Abstract
Colorectal cancer (CRC) is one of the most common malignancies in the world that seriously affects human health. Activation of epithelial-mesenchymal transition (EMT) is a physiological phenomenon during embryonic development that is essential for cell metastasis. EMT participates in various biological processes associated with trauma repair, organ fibrosis, migration, metastasis, and infiltration of tumor cells. EMT is a new therapeutic target for CRC; however, some patients with CRC develop resistance to some drugs due to EMT. This review focuses specifically on the status of treatments that target the EMT process and its role in the therapeutic resistance observed in patients with CRC.
Keywords: EMT, CRC, drug resistance, treatment
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors of the gastrointestinal tract and can be divided into colon and rectal cancer components. The incidence and mortality of CRC both rank second among all cancers that occur globally. 1 Recent statistics from the USA reveal that between 2000 and 2013, the incidence of CRC in individuals younger than 50 years old increased by 22% and the five-year relative survival rate after diagnosis was 65%. 2 The early diagnosis methods for CRC have gradually improved in recent years; however, because of the atypical clinical symptoms in its initial stages of development, such as abdominal pain, bloating, and altered bowel habits, CRC is often undiagnosed and does not receive sufficient attention until it causes bowel obstruction or systemic symptoms such as anemia, weight loss, and other obvious symptoms. This underdiagnosis reduces the effect of treatments available to patients with now middle or late stage CRC, as well as the high mortality of the disease. Surgery alone or combined with radiotherapy, chemotherapy, or emerging targeted therapy remain the cornerstones of CRC treatment. However, CRC recurrence and metastasis during the treatment process cannot be effectively controlled using these traditional methods and recent studies have shown that epithelial-mesenchymal transition (EMT) plays a key role in the infiltration, metastasis, and drug resistance of CRC.
EMT is a reversible cellular process that temporarily transforms an epithelial cell into a mesenchymal cell, 3 characterized by the loss of apical-basal polarity and tight junctions that keep epithelial cells in touch with their neighboring cells and basement membranes. EMT is developmentally necessary, and its underlying processes are reactivated during wound healing, fibrosis, and cancer progression. 4 The resulting mesenchymal cells can be restored to an epithelial state in an opposite process called mesenchymal-epithelial transition (MET). EMT plays an important role in specific stages of embryogenesis, such as gastrula formation, tissue morphogenesis during development, and wound healing in adults. 3 However, as these cells rearrange their cytoskeleton, reprogram metabolism and gene expression, and alter the signaling pathways that define cell shape, individual cell viability can increase and develop an aggressive phenotype. In addition, the malignant progression of many types of cancer may depend on EMT activation in tumor cells.5–7 The process of EMT is accompanied by aberrant expression of molecules, including the reduced expression of epithelial markers, increased expression of mesenchymal markers, and EMT-related transcription factors that involve multiple signaling pathways and molecular regulatory mechanisms.8,9
Academics have found that not all cancer development processes are related to EMT. For example, Fischer et al. 10 found that EMT was not necessary for the development of breast cancer in the MMTV-PyMT mouse model, and Chen et al. 11 found that EMT was not essential for the metastasis of pancreatic ductal carcinoma under certain conditions. EMT can mediate treatment resistance in tumors, but most tumors develop resistance to only one or two clinical treatments. For example, gastric cancer is resistant to chemotherapeutic agents, 12 while non-small cell lung cancer is resistant to chemotherapy and targeted therapy under EMT.13,14 In contrast, CRC can simultaneously develop resistance to chemotherapy, radiotherapy, and targeted therapy and therefore, we hypothesize that the association between CRC and EMT is stronger than that in other tumors.
With the development of modern medicine, therapies for CRC have flourished and this wider variety of treatment raises the hope for better patient care and survival. However, with the diversification of CRC therapies, a series of challenges have arisen in modern clinical medicine practice, the most prominent of which is drug therapy resistance. In this review, we summarize the salient features of CRC and EMT, with a particular focus on effective therapies for CRC and treatment resistance, describing effective treatments for EMT versus EMT-mediated drug resistance and its associated molecular mechanisms.
Characteristics of EMT
Concept of EMT within CRC Development and Treatment Strategies
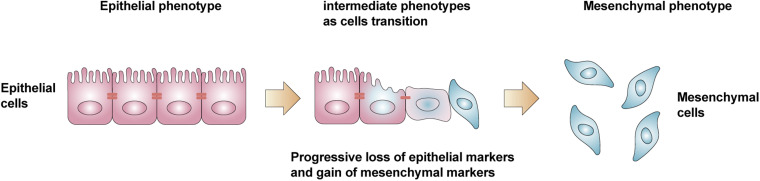
The concept of epithelial-mesenchymal transition (EMT) was first proposed by Greenberg and Hay in 1982, who reported how crystalline epithelial cells lost their original polarity when cultured in gels, extended pseudopods, and exhibited a mesenchymal cell phenotype. 15 EMT refers to the process by which epithelial cells are transformed into mesenchymal cells in response to physiological or pathological factors and is widely known as the process during which epithelial cells lose their polarity and connectivity with adjacent cells, which promotes the acquisition of migration, invasion, proliferation, and antiapoptotic effects 16 (Figure 1). Depending on the biological context, EMT can be classified into three different sub-types by the International Conference on Tumor Invasion and Metastasis, Poland, 2007 17 : Type 1 EMT is associated with implantation, embryogenesis, and organ development, 18 and mediates the transformation of epithelial cells into mesenchymal cells, resulting in a greater diversity of cell types during embryogenesis. Type 2 EMT is associated with wound healing, tissue regeneration, and organ fibrosis by promoting fibroblast proliferation, which leads to repair of damaged tissues or organs. However, this process often results in local fibrosis in organs.19,20 Type 3 EMT is associated with tumorigenesis in which epithelial malignant tumor cells of epithelial origin formed through EMT are prone to migration and metastatic mesenchymal cells and contribute to the development of tumor infiltration and metastasis.21,22
Figure 1.
