Abstract
Mentha spicata, also called Mentha viridis, is a medicinal plant of the Lamiaceae family characterized by its potency to synthesize and secret secondary metabolites, essentially essential oils. Different populations use the aerial parts of this plant for tea preparation, and this tisane has shown several effects, according to ethnopharmacological surveys carried out in different areas around the world. These effects are attributed to different compounds of M. spicata, in which their biological effects were recently proved experimentally. Pharmacological properties of M. spicata extracts and essential oils were investigated for different health benefits such as antioxidant, anticancer, antiparasitic, antimicrobial, and antidiabetic effects. In vitro and in vivo studies showed positives effects that could be certainly related to different bioactive compounds identified in M. spicata. Indeed, volatile compounds seem to be efficient in inhibiting different microbial agents such as bacteria, fungi, and parasites through several mechanisms. Moreover, M. spicata exhibited, according to some studies, promising antioxidant, antidiabetic, anti-inflammatory, and anticancer effects, which show its potential to be used as a source for identifying natural drugs against cellular oxidative stress and its related diseases. Importantly, toxicological investigations of M. spicata show the safety of this species at different doses and several periods of use which justify its use in traditional medicines as tisane with tea. Here, we report, explore, and highlight the data published on M. spicata concerning its botanical description and geographical distribution, its phytochemical compounds, its pharmacological properties, and its toxicological investigations of M. spicata.
1. Introduction
The use of M. spicata is importantly characterized in several populations, including Moroccan population, which has used the aerial parts (with tea) of this plant since time against several diseases including diabetes, digestive and respiratory disorders, throat ailments, and skin disease [1, 2].
Certainly, M. spicata contains molecules biologically active having biological effects, and effective spectroscopic analysis of extracts and essential oils of M. spicata using GC-MS, HPLC, HPLC-MS, and RMN revealed the presence of several phytochemical bioactive compounds belonging to different classes of secondary metabolites in particularly the classes of flavonoids, phenolic acids, and terpenes [3, 4]. Indeed, the distribution of these chemical compounds between different plant parts and collection regions is variable, which explains different traditional uses (with efficacy) of this species according to each region. In addition, the extraction of these chemical compounds depends on used methods and therefore can justify the difference in traditional applications according to used methods of pharmaceutical formulations preparation.
In vitro and in vivo experimental explorations showed that M. spicata extracts and essential oils exhibit remarkable biological activities, including antimicrobial, antiparasitic, antidiabetic, anti-inflammatory, and anticancer effects. Indeed, different organic extracts (rich in bioactive compounds) revealed important antifungal activity by their potency to inhibit the growth of some strains involved in human infections such as Aspergillus niger, Candida albicans, Cryptococcus neoformans, and Microsporum audouinii [5]. Moreover, M. spicata showed antibacterial properties against various bacterial strains, either clinical or reference [6, 7]. It was also revealed that M. spicata extracts target some human complex diseases, including chronic inflammatory diseases, diabetes, and cancers. Plant extracts inhibit or activate targets and/or pathways involved in these pathologies, including membrane receptors, signaling pathways, and molecular targets [8, 9].
To the best of our knowledge, despite numerous investigations that have been carried out until now showing remarkable results, there are now literature reviews exploring M. spicata as a source of potential lead compounds. Therefore, this review aims to explore, discuss, and highlight all data concerning M. spicata and give suggestions about its exploitation as a source for developing bioactive compounds in the pharmaceutical and cosmetic fields.
2. Research Methodology
3. Results and Discussion
3.1. Taxonomy, Botanical Description, Geographic Distribution, and Ecological Factors
Mentha spicata (ID: 29719) is also known as spearmint. There are a couple of heterotypic synonyms for this species including Mentha cordifolia, Mentha crispa var. crispata f. reticulata, Mentha viridis (L.) L., Mentha × cordifolia, and Mentha × villosa var. cordifolia. It is an aromatic plant that belongs to the genus Mentha, family Lamiaceae, subfamily Nepetoideae, placed in Magnoliopsida class, and belongs to order Lamiales. The genus Mentha, one of the most important members of the Lamiaceae family, is represented by 19 species and 13 natural hybrids, and Lamiaceae family consists of over 7000 species and around 260 genera of trees and shrubs [10]. The spearmint, M. spicata, is a hybrid of M. longifolia and M. rotundifolia. This species is widely grown in Europe, North America, and Asia, but nowadays cultivated throughout all regions of the world [11].
M. spicata L. (spearmint) is a creeping rhizomatous, glabrous, and perennial herb with a strong aromatic odor, growing up to 30–100 cm tall with variably hairless to hairy stems and foliage, and a wide spreading fleshy underground rhizome [12]. The leaves are ovate to lancolate, 5–9 cm long and 1.5–3 cm broad, with a serrated margin. Spearmint produces flowers in slender spikes, each flower pink or white, and 2.5–3 mm long and broad. The stem is square-shaped, a trademark of the mint family of herbs [13]. M. spicata L is well adapted to climatic conditions in tropical and subtropical areas. It can be cultivated in wide range of soils and found in back gardens of homesteads [14].
3.2. Medicinal Uses
Mentha viridis is widely used in a variety of applications [15]. Since ancient times, Western and Eastern cultures have practiced Mentha viridis as a medicinal and aromatic plant against several diseases (Table 1) [15]. Ethnobotanical investigations into Mentha viridis have suggested its potential medical applications in different disorders. It has beneficial effects on diabetes, digestive, skin, and respiratory disorders [1, 2, 16–23].
Table 1.
Medicinal use of M. spicata.
| Used part | Dosage form | Traditional use | References |
|---|---|---|---|
| Leaf | Decoction | Diabetes | [1] |
| Leaf | Decoction | Against stomach disorders | [2] |
| Leaf, stem | Infusion | Headache, tiredness | [16] |
| Leaf, stem | Infusion and decoction | Diabetes | [17] |
| Leaf, flower | Infusion and decoction | Asthma, bronchitis, chest pain, lungs disorder, kidney problems, diuretic | [18] |
| Leaf | Infusion | Cold and flu, toothache | [19] |
| Aerial parts | Infusion | Throat affection | [20] |
| Leaf | Powder | Skin diseases | [21] |
| Whole plant | Infusion | Aphrodisiac, cold, flatulence, headache, tonic, toothache | [22] |
| Leaf, stem | Decoction | Against the ailments of intestines | [23] |
In Morocco, Mentha viridis is a medicinal plant most used in the treatment of throat ailments. The use of this plant to treat throat ailments has been demonstrated by Orch et al. [20], who reported the use of aerial parts' infusion of Mentha viridis in Moroccan oriental folklore. The leaves of M. viridis are also administered as a decoction to treat diabetes in the Al Haouz-Rhamna region (Morocco) [1]. Idm' hand et al. [17] showed that the leaves and stems of M. viridis are also used as a decoction and infusion to treat diabetes; on the other hand, El-hilaly et al. [16] showed that these parts were used to treat headache and tiredness. The leaves and flowers of M. viridis have also been widely used to treat asthma, bronchitis, chest pain, lung disorders, kidney problems, and diuretics by decoction or infusion [18]. In addition, the leaves of M. viridis have been used against gastric disorders by decoction, and the stems are used against ailments of intestines [2, 23]. M. viridis whole plant infusions are also used to treat aphrodisiac, cold, flatulence, headache, tonic, and toothache [19, 22]. In another study in Morocco, the powder from the leaves of M. viridis is used to treat skin diseases [21].
3.3. Phytochemical Compounds
Extracts and essential oils extracted from M. spicata (viridis) are considered as valuable source phytochemicals, including natural phenolics and EOS. These volatile compounds are complex mixtures of substances that have been found to create different chemotypes distinguished based on the dominant compound in the essential oil, which depends on the plant species, and within the same variety, the essential oil composition can vary according to the geographical region [24]. In terms of phytochemical content, terpenes and terpenoids are the major components of EOs obtained from aerial parts of M. spicata. Thus, more detailed discussion regarding chemical aspects of EOs of these species is described (Table 2). Previous studies reported the existence of different chemotypes in the chemical composition of M. spicata, naturally grown as cultivated, around the world, and the essential oil mainly composed of carvone, carvacrol, trans-carveol, piperitone oxide, limonene, 1,8-cinéole, camphene, p-cymene, dihydrocarvone, pulegone, β-caryophyllene, germacrene D, menthone, α-pinene, and linalool [3, 5, 26, 27]; whereas, carvone is mentioned as the absolute predominant constituent of M. spicata oil as well as monoterpenes including linalool, piperitone, piperitone oxide, menthone, isomenthone, and pulegone (Figure 1 and Table 2). The composition of M. spicata EOS from Morocco is relatively stable and has strong homogeneity [7, 40, 53, 56]. No significant difference between samples was observed; whatever the locality (region), the main essential oil compounds are carvone and trans-carveol, showing variation in a narrow range of 29–47.3% and 14–20%, respectively [34, 46, 47, 51, 52]. Various chemotypes of M. spicata were also identified for plants cultivated in Italy and Turkey. In plants from Italy, carvone (39.13–59.26%) was detected as the main compound [29], while for the species from Turkey, piperitenone oxide (25.84%), pulegone (24.72%), cis-piperitenone oxide (12.55%), and limonene 1.59% were the principal constituents of the EOS [31]. It is worth noting that chemotype carvone represented the most variation, 79.70% in spearmint M. spicata EOS [24]. Other examples of M. spicata producing EOs with high piperitone oxide content (above 70%) are samples from India [36]. As established in the literature, such compound is one of the most abundant components of M. spicata EO, which offers spearmint its unique smooth characteristic scent [57], and it also varies according to the spearmint oil grown in different countries. Similarly, EOs from Cyprus is reported to possess a higher carvone content (69.23–74.27%) [55].
Table 2.
Chemical compounds of M. spicata.
| Country | Part used | Compounds | Reference |
|---|---|---|---|
| Morocco | Aerial parts | Carvone (33.14%) | [25] |
| trans-Carveol (20.06%) | |||
| β-Caryophyllene (4.41%) | |||
| 1,8-Cineole (3.99%) | |||
| Germacrene D (3.14%) | |||
| Menthone (2.19%) | |||
| α-Pinene (1.06%) | |||
| Aerial parts | Carvone (47.30–69.19%) | [26] | |
| Limonene (4.48–15.43%) | |||
| trans-4-Caranone (0.82–4.63%) | |||
| iso-Dihydrocarveol acetate (0.06–2.66%) | |||
| ρ-Mentha-3,8-diene (0.85–1.32%) | |||
| Aerial parts | Carvone (57.11%) | [27] | |
| Limonene (27.77%) | |||
| 3-Carene (1.01%) | |||
| Germacrene D (0.65%) | |||
| Aerial parts | Carvone (29.00%) | [28] | |
| trans-Carveol (14.00%) | |||
| 1,8-Cineole (7.30%) | |||
| Dihydrocarveol (l4.50%) | |||
| Carvyl acetate-Z (6.70%) | |||
| Germacrene D (3.90%) | |||
|
| |||
| Italy | Aerial parts | Carvone (39.13 to 59.26%) | [29] |
| 1,8-Cineole (1.07–9.02%) | |||
| Dihydrocarveol (2.36–5.94%) | |||
| Germacrene D (1.79–4.11%) | |||
| Limonene (5.9–11.40%) | |||
| trans-Carvyl acetate (0.72–5.90%) | |||
| Aerial parts | p-Cymene (33.9%) | [30] | |
| iso-Piperitone (23.7%) | |||
| Piperitone (6.9%) | |||
| Menthone (21.8) | |||
| p-Cymen-8-ol (19.6) | |||
| β-Linalool (15.2%) | |||
|
| |||
| Czech Republic | Aerial parts | Carvone (0.7–59.1%) | [3] |
| Menthol (1.1%–14.9%) | |||
| p-Menthone (1.1%–4.4%) | |||
| Piperitone oxide (34.1%) | |||
| Germacrene D (14.6%) | |||
| β-Caryophyllene (2.2–3%) | |||
| Dihydrocarvone (11.8–12.7%) | |||
| cis-Jasmone (1.6–1.8%) | |||
|
| |||
| Turkey | Aerial parts | Piperitenone oxide (25.84%) | [31] |
| Pulegone (24.72%) | |||
| cis-Piperitenone oxide (12.55%) | |||
| Limonene (1.59%) | |||
| Aerial parts | Carvone (34.70 to 79.70%) | [24] | |
| 1,8-Cineole (3.40–33.80%) | |||
| β-Pinene (0.87–5.29%) | |||
| Limonene (1.10–22.10%) | |||
| Menthone (0.20–2.73%) | |||
| Pulegone (1.70–9.94%) | |||
| Aerial parts | Carvone (48.6–57.9%) | [32] | |
| ρ-Cymene (9.6–20.5%) | |||
| 1,8-Cineole (14.6–19.3%) | |||
| Carvacrol (0.1–3.5%) | |||
| α-Pinene(2.3–4.3%) | |||
| Phenolic acids | Rosmarinic acid derivatives (88%) | [30] | |
| Salvianolic acids (5.6%) | |||
| Caffeoylquinic acids (1.2%) | |||
| Hydroxycinnamic (1.1%) | |||
| Fatty acids | Palmitic acid (5.11 0.41%) | [33] | |
| Stearic acid (1.92 ± 0.21%) | |||
| Oleic acid (8.19%) | |||
| Linoleic acid (31.14%) | |||
| α-Linolenic acid (48.17%) | |||
| γ-Linolenic acid(2.07%) | |||
| Stearidonic acid (3.02%) | |||
|
| |||
| India | Aerial parts | Carvone (49.62–76.65%) | [34] |
| Limonene (9.57–22.31%) | |||
| 1,8-Cineole (1.32–2.62%) | |||
| trans-Carveol (0.3–1.52%) | |||
| Phenolics | Pentadecanoic acid (7.47%) | [35] | |
| 7-Oxabicyclo[4.1.0] heptane (9.56%) | |||
| 3-Penten-2-one,4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0] hept-1-yl)-,(E)-(12.20%) stigmast-4-EN-3-one (18.99%) | |||
| Aerial parts | trans-Muurola-4 (14%) | [36] | |
| 5-Diene (27.28%) | |||
| Piperitenone oxide (22.22%) | |||
| β-Caryophyllene (10.48%) | |||
| Geranyl propanoate (6.55%) | |||
| Sibirene (3.45%) | |||
| Borneol (1.98%) | |||
| Allo-ocimene (1.71%) | |||
| β-Elemene (1.34%) | |||
| Germacrene D-4-ol (1.02%) | |||
| Aerial parts | Carvone (57.49–72.47%) | [37] | |
| Limonene (10.70–24.81%) | |||
| Myrcene (0.25–4.36%) | |||
| 1,8-Cineole (0.2–2.02%) | |||
| Aerial parts | Carvone (48.60%) | [38] | |
| Limonene (11.30%) | |||
| cis-Carveol (21.30%) | |||
| Linalool (1.30%) | |||
| 1,8-Cineole (2.55%) | |||
| cis-Carvyl acetate (2.10%) | |||
| cis-Dihydrocarvone (1.30%) | |||
| Iran | Aerial parts | Carvone (65.15–74.21%) | [39] |
| Limonene (12.22–20.55%) | |||
| cis-Dihydrocarvone (2.34–11.13%) | |||
| Caryophyllene (1.13–5.06%) | |||
| Aerial parts | Carvone (42.74–54.34%) | [4] | |
| trans-Dihydrocarvone (21.58%) | |||
| 1,8-Cineole (8.41–21.78%) | |||
| Pulegone (6.83%) | |||
| Limonene (5.2–6.1%) | |||
| β-Caryophyllene (3.05%) | |||
| Linalool (5.82%) | |||
| trans-Dihydrocarvone (3.18%) | |||
| Aerial parts | Carvone (49.91%–56.92%) | [40] | |
| Piperitone oxide (10.69%–11.72%) | |||
| 1,8-Cineole (3.78–3.34%) | |||
| Limonene (7.33–6.61%) | |||
| Germacrene D (6.26–1.90%) | |||
| Aerial parts | Carvone (54.34%) | [41] | |
| 1,8-Cineole (8.41–22.71%) | |||
| Piperitenone oxide (58.87%) | |||
| 3,8-Menthadiene (21.58%) | |||
| α-Pinene (0.95–1.68%) | |||
| 2-Cyclohexen (42.74%) | |||
| Borneol (5.82%) | |||
| DL-Limonene (5.2%) | |||
| Pulegone (6.83%) | |||
|
| |||
| Malaysia | Flavonoids leaves | Catechin (14–14.4%) | [42] |
| Epicatechin (15.6–16.3%) | |||
| Rutin 1 (4.8–16.1%) | |||
| Myricetin (4.1–11.7%) | |||
| Luteolin (9.3–65.7%) | |||
| Apigenin (27–39.2%) | |||
| Naringenin (5.4–24.9%) | |||
|
| |||
| Algeria | Leaves | Carvone (59.40%) | [7] |
| Limonene (6.12%) | |||
| 1,8-Cineole, germacrene D (04.66%) | |||
| β-Caryophyllene (2.969%) | |||
| β-Bourbonene (2.796%) | |||
| α-Terpineol (1.986%) | |||
| Terpinene-4-ol (1.120%) | |||
|
| |||
| Brazil | Aerial parts | Carvone (39.42–72.28%) | [43] |
| Pulegone (5.53–10.48%) | |||
| Carveol (3.30–4.98%) | |||
| Cineol (1.49%) | |||
| Leaves | Linalool (58.51%) | [6] | |
| Carvone (15.1%) | |||
| α-Terpineol (1.43%) | |||
| β-Caryophyllene (2.02%) | |||
| Eucalyptol (1.04%) | |||
| Terpinen-4-ol (5.73%) | |||
| Leaves | Piperitone (81.18%) | [44] | |
| Piperitenone (14.75%) | |||
| α-Pinene (0.51%) | |||
| Limonene (1.47%) | |||
| Aerial parts | Limonene (2.04–19.91%), | [45] | |
| Isomenthone (0.46–11.60%) | |||
| Menthone (0.46–11.60%) | |||
| 1,8-Cineole (eucalyptol) (2.98–8.10%) | |||
| d-Carvone (31.35–60.07%) | |||
| β-Pinene (2.41–4.27%) | |||
| Isomenthone (4.46%) | |||
| Pulegone (6.68–53.65%) | |||
|
| |||
| China | Aerial parts | Carvone (46.7–65.4%) | [46] |
| Limonene (0.3–1.8%) | |||
| Linalool (0.6 6.9%) | |||
| Menthone (1.5–4.7%) | |||
| Dihydrocarvone (0.8–15.7%) | |||
| Dihydrocarveol acetate (0.2–7%) | |||
|
| |||
| China | Aerial parts | Carvone (65.33%) | [11] |
| Limonene (18.19%) | |||
| Dihydrocarvone (2.97%) | |||
| Camphene (2.34%) | |||
|
| |||
| Hungary | Leaves | Carvone (35.9–60.5%) | [47] |
| Citronellol (10.1–13.4%) | |||
| Limonene (1.6–9.4%) | |||
| Menthone (3.2–4.4%) | |||
| α-Terpineol (2.1–3%) | |||
| cis-Dihydrocarvone (1.5–2.2%) | |||
| (e)-b-Caryophyllene (1.5–2.1%) | |||
|
| |||
| Jordan | Aerial parts | Carvone (49.5%) | [48] |
| Limonene (16.1%) | |||
| 1,8-Cineole (8.7%) | |||
| cis-Dihydrocarvone (3.9%) | |||
| β-Caryophyllene (2.7%) | |||
| Germacrene D (2.1%) | |||
| β-Pinene (1.1%) | |||
|
| |||
| Abu Dhabi | Leaves | Carvone (14.79–87.11%) | [49] |
| Dihydrocarvone (0.09–0.19%) | |||
| Cineole (0.2–0.6%) | |||
| Limonene (1.94–9.72%) | |||
| Menthol (0.06–0.19%) | |||
| Linalool (0.09–0.23%) | |||
| α-Pinene (0.05–0.3%) | |||
|
| |||
| Romania | Phenolics, 70% ethanol | Ferulic acid (27.32%) | [50] |
| Sinapic acid (6.60%) | |||
| p-Coumaric acid (15.24%) | |||
| Luteolin (4.68%) | |||
|
| |||
| Tunisia | Aerial parts | Carvone (39.21–75.53%) | [51] |
| 1,8-Cineole (7.24–12.49%) | |||
| Limonene (6.07–18.45%) | |||
| cis-Dihydrocarveol (1.17–6.56%) | |||
| trans-Carveol (0–5.22%) | |||
| Pulegone (38.74%) | |||
| Menthone (28.56%) | |||
| Menthol (5.64%) | |||
|
| |||
| Bangladesh | Aerial parts | Carvone (73.29%) | [52] |
| D-Limonene (7.59%) | |||
| Dihydrocarvone (3.83%) | |||
| α-Bourbonene (1.67%) | |||
| trans-Sabinene hydrate (1.57%) | |||
| trans-Carveol (1.25%) | |||
| Dihydrocarveol (1.12%) | |||
| Eucalyptol (1.01%) | |||
|
| |||
| Serbia | Aerial parts | Carvone (49.5%) | [53] |
| Menthone (21.9%) | |||
| Piperitone 0.6%) | |||
| β-Bourbonene (26.8%) | |||
| β-Caryophyllene (0.7%) | |||
| Germacrene A(0.5%) | |||
|
| |||
| Palestine | Aerial parts | Limonene (6.23–9.79%) | [54] |
| Carvone (36.9–76.82%) | |||
| Sabinene (0.14–5.51%) | |||
| cis-Dihydrocarvone (0.65–4.59%) | |||
| β-Caryophyllene (0.81–3.87%) | |||
| Dihydrocarveol (2.27–13.76%) | |||
|
| |||
| Cyprus | Aerial parts | Carvone (ketone: 69.23–74.27%) | [55] |
| Limonene (alkene: 10.42–11.39%) | |||
| 1,8-Cineole (alcohol: 5.28–5.99%) | |||
| β-Pinene (alkene: 1.13–1.25%) | |||
| β-Caryophyllene (alkene: 0.80–1.29%) | |||
| Germacrene D (alkene: 2.09–3.13%) | |||
| Bicyclogermacrene (0.60–1.01%) | |||
|
| |||
| Pakistan | Aerial parts | Carvone (51.7%) | [5] |
| cis-Carveol (24.3%) | |||
| Limonene (5.3%) | |||
| 1,8-Cineol (4.0%) | |||
| cis-Dihydrocarvone (2.2%) | |||
| Carvyl acetate (2.1%) | |||
| cis-Sabinene hydrate (1.0%) | |||
Figure 1.
