Abstract
An in vitro cell culture model was used to investigate the long-term effect of ciprofloxacin and ofloxacin on infection with Chlamydia trachomatis. Standard in vitro susceptibility testing clearly indicated successful suppression of chlamydial growth. To mimic better in vivo infection conditions, extended treatment with the drugs was started after infection in vitro had been well established. Incubation of such established chlamydial cultures with ciprofloxacin and ofloxacin not only failed to eradicate the organism from host cells, but rather induced a state of chlamydial persistence. This state was characterized by the presence of nonculturable, but fully viable, bacteria and the development of aberrant inclusions. In addition chlamydia exhibited altered steady-state levels of key chlamydial antigens, with significantly reduced major outer membrane protein and near constant hsp60 levels. Resumption of overt chlamydial growth occurred after withdrawal of ciprofloxacin, confirming the viability of persisting chlamydia. In vitro ciprofloxacin results are consistent with clinical data, thereby providing an explanation for treatment failures of ciprofloxacin. Parallel in vitro studies with ofloxacin suggest a better correlation between clinical and laboratory-defined efficacy, although the clinical studies on which this assessment is based did not include monitoring of chlamydial persistence. The data presented here clearly demonstrate that under at least some circumstances, standard determination of MICs and minimal bactericidal concentrations for C. trachomatis allows no more than a simple definition of whether an antibiotic has some anti chlamydial activity; however, such testing is not always sufficient to verify that the antibiotic will eliminate the organism in vivo.
Chlamydia trachomatis is an obligate intracellular bacterial parasite whose life cycle involves alternation between the infectious extracellular, metabolically inactive elementary body (EB) form and the intracellular, metabolically active reticulate body form; the latter is the vegetative growth stage of the organism. Depending on the specific serovar involved, human infection with C. trachomatis causes a variety of ocular, pulmonary, and genital diseases. Genital infection with chlamydial serovars D to K is considered to be of major public health importance, since C. trachomatis is the most common sexually transmitted bacterium worldwide (54). Further, acute urogenital infections can progress to persistent infection, which in turn may initiate a pathogenic process leading to chronic diseases, including pelvic inflammatory disease, ectopic pregnancy, tubal factor infertility, and chlamydia-induced arthritis (12, 53). Importantly, C. trachomatis has been shown to be fully viable and metabolically active in both the acute and chronic, persistent infection state. In acute infections, the bacterium can be recovered usually by standard laboratory culture. Chronic chlamydial infections are often characterized by culture negativity, although viability has been demonstrated in this state also. This was shown by detection of unprocessed rRNA transcripts and mRNA from chlamydial genes in synovial tissue of patients with reactive arthritis and tubal specimens from women with tubal factor infertility (20, 21, 32).
The antimicrobial activity of antibiotics against chlamydia or any other organism is usually verified by determination of the MIC and minimal bactericidal concentration (MBC). For C. trachomatis, such in vitro susceptibility testing indicated good activity for ciprofloxacin (22, 38, 45, 46, 49). However, in clinical trials a high rate of treatment failure has been observed with this drug. For example, in the therapy of acute urogenital infections it was shown that C. trachomatis can persist after ciprofloxacin treatment and can result in recurrent infections (23, 52). Ciprofloxacin also has been used in the treatment of chronic reactive arthritis and undifferentiated arthritis, including chlamydia-induced arthritis, but no evidence favoring prolonged use of ciprofloxacin in the latter disease has been forthcoming (48, 51). These and other clinical studies thus suggest that determination of the MIC or MBC may not by itself be a sufficiently accurate predictor for complete eradication of C. trachomatis from any given site of infection.
To mimic in vivo condition long-term incubation of an established in vitro infection of C. trachomatis was done to investigate the antibacterial efficacy of ciprofloxacin. Ofloxacin, an antibiotic with better efficacy in clinical trials than ciprofloxacin, was studied for comparison. In the present work, we demonstrate a significant discrepancy between in vitro susceptibility testing and long-term in vitro treatment for ciprofloxacin and ofloxacin on C. trachomatis. Treatment with either antibiotic is capable of inducing persistent chlamydial infection characterized by the presence of viable, metabolically active organisms.
MATERIALS AND METHODS
Cells.
HEp-2 cells, a human laryngial epidermoid cell line (obtained from the American Type Culture Collection) were maintained at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 10% fetal calf serum (Vitromex, Berlin, Germany), 1% l-glutamine, and 100 μg of gentamicin (Biochrom, Berlin, Germany) per ml.
Growth, purification, and titration of C. trachomatis.
C. trachomatis serovar K/UW-31/Cx (obtained from the Washington Research Foundation, Seattle) was cultured in HEp-2 cells as described (31). Briefly, 48 h postinfection, chlamydia were harvested, purified on a discontinuous renografin gradient (Schering, Berlin, Germany) (9), resuspended in SPG buffer (0.01 M sodium phosphate, pH 7.2; 0.25 M sucrose; 5 mM l-glutamic acid), and stored at −80°C. Infectivity of chlamydia was expressed as inclusion-forming units (IFU) per milliliter.
Determination of MIC and MBC.
Monolayers of HEp-2 cells cultured in antibiotic-free medium were inoculated at a multiplicity of infection (MOI) of 0.05, centrifuged for 20 min at 500 × g, and incubated for 2 h at 37°C. Cells were then washed three times with Hanks' balanced salt solution (HBSS) and overlaid with medium containing 1.0 μg of cycloheximide/ml and various concentrations of ciprofloxacin or ofloxacin. The MIC is defined as the lowest antibiotic concentration required to inhibit development of chlamydial inclusions after 48 h of incubation. Inclusions were visualized by staining with fluorescein-conjugated antibody directed against major outer membrane protein (MOMP) (Syva, Microtrak, Palo Alto, Calif.). The MBC is defined as the lowest concentration of antibiotic necessary to inhibit infectivity as measured by inclusion development after two passages on HEp-2 cells. Harvested cell lysates from monolayers incubated in the presence of the drug were passaged onto antibiotic-free HEp-2 cell monolayers in 96-well microtiter plates. After 48 h, one set of plates for each concentration was used for detection of inclusions by an immunoperoxidase assay (see below). The other set was washed twice in HBSS and frozen at 80°C. These plates were then thawed and inoculated onto fresh HEp-2 cell monolayers and incubated for 48 h, with subsequent staining of chlamydial inclusions.
Infection and antibiotic treatment of cells.
Antibiotic-free HEp-2 cells were seeded into six-well plates. Nearly confluent monolayers were then inoculated with C. trachomatis EBs (MOI = 0.05). Cells were centrifuged for 20 min at 500 × g at room temperature. After 2 h of incubation at 37°C, the inoculum was removed and cells were washed three times with HBSS. Further cultivation was done in antibiotic-free medium containing 0.5 to 1.0 μg of cycloheximide/ml. Two to three days postinfection, ciprofloxacin or ofloxacin was added to infected cell cultures. Medium was replaced every second day. Incubation with the drug was continuous or was stopped at the times indicated below. Infected cells were harvested as indicated over a culture period of 20 or 25 days.
