Abstract
Cancer is a major health problem worldwide and the leading cause of death in many countries. It remains challenging to find anticancer treatments that work efficiently for varying types of cancer cells. Several studies revealed that nuclear factor kappa B (NF-κB) is a family of dimeric transcription factors that induce tumor promotion, progression, and therapeutic resistance, providing evidence that NF-kB may be a promising target for cancer drugs. Some research has found that sea cucumber biocompounds have anticancer properties, but further research is essential to confirm anticancer targets. This manuscript discusses the mechanisms of anticancer targeting the NF-κB signaling pathway induced by sea cucumber-derived compounds. Additional database analysis showed the protein targeted by the compounds involved in several pathways related to the NF-κB network. Moreover, SwissADME predicted druglikeliness properties of the active compounds of sea cucumber. The discussion is expected to provide new insight into the promising potential of these marine natural products for the treatment of many different types of cancers.
Keywords: Drug discovery, nuclear factor kappa B, pharmacokinetics, physicochemical, sea cucumbers
Introduction
NF-kB is an acronym for the nuclear factor kappa light chain enhancer of activated B cells. 1 In mammals, the NF-κB proteins belonging to NF-κB/Rel family consist of five related transcription factors: p50/p105 (NF-κB 1), p52/p100 (NF-κB 2), p65 (RelA), cRel, and RelB.2-4 These 5 proteins share a conserved Rel homology domain (RHD) of 300 amino acid length, which has multiple functions such as dimerization, mediates DNA binding, and interaction with members of the IκB family. 2 NF-κB is a family of dimeric transcription factors that participate in a number of physiological conditions, including innate and adaptive immunity, acute and chronic inflammation, apoptosis, cytoskeletal remodeling, cellular differentiation, adhesion, proliferation, and survival. Misregulation of NF-κB has been implicated in a wide range of diseases, from cancers to inflammatory and immune disorders and metabolic pathologies such as obesity, insulin resistance, and type-2 diabetes.1-3, 5
Many studies have associated NF-κB function with tumor promotion and progression, survival, angiogenesis, metastasis, chemoresistance, and radioresistance, so regulating NF-κB activation pathways is important in cancer prevention and treatment.5-9 It is an important fact that researchers are still struggling to find novel anticancers that cure varying types of cancer efficiently. Various types of drugs with different anticancer mechanisms may be needed for treatments based on the particular characteristics of cancer cells, so novel anticancers from many sources, including sea cucumbers have been explored. 10 This manuscript discusses the mechanisms of anticancer targeting the NF-κB signaling pathway induced by sea cucumber-derived compounds. The discussion is expected to provide new insight into the potential use of these marine natural products for the treatment of many different types of cancer.
NF-kB Signaling Pathway
Two major signaling pathways lead to the activation of NF-κB, namely the classical (canonical) pathway and the alternative (non-canonical) pathway.4,5,8,9,11 It is well studied that the classical pathway is important for immune responses, pro-survival signals, inflammation, and development genes, while the alternative pathway plays an essential role in lymphoid organogenesis, adaptive immunity, and survival.4,5
The classical NF-κB signaling pathway is activated by several receptors, such as tumor necrosis factor receptors (TNFRs), interleukin 1b (IL-1b), toll-like receptor/interleukin-1 (TLR/IL-1R), and T cell receptor (TCR). The alternative NF-κB signaling pathway is triggered by TNFR family receptors including CD40, Epstein-Barr virus (EBV), human T cell leukemia virus (HTLV), B-cell activating factor (BAFF), and lymphotoxin b (LTbR).5,9 Activated NF-κB in turn activates the subunit of IκB kinase (IκK). In both the classical and alternative pathways, activation of IκK leads to proteasomal degradation of IκB protein inhibitor that enables NF-κB protein dimmers (p50/p65 or RelA, c-Rel, RelB, and p50 in the classical pathway and p52/RelB in the alternative pathway) to dissociate from IκB and migrate from the cytoplasm to the nucleus, attached to target DNA sequences to inducing gene expression.4,5,9,11
The Role of NF-κB in Cancer
