Abstract
Purpose:
To study the clinico-pathological profile of breast cancer patients and the prevalence of uterine fibroids in them, their hormonal levels and hormone receptor status.
Patients and methods:
52 patients with breast cancer who attended AIIMS Bhopal from November 2018 to January 2020 were selected, with their clinical details, triple assessment and other investigations for further management being performed and recorded. The presence of uterine fibroids was assessed using ultrasound of the abdomen, and for patients who had undergone hysterectomy, previous medical records were examined to ascertain the history of uterine fibroids. Serum levels of estrogen and progesterone were assessed using chemi-luminescent micro-particle immune assay (CMIA).
Results:
The mean age of patients was 50.35 ± 10.87 years. 36.54% of our patients had uterine fibroids, of whom 15.38% had undergone hysterectomy for the same, and 21.15% was detected on ultrasound of the abdomen during evaluation. Among patients with uterine fibroids, 84.2% were hormone receptor-positive, while in patients without uterine fibroids, only 57.6% had positive receptors. (P = 0.049). Among premenopausal patients, there was a statistically significant difference in serum progesterone values between patients with and without uterine fibroids.
Conclusion:
The prevalence of uterine fibroids in our study group of breast cancer patients was found to be high. The role of estrogen and progesterone in the pathophysiology of both diseases and the common risk factors involved may biologically explain this finding. Breast cancer and other estrogen associated disorders may hold future research prospects.
Keywords: carcinoma breast, fibroid uterus, hormone receptors, hormonal levels, CMIA, estrogen, progesterone
Introduction
Breast cancer is the most common cancer among females in major cities and is second most common in rural areas in India. 1 It accounts for 27.7% of all new cases of cancer among females in India and ranks first as the cause for cancer-related mortality. 2 The risk factors leading to the development of breast cancer may be hormonal or non-hormonal. Estrogen has been found to cause cell proliferation mainly through non-genotoxic pathways, but genotoxic pathways also play a role. 3 Both estrogen and progesterone cause the epithelial cells of the breast to proliferate at an increased rate, hence increasing the chances of mutation. This increased cell production in turn increases the survival of the mutated cells, causing an elevated breast cancer risk. 4
There are a few studies that worked on finding an association between the uterine fibroid and increased breast cancer risk.5–8 Some implicate estrogen and estrogen receptor as the main factor causing uterine myomas, whereas others relate the same to progesterone. 9 Apart from increased hormonal exposure, uterine fibroids also share a few other risk factors in common with breast cancer, like obesity and early menarche, probably due to the hyper-estrogenic state in obesity, and the increased number of cell divisions during the reproductive phase causing more chance of mutations in case of early menarche.5,10
Our aim was to study the clinic-pathological profile of breast cancer patients and the prevalence of uterine fibroids in them. As secondary objectives, we also looked for any possible association between the presence of uterine fibroids in breast cancer patients, and their receptor status, as well as serum levels of estrogen and progesterone.
An increased level of both exogenous and endogenous hormones is associated with a high risk of breast cancer. Only a few studies have looked into disorders associated with breast cancer, along with the hormonal assays, and these studies are all medical record-based.5,6 In our literature search, we could not find any studies that evaluated hormonal levels while investigating the relationship between uterine fibroids and carcinoma breast.
Materials and Methods
The study was a hospital-based, single-center observational study conducted from November 2018 to January 2020. The study protocol was approved by the institutional human ethics committee. Both inpatients and outpatients who consented were screened for the study. Inclusion criteria were patients of 20-80 years age, with tissue proven diagnosis of carcinoma breast, either newly detected or already undergoing treatment. Key exclusion criteria were patients who had already undergone hysterectomy with no documented history of uterine leiomyoma in past medical records and male patients with breast cancer. Our final sample size was 52.
