Abstract
Background:
Intensive lifestyle intervention (ILI) is essential for diabetes management. The Weight Achievement and Intensive Treatment (Why WAIT) program is a 12-week multidisciplinary weight management program that has been implemented in real-world clinical practice since 2005 and has shown long-term maintenance of weight reduction for 5 and 10 years. During the COVID-19 pandemic, the program went virtual using telemedicine and mobile health applications.
Aims:
This retrospective pilot study aims to evaluate the effectiveness of a virtual model of an already established and successful in-person program for diabetes and weight management since 2005.
Methods:
We evaluated 38 patients with diabetes and obesity enrolled in the Why WAIT program between February 2019 and December 2020. Sixteen participants were enrolled in virtual program (VP) and were compared with 22 participants who completed the latest two physical programs (PPs) before COVID-19. We evaluated changes in body weight, A1C, blood pressure (BP), and lipid profile after 12 weeks of ILI.
Results:
Body weight decreased by −7.4 ± 3.6 kg from baseline in VP compared with −6.8 ± 3.5 kg in PP (p = 0.6 between groups). A1C decreased by −1.03% ± 1.1% from baseline in VP, and by −1.0% ± 1.2% in PP (p = 0.9 between groups). BP, lipid profile, and all other parameters improved in both groups with no significant difference between them.
Conclusion:
Virtual multidisciplinary ILI is as effective as the in-person intervention program in improving body weight, A1C, BP, and lipid profile, and in reducing the number of anti-hyperglycemic medications. Results from our study suggest that scaling the Why WAIT program in a virtual format to a larger population of patients with diabetes and obesity is feasible and is potentially as successful as the in-person program.
Keywords: COVID-19, diabetes, digital health, healthcare, intensive lifestyle, m-Health, technology, telemedicine, virtual
Introduction
Diabetes mellitus is estimated to impact more than 463 million people worldwide, becoming one of the greatest risks to public health. Scientists predict that this number will grow exponentially to 578 million by 2030 and to 700 million by 2045. 1 Specifically in the United States, the Centers for Disease Control and Prevention (CDC) estimates that 34.2 million people or 10.5% of the US population has diabetes and 90–95% of those are affected have type 2 diabetes (T2D). 2 It is projected that 40 million US individuals will have T2D by 2030 and 61 million will have it by 2060. 3 Meanwhile, around 88 million adults or 34.5% of the US population are estimated to have prediabetes.2,3 It was estimated that the cost of diabetes in 2017 was $327 billion with $237 billion in direct medical costs and $90 billion in reduced productivity. 4 Most of the diabetes costs are carried out by non-endocrinologists. It is estimated that 85% of diabetes care is primarily delivered by primary care physicians, of whom a shortage of 14,800–49,300 is expected by 2030.5,6 Furthermore, in 2018, the United States had only 7918 endocrinologists, which is translated into a ratio of one endocrinologist per 3800 patients with diabetes. 6 In an effort to tackle this public health crisis, lifestyle modification in the form of dietary intervention and increased physical activity are recommended, especially in the early stages of T2D. Lifestyle intervention programs have demonstrated significant reduction in incidence of T2D among individuals with prediabetes and also resulted in significant improvement in cardiovascular risk factors among overweight and obese patients with T2D.7–10 The landmark Action for Health in Diabetes (Look AHEAD) study, which emphasizes intensive lifestyle intervention, demonstrated that weight reduction improved all cardiovascular risk factors except low-density lipoprotein (LDL)-cholesterol; however, there was a no significant reduction in the incidence of cardiovascular events or mortality in comparison with standard diabetes support and education. 9 The lack of difference between the two groups was probably related to an increased use of lipid-lowering medications and antihypertensive medications in the control group. We previously showed that intensive multidisciplinary weight management (IMWM) in real-world clinical practice can lead to sustained improvement in both A1C and lipid profile among patients who achieved ⩾7% weight loss at 1 year. 11 Traditionally, effective lifestyle intervention programs have been conducted in-person. However, recent technological advances in digital health made it possible to conduct these programs virtually during the current COVID-19 pandemic. These technologies enhance patients’ involvement in their diabetes self-management and lifestyle modifications while maintaining reasonable communications between patients and their healthcare providers using telemedicine platforms.12,13 In addition, telemedicine eliminates the need for patients’ commute to receive multidisciplinary care. In hospitals and clinics, use of telemedicine and mobile health (m-Health) was shown to reduce overhead costs and few other healthcare expenses. 13 The value of applying these technologies was obvious during COVID-19 pandemic. Fortunately, it provided an exceptional opportunity for healthcare providers and patients alike to experience them. 14 Findings from several studies showed that telemedicine and virtual software platforms are convenient, safe, and effective methods in providing optimal medical care to patients while ensuring their safety. They also provided insights to medical practitioners on how to successfully implement telemedicine for future health crisis. 15 Thus, it should be adopted as a proactive measure to improve medical care and should be applied during the pandemic outbreak and beyond.15–17 This pilot study aims to compare the clinical outcomes of a virtual approach to IMWM with a classic in-person physical approach using the same intervention tools and by the same multidisciplinary team and whether it is as effective as the in-person intervention program in reducing body weight and A1C. The primary endpoints are the change in body weight and A1C after 12 weeks of intervention. The secondary endpoints include changes in percentage of glucose time in range (TIR) using continuous glucose monitoring devices (CGMs) and changes in blood pressure (BP), lipid profile, number of diabetes medications, and barriers to exercise.