Epithelial cells lose their polarity and connectivity with adjacent cells, acquire the ability of migration, invasion, proliferation and antiapoptotic effects, and transform into mesenchymal cells.
Relationship between EMT and Tumor Cell Metastasis
The E-calcium adhesion protein (E-cadherin) is an important molecule in the formation of cellular adhesion links and is a key factor in the formation of intercellular contacts. 23 At the onset of EMT in malignant tumors, the expression of E-cadherin is down-regulated or lost, resulting in decreased adhesion among tumor cells and elevated invasion, infiltration, and metastasis of tumor cells. 24 The lower the expression of E-cadherin, the worse the patient prognosis can be; therefore, the downregulation or loss of E-cadherin is considered a hallmark event of EMT. 25 However, it has also been hypothesized that EMT is not required during tumor metastasis, and that downregulation or loss of E-cadherin expression does not necessarily occur at the time of EMT or tumor metastasis. 26 The transcription factor Snail1 enables cells to produce the Snail protein, which can repress E-cadherin transcription by binding to the E-box element proximal to the E-cadherin promoter. Because the downregulation or loss of E-cadherin expression is a hallmark event of EMT, Snail is considered an important switch that controls this process. 27 In the KPC mouse model of pancreatic ductal carcinoma, knockdown of the Snail gene did not effectively inhibit tumor metastasis 28 ; whereas in the MMTV-PyMT breast cancer mouse model, the same knockdown of Snail resulted in a significant reduction in lung metastasis. 29 Therefore, many scholars believe that this may be due to differences in the different tumor microenvironments leading to differences in the EMT process promoting tumor metastasis occurrence. 30
EMT-Mediated Tumor Treatment Resistance
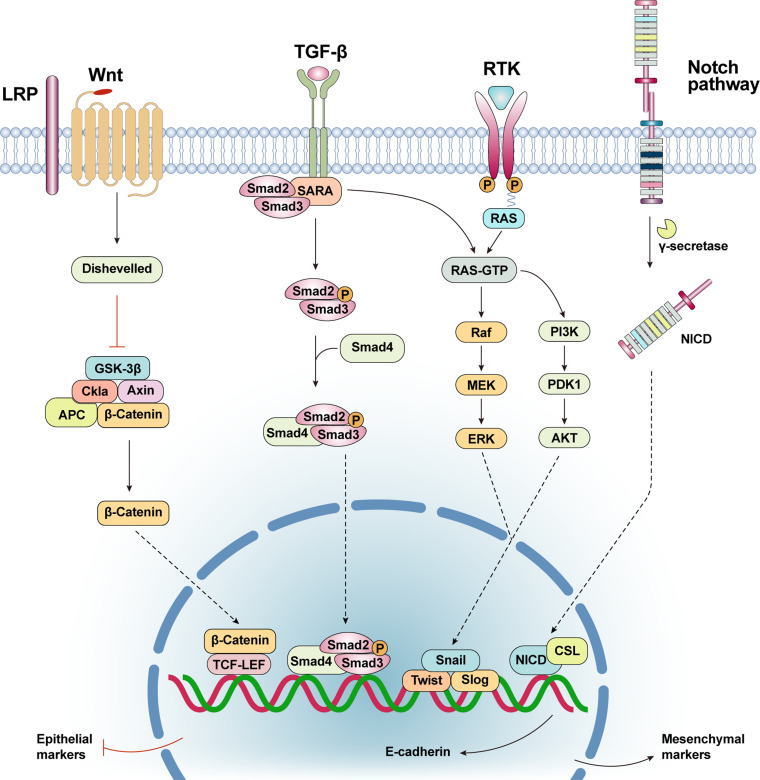
In recent years, it was found that EMT confers tumor cells the ability to adapt to the changing microenvironment during secondary tumor formation, 31 which leads to further enhancement of tumor cell resistance. In general, the occurrence of EMT in tumor cells can simultaneously make them much more resistant to apoptosis, creating a form of apoptotic resistance that can directly reduce the efficacy of radiotherapy, chemotherapy, targeted therapy, and immunotherapy. 32 For example, Mirone et al. 33 found that Notch-1, HES1, and HEY1 were significantly upregulated in tumor cells resistant to regorafenib, and that silencing Notch-1 in resistant cells partially restored sensitivity to regorafenib treatment in vitro. Moreover, Wnt signaling is an important pathway for CRC progression and is also one of the progression factors of EMT, as berrant activation of Wnt/β-catenin signaling is an important cause of CRC development. 34 The Wnt/β-catenin pathway is a classical pathway of the Wnt signaling pathway that consists of a variety of secreted glycoproteins and has an important regulatory role in cell proliferation, differentiation, and migration. Thus, aberrant activation of Wnt/β-catenin signaling can promote EMT activity in CRC cells and tolerance of some cisplatin-based drugs.35,36 Zhou et al. 35 examined miR-506 levels in colon cancer patients to analyze colon cancer cells with different resistance to oxaliplatin. They found that overexpression of miR-506 in HCT116-OxR cells inhibited MDR1/P-gp expression by downregulating the Wnt/β-catenin pathway, which enhanced oxaliplatin sensitivity and indicated that the Wnt/β-catenin pathway can promote EMT occurrence in CRC cells through the expression of MDR1/P-gp and drug resistance, thus providing a theoretical basis for miRNAs-based strategies to reverse oxaliplatin resistance in colorectal cancer cells. In this way, EMT reversal or killing of tumor cells that have already undergone EMT via inhibition or activation of certain signaling pathways, such as the Notch signaling pathway 37 has become a trending topic in modern clinical medicine (Figure 2).
Figure 2.
