Abstract
The early bactericidal activity of the aminoglycoside paromomycin (aminosidine) in doses of 7.5 and 15 mg/kg of body weight was measured in 22 patients with previously untreated smear-positive pulmonary tuberculosis. The fall in log10 CFU per milliliter of sputum per day during the first 2 days of treatment for 7 patients receiving a paromomycin dosage of 7.5 mg/kg/day was 0.066, with a standard deviation (SD) of 0.216 and confidence limits from −0.134 to 0.266, and that for 15 patients receiving 15 mg/kg/day was 0.0924, with an SD of 0.140 and confidence limits from 0.015 to 0.170. The difference between the mean and zero was not significant for the 7.5-mg/kg dose group but was significant for the 15-mg/kg dose group (t = 2.55, P = 0.023). Since paromomycin has no cross-resistance with streptomycin and has no greater toxicity than other aminoglycosides, these results suggest that it has the potential to substitute for streptomycin in antituberculosis regimens and may be a particularly valuable addition to the drug armamentarium for the management of multidrug-resistant tuberculosis.
The early bactericidal activity (EBA) of an antituberculosis drug reflects its ability to kill the rapidly multiplying organisms present in the cavities of pulmonary tuberculosis patients. It is determined by measuring the rate of decrease in viable CFU of Mycobacterium tuberculosis per milliliter of sputum during the first 2 days of treatment with the drug under investigation (10). It has been used to evaluate and compare both new (13, 14) and established (3, 15) antituberculosis drugs and to determine the lowest effective dose of a drug (3).
Paromomycin, also known as aminosidine, is a broad-spectrum aminoglycoside closely related structurally to neomycin and kanamycin and less closely related to streptomycin (5). Although its main use at present is for the management of visceral leishmaniasis (kala-azar) (6, 16), it has been shown, both in vitro (8) and in animal experiments (9), to have considerable activity against M. tuberculosis, including multidrug-resistant strains, and there are anecdotal reports of its use for the management of pulmonary tuberculosis (17). It lacks cross-resistance with streptomycin and other antimycobacterial agents. The MIC of paromomycin for M. tuberculosis ranges from ≤0.09 to ≤1.5 μg/ml (8). In humans, peak concentrations of paromomycin of 11.6 to 25.6 μg/ml 1 h after a 500-mg intramuscular dose have been reported (4). In this pilot study, the EBA of paromomycin at dosages of 7.5 and 15 mg/kg of body weight was evaluated.
MATERIALS AND METHODS
This study was undertaken in the Department of Internal Medicine of Tygerberg Hospital, the teaching hospital of the Faculty of Medicine of the University of Stellenbosch. Tygerberg Hospital serves a number of socioeconomically deprived communities in the Western Cape province of South Africa, a region with a notified tuberculosis incidence of >500 cases/100,000 people in 1998. The study was undertaken between November 1997 and June 1998.
Patients.
Patients had newly diagnosed, previously untreated, pulmonary tuberculosis, were between 18 and 40 years of age, and weighed more than 40 kg. Patients in poor general condition or suffering from other serious medical complications, women who were pregnant or lactating, and patients unable to produce at least 10 ml of sputum overnight were excluded from the study, as were those in whose initial sputum or urine specimens traces of isoniazid or its metabolites could be detected. Also excluded were those with any known hypersensitivity to any aminoglyocoside and those whose initial M. tuberculosis isolates were found to be resistant to isoniazid, rifampin, streptomycin, or paromomycin. Resistance to isoniazid, rifampin, or streptomycin, if found, would indicate that the patient might have received previous antituberculosis treatment. Thirty-two patients were randomized to receive either 15 mg of paromomycin per kg (18 patients) or 7.5 mg of paromomycin per kg (14 patients). The results from 10 patients, 7 from the 7.5-mg/kg group and 3 from the 15-mg/kg group, were excluded from the analysis. The reasons for exclusion were resistance to isoniazid in the isolates for five patients, resistance to isoniazid and inadequate homogenization of the sputum specimen for one patient, and no growth or only very poor growth for four patients.
Sputum collection and drug administration.
Patients were actively encouraged to cough, and a 16-h collection of sputum was done from 1600 on the day of admission to 0800 the next day (S1 sputum sample). Soon after 0800, paromomycin was given by intramuscular injection, and the sputum collection procedure was repeated to obtain sputum specimens following the first and second doses of paromomycin (S2 and S3, respectively).