All patients were given detailed information sheets about the study, and informed consent was taken. Patient history was recorded using a article based proforma. Past history of uterine fibroids, myomectomy, or hysterectomy for uterine fibroids was noted from previous medical records. All women underwent triple assessment, and metastatic investigations were done whenever indicated (according to NCCN practice guidelines). 11 Patients with symptoms suggestive of metastatic disease or with advanced lesions underwent metastatic workup in the form of CECT thorax and abdomen or PET- CT according to clinician discretion. For patients who were already undergoing treatment, medical records were reviewed for the same. Ultrasound abdomen was done as a part of routine metastatic workup. The presence or absence of uterine fibroids was noted in the abdominal imaging of each patient, either ultrasound or cross sectional imaging, whichever was the modality of metastatic work-up done for the patient. Serum estrogen (estradiol and serum progesterone levels of all patients were measured using chemi-luminescent microparticle immune assay (CMIA). Hormonal assays were measured for all patients with carcinoma breast, even if they did not have this correlation with fibroids, to make the study bias-free. Tests of premenopausal patients were done in the early luteal phase of the menstrual cycle. A single blood sample was drawn for the test. Patients who were recruited during the course of their treatment, and had already taken chemotherapy or hormonal therapy were excluded from the hormonal assays.
Data was entered into Microsoft Excel 2016 workbook and analyzed using statistical analysis software SPSS (IBM Corp) Version1.0.0.1327. Mean and the standard deviation were used to describe continuous variable of normal distribution. The median and interquartile range was used for non-normally distributed data. Qualitative variables were analyzed using the Chi-square test. Association between the dependent variable and the continuous independent variable was tested using unpaired t-test if normally distributed and Mann Whitney test if not normally distributed. Type 1 error was set as 5%, hence the level of significance was P value < 0.05.
Results
Patient characteristics
The average age of our patients was a 50.35 ± 10.87 year with the majority falling into the age group of 41-50 years (36.5%). The larger number (53.85%) hailed from a rural background. The most common presentation was breast lump, seen in 98.07% of our patients.
67.3% of patients were postmenopausal. None of the patients in the study group had a history of early menarche (< 12 years) or late menopause (> 50 years) (Table 1).
Table 1.
Patient characteristics.
| PARAMETER | RESULT | |
|---|---|---|
| Average age | 50.35 ± 10.87 years | |
| Background | Urban 24 (46.15%) | |
| Rural 28 (53.85%) | ||
| Menstrual status | Postmenopausal 35 (67.3%) | |
| Premenopausal 17 (32.7%) | ||
| Parity | Multiparous 51 (98.07%) | |
| Nulliparous 1 (1.92%) | ||
| Histological type | Invasive ductal carcinoma NOS 49 (94.23%) | |
| Special type 4 (7.7%) | ||
| Stage of disease | I 4 (7.69%) | |
| II 21 (40.38%) | ||
| III 24 (46.15%) | ||
| IV 3 (5.77%) | ||
| Presence of uterine fibroids | 19 (36.54%) | On ultrasound (21.15%) |
| Hysterectomy for fibroids (15.38%) | ||
| Biologic subtype | Luminal A 28 (53.85%) | |
| Luminal B 7 (13.46%) | ||
| HER2 positive 8 (15.38%) | ||
| Triple negative 9 (17.31%) | ||
98.07% of patients were multiparous, with most patients (52.9%) having their age at first childbirth < 20 years. 98.07% of patients had a history of lactation. 11.54% of patients had a history of abortion.19.23% of women were obese, with a BMI > 27.5.
Only 3.84% of patients had a history of usage of exogenous hormones, and the exposure was more than five years prior to the breast cancer diagnosis in all of them. Only one patient had a history of smoking. None of the patients had history of alcohol abuse.
19.2% of patients had a history of benign breast disease in the past, and none had a past history of breast cancer.
Only 1.92% had a family history of breast cancer, whereas 7.69% of patients had family histories of other cancers (thyroid, prostate, and larynx).
Tumor characteristics
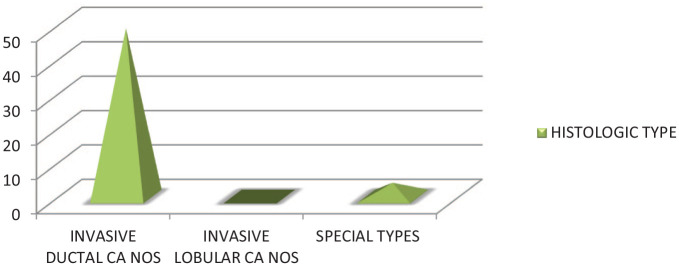
On imaging, 42.31% of patients had BIRADS category 5 lesions. The most common histological type was invasive ductal carcinoma not otherwise specified in 94.23% of patients (Table 1). Most of the patients were found to have histological grade 2 (59.6%) (Graph 2). 92.3% of patients had unifocal and unicentric tumors, while the rest had either multifocal or multicentric tumors.