The first evidence of NF-κB’s role in cancer was proposed by Ballard et al in 1990. They identified the p50 subunit and RelA in humans that have high homology with the oncoprotein v-REL of the avian REL retrovirus. The oncovirus activates the v-Rel oncogene, which is associated with leukemias and lymphomas in chickens. 12 Similarly, certain viral oncoproteins in humans such as the human T cell leukemia virus (HTLV) Tax protein and the hepatitis B virus x protein have the ability to bypass classic and alternative pathways and directly activate the IκK complex and upregulate NF-κB’s downstream proteins.5,7,8 Various evidences have indicated that activation of NF-κB is common in most major cancers for the most part due to aberrant activation of upstream signaling molecules, or through autocrine or paracrine activation by cytokines and growth factors of tumor cells, up-regulation of signaling intermediates, and occasionally by mutations of genes encoding NF-κB and IκB proteins.5,9
Activated NF-κB regulates the transcription of more than 500 target genes. Many NF-κB target genes play a role in tumor cell proliferation, angiogenesis, invasion/metastasis, survival, and therapeutic resistance. NF-κB controls cell proliferation by activating growth factors, including IL-2, CD40 L, and granulocyte monocyte colony stimulator factor. NF-κB also serves as a positive regulator of the cell cycle through upregulation of c-Myc, cyclins D1, D2, D3, and E. NF-κB inhibits programmed cell death, through its inhibition and down-regulation of death receptors, activation of the antiapoptotic proteins, inhibition and degradation of caspases, inhibition of p53 activity, and production of antioxidant molecules.
Rudolf Virchow in 1863 postulated that there is a connection between inflammation and cancer. About 15% of global cancer is related to chronic infections with inflammation as a major component. 13 NF-κB affects carcinogenesis by activating downstream genes involved in the transition from inflammation to cancer growth.5,7 NF-κB activity leads to increased angiogenesis and metastasis through up-regulation of genes related to pro-inflammatory cytokines IL-8 and epithelial-mesenchymal transition (EMT) such as Twist, ICAM-1, ELAM-1, VCAM-1 (migration), CXCR4, and CXCL8 that induce VEGF expression (angiogenesis), and matrix metalloproteinases (MMP) that degrade the extracellular matrix, heparanase, and ROS via iNOS expression (invasion). Finally, the pro-inflammatory gene COX-2, which is regulated by NF-κB, has been shown to be related to cancer progression. COX-2 can be activated by numerous chemotherapeutic agents and radiation and then contributes to therapeutic resistance. NF-κB also is involved in cancer cell resistance to chemotherapy by upregulating the expression of the multidrug resistance 1 (MDR-1) gene. 5 ,7-9
Several studies in the cancer model have linked NF-κB function to tumor promotion and growth in inflammatory cancers. The first of these studies used a dextran-sulfate sodium (DSS)-induced chronic-inflammatory mouse colitis-associated cancer model and knocked down IKKβ in enterocytes, therefore blocking NF-κB activation. The experiment resulted in an 80% reduction in tumor multiplicity but had no effect on tumor size, suggesting that NF-κB was crucial for the early stages of colon cancer development. The study proceeded with the inactivation of IKKβ in myeloid cells of these animal models, believing that macrophages in the lamina propria drove the inflammation. In myeloid cells, blocking NF-κB caused only a 50% reduction in tumor multiplicity but a significant reduction in tumor size, showing that classic NF-kB signaling plays an important role in tumor promotion and progression in the surrounding intestinal stroma. The second important research to study the role of NF-κB in tumor progression used the multidrug resistance 2 gene knockout mouse model, in which the accumulation of bile acid and phospholipid is revealed in hepatocytes, leading to inflammation and formation of hepatocellular carcinoma (HCC). The blockage of NF-κB with a hepatocyte-specific expression of IκB super suppressor (IκB SR, an IκBα mutant that is resistant to phosphorylation and degradation) had no effect in the early stage of the tumor, but treatment later reduced tumor size and incidence, showing that NF-kB is crucial for tumor growth but not initiation.5,6,8 Furthermore, recent studies from many research groups demonstrated that blocking of NF-κB in mutated Kras-induced lung cancer and pancreatic cancer reduced tumor initiation and progression. 6
Sea Cucumber Compounds with Anti-NF-κB Activity
Many studies, including in vitro, in vivo, and in silico, have shown that the bioactive compounds of sea cucumber have anticancer activity. 14 Table 1 provides information about compounds from several species of sea cucumber that have been proven to have anti-NF- κB mechanisms in several cancers.
