Abstract
Objective:
The main aim of this systematic review and meta-analysis is to provide summarized evidence on the prevalence of chronic kidney disease and associated factors among patients with chronic illness in Ethiopia.
Method:
Databases of MEDLINE/PubMed, Embase, Google Scholar, CINAHL, Cochrane library, and ScienceDirect were searched. In addition, gray literatures were searched manually from university repositories. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist was used to select potential studies. Microsoft Excel 2013 sheet template was used to extract data. The quality of included studies was assessed by utilizing the Newcastle-Ottawa Scale. STATA software version 14.0 is used to compute the estimated pooled prevalence and associated factors of chronic kidney disease.
Result:
Twelve articles that fulfilled the inclusion criteria were included. The pooled estimate of chronic kidney disease among patients with chronic illnesses in Ethiopia is 21.71% (95% confidence interval: 17.67, 25.74). The highest prevalence of chronic kidney disease among patients with chronic illnesses is from Oromia (32.55% (confidence interval: 19.91, 45.19)). Glomerular filtration rate showed a comparable pooled prevalence from Cockroft-Gault and MDRD methods; 22.38% (confidence interval: 15.83, 28.92), 22.18 (confidence interval: 18.01, 26.34), respectively. Hypertensives become more likely to have chronic kidney disease compared with normotensive patients, (odds ratio = 3.01, 95% confidence interval: 1.33, 6.81).
Conclusion:
Prevalence of chronic kidney disease among chronic illness patients was significantly high. Hypertension is significantly associated with chronic kidney disease. Hence, we recommend that continuous screening of possible risk factors and proper follow-up and management strategies should be designed.
Keywords: Nephrology, epidemiology/public health, palliative medicine, chronic kidney disease, chronic diseases, prevalence
Introduction
Chronic kidney disease (CKD) is a global public health concern that spreads rapidly all over the world.1,2 It is manifested by albuminuria and glomerular filtration rate (GFR) < 60 mL/min/1.73 m5 for at least 3 months regardless of clinical diagnosis.1,3 Increased incidences of hypertension and diabetes facilitated and escalate the morbidity and mortality rate due to CKD.2,4 The incidence of CKD is usually exacerbated in developing nations due to the co-occurrence of infectious causes that predisposes to CKD like chronic glomerulonephritis and human immunodeficiency virus (HIV).5,6 Consumption of nephrotoxic traditional medicinal plants also amplified the problem in Sub-Saharan African countries.7,8
The increasing pattern of chronic diseases is also a double burden to Africans. 9 Although old age populations are at high risk of developing CKD in the developed world, there are terrifying reports emerged stating young Africans are at high risk of mortality and long-term disabilities when compared with their counterparts of the developed world. 10 This further complicates the situation of economic constraints expended to tackle CKD which demands very expensive interventions including dialysis and kidney transplantation. 11 Since the treatment cost is unbearably high, preventive measures, and early detection were highly suggested mechanisms to mitigate the situation.12,13
In Ethiopia, the emerging burden of noncommunicable disease becomes a serious public health concern which had a great impact on CKD.14,15 This is accompanied by poor knowledge of the early diagnosis of CKD. 16 Besides its economic and medical impact, CKD becomes one of the reasons for psychological affections like depression in Ethiopia. 17
A lot of factors are reported to be associated with the occurrence of CKD. Some of them are cholesterol level, 18 body mass index (BMI),18,19 uncontrolled blood pressure age > 60, duration of noncommunicable diseases,20,21 and residence. 22
Even though CKD is a worrisome situation that can make a country like Ethiopia suffer from the economical and public health aspects, there is a serious information gap in the area.6,23 Therefore, this systematic review and meta-analysis aims at providing summarized evidence on the prevalence of CKD and associated factors among patients with chronic illness in Ethiopia.