Graph 2.
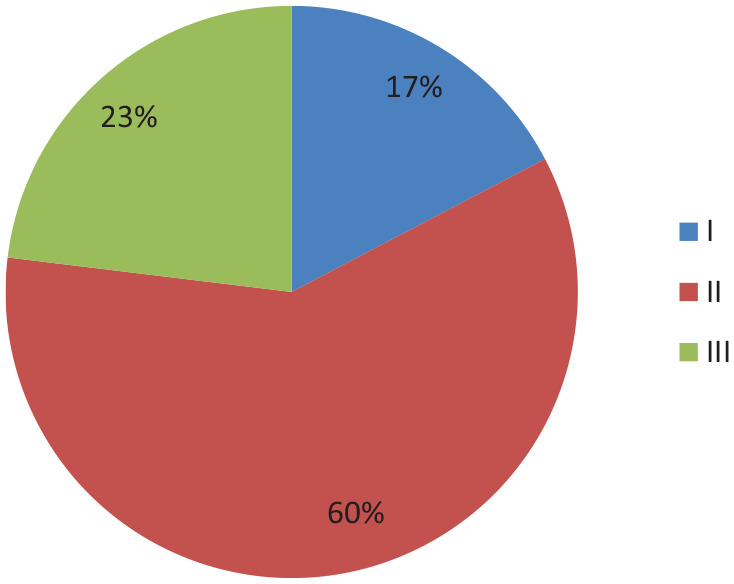
Graph showing frequency distribution of histological grade.
Graph 1.
Graph showing frequency of histological types.
Staging
The most common T stage was T2 in 38.46%, followed by T3 in 25% of patients. N0 and N1 diseases were found in 40.38% of patients each. Only 5.77% of patients had systemic metastases. 48.07% of patients had early breast cancer, 46.15% had locally advanced disease, and 5.77% had advanced breast cancer (Table 1).
Uterine Fibroids
36.54% of our patients had uterine fibroids, of whom 15.38% had undergone hysterectomy for the same, and 21.15% was detected during evaluation with ultrasound. (Table 1), (Graph 3). The presence of uterine leiomyoma was found to have a significant association with thyroid disease (P = 0.011), hormone receptor positivity (P = 0.049), and T stage (P = 0.034).
Graph 3.
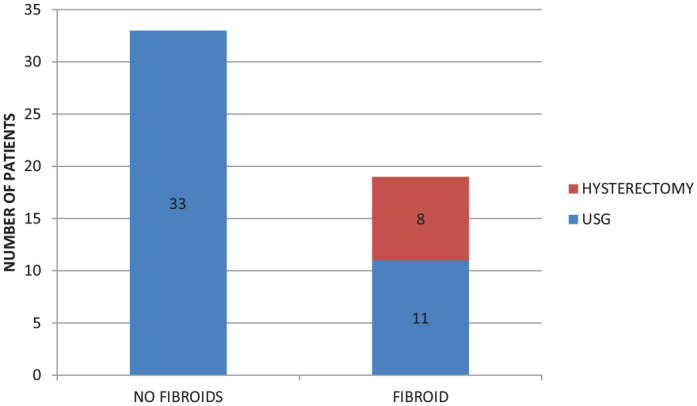
Graph showing prevalence of uterine fibroids in study group.
84.2% of patients with uterine fibroid were hormone receptor-positive, while only 57.6% were hormone receptor-positive in the group without uterine fibroids.
Biologic subtype
The most common biologic subtype was Luminal A in 53.85% of patients, followed by basal-like in 17.31%, HER2 enriched in 15.38%, and Luminal B in 13.46% of patients (Table 1).