Table 1.
Sea cucumber compounds with anti-NF-κB activity.
| Compound | Source | PubChem CID | Molecular formula | Anti-NF-κB mechanism | Neoplasm | Ref. |
|---|---|---|---|---|---|---|
| Ds-echinoside A | Peasonothuria graeffei | 21636250 | C54H88O23 | Induce apoptosis by decreased NF-κB, antimetastatic activity through the specific inhibition of NF-κB-dependent |
Liver | 15 , 16 |
| MMP-9 and VEGF expressions | ||||||
| Frondoside A | Cucumaria frondosa | 23664994 | C60H95NaO29 S | Anti-invasive by antagonize TPA-induced MMP-9 activation via NF-κB and AP-1 signaling, inhibit EGF-induced transcriptional activity of NF-κB |
Breast, epidermal | 17 , 18 |
| Frondoside A | Cucumaria okhotensis | 23664994 | C60H95NaO29 S | Decrease the epidermal growth factor-induced NF- κB-dependent transcriptional activity |
Epidermal | 19 |
| Holothurin A1 (HA1) | Pearsonothuria graeffei | 102057279 | C54H87NaO27 S | Antimetastatic activity through down-regulation the expression level of NF-κB | Liver | 20 |
| Psolusoside А |
Psolus
fabricii |
– | – | Inhibit EGF-induced transcriptional activity of NF-κB |
Epidermal | 17 |
| Stichoposide D | Stichopus chloronotus | – | – | Inhibit EGF-induced transcriptional activity of NF-κB |
Epidermal | 17 |
Abbreviations: MMP, matrix metalloproteinases; NF-κB, nuclear factor kappa B; VEGF, vascular endothelial growth factor.
Cancer is a hyperproliferative abnormality characterized by the up-regulation of genes responsible for the proliferation, transformation, angiogenesis, invasion, and metastasis of cells. These processes are associated with the aberrant expression of NF-κB. 5 ,7-9, 21 For this reason, NF-κB has become an important focus for the discovery of anticancer drug.5,22 Many natural compounds are being studied for their ability to inhibit NF-κB and prevent tumor cell proliferation. 22
It has been reported that Ds-echinoside A of sea cucumber Peasonothuria graeffei has pro-apoptotic and antimetastatic activities in human hepatocellular carcinoma cells Hep G2 by inhibiting the expression of NF-κB-dependent MMP-9 and vascular endothelial growth factor (VEGF). Immunocytochemical analysis showed that Ds-echinoside A significantly decreased the expression level of matrix metalloproteinase-9 (MMP-9) and increased the expression of tissue inhibitor of metalloproteinase-1 (TIMP-1). MMP-9 plays an important role in the degradation of the extracellular matrix in tumor metastasis and angiogenesis, while TIMP-1 is an important activation regulator of MMP-9. Western blot analysis revealed that the expressions of NF-κB and VEGF were found to be remarkably reduced by Ds-echinoside A.15,16
Monosulfated pentaglycoside frondoside A isolated from Cucumaria frondosa and Cucumaria okhotensis decreased the epidermal growth factor–induced NF-κB-dependent transcriptional activity in epidermal cancer.17,19 Psolusoside А from Psolus fabricii and Stichoposide D from Stichopus chloronotus also reported inhibiting epidermal growth factor–induced transcriptional activity of NF-κB in epidermal cancer. 17
Park et al revealed that Frondoside A has the antimetastatic potential for breast cancer treatment. They reported that Frondoside A inhibited TPA-induced activation of NF-κB and AP-1, also reduced TPA-induced activation of PI3 K/Akt, ERK1/2, and p38 MAPK signals resulting in a reduction of MMP-9 expression. 18
Zhao et al 20 identified sulfated triterpene glycosides namely Holothurin A1 (HA1) in sea cucumber Pearsonothuria graeffei. Treatment with HA1 decreased the expression level of NF-κB of human hepatocellular liver carcinoma cells (HepG2), which could be linked to the antimetastatic activity of HA1. HA1 showed an influence on metastasis in vitro and in vivo.