On completion of the study protocol after the S3 sputum collection, the patients were commenced on isoniazid, rifampin, pyrazinamide, and ethambutol as recommended by the South African National Tuberculosis Control Programme.
Microbiologic methods.
Sputum in the S1, S2, and S3 collections was examined conventionally by direct smear, culture, and sensitivity testing. CFU counts on the sputum collections were carried out as described previously (13). Without preliminary centrifugation, 20 μl of the dilutions were set up on thirds of triplicate plates of selective 7H10 medium. Drug resistance did not develop during the 3 days of the study. Analysis of sputum and urine specimens for isoniazid and its metabolites was done by a previously described high-performance liquid chromatography method (12).
Statistical methods.
The EBA for each patient was calculated by first obtaining the mean CFU count (X) at the most appropriate dilution, which was that permitting counting of between 20 and 200 colonies. Then, we used the equation Y = log10 (f X), where f is the dilution factor and Y is log10 CFU per millititer of sputum. This calculation was performed for the S1, S2, and S3 sputum collections to give Y1, Y2, and Y3, respectively, and then EBA was calculated as (Y1 − Y3)/2. The means and standard deviations of Y1, Y2, Y3, and EBA were calculated, together with 95% confidence limits (95% CL). These results were compared to those for three previously described no-drug control groups, all derived from a similar patient population at our institution and evaluated in 1992 (group A) (13) and 1993 (group B) (14) and between July 1996 and April 1998 (group C) (15), and one no-drug control group evaluated between November 1996 and April 1999 (not previously described; group D).
Differences between group means were examined first by one-way analysis of variance, in which between-group variation was regarded as a random effect (the no-drug control groups were from different studies). Since there were considerable differences in the intragroup variances, the group means were further compared using the nonparametric trend test for trends across nonparametric groups using Stata, version 6 (Stata, College Station, Tex). Finally, regression analysis was performed on the group mean values, regarding the no-drug control groups as receiving a dose of 0 mg/kg of body weight, to study the relationship between dose and EBA.
The study protocol was approved by the ethics committee of the Medical Faculty of the University of Stellenbosch. All patients entered in the study gave written informed consent for their participation.
RESULTS
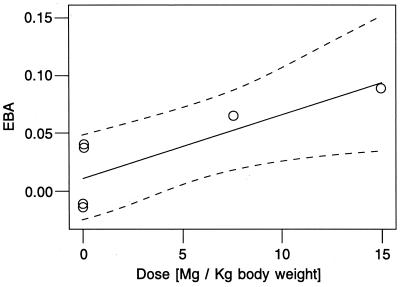
The 22 patients included in the final analysis had a mean age of 36 years and a mean weight of 52 kg. All but one patient were found to have multicavitary disease by chest radiography. The means and standard deviations of the CFU per milliliter sputum for the S1, S2, and S3 sputum samples and the mean EBA, with standard deviations and 95% Cl, are shown in Table 1 and compared to the results obtained for the no-drug control groups. A one-way analysis of variance comparing the mean EBA of the six groups gives an F5.57 of 1.75 and a P value of 0.137, indicating no clear evidence of real differences between group means. Since there was considerable heterogeneity in the intragroup variances (with variances increasing substantially with the timing of the study), the P value reported above may not be reliable. Hence, a nonparametric test for trend with increasing dose was carried out; this test gave an F7 of 2.24 and a P value of 0.03, suggesting that the mean EBA increases with an increasing dose. In addition, the CL for the mean EBA at a dose of 15 mg/kg do not include zero, providing definite evidence of an effect (P = 0.023). There is, however, no evidence that the mean EBA for the 7.5-mg/kg dose differed from zero (P = 0.45). This evidence of a dose effect was examined further using regression analysis. Figure 1 shows a plot of mean EBA against dose for the six groups in Table 1. Fitting a line by weighted least squares, with weights equal to the number of observations per group, gives an intercept of 0.011 with a standard error of 0.013 and a slope of 0.00562 with a standard error of 0.00169. The slope differs significantly from zero, with a t4 of 3.329 and a P value of 0.029. Fitting a line through the six mean points and basing the test of significance on just four degrees of freedom is a very conservative approach, yet the result supports the hypothesis that there is a dose effect. The fitted straight line and 95% confidence band are also shown in Fig. 1.