Among patients with uterine fibroids, 84.2% were hormone receptor-positive, while in patients without uterine fibroids only 57.6% were hormone receptor-positive. On analysis using the chi-square test, we found that there was a statistically significant association between the presence of uterine fibroids and ER PR positivity (P-value 0.049). Regression analysis of the same did not yield any significant results.
Hormone levels
The average serum value of estradiol was 208(51.5-242) pg/ml in premenopausal patients and 17.04 ± 5.29 pg/ml in postmenopausal patients. The average progesterone value was 2.44 (0.97-3.46) ng/ml in premenopausal patients and 0.1 ± 0 ng/ml in postmenopausal patients.
Among pre-menopausal patients, the value of serum progesterone was significantly higher in patients with uterine fibroids, as compared with women without fibroids (Table 2). Although the average values of estrogen were higher among patients with uterine fibroids in both pre and postmenopausal groups, this difference was not statistically significant. The serum progesterone values of all postmenopausal patients were < 0.1 ng/ml, and the exact value was not calibrated. Hence, a comparison of progesterone values has not been done.
Table 2.
Serum hormone values of patients with and without uterine fibroid.
| PRESENCE OF FIBROID UTERUS | PATIENTS WITH FIBROID | PATIENTS WITHOUT FIBROID | p-value |
|---|---|---|---|
| PREMENOPAUSAL | |||
| S. Estrogen | 206.75 ± 85.74 pg/mL | 141(25.25-29.75)pg/mL | 0.120 |
| S. Progesterone | 3.633 ± 1.771 ng/mL | 2.11(0.18-2.87)ng/mL | 0.047 |
| POSTMENOPAUSAL | |||
| S. Estrogen | 18.83 ± 5.65 pg/mL | 15.5 ± 4.60 pg/mL | 0.110 |
| S. Progesterone | 0.1 ± 0 ng/mL | 0.1 ± 0 ng/mL | ——– |
Among postmenopausal patients, the serum estrogen values were more in those who were hormone receptor-positive than in hormone receptor-negative patients, and the difference was statistically significant (Table 3). The progesterone values were not compared, as all patients had values < 0.1 ng/mL, and the exact values were not calibrated.
Table 3.
Serum hormone values of hormone receptor positive and negative patients.
| RECEPTOR STATUS | ER PR + | ER PR- | p-value |
|---|---|---|---|
| PREMENOPAUSAL | |||
| S. Estrogen | 199(23-245)pg/mL | 217(60-241)pg/mL | 0.749 |
| S. Progesterone | 1.98(0.20-3.62)ng/mL | 2.64(1.23-3.41)ng/mL | 0.701 |
| POSTMENOPAUSAL | |||
| S. Estrogen | 18.048 ± 5.25 pg/mL | 12.8 ± 3.033 pg/mL | 0.044 |
| S. Progesterone | 0.1 ± 0 ng/mL | 0.1 ± 0 ng/mL | ——– |
Discussion
The development of both breast cancer and uterine fibroids is closely linked with the hormonal action of estrogen and progesterone.12,13 This led us to do a study on the clinic-pathological factors affecting breast cancer in the women of our region, to find out the prevalence of uterine fibroids in them, as well as the hormone levels in serum, as an objective measurement of their hormonal activity.
Prevalence of uterine fibroids in breast cancer patients
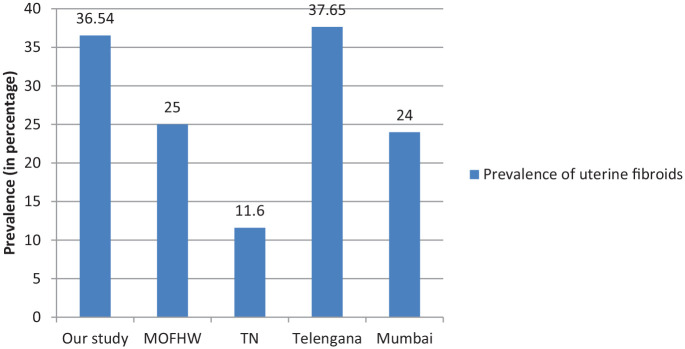
The primary outcome was to find out the prevalence of uterine fibroids in patients with breast cancer. In our study group, 36.54% patients had uterine fibroids either on ultrasound of the abdomen or had previously undergone hysterectomy for uterine fibroids. The prevalence of fibroids is found to be 20% to 30% in the reproductive age group in Indian women. 14 The prevalence varies between different age groups and according to menstrual status as well. 15 There is a considerable difference in prevalence among different racial groups, and women belonging to different geographic locations. 10 A few studies from various parts of India also show a substantial difference in the prevalence rates. Srilatha and Malathi 16 from Telangana have reported a prevalence rate of 11.6%, Munusamy et al 17 from Tamil Nadu found a prevalence of 37.65% in their study, and 24% in Mumbai (Graph 4).