Sea cucumbers intervene NF-κB pathway to inhibiting cancer growth
Genes that play a role in carcinogenesis as sea cucumber active compounds target genes were obtained from references on Table 1. Interaction among the proteins could be used to understand the functions of target genes of the sea cucumber active compounds in the cellular pathway. The results displayed in a network for protein–protein interactions showed correlations of NF-κB with many proteins, which are related to cancer development. The protein has cross-talk and regulates the activation of nuclear factor (NF-κB), which has been found to control multiple cellular processes in cancer including inflammation, transformation, proliferation, angiogenesis, invasion, and metastasis. 23
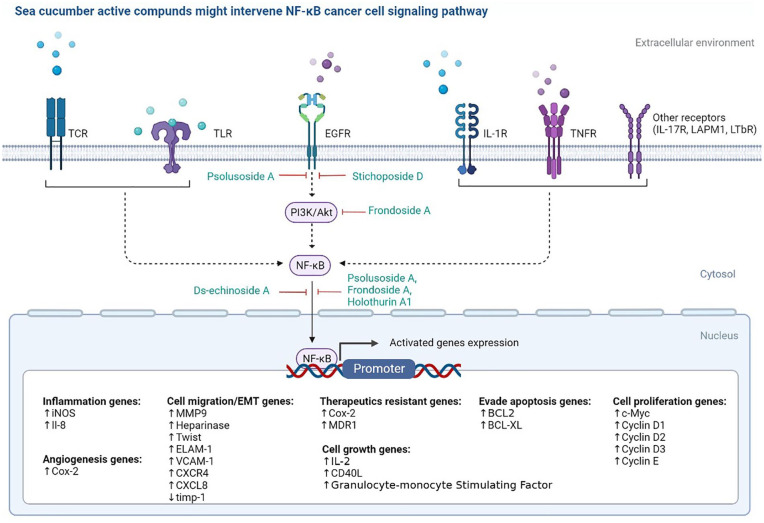
Cell growth signal via T-cell receptor (TCR), toll-like receptor (TCR), epidermal growth factor receptor (EGFR), interleukin-1 receptor (IL-1R), tumor necrotic factor (TNFR), and various other types of receptors which will stimulate cancer growth from the NF-ΚB pathway. This cell growth pathway can be intervened by the active compound of sea cucumber. Based on the illustration from Figure 1, it shows that active compounds of sea cucumber are more dominant in intervening the performance of NF-κB through the EGFR and PI3k/Akt pathways. This shows that sea cucumbers have the best potential to control carcinoma cancer type which is generally the carcinogenesis initiated through the EGFR pathway. Some of these types of cancer include breast and lung cancer.
Figure 1.
Sea cucumber active compounds (green font) might intervene cancer cell growth through EGFR-Pi3 K/AKT dependent NF-κB pathway. The Illustration was Created with BioRender.com application.
Several active compounds such as Psolusoside and Stichoposide D inhibit cancer cell growth signaling at the EGFR level, while Frondoside A acts to intervene on the PI3K/Akt protein. Meanwhile, most of the active compounds, such as Ds-echinoside A, Frondoside A, Holothurin A1 (HA1), and Psolusoside have an activity to reduce NF-κB levels, thereby interfering genes expression were controlled by these regulatory proteins. As we know that NF-κB regulatory proteins can control genes that function in angiogenesis, cell migration, cell cycle, cell growth, and even genes that cause resistance to drugs such as MDR1. In general, this illustrates that sea cucumber has the potential to be developed as an anti-cancer, specifically to inhibit cancer cell progression through the NF-κB pathway.