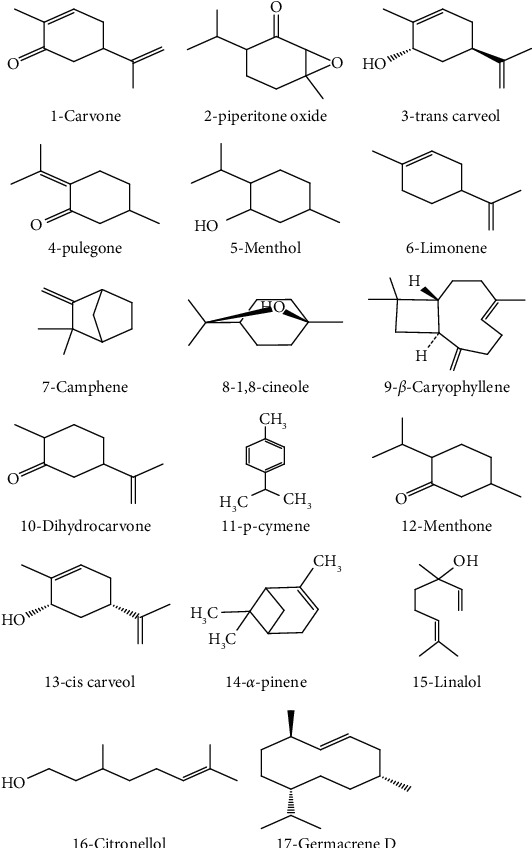
Chemical structures of terpenoids identified.
However, four chemotypes of M. spicata were found in Brazil, characterized by the dominant occurrence of carvone which vary from 39.42% to 72.28% and piperitone presented high level 81.18% [7, 56], Although carvone was constantly present as a chief component among spearmint species, there was one landrace with linalool content up to 58.51%. Since all the studies were carried out in the same environmental conditions, this variation may be triggered by their different genetic backgrounds, having evolved due to complex geographic-environmental differences across Brazil. Interestingly, in most M. spicata EOs, carvone is the major constituent, notably found in quantities above 50% in EOs extracted from plants cultivated in Hungary, Iran, Bangladesh, Serbia, Czech Republic, and Pakistan [3, 5, 40, 46, 47, 52, 53].
Furthermore, the occurrence of huge chemical variations among Mentha accessions collected from diverse countries seems to be due to the divergent climatological and geographical conditions; existing variations in oil content and composition may be attributed to factors related to ecotype and the environment including temperature, relative humidity, irradiance, and photoperiod [34]. Additionally, the reported yields of carvone for M. spicata range from 39.21% to 75.53%, being the highest value found for plants cultivated in Tunisia [51].
As given in Table 2, plants cultivated in several states in Iran usually produce EOs with high (>50%) 1,8-cineole content [39]. Similarly, M. spicata populations in China also show certain stability in essential oils, with carvone chemotype affording high yield 46.7–65.4% above, while dihydrocarveol acetate (0.2–7%) observed in Chinese spearmint is the only oxygenated sesquiterpenes [46]. Also, a large chemical variability is observed among M. spicata essential oil extracted by different methods. Such variation can be attributed to several factors, including genetic, environmental, and their interaction effects, such as plant part, harvest time, extraction method, ecotype, and geographic origin (climate, edaphic, elevation, and topography) [4].
M. spicata has a broad spectrum of bioactive compounds; preliminary screening of M. spicata revealed the presence of polyphenols, flavonoids, tannins, sterols, triterpenes, and glycosides [58]. Besides, the chemical composition of M. spicata methanolic extracts harvested from different regions of India confirmed the presence of alcohols, phenols, alkanes, alkenes, carbonyl, carboxylic acids, and aromatic compounds [35]. Besides, Bimakr et al. identified the flavonoid content from M. spicata leaves by using conventional Soxhlet extraction (CSE) and supercritical carbon dioxide (SC-CO2) extraction [42]. The highest content was obtained with methanol solvent, which extracted seven flavonoids. The highest recovery was recorded for the free aglycone apigenin 27–39.2%, followed by naringenin 5.4–24.9%, epicatechin 15.6–16.3%, catechin 14–14.4%, rutin 14.8–16.1%, myricetin 4.1–11.7%, and luteolin 9.3–65.7% (Figure 2 and Table 2); the same study also identified apigenin as the major isolated flavonoid (6.14 ± 0.76%) from ethanolic and hydroethanolic fractions. Interestingly, supercritical carbon dioxide extract was found to have more main flavonoid compounds and high recovery comparing to the 70% ethanol Soxhlet extraction [42].
Figure 2.
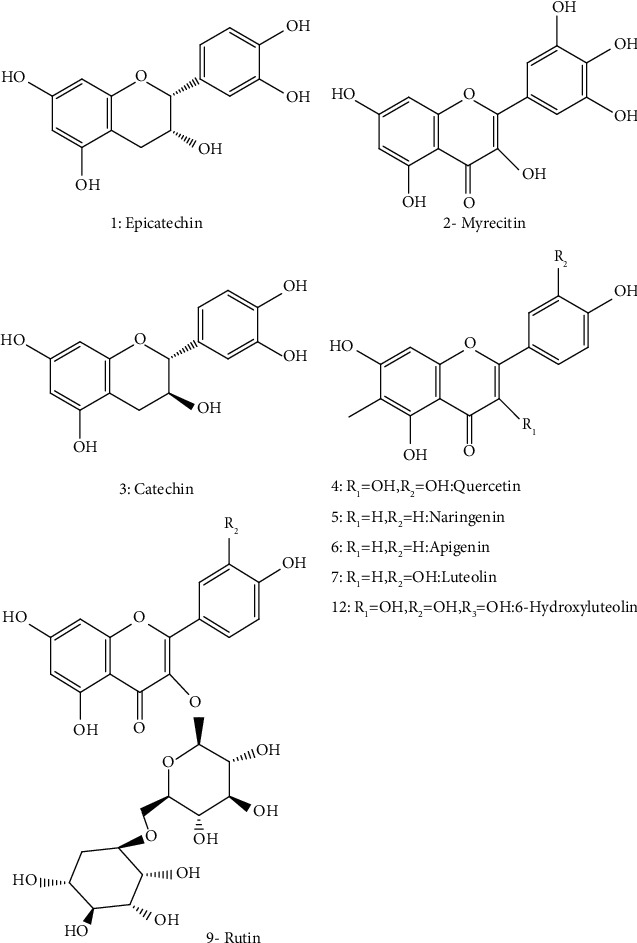
Structures of some flavonoids identified in M. spicata.
The ethanolic extracts of M. spicata contain a large amount of phenolic compounds (polyphenols, flavonoids, and caffeic acid derivatives); ferulic acid was determined in the highest concentration (27.32%), followed by p-coumaric acid (15.24%) and sinapic acid (6.60%). Caftaric acid, caffeic acid, and chlorogenic acid were also identified in low quantities (Figure 2). In addition, luteolin was identified and quantified (4.68%) in M. spicata extract (Figure 2, Table 2) [50]. For fatty acids composition, the EOs produced by M. spicata are the most widely investigated among all Mentha species. Alpha-linolenic acid (48.17%) has been found to be the major polyunsaturated fatty acid of M. spicata. Linoleic acid (31.14%) is the second major polyunsaturated fatty acid in the present study. In comparison, oleic acid (8.19%) and palmitic acid (5.11%) are determined as the major monounsaturated fatty acids, stearidonic acid (3.02%), γ-linolenic acid (2.07%), and stearic acid (1.92%) (Figure 3, Table 2). Various phytosterols including ergosterol (51.42%), stigmasterol (7.6%), and beta-sitosterol (2.86%) (Figure 4) have been found in M. spicata [33]. Moreover, M. spicata contains r-tocopherol (6.11%) and vitamin D3 (31.74%) as lipide-soluble vitamins (Table 2). On the other hand, naringenin (55.44%), naringin (25%), and quercetin (19.38%) have been identified as the major flavonoids in the seeds of M. spicata; while, myricetin and catechin constituents are not detected [33] (Figure 5 and Table 2). Polar extracts of spearmint leaves are characterized mainly by a high content of phenolic compounds; the sum of rosmarinic acid and its derivatives was about 88% of the total amount of detected phenolics, followed by salvianolic acids (5.6%) and caffeoylquinic acids (1.2%). Hydroxycinnamic acids, including caftaric acid, represented about 1.1% of total phenolics. All other detected phenolic groups, such as flavonols, flavanones, flavones, hydroxybenzoic acids, and hydroxyphenyl propanoic acids, represented approximately 1% [30].
Figure 3.
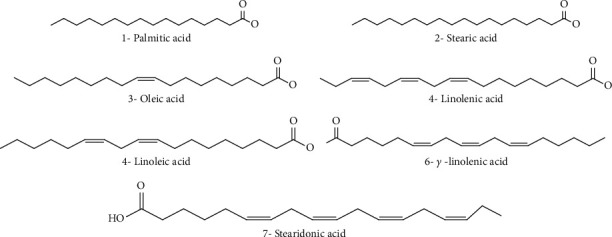
Chemical structures of fatty acids identified in M. spicata.
Figure 4.
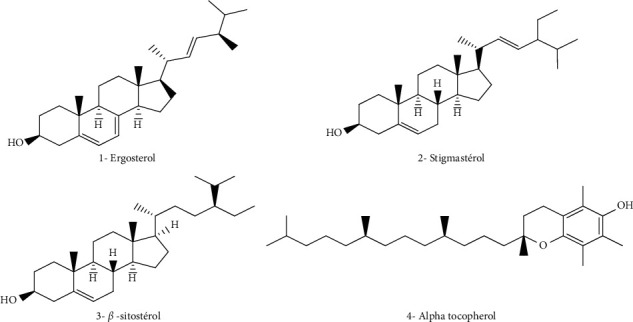
Chemical structures of some sterols identified in M. spicata essential oils.
Figure 5.
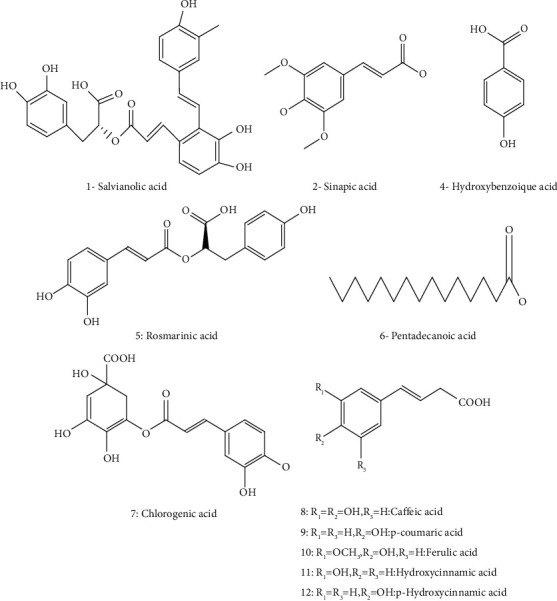
Chemical structures of phenolic acids identified in M. spicata.
3.4. Mineral and Heavy Metal Contents
Mint tea may be an important source of macro and micrometallic elements, which are essential for human health. However, literature reflects enormous variability in determined concentrations. Indeed, Subramanian et al. [59] revealed that total metal concentrations of Fe, Na, Mg, Mn, Pb, Cd, Cu, and Zn in Mentha spicata were 395.74 ± 4.09 mg/kg, 808.09 ± 1.64 mg/kg, 532.72 ± 0.93 mg/kg, 85.72 ± 1.13 mg/kg, 9.89 ± 0.36 mg/kg, 0.74 ± 0.07 mg/kg, 29.83 ± 3.16 mg/kg, and 49.76 ± 4.12 mg/kg, respectively. In another study, Choudhury et al. [60] analyzed ten Mentha spicata leaves samples collected from four different locations in Northwest India for minor and trace elements including heavy toxic metals using thermal neutron activation analysis (TNAA) and atomic absorption spectrophotometry (AAS). The authors revealed that the most elements were found in widely varying amounts depending on the location: Na (0.21–0.86 mg/g), K(12.4–53.3 mg/g), and Ca (5.82–16.8 mg/g); whereas, mean contents of other nutrient elements in mint were as follows: Fe (108 ± 22 μg/g), Mg (4.83 ± 0.92 mg/g), Mn (53.5 ± 9.6 μg/g), P (3.88 ± 0.94 mg/g), Cu (16.9 ± 1.8 μg/g), Zn (21.0 ± 4.7 μg/g), and Se (0.18 ± 0.03 μg/g). The toxic heavy metals such as Hg (97–983 ng/g), Sb (1.8–315 ng/g), Ni (0.37–3.22 ng/g), Cd (15–772 ng/g), and As (98–320 ng/g) are all found at ng/g level only but vary in a wide range. Moreover, aerial parts M. spicata from Iran contains 129.76 μg/g of Fe, 8.52 μg/g of Zn, and 6.8 μg/g of Mn [61].
