Abstract
Introduction:
Women account for 23% of new human immunodeficiency virus diagnoses in the United States, yet remain understudied. Adherence to antiretroviral therapy and consequent viral suppression are keys to preventing human immunodeficiency virus transmission, reducing risk of drug resistance, and improving health outcomes.
Objectives:
This review identified and synthesized peer-reviewed studies in the United States describing factors associated with viral suppression among cisgender women living with human immunodeficiency virus.
Methods:
We searched five databases: Cumulative Index to Nursing and Allied Health (CINAHL), PubMed, Embase, Scopus, and PsycINFO, and reported the findings using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Eligible studies included: (1) peer-reviewed English-language articles published since 2010; (2) includes only cisgender women; (3) participants were at least 18 years of age; (4) reported metrics on viral loads; and (5) conducted in the United States.
Results:
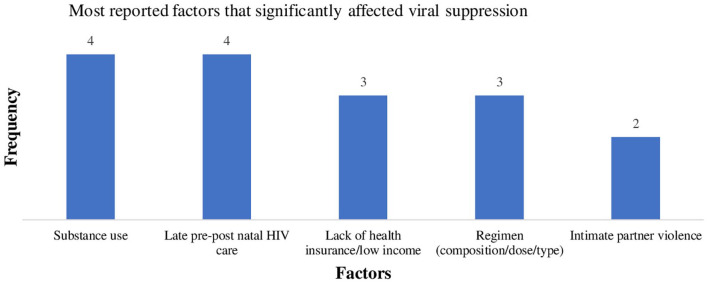
Fourteen studies in total were reviewed. Eight studies had adult women living with human immunodeficiency virus, four recruited only pregnant women, and two included only racial minority women. The most commonly reported factors negatively associated with viral suppression were substance use (n = 4), followed by availability of health insurance, financial constraint, complexity of human immunodeficiency virus treatment regimen (n = 3), and intimate partner violence (n = 2). Other factors were depression, race, and age. In addition, all four studies that included only pregnant women reported early human immunodeficiency virus care engagement as a significant predictor of low viral loads pre- and post-partum.
Conclusion:
Substance use, financial constraint, lack of health insurance, human immunodeficiency virus treatment regimen type, intimate partner violence, and late human immunodeficiency virus care pre–post pregnancy were the most common factors negatively associated with viral suppression. There is a paucity of data on viral suppression factors related to transgender and rural populations. More human immunodeficiency virus research is needed to explore factors associated with human immunodeficiency virus treatment outcomes in transgender women and cisgender women in rural U.S. regions.
Keywords: adherence, antiretroviral therapy, HIV, United States, viral suppression, women
Introduction
Nearly 38 million people live with human immunodeficiency virus (HIV) globally, with women representing over 17 million.1,2 It is estimated that about 1.2 million people aged 13 years or older live with HIV in the United States, 3 with 23% being women. 4 As of 2018, HIV was one of the ten leading causes of death among women ages 25–44 years in the United States. 4 Although the trends of new HIV cases among women in the United States. have been steady since 2014, women still account for about 7000 new HIV diagnoses in the United States annually.5,6 In addition, when compared with other groups, transgender women are 49 times more likely to be infected with HIV. 7
HIV prevention through testing, adherence to treatment, and viral suppression are critical to ending the HIV epidemic. 8 The Centers for Disease Control and Prevention (CDC) defines viral suppression as a condition when HIV Ribonucleic acid (RNA) in the blood is below 200 copies per milliliter of blood. 9 Taking antiretroviral therapy (ART) as prescribed can make HIV viral loads low enough that it is not detected in a standardized blood test, which is referred to as “undetectable viral load.” 9 People living with HIV who take their medication as prescribed and become virally suppressed have a 1% or less risk of transmitting the virus to others, including vertical transmission, as long as the virus remains “undetectable.” 10 Furthermore, viral suppression improves health outcomes, including reduced morbidity, increased longevity, and improved birth outcomes (such as birth weight and term birth) among pregnant women living with HIV.11–13
In 2018, the percentage of women living with HIV in the United States with at least one missed clinic visit was 29% compared with 24% in the overall population. 14 Viral suppression rates of 77% among women versus 84% in men in the United States have also been reported. 15 Some reported factors negatively contributing to lower rates of viral suppression include poor adherence to ART, lack of family support, ART side effects, nondisclosure of HIV status, younger age, female gender, belonging to racial or minority groups, lack of access to healthcare, living with other people, homelessness and stigma, among other factors.2,16–18
The majority of the studies reporting these factors have focused on men living with HIV, recruited both men and women in their research, or were conducted outside the United States.2,12,16 Only a few studies in the United States have focused on women and factors associated with their viral suppression. For instance, a recent systematic review in this area included only Black women in the United States and focused primarily on HIV medication adherence. 19 The purpose of this review is to describe the state of viral suppression in cisgender women in the United States, and provide a current synthesis of factors associated with viral suppression omitted in a previous systematic review. 19
Methods
The literature search
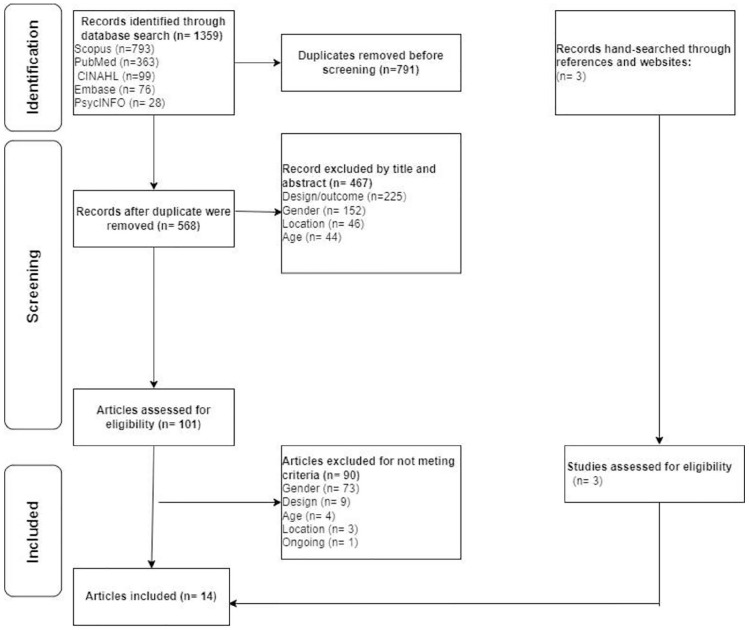
We followed the Whittemore and Knafl methodology for integrative reviews 20 and reported our findings using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 21 On April 9, 2021, we searched five databases: Cumulative Index to Nursing and Allied Health (CINAHL), Embase, PsycINFO, PubMed, and Scopus. In accordance with the PRISMA guidelines and utilizing a search strategy designed by an experienced academic medical librarian, we combined controlled vocabulary terms and free-text words in the title or abstract using the following keywords for our search: “HIV” OR “AIDS,” “female” OR “women,” “factors” OR “predictors,” adherence, “viral suppression” OR “viral load,” “United States.” In addition to the database search, relevant citations and references were hand-searched (see Figure 1) for eligibility based on inclusion and exclusion criteria. The complete search strategies are included in the supplemental material. To minimize bias, we used a broad search strategy to include all adult women within the United States.