Wnt/β-catenin signaling pathway: After activation of the representative protein Wnt, the signal is forwarded through the cytoplasmic protein (GSK-3β, Axin, APC, β-catenin and etc) to activate the nuclear transcription factor(TCF-LEF). Thus, the level of E- cadherin in the cells was downregulated. TGF-β signaling pathway: The TGF-β ligand binds to the type II receptor dimer to form a heterotetramer complex. SARA recruits R-SMAD protein and binds R-SMAD protein to type I receptor. The type I receptor phosphorylates the serine residue of R-SMAD and separates it from the receptor complex and SARA. Phosphorylated R-SMAD binds to CoSMAD(SMAD4) and enters the nucleus, causing the level of E- cadherin in the cells was downregulated. PI3K/Akt signaling pathway: PI3K/Akt signaling pathway can directly induce EMT, mainly by up regulating the expression of transcription factors (Snail, Slug, Twist, ZEB, etc), so as to down regulate the level of intracellular E-cadherin. Notch pathway: Notch signal is cleaved by γ-secretase during activation, releasing the activated form of Notch protein NICD. NICD enters the nucleus and binds to CSL protein to promote downstream gene expression, thereby promoting EMT.
Treatment and Drug Resistance of CRC Associated with EMT
Treatment and Drug Resistance in EMT-Associated CRC During Chemotherapy
Chemotherapy kills cancer cells through the application of chemotherapeutic agents. Chemotherapy is currently one of the most effective means of treating cancer and, along with surgery and radiotherapy, is a primary choice for cancer treatment strategies. Chemotherapy is a systemic treatment option because regardless of the route of administration (oral, intravenous, and body cavity administration, etc), chemotherapeutics spread throughout most organs and tissues through blood circulation. Therefore, chemotherapy is the primary treatment for tumors that tend to disseminate systemically and for mid- to late-stage tumors that have metastasized.
Currently, 5-Fluorouracil (5-Fu) is one of the most effective chemotherapeutic agents for the treatment of CRC. It has been shown that 5-Fu is first converted to 5-fluorouracil nucleoside monophosphate (5-FUMP) and 5-fluorouracil deoxynucleoside monophosphate (5-FdUMP) in vivo. When 5-FdUMP is combined with thymidylate synthase (TS), it forms a stable triplet complex that inhibits TS activity and inhibits TS from efficiently synthesizing deoxythymidine monophosphate (dTMP). In turn, when dTMP is not effectively synthesized, DNA strands cannot replicate properly and DNA repair functions are greatly impaired, leading to the inhibition of cell growth and enhanced tumor cell-killing effects. 38
The molecular mechanisms of chemoresistance are complex, including reduced intracellular drug accumulation, apoptosis inhibition, overexpression of DNA repair enzymes, and activation of DNA damage checkpoint responses. There is growing evidence that EMT enhances 5-Fu resistance in CRC, but the mechanism is unknown. The Snail transcription factor is an important switch that controls EMT; Wang et al. 39 found that CRC cells overexpressing Snail had significantly increased 5-Fu resistance and that Snail could directly bind to the promoter of the ATP-binding cassette transporter protein family B member (ABCB1) and upregulate its transcription. They also found Snail-induced drug resistance to 5-Fu in CRC cells could be significantly attenuated by knocking down the ABCB1 gene, revealing a potential mechanism for EMT and chemoresistance, as well as providing a potential target for clinical treatment of CRC.
Because the induction of EMT promotes the development of conventional chemotherapy resistance in CRC, eliminating or reversing the EMT process is the key to addressing chemoresistance in CRC. Increasing evidence supports the hypothesis that curcumin may inhibit EMT in CRC cells. 40 Toden et al. 41 evaluated the effects of curcumin and 5-Fu alone and in combination on parental and 5-FU-resistant CRC cells. They found that the combined treatment 5-Fu increased apoptosis and inhibited proliferation in both parental and resistant cells; whereas, 5-Fu alone was ineffective in resistant cells. Thus, curcumin has the potential as a new adjuvant chemotherapeutic agent to prevent CRC progression and metastasis. Additionally, Mendoza-Rodríguez et al. 42 found that 5-Fu combined with a transcription activator (STAT-6) inhibitor AS1517499 and trimethylglycine enhanced the effect of chemotherapy. This combination therapy resulted in reduced STAT-6 phosphorylation and increased expression of the epithelial marker E-cadherin and reduced expression of the mesenchymal markers β-catenin and Snail1, which induced apoptosis and increased sensitivity to 5-FU.
Hypoxia-inducible factor-1α (HIF-1α) is closely associated with tumor metastasis, chemotherapy, and poor prognosis. Zhang et al. 43 found that overexpression of HIF-1α promoted EMT during adenovirus infection, facilitating cell invasion and migration in vitro and in vivo. At the molecular level, the α domain of HIF-1 binds directly to the proximal promoter of ZEB1 through the hypoxia response element, thereby increasing ZEB1 trans-activity and expression. In addition, ZEB1 inhibition blocked HIF-1α-induced epithelial mesenchymal transition and cell invasion. Thus, the expression of HIF-1α was highly correlated with ZEB1 expression in normal colorectal epithelial, primary, and metastatic colorectal cancer tissues. Additionally, both HIF-1α and ZEB1 were positively correlated with vimentin, an important mesenchymal marker of EMT, and negatively correlated with E-cadherin expression. These results suggest that HIF-1α promotes endodermal metastasis and tumor metastasis in CRC by binding to the ZEB1 promoter. In contrast, Guo et al. 44 found that ZEB1 expression was significantly increased in colon cancer tissues and correlated with lymph node metastasis and depth of infiltration. Compared with parental colon cancer cells (HCT116), oxaliplatin-resistant HCT116/OXA cells exhibited an EMT phenotype characterized by upregulated expression of ZEB1, vimentin, MMP2, and MMP9, and downregulated expression of E-cadherin. Transfection of Si-ZEB1 into HCT116/OXA cells significantly reversed the EMT phenotype and enhanced oxaliplatin sensitivity both in vitro and in vivo. ZEB1 knockdown was effective in restoring oxaliplatin sensitivity by reversing EMT, which demonstrated that ZEB1 is a potential therapeutic target for the prevention of OXA resistance in colon cancer.