3.5. Pharmacological Properties of M. spicata
M. spicata essential oils and extracts exhibit different biological and pharmacological properties (Figure 6). These properties will be discussed in the following sections.
Figure 6.
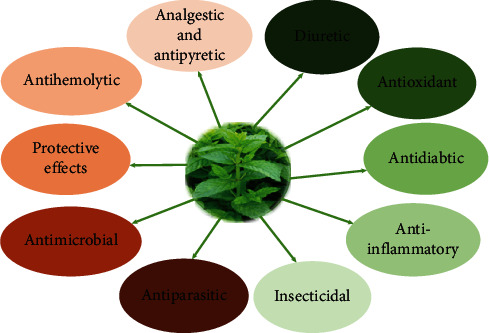
Biological and pharmacological properties of Mentha spicata.
3.5.1. Antifungal Activity
Several studies investigated the antifungal activity of Mentha spicata extracts using different parts of the plant and different methods such as the disc diffusion method, microdilution method, agar well diffusion method, spots method, and microdilution broth susceptibility assay [5, 11, 62, 63].
Table 3 provides all studies that examined the antifungal potential of M. spicata extracts, showing the type of extract, plant part used, used method, tested strains, and key results. Using the disc diffusion method, Alaklabi et al. [62] assessed the antifungal activity of hexane, chloroform, ethyl acetate, methanol, ethanol, toluene, n-butanol, n-propanol, isopropanol, and water extracts from the root of M. spicata against Aspergillus niger, Candida albicans, Cryptococcus neoformans, and Microsporum audouinii. Water extract showed the highest activity against M. audouinii (MIC: 16 μg/mL). It revealed a remarkable antifungal response against other fungal species, A. niger (MIC = 32 μg/mL), C. albicans (MIC = 64 μg/mL), and C. neoformans (MIC = 32 μg/mL). Hexane, chloroform, and ethyl acetate extracts exhibited high antifungal activity against M. audouinii with a MIC equal to 32 μg/mL, 64 μg/mL, and 32 μg/mL, respectively. In contrast, the same extracts did not show a significant effect against the other fungal strains tested. Moreover, C. albicans was significantly inhibited by toluene and n-butanol extracts (MIC = 64 μg/mL), whereas the fungal activity of A. niger was highly reduced by using methanol and ethanol extracts (MIC = 64 μg/mL). Using the same method to screen the antifungal activity of M. spicata root extracts, isopropanol extract was found to be less active for the four fungal strains evaluated [62].
Table 3.
Antifungal activity of Mentha spicata.
| Used part | Extracts | Used method | Tested strains | Key results | References |
|---|---|---|---|---|---|
| Root | Hexane extract | Disc diffusion method | A. niger | MIC > 356 μg/mL | [62] |
| C. albicans | MIC > 356 μg/mL | ||||
| C. neoformans | MIC > 356 μg/mL | ||||
| M. audouinii | MIC = 32 μg/mL | ||||
| Root | Chloroform extract | A. niger | MIC > 356 μg/mL | [62] | |
| C. albicans | MIC > 356 μg/mL | ||||
| C. neoformans | MIC = 64 μg/mL | ||||
| M. audouinii | MIC = 64 μg/mL | ||||
| Root | Ethyl acetate extract | A. niger | MIC = 128 μg/mL | [62] | |
| C. albicans | MIC = 128 μg/mL | ||||
| C. neoformans | MIC = 128 μg/mL | ||||
| M. audouinii | MIC = 32 μg/mL | ||||
| Root | Methanol extract | A. niger | MIC = 64 μg/mL | [62] | |
| C. albicans | MIC = 128 μg/mL | ||||
| C. neoformans | MIC = 128 μg/mL | ||||
| M. audouinii | MIC > 356 μg/mL | ||||
|
| |||||
| Root | Ethanol extract | Disc diffusion method | A. niger | MIC = 64 μg/mL | [62] |
| C. albicans | MIC = 128 μg/mL | ||||
| C. neoformans | MIC = 128 μg/mL | ||||
| M. audouinii | MIC > 356 μg/mL | ||||
| Root | Toluene extract | A. niger | MIC > 356 μg/mL | [62] | |
| C. albicans | MIC = 64 μg/mL | ||||
| C. neoformans | MIC > 356 μg/mL | ||||
| M. audouinii | MIC = 128 μg/mL | ||||
| Root | N-butanol extract | A. niger | MIC = 128 μg/mL | [62] | |
| C. albicans | MIC = 64 μg/mL | ||||
| C. neoformans | MIC > 356 μg/mL | ||||
| M. audouinii | MIC = 128 μg/mL | ||||
| Root | N-propanol extract | A. niger | MIC = 64 μg/mL | [62] | |
| C. albicans | MIC = 64 μg/mL | ||||
| C. neoformans | MIC = 32 μg/mL | ||||
| M. audouinii | MIC = 128 μg/mL | ||||
|
| |||||
| Root | Isopropanol extract | Disc diffusion method | A. niger | MIC = 128 μg/mL | [62] |
| C. albicans | MIC = 128 μg/mL | ||||
| C. neoformans | MIC > 356 μg/mL | ||||
| M. audouinii | MIC > 356 μg/mL | ||||
| Root | Water extract | A. niger | MIC = 32 μg/mL | [62] | |
| C. albicans | MIC = 64 μg/mL | ||||
| C. neoformans | MIC = 32 μg/mL | ||||
| M. audouinii | MIC = 16 μg/mL | ||||
|
| |||||
| Aerial parts | Essential oil | Microdilution method | Candida glabrata | MIC = 256 μg/mL | [48] |
| Aerial parts | Volatile oil | Agar well diffusion method | R. solani | Inhibition = 100% at dose of 12 μL | [64] |
| A. solani | Inhibition = 100% at dose of 12 μL | ||||
| F. oxysporum f.sp. radicis-lycopersici | Inhibition = 100% at dose of 12 μL | ||||
| V. dahliae | Inhibition = 100% at dose of 12 μL | ||||
|
| |||||
| Leaves | Essential oil | Spots method | A. niger 2CA 936 | Ф = 36.0 ± 1.0 mm | [65] |
| A. flavus NRRL 391 | Ф = 43.7 ± 0.6 mm | ||||
| C. albicans (ATCC 1024) | Ф = 44.3 ± 1.1 mm | ||||
|
| |||||
| Leaves | Essential oil | Disc method | A. niger 2CA 936 | Ф = 32.0 ± 1.0 mm | [65] |
| A. flavus NRRL 391 | Ф = 36.0 ± 2.0 mm | ||||
| C. albicans (ATCC 1024) | Ф = 23.3 ± 0.6 mm | ||||
|
| |||||
| Aerial parts | Essential oils | Disc diffusion method Microdilution broth susceptibility assay |
Aspergillus niger | Ф = 26.9 ± 1.2 mm | [5] |
| MIC = 0.07 ± 0.00 μg/mL | |||||
| Mucor mucedo | Ф = 26.2 ± 0.8 mm | ||||
| MIC = 0.08 ± 0.00 μg/mL | |||||
| Fusarium solani | Ф = 25.2 ± 1.0 mm | ||||
| MIC = 0.09 ± 0.00 μg/mL | |||||
| Botryodiplodia theobromae | Ф = 23.0 ± 1.1 mm | ||||
| MIC = 0.11 ± 0.01 μg/mL | |||||
| Rhizopus solani | Ф = 26.3 ± 0.8 mm | ||||
| MIC = 0.09 ± 0.00 μg/mL | |||||
|
| |||||
| Aerial parts | Essential oil | Absidia ramosa | Mycelial inhibition = 100% | [66] | |
| Alternaria alternata | Mycelial inhibition = 100% | ||||
| Aspergillus fumigatus | Mycelial inhibition = 100% | ||||
| Aspergillus glaucus | Mycelial inhibition = 100% | ||||
| Aspergillus luchuensis | Mycelial inhibition = 91.72 ± 0.36% | ||||
| Aspergillus niger | Mycelial inhibition = 100% | ||||
| Aspergillus terreus | Mycelial inhibition = 75.67 ± 0.74% | ||||
| Aspergillus unguis | Mycelial inhibition = 100% | ||||
| Cladosporium cladosporioides | Mycelial inhibition = 100% | ||||
| Curvularia lunata | Mycelial inhibition = 100% | ||||
| Fusarium oxysporum | Mycelial inhibition = 100% | ||||
| Mucor spp. | Mycelial inhibition = 100% | ||||
| Mycelia sterilia | Mycelial inhibition = 100% | ||||
| Penicillium citrinum | Mycelial inhibition = 100% | ||||
| Penicillium italicum | Mycelial inhibition = 100% | ||||
| Penicillium luteum | Mycelial inhibition = 100% | ||||
| Penicillium purpurogenum | Mycelial inhibition = 100% | ||||
| Rhizopus stolonifer | Mycelial inhibition = 100% | ||||
| Spondylocladium australe | Mycelial inhibition = 100% | ||||
|
| |||||
| Essential oils | Disc diffusion method | A. niger | MIC = 6.25 μg/mL MBC = 12.50 μg/mL |
[11] | |
| Leaves | Hexane | Agar well diffusion techniques | Saccharomyces cerevisiae | Ф = 25 | [63] |
| Aspergillus niger | Ф = 26 mm | ||||
| Leaves | Petroleum ether | Agar well diffusion techniques | Saccharomyces cerevisiae | Ф = 24 mm | [63] |
| Aspergillus niger | Ф = 27 mm | ||||
| Aerial parts | Essential oils | Disc diffusion method | Candida albicans | MIC < 3.19 μg/mL | [67] |
| Candida tropicalis | MIC < 3.19 μg/mL | ||||
| Leaves | Ethanol extract | Fusarium oxysporum f.sp. lentis | Inhibition = 1oo% | [68] | |
| Leaves | Essential oil | Agar diffusion method | Aspergillus niger (ATCC 9763) | Ф = 19 mm | [69] |
| Candida albicans (ATCC 7596) | Ф = 18 mm | ||||
|
| |||||
| Essential oil | Agar well diffusion method | Aspergillus niger | Ф = 15.7 ± 0.09 mm | [70] | |
| Aspergillus spp., | Ф = 13 ± 0.13 mm | ||||
| Candida albicans | Ф = 11.8 ± 0.10 mm | ||||
| Rhizopus nigricans | No inhibition | ||||
|
| |||||
| Leaves | Essential oil | Agar diffusion method | Candida albicans | Ф = 16 mm at concentration of 100 mg/mL | [71] |
| Leaves | Essential oil | Agar well diffusion method | Mucor ramamnianus (ATCC 9314) | Ф = 40 mm | [72] |
| Aspergillus ochraceus (NRRL 3174) | Ф = 43 mm | ||||
| Candida albicans (IPA 200) | Ф = 21 mm | ||||
| Saccharomyces cerevisiae (ATCC 4226 A) | Ф = 25 mm | ||||
To investigate the antifungal properties of essential oil isolated from the aerial parts of M. spicata cultivated in the Algerian Saharan Atlas, the results published by Bardaweel et al. [48] showed a lower activity of essential oil of M. spicata against Candida glabrata (MIC = 256 μg/mL) by employing the microdilution method. Nevertheless, in the Turkish study conducted by Bayan et al. [64], the volatile oil from M. spicata extracted of aerial parts exhibited a strong fungitoxicity effect with 100% of inhibition of mycelium growth in F. oxysporum f.sp. radicis-lycopersici (FORL), Verticillium dahliae Kleb (V. dahliae), Alternaria solani (A. solani), and Rhizoctonia solani J.G. Kühn. (R. solani) at a dose of 12 μL petri−1 by using the agar well diffusion method.
In another study from Pakistan, Hussain et al. [5] evaluated the antifungal activity of essential oil of spearmint (Mentha spicata L.) isolated from dried aerial parts against five fungal strains. The results showed that Aspergillus niger was the most responsive fungal species presenting the largest zone of inhibition (26.9 mm) with the MIC value of 0.07 mg/mL, followed by Mucor mucedo (Ф = 26.2 ± 0.8 mm and MIC = 0.08 ± 0.00 μg/mL), Rhizopus solani (Ф = 26.3 ± 0.8 mm and MIC = 0.09 ± 0.00 μg/mL), and Fusarium solani (Ф = 25.2 ± 1.0 mm and MIC = 0.09 ± 0.00 μg/mL). However, B. theobromae was observed to be the most resistant fungus with the smallest inhibition zone (23.0 mm) and a MIC value equal to 0.11 mg/mL by using microdilution broth susceptibility assay.
Additionally, Kedia et al. [66] tested the antifungal potency of essential oil of spearmint against 19 food-deteriorating molds using the poisoned food assay. The fundings showed that the oil of M. spicata has a notable potential to inhibit the fungal growth of all fungi species, causing 100% of mycelial inhibition at 1.0 μL ml−1 excluding Aspergillus luchuensis and Aspergillus terreus, where the percentage of mycelial inhibition was 91.72 ± 0.36% and 75.67 ± 0.74%, respectively. The results of testing the nature toxicity of the oil from M. spicata revealed that spearmint essential oil possessed a fungicidal effect in Cladosporium cladosporioides, Mycelia sterilia, Alternaria alternata, and Curvularia lunata at 1.0 μL mL−1. In their study, Liu et al. [11] investigated the biological properties of the essential oil isolated from aerial parts of M. spicata from China. Using the disc diffusion method, the results of this study showed quite strong antifungal potency against A. niger with an MIC value of 6.25 μg/mL and an MBC value of 12.50 μg/mL. Compared to a study carried out by Şarer et al. [67] from eastern Turkey, the oil of M. spicata subsp. spicata exhibited high antifungal activity against Candida albicans and Candida tropicalis with an MIC value less than 3.19 μg/mL.
Regarding testing the potential antimicrobial effects of M. spicata, [45] investigated the essential oil extracted from air-dried leaves of Algerian spearmint against Candida albicans (ATCC 1024) strain and two Aspergillus species (flavus NRRL 391 and niger 2CA 936). Using the spots method, their finding indicates that Candida albicans (ATCC 1024) was the most sensitive species with a diameter of growth inhibition zones equal to 44.3 ± 1.1 mm, followed by A. flavus NRRL 391 (Ф = 43.7 ± 0.6 mm), and A. niger 2CA 936 (Ф = 36.0 ± 1.0 mm). The disc diffusion method also showed high activity against Aspergillus species, A. flavus NRRL 391 (Ф = 36.0 ± 2.0 mm) and A. niger 2CA 936 (Ф = 32.0 ± 1.0 mm) than C. albicans (ATCC 1024) (Ф = 23.3 ± 0.6 mm).
On the other hand, Ojewumi et al. [63] demonstrated the antimicrobial role of the leaf oil extract of M. spicata from Nigeria by using two types of petroleum ether and hexane extract. They found that the hexane extract showed higher activity against Aspergillus niger (Ф = 26 mm) followed by Saccharomyces cerevisiae (Ф = 25). In addition, they observed that petroleum ether extract showed potent activity against Aspergillus niger (Ф = 27 mm) followed by S. cerevisiae (Ф = 27). Therefore, it was noted that the effectiveness of the two extracts was significantly comparable as the inhibitory zone values are very similar. Furthermore, the ethanolic extract exhibited 100% of inhibition against Fusarium oxysporum f.sp. lentis in the investigation performed by Singh et al. [68] that aimed to study the antifungal activity of M. spicata. The results found were supported by the study conducted in Sudan by Sulieman et al. [69]; they indicated that spearmint oil leaves have demonstrated potent activity against Aspergillus niger (ATCC 9763) with an inhibition zone equal to 19 mm at a high concentration (20%) and (15 mm) at low concentration (5%). In addition, the oil of M. spicata exhibited considerable inhibition capacity against C. albicans with an inhibition zone diameter of 18 mm at higher concentration (20%) and 14 mm at lower concentration (5%). Similarly, the concentration of 100 mg/mL was able to inhibit C. albicans with a diameter of growth inhibition zone reached 16 mm using the agar diffusion method [71].
Zaidi et al. [70] evaluated the antifungal efficiency of oil leaves from M. spicata against four fungal species including A. niger and Aspergillus spp., C. albicans, and Rhizopus nigricans, using the agar well diffusion method. The results showed that Mentha spicata oil exhibited an excellent potential against fungal strains tested but with differing sensitivity. A. niger showed a strong inhibition zone of 15.7 ± 0.09 mm compared to C. albicans, which possessed an inhibition zone of 11.8 ± 0.10 mm. However, M. spicata oil was not able to inhibit the growth of R. nigricans strain. The oil also exhibited an antifungal effect against Aspergillus spp. (13 ± 0.13 mm). In another study, using the agar well diffusion method, essential oil isolated from spearmint was observed to act as a stronger bioactive source against fungal species with a different zone of inhibition. Indeed, inhibition zone diameters for Aspergillus ochraceus (NRRL 3174) (Ф = 43 mm) and Mucor ramamnianus (ATCC 9314) (Ф = 40 mm) were higher than inhibition zone diameters for S. cerevisiae (ATCC 4226 A) (Ф = 25 mm) and C. albicans IPA 200 (Ф = 21 mm) [72].