Methods
Databases and search strategy
Both published and unpublished studies about the magnitude and associated factors of CKD in Ethiopia were thoroughly searched by two authors (Z.A. and G.W.) in the databases of MEDLINE/PubMed, Embase, Google Scholar, CINAHL, Cochrane library, and ScienceDirect. In addition, gray literatures were searched manually from university repositories. Reference lists of eligible studies were checked to maximize the inclusion of relevant studies. The search was not bounded by the year of publication. As a result, all articles published and/or reported up to June 30, 2020 were included. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used to select potential studies. 24 Studies from MEDLINE/PubMed database were searched using the following Medical Science Heading (MeSH) terms: ((((((“prevalence”(All Fields) OR “magnitude”(All Fields)) OR “epidemiology”(All Fields)) OR “incidence”(All Fields)) AND ((“prevalence”(MeSH Terms) OR “epidemiology”(MeSH Terms)) OR “incidence”(MeSH Terms))) AND ((“chronic kidney disease”(All Fields) OR “kidney disease”(All Fields)) OR (“kidney diseases”(MeSH Terms) OR “renal insufficiency”(MeSH Terms)))) AND (((“associated factor”(All Fields) OR “risk factor”(All Fields)) OR “predictors”(All Fields)) OR (“risk factors”(MeSH Terms) OR “predictor”(All Fields)))) AND (“Ethiopia”(All Fields) OR “Ethiopia”(MeSH Terms)). EndNote X7 was also used to manage the duplication of articles.
Inclusion and exclusion criteria
All observational studies conducted in Ethiopia depicting magnitude and associated factors of CKD and written in English language done up to 30 June 2020 were included. However, inaccessible full-text articles due to non-responsiveness of the corresponding authors upon frequent inquiry through email by two authors (Z.A. and G.W.) and articles that did not provide calculably or reported odds ratios (Ors) and 95% confidence intervals (CIs) for associated factors were excluded
Data extraction
Microsoft Excel 2013 sheet template was used to extract data regarding authors name with publication year, study period, study design, study population, study area/region, type of GFR confirmatory equations used, GFR cut points, sample size, numbers of cases with CKD and prevalence of CKD among the total number of chronic patients. A two-by-two table was also used to address odds ratios from studies depicting factors associated with CKD and the factors included were age, sex, BMI, duration of hypertension, hypertension, diabetes mellitus (DM), and residence.
Quality assessment
The quality of included studies was assessed by three authors (Z.A., G.W., and A.M.) utilizing Newcastle-Ottawa Scale which enables to assess the quality of each article by their methodological merit, comparability caliber, and outcome excellence. 25 Arguments between the authors were addressed by discussion and articles were included after the authors reached an agreement.
Statistical analysis
STATA software version 14.0 is used to compute the estimated pooled prevalence of CKD among patients with chronic diseases and odds ratios of the associated factors. Heterogeneity among included studies was assessed using percentage of variance (I2) and P value. Since statistically significant heterogeneity was found with I2 = 89.9% at P value < 0.001, Random effect model was applied to estimate the pooled prevalence of CKD among patients with chronic diseases and summary odds ratios of factors associated with CKD. Sub-group analysis was also calculated based on the type of chronic disease, study region, type of equation used to determine GFR, and stages of CKD.
Protocols and registration
This systematic review and meta-analysis is registered on PROSPERO under the registration number CRD42021224762 and can be accessed at https://www.crd.york.ac.uk/PROSPERO.
Results
Characteristics of studies
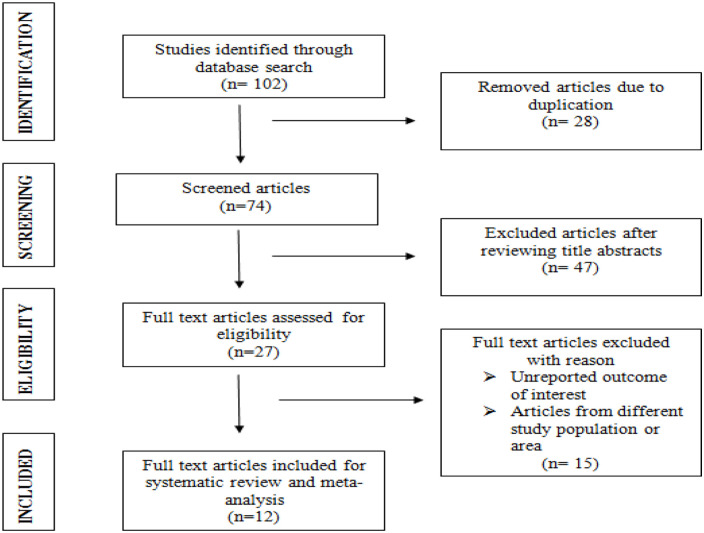
A systematic review and meta-analysis were done on 12 articles that fulfilled the inclusion criteria (Table 1). A total of 3766 patients (ranging from 163 26 to 568 18 ) with chronic illnesses were involved in this study. The included studies were published from 2013 27 up to 2020 20 , 28 (Figure 1). Except one study, 19 all studies used a cross-sectional study design. The highest magnitude of CKD is reported from Jimma University medical center (38.9%) 29 while the least is from Zewditu memorial hospital (12.2%). 30 The studies were conducted in five different regions of the country; 4 from Addis Ababa,19,26,30,31 4 from Amhara,20,21,27,28 2 from Oromia,22,29 and 1 from SNNPR. 32
Table 1.