Figure 1.
PRISMA flow diagram.
Integrated reviews are based on findings from previous studies. Therefore, ethical approval was not required for this review.
Inclusion and exclusion criteria
We included articles if they met the following criteria: (1) peer-reviewed English-language articles published since 2010; (2) included female participants exclusively; (3) study participants were at least 18 years of age; (4) reported on factors associated with viral load; and (5) conducted in the United States. There were no restrictions on study design or sample size. However, ongoing studies and studies solely focused on transgender women were excluded.
Data selection and analysis
All relevant articles were exported to RefWorks citation manager. Duplicates were identified and excluded before exporting the remaining articles to Rayyan, a web application for organizing and reviewing articles. 22 The articles were examined for eligibility based on title, abstract, and fitness for the review. The full-text articles were reviewed to further validate the inclusion and exclusion criteria. Data were extracted into an Excel spreadsheet for detailed review. The extracted data were grouped and summarized for comparison and analysed to identify similar, divergent, and unique findings (see Table 1).
Table 1.
Studies reporting on factors affecting viral suppression among women living with HIV in the United States.
| Authors | Purpose/objectives | Design | Data source and study period | Sample size and characteristics | Intervention/procedure | Variables | Findings |
|---|---|---|---|---|---|---|---|
| Blank et. al. 23 | Prospectively examined factors associated with viral suppression in women of color. | Cohort | Data from eight Health Resources and Services Administration (HRSA)-sponsored HIV programs across the U.S. Study period: not reported. |
921 non-Caucasian or White adult women Average age: 42 years. |
Applied predictive model to determine factors related to HIV care retention and viral suppression 12 months after baseline interviews. | HIV care retention: ⩾ 2 medical care visits ⩾ 90 days apart in 12 months. Viral suppression: most recent viral load in the last 12 months Viral load cut off: < 200 copies/mL. |
Living with someone the past 3 months was associated with less suppression, current substance use, self-reported poor or fair health, and having more than 14 days of limited activity were associated with higher viral loads. However, being African American, medication adherence at baseline, not seeking care at baseline, and concerns about the impact of HIV management on family were associated with viral suppression. Undecided about care at baseline, having children below 18 years, thoughts that nothing can help HIV condition, housed in institutional facilities (such as substance use programs, psychiatry facilities) were associated with less retention. Also, having ⩾ 14 mentally unhealthy days per month and have lived in the United States for ⩾ 5 years were associated with higher retention. |
| Hanna et al. 24 | To compare the effectiveness of single-tablet versus multiple-tablets and how they impact HIV- related outcomes (including adherence and quality of life). | Cohort | Women’s Interagency HIV Study (WIHS), 2006 to 2013 | 1727 adult women participating in WIHS across the United States Average age: 47 years. |
Women self-reported the frequency of their prescribed medication use over the past 6 months. Quality of life was assessed using the Medical Outcomes Study-HIV score, AIDS events were assessed through self-reports and viral suppression at 6-month intervals measured through viral load test results. | ART adherence: ⩾ 95% of medication use. Viral suppression: having < 80 copies/mL. Incidence of AIDS: self-reported or through matches with cancer and tuberculosis registries and death. Viral load cut off: < 80 copies/mL |
Single-tablet regimens were significantly associated with increased treatment adherence and viral suppression. But single-tablet regimens were not significantly associated with quality of life and AIDS-defining events. |
| Kelso et al. 25 | To examine critical consciousness in relation to perceived racial and gender discrimination and HIV-related outcomes. | Cohort | CORE Center, Chicago site of the WIHS, 2009 to 2010 | 67 African American women from the WHIS Chicago who had semi-annual WIHS visits between October 1, 2009, and March 31, 2010 Average age: 46 years. |
Participants participated in structured interviews. Self-reported data on HIV health history, HAART use adherence, perceived racial and gender discrimination, and critical consciousness were also collected. | HAART adherence: ⩾ 95% of medication use. CD4 count: < 350 cells/mm3 was considered poor HIV health. Viral load for detectable or undetectable. Viral load cut off: ⩾ 50 copies/mL |
Higher critical consciousness was associated with a higher CD4 count. High perceived racial discrimination was associated with detectable viral loads. Women with high perceived racial discrimination and high critical consciousness were significantly less likely to have detectable viral load and were more likely to have CD4 above 350 cells/mm3 Perceived racial and perceived gender discrimination were positively correlated with critical consciousness but not significantly related to CD4 and viral loads. |
| Lazenby et al. 13 | To determine the risk of contracting HIV among infants born in rural counties. | Cohort | South Carolina Department of Health and Environmental Control enhanced HIV/AIDS Reporting System (eHARS), 2004 to 2014 |
666 women in South Carolina who had babies between 2004 and 2014 Average age: not reported. |
Evaluated HIV-related maternal data. Compared most recent viral load data before delivery to post-partum data (⩽12 weeks after delivery), then ART use before and during delivery. |
Maternal outcomes: Viral load Neonatal outcomes: preterm birth (< 37 weeks), low birth weight (< 2500 grams), death (demise ⩽ 6 weeks postdelivery Viral load cut off: < 40 copies/mL |
Maternal outcomes: Women in rural counties were less likely to be virally suppressed, but combination ART of ⩾ 3 regimens decreased viral loads. Intra- and post-partum maternal HIV diagnoses, parenteral drug use, and preterm birth were associated with perinatal HIV infection. Neonatal outcomes: 868 pregnancies and 885 babies. 1.5% of the babies died, 1.2% had HIV. Preterm birth and low birth weights between babies delivered in rural and urban were (21% versus 22%) and (28% versus 26%) respectively. |