3.5.2. Antibacterial Activity
For over 60 years, antimicrobial agents have been used to treat infections in humans, animals, and plants. Currently, they are among the most widely used therapeutic agents in human and veterinary medicine [73]. At the start of antibiotic therapy, as resistant strains were low and highly effective antimicrobial agents of different classes were detected, antimicrobial resistance was not considered a major problem. This has forced sensitive bacteria living in close contact with antimicrobial producers to develop mechanisms to bypass the inhibitory effects of antimicrobial agents (Table 4). In the context of this study, several in vitro studies have determined the antibacterial activity of M. spicata essential oils and solvent extracts against various bacterial strains, either clinical or reference, using the agar diffusion methods (disks or well) and the agar and broth dilution methods [5, 74, 89]. In most of these studies, qualitative inhibition was determined by the dilution method, which is used to assess minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values [5, 74, 89]. Indeed, the increased selective pressure imposed by the widespread use of antimicrobial agents has clearly accelerated the development and spread of bacterial resistance to antimicrobial agents [5, 74, 89]. These observations underscore the enormous flexibility of bacteria to resist less favorable environmental conditions by constantly developing new survival strategies.
Table 4.
Antibacterial activity of Mentha spicata.
| Parts used | Extracts | Methods used | Strains tested | Key results | References |
|---|---|---|---|---|---|
| Leaves | Essential oils | Broth microdilution method | Staphylococcus aureus (ATCC 14458) | MIC = 3.2 μL/mL | [6] |
| Staphylococcus epidermidis (ATCC 12228) | MIC = 1.6 μL/mL | ||||
| Bacillus cereus (ATCC 11778) | MIC = 1.6 μL/mL | ||||
| Listeria monocytogenes (ATCC 7644) | MIC = 3.2 μL/mL | ||||
| Escherichia coli (ATCC 11229) | MIC = 3.2 μL/mL | ||||
| Salmonella enterica subsp. enterica serovar typhimurium (ATCC 13311) | MIC = 1.6 μL/mL | ||||
| Salmonella. enterica subsp. enterica serovar typhi (ATCC 19214) | MIC = 1.6 μL/mL | ||||
| Shigella flexneri (ATCC 12022) | MIC = 3.2 μL/mL | ||||
|
| |||||
| Aerial parts | Essential oils | Disc diffusion assay | P. aeruginosa (ATCC 27853) | No inhibition | [7] |
| Escherichia coli (ATCC 25922) | Ф = 9 mm | ||||
| Staphylococcus aureus (ATCC 25923) | Ф = 11 mm | ||||
| Staphylococcus epidermidis | Ф = 10 mm | ||||
| Streptococcus pneumoniae | Ф = 13 mm | ||||
| Streptococcus pyogenes | Ф = 16 mm | ||||
| Klebsiella pneumoniae | Ф = 8 mm | ||||
| Salmonella typhi | Ф = 8 mm | ||||
| Shigella sonnei | Ф = 9 mm | ||||
|
| |||||
| Leaves | Ethanol extract | Disc diffusion assay | Salmonella paratyphi | Ф = 17.00 ± 2.00 mm | [74] |
| Shigella boydii | Ф = 31.67 ± 1.53 mm | ||||
| Staphylococcus aureus | Ф = 23.00 ± 1.00 mm | ||||
| Escherichia coli | Ф = 9.00 ± 1.00 mm | ||||
| Vibrio cholerae | Ф = 12.00 ± 1.00 mm | ||||
| Pseudomonas aeruginosa | Trace activity | ||||
| Enterococcus faecalis | No activity | ||||
| Salmonella typhi | Trace activity | ||||
| Proteus vulgaris | No activity | ||||
| Klebsiella pneumoniae | No activity | ||||
|
| |||||
| Leaves | Hexane fraction | Disc diffusion assay | Salmonella paratyphi | Ф = 25.67 ± 2.08 mm | [74] |
| Shigella boydii | Ф = 36.00 ± 1.00 mm | ||||
| Staphylococcus aureus | Ф = 22.33 ± 1.53 mm | ||||
| Escherichia coli | Ф = 10.67 ± 2.52 mm | ||||
| Vibrio cholerae | Ф = 18.67 ± 0.58 mm | ||||
| Pseudomonas aeruginosa | Trace activity | ||||
| Enterococcus faecalis | No activity | ||||
| Salmonella typhi | No activity | ||||
| Proteus vulgaris | No activity | ||||
| Klebsiella pneumoniae | No activity | ||||
|
| |||||
| Leaves | Chloroform | Disc diffusion assay | Salmonella paratyphi | Ф = 22.67 ± 2.52 mm | [74] |
| Shigella boydii | Ф = 34.00 ± 1.00 mm | ||||
| Staphylococcus aureus | Ф = 24.00 ± 1.00 mm | ||||
| Escherichia coli | Ф = 18.67 ± 1.53 mm | ||||
| Vibrio cholerae | Ф = 16.00 ± 1.00 mm | ||||
| Pseudomonas aeruginosa | Ф = 12.33 ± 1.53 mm | ||||
| Enterococcus faecalis | Ф = 8.33 ± 0.58 mm | ||||
| Salmonella typhi | No activity | ||||
| Proteus vulgaris | No activity | ||||
| Klebsiella pneumoniae | No activity | ||||
|
| |||||
| Leaves | Ethyl acetate fraction | Disc diffusion assay | Salmonella paratyphi | Ф = 20.67 ± 1.53 mm | [74] |
| Shigella boydii | Ф = 32.67 ± 2.52 mm | ||||
| Staphylococcus aureus | Ф = 25.33 ± 0.58 mm | ||||
| Escherichia coli | Ф = 18.33 ± 1.53 mm | ||||
| Vibrio cholerae | Ф = 17.33 ± 1.53 mm | ||||
| Pseudomonas aeruginosa | Ф = 8.00 ± 1.00 mm | ||||
| Enterococcus faecalis | No activity | ||||
| Salmonella typhi | No activity | ||||
| Proteus vulgaris | No activity | ||||
| Klebsiella pneumoniae | No activity | ||||
|
| |||||
| Leaves | Aqueous fraction | Disc diffusion assay | Salmonella paratyphi | Ф = 22.33 ± 2.52 mm | [74] |
| Shigella boydii | Ф = 36.00 ± 1.00 mm | ||||
| Staphylococcus aureus | Ф = 31.00 ± 1.00 mm | ||||
| Escherichia coli | Ф = 21.00 ± 1.00 mm | ||||
| Vibrio cholerae | Ф = 20.33 ± 0.58 mm | ||||
| Pseudomonas aeruginosa | Ф = 10.00 ± 1.00 mm | ||||
| Enterococcus faecalis | No activity | ||||
| Salmonella typhi | Trace activity | ||||
| Proteus vulgaris | No activity | ||||
| Klebsiella pneumoniae | No activity | ||||
|
| |||||
| Aerial parts | Essential oil | Microdilution method | Staphylococcus epidermidis | MIC = 32 μg/mL | [48] |
| Escherichia coli | MIC = 64 μg/mL | ||||
|
| |||||
| Aerial parts | Volatile oil | Disk diffusion method | Xanthomonas spp. ZI378 | Ф = 14 mm | [64] |
| Xanthomonas spp. ZI376 | Ф = 14 mm | ||||
| Xanthomonas spp. ZI375 | Ф = 13 mm | ||||
| Xanthomonas spp. ZI373 | Ф = 13 mm | ||||
| Xanthomonas spp. ZI370 | Ф = 13 mm | ||||
| Xanthomonas spp. ZI368 | Ф = 13 mm | ||||
| Xanthomonas spp. ZI366 | Ф = 12 mm | ||||
| Xanthomonas spp. ZI365 | Ф = 16 mm | ||||
|
| |||||
| Leaves | Essential oil | Agar disc diffusion method Microbroth dilution |
Staphylococcus aureus (ATCC 29213) | Ф = 19 ± 1.73 mm | [75] |
| MIC = 1.25 μg/mL | |||||
| MBC = 1.25 μg/mL | |||||
| Escherichia coli (ATCC 25922) | Ф = 13.66 ± 1.1 mm | ||||
| MIC = 1.25 μg/mL | |||||
| MBC = 2.5 μg/mL | |||||
| Pseudomonas aeruginosa (ATCC 27853) | Ф = 9.5 ± 0.70 mm | ||||
| MIC > 10 | |||||
| MBC > 10 | |||||
|
| |||||
| Leaves | Essential oil | Disc method | MRSA (ATCC 43300) | Ф = 24.0 ± 1.0 mm | [65] |
| Bacillus subtilis (ATCC 6633) | Ф = 17.7 ± 0.6 mm | ||||
| Staphylococcus aureus (NCC B9163) | Ф = 14.3 ± 1.5 mm | ||||
| Escherichia coli (ATCC 25922) | Ф = 11.0 ± 1.0 mm | ||||
| Pseudomonas aeruginosa (ATCC 27853) | Ф = 6.0 ± 0.0 mm | ||||
| Klebsiella pneumonia E47 | Ф = 10.3 ± 0.6 mm | ||||
|
| |||||
| Leaves | Essential oil | Spots method | MRSA (ATCC 43300) | Ф = 22.3 ± 1.5 mm | [65] |
| Bacillus subtilis (ATCC 6633) | Ф = 32.7 ± 0.6 mm | ||||
| Staphylococcus aureus (NCC B9163) | Ф = 20.3 ± 0.6 mm | ||||
| Escherichia coli (ATCC 25922) | Ф = 22.0 ± 1.0 mm | ||||
| Pseudomonas aeruginosa (ATCC 27853) | Ф = 6.0 ± 0.0 mm | ||||
| Klebsiella pneumonia (E47) | Ф = 17.3 ± 0.6 mm | ||||
|
| |||||
| Aerial parts | Essential oil | Disc diffusion method | Escherichia coli | Ф = 14 ± 0.6 mm | [76] |
| Salmonella enterica subsp. enterica | Ф = 10 ± 0.8 mm | ||||
| Pasteurella multocida | Ф = 12 ± 1.0 mm | ||||
| Staphylococcus aureus | Ф = 9 ± 1.1 mm | ||||
|
| |||||
| Essential oils | Disc diffusion assay Microwell dilution assay |
Escherichia coli (O157H7) | Ф = 10 mm | [77] | |
| MIC = 2.26 ± 0.11 μg/mL | |||||
| MBC = 3.66 ± 0.11 μg/mL | |||||
| Listeria monocytogenes | Ф = 11 mm | ||||
| MIC = 1.33 ± 0.11 μg/mL | |||||
| MBC = not observed | |||||
|
| |||||
| Leaves | Essential oils | Disc diffusion technique Checker board technique |
Staphylococcus aureus 29737 | Ф = 10.0 mm | [78] |
| MIC = 10 μg/mL | |||||
| Staphylococcus aureus ML 267 | Ф = 10.0 mm | ||||
| MIC = 10 μg/mL | |||||
| Suillus luteus 9341 | Ф = 11.0 mm | ||||
| MIC = 5 μg/mL | |||||
| Bacillus pumilus 8241 | Ф = 10.0 mm | ||||
| MIC = 10 μg/mL | |||||
| Bacillus subtilis (ATCC) | Ф = 11.0 mm | ||||
| MIC = 10 μg/mL | |||||
| Escherichia coli (ATCC 10536) | Ф = 9.0 mm | ||||
| MIC = 50 μg/mL | |||||
| Escherichia coli VC Sonawave 3 : 37 C | Ф = 9.0 mm | ||||
| MIC = 50 μg/mL | |||||
| Escherichia coli (CD/99/1) | Ф = 9.5 mm | ||||
| MIC = 50 μg/mL | |||||
| Escherichia coli (RP4) | Ф = 9.0 mm | ||||
| MIC = 25 μg/mL | |||||
| Escherichia coli (18/9) | Ф = 9.0 mm | ||||
| MIC = 25 μg/mL | |||||
| Escherichia coli (K88) | Ф = 8.5 mm | ||||
| MIC = 25 μg/mL | |||||
| Shigella dysenteriae L. | Ф = 10.0 mm | ||||
| MIC = 10 μg/mL | |||||
| Shigella sonnei 1 | Ф = 10.0 mm | ||||
| MIC = 10 μg/mL | |||||
| Shigella sonnei BCH 217 | Ф = 12.0 mm | ||||
| MIC = 5 μg/mL | |||||
| Shigella flexneri type 6 | Ф = 9.0 mm | ||||
| MIC = 10 μg/mL | |||||
| Shigella boydii 937 | Ф = 9.5 mm | ||||
| MIC = 10 μg/mL | |||||
| Pseudomonas aeruginosa (ATCC 25619) | Ф = 11.0 mm | ||||
| MIC = 10 μg/mL | |||||
| Vibrio cholerae 2 | Ф = 10.0 mm | ||||
| MIC = 50 μg/mL | |||||
| Vibrio cholerae 785 | Ф = 10.0 mm | ||||
| MIC = 50 μg/mL | |||||
| Vibrio cholerae 1037 | Ф = 9.0 mm | ||||
| MIC = 50 μg/mL | |||||
|
| |||||
| Leaves | Essential oils | Agar well diffusion method Dilution method |
Staphylococcus aureus | Ф = 17 ± 0.01 mm | [79] |
| MIC = 0.4 ± 0.01 μg/mL | |||||
| Escherichia coli | Ф = 14 ± 0.01 mm | ||||
| MIC = 0.5 ± 0.02 μg/mL | |||||
| Erwinia carotovora | Ф = 14 ± 0.01 mm | ||||
| MIC = 0.5 ± 0.02 μg/mL | |||||
| Bacillus subtilis | Ф = 17 ± 0.01 mm | ||||
| MIC = 0.6 ± 0.01 μg/mL | |||||
| Xanthomonas campestris | Ф = 22 ± 0.01 mm | ||||
| MIC = 0.5 ± 0.02 μg/mL | |||||
| Klebsiella pneumoniae | Ф = 20 ± 0.01 mm | ||||
| MIC = 0.4 ± 0.01 μg/mL | |||||
| Leaves | Essential oils | Diffusion method | Bacillus subtilis | Ф = 11.5 ± 0.61 mm | [80] |
| Staphylococcus aureus | Ф = 13 ± 1.52 mm | ||||
| Staphylococcus epidermidis | Ф = 11.2 ± 1.61 mm | ||||
| Escherichia coli | Ф = 21 ± 0.90 mm | ||||
| Pseudomonas aeruginosa | Ф = 16 ± 1.9 mm | ||||
| Salmonella enterica subsp. | Ф = 18 ± 1.33 mm | ||||
|
| |||||
| Aerial parts | Essential oils | Disc diffusion method Microdilution broth assay |
Staphylococcus aureus | Ф = 26.0 ± 1.1 mm | [5] |
| MIC = 0.07 ± 0.00 μg/mL | |||||
| Bacillus subtilis | Ф = 27.1 ± 1.1 mm | ||||
| MIC = 0.05 ± 0.00 μg/mL | |||||
| Pasteurella multocida | Ф = 24.3 ± 0.9 mm | ||||
| MIC = 0.12 ± 0.01 μg/mL | |||||
| Escherichia coli | Ф = 20.3 ± 0.9 mm | ||||
| MIC = 0.21 ± 0.01 μg/mL | |||||
|
| |||||
| Whole plant | Essential oils | Disc diffusion method | Escherichia coli | MIC = 1/250 (V/V) | [81] |
| MBC = 1/250 (V/V) | |||||
|
| |||||
| Not reported | Essential oil | Disc diffusion method | Escherichia coli | MIC = 1.56 μg/mL | [11] |
| MBC = 25 μg/mL | |||||
| Staphylococcus aureus | MIC = 25 μg/mL | ||||
| MBC = 50 μg/mL | |||||
| Saccharomyces cerevisiae | MIC = 0.78 μg/mL | ||||
| MBC = 6.25 μg/mL | |||||
| Penicillium citrinum | MIC = 3.12 μg/mL | ||||
| MBC = 12.50 μg/mL | |||||
|
| |||||
| Leaves | Hexane | Agar well diffusion techniques | Pseudomonas aeruginosa | Ф = 15 mm | [63] |
| Bacillus subtilis | Ф = 10 mm | ||||
| Escherichia coli | Ф = 25 mm | ||||
| Staphylococcus aureus | Ф = 26 mm | ||||
|
| |||||
| Leaves | Petroleum ether | Agar well diffusion techniques | Pseudomonas aeruginosa | Ф = 17 mm | [63] |
| Bacillus subtilis | Ф = 12 mm | ||||
| Escherichia coli | Ф = 26 mm | ||||
| Staphylococcus aureus | Ф = 27 mm | ||||
|
| |||||
| Aerial parts | Essential oils | Disc diffusion method | Staphylococcus aureus | MIC = 15.6 μg/mL | [67] |
| Enterococcus faecalis | MIC = 125 μg/mL | ||||
| Pseudomonas aeruginosa | MIC = 125 μg/mL | ||||
| Escherichia coli | MIC < 3.19 μg/mL | ||||
|
| |||||
| Leaves | Essential oil | Broth microdilution method | Serratia spp. | MIC = 4.75 mg/mL | [82] |
| MBC > 9.5 mg/mL | |||||
| Salmonella spp. | MIC = 2.37 mg/mL | ||||
| MBC > 9.5 mg/mL | |||||
| Kluyvera spp. | MIC = 2.37 mg/mL | ||||
| MBC > 9.5 mg/mL | |||||
| Klebsiella spp. | MIC = 2.37 mg/mL | ||||
| MBC = 9.5 mg/mL | |||||
| Escherichia coli (F5) | MIC = 2.37 mg/mL | ||||
| MBC > 9.5 mg/mL | |||||
| Escherichia coli (F17) | MIC > 9.5 mg/mL | ||||
| MBC > 9.5 mg/mL | |||||
| Escherichia coli (CS31 A) | MIC = 2.37 mg/mL | ||||
| MBC = 9.5 mg/mL | |||||
|
| |||||
| Aerial parts | Essential oil | Disc diffusion method | MRSA | Ф = 17.5 ± 0.7 mm | [31] |
| Staphylococcus aureus (ATCC 6538) | Ф = 11 ± 1.4 mm | ||||
| Pseudomonas aeruginosa | Ф = 21 ± 8.4 mm | ||||
| Escherichia coli Q157 : H7 | Ф = 20.5 ± 2.1 mm | ||||
| Bacillus cereus (CCM99) | Ф = 22.5 ± 0.7 mm | ||||
| Enterococcus faecium (DSM 13590) | Ф = 13 ± 4.2 mm | ||||
|
| |||||
| Leaves | Essential oil | Broth microdilution method | Staphylococcus aureus (ATCC 6538) | MIC = 10 μg/mL | [83] |
| MBC = 10 μg/mL | |||||
| Staphylococcus aureus (ATCC 29213) | MIC = 8 μg/mL | ||||
| MBC = 8 μg/mL | |||||
| Bacillus subtilis (ATCC 6633) | MIC = 2.5 μg/mL | ||||
| MBC = 5 μg/mL | |||||
| Bacillus cereus (ATCC 11774) | MIC = 2.5 μg/mL | ||||
| MBC = 5 μg/mL | |||||
| Listeria monocytogenes (ATCC 19118) | MIC = 2.5 μg/mL | ||||
| MBC = 2.5 μg/mL | |||||
| Salmonella typhimurium (ATCC 14028) | MIC = 10 μg/mL | ||||
| MBC = 10 μg/mL | |||||
| Escherichia coli O157 : H7 (ATCC 10536) | MIC = 10 μg/mL | ||||
| MBC = 10 μg/mL | |||||
|
| |||||