Methods
In-person physical program
Weight Achievement and Intensive Treatment (Why WAIT – WW) is a 12-week Intensive Multidisciplinary Weight Management (IMWM) Program designed and implemented at Joslin Diabetes Center in Boston, MA, since 2005. A full description of the program was previously published. 18 The WW program primarily focuses on weight reduction as an effective tool for diabetes management. Participants are eligible for WW program if they have a body mass index (BMI) between 30 and 45 kg/m2 and have either type 1 diabetes or T2D. WW program usually enrolls 10–15 participants in a group at a time. Throughout the program, participants undergo evaluations by an intervention team consisting of a diabetologist, a registered dietitian (RD), a registered clinical exercise physiologist (RCEP), and a psychologist or behavioral therapist. This interdisciplinary team works toward individualizing patient care in order to achieve an optimal weight reduction and improvement in overall health. Intervention sessions are conducted weekly over 12 consecutive weeks. Each session is 2 h in length. The five major components of the WW program include the following:
Medication adjustments
At the start of the program, diabetologists reviews participants’ medications and work toward reducing or eliminating medications known to contribute to weight gain or potentially cause hypoglycemia during weight reduction. In addition, anti-hyperglycemic medications known to be weight neutral are maintained. 19 Finally, diabetes medications that promote weight loss may be initiated if covered by patients’ insurance plans. Participants are encouraged to diligently monitor their glucose levels using CGMs. Participants’ glucose logs are reviewed weekly by a diabetes nurse practitioner or a certified diabetes educator, and anti-hyperglycemic medications are adjusted accordingly.
Dietary intervention
Dietary evaluations are conducted by a registered dietitian who evaluates participants’ dietary histories, past weight management efforts, and adherence barriers to previous dietary interventions. After evaluations, participants receive meal plans based on their sex and current caloric intake from their 24-h dietary recall. These meal plans are based on 1200, 1500, or 1800 calorie levels and are assigned to participants accordingly. All meal plans are developed based on Joslin Nutrition Guidelines for obese patients with T2D. Meal plans are structured such that 40–45% of daily calories are from carbohydrates, <35% are from fat with saturated fat <10%, protein intake of 1–1.5 g/kg of adjusted body weight, and fiber intake of 14 g/1000 calories.20,21 The WW program utilized diabetes-specific meal replacements (DSMRs) including a choice of BOOST Glucose Control™, BOOST Calorie Smart™ (Nestlé Medical Nutrition®, Minneapolis, Minnesota, USA) or Glucerna Hunger Smart™ (Abbott Nutrition®, Columbus, Ohio, USA). DSMRs are used to replace breakfast and lunch for the first 6 weeks of the program. Two snacks per day are advised between meals, and these could be chosen from lists of 100-calorie and 200-calorie snacks. Participants are provided with 17 different menus for dinners. These dinner choices adhere to the daily 1200-, 1500-, and 1800-calorie meal plans and list the meals’ ingredients, nutrition facts, and cooking instructions. Every meal plan is low in glycemic index and low in sodium (<2300 mg/day). Participants are urged to keep logs of their daily meals and snacks. If participants are unable to achieve 3% weight loss by the fourth week or 5% by the eighth week, total daily caloric intake is advanced to the next lower caloric level. After 6 weeks, menus for breakfast and lunch equivalent in caloric content and composition to DSMRs are introduced. Participants are given the option to continue using the DSMRs or use alternative breakfast and lunch menus.
Exercise intervention
An individualized exercise plan is designed by the program RCEP after evaluation of exercise capacity and barriers to exercise. In addition, the RCEP provides specific corrective exercises to help with muscle imbalance. Exercise intervention includes a balanced mix of aerobic exercise (cross- and interval training) to promote development and maintenance of cardiovascular health; resistance exercise (circuit, pyramid, superset training) to enhance muscular strength and endurance and to improve performance of daily living. The exercise plan also includes core stability training to improve trunk mobility and stabilization, as well as dynamic and static stretching to enhance functional capabilities and reduce risk of injury. The exercise intensity is set above the minimum required to improve participant’s current exercise capacity, but below a level that might evoke abnormal clinical symptoms. The exercise plan includes a weekly 60-min exercise session under the supervision of the RCEP. In addition, each participant is given an individualized exercise plan, addressing specific skeletal muscle issues and diabetes complications to perform independently at home. Participants are instructed to progress gradually over 12 weeks from 20 min of the training plan 4 days/week to 60 min 5–6 days/week. On completion of the initial 12 weeks, participants are encouraged to continue to exercise independently for 60 min/day, 5–6 days/week and maintain ⩾ 300 min/week with focus on preserving or increasing muscle mass.