| Ludema et al. 26 | To estimate the effect of health insurance and income on viral suppression among WHIS participants. | Cohort | WIHS, 2006 to 2009 | 1481 HIV-positive infected women enrolled in WIHS and had viral load measurement at their 24th visit or after six months. Average age: 38 years |
Participants were characterized based on access and type of insurance. Participation in AIDS Drug Assisted Program and income were self-reported by participants during each semi-annual WIHS visits and viral loads were measured during those visits. | Virologic failure: maintaining < 200 copies/mL after confirmed < 80 copies/mL, insurance status, insurance type, and income Viral load cut off: < 200 copies/mL |
Having private insurance was associated with viral suppression. Women with no insurance but were participants of ADAP were more likely to be virally suppressed than Medicaid beneficiaries or uninsured women without ADAP. |
| McFall et al. 27 | To describe racial/ethnic differences in virologic failure then, determine behavioral, psychosocial, socioeconomic, and healthcare-related correlates of virologic failure among HIV-positive women using HAART. | Cohort | WIHS, 2006 to 2011 | 887 women on HAART, virally suppressed six months before the study and were enrolled in WHIS between April 1, 2006, and March 31, 2011 Average age: not reported. |
Data analysis of WIHS HIV-infected women on HAART between April 1, 2006, and March 31, 2011. | Virological failure: not having consistent viral load below 200 copies/mL Viral load cut off: < 200 copies/mL |
Being Hispanic or White was significantly associated with a lower risk of virologic failure compared to being African American. Born in the United States, having a household income below $24,000, depressive symptoms, smoking, alcohol use, and no ADAP participation were significantly associated with virologic failure. |
| McKinney et al. 28 | To evaluate HIV-adapted group prenatal care effects on viral suppression and post-partum retention in HIV- primary care. | Cohort | Primary data from a community-based health center in Houston, 2013 to 2019 |
194 English and Spanish-speaking HIV-positive women at a community-based health center in Houston, Texas, who had babies between September 1, 2013, and July 31, 2019. Average age: 29 years. |
Women self-selected group or individual prenatal care. Both categories received clinical case management and social work services. Participants in group care participated in additional discussions on pregnancy and HIV-related topics. Groups comprised of 4 to 12 participants who had due dates within 8 weeks. Group sessions were 10 two-sessions led by an obstetrician, nurse practitioner, and/or social worker. | Viral suppression within 12 months post-partum and post-partum care retention. Viral load cut off: < 20 copies/mL |
Women who participated in group prenatal care were more likely to be virally suppressed during delivery and within 12 months post-delivery. Parental group care was significantly associated with more prenatal care visits and having at least one primary care visit within 12 months post-partum. |
| Mills et al. 29 | To estimate the effects of cumulative depression on HIV care appointments, ART adherence, and virologic failure. | Cohort | WIHS, 2013 to 2017 | 1491 HIV-infected women enrolled in WIHS between 2013 and 2017. Average age: 48 years |
Participants completed questionnaires related to mental health and HIV during each semi-annual WIHS visits between 2013 and 2017. Afterward, baseline data were compared to final data. | Missed visits: HIV care visits missed in the last six months, ART adherence: 95% of medication use, Virologic failure: ⩾ 20 copies/mL Viral load cut off: < 200 copies/mL |
Forty-six percent of the women had depressive symptoms. Percent of days depressed (PDD) or higher time spent depressed increased the risk of virologic failure. Also, PDD increased the risk of being < 95% adherent to ART and the likelihood of missing HIV care appointments. A twenty-five percent PDD increased the risk of nonadherence by 27%, missed appointments by 16%, and virologic failure by 8%. |
| Okonsky et al. 30 | To examine reasons why women miss their medications and how results can improve patient-centered adherence research. | Cross-sectional | Data from questionnaires administered in Cleveland, Ohio, and San Francisco and Oakland, California from October 2010 through March 2011. | 206 English speaking adult women with HIV diagnosis. Average age: 47 years |
Participants completed 45–60 min questionnaire on demographic and HIV-related clinical information delivered at medical clinics and community support organizations. | Viral loads, CD4 count, ART adherence: < 100% medication use in the last 30 days. Viral load cut off: < 75 copies/mL |
Forgetfulness was associated with not taking pills. Women on protease inhibitor-based regimens had more detectable viral loads, lower CD4 counts, and a higher risk of AIDS diagnosis than those not on protease inhibitor-based. Women on protease inhibitor-based regimen had an adherence level of 99 (79%) versus 107 (91%) among women in non-protease inhibitor-based regimen group. |
| Trimble et al. 31 | To examine ART adherence rates and viral loads among women living with HIV who had experienced IPV during the last 12 months. | Cross-sectional | Survey data collection among women receiving care at a large specialty clinic in southwestern Texas, 2010. | 272 English or Spanish-speaking HIV-positive women who reported having intimate partner relationships, and had been on ART for at least 12 months. Average age: not reported |
Participants completed interviews at the clinic. Two most recent viral load measurements at least 3 months apart were collected to measure adherence scores. |
Adherence to ART, Viral loads were measured as detectable or undetectable Viral load cut off: Not reported |
Fifty-two percent of the women reported physical or sexual IPV in the last 12 months. Experiencing IPV was significantly associated with detectable viral loads and low medication adherence scale scores. |
| Truong et al. 32 | To evaluate virologic and immunological markers in HIV-positive women receiving different ART regimens and assess the correlation between elevated levels of immune activation and viral load. | Cohort | Patients’ medical records from the University of California, Los Angeles, and the Los Angeles Pediatric AIDS Consortium maternal-fetal HIV transmission study data and laboratory results, 1989 to 2003. |
