Abstract
An approach to removing crystal violet (CV) dye from aqueous solutions was investigated by introducing a xanthate group on charred rice husk. The newly prepared charred rice husk (CRH) and xanthated rice husk (XRH) were characterized by XRD, SEM, FTIR, and elemental analysis. A batch technique was used to adsorb CV dye in aqueous suspensions. Different adsorbent quantities, concentrations, pH, and contact times were investigated to find the effect of these parameters. The optimum pH for both CRH and XRH was found to be 10. The adsorption capacity of CV dye onto CRH and XRH was found to be 62.85 mg/g and 90.02 mg/g at pH10, respectively. Langmuir isotherms could be reasonably explained by the experimental data. Within 60 min, equilibrium was achieved. Similarly, the kinetic data are best suited to the pseudo-second-order model. In comparison to XRH with CRH, XRH was found more efficient and can be used as a feasible alternative for removing CV dye from aqueous solutions.
Keywords: Adsorption isotherm, Bio-adsorbent, Crystal violet dye, Xanthated rice husk
Adsorption isotherm; Bio-adsorbent; Crystal violet dye; Xanthated rice husk.
1. Introduction
The environmental pollution and health hazards of dye contamination [1] are of great concern in almost all the parts of the developing world because its users impart color to textiles, paper, leather, and other materials to decorate it and to protect them. A dye is an imperfectly ground solid dissolved in a liquid, such as paint or ink, or mixed with another material. Pigments are finely ground solid dispersed in a liquid [2]. Both natural and artificial dyes are found in widespread use because of their decorative and protective properties.
In ancient times, crude methods allowed the separation of natural dyes from their complex mixtures, but today most are highly unstable and difficult to separate successfully. These dyes also provided a basis for the development of synthetic dyes. Unlike most organic compounds, dyes collect light from the visible spectrum, have chromophores, and exhibit a conjugated structure [3]. The color of a molecule is lost when any of these characteristics are absent. As dye-bearing wastewaters are difficult to treat with conventional methods, removing color from them is a major issue.
All the wastewater from dye manufacturing and textile finishing is discharged into rivers, and these water bodies contain a variety of toxic organic compounds harmful to fish and animals in the aquatic ecosystem [4]. Colors are used by many industries, including dyestuffs, textiles, paper, and plastics. Since they generate significant amounts of colored wastewater byproducts, they end up draining into the water sources [5]. Urbanization and population growth have resulted in such industrial advances, knowingly or unknowingly, increasing pollution levels. To prevent the release of industrial waste effluents into the environment, textile companies must treat their effluents before released into the environment [6]. By contaminating water with synthetic dye molecules, the environment suffers and public health is adversely affected.
Additionally, dyes are an esthetic pollutant and eutrophication. Color is necessary for industries such as textiles, leather, paper, paint, acrylic, cosmetics, plastic, pharmaceuticals, etc., which use dyes to color their products. They also consume considerable amounts of water [7]. The result is that colored wastewater is generated in significant quantities. Therefore, this study was undertaken to eliminate harmful dye contamination in drinking water.
Before wastewater is discharged, dyes must be eliminated. Adsorption methods include alum, lime, ferric sulfate, and ferric chloride, as well as chemical oxidation using chlorine and ozone [8], membrane separation processes [9], and adsorption [10, 11]. Treatments based on adsorption appear to have the greatest potential. The physicochemical techniques of adsorption and electrochemical coagulation have gained popularity over the last few years. As a result of its ease of use and versatility, adsorption is becoming more popular [12, 13].
Several methods have been used to remove dyes, in addition to oxidation with ozone or hydrogen peroxide, biological degradation, membrane filtration, and ion exchange [14], electrochemical oxidation [15], reverse osmosis [16], photocatalytic degradation [17], and adsorption [18], there are several other techniques available. The effective removal of color, the capital costs, and the operating costs of each of these methods vary.
Chakraborty et al. evaluated the adsorption of CV dye on modified rice husk (NMRH) from equilibrium, kinetic, and thermodynamic perspectives in 2011 [19]. Similarly, an experimental batch study was conducted on the adsorption of CV dye on chemically modified rice husk (CMRH) by Das et al. in the year 2012 [20]. The adsorbent CMRH showed the highest adsorption efficiency.
Adsorbent nanoparticles containing rice husk (TARH) were used to remove the CV dye from water [21]. An improved magnetic biochar nanocomposite (MBC) prepared from rice husk and iron oxide nanoparticles (IONPs) was evaluated for removing CV dye from aqueous solutions [22]. NaOH-modified rice husk was investigated for its ability to effectively adsorb CV dye from aqueous solution in laboratory-scale fixed-bed columns [23]. Rice husk carbon (RHC) was shown to adsorb CV dye from aqueous solutions [24].
In one of the experiments, ultrasonic technology was used to obtain bio-nanosilica from rice husk and aqueous effluents were used to adsorb CV dye [25]. Dye-contaminated water was treated using rice husk (RH). A chemical modification was used to synthesize rice husk ash-mediated zeolites (Z-RHA) and a comparative study for the decontamination of aqueous solutions containing CV dye was performed [26]. In the literature [12], there was feasible to remove CV from wastewater using available and affordable RH as an adsorbent. RHC was also used to remove CV dye. In addition, it has been investigated the effects of various parameters included are agitation time, pH, and dosage of adsorbent [27].
It has been reported that rice husk treated with succinic acid can be used to remove dye from aqueous solutions [28]. Moreover, the adsorption pattern of CV dye on rice husk treated with succinic acid was also investigated by using a cyclic voltammetry technique. This revealed an increase in the oxidation and reduction streams, along with the appearance of an oxidation peak.
Van Hung synthesized nanosilica from rice husk and used it to remove CV dye from an aqueous solution [29]. Activated rice husk was prepared by treating rice husk with nitric acid. To decolorize wastewater containing crystal violet, the adsorption capacity was evaluated [30]. Rice husk treated with sodium carbonate and potassium hydrogen phosphate were all found to be effective at removing CV dye from aqueous solutions. The process of dye adsorption onto raw rice husk (RRH) increases the chemical oxygen demand (COD) of the treated wastewater, therefore, it cannot be a great biosorbent. Additionally, they reduce the COD of wastewater treated with them as well as remove dyes from wastewater. COD analysis should be conducted to determine the suitability of the biosorbent [31].
Synthetic dyes like crystal violet are not biodegradable, toxic, mutagenic, and carcinogenic, and can also induce skin cancer and allergies even at low concentrations. It is possible to have nausea, vomiting, diarrhea, and gastritis after consuming crystal violet through the mouth. If a large amount is accidently consumed, it can result in abdominal and chest pain, severe headaches, excessive sweating, mental confusion, painful micturition, and hemoglobinemia. The inhaling crystal violet may experience headaches, dizziness, vomiting, and diarrhea. Mucous membranes and the gastrointestinal tract can be damaged by long-term exposure [32].