Immunofluorescence assays.
Harvested cells were cytocentrifuged (Cytospin; Shandon) and fixed for 10 min in 100% methanol. Visualization of inclusions was done by staining with anti-MOMP or -hsp60-specific antibodies. The anti-MOMP antibody used was a fluorescein-conjugated murine monoclonal antibody directed against a common epitope of chlamydial EB and reticulate body (Syva-Microtrak). hsp60 was stained via an indirect immunofluorescence assay using the anti-hsp60 antibody GP 57-19 as the primary antibody (kindly provided by R. P. Morrison, Hamilton, Mont.); the secondary antibody was a fluorescein isothiocy-anate-labeled goat anti-mouse immunoglobulin G in 1:50 dilution (Dako, Hamburg, Germany). All samples were viewed using an epifluorescence microscope (Leitz, Wetzlar, Germany). Inclusions were counted and expressed as number of inclusion bodies per 105 host cells.
Detection of infectious yields of chlamydia.
Chlamydial infectivity was determined by titration of cell lysates on confluent HEp-2 monolayers (47). After 48 h of incubation inclusions were visualized by an immunoperoxidase assay as described by Nettelnbreker et al. (39). The number of inclusions was expressed as IFU per 105 cells.
SDS-PAGE and immunoblotting.
Protein content of harvested cells was determined by Micro Bradford assay (Bio-Rad, Munich, Germany), using bovine serum albumin as a standard. Samples of 50 μg of total protein were solubilized by boiling in Laemmli sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12 or 15% acrylamide) (34). Separated proteins were transferred electrophoretically to polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). After blocking in nonfat dried milk powder in phospate-buffered saline or Roti-Block (Roth, Karlsruhe, Germany), blots were probed with either anti-hsp60 (GP 57-19), anti-MOMP (LV-21) or antilipopolysaccharide (anti-LPS) (S 25-23; kindly provided by H. Brade, Borstel, Germany) antibodies. Antibody bound to chlamydial antigens was detected using alkaline phosphatase-conjugated rabbit or goat anti-mouse immunoglobulin G (Dianova, Hamburg, Germany) and subsequent staining with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (Sigma, Deisenhofen, Germany).
RT-PCR analysis.
Infected and uninfected cells were harvested by centrifugation, washed twice with HBSS, snap-frozen in liquid N2, and stored at −80°C until use. Total RNA was extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Prior to reverse transcription (RT) reactions, RNA was treated with RNase-free DNase I (Life Technologies, Gaithersburg, Md.). For RT 1 μg total RNA was incubated with 200 U of murine leukemia virus reverse transcriptase (Life Technologies) or Superscript II RNase H− reverse trancriptase (Life Technologies) and 100 pmol of downstream primer, using the buffers and conditions specified by the manufacturer. Amplification of cDNA was carried out in a total volume of 100 μl as described, using the primers described (19). Amplification was performed in a Perkin-Elmer 9600 thermocycler, and amplification products were visualized on standard agarose electrophoretic gels stained with ethidium bromide.
RESULTS
Determination of MIC and MBC for ciprofloxacin and ofloxacin on C. trachomatis.
The MIC, defined as the lowest concentration of the antibiotic required to inhibit chlamydial inclusion formation, and the MBC, the lowest concentration which prevents formation of inclusion bodies after two passages on fresh HEp-2 monolayers, were both determined to be 0.35 μg/ml for ciprofloxacin and 0.5 μg/ml for ofloxacin.
Effect of ciprofloxacin on growth of C. trachomatis.
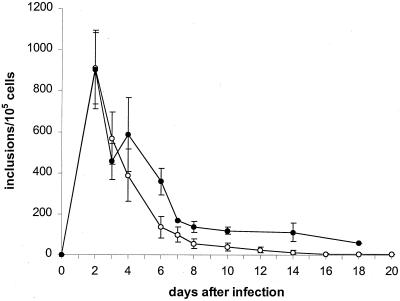
Inoculation of HEp-2 cells with EB at an MOI of 0.05 resulted in complete destruction of the cell monolayer in 10 to 12 days. To simulate the clinical situation more closely, antibiotic treatment was started after chlamydia infection of HEp-2 cell monolayers had been established. That is, ciprofloxacin was added 48 or 72 h postinfection at a concentration of 0.5 μg/ml. The effect of antibiotic treatment on chlamydial growth was assessed by determination of the yield of infectious organism and by the presence of inclusions. The data in Fig. 1 show the influence of ciprofloxacin (0.5 μg/ml) given 2 or 3 days postinfection on infectious progeny. Addition of the antibiotic at 2 days led to a continuous decrease of infectivity, and such treatment resulted in loss of infectivity after 8 days. At 2 days after infection, inclusions had developed in about 0.9 % of host cells, and this decreased by about 85% during the first 6 days of incubation (Fig. 2). Further treatment provided only a slight increase in inhibitory effect. At 20 days following infection, single inclusions were still detectable in 0.003% of host cells, but these were of a distinctly smaller size than those seen in untreated cells. Additionally, a considerable number of extremely small inclusions was observed during incubation with ciprofloxacin; these were present throughout the culture period, although their numbers also decreased. While infectious chlamydiae could not be recovered from cultures at 10 days postinfection, inclusions were present during the entire culture period.
FIG. 1.
Effect of ciprofloxacin (0.5 μg/ml) on infectious chlamydial progeny in HEp-2 cells. HEp-2 cells were infected at an MOI of 0.05. Incubation with ciprofloxacin (0.5μg/ml) was started 2 (○) and 3 (●) days after infection. Data presented are the means ± standard deviations (error bars) of eight (○) and four (●) experiments.
FIG. 2.
Effect of ciprofloxacin (0.5 μg/ml) on chlamydial inclusions in HEp-2 cells. HEp-2 cells were infected at an MOI of 0.05. Incubation with ciprofloxacin (0.5 μg/ml) was started 2 (○) and 3 (●) days after infection. The figure does not include numbers of atypical inclusions. Data presented are the means ± standard deviations (error bars) of 10 (○) and 5 (●) experiments.