96 HIV-positive pregnant women prospectively enrolled between 1989 and 2003 in a maternal-fetal HIV transmission study in California. Average age: 27 years |
Participants had laboratory test measurements of viral loads, CD4/CD8 T cells, and serum activation markers during their third trimester, at delivery, and eight weeks post-delivery. | Viral loads, CD4 counts, and β2-microglobulin. Viral loads cut off: ⩾ 400 copies/mL and 50 copies/ML |
HIV-positive status was associated with increased viral loads, CD4 T cells, and β2-microglobulin post-partum regardless of ART. Immune activation increased viral loads during post-partum regardless of ART. Continued use of zidovudine and HAART post-partum were also associated with an increase in viral loads. |
| Turan et al. 33 | To examine the association of attachment-related avoidance and attachment-related anxiety on ART adherence, viral suppression, CD4 count, and HIV visit adherence. | Cross-sectional | Data from Women’s Adherence and Visit Engagement (WAVE), a substudy nested in WIHS, 2016 to 2017 | 453 HIV-infected women enrolled in WIHS between 2016 and 2017 Average age: 49 years |
Participants completed an interviewer-assisted psychosocial questionnaire during their WIHS visits in addition to WIHS data. | Attachment-related avoidance, and attachment-related anxiety measured with 18-item Experiences in Close relationships tool. ART adherence: ⩾ 95% medication use. Viral suppression: detectable or undetectable, CD4 count, and HIV Visit adherence. Viral load cut off: > 200 copies/mL |
Attachment-related avoidance was a predictor for low ART adherence, viral failure, and low CD4 counts. Attachment-related anxiety was a predictor of missed HIV care visits. The association of attachment-related avoidance with viral failure and low CD4 count was mediated by ART adherence. |
| Wilson et al. 34 | To describe the association between women’s healthcare empowerment and ART adherence and HIV primary care retention. | Longitudinal | WIHS, 2014 to 2016 | 973 WHIS HIV-infected women enrolled between April 2014 to March 2016 Average age: 49 years |
Participants completed the Health Care Empowerment Inventory during their WIHS study visits at six months intervals for four consecutive times from April 2014 to March 2016. Participants self-reported ART adherence during each visit. | Viral load, care retention, ART adherence: > 95% medication use. Illicit substance use, and heavy drinking were assessed through self-reported quantity and frequencies. Burden of depression symptoms. Viral load cut off: < 200 copies/mL |
Being older, having a higher income, and no heavy drinking were associated with viral suppression. Substance use and depression symptoms were not significantly associated with viral suppression but were related to adherence at six month. Healthcare empowerment and viral suppression were mediated by adherence and retention in HIV care. |
| Yee et al. 35 | To determine if IPV experience during pregnancy was associated with factors that increased the risk of vertical transmission of HIV. | Cohort | Northwestern Memorial Hospital, Texas patients’ record. 2007 to 2014 |
197 HIV-positive pregnant women with a history of IPV who received perinatal care at Northwestern Memorial Hospital in Texas between 2007 and 2014. Average age: 29 |
Review and analyses of participants’ medical records and self-reported demographic information. | Medication adherence and viral load. Viral load cut off: not reported |
IPV during pregnancy was associated with poor ART adherence. The time to achieve initial viral suppression among women who experienced IPV during pregnancy was 10 weeks versus 5 weeks among those who did not experience IPV during pregnancy. IPV during pregnancy was associated with high viral loads. Time to achieve stable viral suppression among women who experienced IPV during pregnancy was 16 weeks versus 8.5 weeks among women who did not experience IPV during pregnancy. |
Quality assessment
Two authors independently performed a quality check for the included studies using the Joanna Briggs Institute Checklist for Analytical Cross-Sectional Studies and Checklist for Cohort Studies. 36 Each item included in the checklist was assigned one point, and a half-point was assigned for unclear items. One longitudinal study 34 was assessed as cross-sectional because surveys were administered in waves, not over time to the same sample. After independently reviewing the articles, the two reviewers met to reach a consensus on scores. The authors agreed on the overall score for each article. Any articles that received a score of 80%–100% was considered strong quality, 60%–79% were considered moderate quality, and below 60% were considered low quality (see Tables 2 and 3).
Table 2.
Joanna Briggs Institute critical appraisal checklist for cohort studies.
| Articles | Were the two groups similar and recruited from the same population? | Were the exposures measured similarly to assign people To both exposed and unexposed groups? | To both exposed and unexposed groups? | Was the exposure measured in a valid and reliable way? | Were confounding factors identified? | Were strategies to deal with confounding factors stated? | Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Were the outcomes measured in a valid and reliable way? | Was the follow up time reported and sufficient to be long enough for outcomes to occur? | Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | Were strategies to address incomplete follow up utilized? | Was appropriate statistical analysis used? | Score % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blank et al. 23 | N/A | N/A | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | 67 |
| Hanna et al. 24 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | 96 |
| Kelso et al. 25 | N/A | N/A | N/A | Yes | No | No | Unclear | Yes | Yes | Yes | Yes | Yes | 72 |
| Lazenby et al. 13 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 83 |
| Ludema et al. 26 | N/A | N/A | N/A | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 89 |
| McFall et al. 27 | N/A | N/A | N/A | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 89 |
| McKinney et al. 28 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100 |
| Mills et al. 29 | N/A | N/A | N/A | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 89 |
| Truong et al. 32 | N/A | N/A | N/A | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 78 |
| Yee et al. 35 | N/A | N/A | N/A | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | 78 |
Table 3.