Graph 4.
Prevalence of uterine fibroids in our study with available literature.
There are a few studies that have investigated the correlation between uterine fibroids and breast cancer. In 1990, Lindegard et al studied breast cancer and associated uterine leiomyomata and benign breast disease. Leiomyoma uteri formed an epidemiological cluster with premenopausal breast cancer and showed a significant association with non-fatal breast cancer in their study. 18 Chuang et al in a case-control study in Taiwan in 2015 observed that uterine leiomyoma along with various other estrogen-related factors is associated with subsequent breast cancer risk. They obtained an Odds ratio of 1.20 and a 95% confidence interval of 1.03 to 1.40. 7
Another cohort study published in 2017 reported a higher incidence of breast cancer in women with uterine leiomyoma and a higher risk of developing breast cancer, as compared with women without uterine leiomyoma (adjusted hazard ratio of 1.31; 95% confidence interval = 1.13−1.52). In addition, the mortality and risk of mortality were found to be lower in women with uterine leiomyomata who developed breast cancer. 6 This is in congruence with the results of Lindegard. 18 Tseng et al published a case-control study in 2017, which revealed a significant association between uterine leiomyoma and breast cancer (adjusted OR = 1.14; 95% CI = 1.07–1.21). The association remained irrespective of recent hormone use or surgical removal of uterine fibroids. They found that a myomectomy or hysterectomy for removal of the fibroids did not reduce the risk of subsequent development of breast cancer. 5
There is one prospective cohort study from the United States, which found no association between breast cancer and uterine leiomyomata. Although they did observe a positive association between uterine fibroids diagnosed before the age of 30 years and the risk of premenopausal breast cancer. The study had employed self-reporting of uterine leiomyomata, which could have led to an underestimation of women with uterine fibroids since a majority of them would be asymptomatic. This might explain how their results differ from the above-mentioned studies, regarding overall breast cancer risk in women with uterine leiomyoma. 8
On subgroup analysis, we found that patients with fibroid uterus had an association with ER/PR status, stage of the disease, T stage, and the presence of thyroid disease. On regression analysis of these variables, only thyroid disease seemed to have a significantly increased risk (OR = 11.429, 95%CI = 1.220-107). A study by Kim et al found an association between uterine fibroids and thyroid nodules, with a closer relationship in premenopausal women. The common role and interplay of estrogen have been attributed to, this association. 19
Hormone receptor positivity
The majority of patients in our study group were hormone receptor-positive, constituting 67.3%. The most common molecular subtype was Luminal A constituting 53.85% of patients of the entire study group. 15.38% of patients were HER 2 positive, and 17.3% patients were negative for hormone receptors as well as HER2.
We found an association between uterine leiomyoma and hormone receptor positivity (P = 0.049). The majority of the patients with uterine leiomyoma were ER/PR positive (84.2% in patients with fibroids vs. 57.6% in patients without fibroids), falling under the Luminal A subtype. However, the regression analysis did not show any significant results. Shen et al observed a similar result in their study, where more of the breast cancer patients with uterine leiomyoma were hormone receptor-positive (69.6%), as compared with breast cancer patients without uterine fibroid (64.9%). They observed this in terms of whether the patient has received hormonal therapy for ER PR. However, this result was not statistically significant. 6
Wise et al also observed a slightly increased proportion of patients in the uterine leiomyoma group who were ER/PR positive (51.9%), than in the leiomyoma free group (47.5%) although not statistically significant. 8
The basis of investigating the association of breast cancer with uterine leiomyoma is the complex interplay of the sex steroid hormones regulating both the disease and is sometimes attributed to the common risk factors they share as well. The hormone receptor-positive cancers are known to be less aggressive and respond better to hormonal therapy.