TABLE 1.
Viable counts of CFU of tubercle bacilli in sputum collections S1, S2, and S3 and in groups receiving no drug
| Dosage (mg/kg) or no-drug group (reference no.) | Number of patients | Log10 CFU/ml of sputum (mean [SD]) for:
|
EBA
|
|||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | Mean (SD) | 95% Confidence | ||
| 7.5 | 7 | 6.513 (0.898) | 6.449 (0.715) | 6.381 (0.774) | 0.066 (0.216)a | −0.134–0.266 |
| 15 | 15 | 6.961 (0.611) | 6.956 (0.761) | 6.777 (0.754) | 0.092 (0.140)a | 0.015–0.170 |
| A (13) | 13 | 6.762 (0.461) | 6.770 (0.460) | 6.791 (0.464) | −0.015 (0.013) | −0.022–0.007 |
| B (14) | 10 | 6.924 (0.326) | 6.935 (0.320) | 6.949 (0.361) | −0.011 (0.052) | −0.082–0.026 |
| C (15) | 8 | 6.236 (1.177) | NDb | 6.161 (1.206) | 0.038 (0.047) | −0.002–0.077 |
| D | 10 | 7.002 (0.757) | 6.827 (0.757) | 6.921 (0.753) | 0.041 (0.113) | −0.040–0.122 |
The difference between the means and zero was nonsignificant for the 7.5-mg/kg group but significant (t = 2.55, P = 0.023) for the 15-mg/kg group.
ND, not determined.
FIG. 1.
Plot showing linear regression of EBA of the 7.5- and 15-mg/kg paromomycin groups and the no-drug groups.
Of the patients excluded from analysis because of resistance to isoniazid, three received a dose of 7.5 mg/kg, and their EBA results were 0.053, 0.019, and 0.156 (mean, 0.076). The remaining two isoniazid-resistant patients received a dose of 15 mg/kg and had EBAs of 0.049 and 0.102 (mean, 0.076).
DISCUSSION
In this study, paromomycin produced a small but statistically significant increase in EBA, as shown by both the nonparametric trend test (P = 0.03) and the regression analysis on the group means (P = 0.029). Streptomycin, given in a dosage of approximately 20 mg/kg of body weight to a group of four patients, had a similar EBA, 0.094 (7). The CL of both estimates are so wide that comparison is inconclusive. The finding that paromomycin at a dose of 7.5 mg/kg did not have a detectable effect compared to zero suggests that the antituberculosis activity may be limited.
Our findings and the results of earlier in vitro (8) and in vivo (9) studies suggest, but do not prove, that paromomycin has an antituberculosis activity similar to that of streptomycin. Importantly, paromomycin has no cross-resistance with streptomycin and does not appear to have any higher incidence of ototoxicity or nephrotoxicity than that found with other aminoglycosides (1, 2, 6, 11). Paromomycin may therefore be a valuable addition to the antituberculosis drug armamentarium, particularly for the management of multidrug-resistant tuberculosis.
ACKNOWLEDGMENT
This study, FD-R-001167-01, was funded by an orphan products grant from the U.S. food and Drug Administration, Office of Orphan Products.