Prediction of the Active Compounds of Sea Cucumber as Drug Candidates
Many studies have revealed the resistance of cancer cells to synthetic drugs. 24 Therefore, there is a need to explore natural compounds with pharmacological potential for cancer treatment. The important things in drug discovery are the prediction of the permeability and solubility of drug candidates, which affect the ability of the drug to permeate the gastrointestinal membrane and be distributed through the systemic circulation and brain penetration. 25 Drug molecules must reach target cells in sufficient concentration, stay in bioactive forms until biological activities occur, and also have adequate metabolism and excretion from the body to prevent the toxic effect.26,27 The study of the absorption, distribution, metabolism, and excretion/elimination (ADME) of the drug candidate compound is important. In this study, the SwissADME web tool is used to predict the physicochemical properties, pharmacokinetics, druglikeness, and medicinal chemistry friendliness of sea cucumber compounds. The physicochemical profiles indicate that the six sea cucumber compounds need to be modified to obtain suitable properties for good drug agents (Table 2).
Table 2.
ADME prediction of active compounds from sea cucumber.
| Molecule | Ds-echinoside A | Frondoside A | Frondoside A | Holothurin A1 | Psolusoside А | Stichoposide D |
|---|---|---|---|---|---|---|
| Physicochemical properties | ||||||
| Num. heavy atoms | 77 | 91 | 91 | 83 | 89 | 101 |
| Num. aromatic heavy atoms | 0 | 0 | 0 | 0 | 0 | 0 |
| Fraction Csp3 | 0.94 | 0.93 | 0.93 | 0.94 | 0.89 | 0.94 |
| Num. rotatable bonds | 15 | 20 | 20 | 17 | 21 | 24 |
| Num. H-bond acceptors | 23 | 29 | 29 | 27 | 29 | 33 |
| Num. H-bond donors | 12 | 10 | 10 | 12 | 8 | 15 |
| MR | 266.22 | 303.81 | 303.81 | 275.99 | 293.61 | 339.45 |
| TPSA (Å2) | 352.13 | 431.24 | 431.24 | 426.94 | 447.13 | 485.27 |
| Lipophilicity | ||||||
| iLOGP | 4.14 | −9.13 | −9.13 | −9.08 | 0 | 5.6 |
| XLOGP3 | −0.26 | −0.52 | −0.52 | −1.25 | −1.22 | −2.31 |
| WLOGP | −1.22 | −0.1 | −0.1 | −1.69 | 1.18 | −3.85 |
| MLOGP | −3.63 | −3.94 | −3.94 | −4.58 | −3.96 | −6.99 |
| Consensus Log P | −0.45 | −3.33 | −3.33 | −4.04 | −1.47 | −2.28 |
| Water solubility | ||||||
| ESOL Log S | −5.54 | −6.47 | −6.47 | −5.52 | −5.96 | −5.83 |
| Ali Log S | −6.68 | −8.07 | −8.07 | −7.22 | −7.67 | −7.34 |
| Silicos-IT class | Soluble | Soluble | Soluble | Soluble | Soluble | Soluble |
| Pharmacokinetics | ||||||
| GI absorption | Low | Low | Low | Low | Low | Low |
| BBB permeant | No | No | No | No | No | No |
| Pgp substrate | Yes | Yes | Yes | Yes | Yes | Yes |
| CYP1A2 inhibitor | No | No | No | No | No | No |
| log Kp (cm/s) | −13.23 | −14.82 | −14.82 | −14.65 | −15.31 | −16.82 |
| Druglikeness | ||||||
| Lipinski | 3 | 3 | 3 | 3 | 3 | 3 |
| Ghose | 4 | 3 | 3 | 4 | 3 | 4 |
| Veber | 2 | 2 | 2 | 2 | 2 | 2 |
| Egan | 1 | 1 | 1 | 1 | 1 | 1 |
| Muegge | 5 | 6 | 6 | 6 | 6 | 7 |
| Bioavailability score | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| Medicinal chemistry | ||||||
| PAINS | 0 | 0 | 0 | 0 | 0 | 0 |
| Brenk | 2 | 4 | 4 | 4 | 4 | 2 |
| Leadlikeness | 2 | 2 | 2 | 2 | 2 | 2 |
Abbreviations: BBB, blood-brain barrier; GI, gastrointestinal; MR, molar refractivity; PAINS, pan assay interference compounds; TPSA, topological polar surface area.