General characteristic of the included articles for systematic review and meta-analysis pertaining to the magnitude and associated factors of CKD among patients with chronic illnesses in Ethiopia.
| Article | Study period | Study design | Study population | Study area | Region | Equation used | Sample size | Cases | Prevalence | Newcastle-Ottawa scale |
|---|---|---|---|---|---|---|---|---|---|---|
| Geletu et al. 19 | January 2008–November 2017 | Cohort | Diabetes mellitus patients | St. Paul’s Hospital | Addis Ababa | Cockcroft-Gault | 435 | 62 | 14.3% | 8 |
| Bahrey et al. 18 | February–April, 2018 | Cross-sectional | Hypertensive patients | Tigray hospitals | Tigray | Cockcroft-Gault | 578 | 128 | 22.1% | 7 |
| Kore et al. 30 | 20 October 2017–10 December 2017. | Cross-sectional | Nephrologic patients | Zewditu hospital | Addis Ababa | CKD-EPI | 320 | 39 | 12.2% | 7 |
| Chala et al. 26 | September–November 2017 | Cross-sectional | Cardiovascular patients | Tikur Anbesa Hospital | Addis Ababa | MDRD equation | 163 | 39 | 23.9% | 7 |
| Alemu et al. 20 | 2 April–31 July 2018 | Cross-sectional | Diabetes mellitus patients | University of Gondar hospital | Amhara | MDRD equation | 272 | 47 | 17.3% | 7 |
| Abdulkadr et al. 31 | October–December 2017 | Cross-sectional | Diabetes mellitus patients | Police hospital | Addis Ababa | Cockcroft-Gault | 362 | 53 | 14.65% | 7 |
| Damtie et al. 21 | February–April 2016 | Cross-sectional | Diabetes mellitus patients | University of Gondar hospital | Amhara | MDRD equation | 229 | 50 | 21.8% | 7 |
| Fiseha and Tamir 28 | 1 February–30 July 2016 | Cross-sectional | Diabetes mellitus patients | Dessie Referral Hospital | Amhara | MDRD equation | 323 | 85 | 26.3% | 7 |
| Fiseha et al. 32 | 1 September–31 October 2013 | Cross-sectional | Diabetes mellitus patients | Butajira Hospital | SNNPR | Cockcroft-Gault | 214 | 51 | 23.8% | 7 |
| Kahsu et al. 27 | 1 January–30 May 2012 | Cross-sectional | HIV patients | Felege Hiwot Referral Hospital | Amhara | Cockcroft-Gault | 307 | 66 | 21.5% | 7 |
| Kumela Goro et al. 29 | – | Cross-sectional | Diabetes mellitus and hypertensive patients | Jimma University Medical Center | Oromia | CKD-EPI | 208 | 54 | 26.0% | 7 |
| Adugna et al. 22 | 1 November 2016–30 April 2017 | Cross-sectional | Admitted patients | Jimma University Medical Center | Oromia | Cockcroft-Gault | 355 | 138 | 38.9% | 7 |
CKD: chronic kidney disease; CKD-EPI: chronic kidney disease epidemiology collaboration; HIV: human immunodeficiency virus; MDRD: modification of diet in renal disease; SNNPR: Southern Nation Nationalities and Peoples region.
Admitted patient prevalence of CKD indicated inpatient prevalence of CKD and hospital patient prevalence of CKD indicated out-patient prevalence of CKD.
Figure 1.
PRISMA chart flow depicting article selection process for systematic review and meta-analysis on the magnitude and associated factors among patients with chronic diseases.
Meta-analysis
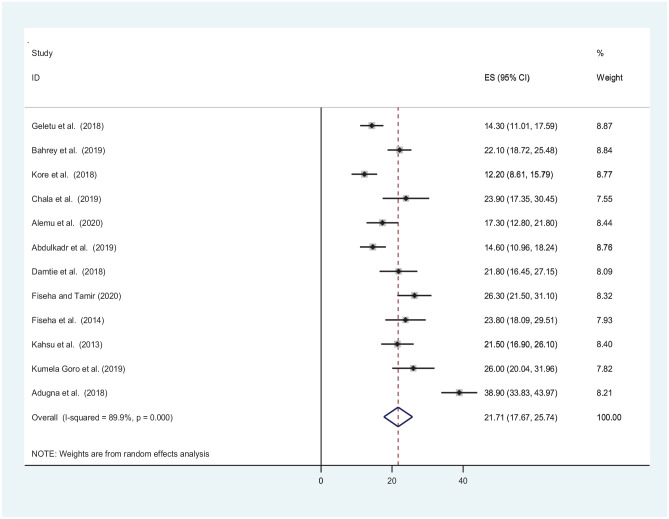
The pooled estimate of CKD among patients with chronic illnesses in Ethiopia is 21.71% (95% CI: 17.67, 25.74, P < 0.001). The meta-analysis showed significant heterogeneity across the studies with I2 = 89.9%, P < 0.001. As a result, the random effect model was used to compute the pooled estimate of CKD (Figure 2).