The Wnt pathway can promote EMT in CRC cells by activating MDR1, which in turn increases CRC resistance to oxaliplatin, but is there a targeted therapeutic agent for the Wnt pathway? Wu et al. 45 reported the synergistic effects of cinnamaldehyde and L-OHP inhibited hypoxia-activated Wnt/β-catenin signaling, reversed EMT, activated CsC, and reduced the development of L-OHP resistance. CRC patients with KRAS mutations are insensitive to cetuximab and panitumumab, 46 and the potent and selective Wnt/β-catenin inhibitor KYA1797K activates GSK3β and degrades small β-catenin and RAS molecules to increase the sensitivity of tumors with KRAS mutations to cetuximab and panitumumab. These results suggested that Wnt signaling leads to chemoresistance in CRC and that the use of Wnt inhibitors affects cellular chemosensitivity to other drugs, providing a new avenue for the clinical treatment of colorectal cancer.
Radiotherapy Treatment and Resistance in CRC is Associated with EMT
Radiation therapy (RT) for tumors is a local or regional treatment that uses radiation to destroy tumor cells. Radiation includes alpha, beta, and gamma rays produced by radioisotopes and x-rays, electrons, proton beams, or other particle beams produced by several types of x-ray therapy machines or accelerators. Approximately 70% of patients with cancer require radiation therapy to varying degrees, which can be administered radiation alone or in combination with surgery, chemotherapy, or targeted therapy, in the treatment of cancer. Approximately 40% of cancers can be cured radically with radiation therapy. The fundamental goal of radiotherapy is to kill tumor cells by focusing the maximum amount of radiation on the target area (ie, lesion) while sparing the surrounding normal tissues or organs as much as possible from unnecessary exposure. The role and status of radiotherapy as a tumor treatment is becoming increasingly prominent and one of the three major tools of tumor treatment (ie, chemotherapy, radiotherapy, and surgical treatment).
The mitochondrial pyruvate carrier (MPC) is found in the inner membrane of mitochondria and is a membrane protein that transports pyruvate into the mitochondria. As the only entry point for pyruvate into the mitochondrial matrix, MPC is important for coordinating glycolysis and mitochondrial activity and critical in the regulation of cellular energy production and metabolism. 47 Recent studies have shown that MPC1 is usually lowly expressed, while MPC2 is more highly expressed in tumors compared to normal cells; however, the survival of colon, kidney, and lung cancers with low MPC1 expression tend to be poor. 48 Yuji Takaoka et al. 49 found that EMT was induced by glutamine (GLS) in cells with inhibited MPC1 cultured in a medium lacking glutamine, suggesting the metabolization of glutamine into glutamate increased mitochondrial intake. 48 In addition, MPC1 expression induced EMT in pancreatic cancer (PC) and contributed to their acquisition of therapeutic resistance to radiotherapy.
Wang et al. 39 found that the tumor-associated non-coding RNA microRNA-310A (miR-130a) could further induce EMT and CRC radiotherapy resistance by affecting the related proteins NBS1, p-ATM, and γH2AX and their associated signaling pathways by inhibiting the target protein SOX4. This finding provides a potential therapeutic target and preoperative prognostic marker for CRC radiotherapy. In addition, the bioactive form of vitamin D, 1,25-dihydroxyvitamin D3 (VD3), can also act as a sensitizer of CRC to radiotherapy. 50 VD3 not only has antitumor effects on CRC, but also enhances the sensitivity of CRC cells to ionizing radiation (IR). VD3-induced recovery of radiosensitivity is associated with a range of phenotypes, including apoptosis, autophagy, and EMT. Studies suggest that VD3 can act synergistically with IR both in vitro and in vivo, as well as enhance radiosensitivity by modulating EMT, thus providing a new direction for improving the efficacy of CRC radiation therapy.
Targeted Therapy for Treatment and Drug Resistance in EMT-Associated CRC
Targeted therapy is a treatment modality that treats an already defined oncogenic site at the cellular level. In addition to conventional chemotherapy, radiotherapy, and surgery, various targeted therapies can be used to treat tumors at the organ-tissue and molecular levels. The targets of molecular targeted therapy are phenotypic molecules associated with malignant tumor cells that often act on specific cellular receptors, signaling, and other channels that promote tumor growth and metastasis, neovascularization, and cell cycle regulation to inhibit tumor cell growth or promote apoptosis. Unlike traditional chemotherapy, which is toxic to cells, targeted therapy has specific anti-tumor effects and significantly reduced toxicity that have pioneered the field of tumor therapy.