| Not reported | Essential oil | Microdilution method | Staphylococcus aureus | MIC = 0.005 μg/mL | [84] |
| Bacillus subtilis | MIC = 0.005 μg/mL | ||||
| Bacillus cereus | MIC = 0.005 μg/mL | ||||
| Listeria monocytogenes | MIC = 0.005 μg/mL | ||||
| Salmonella typhimurium | MIC = 0.005 μg/mL | ||||
| Escherichia coli O157 : H7 | MIC = 0.005 μg/mL | ||||
|
| |||||
| Leaves | Essential oil | Agar diffusion method | Escherichia coli (ATCC 25922) | Ф = 17 mm | [69] |
| Bacillus subtilis (NCTC 8236) | Ф = 16 mm | ||||
|
| |||||
| Not reported | Decanted essential oil | Disc diffusion assay | Staphylococcus epidermidis | Ф = 2 mm | [85] |
| Enterococcus faecalis | Ф = 5 mm | ||||
| Streptococcus mutans | Ф = 5 mm | ||||
| Escherichia coli | Ф = 6 mm | ||||
| Pseudomonas aeruginosa | No inhibition | ||||
|
| |||||
| Not reported | Recovered essential oil | Disc diffusion assay | Staphylococcus epidermidis | Ф = 2 mm | [85] |
| Enterococcus faecalis | Ф = 4 mm | ||||
| Streptococcus mutans | Ф = 5 mm | ||||
| Escherichia coli | Ф = 6 mm | ||||
| Pseudomonas aeruginosa | No inhibition | ||||
|
| |||||
| Not reported | Essential oil | Agar well diffusion method | Escherichia coli | Ф = 14 ± 0.05 mm | [70] |
| Salmonella typhi | No inhibition | ||||
| Salmonella paratyphi | No inhibition | ||||
| Staphylococcus aureus | Ф = 21 ± 0.09 mm | ||||
| Klebsiella pneumoniae | Ф = 12.7 ± 0.07 mm | ||||
| Pseudomonas aeruginosa | No inhibition | ||||
| Acinetobacter spp. | Ф = 18 ± 0.11 mm | ||||
|
| |||||
| Leaves | Essential oil | Agar diffusion method | Bacillus subtilis | Ф = 15 mm at a concentration of 100 mg/mL | [71] |
| Escherichia coli | Ф = 17 mm at concentration of 100 mg/mL | ||||
| Staphylococcus aureus | Ф = 16 mm at a concentration of 100 mg/mL | ||||
| Pseudomonas aeruginosa | Ф = 16 mm at a concentration of 100 mg/mL | ||||
|
| |||||
| Aerial parts | Essential oil | Agar diffusion method | Pseudomonas aeruginosa (ATCC 27853) | No inhibition | [7] |
| Escherichia coli (ATCC 25922) | Ф = 9 mm | ||||
| Staphylococcus aureus (ATCC 25923) | Ф = 11 mm | ||||
| Staphylococcus epidermidis | Ф = 10 mm | ||||
| Streptococcus pneumoniae | Ф = 13 mm | ||||
| Streptococcus pyogenes | Ф = 16 mm | ||||
| Klebsiella pneumoniae | Ф = 8 mm | ||||
| Salmonella typhi | Ф = 8 mm | ||||
| Shigella sonnei | Ф = 9 mm | ||||
|
| |||||
| Leaves | Essential oil | Agar-well diffusion assay Broth microdilution assay |
Staphylococcus aureus | Ф = 32.00 ± 2.65 mm | [86] |
| MIC = 0.25% (v/v) | |||||
| MBC = 0.25% (v/v) | |||||
| Pseudomonas aeruginosa | Ф = 13.33 ± 1.53 mm | ||||
| MIC = 0.5% (v/v) | |||||
| MBC = 2% (v/v) | |||||
| Listeria monocytogenes | Ф = 26.67 ± 2.08 mm | ||||
| MIC = 0.25% (v/v) | |||||
| MBC = 0.25% (v/v) | |||||
| Bacillus subtilis | Ф = 17.00 ± 2.00 mm | ||||
| MIC = 1% (v/v) | |||||
| MBC = 1% (v/v) | |||||
| Proteus mirabilis | Ф = 29.33 ± 1.53 mm | ||||
| MIC = 0.5% (v/v) | |||||
| MBC = 1% (v/v) | |||||
| Escherichia coli | Ф = 15.33 ± 1.89 mm | ||||
| MIC = 2% (v/v) | |||||
| MBC > 2% (v/v) | |||||
|
| |||||
| Whole plant | Staphylococcus aureus (MBLA) | Ф = 18 ± 1.34 mm | [87] | ||
| MIC = 4% (v/v) | |||||
| MBC ˃ 8% (v/v) | |||||
| Staphylococcus aureus 976 | Ф = 9 ± 1.9 mm | ||||
| Listeria monocytogenes | Ф = 21 ± 3.11 mm | ||||
| MIC = 1% (v/v) | |||||
| MBC = 4% (v/v) | |||||
| Staphylococcus aureus 994 | Ф = 7 ± 0.66 mm | ||||
| Bacillus subtilis 6633 | Ф = 15 ± 0.80 mm | ||||
| Escherichia coli K12 | Ф = 9 ± 0.65 mm | ||||
| Pseudomonas aeruginosa IH | No activity | ||||
| Proteus mirabilis | Ф = 19 ± 0.41 mm | ||||
| MIC = 4% (v/v) | |||||
| MBC ˃ 8% (v/v) | |||||
|
| |||||
| Leaves | Essential oil | Agar well diffusion method | Klebsiella pneumoniae (CIP8291) | Ф = 25 mm | [72] |
| Escherichia coli (ATCC10536) | No activity | ||||
| Staphylococcus aureus (CIP7625) | No activity | ||||
| Listeria monocytogenes (Scott A 724) | Ф = 29 mm | ||||
|
| |||||
| Leaves | Essential oil | Agar well diffusion method | Escherichia coli | Ф = 8 mm at a concentration of 500 μL/mL | [88] |
| Salmonella choleraesuis | Ф = 13 mm at a concentration of 500 μL/mL | ||||
| Staphylococcus aureus | Ф = 11 mm at a concentration of 500 μL/mL | ||||
| Listeria monocytogenes | Ф = 9.5 mm at a concentration of 500 μL/mL | ||||
|
| |||||
| Aerial parts | Essential oil | Disc diffusion method | Escherichia coli | MIC = 2.5 μL/mL | [89] |
| MBC = 2.5 μL/mL | |||||
| Streptococcus D | MIC = 2.5 μL/mL | ||||
| MBC = 2.5 μL/mL | |||||
| E. faecalis | MIC = 2.5 μL/mL | ||||
| MBC = 2.5 μL/mL | |||||
| K. pneumoniae | MIC = 2.5 μL/mL | ||||
| MBC = 2.5 μL/mL | |||||
3.5.3. Antiparasitic Activity
Table 5 provides investigations interested in the antiparasitic effect of spearmint [90, 91]. Zandi-Sohani and Ramezani [90] investigated the antiparasitic effect of essential oil isolated from spearmint leaves collected from southwestern Iran against Tetranychus turkestani. They discovered that the essential oil of spearmint exhibited acaricidal potential and can be employed to protect against Tetranychus turkestani, which showed to cause 100% adult mortality at a concentration of 20 μL/L. The lethal concentration values (LC50 and LC95) for essential oil spearmint were estimated to be 15.3 μL.L−1 and 23.4 μL.L−1, respectively. However, the study conducted by Koumad and Berkani [91] demonstrated that spearmint leaves revealed the lowest acaricidal activity against Varroa destructor by smoke. Results showed that spearmint killed 26.20% of Varroa destructor and reduced the infestation rate by 2.35%. The mortality rate was estimated at 30.65%, and infestation rate was 13.18%.
Table 5.
Antiparasitic activity of Mentha spicata.
| Part used | Extracts | Tested strains | Key results | Reference |
|---|---|---|---|---|
| Leaves | Dried plant | Varroa destructor | Killed 26.20% of Varroa | [90] |
| Infestation rates = 13.18% | ||||
| Reduced the infestation rate of 2.35% | ||||
| Mortality rate = 30.65% | ||||
|
| ||||
| Leaves | Essential oils | Tetranychus turkestani | LC50 = 15.3 mL/L | [91] |
| LC95 = 23.4 mL/L | ||||
| Mortality = 100% at concentration of 20 μL/L | ||||
3.5.4. Insecticidal Activity
Several investigations reported that extracts and essential oils from M. spicata have insecticidal activities against some pathogenic microorganisms [3, 92, 93] (Table 6).
Table 6.
Insecticidal activity of Mentha spicata.
| Part used | Extracts | Tested strains | Key results | Reference |
|---|---|---|---|---|
| Leaves | Essential oil | Rhyzopertha dominica | LD50 = 6.1 μL/mL | [65] |
| Mortality = 43% after 96 hours at a concentration of 2 μL/mL | ||||
| Repellent effect = 56.2% at a concentration of 2 μL/mL | ||||
|
| ||||
| Leaves | Essential oil | Rice weevil Sitophilus oryzae | LC50 = 100.16 μL/L air; LC95 = 192.197 μL/L air; mortality = 22% at a concentration of 71.43 μL/L air; LT50 = 45.52 h | [93] |
| Aerial parts | Essential oil | Callosobruchus chinensis | Mortality = 100% after 12 h at concentration of 0.1 μL/mL air | [66] |
| LC50 = 0.003 μL/mL air | ||||
| LC90 = 0.005 μL/mL air | ||||
| Repellency value = 100% at 0.025 µL/mL air of oil concentration | ||||
| 98% oviposition deterrence at 0.1 μL/mL concentration | ||||
|
| ||||
| Essential oil | Sitophilus granarius | Mortality = 43% at the 24 h exposure test | [94] | |
| Mortality = 80% at the 48 h exposure test | ||||
|
| ||||
| Whole flowering plants | Essential oil | Acanthoscelides obtectus | LC50 = 1.2 mL/L air, for males | [95] |
| LC50 = 4.4 mL/L air, for females | ||||
| Essential oil | Boophilus annulatus | Embryonated eggs (LC50 = 1.20%); unfed larvae (LC50 = 0.90%); fed females (LC50 = 10.57%) | [96] | |
| Leaves | oil | Callosobruchus maculatus | LC50 = 235 ppm | [92] |
| Essential oil | Culex quinquefasciatus Say | LC50 = 92 mg/L | [3] | |
| LC90 = 160 mg/L | ||||
|
| ||||
| Leaves | Essential oil | C. quinquefasciatus | LC50 = 62.62 ppm; LC90 = 118.70 ppm | [38] |
| Mortality = 96.8 ± 1.2% at a concentration of 125 ppm | ||||
| A. aegypti | LC50 = 56.08 ppm; LC90 = 110.28 ppm | |||
| Mortality = 98.1 ± 1.2% at a concentration of 125 ppm | ||||
| A. stephensi | LC50 = 49.71 ppm; LC90 = 100.99 ppm | |||
| Mortality = 99.6 ± 1.6% at a concentration of 125 ppm | ||||
Brahmi et al. [65] studied the impact of essential oil from M. spicata leaves against Rhyzopertha dominica. This study revealed that the essential oil from M. spicata leaf was effectively toxic against Rhyzopertha dominica adults. At a high concentration of 2 μL/mL, M. spicata oil showed high repellent activity against Rhyzopertha dominica (56.2% at 30 minutes), and the mortality rate was 43% after 96 hours of treatment. Furthermore, the toxicity contact assay showed that spearmint oil showed a low insecticidal effect with DL50 equal to 6.1 μL/mL. In another study, Kedia et al. [66] discovered the possibility of using essential oil extracted from aerial parts of M. spicata as a pesticide against the insect pest Callosobruchus chinensis. According to their findings, treatment with essential oil from M. spicata caused 100% mortality to C. chinensis after 12 h at a concentration of 0.1 μL/mL air using the fumigation toxicity test, and 100% repellency was observed at 0.025 μL/mL oil concentration in air during repellent activity assay. Using the probit model, the LC50 and LC90 values obtained were 0.003 and 0.005 μL/mL air concentrations, respectively. Furthermore, the essential oil from M. spicata at 0.1 μL/mL concentration has been reported as the effective fumigant with an oviposition deterrence value estimated at 98%.
In an effort to identify biopesticides for granary weevil to avoid losses of crops caused by insects, Lamiri et al. [94] screened a variety of essential oils for their pesticide effects against Sitophilus granarius. They discovered that essential oil of spearmint caused 80% and 43% mortality after 24 h and 48 h of exposure, respectively. These findings indicate that the rate of adult mortality rises as the concentration of oil used in the test increases. The study by Papachristos and Stamopoulos [95] assessed the repellent effects of essential oil extracted from whole flowering plants of spearmint against Acanthoscelides obtectus. The results showed that this oil exhibited a highly toxic effect in both males and females with LC50 values of 1.2 mL/L air for males and 4.4 mL/L air for females, where males are more affected than females. Also, the oil of spearmint exhibited the most repellent property against Acanthoscelides obtectus and appears to be more promising for potential use against this pest.
Abdel-Shafy and Soliman [96] in their research hypothesized that essential oil of spearmint (M. viridis) possesses the toxicity effect against embryonated eggs, larvae, and fed females of the cattle tick Boophilus annulatus (Acari: Ixodida: Amblyommidae) in Egypt. It was found that oil spearmint (M. viridis) was less toxic on embryonated eggs (LC50 = 1.20%) as well as on unfed larvae (LC50 = 0.90%) and fed females (LC50 = 10.57%) than other oils tested, including peppermint (Mentha piperita), marjoram (Majorana hortensis), lavender (Lavandula officinalis), and sweet basil (Ocimum basilicum). Compared to the study performed by Derbalah and Ahmed [92], spearmint oil leaf was highly effective against Callosobruchus maculatus with an LC50 value of 235 ppm. The results showed that oil spearmint could be used as a botanical product to control C. maculatus insect in cowpea seeds.
Pavela et al. [3] showed the effects of a variety of essential oils from the genus Mentha L., including M. spicata, against the larvae and adults of Culex quinquefasciatus Say (Diptera: Culicidae). Their finding indicates that the oil of M. spicata revealed lower larvicidal efficacy against C. quinquefasciatus compared to other oils tested. The lethal response of the oil towards the larvae for LC50 was estimated as 92 mg/L and for LC90 was estimated as 160 mg/L. Similarly, the study carried out by Govindarajan et al. [38] focused on the possible larvicidal properties of essential oil from M. spicata against three larvae species: A. stephensi, C. quinquefasciatus, and A. aegypti. After the exposure of treatment (24 h), the essential oil from M. spicata leaves showed a significant larvicidal effect against A. stephensi, C. quinquefasciatus, and A. aegypti, with LC50 and LC90 values of 49.71 versus 100.99 ppm, 62.62 versus 118.70 ppm, and 56.08 versus 110.28 ppm, respectively. Also, the essential oil of M. spicata caused 99.6 ± 1.6% mortality for A. stephensi and 98.1 ± 1.2% for both C. quinquefasciatus and A. aegypti at a concentration of 125 ppm.
To test the application for alone or combined, three essential oils were isolated from three medicinal plant species belonging to the Mentha genus to manage the rice weevil Sitophilus oryzae (Curculionidae). The study conducted by Haouel-Hamdi et al. [93] showed that binary combined Tunisian spearmint oils from M. rotundifolia, M. viridis, and M. longifolia leaves have exerted an important anti-insecticide activity against Sitophilus oryzae. However, Mentha essential oils alone revealed the lowest repellent activity to S. oryzae adults. After 24 days of exposure, LC50 and LC95 values of fumigant toxicity of M. viridis essential oils alone was 100.16 μL/L air and 192.197 μL/L air, respectively, against S. oryzae adults. In addition, the LT50 value was 45.52 h for M. viridis, and the percentage of mortality was 22% at a concentration of 71.43 μL/L air.