Figure 2.
Forest plot depicting pooled prevalence of CKD among patients with chronic diseases in Ethiopia.
Publication bias
Begg’s rank test and Egger’s regression intercept tests were also carried out to indicate the correlation between the effect sizes and sampling variance to determine publication bias. 33 Based on Begg’s and Egger’s test results, the absence of significant publication bias was declared objectively (P > 0.05)
Sub-group analysis
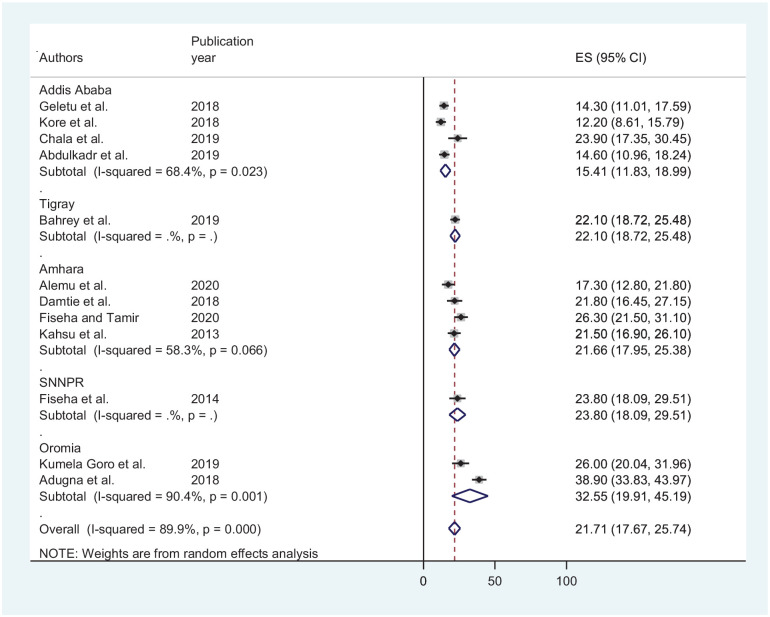
A sub-group analysis based on study region revealed that the highest prevalence of CKD among patients with chronic illnesses is from Oromia (32.55% (CI: 19.91, 45.19)) while the least is from Addis Ababa (15.41% (CI: 11.83, 18.99)) (Figure 3).
Figure 3.
A forest plot showing sub-group analysis based on regions.
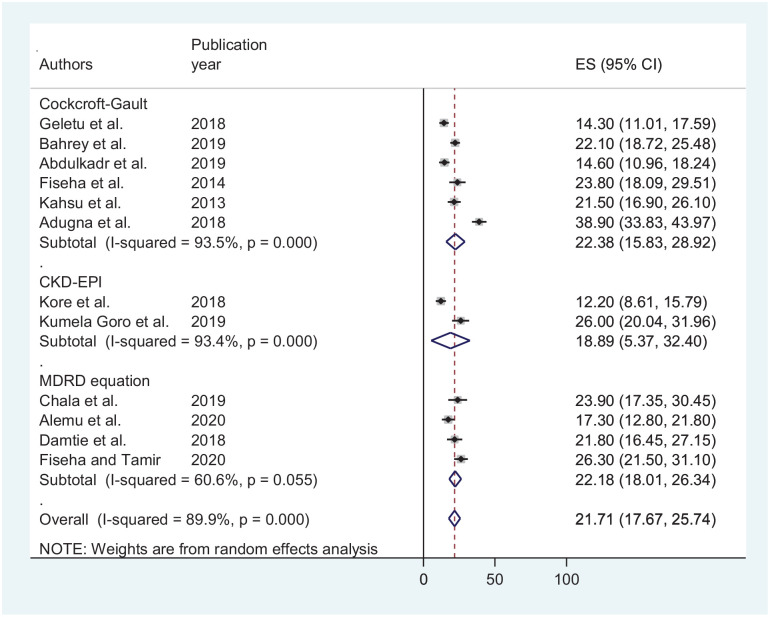
Another sub-group analysis based on equations used to determine GFR showed a comparable pooled prevalence among Cockroft-Gault and MDRD methods; 22.38% (CI: 15.83, 28.92), 22.18 (CI: 18.01, 26.34), respectively (Figure 4).