There was no research regarding CRH and XRH for removing CV dye from an aqueous solution. In this research, agricultural waste material rice husk with no cost is used to prepare a low-cost eco-friendly and biodegradable adsorbent to sequester CV dye from contaminated water. In rice husk, there are 32.24 % cellulose, 21.34% hemicellulose, 21.44% lignin, and 15.05 % mineral ash [33]. Rice husks can be pretreated with acid to remove lignin, hemicellulose, and crystallinity to boost porosity and surface area. A good adsorbent for wastewater treatment is modified rice husk, which is insoluble in water, has good chemical stability and is highly mechanically stable. A comparison of the effectiveness between pretreated (CRH) and chemically modified (XRH) rice husk for removing CV dye from an aqueous solution has also been examined in this paper.
2. Experimental
2.1. Materials
Crystal violet dye, concentrated sulphuric acid, sodium hydroxide and buffer solution were used of AR grades manufactured from Merck, India without further purification. Laboratory grinder, sieve of 250 μm size, electronic balance (Phoenix, PH2204C, India), laboratory oven, pH meter (Labtronics, India), mechanical shaker, and UV-VIS spectrophotometer double beam (Labtronics, LT-2802, India) were used.
2.2. Methods
2.2.1. Analytical procedure of XRD
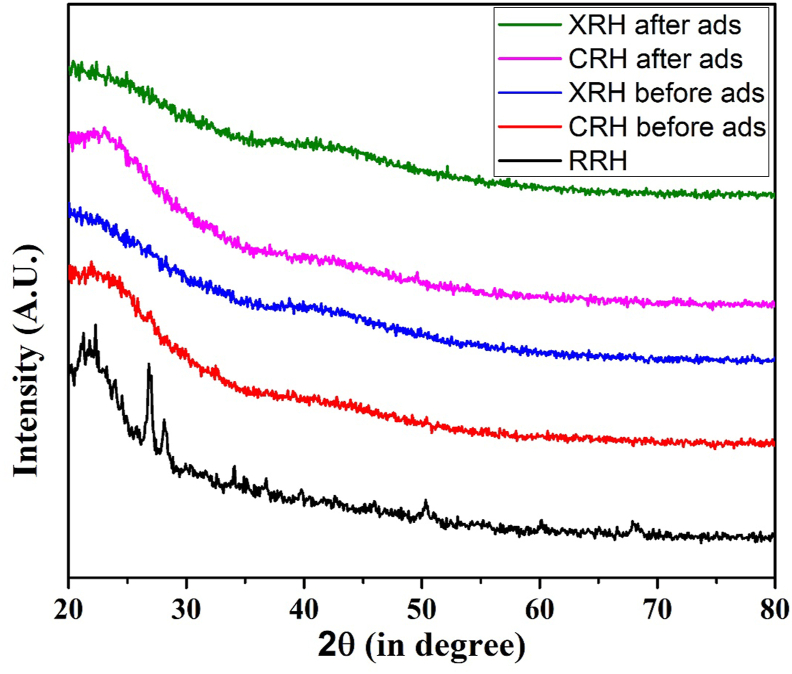
The XRD patterns were achieved from the Nepal Academy of Science and Technology (NAST) where the X-ray is generated from the copper tube with the wavelength of 1.544 Å The X-ray source is operating at 30kV/10mA. Both types of pyrolysis (treating with conc. H2SO4 and heating in an inert atmosphere of N2) change the structure of CRH and XRH which can be noticed without sharp peaks. It was found in the literature [34] that the XRD spectrum of rice husk silica showed a broad peak at the 2θ value of 22°, a characteristic of silica in the amorphous form which is matched with our findings. Silica was washed out from the adsorbent at the time of modification, changing it in the form of soluble sodium silicate.
2.2.2. Analytical procedure of FE-SEM
Field-Emission Scanning Electron Microscopy (FE-SEM) can obtain topographical and elemental data at magnifications ranging between 10 × and 300,000×. FE-SEM offers three to six times better spatial resolution than conventional SEM and yields clearer, less electrostatically distorted images [35]. An FE-SEM can also use low accelerating voltages to obtain high-quality images without causing significant damage to samples (accelerating voltage under 0.5–30 kV). FE-SEM is unique in that it does not require coating conductors with insulating materials [36].
2.2.3. Preparation of charred rice husks (CRH)
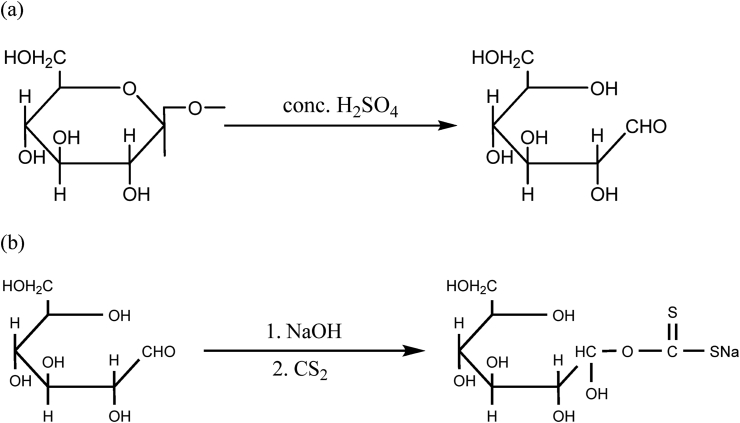
Raw rice husks were collected from the local rice mill located at Syuchatar, Kalanki, Kathmandu, Nepal. 200 g of raw rice husk was placed in a 5L bucket, then concentrated sulphuric acid was added, stirring it with a wooden spatula until it turns completely black. The materials were left for 24 h to complete the reaction. To remove excess acid and any other soluble materials, the item was first washed with deionized water until neutral, followed by drying. The dried charcoal was ground and sieved with 250 μm sieves, then again were put in an oven at 80 °C till it resumes constant weight. Eventually, the charred rice husk was kept in the desiccator and it was ready for experiments as well as for further modification. Thus, the prepared rice husk is known as charred rice husk (CRH). Acid treatment with biomaterials creates suitable active sites with the opening of the ring [37] of the biopolymers proposed in Scheme 1(a) and xanthation in Scheme 1(b). A plausible mechanism of CV ions onto XRH is shown in Scheme 2.
Scheme1.
(a) Cellulose monomer ring-opening proposed in charring process of rice husk followed by (b) xanthation.
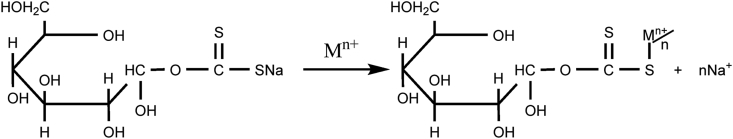
Scheme 2.