3.5.5. Anti-Inflammatory Activity
Table 7 provides studies focused on the anti-inflammatory propriety of the M. spicata in different in vivo experiments [97, 99]. Using the carrageen-induced paw edema method, Yousuf et al. [97] showed that methanol extract from the whole plant of M. spicata exhibited a strong anti-inflammatory activity which presenting at both doses 250 and 500 mg/kg of methanol extract a significant dose-dependent reduction of paw edema. Furthermore, the anti-inflammatory action of the extract remained significant until the 6th hour of the test. In another study, Arumugam et al. [98] evaluated in vivo anti-inflammatory effect of different solvent fractions of the ethanolic extract of the dried leaves of M. spicata on rats with acute and chronic inflammation by using two experimental approaches, carrageenan and cotton pellet-induced inflammation models. The finding showed that ethyl acetate extract and aqueous fraction were potent in cotton pellet (chronic) induced inflammation where the rate of inflammation was reduced by 65% and 54%, respectively. However, inflammation was reduced with less effectiveness in hexane extract (0–20%) and aqueous fraction (7–11%); only the ethyl acetate fraction was found to be effective in carrageenan (acute) induced inflammation, while chloroform fraction has not been able to decrease inflammation.
Table 7.
Anti-inflammatory activity of Mentha spicata.
| Used part | Extracts | Experimental approach | Key results | References |
|---|---|---|---|---|
| Whole plant | Methanol extract | Carrageen-induced paw edema method | Significant dose-dependent reduction of paw edema | [97] |
| Leaves | Hexane extract | Carrageenan-induced paw edema in rats | Reduced the inflammation with less effectiveness | [98] |
| Reduced the inflammation by 0–20% | ||||
| Ethyl acetate extract | Reduced the inflammation by 9–85% | |||
| Chloroform fraction | The inflammation did not decrease | |||
| Aqueous fraction | Enhances inflammation by about 7–11% | |||
| Hexane extract | Cotton pellet-induced granuloma in rats | Reduced inflammation with 20% | ||
| Ethyl acetate extract | Reduced inflammation with 65% | |||
| Chloroform fraction | Reduced inflammation with 20% | |||
| Aqueous fraction | Reduced inflammation with 54% | |||
|
| ||||
| Leaves | Methanol extract | Irinotecan-induced mucositis in mice | Significantly decreased both jejunal tissue IL-1β and fecal β-glucuronidase activity | [99] |
| Improvements in mucositis features | ||||
The study conducted by Jabbar and Kathem [99] evaluated the preventive effect of ethanolic extract of leaves from M. spicata on irinotecan-induced mucositis in mice. The results revealed that the ethanolic extract of M. spicata markedly reduced jejunal tissue IL-1β (3.47 ± 1.23 vs. 6.5 ± 0.36 ng/mL), and fecal β-glucuronidase activity (79.78 ± 10.7 vs. 120.6 ± 8.3 U) compared to no-treated mice. In addition, histological investigation of the jejunum section of the animal after administration of irinotecan and ethanolic extract of M. spicata showed enhancements in mucositis features.
3.5.6. Antidiabetic Activity
Diabetes mellitus is a metabolic disease that affects the endocrine system, often occurring when the pancreas does not secrete enough insulin or when the body cannot use this hormone effectively, resulting in chronic hyperglycemia with disruptions in protein, lipid, and carbohydrate metabolism.
In order to understand the mechanism of antidiabetic action of M. spicata better, several recent studies (in vivo and in vitro) performed in chronological order were discussed in this review [8, 100, 101] (Table 8).
Table 8.
Antidiabetic effects of Mentha spicata.
| Part used | Extracts | Dose | Model | Keys results | References |
|---|---|---|---|---|---|
| Leaves | Aqueous ethanolic extract | 200 mg/kg and 400 mg/kg bodyweight | Alloxan-induced hyperglycemic rats | Reduced blood glucose level, reduced serum cholesterol, triglycerides, LDL, and VLDL and increased bodyweights and HDL levels | [101] |
| Leaves | Phenolic extract | 200 mg/kg bodyweight | Alloxan-induced hyperglycemic rats | Significant decrease in glucose concentration of blood serum; significant decrease in cholesterol and TG; significant increase in plasma HDL; significant decrease in plasma LDL, VLDL | [100] |
| Leaves | Aqueous extract | 300 mg/kg bodyweight | Alloxan-induced hyperglycemic rats | Decreased blood glucose level; decreased bodyweight; significant reduction of total cholesterol, triglyceride, and LDL-cholesterol levels; significant increase in plasma HDL; significant reduction in the level of MDA | [13] |
| Roots | Butanol extract | 100 mg/kg bodyweight | Streptozotocin-induced hyperglycemic rats | Increased bodyweight; reduced blood glucose | [8] |
| Leaves | Essential oil | 200 μL | α-Glucosidase inhibitory assay | IC50 = 86.93 ± 2.43 μg/mL | [86] |
| 250 μL | α-Amylase inhibitory assay | IC50 = 101.72 ± 1.86 μg/mL |
Regarding in vivo studies, Al-Fartosi and collaborators evaluated this activity on male rats rendered diabetic by alloxan intraperitoneal injection (125 mg/kg b.w) and treated with phenolic compounds (200 mg/kg b.w) extracted from the leaves of this plant [100]. During 14 days of daily treatment, a decrease in the level of blood glucose, triglycerides, cholesterol, plasma LDL, and VLDL and a significant increase in plasma HDL levels were recorded. This work confirmed the potential of M. spicata in the management of diabetes and its complications. In 2017, two similar studies verified these findings on the same animal model. Indeed, the aqueous ethanolic extract (200 and 400 mg/kg b.w) [101] and the aqueous extract (300 mg/kg b.w) [13] of the leaves of this species presented the same results as the previous study. The following year, 40 streptozotocin-induced diabetic rats were treated for 4 weeks with butanol extract from M. spicata roots [8]. At the end of this period, the authors observed antidiabetic properties represented by a decrease in blood glucose level and an increase in bodyweight.
A very recent investigation tested this powder on two carbohydrate hydrolyzing enzymes, namely, α-amylase and α-glucosidase [86]. In fact, inhibiting these two enzymes prevents the digestion of carbohydrates, which is a promising strategy in the treatment of diabetes. The results of this study showed that the leaf essential oil of this herb at doses of 200 and 250 μL was able to inhibit α-amylase (IC50 = 101.72 ± 1.86 μg/mL) and α-glucosidase (IC50 = 86.93 ± 2.43 μg/mL), respectively.
From these studies, it can be inferred that M. spicata may be used as an antidiabetic agent; however, further investigations, as well as clinical trials, must be carried out to evaluate this benefit in humans.
3.5.7. Antioxidant Activity
Oxidative stress corresponds to an attack on cells by free radicals, also called reactive oxygen species (ROS), produced continuously from oxygen in the cell, particularly in the mitochondrial respiratory chain. ROS are reactive and very toxic substances. Oxidative stress is caused by an imbalance between the production of prooxidant free radicals and antioxidants. Regarding M. spicata, many studies have evaluated its antioxidant activity either by measuring its effectiveness in scavenging free radicals or by directly assaying the products formed using photometric techniques [5, 78, 102] (Table 9). Indeed, Getahun et al. [78] obtained essential oils by hydrodistillation from M. spicata leaves to determine their radical scavenging potentials in vitro in DPPH and deoxyribose degradation assays. These oils exhibited potent radical scavenging activities, with IC50 values of 5.96 and 0.57 μL/mL in the DPPH and deoxyribose degradation assays, respectively. In the same year, Nickavar et al. [102] found that the ethanolic extract of M. spicata aerial parts showed IC50 values of 87.89 and 173.80 μg/mL by the DPPH• and ABTS•+ assays, respectively. The following year, using the same methods, Mkaddem et al. [72] showed that the essential oil from the leaves of this plant has significant anti-free radical potential.
Table 9.
Antioxidant activity of Mentha spicata.
| Parts used | Extracts | Methods used | Key results | References |
|---|---|---|---|---|
| Whole plant | Methanol extract | DPPH | IC50 = 65.13 ± 1.29 μg/mL | [103] |
| ABTS | IC50 = 52.31 ± 0.81 μg/mL | |||
| Aerial parts | Ethanol extract | DPPH | IC50 = 87.89 μg/mL | [102] |
| ABTS | IC50 = 173.80 μg/mL | |||
| Aerial parts | Essential oil | DPPH | IC50 = 3450 ± 172.5 μg/mL | [48] |
| ABTS | IC50 = 40.2 ± 0.2 μg/mL | |||
| FRAP | IC50 = 215 ± 4.50 μg/mL | |||
| Leaves | Essential oil | DPPH | IC50 = 9544.6 ± 196.2 μg/mL | [65] |
| ABTS | IC50 = 36.2 ± 3.2 μg/mL | |||
| Reducing power | RP50 = 452.3 ± 0.4 μg/mL | |||
| Phosphomolybdate | RP50 = 53.3 ± 2.8 μg/mL | |||
| Leaves | Essential oil | DPPH | IC50 = 10 ± 0.24 μg/mL | [104] |
| Superoxide anion | IC50 = 1.33 ± 0.10 μg/mL | |||
| Aerial parts | Ethanol-water extract | DPPH | IC50 = 105.8 ± 3.98 μg/mL | [9] |
| Nitric oxide radical scavenger | IC50 = 210.6 ± 7.7 μg/mL | |||
| Metal chelating | IC50 = 757.4 ± 29.5 μg/mL | |||
| Scavenging of H2O2 | IC50 = 631.1 ± 26.0 μg/mL | |||
| Seeds | Methanol extract | DPPH | Inhibition = 89.91 ± 2.12% | [33] |
| Leaves | Ethanol extract | DPPH | IC50 = 16.2 ± 0.2 μg/mL | [105] |
| ABTS | IC50 = 10.3 ± 0.9 μg/mL | |||
| TEAC | TEAC = 0.90 ± 0.07 mM | |||
| Leaves | Essential oils | DPPH | IC50 = 8.81 μL/mL | [78] |
| Leaves | Essential oils | DPPH | IC50 = 41,23 μL/mL | [80] |
| Aerial parts | Essential oils | DPPH | IC50 = 13.3 ± 0.6 μL/mL | [5] |
| Essential oil | DPPH | IC50 = 72.07 ± 0.34 mg/mL | [11] | |
| Aerial parts | Water extract | DPPH | Inhibition = 74.2 ± 0.2% | [106] |
| β-Carotene | Inhibition = 79.1 ± 2.4% | |||
| Leaves | Water extract | DPPH | EC50 = 336 ± 3 μg/mL | [107] |
| Reducing power | EC50 = 198 ± 2 μg/mL | |||
| TBARS | EC50 = 152 ± 5 μg/mL | |||
| Leaves | Essential oil | DPPH | IC50 = 21.19 ± 7.17 μg/mL | [82] |
| Reducing power | IC50 = 2.28 ± 0.68 μg/mL | |||
| DPPH | Inhibition = 30.52 ± 0.09% at a concentration of 10 μg/mL | |||
| Aerial parts | Essential oil | DPPH | IC50 = 3.08 ± 0.07 μg/mL | [108] |
| Reducing power | EC50 = 2.49 ± 0.07 μg/mL | |||
| Chelating power | IC50 = 6.33 ± 0.12 μg/mL | |||
| β-Carotene | IC50 = 6.4 ± 0.07 μg/mL | |||
| Leaves | Essential oil | β-Carotene-linoleic acid | Antioxidant activity = 25.37% at 500 μg/mL | [44] |
| DPPH | Antioxidant activity = 13.81% at 500 μg/mL | |||
| Aerial parts | Ethanol extract | DPPH | Radical scavenging effect = 18.34 ± 2.2% at a concentration of 0.4 mg/mL | [50] |
| Essential oil | DPPH | IC50 ˃ 2.5 μg/mL | [87] | |
| Leaves | Essential oil | DPPH | IC50 = 80.45 ± 1.86 μg/mL | [86] |
| FRAP | IC50 = 101.78 ± 3.14 μg/mL | |||
| ABTS | IC50 = 139.59 ± 3.12 μg/mL | |||
| Leaves | Essential oil | ABTS | IC50 = 195.1 ± 4.2 mg/L | [72] |
| DPPH | IC50 = 3476.3 ± 133 mg/L |
By respecting the chronology of the studies carried out over time, Ebrahimzadeh et al. [9] examined the antioxidant capacity of M. spicata aerial parts in vitro using eight assay systems. They recorded the best activity with the DPPH test (IC50 = 105.8 ± 3.98 μg/mL), followed by the assay of nitric oxide-scavenging activity (IC50 = 210.6 ± 7.7 μg/mL) and scavenging of H2O2 (IC50 = 631.1 ± 26.0 μg/mL). In addition, good antioxidant activity has been demonstrated by Hussain et al. [5] (IC50 = 13.3 ± 0.6 μL/mL) and by Liu et al. [11] (IC50 = 72.07 ± 0.34 mg/mL), using DPPH free radical-scavenging ability. Moreover, the antioxidant power of M. spicata aerial parts has been tested by Benedec et al. [50] using only the DPPH radical scavenging assay, which showed a value of 18.34 ± 2.2% at the concentration of 0.4 mg/mL. A Tunisian research team also confirmed this when they recorded an important antiradical (IC50 = 10 ± 0.24 μg/mL) and superoxide anion (IC50 = 1.33 ± 0.10 μg/mL) scavenging ability [104]. Furthermore, according to Teixeira and collaborators, the essential oil of this plant was shown to be a potent antioxidant by exhibiting a dose-dependent antioxidant effect at the concentrations tested (25, 50, 100, 150, 200, 250, 300, and 500 μg/mL), determined by the sequestration of the DPPH radical and by the β-carotene-linoleic acid method [44].
Using the same methods as previous studies, other more recent investigations have confirmed the important antioxidant activity of M. spicata, regardless of its harvest region or parts used (Table 9).
The antioxidant activity of different parts of M. spicata is certainly attributed to its major compounds. Indeed, L-menthone (32.74%) and pulegone (26.67%) were the main volatiles of its essential oil, while apigenin (38.4 mg/100 g dry weight) was the main flavonoid in methanolic extracts [104]. These molecules are renowned for their antioxidant potential [109].
3.5.8. Diuretic Activity
The in vivo study performed by Aziz et al. [110] assessed the diuretic property of the aqueous methanol extract from aerial parts of spearmint in rat models. The treatment administered to experimental rats at dose 100 mg/kg revealed significant diuresis (3.74 ± 0.41 mL). The values obtained are more or less close to the reference standard (furosemide, 4.05 ± 0.34 mL) (p < 0.05). Also, the extract of spearmint significantly increased the excretion of potassium and sodium (p < 0.05), while a significant change in the pH has not been observed after administration of M. viridis extract.
3.5.9. Analgesic and Antipyretic Activities
For testing the analgesic and antipyretic effects of methanol extract from M. spicata, Yousuf et al. [97] in their study demonstrated that the methanol extract from the whole plant of M. spicata had markedly increased the reaction time of mice in a dose-dependent manner by the hot-plate test (p < 0.001) proving its marked analgesic effect. In addition, using the acetic acid-induced writhing method, the methanol extract of M. spicata also exhibited a significant analgesic action. The inhibition at the dose of 500 mg/kg was estimated at 60.30%. On the other hand, using Brewer's yeast-induced pyrexia in rats, the methanol extract of M. spicata was revealed to exert a strong marked (p < 0.01) antipyretic activity at the dose of 500 mg/kg at 3 h than at a dose of 100 mg/kg at 2 h.
3.5.10. Antihemolytic Activity
In order to investigate the biological functions of M. spicata, Ebrahimzadeh et al. [9] decided to study the antihemolytic effect of ethanol-water extract from aerial parts of M. spicata. The results showed that this extract possesses a weak inhibiting effect with an IC50 = 1250.7 ± 46.1 μg/mL by H2O2-induced membrane damage and hemolysis.
3.5.11. Protective Effects
In their research, Saad et al. [111] were interested in studying the protective activity of M. spicata treatment against nicotine-induced oxidative damage in the liver and erythrocytes Wistar rats. The findings showed that aqueous extract from aerial parts of M. spicata exhibited a strong protective action. On the hematological parameters, it was found to restore to normal levels the levels of erythrocytes, haematocrit, hemoglobin, and white blood cells. However, on hepatic dysfunction parameters, the aqueous extract of spearmint significantly decreased ALT and ALP activities resulting in a decrease in liver toxicity. Furthermore, the aqueous extract of M. spicata to nicotine-treated rats provided a statistically significant (p ≤ 0.01) enhancement of antioxidant enzyme capacities, including CAT, SOD, and GPX activities, suggesting an improvement in antioxidant status. According to liver histological analysis, the treatment with the aqueous extract of M. spicata showed considerable recovery in the form of hepatic histoarchitecture. Similarly, Saad et al. [111] aimed to screen the in vivo and in vitro antioxidative effect of M. spicata extract against nicotine-induced oxidative injury in the kidney and brain of rats. The in vivo results obtained reported that Mentha extract significantly increased the bodyweight of rats as well as exhibited a significant increase in testis, brain, and accessory sex organ weights. In addition, treatment with the aqueous extract of M. spicata had a significant decrease in the MDA levels, but no significant changes in brain AChE were recorded. Also, M. spicata extract supplementation could restore the antioxidant enzymes activities to normal levels and participate to ameliorate cerebral cortex histological pictures and histological damages.