The active compounds of sea cucumbers in Table 2 have physicochemical properties, number of heavy atoms, molecular weight (MR), and topological polar surface area (TPSA), which are not the best fit with drug properties. Although in various in vitro studies, the active compound has excellent potential as an anticancer, it may need optimization for applications in humans therapeutics. The TPSA score could be used to measure its ability to penetrate cell membranes 28 and the ability to penetrate the blood–brain barrier. 29 The solubility in lipids and water is also a determining factor for the efficiency of molecules to work in tissues or cells. The results of the analysis of the solubility of several sea cucumber active compounds show that these compounds have good water solubility (Table 2), so they can facilitate the distribution process in the bloodstream. Lipophilicity, another physicochemical property of the drug, is important in the transport of substances across membrane into the cell. 30 Lipophilicity is predicted by multiple computational methods (iLOGP, XLOGP3, WLOGP, MLOGP, and Consensus Log P) that were used to predict values of n-octanol/water partition coefficient (log Po/w). 26 Based on pharmacokinetic parameters, all the compounds tested have low gastrointestinal (GI) absorption and no blood–brain barrier (BBB) permeant. These data suggested that the compounds need some adjuvant for oral application or might be more suitable for intravenous administration. However, the compounds were unable to enter the blood brain barrier, which is not suitable for treating brain disease.
All of the sea cucumber compounds (Table 2) show the potential ability to be P-gp substrates that can inhibit CYP. On the contrary, no inhibition of CYP1A2 might not be expected to interfere with the function of these enzymes in the xenobiotic clearance that are at risk of adverse effects. 26 Druglikeness is a prediction that specifies whether a pharmacological agent has properties that can be considered as oral drug candidates. 31 The druglikeness properties were established according to the Lipinski (Pfizer) rule of five, Ghose (Amgen), Veber (GSK), Egan (Pharmacia), and Muegge (Bayer).26,32 According to the five Lipinski’s rules, the physicochemical parameters with the following ranges (MW ⩽ 500 Da, log P ⩽ 5, number of hydrogen bond donors ⩽ 5 and number of hydrogen bond acceptors ⩽ 10) define good absorption or permeation. Compounds that are substrates for biological transporters are exceptions to the rule. 33 A study of medicinal chemistry was conducted using the pan assay interference compounds (PAINS) test and the Brenk test to determine whether there were ligand parts that could provide a bogus (false positive) biological response. 34 Leadlikeness is subjected to chemical modifications that will make compounds possess the structural and physicochemical profile of an optimal quality lead, such as increased size and lipophilicity.26,35 The data suggested that the predicted active compounds have properties that do not fit the druglikeness and medicinal chemistry criteria. Therefore, optimization and improvement of the performance of these compounds are required. Research on the metabolism of this drug in vivo by oral administration is needed to see the kinetics and dynamics of the compounds.
Conclusion
Abnormal regulation of the NF-κB signaling pathway is directly linked to many human cancers. The active compound of sea cucumber targeted some genes that are related to the NF-κB and cancer pathways. Some of the active compounds of sea cucumber have potency for anticancer, but they still need optimization to improve their properties to match druglikeness.
Footnotes
Author Contributions: TLW and NW designed the study. TLW, HR, and NW conducted the research and prepared the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the Ministry of Research and Technology/National Research and Innovation Agency of the Republic of Indonesia [8/E1/KPT/2020]. The work was supported by the Ministry of Research and Technology/National Research and Innovation Agency of the Republic of Indonesia Grant in 2020.