For treatment with ciprofloxacin at 3 days postinfection, the length of the chlamydial growth cycle varied from 48 h to more than 72 h; this was demonstrated by a 50% decrease in and concurrent enlargement of inclusions, as well as an increase of infectious progeny. Despite addition of the drug 3 days postinfection, inclusion number increased by approximately 28% at 4 days postinfection (Fig. 2). Hence, the rate of infection of HEp-2 cells was at a higher level overall when antibiotic treatment was started at 3 days postinfection than in cells treated with ciprofloxacin from 2 days after infection. This difference was maintained over the entire incubation period. At 18 days after infection, inclusions were still detectable in 0.05% of cells, whereas cells treated from 2 days postinfection showed only a 0.004% infection rate. Further experiments were done to investigate whether a higher concentration of this drug would result in more efficient inhibition of chlamydial growth when added at 2 days postinfection; however, a 1.0-μg/ml concentration of ciprofloxacin produced the same effect on chlamydial growth as did the lower concentration. Infectious chlamydia were detectable for 8 days and the number of inclusions present in host cells 20 days after infection was identical at both high and low concentrations (data not shown).
Analysis of chlamydial antigens during treatment with ciprofloxacin.
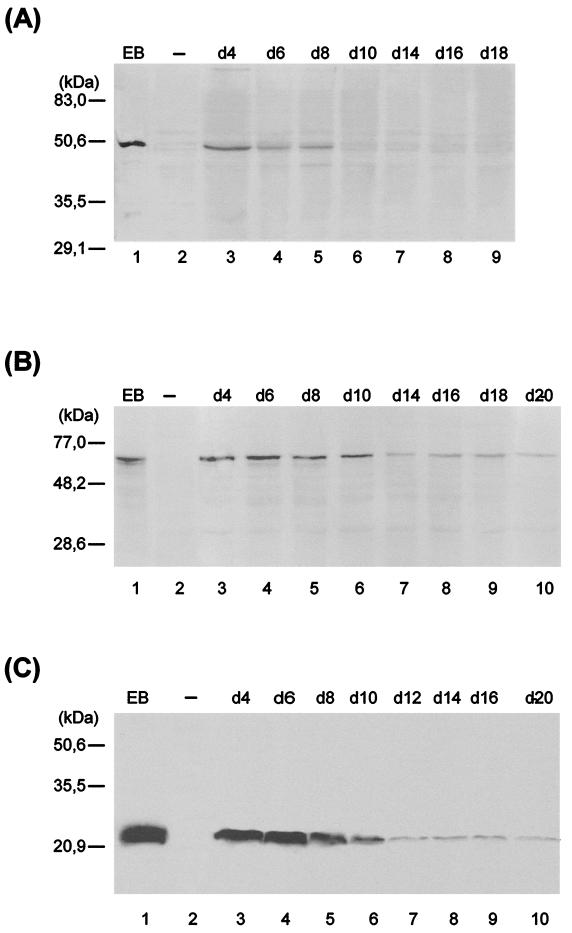
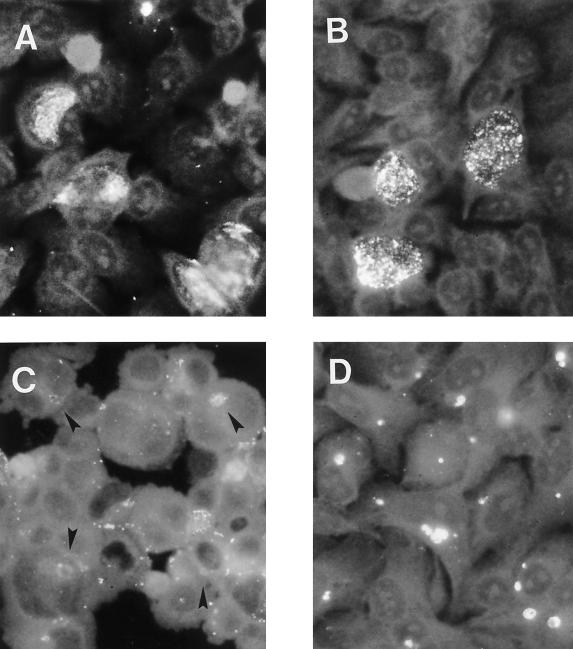
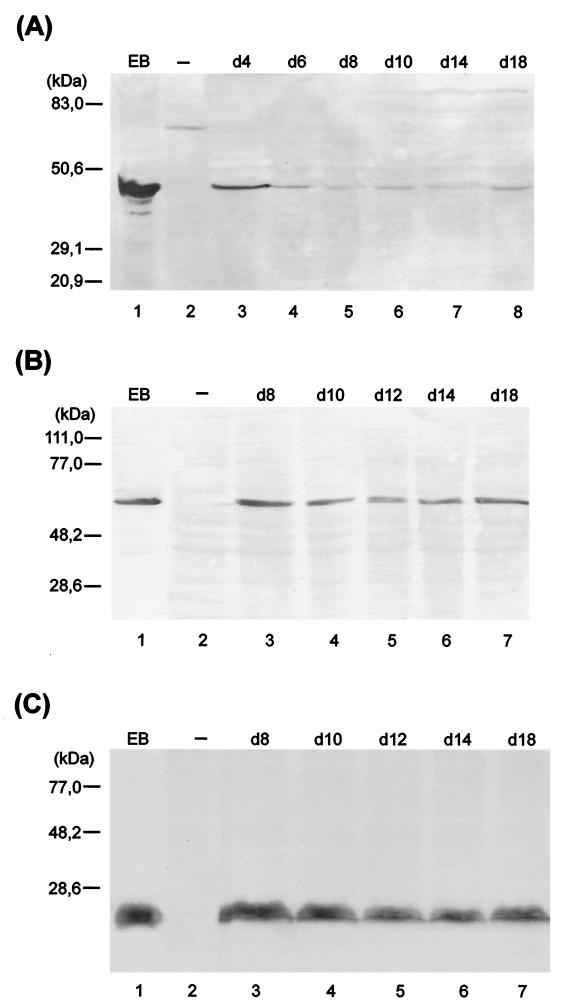
The effect of ciprofloxacin treatment during infection on levels of key chlamydial antigens was determined by imunoblotting and immunfluorescence analyses. The antigens assessed were MOMP, hsp60, and LPS. hsp60 and LPS have been implicated in strong elicitation of immunopathogenic reactions (26, 36, 37). MOMP, a major structural constituent of the chlamydial outer envelope, is thought to play a role in protective immunity (8, 14, 50, 55). Deviation from typical chlamydial growth upon ciprofloxacin treatment was associated also with alterations in steady-state levels of chlamydial antigens (Fig. 3). For example, a significant reduction of MOMP staining was observed, and no MOMP could be detected by immunoblotting from day 10 postinfection. hsp60 and LPS, however, were clearly demonstrable throughout the culture period of 20 days. The culture-negative state (between day 10 and 20 postinfection) was characterized by nearly constant levels of these two antigens. An increase of antibiotic concentration to 1.0 μg/ml did not alter these results (data not shown). To confirm differences in protein levels in untreated and ciprofloxacin-treated cells, immunofluorescence analyses were done. Normal inclusions stained with monoclonal antibodies targeting MOMP and hsp60 showed similar intensities of fluorescence (Fig. 4A and B). The atypical small inclusions found in cells treated with ciprofloxacin stained only weakly with anti-MOMP antibody (Fig. 4C), but these stained intensely with anti-hsp60 antibody (Fig. 4D). As mentioned, the number of such aberrant inclusions decreased during treatment with ciprofloxacin. Although this reduction was clear, atypical small inclusions were still present in host cells 20 days after infection. Cells incubated with 1.0 μg of ciprofloxacin per ml gave similar results (data not shown).