Serum levels of estrogen and progesterone
All patients in our study had normal levels of serum estrogen and progesterone with respect to their their age and menopausal status. Several studies have proved that the level of endogenous estrogens is relatively more in women who develop breast cancer than those who do not.3,20,21 In the study by Toniolo et al, the level of hormones of all the women fell under the conventional normal limits, but the estrone, total estradiol, and free estradiol of women who subsequently developed breast cancer were higher than those of women who did not. 20 However, Beattie 22 did not find any difference in the hormone levels of patients and control group in their study. Although there is a strong positive association between serum estrogen levels and the risk of breast cancer, the lack of a standardized and reproducible laboratory technique limits its use as a clinical marker for risk identification.3,23
Hormone levels and uterine fibroids
The serum estrogen and progesterone values of the women with uterine fibroids were more than those in women without uterine fibroids, in our study. The difference was statistically significant (P = 0.047) in the case of progesterone in the premenopausal group. In the postmenopausal group, the difference in estrogen values was not significant, and progesterone was not compared because all patients had a value < 0.1 ng/ml.
The origin of uterine fibroids is probably linked with chromosomal aberrations and genetic mutations, but their development is intricately linked with ovarian steroid hormones. When fibroid cells proliferate in response to estrogen and progesterone, Ki67 is co-expressed with ERα and PR, indicating their direct effect on promoting the proliferation of fibroid cells. The hormone-dependent increase in the size of fibroids is through various mechanisms, cellular proliferation, cellular hypertrophy, and extracellular matrix accumulation, which is mainly under the control of progesterone, with a permissive role of estrogen. 24
There are conflicting findings regarding serum estrogen and progesterone levels in patients with uterine leiomyoma as compared with those without. Some studies suggest that the levels are not elevated in people with uterine leiomyoma, whereas some studies have found higher levels of urinary estrogens. The tissue concentration of ovarian hormones and their receptors have, however, been found to be at higher concentrations in myoma tissue, as compared with the surrounding normal myometrium.10,25 Wong et al 26 found women with higher levels of circulating testosterone and estradiol to have an elevated risk of developing fibroids.
Hormone levels and ER/PR status
In our study, there was no statistically significant difference in serum estrogen (P = 0.749) and progesterone levels (p = 0.701) of premenopausal patients who were ER/PR + and ER/PR-. The serum estrogen values of postmenopausal patients were more in the ER/PR + group than in the ER/PR negative group, and this difference was statistically significant (p = 0.044).
Exposure to higher levels of both endogenous and exogenous hormones is a risk factor for breast cancer. Various studies have compared the level of endogenous hormones with the risk of developing breast cancer, and more recently, with the ER/PR status. The data regarding the same is stronger for postmenopausal women than for the premenopausal group. Circulating estrogen in various forms has been linked to an increased risk of development of breast cancer. These include total estradiol, free estradiol, estrone, and estrone sulfate. The circulating androgens also have a similar risk relationship, but the role of endogenous progesterone in raising breast cancer risk has not yet been proven. 27 Missmer et al 28 proved that the level of circulating estrogens and androgens are strongly associated with ER/PR + breast cancer. In another study, ER + breast cancer risk was two-fold higher in women with higher estradiol levels. The ER + patients had higher mean levels of estradiol and testosterone as compared with controls, whereas ER- patients did not. The difference in the former group was statistically significant. 29 A study on premenopausal women found that luteal phase estradiol was significantly associated with an increase in the risk of developing ER/PR + breast cancer. Other forms like estrone, free estradiol, and progesterone in the luteal phase were not associated with an increased risk. None of the hormones measured in the follicular phase showed a significant risk association. 30
The data for premenopausal women is less robust than that for postmenopausal patients, due to inter, and intra-individual variability in hormone levels during different phases of the menstrual cycle. In the case of postmenopausal women, laboratory assays need to be ultra-sensitive for detecting the low levels of estradiol present in them. However, even with the inter-laboratory variability, the risk association of estradiol across different studies is evident, emphasizing the importance of this association.