Joanna Briggs Institute critical appraisal checklist for cross-sectional studies.
| Items | Okonsky et al. 30 | Turan et al. 33 | Wilson et al. 34 |
|---|---|---|---|
| Were the criteria for inclusion in the sample clearly defined? | Yes | Yes | Yes |
| Were the study subjects and the setting described in detail? | Yes | Yes | Yes |
| Was the exposure measured in a valid and reliable way? | Unclear | Yes | Yes |
| Were objective, standard criteria used for measurement of the condition? | Yes | Yes | Yes |
| Were confounding factors identified? | No | No | No |
| Were strategies to deal with confounding factors stated? | No | No | No |
| Were the outcomes measured in a valid and reliable way? | Yes | Yes | Yes |
| Was appropriate statistical analysis used? | Yes | Yes | Yes |
| Score | 69% | 75% | 75% |
Results
The literature search yielded 1359 articles (see Figure 1), in which 791 articles were identified as duplicates, resulting in 568 unique articles. All 568 articles were examined for eligibility based on title, abstract, and appropriateness for this review. A total of 467 articles were excluded for a total of 101 remaining articles. Three hand-searched articles were added for a new total of 104 articles. The full texts articles were reviewed, where 90 articles were excluded. A total of 14 articles were included in the review. Table 1 provides a summary of each article. Ten were cohort studies,13,23–29,32,35 three were cross-sectional design,30,31,33 and one had a longitudinal design. 34 Eight of the studies included adult women living with HIV regardless of demographic factors,24,26,27,29–31,33,34 four included only pregnant women,13,28,32,35 one included only African American women, 25 and one included women who identified with races or ethnicities other than Caucasian or White. 23 The sample sizes for the included studies ranged from 67 to 1727, and the study year was from 1989 to 2019 (see Table 1) as some studies were retrospective.13,24,26–28,32,35 Figure 2 shows the most commonly reported factors associated with viral suppression. For the purpose of this review, studies associated with each factor were described.
Figure 2.
Most commonly reported biological, behavioral, or psychosocial factors affecting viral suppression.
Behavioral- and psychological-related factors
Substance abuse
Substance abuse is defined as the use of cannabis, cocaine, methamphetamine, inhalants, hallucinogens, opioids, alcohol, or nicotine in excess of directions or for nonmedical use. 37 The use of substances was the most commonly reported behavioral factor negatively associated with viral suppression (see Figure 2). Four studies23,27,30,34 reported substance use as a factor negatively associated with viral suppression. Wilson et al. 34 found that women who were not heavy drinkers were more likely to be virally suppressed than heavy drinkers. Although Wilson and colleagues did not find a significant association between substance use and viral suppression, substance use was negatively related to ART adherence. 34 Among the 921 women of color (non-White or non-Caucasian) in the Blank et al. 23 study, substance use was associated with high viral load and lower HIV care retention. 23 McFall et al. 27 reported cigarette smoking and alcohol use were significantly associated with virologic failure, a condition when ART fails to suppress and keep the virus below 200 copies/mL. 34 In another study, perinatal HIV transmission was significantly associated with drug use. 13 However, drug use was reportedly higher among women who resided in urban regions compared with women in rural regions. 13
Depression
Depression and other mental health-related conditions were negatively associated with viral suppression in two studies.28,29 Depression was as high as 46% among the 1491 women in Mills et al. 29 study. The odds of virologic failure, missed healthcare visits, and poor ART adherence was positively associated with longer time of depression. Mills and colleagues assessed levels of depression using the Center for Epidemiologic Study Depression scale where scores range from 0 to 60, with a score of 33 or more classified as fully depressive symptomology. 29 According to the Mills et al. 29 study, women who reported being depressed 25% of the time were 27% more likely to report less than 95% adherence to ART, 16% more likely to miss their HIV care appointments, and 8% more likely to experience virologic failure than women who reported not being depressed. 29 Furthermore, ART adherence below 95% mediated the relationship between the percent of days depressed and virologic failure. 28
In contrast, experiencing 2 weeks or more of mentally unhealthy periods were positively associated with higher HIV care retention. 23 Wilson et al. 34 however, found no significant association between depression and viral suppression but reported a negative relationship between depression and ART adherence. 34
Social-related factors
Income and health insurance
Financial constraints and lack of health insurance were also reported as significant factors negatively associated with viral suppression in three studies (see Figure 2).26,27,34 These studies recruited participants from the same cohort, the Women’s Interagency HIV Study (WIHS). High income and private health insurance were significantly associated with viral suppression. One of the studies 27 found annual income levels below $24,000 was negatively associated with virologic failure. 27 In addition, two studies27,26 reported on women who participated in the AIDS Drugs Assisted Program (ADAP), a federal ART subsidy program based upon income thresholds. Women who participated in ADAP were significantly more likely to experience viral suppression than women with similar income but no ADAP participation. Also, women who had no health insurance but participated in ADAP were more likely to be virally suppressed than women with no health insurance and no ADAP.27,26
Intimate partner violence
Any form of physical or sexual violence, sexual threats, stalking, or aggression on either a former or current intimate partner is considered intimate partner violence (IPV). 35 IPV was positively associated with significantly increased viral loads in two studies (see Table 1),31,35 and associated with an increased risk of vertical transmission of HIV. 35 Women who experienced IPV during pregnancy took two times longer to achieve viral suppression and become virally stable than women who did not experience IPV during pregnancy. 35 Similarly, more than half the 272 women in Trimble et al. 31 study reported experiencing IPV in the last 12 months and 65% of those who experienced IPV had significantly higher (p < .001) viral loads than women who did not experience IPV in the last 12 months. 31 Turan et al. 33 examined the association of attachment-related avoidance and attachment-related anxiety on HIV-markers (i.e. viral load, ART adherence, and CD4 counts). Attachment-related avoidance was defined as avoiding intimacy with others and avoiding negative emotions, while attachment-related anxiety feeling was defined as the inability to handle stress in the absence of others. 33 Attachment-related avoidance was significantly positively associated with virologic failure, CD4 count, and ART adherence. Also, ART adherence mediated the relationship between attachment-related avoidance and viral failure and CD4 count. In contrast, attachment-related anxiety was a predictor for missed HIV care visits but not associated with virologic failure. 33
Race, despite being identified and controlled for by most of the studies, showed a mixed impact. For instance, being African American was a significant predictor for virologic failure compared to Hispanic and White/Caucasian women. 27 After adjusting for confounders (i.e. age, AIDS history, and time when ART was initiated), race was no longer significantly associated with virologic failure. 27 Even though, between 2007 and 2010, the absolute difference in the proportion of women with virologic failure was 11% higher among African American women when compared with White/Caucasian women and 5% higher when compared to Hispanic women. 27 The only study included in this review that examined African American women 25 exclusively reported that women who perceived both high racial discrimination and critical consciousness (i.e. way of coping with stress) were significantly more likely to have undetectable viral loads and more likely to have CD4 above 350 cells/mm3 when compared to African American women who perceived high racial discrimination but had low critical consciousness. 25 Furthermore, African American women were also more likely to be virally suppressed than other women of color in the Blank et al. 23 study.