FIG. 3.
Immunoblot analysis of ciprofloxacin-treated HEp-2 cells with anti-MOMP (A), anti-hsp60 (B), and anti-LPS (C) antibodies. HEp-2 cells were infected at an MOI of 0.05 and treated with ciprofloxacin (0.5 μg/ml) starting 2 days postinfection. Lanes: 1, C. trachomatis serovar K EBs; 2, uninfected (−) cells; 3 to 10, chlamydia-infected cells treated with ciproflaxin (0.5 μg/ml) on the indicated day (d).
FIG. 4.
Immunofluorescent staining of untreated cells 2 days postinfection (A and B) and ciprofloxacin treated HEp-2 cells 8 days postinfection (C and D). Chlamydial inclusions were stained with either anti-MOMP (A and C) or anti-hsp60 (B and D) monoclonal antibodies. Arrowheads indicate atypical small inclusions. Magnification, ×1,300.
Chlamydial viability during treatment with ciprofloxacin.
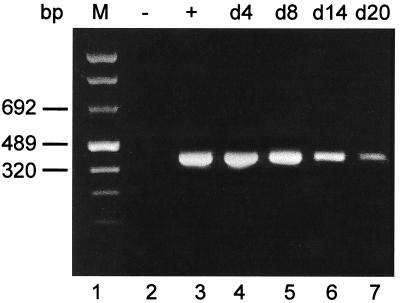
The failure to detect infectious organism and the presence of small aberrant inclusions together indicate an abrogation of or deviation from the typical intracellular developmental cycle of C. trachomatis. This state, however, does not necessarily exclude the viability of bacteria. Therefore, we used RT-PCR targeting unprocessed rRNA transcripts to investigate the metabolic state of intracellular chlamydiae during ciprofloxacin treatment of infected HEp-2 cells. Such transcripts are detectable only in viable, metabolically active organisms and are processed to functional 16S and 23S rRNA rapidly (3, 10, 11, 18, 29, 40); thus, their presence in a given preparation of total RNA from infected cells indicates the viability of the chlamydiae infecting those host cells (19). RT-PCR assays clearly demonstrated the continuous presence of unprocessed chlamydial primary rRNA gene transcripts throughout the culture period of 20 d, indicating viability of the organism despite ciprofloxacin treatment (Fig. 5). Treatment of infected cells with ciprofloxacin (1.0 μg/ml) did not result in more effective suppression of primary transcript production, consistent with data given above for infectivity, inclusion number, and chlamydial antigen presence (data not shown).
FIG. 5.
RT-PCR analysis of HEp-2 cells treated with ciprofloxacin. Cells were infected at an MOI of 0.05 and treated with ciprofloxacin (0.5 μg/ml) starting 2 days postinfection. RNA from cell pellets was prepared and analyzed by RT-PCR as described in Materials and Methods. Lanes: 1, marker; 2, uninfected cells; 3, control cells 2 days postinfection; 4 to 12, chlamydia-infected cells treated with ciprofloxacin on the indicated day (d).
Recovery of infectious chlamydiae after removal of ciprofloxacin.
We reasoned that if treatment with ciprofloxacin resulted in a persistent infection, then recovery of infectious chlamydiae would be expected upon removal of the drug from cultures. Therefore, infected HEp-2 cells were incubated with ciprofloxacin at a concentration of 0.5 μg/ml for various times; the start of drug treatment again was 2 days postinfection. When antibiotic-containing medium was replaced after 4 days with medium lacking ciprofloxacin, a rapid increase in chlamydial growth ensued. Two days after drug removal, inclusion numbers had increased by 50%, and infectious yield had increased by three- to four-fold. Longer cultivation resulted in destruction of host cell monolayers.
Treatment of infected cultures with ciprofloxacin for 6 days provided a different result. Although relatively few infectious chlamydiae were detectable at 8 days postinfection, removal of ciprofloxacin from the culture medium resulted in a constant, low level of replicative infection. Immunofluorescence demonstrated reorganization of aberrant inclusions to typical inclusions, and thus a slight increase in inclusion number (Fig. 6) and infectivity (Table 1) were noted after drug withdrawal. This low level of replicative infection was accompanied by constant levels of chlamydial antigens, as shown by immunoblotting (Fig. 7). In this situation, chlamydial MOMP was detectable, and the hsp60/MOMP ratio showed a predominance of hsp60. These results were confirmed by immunfluorescence staining; that is, the fluorescence level for small atypical inclusions was much more intense in cells stained with anti-hsp60 antibody than in those stained with the MOMP-specific antibody (data not shown).
FIG. 6.
Chlamydial inclusions in HEp-2 cells, treated with ciprofloxacin (0.5 μg/ml) for 6 days. Data presented are the means ± standard deviations (error bars) of five experiments.
TABLE 1.
Recovery of infectious chlamydiae from persistently infected HEp-2 cellsa
| Days after infection | Mean IFU/105 cells after treatment lasting:
|
||
|---|---|---|---|
| 6 days | 8 days | 12 days | |
| 8 | 100 ± 78 | 63 | 35 |
| 10 | 654 ± 310 | 0 | 0 |
| 12 | 2,491 ± 939 | 19 | 0 |
| 14 | 2,713 ± 1,819 | NDb | 0 |
| 16 | 3,268 ± 1,470 | 9 | ND |
| 18 | 5,225 ± 1,442 | 185 | 102 |
| 20 | 890 ± 812 | 243 | 107 |
Data presented are mean IFU per 105 cells determined in quadruplicate (6-day treatment) or duplicate experiments.
ND, not done.
FIG. 7.
Imunoblot analysis of HEp-2 cells treated for 6 days with ciprofloxacin (0.5 μg/ml). (A) MOMP; (B) hsp60; (C) LPS. Lanes: 1, C. trachomatis serovar K EBs; 2, uninfected cells; 3 to 8, chlamydia-infected cells treated for 6 days with ciprofloxacin (0.5 μg/ml). d, day.
At day 10 or 14 postinfection, infectious chlamydiae were no longer detectable. When ciprofloxacin was removed at these time points, infectious organisms were rescued within 2 days, although no development of typical inclusions was observed (Table 1). Further cultivation in antibiotic-free medium did not result in any significant increase of infectivity; rather, infectivity was maintained at a relatively constant level.
Effect of ofloxacin on chlamydial infection.