Cognitive-behavioral intervention
Intervention sessions focus primarily on self-monitoring of eating and exercise, behavioral goal setting, stimulus control techniques, cognitive restructuring, assertive communication skills, stress management, and relapse prevention.22–24 These components had been previously validated for weight management in other clinical trials.25,26 Sessions are conducted by a clinical psychologist or a behavioral therapist. Sessions are held throughout the 12 weeks of WW program.
Group education
Throughout the WW program, group didactic sessions are held. The topics vary each week but are relevant to weight and diabetes management. The program’s RCEPs and RDs lead these sessions and provide educational handouts to participants at the end of each session.
Virtual program
Group education, intervention, and logging of diet and exercise were conducted through a combination of a telemedicine, using the GoToMeeting program (LogMeIn Inc., Boston, MA) and m-health application of WW program (Healthimation Inc®, Boston, MA) with an option of using Good Measure m-health application for nutrition information, especially for type 1 patients (Good Measure Inc®, Boston, MA). Virtual visits were conducted every Wednesday from 3–5 p.m., where participants received a link to log-in 15 min before each session. Nutrition intervention included review of food logs using m-health applications, instruction to participants during telemedicine sessions, communication with participants in between sessions using the chat functionality of WW m-health applications, and portal messaging through electronic health records. Exercise sessions were conducted virtually and include demonstration of exercise through telemedicine and instructions to follow an exercise plan through WW m-health applications. Through WW m-health applications, participants were able to see each type of exercise and its duration. Logging of exercise was reviewed from the coaching module of the WW m-health application. RCEP communicated with participants in between sessions using the chat functionality of the WW m-health applications and portal messaging through Joslin electronic health records. Participants were instructed to upload their CGM data each week using the corresponding web programs (Abbott freestyle Libre® or Dexcom®). Glucose data were reviewed weekly by the program’s nurse practitioner who adjusted anti-hyperglycemic medications accordingly. Cognitive-behavioral support was conducted through telemedicine and through behavioral tips and videos from the WW m-health applications.
Study participants and design
This retrospective study was approved by the Committee on Human Studies (CHS) at the Joslin Diabetes Center. Sixteen participants were included in the virtual version of the program (13 with T2D and 3 with type 1 diabetes). These participants were enrolled as a consecutive series to the in-person program. The virtual program (VP) was conducted between April 2020 and December 2020. Twenty-two participants were included in the in-person physical program (PP), which comprised participants in the last 2 programs before the COVID-19 pandemic during the period from February 2019 to December 2019.
Study endpoints
The primary endpoints of this study were the changes in body weight and A1C after 12 weeks of intervention. The secondary endpoints included changes in percentage of glucose TIR from CGMs, changes in BP, lipid profile, and number of anti-hyperglycemic medications. In VP only, we compared barriers to exercise after versus before the VP. This survey was not part of the in-person PP in the past and was only introduced in the VP.
Statistical analysis
Demographic and baseline characteristics were evaluated using descriptive statistics. Continuous variables are expressed as mean ± standard deviation (SD) or as mean [95% confidence interval (CI)]. Categorical variables are expressed as percentages. Chi-square test and paired t-test were used to compare baseline characteristics and within-group differences in endpoints at 12 weeks. Unpaired t-test was used to compare quantitative differences between the two groups at 12 weeks. A p value of < 0.05 was considered statistically significant. All analyses were performed using STATA Special Edition 15.0 for Windows® (StataCorp ®, College Station, Texas, USA 2017).
Results
VP included 16 participants (42% of the total WW program participants in this study) and PP included 22 participants (58% of the total WW program participants in this study). At baseline, participants in the PP were non-significantly heavier than those in VP with an initial average weight of 104.7 ± 17.7 kg versus 99 ± 20.8 kg, respectively (p = 0.4), and had non-significantly higher mean A1C of 7.9% ± 1.09% versus 7.7% ± 1.3% in VP (p = 0.5) (Table 1). In addition, participants in the PP had non-significantly higher baseline high sensitivity C-reactive protein (hs-CRP) of 7.2 ± 6.2 compared with 3 ± 3.8 in the VP (p = 0.09). There were no differences between the two groups in other cardiovascular risk factors (BP, lipid profile), urinary microalbumin/creatinine ratio, or glucose TIR (Table 1).
Table 1.
Baseline characteristics of participants in real-world intensive lifestyle intervention.