Biological-related factors
HIV treatment regimen
HIV treatment regimens were associated with viral loads and ART adherence, as reported by three studies (see Figure 2).13,24,30 One 24 of these studies reported that protease inhibitor-based regimens were associated with high viral loads, low CD4 count, and incidence of AIDS. Another study reported that women on protease inhibitor-based regimens were less likely to be virally suppressed than those not on protease inhibitor-based regimens. 30 As protease inhibitors are often used as second line or salvage therapy, there may be a bias toward individuals with a history of non-suppression utilizing protease inhibitor therapy and comparisons to other classes of ART should be viewed with this regard. Hanna et al. 24 examined a cohort of 1727 women on a single-tablet HIV treatment regimen over a 7-year period. They reported an increased adherence rate and viral suppression from 78% to 85% among the participants on single-tablet regimen. Although not statistically significant, a single-tablet regimen was associated with improved quality of life and lower incidence of AIDS compared with those on multiple tablet regimens. 24 Similarly, Lazenby et al. 13 examined women on single or multiple HIV treatment regimens and found that those on three or more had higher viral loads than women on fewer.
Age was another common confounder controlled for by most of the studies. Despite being identified as a confounder, two studies found increased age23,34 negatively associated with viral suppression.
Pregnant women-specific factors
Four of the 14 articles examined factors affecting viral suppression during pregnancy and post-partum (see Table 1).13,28,32,35All four articles reported being in HIV care; specifically, early initiation of HIV care as a factor associated with viral suppression during delivery and post-partum. Also, early engagement in HIV care during pregnancy was found to be associated with HIV care retention post-partum. 28 However, one of the studies 32 found the post-partum period to be positively associated with high viral load regardless of ART due to immune activation. Truong et al. 32 specifically found continued use of zidovudine and ART post-partum were positively associated with an increase in viral loads. 32 One 28 of the studies and the only interventional study included in this review reported that group prenatal care was significantly associated with viral suppression at the time of delivery and up to 12 months retention in HIV care postdelivery. 28
Findings from another study 13 reported location as a predictor for viral loads. Women in rural regions were less likely to be virally suppressed compared with women in urban regions. 13 Although, this was reported by one of the studies that examined 666 pregnant women (see Table 1). 13
Discussion
We examined a total of 14 articles to identify and synthesize factors associated with viral suppression in women living with HIV in the United States. These factors were psychologically, socially, and biologically related, while some were specific among pregnant women. The most commonly reported factors negatively associated with viral suppression were substance use, suboptimal or late pre- or post-partum HIV care. Lack of health insurance, financial constraint, and complexity of HIV treatment regimen were the next most common set of factors, followed by IPV, depression, location, age, and race.
This review was the first to report on factors associated with viral suppression among women of all races living with HIV in the United States. Although a previous systematic review 19 included three25,27,33 of the 14 articles in this review, it primarily focused on adherence and retention among African American women. Substance use was the most common (4 out of the 14 studies) reported significant factor negatively associated with viral suppression, found among pregnant and nonpregnant women. Consistent with another systematic review, 38 substance use was significantly associated with ART adherence and viral suppression gaps among female sex workers living with HIV in sub-Saharan Africa. 38
Findings from all four studies that recruited pregnant women were consistent with another systematic review. These four studies found late HIV care engagement pre- and post-partum negatively associated with viral suppression. Similarly, late pre- and postnatal HIV care were negatively associated with ART adherence and viral suppression among pregnant women in Africa. 39 However, this was linked to stigma and discrimination. 39
History and experience of IPV was a common factor that was negatively associated with viral suppression among pregnant and nonpregnant women. The findings from this review on IPV corroborate other systematic reviews.39,40 One systematic review reported IPV as a significant factor associated with lower odds of viral suppression and poor ART adherence in women. 40 Also, IPV and financial constraints were strong factors associated with ART adherence during pregnancy in another review. 39
Of note, half of the 14 studies included in this review (see Table 1) recruited participants from the WIHS, the largest multicenter prospective observational study of HIV-infected and noninfected women in the United States. 41 The WIHS study sites are located in big cities across the United States suggesting less research focus on women living with HIV in rural regions. In fact, only one 13 of the 14 studies in this review focused on women in the rural U.S. Women in the WIHS might not be generalizable or represent the entire U.S. population of women living with HIV, highlighting the need for more studies in the rural United States. In addition, the current state of HIV research among women living with HIV in the United States reveals a lack of interventional studies in this area. Of the 14 studies reviewed, only one 28 study was interventional, suggesting directions for future interventional studies.
There were inconsistencies in viral suppression criteria or viral load cut-offs across all studies despite CDC’s recommended < 200 copies/mL of HIV RNA in the blood. 8 This may have been related to differences in testing capabilities at the time of study conduct. For example, most U.S. laboratories currently utilize tests with detection limits of 50 or even 20 copies per mL. Many clinics now use these thresholds as limits for “undetectable” viral load. The differences in detection limits of assays can make it difficult to compare groups utilizing different cut-offs. However, for the purpose of this review, classification of viral suppression was unlikely to be influenced by virologic blips in individuals utilizing assays with lower limits of detection.
Transgender people make up a significant part of the U.S. population, with about 1 million individuals identifying as transgender in the United States in 2018. 42 Also, in 2018, transgender women accounted for 92% of new HIV diagnoses among transgender people in the United States. 42 A more recent survey of transgender women by the CDC in 2020 revealed that four out of ten transgender women had HIV. 43 Despite the burden of HIV among transgender women, there is a paucity of data about this population. 44 For instance, only one transgender women U.S. study 7 with a focus on this research area was found during the literature search. The study was excluded due to limited data on levels in gender transition (such as hormone, surgery, or full transition) and factors associated with viral suppression in different stages in gender transition. Furthermore, factors associated with viral suppression among transgender and cisgender women may differ. In the Swiss HIV Cohort Study, for example, transgender women experienced twice the rates of previous psychological-related hospitalization than cisgender women (11.2% versus 5.1%). 44 However, there are limited studies in this area. Hence, the state of science on transgender women underscores the need for research to focus on HIV prevention and care among this population.