A plausible mechanism for the adsorption of CV ions onto monomeric cellulose units in XRH.
2.2.4. Preparation of xanthated rice husks (XRH)
Using the previous article as a guide, the preparation process was followed [38]. Dehydrating sulphuric acid can facilitate chemical modifications. 50 g of Charred rice husk was mixed into 250 mL of 15% of NaOH and stirred for 1h where any silica present is changed to soluble sodium silicate in the alkaline medium. Then 25 mL of CS2 was mixed and shook for 3 h then left for the night. The suspension was cleaned many times with deionized water to make it neutral. Lastly, the materials were dried at 80 °C in an oven till it resumes constant weight and the sample was placed in a desiccator. The materials are called xanthated rice husk (XRH) that are ready for the adsorption studies and it is shown in schematic diagram 1(b).
2.2.5. Preparation of chemicals
Dissolution of 1g of CV dye in 1000 mL volumetric flask with pure water to prepare a stock solution of 1000 ppm was performed. Similarly, other working solutions of required concentrations were diluted using 0.1M sodium hydroxide or hydrochloric acid.
2.2.6. Adsorption experiments
25 mg of dried adsorbent was kept in a 50 mL Erlenmeyer flask with 25 mL of 50 mg/L test solution and then shaken the flask at room temperature for 24 h until equilibrium was attained. By using a spectrophotometer, the dye ions were measured at their initial and equilibrium concentrations.
The adsorption of dye ion onto adsorbent is affected by different parameters, viz; pH of dye solution, adsorbent doses (mg), initial dye concentration (mg/L) and contact time (min). The effect of each parameter can be studied by altering any one of the above parameters and keeping others constant. The amount of dye ion adsorbed onto adsorbent is calculated using Eq. (1):
| (1) |
To calculate the adsorption efficiency (A%) of the dye ion, Eq. (2) can be used as:
| (2) |
Here q shows the amount of dye ion taken up per unit mass (mg/g), V is the volume of adsorbate solution in liter, W is mass (g) of dry adsorbent, Ci and Ce are the initial and equilibrium concentrations (mg/L) of adsorbate, respectively.
3. Results and discussion
3.1. X-ray diffraction (XRD) analysis of adsorbents
In Figure 1, XRD patterns for RRH, CRH, and XRH show weak and unresolved peaks, indicating amorphous nature. The amorphous nature of the biosorbent made its use in biosorption possible, which allows the dye ions to penetrate the surface rapidly. Reddy et al. [39] claimed that the biosorbent's amorphous nature is due to a high content of lignin, cellulose, and hemicellulose. There is a notable difference between the XRD patterns of RRH before modification and after modification forming adsorbents such as CRH and XRH. The XRD patterns showed that there is no noticeable change in the peak intensity after the modification of CRH and XRH even after dye ions adsorption, which may be due to the amorphous character of lignin, cellulose, and hemicellulose.
Figure 1.
X-ray diffraction patterns of RRH, CRH, CRH (ads), XRH, and XRH (ads).
3.2. FE-SEM analysis
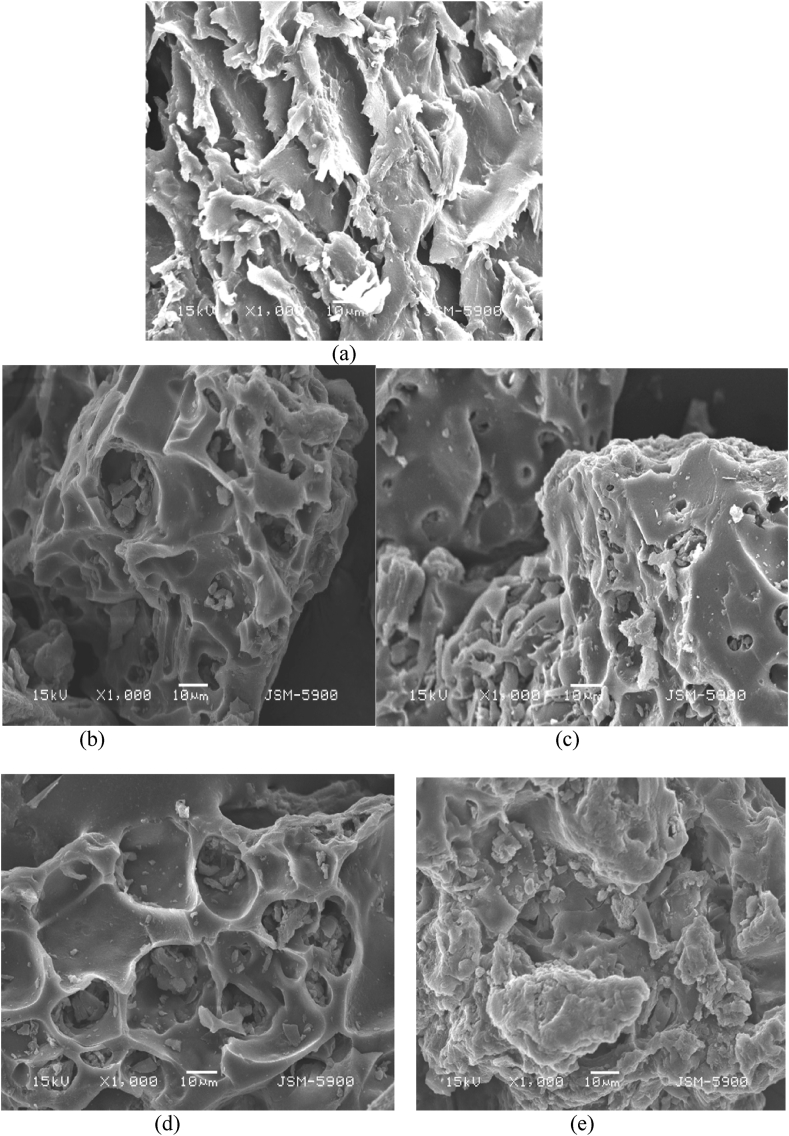
In this study, FE-SEM was used to examine the surface morphology of RRH, CRH, CRH (ads) before and after the chemical modification of XRH and XRH (ads). As a result of the hydrolysis reaction caused by concentrated sulphuric acid on the surface of RRH, the fiber surface of CRH became rough and non-uniform. The surface morphologies of RRH, CRH, CRH (ads), and XRH (ads) were characterised by FE-SEM images shown in Figure 2 (a), (b), (c), (d), and (e). A charred rice husk fabricated in a xanthation process shows a remarkable increase in surface textural property, chemical composition, and biodegradability, which can vary depending on the type of side chains. FE-SEM analysis of CRH showed that its irregular honeycomb structure was changed into non-uniform, rough surfaces in XRH. Before adsorption, the surface of both the adsorbents CRH and XRH was found to be rough and irregular in shape.