The TGF-β signaling pathway mediates tumor suppression during the initial stages of tumor growth, but it can also promote tumor progression by inducing tumorigenic EMT in mid- to late-stages of tumor development. A current study found that inhibition of endogenous TGF-β cytokine levels induced by Smad4 gene expression can lead to enhanced TGF-β signaling and EMT development. 51 Several receptor kinase inhibitors are effective in blocking the downstream effectors of the TGF-β signaling pathway, such as LY2109761, a small molecule that inhibits TβR I and TβR II kinase activity, which inhibits CRC transfer in a mouse model.52,53 The Notch signaling pathway is associated with development and differentiation in all postnatal animals; it controls proper germ layer formation and embryonic segmentation and controls the timing and duration of differentiation events in a dynamic manner. 54 Studies have shown that the Notch pathway can lead to the loss of epithelial cell polarity and induces the onset of EMT in CRC. 55 The Notch pathway reduces the adhesion between epithelial cells by downregulating E-cadherin expression, which enhances the metastatic ability of CRC. 56 Non-selective GSIs are often referred to as Notch inhibitors because they can inhibit tumor metastasis and EMT development by the suppressing the Notch pathway. 57 Currently, GIS has demonstrated antitumor effects in a number of preclinical models. 58
Cetuximab (Erbitux, C225) is a closed monoclonal antibody for the epidermal growth factor receptor (EGFR) 59 that competitively blocks the EGFR signaling pathway and inhibits the proliferation of tumor cells; therefore, it is an effective targeted therapeutic agent for the treatment of CRC. The exogenous expression of a tumor non-coding small RNA molecule (miR-141-3p) can increase the expression of E-cadherin, but decrease the expression of N-cadherin, Snail, and vimentin in CRC cells. The downregulation of miR-141-3p decreased the expression levels of E-cadherin in colorectal cancer cells and increased the expression levels of expression levels of N-cadherin, Snail, and Vimentin. Therefore, miR-141-3p is thought to be an important miRNA that affects EMT because it can inhibit the expression of EGFR in CRC cells. As a target drug used to inhibit the EGFR pathway, miR-141-3p can also effectively enhance the sensitivity of CRC cells to cetuximab. 60 Thus, miR-141-3p could be a potential biomarker for enhancing the efficacy of cetuximab and inhibiting the occurrence of EMT in CRC.
Misale et al. 61 found that continuous administration of cetuximab induced mutations in the KRAS gene in CRC cells. When KRAS is mutated, aberrant KRAS proteins are produced, which are permanently activated and cannot be regulated by the ligand EGFR; therefore, downstream pathways are continuously active and can stimulate unregulated CRC cell growth. β-elemene is a bioactive compound isolated from one herb with broad-spectrum anticancer effects. 62 Chen et al. 63 found that β-elemene in combination with cetuximab was effective in reducing the growth of CRC cells because it decreased the expression of the mesenchymal markers vimentin, N-cadherin, Slug, Snail, and MMP-9, as well as promoted the expression of E-cadherin to inhibit EMT. These processes, in turn, inhibited tumor metastasis, growth, and lymph node metastasis and reduced cetuximab resistance in CRC with KRAS mutations. Thus, β-elemene is potentially a novel targeted therapeutic adjuvant for KRAS-mutated tumors.
AXL is a tyrosine kinase receptor associated with chemotherapy resistance. Studies suggest that AXL is associated with the development of resistance to anticancer drugs, including paclitaxel, 64 doxorubicin, 65 and vincristine. 66 Its downstream signaling is mediated by Twist1, which activates the expression of gene programs that may lead to PLK1 inhibitor resistance via EMT and activates the transcriptional regulation of ABCB1, which encodes the multidrug resistance protein MDR1, also known as P-glycoprotein (P-gp). Solanes-Casado et al. 67 further confirmed the EMT process in drug-resistant colorectal cancer cells is driven by AXL signaling and Twist1 as part of their acquired drug resistance mechanism by assessing the expression levels of Ax1, Twist1, E-calmodulin, vimentin, ABCB1, and MDR1 in colorectal cancer cell lines that are PLK inhibitor BI2536-resistant and parental cell lines without detectable mutations in HT29, SW837, and HCT116. In addition, Solanes-Casado et al. 67 found that mdr1 expression was significantly higher in BI2536-resistant cell lines than in parental cell lines at both the mRNA and protein levels. The variation in protein increased 6- to 50-fold, while mRNA expression was 200-fold in the BI2536-resistant lines compared to the parental lines. The correlation of AXL with MDR1 upregulation in BI2536-resistant cell lines has been previously reported, and these results are consistent with the significant role MDR1 plays in the acquisition of resistance to PLK inhibitors.
During EMT, tumor cells meet their increased bioenergetic demands during metastasis by regulating their metabolic circuits. 68 Therefore, understanding the metabolic regulatory processes of EMT would provide a novel therapeutic target for the treatment of tumors. As mentioned, activation of signaling pathways such as TGF-β, Wnt, and Notch, can induce and mediate EMT by rewiring metabolic circuits within tumor cells; TGF-β can increase 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase to promote the development of EMT. Simvastatin is a competitive inhibitor of HMG-CoA reductase and an FDA-approved drug used to treat hyperlipidemia. Some studies69,70 have found that simvastatin silences the TGF-β-induced EMT process and there are clinical trials 71 demonstrating that the inhibition of this signaling pathway in advanced rectal cancer, which provides a promising potential treatment option for patients with colorectal cancer.
Immunotherapy Strategies for Treatment and Drug Resistance Associated with EMT
Immunotherapy is a treatment that artificially enhances or suppresses immune function in response to low or hyperactive immune status to treat disease. Unlike the previous surgeries mentioned above, the target of immunotherapy is not tumor cells, but the body's own immune system.
The expression of the waveform protein, which is an associated protein of EMT, is associated with poor prognosis. 72 Phosphorylation of waveform proteins promotes the growth of metastatic tumors and the high expression of waveform proteins indicates the activation of EMT in malignant tumors. Ohara et al. 73 reported that a novel phosphorylated helper peptide epitope in the waveform protein could induce sufficient T cell responses, suggesting that immunotherapy targeting phosphorylated waveform proteins could be a promising treatment strategy for patients with metastatic colorectal cancer.
Conclusion and Outlook
CRC is one of the most prevalent neoplasms that occurs worldwide and has been explored and innovated in the past decades. Currently, targeted therapy has been added to the three conventional therapeutic tools (chemotherapy, radiotherapy, and surgery) as a potential treatment option. However, as with the other treatments, there is the challenge of drug resistance. The mechanisms of drug resistance in CRC are very complex and differ from person to person, including EMT, autophagy, apoptosis, and self-repair of DNA damage. In the future, there is a greater need to comprehensively explore the molecular mechanisms by which CRC generates drug resistance.