There are limitations in this review. First, recent studies related to this topic might have been missed during our search and omitted in this review. Second, articles were limited to English-language articles, which might have prevented us from capturing relevant Spanish studies as Hispanics and Latinos make up 29% of people living with HIV in the United States and 18.5% of the national population.45,46 Third, factors associated with viral suppression in women might have been missed by excluding studies that recruited females and other genders.
This review highlights the importance of considering substance use, pre- and post-partum HIV care, health insurance, and IPV on viral suppression, which is corroborated in previous reviews. Most of the included studies were cross-sectional; only one was interventional. Future interventional studies in this area are needed to determine the causation between viral suppression and the factors identified in this review. In addition, we recommend future HIV research compare urban and rural regions in the United States as most of the studies included in this review were conducted in densely populated, urban areas. Finally, there are limited HIV research data on transgender women, making it difficult to draw a conclusion on HIV among this population. Of the 568 articles retrieved during the database search for this review, only one study 7 exclusively focused on this area of HIV research among transgender women in the United States. More HIV studies on transgender women are needed for sufficient data for future HIV prevention and management research among this population.
Conclusion
Included in this review is a synthesis of factors associated with viral suppression among women living with HIV in the United States. Although viral load cut-offs were inconsistent across all studies, substance use, complexity of HIV treatment regimen, lack of health insurance, financial constraints, suboptimal or late pre- and post-partum HIV care, IPV, younger age, depression, and belonging to racial or ethnic minority groups were the most commonly reported factors negatively associated with viral suppression. It is strongly recommended that future interventional studies are designed to address the aforementioned factors among women living with HIV in the United States, particularly in rural regions.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057221092267 for Factors associated with viral suppression among cisgender women living with human immunodeficiency virus in the United States: An integrative review by Titilola O Labisi, Anthony T Podany, Nada A Fadul, Jason D Coleman and Keyonna M King in Women’s Health
Acknowledgments
The authors acknowledge the support of the following individuals: Myra Schmaderer, PhD; Robin Lally, PhD; and Kimberly Harp, MLS, and thank them for their contributions by supervising, editing, and providing feedback on this review.
Footnotes
Author contribution(s): Titilola O Labisi: Conceptualization; Data curation; Formal analysis; Methodology; Software; Validation; Visualization; Writing – original draft.
Anthony T Podany: Conceptualization; Methodology; Supervision; Validation; Visualization; Writing – review and editing.
Nada A Fadul: Conceptualization; Investigation; Supervision; Visualization; Writing – review and editing.
Jason D Coleman: Conceptualization; Methodology; Supervision; Visualization; Writing – review and editing.
Keyonna M King: Conceptualization; Methodology; Validation; Visualization; Writing – original draft; Writing – review and editing.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Titilola O Labisi  https://orcid.org/0000-0002-0379-557X
https://orcid.org/0000-0002-0379-557X
Supplemental material: Supplemental material for this article is available online.
References
- 1. HIV gov. Global statistics, https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics (2021, accessed September 2021).
- 2. Waldron EM, Burnett-Zeigler I, Wee V, et al. Mental health in women living with HIV: the unique and unmet needs. J Int Assoc Provid AIDS Care 2021; 20: 2325958220985665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Healthy People 2020. HIV, https://www.healthypeople.gov/2020/topicsobjectives/topic/hiv (2020, accessed 23 September 2021).
- 4. Kaiser Family Foundation. Women and HIV in the United States, https://www.kff.org/hivaids/fact-sheet/women-and-hivaids-in-the-united-states/ (2020, accessed 23 September 2021).
- 5. Centers for Disease Control Prevention. HIV and women https://www.cdc.gov/hiv/group/gender/women/index.html (2021, accessed 23 September 2021).
- 6. Centers for Disease Control Prevention. CDC fact sheet HIV incidence: Estimated annual infections in the U.S. 2014–2018, https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf (2020, accessed 23 September 2021).
- 7. Sevelius JM, Saberi P, Johnson MO. Correlates of antiretroviral adherence and viral load among transgender women living with HIV. AIDS Care 2014; 26(8): 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. United Nations Programme on HIV and AIDS. Testing viral suppression, https://www.unaids.org/en/resources/presscentre/featurestories/2016/november/20161114_viral-loa (2016, accessed 23 September 2021).
- 9. Centers for Disease Control Prevention. HIV treatment as prevention: Overview, https://www.cdc.gov/hiv/risk/art/index.html#:~:text=This%20is%20called%20viral%20suppression, called%20an%20undetectable%20viral%20load (2021, accessed 23 September 2021).
- 10. Centers for Disease Control Prevention. Evidence of HIV treatment and viral suppression in preventing the sexual transmission of HIV, https://www.cdc.gov/hiv/pdf/risk/art/cdc-hiv-art-viral-suppression.pdf (2020, accessed 23 September 2021).
- 11. Fox MP, Pascoe S, Huber AN, et al. Adherence clubs and decentralized medication delivery to support patient retention and sustained viral suppression in care: Results from a cluster-randomized evaluation of differentiated ART delivery models in South Africa. PLoS Med 2019; 16(7): e1002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chagomerana MB, Miller WC, Tang JH, et al. Optimizing prevention of HIV mother to child transmission: duration of antiretroviral therapy and viral suppression at delivery among pregnant Malawian women. PLoS ONE 2018; 13(4): e0195033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lazenby GB, Powell AM, Sullivan SA, et al. The impact of delivery in a rural county on a cohort of women living with HIV infection and their infants. J Rural Health 2019; 35(3): 319–329. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control Prevention. HIV and women: viral suppression, https://www.cdc.gov/hiv/group/gender/women/viral-suppression.html (2021, accessed 23 September 2021).