Figure 2.
SEM images of (a) RRH, (b) CRH, (c) CRH (ads), (d) XRH and (e) XRH (ads).
According to the XRH, isolated and irregular pores were formed due to the increased surface area of effective diffusion, which provided the additional surface-active binding sites and finally enhanced the adsorption capacity of the modified rice husks. In addition, both the CRH (ads) and XRH (ads) showed that honeycomb-like structures were filled after the adsorption of dye, signifying its efficiency. In contact with CV dye ions, the biosorbent surface developed flakes-like deposits, and interactions with the ions smoothed out the surface. As a result of physicochemical interactions between the target ions and functional groups on the biosorbents surface, the surface of the biosorbent became bright and smooth following dye ion adsorption.
3.3. FTIR analysis
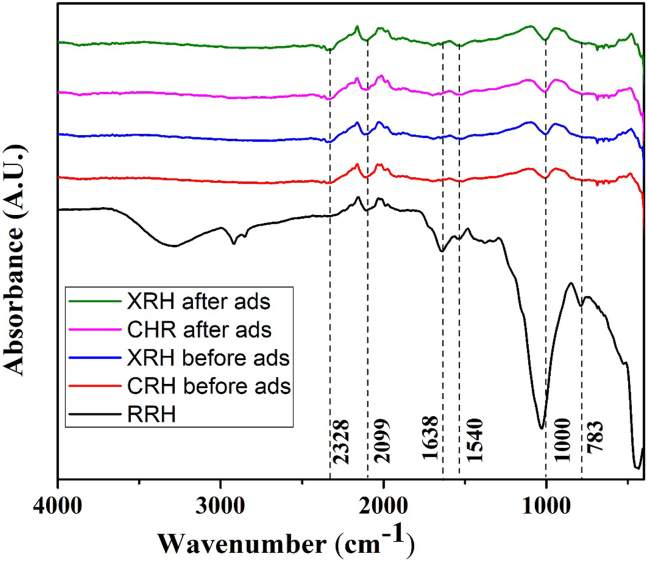
The combined Fourier Transformation Infrared Spectrum (FTIR) is shown in Figure 3. FTIR spectra of the adsorbents were obtained using IR Tracer between 400-4000cm−1. FTIR spectrum analysis shows that the broadband between 3200-3600cm−1is due to OH vibrations in alcohols, phenols, and carboxylic acids. RAW samples have an intense band, but modified samples have a reduced intensity. Therefore, the amount of carbon in the sample decreased after treatment due to the drastic decrease in moisture content. Medium adsorption near 3400 cm−1, sometimes showing a double peak, indicates the presence of N–H. An observed broad, intense peak at 3410 cm−1 is the result of bound hydroxyl groups.
Figure 3.
FTIR images showing the surface morphologies of RRH, CRH, and XRH of before and after adsorption.
C–H peaks are observed at 2919 cm−1, and peaks around 1034.48 cm−1 (RRH), 1211.63cm−1(CRH),1108.65 cm−1(CRH ads.), 1208cm−1 (XRH), and 1107.70cm−1 (XRH ads.) are the characteristic of the primary hydroxyl group is the C–O group. When CRH is not modified, the CHO group of this band was observed at 1704 cm−1. Once CRH is modified to XRH, the peak disappears and broadband at 1610.7cm−1 is depicted. The range of primary amine is 3100–3500cm−1and from the result extracted, it appears that the band 3406.28cm−1 of CRH has been changed to 3384.35cm−1, indicating that an amine group is now present on CRH. As can be seen from the graph, XRH, which contains nitrogen, was more effective than CRH as a bio-adsorbent in removing dye. After adsorption of cationic dye ions, some of the peaks in the absorbance bands between 2400 and 1500 cm−1 were less intense, and some peaks disappeared, indicating that carboxyl groups play a role similar to that of metal ions [40]. These results clearly show that NH, –OH, C–H, -O-C, and –C=O play an essential role in dye ion adsorption.
3.4. Elemental analysis
We used the element detector (Nepal Bureau of Standard and Metrology) to analyze the adsorbent sample to detect elements in it.
RRH, CRH, and XRH have different percentages of carbon, hydrogen, and sulphur, which indicates that the sample has been altered effectively (Table 1). Because carbon-disulphide was used during xanthation, XRH contains a higher sulphur percentage than raw and charred rice husk. As the percentage of sulphur increases onto XRH adsorbent, the adsorption of CV will be more in the experiments.
Table 1.
A comparative study of rice husks before and after modification.
| Samples | Carbon (%) | Hydrogen (%) | Sulphur (%) |
|---|---|---|---|
| RRH | 41.17 | 0.22 | 0.24 |
| CRH | 50.35 | 0.50 | 1.80 |
| XRH | 44.68 | 0.25 | 5.31 |
3.5. Point of zero charge
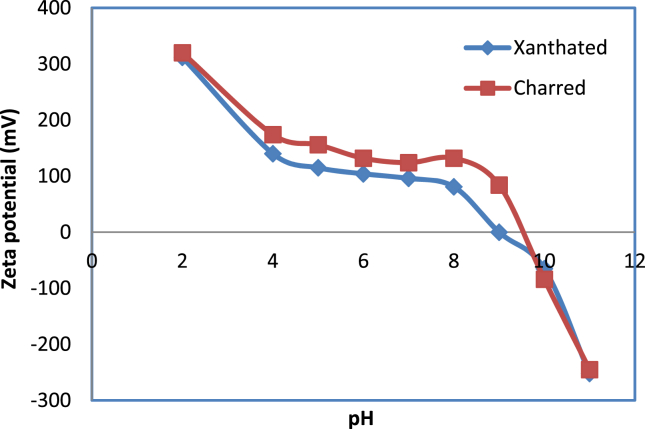
Based on point of zero charge , we can find the optimum pH of the solution. A pH at which an adsorbent surface has no net charge is as shown in Figure 4. Above the pHpzc, the surface charge for both adsorbents is negative.
Figure 4.
A typical plot of zeta potential vs. pH.
Adsorption of dye ions increases above pH 9 for XRH and pH10 for CRH. When pH is lower than pHpzc, then the surface charge becomes positive and there will repulsion between cationic CV dye and adsorbents, hence dye adsorption is decreased.
As a result of the interaction between cationic dye ions (Mn+) and negatively charged adsorbent surface, above the pHpzc adsorption found maximum. Similarly, dye ion adsorption decreases at pH values below pHpzc. Consequently, the dye ions (Mn+) can repel an adsorbent surface that has the same charge as dye ions. Therefore, dye ions adsorb via ion exchange mechanism.
