Clin. Cardiol. 2016;39(1):56–61. doi: 10.1002/clc.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.