Figure 4.
Forest plot depicting a sub-group analysis based on equations used to determine GFR.
Stages of CKD
Based on the results from GFR, the pooled prevalence of patients with Stage-2 CKD turns out to be the most prevalent followed by Stage 1 accounting for 30.76% and 26.16%, respectively. The least pooled prevalence is reported from patients with Stage-5 CKD (Table 2).
Table 2.
Pooled prevalence of CKD stages among patients with chronic diseases in Ethiopia.
| Stages of CKD based on GFR | GFR cut point (mL/min/1.73 m2) | Description | No. of studies | Pooled prevalence (95% CI) | I 2 | P values | |
|---|---|---|---|---|---|---|---|
| Stage 1 | ⩾90 | Normal or high | 6 | 26.16% (12.16, 40.16) | 98.4% | <0.001 | |
| Stage 2 | 60–89 | Mild | 6 | 30.76% (13.42, 48.10) | 98.8% | <0.001 | |
| Stage 3 | A | 45–59 | Mild to moderate | 7 | 9.94% (5.97, 13.91) | 89.4% | <0.001 |
| B | 30–44 | Moderate to severe | 7 | 3.77% (2.11, 5.42) | 73.9% | <0.001 | |
| Total | 10 | 13.88% (9.92, 17.85) | 91% | <0.001 | |||
| Stage 4 | 15–29 | Severe | 9 | 1.19% (0.76, 1.61) | 90% | <0.001 | |
| Stage 5 | <15 | Kidney failure | 5 | 0.4% (0.05, 0.74) | 88% | <0.001 | |
CKD: chronic kidney disease; CI: confidence interval; GFR: glomerular filtration rate.
Stage-5 CKD patients included predialysis patients and excluded patients with renal transplantation.
Associated factors
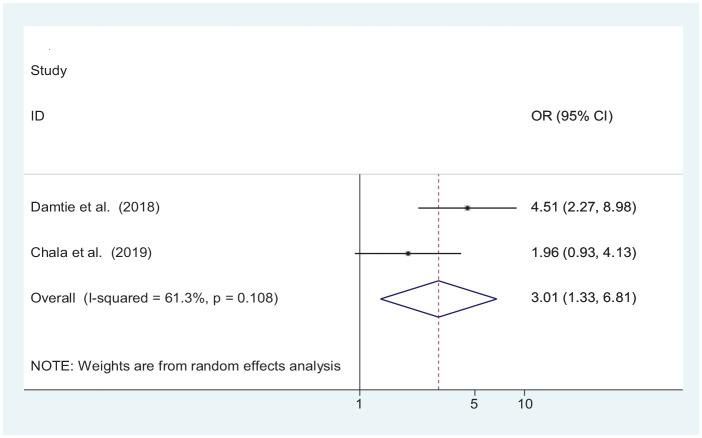
Although individual studies depicted age,21,26 BMI,18,21,26 and the presence of hypertension as a significantly associated factor to develop CKD. Our meta-analysis found only presence of hypertension being associated significantly. According to our finding, the probability of developing CKD among hypertensive patients is three times higher when compared with normotensive patients (OR = 3.01 (1.33, 6.81)) (Figure 5).
Figure 5.
Forest plot indicating association of hypertension with CKD among patients with chronic illnesses in Ethiopia.
Discussion
Chronic diseases are becoming a double burden public health issue both in developed and developing countries. However, the prevalence is rising in developing countries when compared with developed nations.34–36 This systematic review and meta-analysis provided an update on pooled estimates of CKD in patients with chronic diseases in Ethiopia which will give invaluable information to policymakers, health planners, and the community itself.