The effect of ofloxacin was examined on in vitro chlamydial infection in order to compare the effect of this antibiotic with that of ciprofloxacin. The latter showed poor efficacy in the experiments given above, whereas ofloxacin is considered to be reliably active against acute urogenital C. trachomatis infection (16, 24, 30, 43). Two concentrations of ofloxacin, 1.0 and 2.0 μg/ml, were used, and these were added to culture media at 2 days postinfection. Cells were harvested at 4, 8, 14, and 20 days postinfection, and these were investigated for infectious organism, inclusion formation, and unprocessed primary rRNA transcripts.
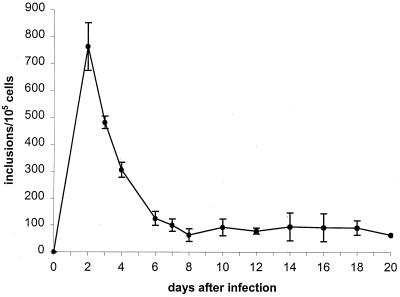
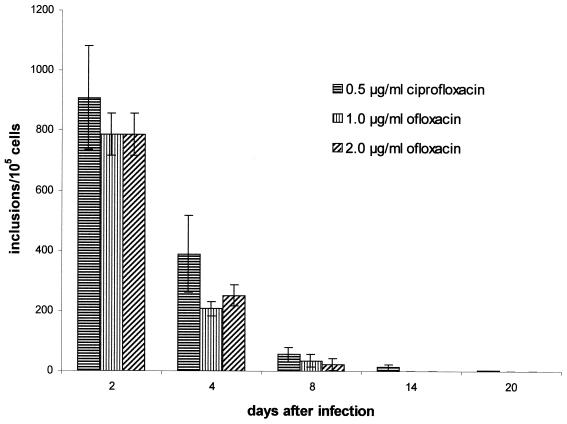
Chlamydial infectivity was significantly reduced after addition of ofloxacin, as shown by a decrease of about 85 to 87% at 4 days and 99.9% at 8 days postinfection; at 14 days postinfection, no infectious chlamydiae were detectable. The effect of ofloxacin on chlamydial inclusion formation is shown in Fig. 8. Treatment resulted in reduction in inclusion number by about 95% on day 8 postinfection. Host cells at 14 days postinfection were free of typical inclusions. However, a significant number of atypical small inclusions were observed during incubation in the presence of ofloxacin. These structures already had appeared 4 days postinfection, and they remained present throughout the culture period, although their number decreased during treatment. MOMP- and hsp60-specific staining of ofloxacin-treated cells revealed different staining patterns of aberrant small inclusions. Fluorescence was significantly weaker when cells were stained with anti-MOMP antibody than when they were stained with anti-hsp60 antibody (data not shown). RT-PCR analyses for detection of short-lived 16S rRNA transcripts were done to investigate the viability of intracellular chlamydiae during ofloxacin treatment. Figure 9 shows the continuous presence of unprocessed rRNA in cells treated with ofloxacin, indicating the continued viability of chlamydiae under these culture and antibiotic treatment conditions. Both concentrations of the drug used for long-term treatment of C. trachomatis-infected HEp-2 cells, i.e., 1.0 and 2.0 μg/ml, showed the same effect on infectivity, presence of inclusions, development of aberrant inclusions during treatment, and production of unprocessed 16S rRNA transcripts.
FIG. 8.
Effect of ofloxacin and ciprofloxacin on chlamydial inclusions in HEp-2 cells. HEp-2 cells were infected at an MOI of 0.05. Incubation with ofloxacin or ciprofloxacin was started 2 days postinfection. The figure does not include numbers of atypical small inclusions. Data presented are the means ± standard deviations (error bars) of 10 (ciprofloxacin) and 4 (ofloxacin) experiments.
FIG. 9.
RT-PCR analysis of HEp-2 cells treated with ofloxacin. Cells were infected at an MOI of 0.05 and incubated with 2 μg of ofloxacin per ml starting 2 days postinfection. RNA from cell pellets was prepared and analyzed by RT-PCR as described in Materials and Methods. Lanes: 1, marker; 2, uninfected cells; 3, control cells 2 days postinfection; 4 to 7, chlamydia-infected cells treated with ofloxacin on the indicated day (d).
DISCUSSION
Clinical trials have shown different efficacies for treatment with ciprofloxacin and ofloxacin of acute urogenital C. trachomatis infection. Ofloxacin has proved to be reliably active, whereas ciprofloxacin has shown relatively poor efficacy in clinical trails. In vitro susceptibility testing has clearly indicated successful suppression of chlamydial growth by both drugs, as demonstrated by a MIC and MBC of 0.35 μg/ml for ciprofloxacin and 0.5 μg/ml for ofloxacin. These results are in good accord with those published by others (4, 7, 22, 28, 38, 42, 44, 45, 46, 49). Treatment failures observed for ciprofloxacin, however, present an apparent contradiction to results of determination of MIC and MBC, whereas data of ofloxacin appeared to be consistent.
The basis for this lack of congruence between in vitro MIC and MBC test results for C. trachomatis susceptibility to ciprofloxacin and the apparent lack of efficacy for this drug in the clinical settings is not known. However, given results from our cell culture model, we are now able to propose an explanation for this discrepancy. In our studies, the long-term effect of ciprofloxacin on ongoing C. trachomatis infection of HEp-2 cells was characterized by two different stages. During the first, generation of new infectious EB is restricted, as shown by loss of infectivity 10 days after infection. However, further ciprofloxacin treatment not only failed to eradicate chlamydiae from host cells, but also appeared to induce persistent infection which was maintained during the subsequent culture-negative second phase. The persistent infection was characterized by a low number of inclusions, differentially regulated synthesis of key chlamydial antigens, and presence of short-lived metabolic products. Importantly, the persistent chlamydiae retained viability and metabolic activity, as indicated by detection of unprocessed (primary) transcripts from the chlamydial rRNA operons. Decrease in the number of atypical inclusions during antibiotic treatment suggests to us that chlamydiae are indeed susceptible to ciprofloxacin, providing yet another indication of the viability of persisting chlamydiae. Immunofluorescence analyses revealed deviations from the typical chlamydial growth cycle, as demonstrated by the generation of morphologically aberrant small inclusions. In these experiments, alteration in chlamydial growth was concomitant with aberrant steady-state levels of key chlamydial antigens. Specifically, a substantial decrease in expression of MOMP was noted, while the production of hsp60 and LPS was minimally affected; aberrant inclusions exhibited a pronounced imbalance between MOMP and hsp60, with a clear predominance of the latter. Another striking piece of evidence for the viability of persisting chlamydiae comes from experiments in which ciprofloxacin was removed after treatment inhibited development of new infectious organism. In these studies C. trachomatis retained the ability to differentiate to infectious EB, as demonstrated by recovery of infectious material following removal of ciprofloxacin 10 or 14 days postinfection. Thus, viable organisms are temporarily arrested in a nonproductive but metabolically active state of growth.