| All participants | Physical program (PP) | Virtual program (VP) | p value* | |
|---|---|---|---|---|
| Age (years) | 57.1 (10) | 56.3 (10.9) | 58.2 (8.9) | 0.5 |
| Female sex (%) | 52.6 | 68.2 | 31.2 | 0.024 |
| Type 2 diabetes (%) | 71.0 | 63.6 | 81.2 | 0.23 |
| Duration of diabetes (years) | 17.9 (12.6) | 20.2 (15.2) | 14.9 (7.4) | 0.2 |
| Weight (kg) | 102.4 (19) | 104.7 (17.7) | 99 (20.8) | 0.4 |
| Body mass index (kg/m2) | 35 (5.7) | 36.1 (5.06) | 33.4 (6.4) | 0.18 |
| A1C (%) | 7.8 (1.2) | 7.9 (1.09) | 7.7 (1.3) | 0.5 |
| Systolic blood pressure (mmHg) | 129 (14.6) | 128.4 (15.8) | 129.7 (12.5) | 0.79 |
| Diastolic blood pressure (mmHg) | 76.2 (10.4) | 76.3 (10.8) | 76 (9.8) | 0.9 |
| Total cholesterol (mg/dl) | 153 (30.7) | 158.7 (29) | 144 (32.2) | 0.2 |
| LDL-cholesterol (mg/dl) | 82.8 (27.7) | 83.4 (28.7) | 81.7(27.3) | 0.8 |
| HDL-cholesterol (mg/dl) | 49.5 (15.2) | 51.9 (15.3) | 46 (14.8) | 0.3 |
| Triglycerides (mg/dl) | 176.3 (172) | 173 (164.5) | 181.1 (189) | 0.9 |
| UACR (µg/mg) | 118 (257) | 107.8 (227.5) | 134.4 (314) | 0.8 |
| hs-CRP (mg/l) | 4.8 (5.3) | 7.2 (6.2) | 3 (3.8) | 0.09 |
| Number of diabetes medications | 2.65 (0.9) | 2.5 (0.9) | 2.8 (0.98) | 0.4 |
| Number of antihypertensive medications | 1.18 (0.98) | 1.2 (0.9) | 1.06 (0.9) | 0.5 |
| Time in range (%) | 72 (24) | 69 (27) | 77 (20) | 0.3 |
| <70 mg/dl (%) | 2.1 (3.4) | 2 (3.5) | 2 (3.4) | 0.9 |
| >180 mg/dl (%) | 15 (18) | 15 (15) | 15 (21) | 0.9 |
| TDD (units) | 59.5 (44.9) | 56.5 (28.4) | 64.5 (66) | 0.7 |
Data are given as mean (SD) or %. hs-CRP, high sensitivity C-reactive protein; HDL: high-density lipoprotein; LDL: low-density lipoprotein; p value: PP versus VP; TDD, total daily dose of insulin; UACR, urine albumin-to-creatinine ratio.
Two-sample t-test or chi-square test.
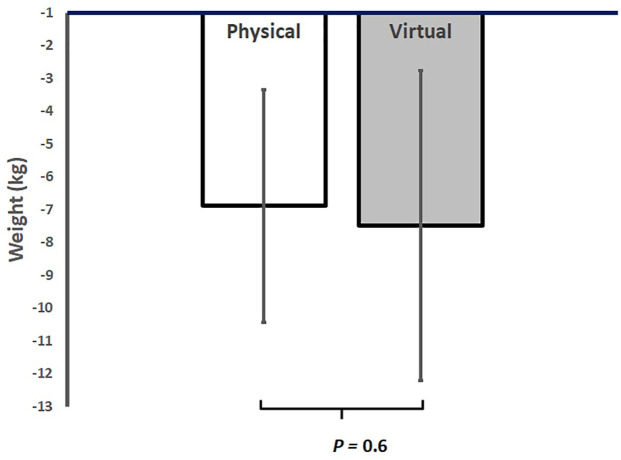
After 12 weeks, participants in VP lost on average −7.4 ± 3.6 kg (95% CI, −9.4 to −5.5 kg), which corresponds to a weight loss of 7.4% ± 3% (p < 0.001 from baseline). Participants in PP lost on average −6.8 ± 3.5 kg (95% CI, −8.4 to −5.3 lbs.), which corresponded to a weight loss of 6.4% ± 3% (p < 0.001 from baseline) (Table 2). There was no significant difference in weight loss between the two groups (p = 0.6) (Figure 1).
Table 2.
Changes in metabolic and cardiovascular risk factors after 12 weeks of intensive lifestyle intervention in real-world clinical practice.