This systematic review and meta-analysis revealed that the pooled prevalence of CKD among patients with chronic illnesses in Ethiopia was 21.71% (95% CI: 17.67–25.74) which was consistent with studies conducted in the United States on HIV patients (23.7%), Chinese HTN patients (22.0%), Thai DM patients (24.4%), Japanese type-I DM patients (19.1%), Chinese DM patients (27.1%) Ghanaian DM only, both DM and HTN and hypertensive patients (28.5%, 26.3%, and 16.1%), respectively.37–39 However, the finding of this meta-analysis was lower than previous studies in Brazil (38.6%), Burundi (45.7%), Japanese type-II DM patients (46%), Ghana (46.9%), and Finland (68.6%).40–44 On the other hand, our finding is higher than studies conducted on the general population Senegal (4.9%), China (9.88%), Asian HIV patients (13%), and Botswanan admitted patients (13.5%).35,45–47 The possible reason for this discrepancy might be attributed to the time of the study, the age group of the population studied, the type of chronic illnesses, the diagnosis criteria for CKD, the study setting, and the study population.
The sub-group analysis by region revealed, the highest prevalence of CKD among patients with chronic illnesses was from Oromia (32.55%) while the least is from Addis Ababa in the capital city of Ethiopia (15.41%) was observed. This can be due to significant differences in socioeconomic characteristics, lifestyles, controlling and follow-up of chronic illnesses, climatic conditions, medical care, and economic development between regions that may cause the differences in CKD prevalence among chronically ill patients.
In another sub-group analysis using the stage of CKD, the pooled prevalence of patients with Stage 2 turns out to be the most prevalent followed by Stages 1 and 3 accounting 30.76%, 26.16%, and 13.88%, respectively. The least pooled prevalence is reported for stage 5 (0.4%). The finding of this systematic review and meta-analysis was in line with studies from Ghana and Botswana.47,48 Similarly study among Kenyan medical inpatients reported type II (30.5%) was dominant followed by type III (28.8%). 16 Whereas type III was the most prevalent stage (18.2%) and the least was Stage 5 (1.6 %) in Thai type-II DM patients. 17 While in the Chinese DM patients’ Stage 1 was most prevalent followed by Stage 2. 39
This systematic review and meta-analysis also identified factors associated with CKD among chronic illness patients in Ethiopia. In random-effects model pooled estimates, hypertension was significantly associated with CKD. The pooled estimates of developing CKD among hypertensive patients are three times higher when compared with normotensive patients. This finding was similar with studies conducted in Finland, Senegal, Singapore, and China.35,44,49 The possible reason might be hypertension is highly related with end organ damage and may result in kidney injury. 50 But factors like duration of hypertension, current blood pressure, duration of DM, poor control of blood pressure, drug adherence to antihypertensive medication, DM, and cardiovascular disease should not be neglected.23,34,39,41
Strength and limitation
This study follows some strengths and limitations. Our review adds considerable knowledge of the updated prevalence of CKD among patients with chronic illnesses in Ethiopia. Sub-group analysis was performed to minimize statistical heterogeneity. Multiple factors were also included to identify the significant factors for CKD but the lack of important risk factors in the analysis was the limitation. However, substantial statistically significant heterogeneity was observed across studies which undermines the pooled estimate of CKD suggests that chance could be responsible for between-study variability. Sub-group analysis could not identify the source of heterogeneity. The limitations were the included Studies used different criteria to assess CKD, and all the included studies were cross-sectional which undermines the assessment of risk factors.
Conclusion
In this systematic review, the pooled prevalence of CKD among chronic illness patients was significantly high. Hypertension was significantly associated with CKD. Based on the finding of this review, we recommend that continuous screening of renal function, identification of the possible risk factors, and proper follow-up and management strategy should be designed to halt CKD among patients with chronic illnesses.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Zelalem Animaw  https://orcid.org/0000-0001-9270-5558
https://orcid.org/0000-0001-9270-5558
Gashaw Walle Ayehu  https://orcid.org/0000-0001-7333-152X
https://orcid.org/0000-0001-7333-152X
References
- 1. Levey A, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives: a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007; 72(3): 247–259. [DOI] [PubMed] [Google Scholar]
- 2. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379(9818): 815–822. [DOI] [PubMed] [Google Scholar]