3.6. Adsorption dose
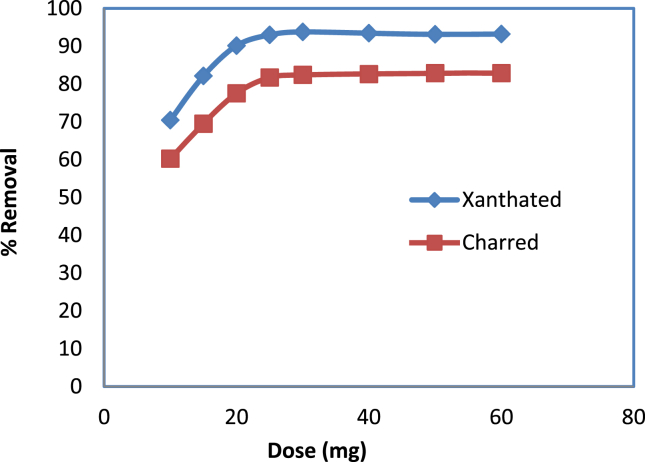
Adsorption at constant temperature was carried out with different adsorbent dosages to optimize adsorbent dosage for removal of CV dye from aqueous solution. Adsorption efficiency of CV uptake increases up to a certain level then it remains constant with increasing dosage of adsorbent varied from 10 mg to 60 mg at fixed pH, temperature, and adsorbate concentration as shown in Figure 5. Initially, the number of active sites of adsorbent is less at low dosages to uptake the dye ion and as the amount of adsorbent dosages increases then the percentage of removal also increases with the increase of active sites. The removal percentage increases up to 25 mg adsorbent dose then the removal remains constant as the increase of adsorbent dose. Hence, the highest adsorption for 50 ppm adsorbate of CV dye is found to be 25mg adsorbent. There is no chance of adsorption when most of the adsorbate molecules occupy the active sites of the adsorbent, no matter how large the dosage of the adsorbent is.
Figure 5.
Removal versus adsorbent dose (mg).
3.7. Effect of pH
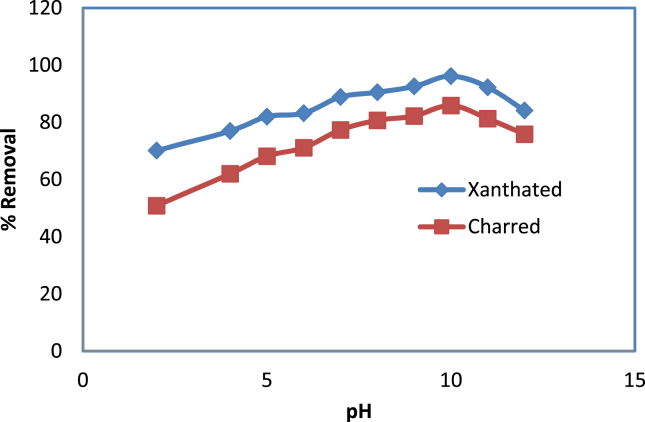
Figure 6 shows the effect of pH on the adsorption of CV dyes at initial concentrations of 25ppm onto charred rice husks and xanthated rice husks at room temperature simultaneously. Since pH influences the surface charge of adsorbents and the ionization of adsorbates, the pH of the solution impacts CV dye uptake. Both charred rice husks and xanthated rice husks with CV dye were tested for the effect of pH on the biosorption of CV dye at different pH values between 1.0 and 12.0. The percentage removal of CV dye by CRH increased from 50.85% to 85.89% and then again decreased whereas XRH increased from 70.11% to 96.16% and then again got decreased when the pH of the CV dye solution increased from 1.0 to 10.0.
Figure 6.
Effect of pH for adsorption of CV dye onto CRH and XRH.
pH studies showed that CRH and XRH were optimum at 10.0. XRH is also found to be more effective than CRH at 98.16 % removal, while 85.89 % at CRH. The adsorption rate increases rapidly near optimum pH. Because of the sharp increase in pH of the final solution, the maximum amount of dye adsorption occurred at the optimum pH level.
3.8. Effect of concentration
The adsorption capacity of CRH and XRH can be evaluated with the help of an adsorption isotherm for the adsorption of CV dye onto these adsorbents. As the concentration of dye ions increases, the adsorption of dye ions increases. It is due to the limited number of active sites in an adsorbent, and a plateau of isotherm is reached. Adsorbents have sufficient active sites at low dye concentrations, whereas at high concentrations the active sites are significantly diminished as compared to dye ions. Equal amounts of adsorbents were placed in the flask to conduct an adsorption isotherm experiment. The absorbance of the supernatant clear liquid is measured spectrophotometrically at the dye's maximum wavelength after 4 h equilibration interval. A graph was used to calculate the amount of dye adsorbed. The correlation coefficient values for Freundlich and Langmuir isotherms were used to determine the applicability of the isotherms. Adsorption isotherms were calculated utilizing Langmuir and Freundlich's relation by Eqs. (3) and (4), respectively.
| (3) |
| (4) |
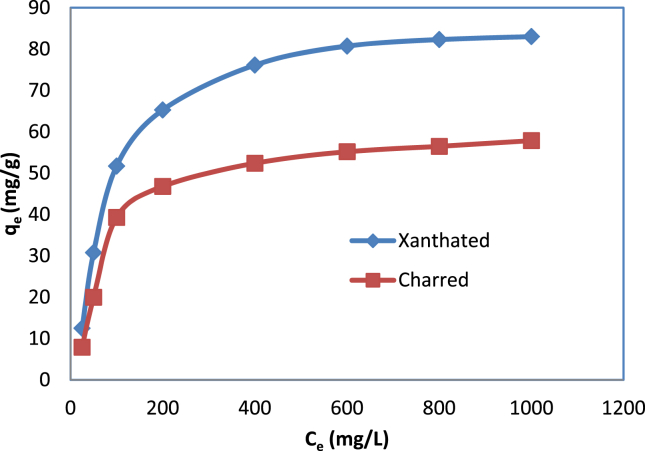
Here, Ce (mg/L) = concentration of adsorbate at equilibrium, qe (mg g−1) = amount of adsorbate adsorbed by an adsorbent, qm (mg g−1) = maximum adsorption capacity, and b (L/mg) = binding constant. The qm and b can be evaluated by plotting Ce/qe against Cewhich provide a linear line of 1/qm slope and 1/qm b intercept. KF = adsorption capacity in L/mg, and 1/n = adsorption intensity, which also represents the relative energy distribution and adsorption site heterogeneity. The maximum adsorption capacities of experimental results shown in Figure 7 are 90.02 mg/g and 62.85 mg/g at 1000 mg/L for CV dye onto XRH and CRH, respectively, as its optimum pH is 10. According to the results extracted, effective adsorption occurs at XRH rather than CRH. Adsorption capacity may be increased by higher dye ion concentrations at the start of the process. As the concentration increases, the adsorbent becomes saturated.