ORCID iD: Teresa Liliana Wargasetia  https://orcid.org/0000-0002-3433-3300
https://orcid.org/0000-0002-3433-3300
References
- 1. Dąbek J, Kułach A, Gąsior Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): a new potential therapeutic target in atherosclerosis. Pharmacol Rep. 2010;62:778-783. doi: 10.1016/S1734-1140(10)70338-8. [DOI] [PubMed] [Google Scholar]
- 2. Moretti M, Bennett J, Tornatore L, Thotakura AK, Franzoso G. The international journal of biochemistry cancer: NF-kB regulates energy metabolism. Int J Biochem Cell Biol. 2012;44:2238-2243. doi: 10.1016/j.biocel.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8:227-241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain H, Dhingra N, Narsinghani T, Sharma R. Insights into the mechanism of natural terpenoids as NF-κB inhibitors: an overview on their anticancer potential. Exp Oncol. 2016;38:158-168. doi: 10.31768/2312-8852.2016.38(3):158-168. [DOI] [PubMed] [Google Scholar]
- 5. Erstad DJ, Cusack JC. Targeting the NF-κB pathway in cancer therapy. Surg Oncol Clin N Am. 2013;22:705-746. doi: 10.1016/j.soc.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 6. Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2:823-830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gambhir S, Vyas D, Hollis M, Aekka A, Vyas A. Nuclear factor kappa B role in inflammation associated gastrointestinal malignancies. World J Gastroenterol. 2015;21:3174-3183. doi: 10.3748/wjg.v21.i11.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin Y, Bai L, Chen W, Xu S. The NF-κB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14:45-55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li F, Zhang J, Arfuso F, et al. NF-κB in cancer therapy. Arch Toxicol. 2015;89:711-731. doi: 10.1007/s00204-015-1470-4. [DOI] [PubMed] [Google Scholar]
- 10. Wargasetia TL, Permana S, Widodo N. Potential use of compounds from sea cucumbers as MDM2 and CXCR4 inhibitors to control cancer cell growth. Exp Ther Med. 2018;16:2985-2991. doi: 10.3892/etm.2018.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jing H, Lee S. NF-κB in cellular senescence and cancer treatment. Mol Cells. 2014;37:189-195. doi: 10.14348/molcells.2014.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ballard DW, Walker WH, Doerre S, et al. The v-rel oncogene encodes a κB enhancer binding protein that inhibits NF-κB function. Cell. 1990;63:803-814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- 13. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 14. Wargasetia TL, Permana S, Widodo. The role of sea cucumber active compound and its derivative as an anti-cancer agent. Curr Pharmacol Reports. 2018;4:27-32. doi: 10.1007/s40495-018-0121-x. [DOI] [Google Scholar]
- 15. Zhao Q, Xue Y, Wang J, et al. In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pearsonothuria graeffei. J Sci Food Agric. 2012;92:965-974. doi: 10.1002/jsfa.4678. [DOI] [PubMed] [Google Scholar]
- 16. Zhao Q, Liu ZD, Xue Y, et al. Ds-echinoside A, a new triterpene glycoside derived from sea cucumber, exhibits antimetastatic activity via the inhibition of NF-κB-dependent MMP-9 and VEGF expressions. J Zhejiang Univ Sci B. 2011;12:534-544. doi: 10.1631/jzus.B1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fedorov SN, Dyshlovoy SA, Kuzmich AS, et al. In vitro anticancer activities of some triterpene glycosides from holothurians of cucumariidae, stichopodidae, psolidae, holothuriidae and synaptidae families. Nat Prod Commun. 2016;11:1239-1242. doi: 10.1177/1934578x1601100911. [DOI] [PubMed] [Google Scholar]
- 18. Park SY, Kim YH, Kim Y, Lee SJ. Frondoside A has an anti-invasive effect by inhibiting TPA-induced MMP-9 activation via NF-κB and AP-1 signaling in human breast cancer cells. Int J Oncol. 2012;41:933-940. doi: 10.3892/ijo.2012.1518. [DOI] [PubMed] [Google Scholar]