REFERENCES
- 1.Arcamone F, Bertazzoli C, Buogo A, Cassinelli G, Vigevani A. New studies in the field of aminoglycosidic aminocyclitol antibiotics. Panminerva Med. 1974;16:9–24. [PubMed] [Google Scholar]
- 2.Chunge C N, Owate J, Pamba H O, Donno L. Treatment of visceral leishmaniasis in Kenya by aminosidine alone or combined with sodium stibogluconate. Trans R Soc Trop Med Hyg. 1990;84:221–225. doi: 10.1016/0035-9203(90)90263-e. [DOI] [PubMed] [Google Scholar]
- 3.Donald P R, Sirgel F A, Botha F J, Selfart H I, Parkin D P, Van den Plas M, Van de Wal B W, Maritz J S, Mitchison D A. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156:895–900. doi: 10.1164/ajrccm.156.3.9609132. [DOI] [PubMed] [Google Scholar]
- 4.Eastwood J. Paromomycin: serum levels following parenteral administration. Antibiot Chemother. 1962;12:77–84. [PubMed] [Google Scholar]
- 5.Fisher M W, Manning M C, Gagliardi L A, Gaetz M R, Erlandson A L. Paromomycin: experimental antibacterial activity. Antibiot Annu. 1960;7:293–303. [PubMed] [Google Scholar]
- 6.Jha T K, Olliaro P, Thakur C P, Kanyok T P, Singhania B L, Singh I J, Singh N K, Akhoury S, Jha S. Randomised controlled trial of aminosidine (paromomycin) v. sodium stibogluconate for treating visceral leishmaniasis in North Bihar, India. Br Med J. 1998;316:1200–1205. doi: 10.1136/bmj.316.7139.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jindani A, Aber V R, Edwards E A, Mitchison D A. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–949. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 8.Kanyok T P, Reddy M V, Chinnaswamy J, Danziger L H, Gangadharam P R J. Activity of aminosidine (paromomycin) for Mycobacterium tuberculosis and Mycobacterium avium. J Antimicrob Chemother. 1994;33:323–327. doi: 10.1093/jac/33.2.323. [DOI] [PubMed] [Google Scholar]
- 9.Kanyok T P, Reddy M V, Chinnaswamy J, Danziger L H, Gangadharam P R J. In vivo activity of paromomycin against susceptible and multidrug-resistant Mycobacterium tuberculosis and M. avium complex strains. Antimicrob Agents Chemother. 1994;38:170–173. doi: 10.1128/aac.38.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchison D A. Basic concepts in chemotherapy of tuberculosis. In: Gangadharam P R J, Jenkins P A, editors. Mycobacteria: chemotherapy. Vol. 2. New York, N.Y: Chapman Hall; 1998. pp. 15–50. [Google Scholar]
- 11.Scott J A G, Davidson R N, Moody A L T, Grant H R, Felmingham D, Scott G M S. Aminosidine (paromomycin) in the treatment of leishmaniasis imported into the United Kingdom. Trans R Soc Trop Med Hyg. 1992;86:617–619. doi: 10.1016/0035-9203(92)90151-2. [DOI] [PubMed] [Google Scholar]
- 12.Seifart H I, Gent W L, Parkin D P, Van Jaarsveld P P, Donald P R. High performance liquid chromatographic determination of isoniazid, acetylisoniazid and hydrazine in biological fluids. J Chromatogr B. 1995;674:269–275. doi: 10.1016/0378-4347(96)82886-6. [DOI] [PubMed] [Google Scholar]
- 13.Sirgel F A, Botha F J H, Parkin D P, Van de Wal B W, Donald P R, Clark P K, Mitchison D A. The early bactericidal activity of rifabutin in patients with pulmonary tuberculosis measured by sputum viable counts: a new method of drug assessment. J Antimicrob Chemother. 1993;32:867–875. doi: 10.1093/jac/32.6.867. [DOI] [PubMed] [Google Scholar]
- 14.Sirgel F A, Botha F J H, Parkin D P, Van de Wal B W, Schall R, Donald P R, Mitchison D A. The early bactericidal activity of ciprofloxacin in patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156:901–905. doi: 10.1164/ajrccm.156.3.9611066. [DOI] [PubMed] [Google Scholar]
- 15.Sirgel F A, Donald P R, Odhiambo J, Githui W, Umqpathy K C, Paramasivan C N, Tam C M, Kam K M, Lam C W, Sole K M, Mitchison and the EBA Collaborative Study Group D A. A multicentre study of the early bactericidal activity of antituberculosis drugs. J Antimicrob Chemother. 2000;45:859–870. doi: 10.1093/jac/45.6.859. [DOI] [PubMed] [Google Scholar]
- 16.Thakur C P, Olliaro P, Gothoskars, Bhowmick S, Choudhury B K, Prasad S, Kumar M, Verma B B. Treatment of visceral leishmaniasis (kala-azar) with aminosidine (paromomycin)-antimonial combinations, a pilot study in Bihar, India. Trans R Soc Trop Med Hyg. 1992;86:615–616. doi: 10.1016/0035-9203(92)90150-b. [DOI] [PubMed] [Google Scholar]
- 17.Tokuoka S, Oka S, Narusawa T, Kondo J. A study of the effectiveness of aminosidine against pulmonary tuberculosis. Jpn J Chest Dis. 1970;29:550–553. [Google Scholar]