| Physical program (PP) | Virtual program | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Change from baseline | Baseline | 12 weeks | Change from baseline | p value | |
| Weight (kg) | 104.7 (17.7) | 97.9 (16.6)* | −6.9 (−8.4 to −5.3) | 99 (20.8) | 91.6 (18.87)* | −7.4 (−9.4 to −5.5) | 0.6 |
| Body mass index (kg/m2) | 36.1 (5.06) | 33.78 (5.02)* | −2.3 (−2.8 to −1.8) | 33.4 (6.3) | 31 (5.9)* | −2.4 (−3 to −1.9) | 0.7 |
| A1C (%) | 7.9 (1.09) | 6.9 (0.62)** | −1 (−1.5 to −0.45) | 7.7 (1.3) | 6.7 (0.55)** | −1.03 (−1.6 to −0.4) | 0.9 |
| Systolic blood pressure (mmHg) | 128.4 (15.8) | 123.18 (11.8) | −5.18 (−11.3 to 0.97) | 129.7 (12.5) | 121.3 (10.3) | −8.4 (−19.5 to 2.5) | 0.5 |
| Diastolic blood pressure (mmHg) | 76.3 (10.8) | 74.9 (8.5) | −1.36 (−6 to 3.3) | 76 (9.8) | 72 (7.7) | −4.8 (−9.6 to 1.29) | 0.4 |
| Total cholesterol (mg/dl) | 158.7 (29) | 151.6 (32.9) | −6.8 (−18.4 to 4.7) | 144 (32.2) | 127.7 (26)** | −15.4 (−28.4 to −2.3) | 0.3 |
| LDL-cholesterol (mg/dl) | 83.4 (28.7) | 81 (31.7) | −2.26 (−12.8 to 8.3) | 81.7 (27.3) | 67.9 (18) | −13.8 (−32 to 4.4) | 0.2 |
| HDL-cholesterol (mg/dl) | 51.9 (15.3) | 51.9 (16.6) | 0 (−3.17 to 3.17) | 46 (14.8) | 48 (14.7) | 2 (−1 to 5) | 0.3 |
| Triglycerides (mg/dl) | 173 (164.5) | 119.5 (76.3) | −53.5 (−117.4 to 10.3) | 181.1 (189) | 121 (81.5) | −60 (−125.5 to 5.5) | 0.8 |
| UACR (µg/mg) | 107.8 (227.5) | 36.1 (46) | −60 (−147 to 27) | 134.4 (314) | 57 (131) | −70 (−195 to 55) | 0.8 |
| hs-CRP (mg/l) | 7.2 (6.2) | 5.3 (3.25) | −1.9 (−5.8 to 1.95) | 1.8 (1.7) | 1.92 (2.16) | 0.114 (−1.06 to 1.29) | 0.3 |
| Number of diabetes medications | 2.5 (0.9) | 1.77 (0.97)* | −0.8 (−1.13 to −0.4) | 2.8 (0.98) | 1.9 (1)* | −0.9 (−1.3 to −0.5) | 0.5 |
| Number of antihypertensive medications | 1.2 (0.9) | 1.09 (1.01)** | −0.18 (−0.35 to −0.006) | 1.06 (0.9) | 1 (1.03) | −0.06 (−0.2 to 0.07) | 0.3 |
| Time in range (%) | 69 (27) | 72 (23) | 3.2 (−5.2 to 11) | 77 (20) | 87 (14) | 10.2 (−1.09 to 21) | 0.3 |
| Patients on insulin (%) | 68.2 (47) | 45.5 (50) | −22.7 (−42 to −3.7) | 62.5 (50) | 31.2 (48) | −31.2 (−56.7 to −5.7) | 0.6 |
| <70 mg/dl (%) | 15 (15) | 46 (90) | 31 (−15 to 77) | 15 (21) | 12 (13) | −3.7 (−16 to 8.3) | 0.1 |
| >180 mg/dl (%) | 56.5 (28.3) | 27.1 (26)* | −29 (−41.8 to −17) | 64.5 (65) | 10.6 (13)** | −54 (−103 to −4.6) | 0.3 |
| TDD (units) | 56.5 (28.4) | 27.1 (26.5)* | −29 (−41.8 to −17) | 64.5 (66) | 10.6 (13)* | −54 (−103 to −4.6) | 0.3 |
Data are given as mean (SD) or mean (95% CI). P value = change from baseline between PP and VP. HDL: high-density lipoprotein; LDL: low-density lipoprotein.
p < 0.001 compared with baseline. **p < 0.05 compared with baseline.
Figure 1.
Average reduction in body weight in physical versus virtual programs after 12 weeks in a real-world intensive lifestyle intervention. All participants N = 38, physical group n = 22, virtual group n = 16, p = change from baseline between PP and VP.
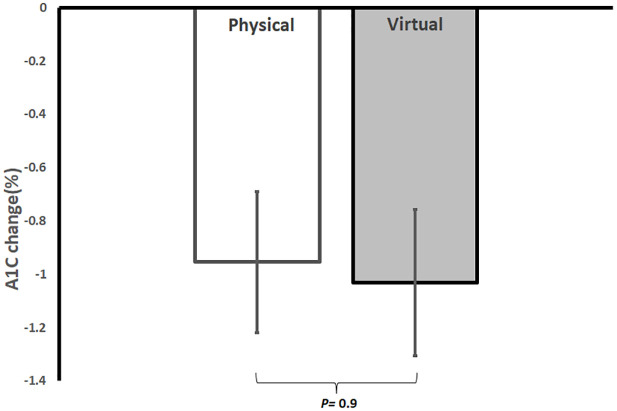
Glycemic control improved in both groups compared with baseline with an average reduction in A1C of −1.03% ± 1.1% (95% CI, −1.6 to −0.4; p < 0.05 from baseline) in VP and an average reduction of −1% ± 1.2% (95% CI, −1.5 to −0.45; p = 0.001 from baseline) in PP. There was no significant difference in A1C reduction between the two groups (p = 0.9) (Figure 2). Glucose TIR was 87% ± 14% at the end of VP and 72% ± 23% at the end of PP, with no significant difference between the two groups (p = 0.3). Patients in VP reported reduced barriers to being active from a score of 17.2 ± 12.2 at week 1 to a score of 4.6 ± 3.9 at week 12 of the program (p < 0.001). Among insulin-treated patients with T2D, five patients (50%) in VP and five patients (33.3%) in PP stopped insulin at 12 weeks; however, these changes were not statistically significant (p = 0.4). Total daily dose (TDD) of insulin decreased significantly in VP from 64.5 ± 66 units at baseline to 10.6 ± 13 units at 12 weeks (p < 0.05) and in PP from 56.5 ± 28.4 units at baseline to 27.1 ± 26 units at 12 weeks (p < 0.001). The number of anti-hyperglycemic medications reduced by 36% ± 28.6% in VP and by 28% ± 29.6% in PP (p = 0.4 between them). There were no significant changes in BP, renal function, lipid profile, hs-CRP, percentage of time in low interstitial glucose (<70 mg/dl), percentage of time in high interstitial glucose (>180 mg/dl), and utilization of antihypertensive medications between the two groups at 12 weeks (Table 2).