EMT is an important mechanisms that mediating drug resistance in CRC that has been uncovered by researchers. Although EMT can enhance the resistance of CRC to various treatments through different molecular mechanisms (eg, Snail gene expression, MPC effects, KRAS gene mutations, etc), there are new therapeutic agents or potential targets that can counteract these mechanisms (eg, curcumin, VD3, β-elemene, etc). However, these therapeutic agents or potential targets have not yet been put into clinical use and it is therefore, still unknown if they can inhibit EMT without affecting other normal tissues and organs in humans. Exploring the mechanisms of EMT resistance and innovative therapeutics is not only limited to the efficacy of a treatment method on CRC cells outside the human body or individual, it also needs to combine basic research conclusions with its clinical applicability. To provide new options for CRC treatment, the priority should be to transform the molecules or compounds of effective treatment we found in experiments into treatments that are clinically available and less toxic.
Glossary
Abbreviations
- CRC
Colorectal Cancer
- EMT
epithelial-mesenchymal transition
- MET
mesenchymal-pithelial transition;5-Fu,5-Fluorouracil;5-FUMP,5-fluorouracil nucleoside monophosphate;5-FdUMP,5-fluorouracil deoxynucleoside monophosphate
- TS
thymidylate synthase
- dTMP
deoxythymidine monophosphate
- MPC
Mitochondrial pyruvate carrier
- PC
pancreatic cancer
- EGFR
epidermal growth factor receptor
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- MDR1
multidrug resistance protein
- P-gp
P-glycoprotein
Footnotes
Author Contributions: Qianyang Ni and Meng Li contributed equally to this study. Qianyang Ni contributed to its conception and wrote the original manuscript with Meng Li. Suyang Yu contributed to the review of the manuscript. Suyang Yu designed the project and edited the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Qianyang Ni https://orcid.org/0000-0002-7291-2602
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177-193. doi: 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 3.Nieto MA. Epithelial-mesenchymal transitions in development and disease: old views and new perspectives. Int J Dev Biol. 2009;53(8-10):1541-1547. doi: 10.1387/ijdb.072410mn [DOI] [PubMed] [Google Scholar]
- 4.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178-196. doi: 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1-2):349-361. doi: 10.1016/j.cell.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye X, Tam WL, Shibue T, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525(7568):256-260. doi: 10.1038/nature14897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krebs AM, Mitschke J, Lasierra Losada M, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518-529. doi: 10.1038/ncb3513 [DOI] [PubMed] [Google Scholar]
- 8.Lindner P, Paul S, Eckstein M, et al. EMT Transcription factor ZEB1 alters the epigenetic landscape of colorectal cancer cells. Cell Death Dis. 2020;11(2):147. doi: 10.1038/s41419-020-2340-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha D, Saha P, Samanta A, Bishayee A. Emerging concepts of hybrid epithelial-to-mesenchymal transition in cancer progression. Biomolecules. 2020;10(11):1561. doi: 10.3390/biom10111561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527(7579):472-476. doi: 10.1038/nature15748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, LeBleu VS, Carstens JL, et al. Dual reporter genetic mouse models of pancreatic cancer identify an epithelial-to-mesenchymal transition-independent metastasis program. EMBO Mol Med. 2018;10(10):e9085. doi: 10.15252/emmm.201809085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Xu Z, Sun J, et al. Cisplatin resistance in gastric cancer cells is involved with GPR30-mediated epithelial-mesenchymal transition. J Cell Mol Med. 2020;24(6):3625-3633. doi: 10.1111/jcmm.15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin S, Zhang R, An X, et al. LncRNA HOXA-AS3 confers cisplatin resistance by interacting with HOXA3 in non-small-cell lung carcinoma cells. Oncogenesis. 2019;8(11):60. doi: 10.1038/s41389-019-0170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Wang T, Lv D, et al. Acquired resistance to EGFR TKIs mediated by TGFβ1/integrin β3 signaling in EGFR-mutant lung cancer. Mol Cancer Ther. 2019;18(12):2357-2367. doi: 10.1158/1535-7163.MCT-19-0181 [DOI] [PubMed] [Google Scholar]
- 15.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95(1):333-339. doi: 10.1083/jcb.95.1.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. 2015;25(11):675-686. doi: 10.1016/j.tcb.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420-1428. doi: 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owusu-Akyaw A, Krishnamoorthy K, Goldsmith LT, Morelli SS. The role of mesenchymal-epithelial transition in endometrial function. Hum Reprod Update. 2019;25(1):114-133. doi: 10.1093/humupd/dmy035 [DOI] [PubMed] [Google Scholar]
- 19.VanDussen KL, Stojmirović A, Li K, et al. Abnormal small intestinal epithelial microvilli in patients with Crohn’s disease. Gastroenterology. 2018;155(3):815-828. doi: 10.1053/j.gastro.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill C, Li J, Liu D, et al. Autophagy inhibition-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in pulmonary fibrosis. Cell Death Dis. 2019;10(8):591. doi: 10.1038/s41419-019-1820-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou S, Zhang M, Zhou C, Wang W, Yang H, Ye W. The role of epithelial-mesenchymal transition in regulating radioresistance. Crit Rev Oncol Hematol. 2020;150:102961. doi: 10.1016/j.critrevonc.2020.102961 [DOI] [PubMed] [Google Scholar]