Despite the apparently better clinical activity of ofloxacin, long-term treatment in vitro of chlamydia-infected cells showed insufficient inhibition of the organism by the drug, as for ciprofloxacin. Although ofloxacin was able to restrict productive infection, chlamydiae remained viable and metabolically active during the entire treatment period. Typical inclusions were eliminated from host cells by ofloxacin treatment, but deviation from typical chlamydial growth occurred with generation of aberrant small inclusions. These structures exhibited differential expression of chlamydial antigens, with a predominance of hsp60 compared to MOMP, as was the case also for ciprofloxacin treatment.
Published studies have established cell culture models in which various host-elaborated factors, as well as environmental influences, have been shown to induce persistent chlamydial infection; these factors include gamma interferon (IFN-γ), penicillin treatment, and depletion of essential nutrients or iron (5, 6, 13, 27, 33, 35, 41). All these models show features similar to those observed for ciprofloxacin- and ofloxacin-arrested growth of C. trachomatis in HEp-2 cells. Specifically, factors such as IFN-γ lead to suppression of chlamydial growth and to development of aberrant chlamydial forms. These atypical bacteria are noninfectious, or infectivity is reduced significantly. Once the factors stimulating persistence are removed, resumption of overt growth usually occurs, providing evidence for viability of the persisting chlamydiae. Persistent chlamydiae which develop in the presence of IFN-γ or as a result of iron depletion also display altered expression of chlamydial antigens, with significantly reduced MOMP and near normal levels of hsp60 (5, 6, 41). Such characteristics of antigenic expression may have important consequences in terms of the sequelae known to follow chlamydial infection. For example, MOMP is known to act as an antigen that stimulates protective immunity (50, 55). Host immune responses to hsp60 have been discussed as immunopathogenic, a process which may lead to development of chronic disease. That is, Morrison et al. (36) showed that chlamydial hsp60 induces a delayed-type hypersensitivity response in immune guinea pigs. This reaction was characterized by a submucosal cellular infiltrate of lymphocytes and monocytes. Ingalls et al. (26) observed that chlamydial LPS is a weak inducer of tumor necrosis factor alpha. Thus, chlamydial LPS probably plays a role in the pathogenesis of chlamydial infections by eliciting proinflammatory cytokine responses.
The question of whether results obtained for antichlamydial antibiotic activity as determined from cell culture models have any useful implications for natural infections is, of course, a critical one. The most critical issue of such culture models is lack of an immune system, which may support antibiotic-mediated elimination of chlamydiae. Nevertheless, the in vitro data for ciprofloxacin treatment given here generally show good agreement to the results of most clinical trials. Specifically, a high rate of recurrent infection was observed after treatment of acute urogenital chlamydial infections with ciprofloxacin (1, 2, 17, 23, 52). Treatment failure did not correlate with resistance to the drug, as demonstrated by antimicrobial susceptibility testing of reisolated chlamydial strains (23, 52). Serotyping of initial and recurrent chlamydia strains was done to determine whether the reisolated organism derived from persistent infection or reinfection (23, 52). In these studies, each of the recovered strains tested was identical in serotype to the original infecting strain. Moreover, Hooton et al. (23) found a strong correlation between recurrence and the number of infectious chlamydiae present before treatment. These observations suggest that recurrent infection was a result of persisting organisms rather than reinfection. Importantly, comparison of results from clinical investigations and long-term in vitro incubation with ciprofloxacin reveals a number of common features. For example, ciprofloxacin treatment of chlamydia-infected cells suppresses overt growth of the organism but does not eradicate viable bacteria completely. Cessation of drug treatment, i.e., removal of ciprofloxacin from the growth medium, allows the organism to resume normal growth, reactivating infection. Thus, the data for ciprofloxacin from our in vitro testing system does appear to provide a reasonably compelling explanation for the clinical observations with regard to this antibiotic.
Given the clinical observation that ciprofloxacin treatment does not effectively inhibit acute chlamydial infection, one must assume that treatment of chronic infections presents a much more difficult problem. As mentioned earlier, clinical studies investigating ciprofloxacin for treatment of chronic reactive arthritis and undifferentiated arthritis, including chlamydia-induced arthritis, did not demonstrate a beneficial effect of prolonged treatment with ciprofloxacin (48, 51). In this instance, the lack of efficacy may be related to the altered metabolic state of the persisting organism; i.e., C. trachomatis present at the site of synovial inflammation has been shown to be metabolically active, although culture of the organism was not successful using normal microbiological techniques (20). In addition, persistent chlamydiae exhibit an aberrant morphology and a significantly reduced rate of metabolic activity, possibly rendering these organisms less susceptible to antibiotics. It may not be surprising, therefore, that ciprofloxacin was not effective in treatment of chronic chlamydia-induced arthritis. Data from an animal model for this disease are in accordance with this assumption. Treatment with ciprofloxacin of experimentally infected rats which developed a synovitis, was neither effective in modifying the arthritis nor eliminating live chlamydiae from the joints (25).
Although ofloxacin has been proved effective in in vitro susceptibility testing and in some clinical trials, long-term in vitro treatment as given here demonstrates inconsistent results. Our observations may result from two possible causes. First, our in vitro model may not represent a system accurate enough to mimic fully in vivo conditions. However, the high level of accord between in vitro data and in vivo observation for ciprofloxacin does not support this possibility. A second possible explanation for the apparent nonconcordance between in vivo data and the results given here might derive from a substantially lower efficacy of ofloxacin treatment than has been deduced from clinical studies and determination of in vitro susceptibilities. A critical point that must be kept in mind for clinical trials investigating the effect of ofloxacin on acute C. trachomatis infection is that tests for eradication of chlamydia are done primarily by laboratory culture (16, 24). The present study, however, clearly indicates that a culture negative state does not necessarily exclude presence of viable chlamydia. C. trachomatis has been shown to be fully viable and metabolically active during in vivo infections even when culture detection failed (20, 21). Additionally, there is a growing body of evidence for persistence of live chlamydiae in the presence of adequate antibiotic treatment. Dean et al. (15) suggest that certain C. trachomatis may persist in the cervix despite appropriate antimicrobial therapy.
The important message of the present study lies in the differential effects of ciprofloxacin and ofloxacin on chlamydial biology, which resulted from the differing experimental conditions employed. That is, only the long-term in vitro tests used appear to be truly reflective of the situation in vivo for chlamydial infection (i.e., persistence as defined by continuation of PCR positivity for the organism posttreatment), since the 48- or 72-h incubation periods before addition of the drug allows the infection to become established. Under normal circumstances, the determination of the MIC and the MBC is done by addition of the target antibiotic immediately after culture infection. Thus, these tests measure the prevention of formation of inclusions, rather than elimination of inclusions and viable bacteria from host cells. Optimal growth conditions during antibiotic susceptibility testing for C. trachomatis thus do not consider the possibility of persistence. In our view, the validity of using the term MBC to express the ability of a given antibiotic to kill chlamydiae should therefore be revised. The determination of the MIC and the MBC for C. trachomatis allows the definition of whether an antibiotic has some antichlamydial effect, but these are not sufficient to verify the efficacy of antibiotics on natural chlamydial infections. To address this critical issue, cell culture studies must be done over a prolonged period, with the addition of antibiotic to an established infection.