Figure 7.
Effect of concentration on adsorption of CV dye onto CRH and XRH.
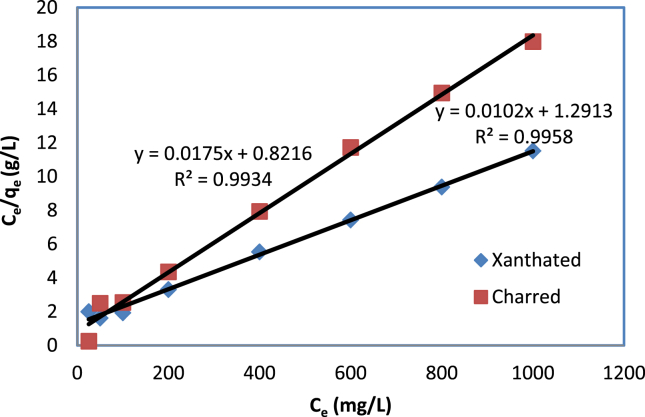
In Table 2, we present the Langmuir constants and their correlation coefficients based on the isotherm. Langmuir correlation coefficient (R2 = 0.9958 for XRH and 0.9934 for CRH) was found to be better than Freundlich isotherm on both CRH and XRH and found to be near unity. Hence a Langmuir isotherm model appears to be the best fit.
Table 2.
Parameters associated with Langmuir adsorption isotherms.
| Dye (CV) | (mg/g) | b (L/mg) | |
|---|---|---|---|
| CV onto XRH | 90.02 | 0.0102 | 0.9958 |
| CV onto CRH | 62.85 | 0.0175 | 0.9934 |
Additionally, in Langmuir's isotherm, the equilibrium parameter is a dimensionless constant (KL), given by Eq. (5):
| (5) |
Where, b = Langmuir constant and Ci = initial dye ion concentration. KL values show the nature of the isotherm and its value evaluated between 0 and 1 indicates favorable adsorption. The KL values calculated (data not given) from b values and different concentrations of dye ions were found to be between 0 and 1, which the Langmuir model to be supported.
After analysis, Figure 8 of the Langmuir plot for CV dye adsorption onto CRH and XRH, the correlation coefficient and Langmuir sorption capacity (qm) values are given in Table 2.
Figure 8.
Langmuir isotherm plot for CV dye adsorption onto CRH and XRH.
This study compared the maximum adsorption value of (mg/g) to other sorbents for crystal violet (CV) adsorption (Table 3). Results of this comparison showed that charred rice husk (CRH) and xanthated rice husk (XRH) are effective sorbents for removing crystal violet in aqueous systems. The adsorption capacity from our work competes with other studied sorbents which prove from higher values that are compared to other investigations, these results are significantly more reasonable.
Table 3.
Comparison of Langmuir sorption capacity for the adsorption of CV onto rice husk.
| Sorbents | (mg/g) | References |
|---|---|---|
| Charred Rice Husks (CRH) Xanthated Rice husks (XRH) |
62.85 90.02 |
our work |
| Rice husk ash | 125.43 | [10] |
| Sulfuric acid-activated rice husk carbon (RHS) Zinc chloride activated rice husk carbon (RHZ) |
64.875 61.575 |
[11] |
| Nascent Rice Husk | 24.4781 | [12] |
| Crosslinked poly tartaric acid-treated rice husk ash | 93.45 | [21] |
| Rice Husk Biochar-Based Magnetic Nanocomposite | 185.6 | [22] |
| Rice Husk ash (RHA) Carbon embedded silica from rice husk (CES) Carbon embedded zeolite from rice husk (Z-RHA) |
8.3 18.78 19.28 |
[26] |
| Activated Carbon of Lemon Wood (ACL)and Activated Carbon/Fe3O4 | 23.6 35.64 |
[41] |
3.9. Adsorption kinetics
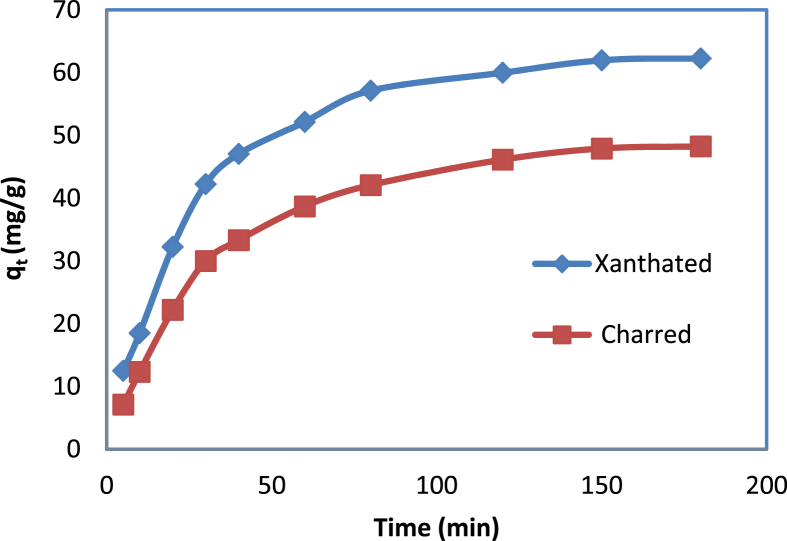
The dye adsorption rate plays an important role in determining the field adsorption application. Based on an initial concentration of 25 mg/L solution, the sorption kinetics of CV dye onto XRH and CRH has been analyzed for time. As dye ions were initially adsorbing rapidly with increasing time, they became slow and finally stable with time. Figure 9 shows that the amount of adsorption increased from 5 min up to 70 min, and then remained constant up to 180 min. We tested (data not given) all the CV dye ion kinetics using the pseudo-first-order rate equation and a very low Lagergren correlation coefficient (R2) value was found. Therefore, we evaluated the experimental kinetic adsorption data using a pseudo-second-order kinetics model.
Figure 9.
Adsorption kinetics of CV dye onto CRH and XRH.