- 3. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl. 1): S1–S266. [PubMed] [Google Scholar]
- 4. Jha V, Wang AY-M, Wang H. The impact of CKD identification in large countries: the burden of illness. Nephrol Dial Transplant 2012; 27(Suppl. 3): iii32–iii38. [DOI] [PubMed] [Google Scholar]
- 5. Wyatt CM, Klotman PE, D’Agati VD. (eds). HIV-associated nephropathy: clinical presentation, pathology, and epidemiology in the era of antiretroviral therapy. Semin Nephrol 2008; 28(6): 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2014; 2(3): e174–e181. [DOI] [PubMed] [Google Scholar]
- 7. Colson CR, De Broe ME. Kidney injury from alternative medicines. Adv Chronic Kidney Dis 2005; 12(3): 261–275. [DOI] [PubMed] [Google Scholar]
- 8. Bagnis CI, Deray G, Baumelou A, et al. Herbs and the kidney. Am J Kidney Dis 2004; 44(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 9. Moosa M, Van der Walt I, Naicker S, et al. Important causes of chronic kidney disease in South Africa. S Afr Med J 2015; 105(4): 320–327. [DOI] [PubMed] [Google Scholar]
- 10. Bamgboye EL. End-stage renal disease in sub-Saharan Africa. Ethn Dis 2006; 16(2 Suppl. 2): S259. [PubMed] [Google Scholar]
- 11. Barsoum RS. Burden of chronic kidney disease: North Africa. Kidney Int Suppl 2013; 3(2): 164–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dirks JH, De Zeeuw D, Agarwal SK, et al. Prevention of chronic kidney and vascular disease: toward global health equity: The Bellagio 2004 Declaration. Kidney Int 2005; 68: S1–S6. [DOI] [PubMed] [Google Scholar]
- 13. Krzesinski J-M, Sumaili KE, Cohen E. How to tackle the avalanche of chronic kidney disease in sub-Saharan Africa: the situation in the Democratic Republic of Congo as an example. Nephrol Dial Transplant 2007; 22: 332–335. [DOI] [PubMed] [Google Scholar]
- 14. Nshisso LD, Reese A, Gelaye B, et al. Prevalence of hypertension and diabetes among Ethiopian adults. Diabetes Metab Syndr 2012; 6(1): 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tefera YG, Abegaz TM, Abebe TB, et al. The changing trend of cardiovascular disease and its clinical characteristics in Ethiopia: hospital-based observational study. Vasc Health Risk Manag 2017; 13: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolide AD, Kumela K, Kerga F, et al. Health sciences students knowledge, attitude and practices with chronic kidney disease in Jimma University, Ethiopia: cross-sectional study. BMC Res Notes 2019; 12(1): 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Assefa B, Duko B, Ayano G, et al. Prevalence and factors associated with depressive symptoms among patient with chronic kidney disease (CKD) in Black Lion Specialized Hospital and Saint Paulo’s Hospital Millennium Medical College, Addis Ababa, Ethiopia: cross sectional study. J Psychiatry 2016; 19: 390. [Google Scholar]
- 18. Bahrey D, Gebremedhn G, Mariye T, et al. Prevalence and associated factors of chronic kidney disease among adult hypertensive patients in Tigray teaching hospitals: a cross-sectional study. BMC Res Notes 2019; 12(1): 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geletu AH, Teferra AS, Sisay MM, et al. Incidence and predictors of chronic kidney diseases among type 2 diabetes mellitus patients at St. Paul’s Hospital, Addis Ababa, Ethiopia. BMC Res Notes 2018; 11(1): 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alemu H, Hailu W, Adane A. Prevalence of chronic kidney disease and associated factors among patients with diabetes in northwest Ethiopia: a hospital-based cross-sectional study. Curr Ther Res Clin Exp 2020; 92: 100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Damtie S, Biadgo B, Baynes HW, et al. Chronic kidney disease and associated risk factors assessment among diabetes mellitus patients at a tertiary hospital, northwest Ethiopia. Ethiop J Health Sci 2018; 28(6): 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adugna T, Merga H, Gudina EK. Impaired glomerular filtration rate, high grade albuminuria and associated factors among adult patients admitted to tertiary Hospital in Ethiopia. BMC Nephrol 2018; 19(1): 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abd ElHafeez S, Bolignano D, D’Arrigo G, et al. Prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: a systematic review. BMJ Open 2018; 8(1): e015069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta-analysis: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal 2017; 5: 80–84. [Google Scholar]
- 26. Chala G, Sisay T, Teshome Y. Chronic kidney disease and associated risk factors among cardiovascular patients. Int J Nephrol Renovasc Dis 2019; 12: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kahsu G, Birhan W, Addis Z, et al. Renal function impairment and associated risk factors among human immunodeficiency virus positive individuals at Flege Hiwot Referral Hospital, northwest Ethiopia. J Interdiscip Histopathol 2013; 1(5): 252–260. [Google Scholar]