ACKNOWLEDGMENTS
This study was supported by the German Ministry of Technology, grant 01VM9708/4, and grant AR-42541 from the U.S. National Institutes of Health.
REFERENCES
- 1.Ahmed-Jusuf I H, Arya O P, Hobson D, Pratt B C, Hart C A, How S J, Tait I A, Rao P M S. Ciprofloxacin treatment of chlamydial infections of urogenital tracts of women. Genitourin Med. 1988;64:14–17. doi: 10.1136/sti.64.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arya O P, Hobson D, Hart C A, Bartzokas C, Pratt B C. Evaluation of ciprofloxacin 500 mg twice daily for one week in treating uncomplicated gonococcal, chlamydial, and non-specific urethritis in men. Genitourin Med. 1986;62:170–174. doi: 10.1136/sti.62.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aviv M, Giladi H, Oppenheim A B, Glaser G. Analysis of the shut-off of ribosomal RNA promotors in Escherichia coli upon entering the stationary phase of growth. FEMS Microbiol Lett. 1996;140:71–76. doi: 10.1111/j.1574-6968.1996.tb08317.x. [DOI] [PubMed] [Google Scholar]
- 4.Bailey J M, Heppleston C, Richmond S J. Comparison of the in vitro activities of ofloxacin and tetracycline against Chlamydia trachomatis as assessed by indirect immunofluorescence. Antimicrob Agents Chemother. 1984;26:13–16. doi: 10.1128/aac.26.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatty W L, Byrne G I, Morrison R P. Immunoelectron-microscopic quantitation of differential levels of chlamydial proteins in a cell culture model of persistent Chlamydia trachomatis infection. Infect Immun. 1994;62:4059–4062. doi: 10.1128/iai.62.9.4059-4062.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty W L, Byrne G I, Morrison R P. Morphologic and antigenic characterization of interferon γ-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchi A, Scieux C, Salmeron C M, Casin I, Perol Y. Rapid determination of MICs of 15 antichlamydial agents by using an enzyme immunoassay (Chlamydiazyme) Antimicrob Agents Chemother. 1988;32:1350–1353. doi: 10.1128/aac.32.9.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne G I, Stephens R S, Ada G, Caldwell H D, Su H. Workshop on in vitro neutralization of Chlamydia trachomatis: summary of proceedings. J Infect Dis. 1993;168:415–420. doi: 10.1093/infdis/168.2.415. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cangelosi G A, Brabant W H, Britschgi T B, Wallis C K. Detection of rifampicin- and ciprofloxacin-resistant Mycobacterium tuberculosis by using species-specific assays for precursor rRNA. Antimicrob Agents Chemother. 1996;40:1790–1795. doi: 10.1128/aac.40.8.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cangelosi G A, Brabant W H. Depletion of pre-16S rRNA in starved Escherichia coli cells. J Bacteriol. 1997;179:4457–4463. doi: 10.1128/jb.179.14.4457-4463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cates W, Wasserheit J H. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstet Gynecol. 1991;164:1771–1781. doi: 10.1016/0002-9378(91)90559-a. [DOI] [PubMed] [Google Scholar]
- 13.Coles A M, Reynolds D J, Harper A, Devitt A, Pearce J H. Low-nutrient induction of abnormal chlamydial development: a novel component of chlamydial pathogenesis. FEMS Microbiol Lett. 1993;106:193–200. doi: 10.1111/j.1574-6968.1993.tb05958.x. [DOI] [PubMed] [Google Scholar]
- 14.Cotter T W, Meng Q, Shen Z L, Zhang Y X, Su H, Caldwell H D. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean D, Suchland R J, Stamm W E. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. 1998. Apparent long-term persistence of Chlamydia trachomatis cervical infections—analysis by OMP1 genotyping. Chlamydial Infections; pp. 31–34. [Google Scholar]
- 16.Faro S, Martens M G, Maccato M, Hammill H A, Roberts S, Riddle G. Effectiveness of ofloxacin in the treatment of Chlamydia trachomatis and Neisseria gonorrhoeae cervical infection. Am J Obstet Gynecol. 1991;164:1380–1383. doi: 10.1016/0002-9378(91)91476-d. [DOI] [PubMed] [Google Scholar]
- 17.Fong I W, Linton W, Simbul M, Thorup R, Mclaughlin B, Rahm T, Quinn P A. Treatment of nongonococcal urethritis with ciprofloxacin. Am J Med. 1987;82(Suppl. 4A):311–316. [PubMed] [Google Scholar]
- 18.Gegenheimer P, Watson N, Apiron D. Multiple pathways for primary processing of ribosomal RNA in Escherichia coli. J Biol Chem. 1977;252:3064–3073. [PubMed] [Google Scholar]
- 19.Gerard H C, Whittum-Hudson J A, Hudson A P. Genes required for assembly and function of the protein synthetic system in Chlamydia trachomatis are expressed early in elementary to reticulate body transformation. Mol Gen Genet. 1997;255:637–642. doi: 10.1007/s004380050538. [DOI] [PubMed] [Google Scholar]
- 20.Gérard H C, Branigan P J, Schuhmacher H R, Hudson A P. Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiter's syndrome are viable but show aberrant gene expression. J Rheumatol. 1998;25:734–742. [PubMed] [Google Scholar]
- 21.Gérard H C, Branigan P J, Balsara G R, Heath C, Minassian S S, Hudson A P. Viability of Chlamydia trachomatis in fallopian tubes of patients with ectopic pregnancy. Fertil Steril. 1998;70:945–948. doi: 10.1016/s0015-0282(98)00304-5. [DOI] [PubMed] [Google Scholar]
- 22.Heessen F W A, Muytjens H L. In vitro activity of ciprofloxacin, norfloxacin, pipemidic acid, cinoxacin and nalidixic acid against Chlamydia trachomatis. Antimicrob Agents Chemother. 1984;25:123–124. doi: 10.1128/aac.25.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooton T M, Rogers M E, Medina T G, Kuwamura L E, Ewers C, Roberts P L, Stamm W E. Ciprofloxacin compared with doxycycline for nongonococcal urethritis. JAMA. 1990;264:1418–1421. [PubMed] [Google Scholar]
- 24.Hooton T M, Batteiger B E, Judson F N, Spruance S L, Stamm W E. Ofloxacin versus doxycycline for treatment of cervical infection with Chlamydia trachomatis. Antimicrob Agents Chemother. 1992;36:1144–1146. doi: 10.1128/aac.36.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inman R D, Chiu B. Synoviocyte-packaged Chlamydia trachomatis induces a chronic aseptic arthritis. J Clin Investig. 1998;102:1776–1782. doi: 10.1172/JCI2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingalls R R, Rice P A, Qureshi N, Takayama K, Lin J S, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson F W A, Hobson D. The effect of penicillin on genital strains of Chlamydia trachomatis in tissue culture. J Antimicrob Chemother. 1977;3:49–56. doi: 10.1093/jac/3.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Jones R B, van Der Pol B, Johnson R B. Susceptibility of Chlamydia trachomatis to trovafloxacin. J Antimicrob Chemother. 1997;39(Suppl. B):63–65. doi: 10.1093/jac/39.suppl_2.63. [DOI] [PubMed] [Google Scholar]