Figure 2.
Average reduction in A1C in physical versus virtual programs after 12 weeks in a real-world intensive lifestyle intervention. All participants N = 38, physical group n = 22, virtual group n = 16, p = change from baseline between PP and VP.
Discussion
Multidisciplinary intensive lifestyle intervention is costly and labor-intensive. The WW program, a 12-week intensive weight management program, costs health insurance between $5000 and $7000 per participant. Although it is the only weight management program for patients with diabetes that showed sustained weight loss of 6.4% after 5 years and 6.9% after 10 years,11,27 it is very difficult to scale it to other patients with diabetes who are in dire need for long-term weight management. Currently, the WW program is only conducted at the Joslin Diabetes Center in Boston since 2005. This study showed that a virtual version of the WW program, using a combination of telemedicine and m-health application, resulted in similar weight reduction and comparable improvement in A1C and other cardiovascular risk factors to the in-person PP. It also showed that barriers to exercise significantly decreased after the virtual WW program. In the virtual version of the WW program, participants lost an average of 7.4 ± 3.6 kg or 7.4% ± 3% of their initial body weight, while participants in the in-person physical version of the program lost an average of 6.8 ± 3.5 kg or of 6.4% ± 3% of their initial body weight. A1C decreased by −1.03% ± 1.1% in VP and by −1% ± 1.2% in PP. The differences in weight loss and A1C reduction were not significant between the two groups. Glucose TIR was 87% at the end of VP and 72% at the end of PP with no significant difference between the two groups. These results may point to the possibility of scaling this successful multidisciplinary program to other patients with diabetes who seek weight management, irrespective of their location. Despite achieving modest weight loss, previous online versions for lifestyle intervention programs showed that adults were more likely to enroll but less likely to remain engaged when compared with the in-person programs, thus leading to a lack of comparable effectiveness of the online and in-person lifestyle change programs. 28 The superior effectiveness of the virtual WW program is most likely related to the multidisciplinary approach and the unique components of the WW program.
Recent advances in digital health made it possible that group intervention can be conducted using web or mobile platforms. 29 Although physical interaction remains an important component of any multidisciplinary weight management program, digital platforms allow participants to interact within the cyberspace and from the convenience of their homes. In this study, logs of diet and exercise were captured electronically. The m-health applications provided an advantage of helping participants to maintain accurate records of dietary, exercise, and behavioral interventions, so participants can review them whenever needed. Visual demonstration of each exercise and its timing were additional valuable tools that might increase patients’ adherence to the recommended exercise program to achieve similar results. These options were not available in the in-person PP, as participants depended on their memory for recalling every exercise, especially those used for strength training.
With the current advances in technology, participants were provided with cellular-connected scales and BP kits that captured vital signs remotely, without the need for wired connections or manual recordings. CGM software were also valuable tools, as remote upload of glucose data was easily conducted. These software come as standard features of all CGM devices currently on the market. This valuable information allowed the WW intervention team to adjust anti-hyperglycemic medications with ease on a weekly basis, as done in PP. Although CGMs were used in both versions of the program, we noticed that the percentage of glucose TIR is trending higher in VP in comparison with PP. We think that the ability of the WW coaches to electronically chat with participants on a continuous basis might help in keeping glucose in range. However, the differences between the two groups in TIR, time in low (glucose < 70 mg/dL) and time in high (glucose > 180 mg/dL), were not statistically significant. It is known that proper coaching enhances weight management, increases adherence to intervention programs, and improves patients’ satisfaction. 26 Providing coaches with virtual and digital tools to easily connect or chat with participants during intensive lifestyle modification were extremely valuable in VP over PP as well as showing superior effectiveness when compared with other online lifestyle intervention programs.28,30 Meanwhile, m-health applications provide coaching modules where coaches can easily see participants’ progress and monitor their level of adherence and compliance. Although using technology is still a constant challenge for some patients, especially elderly participants, proper selection of participants, training them on using virtual tools, and frequent troubleshooting may overcome this barrier. We started to see more participants above the age of 60, who are comfortable with using mobile applications and telemedicine, especially during the COVID-19 pandemic. Although it has been a challenge to conduct such a comprehensive and intensive program in a virtual manner, patients’ safety was preserved during the COVID-19 pandemic through this remote method. The study has many limitations as the sample size was relatively small. However, this pilot feasibility study may encourage us to expand this method and test it in a randomized-controlled study among a larger sample size of patients with diabetes in a real-world clinical practice. Some