3.6. Toxicity Investigations
In pharmacology, the efficacy of a plant or a natural constituent is not sufficient to justify its therapeutic use. Indeed, each bioactive substance is likely to have deleterious effects for human health, at least in high doses and over long periods [112]. In addition to efficacy, the active dose must be free from any toxicity and demonstrate safety. Therefore, in the therapeutic indication of any substance, it is imperative to define its risk-benefit ratio.
Despite the data paucity on its safety profile and given its wide use, the acute and subacute toxicities of M. spicata have been tested in four studies to optimize its use [66, 113, 114] (Table 10).
Table 10.
Toxicity study of Mentha spicata.
| Parts used | Extracts | Experimental approaches | Key results | References |
|---|---|---|---|---|
| Aerial parts | Methanol extract | The animals treated with a single dose of 5000 mg/kg of M. spicata extract by oral gavage | No mortality during the observation period | [113] |
| No toxicologically significant hematological and biochemical changes | ||||
| Any morphological changes in the heart, liver, kidney, and lung tissues of the rats | ||||
| LD50 is considered to be >5000 mg/kg | ||||
| Leaves | Ethanolic extract | The animals treated with a single dose of 10000, 12000, 14000, 16000, and 18000 mg/kg BW of M. spicata extract by oral gavage | NOEL dose for M. spicata was 10000 mg/kg | [114] |
| 100% mortality at 18000 mg/kg BW | ||||
| LD50 = 13606 mg/kg BW | ||||
|
| ||||
| Leaves | Ethanolic extract | The animals treated with 500, 1000, and 1500 mg/kg BW daily for 28 days | No signs of toxicity; no mortalities; no significant change in bodyweight; significant increase in WBC, Lym, and MCHC levels; significant reduction in HCT level; significant increase in AST levels; unaffected serum creatinine and urea; no significant histopathological change | [114] |
| Whole plant | Methanol extract | Mice treated with a single dose of 500, 1000, and 2000 mg/kg of M. spicata extract by oral gavage | No mortality during the observation period | [97] |
| Aerial parts | Essential oil | EO (0.05–0.5 mL) orally administered to mice (Mus musculus L., average weight 30.0 g, age 3 months) | LD50 = 8342.33 μL/kg | [66] |
| Leaves | Ethanolic extract | The animals treated with 500, 1000, and 1500 mg/kg BW daily for 28 days | No signs of toxicity; no mortalities; no significant change in bodyweight; significant increase in WBC, Lym, and MCHC levels; significant reduction in HCT level; significant increase in AST levels; unaffected serum creatinine and urea; no significant histopathological change | [114] |
| Whole plant | Methanol extract | Mice treated with a single dose of 500, 1000, and 2000 mg/kg of M. spicata extract by oral gavage | No mortality during the observation period | [97] |
| Aerial parts | Essential oil | EO (0.05–0.5 mL) orally administered to mice (Mus musculus L., average weight 30.0 g, age 3 months) | LD50 = 8342.33 μL/kg | [66] |
Initially, Yousuf et al. [97] orally administered single doses of 500, 1000, and 2000 mg/kg of whole plant methanolic extract to mice of both sexes. After 24 hours of observation, no mortality or signs of toxicity were noticed. One year later, aerial parts of the same extract at a dose of 5000 mg/kg of extract (the limit test dose according to OECD guidelines 425) showed similar results in female rats [113]. Indeed, during the 14 days of oral gavage, no mortality was recorded, considering the LD50 to be greater than 5000 mg/kg. In addition, no changes in the behavior and the bodyweight of the animals were observed. At the end of the experiment and after sacrificing animals, there were no toxicologically significant biochemical and hematological changes compared to the control group. The histological evaluation did not reveal any morphological changes or gross lesions in the lung, kidney, liver, and heart tissues. These results corroborate those obtained by Kedia et al. [66]. They recorded low toxicity (LD50 = 8342.33 μL/kg) of the essential oil of M. spicata aerial parts following oral administration of different doses (0.05–0.5 mL) to mice (Mus musculus L.).
In the same year, Mugisha and colleagues tested the acute and subacute toxicities of the leaves of this plant in Swiss mice and Wistar albino rats, respectively [114]. For acute toxicity, animals received intragastrically over 72 hours, doses of 10000, 12000, 14000, 16000, and 18000 g/kg b.w of the 70% ethanolic extract. Therefore, a death rate of 100% was obtained at the highest dose with some signs of toxicity (convulsions, abdominal muscle contractions, and hyperurination) above 12000 mg/kg b.w. The LD50 value was 13606 mg/kg b.w. Regarding subacute toxicity (28 days), ethanol leaf extract (500, 1000, and 1500 mg/kg b.w) caused no mortality or signs of toxicity. However, it significantly increased the levels of mean corpuscular hemoglobin concentration, lymphocytes, blood cells count, and aspartate transferase and significantly reduced haematocrit. At the same time, serum urea and creatinine levels were not affected, confirmed by histopathological data.
From these toxicological investigations, it can be declared that M. spicata is an experimentally safe plant, thus justifying its use in treating numerous abnormalities. However, prolonged treatment in high doses can lead to specific problems. For this, other studies on this plant's chronic toxicity are necessary to complete its toxicological profile.
4. Conclusion and Perspectives
In this work, we reported the ethnobotanical, phytochemical, and pharmacological aspects of M. spicata (M. viridis). This medicinal plant is frequently used in traditional practices to treat certain diseases and showed interesting biological properties in various scientific investigations. Phytochemical studies of this species showed its richness in numerous bioactive compounds in particularly terpenoid components, exhibiting important biological effects. Pharmacological biology explorations demonstrated that extracts and essential oils of M. spicata showed different pharmacological properties such as antibacterial, antiparasitic activity, insecticidal, anti-inflammatory, antidiabetic, antioxidant, diuretic, analgesic, antipyretic, antihemolytic, and protective activities. However, these effects were evaluated often using in vitro and in vivo approaches, and therefore, further investigations to validate these activities with determining mechanisms of their actions are needed. Toxicological investigation of M. spicata extracts was examined by some studies and showed a safety of this plant. However, clinical trials were not conducted, and there is an urgent need to perform such trials to promote the use of the plant especially after proving its excellent safety profile in the toxicological investigation. Indeed, bioactive compounds of M. spicata need further investigations concerning the pharmacodynamic and pharmacokinetic aspects to determine their bioavailability and their mechanisms of action of different targets.
Acknowledgments
The authors wish to thank the Research Center at College of Pharmacy and Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia, for providing financial support and access to online database for literature survey.
Contributor Information
Riaz Ullah, Email: rullah@ksu.edu.sa.
Abdelhakim Bouyahya, Email: boyahyaa-90@hotmail.fr.
Data Availability
The data used to support this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Graphical abstract of this study is attached in supplementary file.
References
- 1.Benkhnigue O., Fatiha B. A., Salhi S., Mohamed F., Douira A., Zidane L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d’Al Haouz-Rhamna (Maroc) The Journal of Animal and Plant Sciences . 2014;23(1):3539–3568. [Google Scholar]
- 2.Bouyahya A., Abrini J., Et-Touys A., Bakri Y., Dakka N. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. European Journal of Integrative Medicine . 2017;13:9–25. doi: 10.1016/j.eujim.2017.06.004. [DOI] [Google Scholar]
- 3.Pavela R., Kaffková K., Kumšta M. Chemical composition and larvicidal activity of essential oils from different Mentha L. and Pulegium species against Culex quinquefasciatus say (Diptera: Culicidae) Plant Protection Science . 2014;50(1):36–42. doi: 10.17221/48/2013-pps. [DOI] [Google Scholar]
- 4.Print I., Golparvar A. R., Hadipanah A., Mehrabi A. M. Diversity in chemical composition from two ecotypes of (Mentha longifolia L.) and ( Mentha spicata L. in Iran climatic conditions) Journal of Biodiversity and Environmental Sciences JBES. . 2015;6(4):26–33. [Google Scholar]
- 5.Hussain A. I., Anwar F., Shahid M. Chemical composition, and antioxidant and antimicrobial activities of essential oil of spearmint (mentha spicata L.) from Pakistan. Journal of Essential Oil Research . 2010;22(1):78–84. [Google Scholar]
- 6.Franciscato L. M. S. S., Silva M. R., Andrich F., et al. Chemical characterization and antimicrobial activity of essential oils of mint (mentha spicata L.) and surinam cherry (eugenia uniflora L.) Orbital - The Electronic Journal of Chemistry . 2018;10(6):475–481. doi: 10.17807/orbital.v10i6.1008. [DOI] [Google Scholar]
- 7.Boukhebti H., Chaker A. N., Belhadj H., Sahli F., Laouer H., Harzallah D. Chemical composition and antibacterial activity of Mentha pulegium L. and Mentha spicata L. essential oils. Der Pharmacia Lettre . 2011;3(4):p. 10. [Google Scholar]
- 8.Rakebizadeh M., Zahedizadeh M., Panah Y. E. Supplemental effect of zinc oxide nanoparticles and mentha spicata butanol extract on blood glucose of diabetic wistar rats. Journal of Science, Engineering & Technology . 2018;3(5) [Google Scholar]
- 9.Ebrahimzadeh M. A., Nabavi S. M., Nabavi S. F. Biological Activities of Me Tha Spicata L. Vol. 1. Pharmacology Online; 2010. pp. 841–848. [Google Scholar]
- 10.Mahendran G., Rahman L. U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha × piperita L.)-A review. Phytotherapy Research . 2020;34(9):2088–2139. doi: 10.1002/ptr.6664. [DOI] [PubMed] [Google Scholar]
- 11.Liu K. H., Zhu Q., Zhang J. J., Xu J. F., Wang X. C. Chemical composition and biological activities of the essential oil of Mentha spicata Lamiaceae. Advanced Materials Research . 2012;524-527:2269–2272. doi: 10.4028/www.scientific.net/amr.524-527.2269. [DOI] [Google Scholar]
- 12.Kunwar G., Pande C., Tewari G. Essential oil composition of the aerial parts of mentha spicata L. Journal of essential oil-bearing plants JEOP . 2017;13(3):353–356. [Google Scholar]
- 13.Bayani M., Ahmadi-hamedani M., Jebelli Javan A. Study of hypoglycemic, hypocholesterolemic and antioxidant activities of Iranian mentha spicata leaves aqueous extract in diabetic rats. Iranian Journal of Pharmaceutical Research: IJPR . 2017;16(8):75–82. [PMC free article] [PubMed] [Google Scholar]
- 14.Kassahun B. M., Egata D. F., Lulseged T., Yosef W. B., Tadesse S. Variability in agronomic and chemical characteristics of spearmint (mentha spicata L.) genotypes in Ethiopia. International Journal of Advanced Biological and Biomedical Research . 2014;2(10):2704–2711. [Google Scholar]
- 15.Kee L. A., Shori A. B., Baba A. S. Bioactivity and health effects of Mentha spicata. Integrative Food, Nutrition and Metabolism . 2017;5(1):1–2. doi: 10.15761/ifnm.1000203. [DOI] [Google Scholar]
- 16.El-hilaly J., Hmammouchi M., Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco) Journal of Ethnopharmacology . 2003;86(2-3):149–158. doi: 10.1016/s0378-8741(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 17.Idm’hand E., Msanda F., Cherifi K. Ethnopharmacological review of medicinal plants used to manage diabetes in Morocco. Clin. Phytoscience. . 2020;6(1) [Google Scholar]
- 18.Fakchich J., Elachouri M. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. Journal of Ethnopharmacology . 2014;154(1):76–87. doi: 10.1016/j.jep.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Labiad H., Et-Tahir A., Ghanmi M., et al. Ethnopharmacological survey of aromatic and medicinal plants of the pharmacopoeia of northern Morocco. Ethnobotany Research and Applications . 2020;19:1–16. doi: 10.32859/era.19.45.1-16. [DOI] [Google Scholar]
- 20.Orch H., Zidane L., Douira A. Ethnobotanical study of plants used in the treatment of respiratory diseases in a population bordering the forest of Izarène. Journal of Pharmacy & Pharmacognosy Research . 2020;8(5):392–409. [Google Scholar]
- 21.Salhi N., Bouyahya A., Fettach S., Zellou A., Cherrah Y. Ethnopharmacological study of medicinal plants used in the treatment of skin burns in Occidental Morocco (area of Rabat) South African Journal of Botany . 2019;121:128–142. doi: 10.1016/j.sajb.2018.10.038. [DOI] [Google Scholar]
- 22.El Abbouyi P. A., Filali-Ansari N., Khyari P. S., Loukili H. Inventory of medicinal plants prescribed by traditional healers in El Jadida city and suburbs (Morocco) International Journal of Green Pharmacy . 2014;8(4):242–251. doi: 10.4103/0973-8258.142681. [DOI] [Google Scholar]
- 23.El Hafian M., Benlandini N., Elyacoubi H., Zidane L., Rochdi A. Étude floristique et ethnobotanique des plantes médicinales utilisées au niveau de la préfecture d’Agadir-Ida-Outanane (Maroc) Journal of Applied Biosciences . 2014;81(1):7198–7213. doi: 10.4314/jab.v81i1.8. [DOI] [Google Scholar]
- 24.Telci I., Sahbaz N., Yilmaz G., Tugay M. E. Agronomical and chemical characterization of spearmint (Mentha spicata L.) originating in Turkey. Economic Botany . 2004;58(4):721–728. doi: 10.1663/0013-0001(2004)058[0721:aaccos]2.0.co;2. [DOI] [Google Scholar]
- 25.Ainane A. Chemical study by GC-MS of the essential oils of certain mints grown in the region of settat (Morocco): mentha piperita, mentha pulegium and mentha spicata. Drug Designing & Intellectual Properties International Journal . 2018;1(4) doi: 10.32474/ddipij.2018.01.000120. [DOI] [Google Scholar]
- 26.Zekri N., Elazzouzi H., El Makhoukhi F., Alaoui El Belghiti M., Zair T. Drying effect on yields and chemical composition of essential oils extracted from the Moroccan mentha spicata (L.) aerial parts. Journal of Essential Oil Bearing Plants . 2019;22(3):789–798. doi: 10.1080/0972060x.2019.1632746. [DOI] [Google Scholar]
- 27.Bensabah F., Houbairi S., Essahli M., Lamiri A., Naja J. Chemical composition and inhibitory effect of the essential oil from Mentha Spicata irrigated by wastewater on the corrosion of aluminum in 1 molar hydrochloric acid. Portugaliae Electrochimica Acta . 2013;31(4):195–206. doi: 10.4152/pea.201304195. [DOI] [Google Scholar]
- 28.Znini M., Bouklah M., Majidi L., et al. Chemical composition and inhibitory effect of Mentha spicata essential oil on the corrosion of steel in molar hydrochloric acid. International Journal of Electrochemical Science . 2011;6(3):691–704. [Google Scholar]
- 29.Maffei M., Codignola A., Fieschi M. Essential oil from Mentha spicata L. (spearmint) cultivated in Italy. Flavour and Fragrance Journal . 1986;1(3):105–109. [Google Scholar]
- 30.Cirlini M., Mena P., Tassotti M., et al. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules . 2016;21(8):1–15. doi: 10.3390/molecules21081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sevi̇ndi̇k E., Yamaner Ç., Kurtoğlu C., Ti̇n B. Chemical composition of mentha spicata L. Subsp. tomentosa and M. Pulegium L., and their antimicrobial activity on strong pathogen microorganisms. Notulae Scientia Biologicae . 2017;9 [Google Scholar]
- 32.Kizil S., Tonçer Ö. Influence of different harvest times on the yield and oil composition of spearmint (Mentha spicata L. var. spicata) Journal of Food Agriculture and Environment . 2006;4(3–4):135–137. [Google Scholar]
- 33.Emre İ., Kurşat Y., Kurşat M., Yilmaz Ö., Erecevit P. Chemical compositions, radical scavenging capacities and antimicrobial activities in seeds of satureja hortensis l. And mentha spicata l. subsp. spicata from Turkey. Brazilian Journal of Biology . 2021;81(1):144–153. doi: 10.1590/1519-6984.224654. [DOI] [PubMed] [Google Scholar]
- 34.Chauhan R. S., Kaul M. K., Shahi A. K., Kumar A., Ram G., Tawa A. Chemical composition of essential oils in Mentha spicata L. accession [IIIM(J)26] from North-West Himalayan region, India. Industrial Crops and Products . 2009;29(2–3):654–656. doi: 10.1016/j.indcrop.2008.12.003. [DOI] [Google Scholar]
- 35.Jain P. K., Soni A., Jain P., Bhawsar J. Phytochemical analysis of Mentha spicata plant extract using UV-VIS, FTIR and GC/MS technique. Journal of Chemical and Pharmaceutical Research . 2016;8(2):1–6. [Google Scholar]
- 36.Tewari G. Essential oil composition of the aerial parts of mentha spicata L. World Journal of Pharmaceutical Research . 2017;6:853–859. doi: 10.20959/wjpr20176-8518. [DOI] [Google Scholar]
- 37.Aziz M. A., Adnan M., Khan A. H., Shahat A. A., Al-Said M. S., Ullah R. Traditional uses of medicinal plants practiced by the indigenous communities at Mohmand Agency, FATA, Pakistan. Journal of Ethnobiology and Ethnomedicine . 2018;14(1):1–16. doi: 10.1186/s13002-017-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Govindarajan M., Sivakumar R., Rajeswari M., Yogalakshmi K. Chemical composition and larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species. Parasitology Research . 2012;110(5):2023–2032. doi: 10.1007/s00436-011-2731-7. [DOI] [PubMed] [Google Scholar]
- 39.Mahmoodi M., Sourestani M. M. Oil Content, Yield and Composition of Spearmint (Mentha Spicata L. Var. Kashan) at Different Cuts the First Iranian Congress of Essential Oil, Oil Content, Yield and Composition of Spearmint (Mentha Spicata L. Var. Kashan) at Different Cuts . Berlin, Germany: Researchgate.Net; 2016. [Google Scholar]
- 40.Farahbakhsh J., Najafian S., Hosseinifarahi M., Gholipour S. Essential oil storage conditions affect the chemical composition in cultivated Mentha spicata. Iranian Journal of Plant Physiology . 2020;11 [Google Scholar]
- 41.Golparvar A. R., Hadipanah A., Gheisari M. M. Comparative analysis of chemical composition of three ecotypes of spearmint (Mentha spicata L. (in Isfahan province. 2013. pp. 2011–2013.