- 29.King T C, Sirdeskmukh R, Schlessinger D. Nucleolytic processing of ribonucleic acid transcripts in prokaryotes. Microbiol Rev. 1986;50:428–451. doi: 10.1128/mr.50.4.428-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitchen V S, Donegan C, Ward H, Thomas B, Harris J R W, Taylor-Robinson D. Comparison of ofloxacin with doxycyclin in the treatment of non-gonococcal urethritis and cervical chlamydial infection. J Antimicrob Chemother. 1990;26(Suppl. D):99–105. doi: 10.1093/jac/26.suppl_d.99. [DOI] [PubMed] [Google Scholar]
- 31.Köhler L, Nettelnbreker E, Hudson A P, Ott N, Gérard H C, Branigan P J, Schumacher H R, Drommer W, Zeidler H. Ultrastructural and molecular analyses of the persistence of Chlamydia trachomatis (serovar K) in human monocytes. Microb Pathog. 1997;22:133–142. doi: 10.1006/mpat.1996.0103. [DOI] [PubMed] [Google Scholar]
- 32.Köhler L, Zeidler H, Hudson A P. Aetiological agents: their molecular biology and phagocyte-host interaction. Bailliere's Clin Rheumatol. 1998;12:589–609. doi: 10.1016/s0950-3579(98)80039-3. [DOI] [PubMed] [Google Scholar]
- 33.Kramer M J, Gordon F B. Ultrastructural analysis of the effects of penicillin and chlortetracycline on the development of a genital tract Chlamydia. Infect Immun. 1971;3:333–341. doi: 10.1128/iai.3.2.333-341.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto A, Manire G P. Electron microscopic observation on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970;101:278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison R P, Lyng K, Caldwell H D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989;169:663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison R P, Belland R J, Lyng K, Caldwell H D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989;170:1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagayama A, Nakao T, Taen H. In vitro activities of ofloxacin and four other new quinolone-carboxylic acids against Chlamydia trachomatis. Antimicrob Agents Chemother. 1988;32:1735–1737. doi: 10.1128/aac.32.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nettelnbreker E, Zeidler H, Bartels H, Dreses-Werringloer U, Däubener W, Holtmann H, Köhler L. Effect of IFN-γ persistent infection by Chlamydia trachomatis serovar K in TPA- differentiated U937 cells. J Med Microbiol. 1997;46:1–9. doi: 10.1099/00222615-47-2-141. [DOI] [PubMed] [Google Scholar]
- 40.Nomura M, Gourse R, Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- 41.Raulston J E. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect Immun. 1997;65:4539–4547. doi: 10.1128/iai.65.11.4539-4547.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice R J, Bhullar V, Mitchell S H, Bullard J, Knapp J S. Susceptibilities of Chlamydia trachomatis isolates causing uncomplicated female genital tract infections and pelvic inflammatory disease. Antimicrob Agents Chemother. 1995;39:760–762. doi: 10.1128/AAC.39.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridgeway G L. Treatment of chlamydial genital infection. J Antimicrob Chemother. 1997;40:311–314. doi: 10.1093/jac/40.3.311. [DOI] [PubMed] [Google Scholar]
- 44.Sambri V, Rumpianesi F, Xerri L, Cevenini R. In vitro activity of ofloxacin against Chlamydia trachomatis J. Chemother. 1989;1:231–232. doi: 10.1080/1120009x.1989.11738897. [DOI] [PubMed] [Google Scholar]
- 45.Schachter J, Monada J. In vitro activity of ciprofloxacin against Chlamydia trachomatis. Am J Med. 1987;82(Suppl. 2):42. [Google Scholar]
- 46.Segreti J, Hirsch D J, Harris A A, Kapell K S, Orbach H, Kessler H A. In vitro activity of tosufloxacin (A-61827; T-3262) against selected genital pathogens. Antimicrob Agents Chemother. 1990;34:971–973. doi: 10.1128/aac.34.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shemer Y, Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985;48:592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sieper J, Fendler C, Laitko S, Sörenson H, Gripenberg-Lerche C, Hiepe F, Alten R, Keitel W, Groh A, Uksila J, Eggens U, Grainfors K, Braun J. No benefit of long-term ciprofloxacin treatment in patients with reactive arthritis and undifferentiated oligoarthritis. Arthr Rheum. 1999;42:1386–1396. doi: 10.1002/1529-0131(199907)42:7<1386::AID-ANR12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 49.Slaney L, Chubb H, Ronald A, Brunham R. In vitro activity of azithromycin, erythromycin, ciprofloxacin and norfloxacin against Neisseria ghonorrhoea, Haemophilus ducreyi and Chlamydia trachomatis. J Antimicrob Chemother. 1990;25(Supp. A):1–5. doi: 10.1093/jac/25.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 50.Su H, Watkins N G, Zhang Y X, Caldwell H D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toivanen A, Yli-Kerttulla T, Luukainen R, Merilahti-Palo R, Granfors K, Seppälä J. Effect of antimicrobial treatment on chronic reactive arthritis. Clin Exp Rheumatol. 1993;11:301–307. [PubMed] [Google Scholar]
- 52.Van der Willigen A H, Polak-Vogelzang A A, Habbema L, Waagenvoort J H T. Clinical efficacy of ciprofloxacin versus doxycycline in the treatment of non-gonococal urethritis in males. Eur J Clin Microbiol Infect Dis. 1988;7:658–661. doi: 10.1007/BF01964246. [DOI] [PubMed] [Google Scholar]
- 53.Wollenhaupt J, Zeidler H. Chlamydia-induced arthritis. EULAR Bull. 1990;3:72–77. [Google Scholar]
- 54.World Health Organization. Global prevalence and incidence of selected curable sexually transmitted diseases: overview and estimates. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 55.Zhang Y S, Stewart S, Joseph T, Taylor H R, Caldwell H D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987;138:575–581. [PubMed] [Google Scholar]