- 15. Haider MR, Brown MJ, Harrison S, et al. Sociodemographic factors affecting viral load suppression among people living with HIV in South Carolina. AIDS Care 2021; 33(3): 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thakarar K, Morgan JR, Gaeta JM, et al. Homelessness, HIV, and incomplete viral suppression. J Health Care Poor Underserved 2016; 27(1): 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kunzweiler CP, Bailey RC, Mehta SD, et al. Factors associated with viral suppression among HIV-positive Kenyan gay and bisexual men who have sex with men. AIDS Care 2018; 30(suppl. 5): S76–S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chesney MA. Factors affecting adherence to antiretroviral therapy. Clin Infect Dis 2000; 30(Suppl. 2): S171–S176. [DOI] [PubMed] [Google Scholar]
- 19. Lambert CC, Mugavero MJ, Najjar YS, et al. The state of adherence to HIV care in black women. J Assoc Nurses AIDS Care 2018; 29(4): 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs 2005; 52(5): 546–553. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210–016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blank AE, Fletcher J, Verdecias N, et al. Factors associated with retention and viral suppression among a cohort of HIV+ women of color. AIDS Patient Care STDS 2015; 29(Suppl. 1): S27–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr 2014; 65: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelso GA, Cohen MH, Weber KM, et al. Critical consciousness, racial and gender discrimination, and HIV disease markers in African American women with HIV. AIDS Behav 2014; 18(7): 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ludema C, Cole SR, Eron JJ, et al. Impact of Health Insurance, ADAP, and Income on HIV viral suppression among US Women in the Women’s Interagency HIV Study, 2006-2009. J Acquir Immune Defic Syndr 2016; 73: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McFall AM, Dowdy DW, Zelaya CE, et al. Understanding the disparity: predictors of virologic failure in women using highly active antiretroviral therapy vary by race and/or ethnicity. J Acquir Immune Defic Syndr 2013; 64: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKinney J, Jackson J, Sangi-Haghpeykar H, et al. HIV-adapted group prenatal care: assessing viral suppression and postpartum retention in care. AIDS Patient Care STDS 2021; 35(2): 39–46. [DOI] [PubMed] [Google Scholar]
- 29. Mills JC, Pence BW, Edmonds A, et al. The impact of cumulative depression along the HIV care continuum in women living with HIV during the era of universal antiretroviral treatment. J Acquir Immune Defic Syndr 2019; 82: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okonsky JG, Webel A, Rose CD, et al. Appreciating reasons for nonadherence in women. Health Care Women Int 2015; 36(9): 1007–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trimble DD, Nava A, McFarlane J. Intimate partner violence and antiretroviral adherence among women receiving care in an urban Southeastern Texas HIV clinic. J Assoc Nurses AIDS Care 2013; 24(4): 331–340. [DOI] [PubMed] [Google Scholar]
- 32. Truong HM, Sim MS, Dillon M, et al. Correlation of immune activation during late pregnancy and early postpartum with increases in plasma HIV RNA, CD4/CD8 T cells, and serum activation markers. Clin Vaccine Immunol 2010; 17(12): 2024–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turan B, Crockett KB, Kempf MC, et al. Internal working models of attachment relationships and HIV outcomes among women living with HIV. J Acquir Immune Defic Syndr 2019; 80: e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson TE, Kay ES, Turan B, et al. Healthcare empowerment and HIV viral control: mediating roles of adherence and retention in care. Am J Prev Med 2018; 54(6): 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yee LM, Crisham Janik M, Dorman RM, et al. Relationship between intimate partner violence and antiretroviral adherence and viral suppression in pregnancy. Sex Reprod Healthc 2018; 17: 7–11. [DOI] [PubMed] [Google Scholar]
- 36. Joanna Briggs Institute. Critical Quality Appraisal tools, https://jbi.global/critical-appraisal-tools (accessed 1 August 2021).
- 37. National Institute on Drug abuse. Screening for drug use in general medical settings: Quick reference, https://www.drugabuse.gov/sites/default/files/pdf/screening_qr.pdf. (2011, accessed 4 September 2021).
- 38. Lancaster KE, Cernigliaro D, Zulliger R, et al. HIV care and treatment experiences among female sex workers living with HIV in sub-Saharan Africa: a systematic review. Afr J AIDS Res 2016; 15(4): 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Omonaiye O, Kusljic S, Nicholson P, et al. Medication adherence in pregnant women with human immunodeficiency virus receiving antiretroviral therapy in sub-Saharan Africa: a systematic review. BMC Public Health 2018; 18: 805–018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hatcher AM, Smout EM, Turan JM, et al. Intimate partner violence and engagement in HIV care and treatment among women: a systematic review and meta-analysis. AIDS 2015; 29: 2183–2194. [DOI] [PubMed] [Google Scholar]
- 41. Adimora AA, Ramirez C, Benning L, et al. Cohort profile: the women’s interagency HIV study (WIHS). Int J Epidemiol 2018; 47: 393–394; i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Centers for Disease Control Prevention. HIV and transgender people, https://www.cdc.gov/hiv/group/gender/transgender/index.html (2021, accessed 23 September 2021).
- 43. Centers for Disease Control Prevention. Transgender women urgently need more HIV prevention and treatment services, new CDC data Show, https://www.cdc.gov/media/releases/2021/p0414-trans-HIV.html (2021, accessed 14 September 2021).
- 44. Nguyen H, Hampel B, Nuñez DG, et al. Identifying and characterizing Trans women in the Swiss HIV cohort study as an epidemiologically distinct Risk Group. Clin Infect Dis 2021; 2021: ciab628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. United States Census Bureau. QuickFacts United states, https://www.census.gov/quickfacts/fact/table/US/RHI725219 (2021, accessed 10 September 2021).
- 46. Centers for Disease Control Prevention. HIV and Hispanic/Latino people: HIV incidence, https://www.cdc.gov/hiv/group/racialethnic/hispanic-latino/incidence.html (2021, accessed 4 January 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057221092267 for Factors associated with viral suppression among cisgender women living with human immunodeficiency virus in the United States: An integrative review by Titilola O Labisi, Anthony T Podany, Nada A Fadul, Jason D Coleman and Keyonna M King in Women’s Health