limitations in interpreting results between patients with type 1 diabetes and patients with T2D is related to the frequency of insulin use, which is definitely much less in T2D patients, and despite the higher percentage of type 1 diabetes in PP, there was no significant difference between the two groups (p = 0.23). Definitely, the absence of personal face-to-face interaction in the clinic will remain a drawback of any virtual interventions. However, elimination of the complexity of attending in person at a specific time each week with its associated cost, while providing easy communication tools from the convenience of participants’ home may be an equitable trade-off. Evaluation of cost-effectiveness of VP and its impact on participants’ quality of life should be thoroughly investigated in a larger clinical study. Another limitation is the inability of participants in VP to come to the Joslin gymnasium and use professional exercise equipment, especially for interval training. However, we mailed participants resistance stretching rubber bands to use for strength exercises. Meanwhile, RCEP was able to remotely demonstrate all the possible exercises using these bands and other available home equipment, such as free weights and kettlebells. It was not possible to remotely evaluate body composition or measure amount of visceral fat as we used to do in PP. Missing these data was a drawback. In VP, despite the elimination of the exercise-capacity testing (6-min walk test) as it requires physical presence at Joslin, the RCEP was able to capture a lot of information regarding skeletal muscle issues and worked closely with each participant to address specific skeletal muscle injuries such as plantar fasciitis, sciatica, and weakness in core stabilization. Once the COVID-19 pandemic is over, we think a hybrid model may be evaluated for some patients, where participants may come for initial evaluations and measurements and conduct the rest of the program virtually, from the convenience of their home or work.
Conclusion
Virtual multidisciplinary intensive lifestyle intervention program using telemedicine and m-health seems to be as effective as the costly in-person PP in improving body weight, A1C, BP, and lipid profile, and in reducing number of anti-hyperglycemic medications for patients with diabetes and obesity. This study may have a significant implication on how we may conduct multidisciplinary intensive lifestyle intervention in the future, as technology potentially reduces cost and allows for scalability of the program to reach patients who are equally motivated but otherwise cannot join the in-person for the barriers of distance or other personal reason. However, the VP has many limitations; first, it is not suitable for patients who are technologically challenged; second, it eliminates the essential personal connection; and third, it risks that some of the measurements at home may not be accurate such as BP values. The virtual WW program can be scalable to overweight and obese patients with diabetes, irrespective of their location, and is potentially as successful as the in-person WW program.
Acknowledgments
The authors would like to thank the clinical and administrative staff of the Why WAIT program at the Joslin Diabetes Center. They would also like to thank Mariam Ibrahim for her contribution to data collection. Data from this work was presented at the 81st Scientific Sessions of the American Diabetes Association, 25–29 June 2021, Virtual.
Footnotes
Author contributions: Marwa Al-Badri: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Cara L. Kilroy: Data curation; Investigation; Methodology; Resources; Validation; Writing – review & editing.
Jacqueline Ifat Shahar: Data curation; Investigation; Methodology; Resources; Validation; Writing – review & editing.
Shaheen Tomah: Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Writing – review & editing.
Hannah Gardner: Investigation; Methodology; Resources; Validation; Writing – review & editing.
Mallory Sin: Investigation; Methodology; Resources; Validation; Writing – review & editing.
Jennie Votta: Methodology; Resources; Validation; Writing – review & editing.
Aliza Phillips-Stoll: Investigation; Methodology; Resources; Validation; Writing – review & editing.
Aaron Price: Investigation; Methodology; Resources; Validation; Writing – review & editing.
Joan Beaton: Investigation; Methodology; Resources; Validation; Writing – review & editing.
Chandra Davis: Investigation; Methodology; Resources; Validation; Writing – review & editing.
Jo-Anne Rizzotto: Investigation; Methodology; Resources; Validation; Writing – review & editing.
Shilton Dhaver: Investigation; Methodology; Resources; Software; Validation; Writing – review & editing.
Osama Hamdy: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.A.-B., C.L.K., J.I.S., M.S., A.P.-S., J.B., C.D., H.G., J.-A.R., and S.D. have nothing to disclose. S.T. reports consulting for Research America; shareholder of Amarin corp. O.H. is consultant to Abbott Nutrition, Sanofi Aventis, AstraZeneca; his employer Joslin Diabetes Center receives research grants from Novo-Nordisk, Eli-Lilly, Gilead, and National Dairy Council. On scientific advisory board of Twin and L-Neutra and stock holder of Healthimation.
Ethics approval: The Joslin Diabetes Center, Committee on Human Studies approved this study (IRB approval ID: STUDY00000133) with a waiver of informed consent.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Marwa Al-Badri  https://orcid.org/0000-0002-4998-9708
https://orcid.org/0000-0002-4998-9708
Data sharing: The data contained in this manuscript are held at the Joslin Diabetes Center clinical research center.
Contributor Information
Marwa Al-Badri, Joslin Diabetes Center, Affiliated with Harvard Medical School, One Joslin Place, Room 316, Boston, MA 02215, USA.