- 19. Silchenko AS, Avilov SA, Kalinin VI, et al. Constituents of the sea cucumber cucumaria okhotensis. J Nat Prod. 2008;71:351-356. [DOI] [PubMed] [Google Scholar]
- 20. Zhao Q, Xue Y, Liu ZD, et al. Differential effects of sulfated triterpene glycosides, holothurin A1, and 24-dehydroechinoside A, on antimetastasic activity via regulation of the MMP-9 signal pathway. J Food Sci. 2010;75:H280-H288. doi: 10.1111/j.1750-3841.2010.01837.x. [DOI] [PubMed] [Google Scholar]
- 21. Nair AS, Shishodia S, Ahn KS, Kunnumakkara AB, Sethi G, Aggarwal BB. Deguelin, an akt inhibitor, suppresses IκBα kinase activation leading to suppression of NF-κB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J Immunol. 2006;177:5612-5622. doi: 10.4049/jimmunol.177.8.5612. [DOI] [PubMed] [Google Scholar]
- 22. Careaga VP, Bueno C, Muniain C, Alché L, Maier MS. Antiproliferative, cytotoxic and hemolytic activities of a triterpene glycoside from Psolus patagonicus and its desulfated analog. Chemotherapy. 2009;55:60-68. doi: 10.1159/000180340. [DOI] [PubMed] [Google Scholar]
- 23. Chaturvedi MM, Sung B, Yadav VR, Kannappan RAB. NF-κB addiction and its role in cancer: “one size does not fit all.” Oncogene. 2011;30:1615-1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714-726. doi: 10.1093/nar/gku1003. [DOI] [PubMed] [Google Scholar]
- 25. Feng Z, Cao J, Zhang Q, Lin L. The drug likeness analysis of anti-inflammatory clerodane diterpenoids. Chinese Med. 2020;15:1-13. doi: 10.1186/s13020-020-00407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:1-13. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al-Nour MY, Ibrahim MM, Elsaman T. Ellagic acid, kaempferol, and quercetin from acacia nilotica: promising combined drug with multiple mechanisms of action. Curr Pharmacol Reports. 2019;5:255-280. doi: 10.1007/s40495-019-00181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. Neurorx. 2005;2:541-553. doi: 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hitchcock SA, Pennington LD. Structure−brain exposure relationships. J Med Chem. 2006;49:7559-7583. doi: 10.1021/jm060642i. [DOI] [PubMed] [Google Scholar]
- 30. Tylińska B, Wiatrak B, Czyżnikowska Ż, Cieśla-Niechwiadowicz A, Gębarowska E, Janicka-Kłos A. Novel pyrimidine derivatives as potential anticancer agents: synthesis, biological evaluation and molecular docking study. Int J Mol Sci. 2021;22:3825. doi: 10.3390/ijms22083825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oduselu GO, Ajani OO, Ajamma YU, Brors B, Adebiyi E. Homology modelling and molecular docking studies of selected substituted benzo[d]imidazol-1-yl)methyl)benzimidamide scaffolds on plasmodium falciparum adenylosuccinate lyase receptor. Bioinform Biol Insights. 2019;13:1177932219865533. doi: 10.1177/1177932219865533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mokgautsi N, Wang Y-C, Lawal B, et al. Network pharmacological analysis through a bioinformatics cancer types. Cancers (Basel). 2021;13:2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;64:4-17. doi: 10.1016/j.addr.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 34. Nazemi M, Khaledi M, Golshan M, et al. Cytotoxicity activity and druggability studies of sigmasterol isolated from marine sponge dysidea avara against oral epithelial cancer cell (KB/C152) and t-lymphocytic leukemia cell line (Jurkat/ E6-1). Asian Pacific J Cancer Prev. 2020;21:997-1003. doi: 10.31557/APJCP.2020.21.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Verheij HJ. Leadlikeness and structural diversity of synthetic screening libraries. Mol Divers. 2006;10:377-388. doi: 10.1007/s11030-006-9040-6. [DOI] [PubMed] [Google Scholar]