Kinetic studies in the batch adsorption process reveal optimum conditions, sorption mechanisms, and potential rate-controlling steps. Ho and Mckay [42] gave the following integrated form of Eq. (6) for the pseudo-second-order model to explain adsorption kinetics:
| (6) |
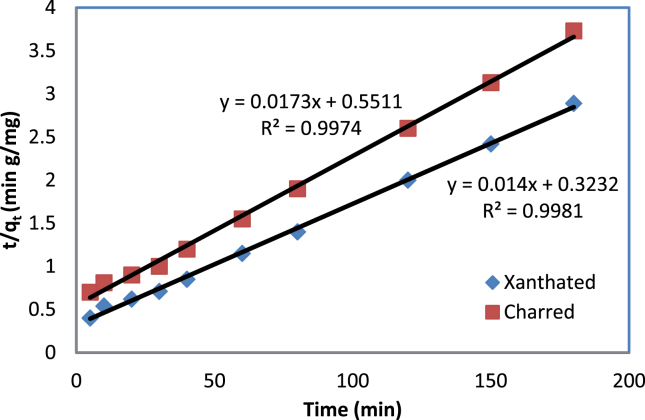
Here qt (mg g−1) = amount of the adsorption at time t (min), K2 (g mg−1 min−1) = rate constant of the pseudo-second-order kinetic adsorption. Using the intercept and slope of the plot of the experimental t/qt versus t, we can calculate K2 and qe. A pseudo-second-order kinetic model can explain the experimental data shown in Figure 10, where the correlation coefficient R2 for dye ions is almost unity (0.99). Based on a correlation coefficient of greater than 0.99, the model confirms that dye ions are chemisorbed to XRH. As a result of the kinetics experiment, we concluded that XRH adsorbent might effectively remove CV dye from aqueous solution within 70 min.
Figure 10.
Adsorption of CV dye onto XRH and CRH in pseudo-second-order.
After analysis, Figure 10 of adsorption of CV dye onto CRH and XRH, the correlation coefficient values noted higher than 0.9 which are given in Table 4.
Table 4.
Modeling pseudo-second-order kinetics of dye adsorption onto CRH and XRH.
| Dye (CV) | Slope | |
|---|---|---|
| CV onto XRH | 0.0140 | 0.9981 |
| CV onto CRH | 0.0173 | 0.9974 |
3.10. Plausible adsorption mechanism
XRH could react with cationic CV dye by electrostatic interaction, ion exchange, or chemical reaction. Cations from xanthate groups contained within the monomeric units of cellulose are exchanged by cationic CV dye ions in the solution. Using schematic diagram 2, we can see how the CV ion is interacting with the xanthate group via ion exchange to form the composite dye, where Mn+ represents the cationic CV dye. Here heavy and larger CV dye ion easily displaces smaller and lighter Na+ ion forming the new complex molecule. It has been suggested that a xanthate group is a soft base that tends to form stable complexes with soft acids such as CV cationic dye ions, according to the HESB theory by Pearson [43].
4. Conclusions
Based on the results of this study, rice husks can be chemically modified to enhance their performance in removing CV dye from contaminated aqueous solutions. CRH and XRH both performed well in adsorption experiments conducted in this study to remove CV dye.
Both CRH and XRH exhibited maximum dye uptake at pH 10 but XRH had an improved percentage removal compared to CRH i.e., 96.16% and 85.89% respectively with an adsorbent dose of 0.025 g and agitation speed of 190 rpm. Data were fit to Langmuir isotherm with high correlation coefficient values: 0.9958 and 0.9934 for XRH and CRH, respectively. It is concluded that CRH and XRH both follow pseudo-second-order kinetics, which is also indicated by larger correlation coefficient values: 0.9981 and 0.9974 for XRH and CRH, respectively. Additionally, the results revealed that XRH as a bio adsorbent may be a promising alternative for removing CV dye from aqueous solutions compared to CRH.
Declarations
Author contribution statement
Puspa Lal Homagai: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Rachana Poudel, Sujan Poudel: Performed the experiments; Analyzed and interpreted the data.
Ajaya Bhattarai: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Dr. Kamal Prasad Sapkota, Chonbuk National University (CBNU), South Korea for the opportunity to do some characterizations of the bioadsorbents.
References
- 1.Lellis B., Fávaro-Polonio C.Z., Pamphile J.A., Polonio J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019;3(2):275–290. [Google Scholar]
- 2.Hielscher K. Ultrasonic milling and dispersing technology for nano-particles. MRS Proceed. 2012;1479:21–26. [Google Scholar]
- 3.Abrahart E.N. Chemical Publishing; New York: 1977. Dyes and Their Intermediates; pp. 1–12. [Google Scholar]
- 4.Zabłocka-Godlewska E., Przystaś W., Grabińska-Sota E. Possibilities of obtaining from highly polluted environments: new bacterial strains with a significant decolorization potential of different synthetic dyes. Water, Air, Soil Pollut. 2018;229(6) doi: 10.1007/s11270-018-3829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ismail M., Akhtar K., Khan M.I., Kamal T., Khan M.A., Asiri M.A., Seo J., Khan S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharmaceut. Des. 2019;25(34):3645–3663. doi: 10.2174/1381612825666191021142026. [DOI] [PubMed] [Google Scholar]
- 6.Slama H.B., Chenari Bouket A., Pourhassan Z., Alenezi F.N., Silini A., Cherif-Silini H., Oszako T., Luptakova L., Golińska P., Belbahri L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021;11(14):6255. [Google Scholar]
- 7.Khattab T.A., Abdelrahman M.S., Rehan M. Textile dyeing industry: environmental impacts and remediation. Environ. Sci. Pollut. Control Ser. 2019;27(4):3803–3818. doi: 10.1007/s11356-019-07137-z. [DOI] [PubMed] [Google Scholar]
- 8.Brik M., Chamam B., Schöberl P., Braun R., Fuchs W. Effect of ozone, chlorine and hydrogen peroxide on the elimination of colour in treated textile wastewater by MBR. Water Sci. Technol. 2004;49(4):299–303. [PubMed] [Google Scholar]
- 9.Thamaraiselvan C., Noel M. Membrane processes for dye wastewater treatment: recent progress in fouling control. Crit. Rev. Environ. Sci. Technol. 2015;45(10):1007–1040. [Google Scholar]
- 10.Sivalingam S., Sen S. Rice husk ash derived nanocrystalline ZSM-5 for highly efficient removal of a toxic textile dye. J. Mater. Res. Technol. 2020;9(6):14853–14864. [Google Scholar]
- 11.Mohanty K., Naidu J.T., Meikap B.C., Biswas M.N. Removal of crystal violet from wastewater by activated carbons prepared from rice husk. Ind. Eng. Chem. Res. 2006;45(14):5165–5171. [Google Scholar]
- 12.Quansah J.O., Hlaing T., Lyonga F.N., Kyi P.P., Hong S.-H., Lee C.-G., Park S.-J. Nascent rice husk as an adsorbent for removing cationic dyes from textile wastewater. Appl. Sci. 2020;10(10):3437. [Google Scholar]
- 13.Avramiotis E., Frontistis Z., Manariotis I.D., Vakros J., Mantzavinos D. Oxidation of sulfamethoxazole by rice husk biochar-activated persulfate. Catalysts. 2021;11(7):850. [Google Scholar]