- 22.Li L, Liu J, Xue H, et al. A TGF-β-MTA1-SOX4-EZH2 signaling axis drives epithelial-mesenchymal transition in tumor metastasis. Oncogene. 2020;39(10):2125-2139. doi: 10.1038/s41388-019-1132-8 [DOI] [PubMed] [Google Scholar]
- 23.Zhao P, Guo S, Tu Z, et al. Grhl3 induces human epithelial tumor cell migration and invasion via downregulation of E-cadherin. Acta Biochim Biophys Sin (Shanghai). 2016;48(3):266-274. doi: 10.1093/abbs/gmw001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kourtidis A, Lu R, Pence LJ, Anastasiadis PZ. A central role for cadherin signaling in cancer. Exp Cell Res. 2017;358(1):78-85. doi: 10.1016/j.yexcr.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174(5):1588-1593. doi: 10.2353/ajpath.2009.080545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Kang Y. Probing the fifty shades of EMT in metastasis. Trends Cancer. 2016;2(2):65-67. doi: 10.1016/j.trecan.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280(12):11740-11748. doi: 10.1074/jbc.M413878200 [DOI] [PubMed] [Google Scholar]
- 28.Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525-530. doi: 10.1038/nature16064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran HD, Luitel K, Kim M, Zhang K, Longmore GD, Tran DD. Transient SNAIL1 expression is necessary for metastatic competence in breast cancer. Cancer Res. 2014;74(21):6330-6340. doi: 10.1158/0008-5472.CAN-14-0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019;216(5):1016-1026. doi: 10.1084/jem.20181827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams ED, Gao D, Redfern A, Thompson EW. Controversies around epithelial–mesenchymal plasticity in cancer metastasis. Nat Rev Cancer. 2019;19(12):716-732. doi: 10.1038/s41568-019-0213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blazquez R, Rietkötter E, Wenske B, et al. LEF1 supports metastatic brain colonization by regulating glutathione metabolism and increasing ROS resistance in breast cancer. Int J Cancer. 2020;146(11):3170-3183. doi: 10.1002/ijc.32742 [DOI] [PubMed] [Google Scholar]
- 33.Mirone G, Perna S, Shukla A, Marfe G. Involvement of Notch-1 in resistance to regorafenib in colon cancer cells. J Cell Physiol. 2016;231(5):1097-1105. doi: 10.1002/jcp.25206 [DOI] [PubMed] [Google Scholar]
- 34.Ren Y, Tao J, Jiang Z, Guo D, Tang J. Pimozide suppresses colorectal cancer via inhibition of Wnt/β-catenin signaling pathway. Life Sci. 2018;209:267-273. doi: 10.1016/j.lfs.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 35.Zhou H, Lin C, Zhang Y, et al. miR-506 enhances the sensitivity of human colorectal cancer cells to oxaliplatin by suppressing MDR1/P-gp expression. Cell Prolif. 2017;50(3):e12341. doi: 10.1111/cpr.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikehata M, Yamada A, Morimura N, et al. Wnt/β-catenin signaling activates nephronectin expression in osteoblasts. Biochem Biophys Res Commun. 2017;484(2):231-234. doi: 10.1016/j.bbrc.2017.01.053 [DOI] [PubMed] [Google Scholar]
- 37.Lu HY, Zu YX, Jiang XW, et al. Novel ADAM-17 inhibitor ZLDI-8 inhibits the proliferation and metastasis of chemo-resistant non-small-cell lung cancer by reversing Notch and epithelial mesenchymal transition in vitro and in vivo. Pharmacol Res. 2019;148:104406. doi: 10.1016/j.phrs.2019.104406 [DOI] [PubMed] [Google Scholar]
- 38.Baba H, Teramoto K, Kawamura T, Mori A, Imamura M, Arii S. Dihydropyrimidine dehydrogenase and thymidylate synthase activities in hepatocellular carcinomas and in diseased livers. Cancer Chemother Pharmacol. 2003;52(6):469-476. doi: 10.1007/s00280-003-0695-8 [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Li JM, Wei W, et al. Regulation of ATP-binding cassette subfamily B member 1 by snail contributes to chemoresistance in colorectal cancer. Cancer Sci. 2020;111(1):84-97. doi: 10.1111/cas.14253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bahrami A, Majeed M, Sahebkar A. Curcumin: a potent agent to reverse epithelial-to-mesenchymal transition. Cell Oncol (Dordr). 2019;42(4):405-421. doi: 10.1007/s13402-019-00442-2 [DOI] [PubMed] [Google Scholar]
- 41.Toden S, Okugawa Y, Jascur T, et al. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015;36(3):355-367. doi: 10.1093/carcin/bgv006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendoza-Rodríguez MG, Sánchez-Barrera CÁ, Callejas BE, et al. Use of STAT6 phosphorylation inhibitor and trimethylglycine as new adjuvant therapies for 5-fluorouracil in colitis-associated tumorigenesis. Int J Mol Sci. 2020;21(6):2130. doi: 10.3390/ijms21062130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Shi X, Peng Y, et al. HIF-1α promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLOS ONE. 2015;10(6):e0129603. doi: 10.1371/journal.pone.0129603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo C, Ma J, Deng G, et al. ZEB1 promotes oxaliplatin resistance through the induction of epithelial—mesenchymal transition in colon cancer cells. J Cancer. 2017;8(17):3555-3566. doi: 10.7150/jca.20952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu CE, Zhuang YW, Zhou JY, Liu SL, Wang RP, Shu P. Cinnamaldehyde enhances apoptotic effect of oxaliplatin and reverses epithelial-mesenchymal transition and stemnness in hypoxic colorectal cancer cells. Exp Cell Res. 2019;383(1):111500. doi: 10.1016/j.yexcr.2019.111500 [DOI] [PubMed] [Google Scholar]