Cara L. Kilroy, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA
Jacqueline Ifat Shahar, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Shaheen Tomah, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Hannah Gardner, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Mallory Sin, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Jennie Votta, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Aliza Phillips-Stoll, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Aaron Price, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Joan Beaton, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Chandra Davis, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Jo-Anne Rizzotto, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Shilton Dhaver, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
Osama Hamdy, Joslin Diabetes Center, Affiliated with Harvard Medical School, Boston, MA, USA.
References
- 1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 2. CDC. National diabetes statistics report 2020, https://www.cdc.gov/diabetes/data/statistics/statistics-report.html
- 3. Lin J, Thompson TJ, Cheng YJ, et al. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr 2018; 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC. National diabetes statistics report 2017, https://www.cdc.gov/diabetes/data/statistics/statistics-report.html
- 5. Dall T, Reynolds R, Chakrabarti R, et al. The complexities of physician supply and demand: projections from 2016 to 2030. Washington, DC: American Association of Medical Colleges, 2018. [Google Scholar]
- 6. American Board of Internal Medicine (ABIM) Number of certificates issued – all candidates, 2018, https://www.abim.org/Media/vaqdilmh/1_candidates-certified-all-candidates-050121.pdf
- 7. Long-term effects of lifestyle intervention or metformin on diabetes development microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015; 3: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010; 170: 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Eng J Med 2013; 369: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. 5 Lifestyle management: standards of medical care in diabetes – 2019. Diabetes Care 2019; 42(Suppl. 1): S46–S60. [DOI] [PubMed] [Google Scholar]
- 11. Hamdy O, Mottalib A, Morsi A, et al. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: a 5-year longitudinal study. BMJ Open Diabetes Res Care 2017; 5: e000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Po YM. Telemedicine to improve patients’ self-efficacy in managing diabetes. J Telemed Telecare 2000; 6: 263–267. [DOI] [PubMed] [Google Scholar]
- 13. Ashrafzadeh S, Hamdy O. Patient-driven diabetes care of the future in the technology era. Cell Metab 2019; 29: 564–575. [DOI] [PubMed] [Google Scholar]
- 14. Anthony Jnr B. Implications of telehealth and digital care solutions during COVID-19 pandemic: a qualitative literature review. Inform Health Soc Care 2021; 46: 68–83. [DOI] [PubMed] [Google Scholar]
- 15. Bokolo Anthony J. Use of telemedicine and virtual care for remote treatment in response to COVID-19 pandemic. J Med Syst 2020; 44: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doshi A, Platt Y, Dressen JR, et al. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med 2020; 15: 302–304. [DOI] [PubMed] [Google Scholar]
- 17. Bokolo AJ. Exploring the adoption of telemedicine and virtual software for care of outpatients during and after COVID-19 pandemic. Ir J Med Sci 2021; 190: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamdy O, Carver C. The Why WAIT program: improving clinical outcomes through weight management in type 2 diabetes. Curr Diab Rep 2008; 8: 413–420. [DOI] [PubMed] [Google Scholar]
- 19. Mitri J, Hamdy O. Diabetes medications and body weight. Exp Opin Drug Safe 2009; 8: 573–584. [DOI] [PubMed] [Google Scholar]
- 20. Giusti J, Rizzotto JA. Interpreting the Joslin Diabetes Center and Joslin Clinic clinical nutrition guideline for overweight and obese adults with type 2 diabetes. Curr Diab Rep 2006; 6: 405–408. [DOI] [PubMed] [Google Scholar]
- 21. Campbell A. Tackling ‘diabesity’ head-on. Diabetes Self Manag 2005; 22: 4042–4044. [PubMed] [Google Scholar]
- 22. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Eng J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wadden TA, Osei S. The treatment of obesity: an overview. Handbook Obes Treat 2002; 229: 248. [Google Scholar]
- 24. Wadden TASA. Handbook of obesity treatment. New York: Guilford Press, 2002. [Google Scholar]
- 25. Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring, Md) 2006; 14: 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The Diabetes Prevention Program (DPP). Description of lifestyle intervention. Diabetes Care 2002; 25: 2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang H, Tomah S, Mottalib A, et al. 238-OR: long-term effects of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: a 10-year longitudinal study. Diabetes 2019; 68(Suppl. 1): 238. [Google Scholar]
- 28. Golovaty I, Wadhwa S, Fisher L, et al. Reach, engagement and effectiveness of in-person and online lifestyle change programs to prevent diabetes. BMC Public Health 2021; 21: 1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christian J, Dasgupta N, Jordan M, et al. Digital health and patient registries: today, tomorrow, and the future. In: Gliklich RE, Dreyer NA, Leavy MB, et al. (eds) 21st century patient registries: registries for evaluating patient outcomes: a user’s guide. 3rd ed. Rockville, MD: Agency for Healthcare Research and Quality, 2018, https://www.ncbi.nlm.nih.gov/books/NBK493822/ [PubMed] [Google Scholar]
- 30. Azar KM, Aurora M, Wang EJ, et al. Virtual small groups for weight management: an innovative delivery mechanism for evidence-based lifestyle interventions among obese men. Transl Behav Med 2015; 5: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]