- 14.Karcher S., Kornmüller A., Jekel M. Anion exchange resins for removal of reactive dyes from textile wastewaters. Water Res. 2002;36(19):4717–4724. doi: 10.1016/s0043-1354(02)00195-1. [DOI] [PubMed] [Google Scholar]
- 15.Sala M., Gutiérrez-Bouzán M.C. Electrochemical techniques in textile processes and wastewater treatment. Int. J. Photoenergy. 2012:1–12. 2012. [Google Scholar]
- 16.Abid M.F., Zablouk M.A., Abid-Alameer A.M. Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. Iran. J. Environ. Health Sci. Eng. 2012;9(1) doi: 10.1186/1735-2746-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azzaz A.A., Jellali S., Hamed N.B.H., El Jery A., Khezami L., Assadi A.A., Amrane A. Photocatalytic treatment of wastewater containing simultaneous organic and inorganic pollution: competition and operating parameters effects. Catalysts. 2021;11(7):855. [Google Scholar]
- 18.Niculescu V.-C., Raboaca M. Efficient rice-husk-derived silica nanocatalysts for organic dye removal from water. Catalysts. 2021;11(7):815. [Google Scholar]
- 19.Chakraborty S., Chowdhury S., Das Saha P. Adsorption of Crystal Violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011;86(4):1533–1541. [Google Scholar]
- 20.Das Saha P., Chakraborty S., Das S. Optimization of hazardous crystal violet by chemically treated rice husk: using central composite response surface methodology. Arch. Environ.Sci. 2012;6:57–61. [Google Scholar]
- 21.Masoumi A., Hemmati K., Ghaemy M. Low-cost nanoparticles sorbent from modified rice husk and a copolymer for efficient removal of Pb(II) and crystal violet from water. Chemosphere. 2016;146:253–262. doi: 10.1016/j.chemosphere.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Luyen N.T., Linh H.X., Huy T.Q. Preparation of rice husk biochar-based magnetic nanocomposite for effective removal of crystal violet. J. Electron. Mater. 2019;49(2):1142–1149. [Google Scholar]
- 23.Chowdhury S., Chakraborty S., Saha P.D. Response surface optimization of a dynamic dye adsorption process: a case study of crystal violet adsorption onto NaOH-modified rice husk. Environ. Sci. Pollut. Control Ser. 2012;20(3):1698–1705. doi: 10.1007/s11356-012-0989-7. [DOI] [PubMed] [Google Scholar]
- 24.Verma V.K., Mishra A.K. Kinetic and isotherm modeling of adsorption of dyes onto rice husk carbon. Global NEST J. 2010;12(2):190–196. [Google Scholar]
- 25.Peres E.C., Favarin N., Slaviero J., Almeida A.R.F., Enders M.P., Muller E.I., Dotto G.L. Bio-nanosilica obtained from rice husk using ultrasound and its potential for dye removal. Mater. Lett. 2018;231:72–75. [Google Scholar]
- 26.Islam T., Liu J., Shen G., Ye T., Peng C. Synthesis of chemically modified carbon embedded silica and zeolite from rice husk to adsorb crystal violet dye from aqueous solution. Appl. Ecol. Environ. Res. 2018;16(4):3955–3967. [Google Scholar]
- 27.Verma V.K., Mishra A.K. Removal of dyes using low-cost adsorbents. Indian J. Chem. Technol. 2008;15:140–145. [Google Scholar]
- 28.Removal and electrochemical investigation of crystal violet dye in aqueous solutions by using rice husk treated with succinic acid. Int. J. Pharm. Res. 2020;12(2) [Google Scholar]
- 29.Van Hung N. Synthesis of nanosilica from rice husk and optimization of the removal of crystal violet dye from aqueous solution. Vietnam J. Sci. Technol. 2018;56(1A):189. 189. [Google Scholar]
- 30.Madhamshettiwar S.V. A study on adsorption process by activated rice husk by using crystal violet as dye by spectrophotometric method. Int. J. Res. Biosci. Agricult. Techn. Special Issue. Feb 2015;1:284–291. [Google Scholar]
- 31.Kumar B., Kumar U., Pandey K.M. Suitability of rice husk as biosorbent for removal of dyes from aqueous solution on the basis of chemical oxygen demand analysis. Global J. Res. Eng.: Civil Structur. Engin. 2014;14(6):1–6. [Google Scholar]
- 32.Jain Seema, Jayaram Radha V. Department of Chemistry, Institute of Chemical Technology, Nathalal Parekh Marg, Matunga, Mumbai-400 019; India: 2010. Removal of Basic Dyes from Aqueous Solution by Low-Cost Adsorbent: Wood Apple Shell (Feronia Acidissima) Desalination 250 921–927. [Google Scholar]
- 33.Rahman I.A., Ismail J., Osman H. Effect of nitric acid digestion on organic materials and silica in rice husk. J. Mater. Chem. 1997;7:1505–1509. [Google Scholar]
- 34.Rintrammee K., Prayoonpokarach S., Wittayakun J. Properties of silica from rice husk and rice husk ash and their utilization for zeolite Y synthesis. Quim. Nova. 2011;34(8):1394–1397. [Google Scholar]
- 35.Mayeen A., Shaji L.K., Nair A.K., Kalarikkal N. Morphological characterization of nanomaterials. Character. Nanomater. 2018:335–364. [Google Scholar]
- 36.Todokoro, H. M., Hitachi, Ezumi L. (1999). Scanning electron microscope, U.S. Patent 5,872,358.
- 37.Morrison R.T., Byod R.N. sixth ed. Printice – Hall of India Private Ltd; New Delhi: 1994. Organic Chemistry. [Google Scholar]
- 38.Homagai P.L., Ghimire K.N., Inoue K. Adsorption behavior of heavy metals onto chemically modified sugarcane bagasse. Bioresour. Technol. 2010;10:2067. doi: 10.1016/j.biortech.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 39.Reddy D.H., Seshaiah K., Reddy A.V., Rao M.M., Wang M.C. Biosorption of Pb2+ from aqueous solutions by Moringa oleifera bark: equilibrium and kinetic studies. J. Hazard Mater. 2010;174:831–838. doi: 10.1016/j.jhazmat.2009.09.128. 2010. [DOI] [PubMed] [Google Scholar]
- 40.Maina I.W., Obuseng V., Nareetsile F. Use of Moringa oleifera (Moringa) seed pods and Sclerocarya birrea (Morula) nut shells for removal of heavy metals from wastewater and borehole water. J. Chem. 2016:1–13. [Google Scholar]
- 41.Rauf F., Seyed J.P., Seyed H.P., Mirian P., Jose M.L. Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from aqueous solutions: a kinetic, equilibrium and thermodynamic study. Molecules. 2021;26:1–19. doi: 10.3390/molecules26082241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho Y.S., McKay G. Pseudo-second order model for sorption process. Process Biochem. 1999;34:451. [Google Scholar]
- 43.Winter M.J. D-Block Chemistry. Oxford University Press; New York: 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.