- 46.Zhao B, Wang L, Qiu H, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2017;8(3):3980-4000. doi: 10.18632/oncotarget.14012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zangari J, Petrelli F, Maillot B, Martinou JC. The multifaceted pyruvate metabolism: role of the mitochondrial pyruvate carrier. Biomolecules. 2020;10(7):1068. doi: 10.3390/biom10071068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schell JC, Olson KA, Jiang L, et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56(3):400-413. doi: 10.1016/j.molcel.2014.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takaoka Y, Konno M, Koseki J, et al. Mitochondrial pyruvate carrier 1 expression controls cancer epithelial-mesenchymal transition and radioresistance. Cancer Sci. 2019;110(4):1331-1339. doi: 10.1111/cas.13980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu X, Wang Q, Liu B, Zhang N, Cheng G. Vitamin D enhances radiosensitivity of colorectal cancer by reversing epithelial-mesenchymal transition. Front Cell Dev Biol. 2021;9:684855. doi: 10.3389/fcell.2021.684855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun J, Li Q, Lian X, et al. MicroRNA-29b mediates lung mesenchymal-epithelial transition and prevents lung fibrosis in the silicosis model. Mol Ther Nucleic Acids. 2019;14:20-31. doi: 10.1016/j.omtn.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H, Gao J, Du Z, Zhang X, Yang F, Gao W. Expression of factors and key components associated with the PI3K signaling pathway in colon cancer. Oncol Lett. 2018;15(4):5465-5472. doi: 10.3892/ol.2018.8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korpal M, Yan J, Lu X, Xu S, Lerit DA, Kang Y. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat Med. 2009;15(8):960-966. doi: 10.1038/nm.1943 [DOI] [PubMed] [Google Scholar]
- 54.Masoudi M, Yamini N, Salehi F, et al. Notch signaling pathway in cumulus cells reflecting zygote and embryo quality in polycystic ovary syndrome. Arch Gynecol Obstet. 2021;304(4):1097-1105. doi: 10.1007/s00404-021-06039-1 [DOI] [PubMed] [Google Scholar]
- 55.Zhang T, Chen S, Peng Y, et al. NOVA1-mediated SORBS2 isoform promotes colorectal cancer migration by activating the Notch pathway. Front Cell Dev Biol. 2021;9:673873. doi: 10.3389/fcell.2021.673873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugiyama M, Oki E, Nakaji Y, et al. High expression of the Notch ligand jagged-1 is associated with poor prognosis after surgery for colorectal cancer. Cancer Sci. 2016;107(11):1705-1716. doi: 10.1111/cas.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichrath J, Reichrath S. Notch signaling in prevention and therapy: fighting cancer with a two-sided sword. Adv Exp Med Biol. 2021;1287:1-7. doi: 10.1007/978-3-030-55031-8_1 [DOI] [PubMed] [Google Scholar]
- 58.Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol Ther. 2013;139(2):95-110. doi: 10.1016/j.pharmthera.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fornasier G, Francescon S, Baldo P. An update of efficacy and safety of cetuximab in metastatic colorectal cancer: a narrative review. Adv Ther. 2018;35(10):1497-1509. doi: 10.1007/s12325-018-0791-0 [DOI] [PubMed] [Google Scholar]
- 60.Xing Y, Jing H, Zhang Y, Suo J, Qian M. MicroRNA-141-3p affected proliferation, chemosensitivity, migration and invasion of colorectal cancer cells by targeting EGFR. Int J Biochem Cell Biol. 2020;118:105643. doi: 10.1016/j.biocel.2019.105643 [DOI] [PubMed] [Google Scholar]
- 61.Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486(7404):532-536. doi: 10.1038/nature11156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhai B, Zhang N, Han X, et al. Molecular targets of β-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: a review. Biomed Pharmacother. 2019;114:108812. doi: 10.1016/j.biopha.2019.108812 [DOI] [PubMed] [Google Scholar]
- 63.Chen P, Li X, Zhang R, et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10(11):5107-5119. doi: 10.7150/thno.44705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palisoul ML, Quinn JM, Schepers E, et al. Inhibition of the receptor tyrosine kinase AXL restores paclitaxel chemosensitivity in uterine serous cancer. Mol Cancer Ther. 2017;16(12):2881-2891. doi: 10.1158/1535-7163.MCT-17-0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin JZ, Wang ZJ, De W, et al. Targeting AXL overcomes resistance to docetaxel therapy in advanced prostate cancer. Oncotarget. 2017;8(25):41064-41077. doi: 10.18632/oncotarget.17026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keating AK, Kim GK, Jones AE, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9(5):1298-1307. doi: 10.1158/1535-7163.MCT-09-0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solanes-Casado S, Cebrián A, Rodríguez-Remírez M, et al. Overcoming PLK1 inhibitor resistance by targeting mevalonate pathway to impair AXL-TWIST axis in colorectal cancer. Biomed Pharmacother. 2021;144:112347. doi: 10.1016/j.biopha.2021.112347 [DOI] [PubMed] [Google Scholar]
- 68.Campbell SL, Wellen KE. Metabolic signaling to the nucleus in cancer. Mol Cell. 2018;71(3):398-408. doi: 10.1016/j.molcel.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 69.Yang T, Chen M, Sun T. Simvastatin attenuates TGF-β1-induced epithelial-mesenchymal transition in human alveolar epithelial cells. Cell Physiol Biochem. 2013;31(6):863-874. doi: 10.1159/000350104 [DOI] [PubMed] [Google Scholar]
- 70.Wang G, Cao R, Wang Y, et al. Simvastatin induces cell cycle arrest and inhibits proliferation of bladder cancer cells via PPARγ signalling pathway. Sci Rep. 2016;6:35783. doi: 10.1038/srep35783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramesh V, Brabletz T, Ceppi P. Targeting EMT in cancer with repurposed metabolic inhibitors. Trends Cancer. 2020;6(11):942-950. doi: 10.1016/j.trecan.2020.06.005 [DOI] [PubMed] [Google Scholar]
- 72.Du L, Li J, Lei L, et al. High vimentin expression predicts a poor prognosis and progression in colorectal cancer: a study with meta-analysis and TCGA database. BioMed Res Int. 2018;2018:6387810. doi: 10.1155/2018/6387810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohara M, Ohara K, Kumai T, et al. Phosphorylated vimentin as an immunotherapeutic target against metastatic colorectal cancer. Cancer Immunol Immunother. 2020;69(6):989-999. doi: 10.1007/s00262-020-02524-9 [DOI] [PMC free article] [PubMed] [Google Scholar]