- 28. Fiseha T, Tamir Z. Prevalence and awareness of chronic kidney disease among adult diabetic outpatients in Northeast Ethiopia. BMC Nephrol 2020; 21: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumela Goro K, Desalegn Wolide A, Kerga Dibaba F, et al. Patient awareness, prevalence, and risk factors of chronic kidney disease among diabetes mellitus and hypertensive patients at Jimma University Medical Center, Ethiopia. Biomed Res Int 2019; 2019: 2383508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kore C, Tadesse A, Teshome B, et al. The magnitude of chronic kidney disease and its risk factors at Zewditu Memorial Hospital, Addis Ababa, Ethiopia. J Nephrol Ther 2018; 8(3): 313. [Google Scholar]
- 31. Abdulkadr M, Merga H, Abdissa B, et al. Epidemiology of chronic kidney diseases in Ethiopian Police Hospital: institutional based cross sectional study. Res Sq. Epub ahead of print 9 August 2019. DOI: 10.21203/rs.2.12582/v1. [DOI] [Google Scholar]
- 32. Fiseha T, Kassim M, Yemane T. Chronic kidney disease and underdiagnosis of renal insufficiency among diabetic patients attending a hospital in southern Ethiopia. BMC Nephrol 2014; 15(1): 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2018; 74(3): 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim S, Lim CS, Han DC, et al. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J Korean Med Sci 2009; 24(Suppl. 1): S11–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seck SM, Doupa D, Gueye L, et al. Prevalence of chronic kidney disease and associated factors in senegalese populations: a community-based study in Saint-Louis. Nephrourol Mon 2014; 6(5): e19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Shdaifat E, Manaf MR. The economic burden of hemodialysis in Jordan. Indian J Med Sci 2013; 67(5–6): 103–116. [PubMed] [Google Scholar]
- 37. Fernando SK, Finkelstein FO, Moore BA, et al. Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci 2008; 335(2): 89–94. [DOI] [PubMed] [Google Scholar]
- 38. Xie K, Bao L, Jiang X, et al. The association of metabolic syndrome components and chronic kidney disease in patients with hypertension. Lipids Health Dis 2019; 18(1): 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo K, Zhang L, Zhao F, et al. Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: cross-sectional study. J Diabetes Complications 2016; 30(5): 803–810. [DOI] [PubMed] [Google Scholar]
- 40. Ohta M, Babazono T, Uchigata Y, et al. Comparison of the prevalence of chronic kidney disease in Japanese patients with type 1 and type 2 diabetes. Diabet Med 2010; 27(9): 1017–1023. [DOI] [PubMed] [Google Scholar]
- 41. da Silva LS, Cotta RMM, Moreira TR, et al. Hidden prevalence of chronic kidney disease in hypertensive patients: the strategic role of primary health care. Public Health 2016; 140: 250–257. [DOI] [PubMed] [Google Scholar]
- 42. Cailhol J, Nkurunziza B, Izzedine H, et al. Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: a cross-sectional study. BMC Nephrol 2011; 12(1): 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Osafo C, Mate-Kole M, Affram K, et al. Prevalence of chronic kidney disease in hypertensive patients in Ghana. Ren Fail 2011; 33(4): 388–392. [DOI] [PubMed] [Google Scholar]
- 44. Metsärinne K, Bröijersen A, Kantola I, et al. High prevalence of chronic kidney disease in Finnish patients with type 2 diabetes treated in primary care. Prim Care Diabetes 2015; 9(1): 31–38. [DOI] [PubMed] [Google Scholar]
- 45. Lin B, Shao L, Luo Q, et al. Prevalence of chronic kidney disease and its association with metabolic diseases: a cross-sectional survey in Zhejiang province, Eastern China. BMC Nephrol 2014; 15(1): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nishijima T, Kawasaki Y, Mutoh Y, et al. Prevalence and factors associated with chronic kidney disease and end-stage renal disease in HIV-1-infected Asian patients in Tokyo. Sci Rep 2017; 7(1): 14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rwegerera G, Bayani M, Taolo E, et al. The prevalence of chronic kidney disease and associated factors among patients admitted at princess marina hospital, Gaborone, Botswana. Niger J Clin Pract 2017; 20(3): 313–319. [DOI] [PubMed] [Google Scholar]
- 48. Tannor EK, Sarfo FS, Mobula LM, et al. Prevalence and predictors of chronic kidney disease among Ghanaian patients with hypertension and diabetes mellitus: a multicenter cross-sectional study. J Clin Hypertens 2019; 21(10): 1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ji A, Pan C, Wang H, et al. Prevalence and associated risk factors of chronic kidney disease in an elderly population from eastern China. Int J Environ Res Public Health 2019; 16(22): 4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmieder RE. End organ damage in hypertension. Dtsch Ärztebl Int 2010; 107(49): 866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]