A similar association was also found for ER/PR + patients in our study, compared with the ER/PR- group in postmenopausal patients only.
Limitations of the study
The cross-sectional observational study design does not permit evaluation of the statistical significance of the increased prevalence of uterine fibroids in breast cancer patients that we found in our study. Also, our sample size was limited, hence a study based on a larger population with more variables may be an appropriate representation of the general population
The standardization of laboratory assessment of steroid hormones in serum is difficult, especially when in small quantities, and needs to be standardized, to avoid research errors.23,31,32
The clinical implications of the presence of uterine fibroids and risk of breast cancer may be observed in cohort studies, and the prognostic effects if any assessed.
Conclusion
The prevalence of uterine fibroids in our study group of breast cancer patients was high. The patients with uterine fibroids were mostly hormone receptor-positive than those who did not have uterine fibroids. The role of estrogen and progesterone in the patho-physiology of both diseases and the common risk factors involved may biologically explain this finding.
The objective assessment of hormonal activity in breast cancer and associated disorder, the role of hormone receptors and the interplay of these factors is an interesting area of research. From the observations in our study, we feel that future research prospects include breast cancer and other estrogen associated disorders in the Indian population, their role in risk modification and prognosis. The prevalence of well-known and commonly studied risk factors for breast cancer was relatively less in our study group. Development of reproducible, standardized laboratory techniques for hormone assays and the assessment of their role in breast cancer prognosis and management, and the possibility of its use as a tumor marker, would be a welcome area for research.
Acknowledgments
Specially to my fellow colleagues Dr. Ankit Lalchandani and Dr. Md Masoom Parwez whose invaluable contributions, allowed this project to see the light of the day.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Dr. Anjaly is the chief author and investigator. Dr. Vinay is the Surgical Oncology chief, whose contribution was in recruiting his surgical patients and guiding Anjaly through the intricacies of completing her project. Dr. Swagata was Anjaly’s co-guide from the department who helped her in compiling her data and details. Dr. Bharati was Anjaly’s chief guide and helped her formulating the research question as well as with the sequencing and discussion of the project.
ORCID iDs: Anjaly Mohan  https://orcid.org/0000-0003-3887-8321
https://orcid.org/0000-0003-3887-8321
Bharati Pandya  https://orcid.org/0000-0002-9574-3688
https://orcid.org/0000-0002-9574-3688
References
- 1. ICMR. Consolidated report of the hospital based cancer registries Bengaluru, India, 2016, http://www.ncdirindia.org/Download/AR_2016_2017.pdf
- 2. Latest global cancer data. World Health Organization. IARC. 1 354 051 855. Int Agency Res Cancer. 2019;468:2018-2019. [Google Scholar]
- 3. Colditz GA. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst. 1998;90:814-823. [DOI] [PubMed] [Google Scholar]
- 4. Blamey R, Collins J, Crosignani PG, et al. Hormones and breast cancer. Hum Reprod Update. 2004;10:281-293. [DOI] [PubMed] [Google Scholar]
- 5. Tseng JJ, Chen YH, Chiang HY, Lin CH. Increased risk of breast cancer in women with uterine myoma: a nationwide, population-based, case-control study. J Gynecol Oncol. 2017;28:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen TC, Hsia TC, Hsiao CL, et al. Patients with uterine leiomyoma exhibit a high incidence but low mortality rate for breast cancer. Oncotarget. 2017;8:33014-33023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chuang SC, Wu GJ, Lu YS, Lin CH, Hsiung CA. Associations between medical conditions and breast cancer risk in Asians: a nationwide population-based study in Taiwan. PLoS ONE. 2015;10:e0143410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wise LA, Radin RG, Rosenberg L, Adams-Campbell L, Palmer JR. History of uterine leiomyomata and incidence of breast cancer. Cancer Causes Control. 2015;26:1487-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans JM. An integrative approach to fibroids, endometriosis, and breast cancer prevention. Integr Med. 2008;7:28-31. [Google Scholar]
- 10. Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Treatment by Cancer Type: Breast cancer. NCCN clinical practice guidelines in oncology version1. 2020, 2020. [Google Scholar]
- 12. Yue W, Wang JP, Li Y, et al. Effects of estrogen on breast cancer development: role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127:1748-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carol A, Lange DY. Progesterone and breast cancer Carol. Womens Heal (L Engl). 2008;4:151-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ministry of Health and Family Welfare. Standard Treatment Guidelines Obstetrics & Gynaecology (ed Ashley J D’cruz, Garima Arora Gandhi and Sharath Damodhar BN). 2014:1–101. http://clinicalestablishments.gov.in/WriteReadData/4571.pdf.
- 15. Khyade RL. A study of menstrual disturbance in cases of fibroid uterus. Int J Reprod Contraception Obstet Gynecol. 2017;6:2494. [Google Scholar]
- 16. Srilatha J, Malathi V. Prevalence of fibroids: a study in a semiurban area in Telangana, India. Int J Reprod Contraception Obstet Gynecol. 2017;6:5247. [Google Scholar]
- 17. Munusamy MM, Sheelaa WG, Lakshmi VP. Clinical presentation and prevalence of uterine fibroids: a 3-year study in 3-decade rural South Indian women. Int J Reprod Contraception Obstet Gynecol. 2017;6:5596. [Google Scholar]
- 18. Lindegard B. Breast cancer among women from Gothenburg with regard to age, mortality and coexisting benign breast disease or leiomyoma uteri. Oncology. 1990;47:369-375. [DOI] [PubMed] [Google Scholar]
- 19. Kim MH, Park YR, Lim DJ, et al. The relationship between thyroid nodules and uterine fibroids. Endocr J. 2010;57:615-621. [DOI] [PubMed] [Google Scholar]
- 20. Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87:190-197. [DOI] [PubMed] [Google Scholar]
- 21. Moore SC, Matthews CE, Ou Shu X, et al. Endogenous estrogens, estrogen metabolites, and breast cancer risk in postmenopausal Chinese women. J Natl Cancer Inst. 2016;108:djw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beattie MS. Endogenous sex hormones, breast cancer risk, and tamoxifen response: an ancillary study in the NSABP breast cancer prevention trial (P-1). J Natl Cancer Inst. 2006;98:110-115. [DOI] [PubMed] [Google Scholar]
- 23. Hankinson SE, Manson JE, London SJ, Willett WC, Speizer FE. Laboratory reproducibility of endogenous hormone levels in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1994;3:51-56. [PubMed] [Google Scholar]
- 24. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Omar M, Laknaur A, Al-Hendy A, Yang Q. Myometrial progesterone hyper-responsiveness associated with increased risk of human uterine fibroids. BMC Womens Health. 2019;19:92. https://bmcwomenshealth.biomedcentral.com/articles/10.1186/s12905-019-0795-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wong JYY, Gold EB, Johnson WO, Lee JS. Circulating sex hormones and risk of uterine fibroids: study of women’s health across the nation (swan). J Clin Endocrinol Metab. 2016;101:123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen WY. Exogenous and endogenous hormones and breast cancer. Best Pr Res Clin Endocrinol Metab. 2008;22:573-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856-1865. [DOI] [PubMed] [Google Scholar]
- 29. Farhat GN, Cummings SR, Chlebowski RT, et al. Sex hormone levels and risks of estrogen receptor-negative and estrogen receptor-positive breast cancers. J Natl Cancer Inst. 2011;103:562-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fortner RT, Eliassen AH, Spiegelman D, Willett WC, Barbieri RL, Hankinson SE. Premenopausal endogenous steroid hormones and breast cancer risk: results from the Nurses’ Health Study II. Breast Cancer Res. 2013;15:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention National Centre for Environment Health Division of Laboratory Science. Standardizing Hormone Measurements National Center for Environmental Health Division of Laboratory Sciences. https://www.cdc.gov/labstandards/pdf/hs/HoSt_Brochure.pdf. [Google Scholar]
- 32. Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab. 2013;98:1376-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]