- 42.Bimakr M., Rahman R. A., Taip F. S., et al. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food and Bioproducts Processing . 2011;89(1):67–72. doi: 10.1016/j.fbp.2010.03.002. [DOI] [Google Scholar]
- 43.Almeida P. P., Mezzomo N., Ferreira S. R. S. Extraction of mentha spicata L. Volatile compounds: evaluation of process parameters and extract composition. Food and Bioprocess Technology . 2012;5(2):548–559. doi: 10.1007/s11947-010-0356-y. [DOI] [Google Scholar]
- 44.Teixeira M. L., Cardoso M. d. G., Figueiredo A. C. S., et al. Essential oils from lippia origanoides kunth. And mentha spicata L.: chemical composition, insecticidal and antioxidant activities. American Journal of Plant Sciences . 2014;05(09):1181–1190. doi: 10.4236/ajps.2014.59131. [DOI] [Google Scholar]
- 45.de Sousa Barros A., de Morais S. M., Ferreira P. A. T., et al. Chemical composition and functional properties of essential oils from Mentha species. Industrial Crops and Products . 2015;76:557–564. doi: 10.1016/j.indcrop.2015.07.004. [DOI] [Google Scholar]
- 46.Zhao D., Xu Y. W., Yang G. L., Husaini A. M., Wu W. Variation of essential oil of Mentha haplocalyx Briq. and Mentha spicata L. from China. Industrial Crops and Products . 2013;42(1):251–260. doi: 10.1016/j.indcrop.2012.06.010. [DOI] [Google Scholar]
- 47.Antal T., Figiel A., Kerekes B., Sikolya L. Effect of drying methods on the quality of the essential oil of spearmint leaves (mentha spicataL.) Drying Technology . 2011;29(15):1836–1844. doi: 10.1080/07373937.2011.606519. [DOI] [Google Scholar]
- 48.Bardaweel S. K., Bakchiche B., ALSalamat H. A., Rezzoug M., Gherib A., Flamini G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complementary and Alternative Medicine . 2018;18(1):201–207. doi: 10.1186/s12906-018-2274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Marzouqi A. H., Rao M. V., Jobe B. Comparative evaluation of SFE and steam distillation methods on the yield and composition of essential oil extracted from spearmint (Mentha spicata) Journal of Liquid Chromatography & Related Technologies . 2007;30(4):463–475. [Google Scholar]
- 50.Benedec D., Vlase L., Oniga I., et al. LC-MS analysis and antioxidant activity of phenolic compounds from two indigenous species of Mentha. Farmacia . 2013;61(2):262–267. [Google Scholar]
- 51.Soilhi Z., Rhimi A., Heuskin S., Fauconnier M., Mekki M. Essential oil chemical diversity of Tunisian mentha spp. collection. Industrial Crops and Products . 2018;131 [Google Scholar]
- 52.Chowdhury J. U., Nandi N. C., Uddin M., Rahman M. Chemical constituents of essential oils from two types of spearmint (Mentha spicata L. And M. cardiaca L.) introduced in Bangladesh. Bangladesh Journal of Scientific & Industrial Research . 1970;42(1):79–82. doi: 10.3329/bjsir.v42i1.359. [DOI] [Google Scholar]
- 53.Soković M. D., Vukojević J., Marin P. D., Brkić D. D., Vajs V., Van Griensven L. J. Chemical composition of essential oils of Thymus and mentha species and their antifungal activities. Molecules . 2009;14(1):238–249. doi: 10.3390/molecules14010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali-Shtayeh M. S., Jamous R. M., Abu-Zaitoun S. Y., Khasati A. I., Kalbouneh S. R. Biological properties and bioactive components of mentha spicata L. Essential oil: focus on potential benefits in the treatment of obesity, alzheimer’s disease, dermatophytosis, and drug-resistant infections. Evidence-Based Complementary and Alternative Medicine . 2019;2019 doi: 10.1155/2019/3834265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chrysargyris A., Xylia P., Botsaris G., Tzortzakis N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Industrial Crops and Products . 2016;103:202–212. [Google Scholar]
- 56.Barbosa L. C. A., Filomeno C. A., Teixeira R. R. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules . 2016;21(12) doi: 10.3390/molecules21121671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laggoune S., Öztürk M., Erol E., et al. Chemical composition, antioxidant and antibacterial activities of the essential oil of Mentha spicata L. from Algeria. BMC Complementary and Alternative Medicine . 2016;18 doi: 10.1186/s12906-018-2274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.EL-Haoud H., Boufellous M., Berrani A., Tazougart H., Bengueddour R. Screening phytochimique D’ une plante medicinale: mentha spicata L. American Journal of Innovative Research & Applied Sciences . 2018;7(4):226–233. [Google Scholar]
- 59.Subramanian R., Gayathri S., Rathnavel C., Raj V. Analysis of mineral and heavy metals in some medicinal plants collected from local market. Asian Pacific Journal of Tropical Biomedicine . 2012;2(1):S74–S78. doi: 10.1016/s2221-1691(12)60133-6. [DOI] [Google Scholar]
- 60.Choudhury R. P., Kumar A., Garg A. N. Analysis of Indian mint (Mentha spicata) for essential, trace and toxic elements and its antioxidant behaviour. Journal of Pharmaceutical and Biomedical Analysis . 2006;41(3):825–832. doi: 10.1016/j.jpba.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 61.Ebrahimzadeh M. A., Eslami S., Nabavi S. M., Nabavi S. F., Moghaddam A. H., Bekhradnia A. R. Estimation of essential and toxic mineral elements in mentha species. Asian Journal of Chemistry . 2011;23(4):p. 3. [Google Scholar]
- 62.Alaklabi A., Arif I. A., Ahamed A., Manilal A., Surendrakumar R., Idhayadhulla A. Larvicidal, nematicidal, antifeedant and antifungal, antioxidant activities of Mentha spicata (Lamiaceae) root extracts. Tropical Journal of Pharmaceutical Research . 2016;15(11):p. 2383. doi: 10.4314/tjpr.v15i11.12. [DOI] [Google Scholar]
- 63.Ojewumi M. E., Adedokun S. O., Omodara O. J., Oyeniyi E. A., Taiwo O. S., Ojewumi E. O. Phytochemical and antimicrobial activities of the leaf oil extract of mentha spicata and its efficacy in repelling mosquito. International Journal of Pharmaceutical Research and Allied Sciences . 2017;6(4):p. 12. [Google Scholar]
- 64.Bayan Y., Küsek M., Küsek M. Chemical composition and antifungal and antibacterial activity of mentha spicata L. Volatile oil. Ciencia e investigación agraria . 2018;45(1):64–69. doi: 10.7764/rcia.v45i1.1897. [DOI] [Google Scholar]
- 65.Brahmi F., Adjaoud A., Marongiu B., et al. Chemical and biological profiles of essential oils fromMentha spicataL. leaf from Bejaia in Algeria. Journal of Essential Oil Research . 2016;28(3):211–220. doi: 10.1080/10412905.2015.1118411. [DOI] [Google Scholar]
- 66.Kedia A., Prakash B., Mishra P. K., Chanotiya C. S., Dubey N. K. Antifungal, antiaflatoxigenic, and insecticidal efficacy of spearmint (Mentha spicata L.) essential oil. International Biodeterioration & Biodegradation . 2014;89:29–36. doi: 10.1016/j.ibiod.2013.10.027. [DOI] [Google Scholar]
- 67.Şarer E., Toprak S. Y., Otlu B., Durmaz R. Composition and antimicrobial activity of the essential oil from mentha spicata L. subsp. spicata. Journal of Essential Oil Research . 2014;23 [Google Scholar]
- 68.Singh J., Dubeyd A. K., Tripathi N. N. Antifungal activity of mentha spicata. International Journal of Pharmacognosy . 1994;32(4):314–319. doi: 10.3109/13880209409083009. [DOI] [Google Scholar]
- 69.Sulieman A. M., Abdelrahman S. E., Abdel Rahim A. M. Phytochemical analysis of local spearmint (mentha spicata) leaves and detection of the antimicrobial activity of its oil. Journal of Microbiology Research . 2012;1(1):1–4. doi: 10.5923/j.microbiology.20110101.01. [DOI] [Google Scholar]
- 70.Zaidi S., Dahiya P. In vitro antimicrobial activity, phytochemical analysis and total phenolic content of essential oil from Mentha spicata and Mentha piperita. International Food Research Journal . 2015;22 [Google Scholar]
- 71.Yahia Balla O. Chemical composition and antimicrobial activity of essential oil of mentha viridis. Biochemistry and Molecular Biology . 2017;2(5):p. 60. doi: 10.11648/j.bmb.20170205.12. [DOI] [Google Scholar]
- 72.Mkaddem M., Bouajila J., Ennajar M., Lebrihi A., Mathieu F., Romdhane M. Chemical composition and antimicrobial and antioxidant activities of mentha (longifolia L. And viridis) essential oils. Journal of Food Science . 2009;74 doi: 10.1111/j.1750-3841.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 73.Schwarz S., Chaslus-Dancla E. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Veterinary Research . 2001;32(3–4):201–225. doi: 10.1051/vetres:2001120. [DOI] [PubMed] [Google Scholar]
- 74.Arumugam P., Murugan R., Subathra M., Ramesh A. Superoxide radical scavenging and antibacterial activities of different fractions of ethanol extract of Mentha spicata (L.) Medicinal Chemistry Research . 2010;19(7):664–673. doi: 10.1007/s00044-009-9221-9. [DOI] [Google Scholar]
- 75.Bensabah F., Sbayou H., Amghar S., Lamiri A., Naja J. Chemical composition and antibacterial activity of essential oils of two aromatic plants: mentha spicata and lippia citriodora irrigated by urban wastewater. International Journal of Engineering Research . 2013;2(12):p. 11. [Google Scholar]
- 76.Chauhan S. S., Agarwal R. Evaluation of antibacterial activity of volatile oil from mentha spicata l. Journal of Drug Delivery and Therapeutics . 2013;3(4):120–121. doi: 10.22270/jddt.v3i4.580. [DOI] [Google Scholar]
- 77.Ekhtelat M., Bahrani Z., Siahpoosh A., Ameri A. Evaluation of antibacterial effects of mentha spicata L., cuminum cyminum L. And mentha longifolia L. Essential oils individually and in combination with sodium benzoate against Escherichia coli O157:H7 and Listeria monocytogenes. Jundishapur Journal of Natural Pharmaceutical Products . 2019;14(3) doi: 10.5812/jjnpp.59092. [DOI] [Google Scholar]
- 78.Getahun Z., Asres K., Mazumder A. Essential oil composition, antibacterial and antioxidant activities of four mentha species growing in Ethiopia. Ethiopian Pharmaceutical Journal . 2008;25(2) doi: 10.4314/epj.v25i2.35123. [DOI] [Google Scholar]
- 79.Githaiga B. M., Gathuru E. M., Gathuru E. M., Waithaka P. N., Kiarie L. W. Determination of antibacterial activity of essential oils from mint (Mentha spicata) leaves on selected pathogenic bacteria. Journal of Drugs and Pharmaceutical Science . 2018;2(2):8–14. doi: 10.31248/jdps2018.015. [DOI] [Google Scholar]
- 80.Ha N., Durić K., meragić E., He N., Muratović S., Bečić F. Chemical characterization, antimicrobial and antioxidant properties of Mentha spicata L. (Lamiaceae) essential oil. Bulletin of the Chemists and Technologists of Bosnia and Herzegovina . 2018;50 [Google Scholar]
- 81.Jaber H., Oubihi A., Ouryemchi I., et al. Chemical composition and antibacterial activities of eight plant essential oils from Morocco against Escherichia coli strains isolated from different Turkey organs. Biochemistry Research International . 2021;2021 doi: 10.1155/2021/6685800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Selles S. M. A., Kouidri M., Bellik Y., et al. Chemical composition, antioxidant and in vitro antibacterial activities of essential oils of mentha spicata leaf from tiaret area (Algeria) Dhaka University Journal of Pharmaceutical Sciences . 2018;17(1):87–96. doi: 10.3329/dujps.v17i1.37123. [DOI] [Google Scholar]
- 83.Shahbazi Y. Chemical composition andIn VitroAntibacterial activity ofMentha spicataEssential oil against common food-borne pathogenic bacteria. Journal of Pathogens . 2015;2015:1–5. doi: 10.1155/2015/916305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shahbazi Y. Antibacterial effects of ziziphora clinopodioides and mentha spicata essential oils against common food-borne pathogen biofilms on stainless steel surface. Bulgarian Journal of Veterinary Medicine . 2020;23 [Google Scholar]
- 85.Verma R. S., Pandey V., Padalia R. C., Saikia D., Krishna B. Chemical composition and antimicrobial potential of aqueous distillate volatiles of Indian peppermint (mentha piperita) and spearmint (mentha spicata) Journal of Herbs Spices & Medicinal Plants Spices & Medicinal Plants . 2011;17 [Google Scholar]
- 86.Bouyahya A., Lagrouh F., El Omari N., et al. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatalysis and Agricultural Biotechnology . 2020;23:p. 101471. doi: 10.1016/j.bcab.2019.101471. [DOI] [Google Scholar]
- 87.Bouyahya A., Bakri Y., Et-Touys A., et al. In vitro screening of antibacterial and antioxidant activities of essential oils from four Moroccan medicinal plants. Microbiology Research Journal International . 2017;18(4):1–10. doi: 10.9734/mrji/2017/30073. [DOI] [Google Scholar]
- 88.Silva L. F., Cardoso M. d. G., Batista L. R., et al. Chemical characterization, antibacterial and antioxidant activities of essential oils of mentha viridis L. And mentha pulegium L. (L) American Journal of Plant Sciences . 2015;06(05):666–675. doi: 10.4236/ajps.2015.65072. [DOI] [Google Scholar]
- 89.Talbaoui A. Chemical composition and antibacterial activity of essential oils from six Moroccan plants. Journal of Medicinal Plants Research . 2012;6(31) doi: 10.5897/jmpr10.078. [DOI] [Google Scholar]
- 90.Zandi-Sohani N., Ramezani L. Evaluation of five essential oils as botanical acaricides against the strawberry spider mite Tetranychus turkestani Ugarov and Nikolskii. International Biodeterioration & Biodegradation . 2015;98:101–106. doi: 10.1016/j.ibiod.2014.12.007. [DOI] [Google Scholar]
- 91.Koumad S., Berkani M. L. Assessment of the efficacy of four medicinal plants as fumigants against Varroa destrutor in Algeria. Animal Husbandry Archives . 2019;68 [Google Scholar]
- 92.Derbalah A., Ahmed S. Efficacy of spearmint oil and powder as alternative of chemical control against C. maculatus in Cowpea Seeds. Egyptian Academic Journal of Biological Sciences, F. Toxicology & Pest Control . 2010;2(1):53–61. doi: 10.21608/eajbsf.2010.17463. [DOI] [Google Scholar]
- 93.Haouel-Hamdi S., Soltani A., Jmal R., et al. Use of binary mixtures of three Mentha essential oils for the control of rice weevil Sitophilus oryzae (Curculionidae) International Journal of Tropical Insect Science . 2021;41(2):1333–1342. doi: 10.1007/s42690-020-00326-1. [DOI] [Google Scholar]
- 94.Lamiri A., Lhaloui S., Benjilali B., Berrada M. Fumigant toxic activity of essential oils on Sitophilus granarius (linné) Physical and Chemical News . 2001;1 [Google Scholar]
- 95.Papachristos D. P., Stamopoulos D. C. Repellent, toxic and reproduction inhibitoryeffects of essential oil vapours on Acanthoscelides obtectus (Say) (Coleoptera: bruchidae) Journal of Stored Products Research . 2002;38 [Google Scholar]
- 96.Abdel-Shafy S., Soliman M. M. M. Toxicity of some essential oils on eggs, larvae and females of Boophilus annulatus (Acari: Ixodida: Amblyommidae) infesting cattle in Egypt. Open Science in Acarology . 2004;44 [Google Scholar]
- 97.Yousuf P. H., Noba N. Y., Shohel M., Bhattacherjee R., Das B. K. Analgesic, anti-inflammatory and antipyretic effect of mentha spicata (spearmint) British Journal of Pharmaceutical Research . 2013;11 doi: 10.9734/bjpr/2013/4640. [DOI] [Google Scholar]
- 98.Arumugam P., Priya N. G., Subathra M., Ramesh A. Anti-inflammatory activity of four solvent fractions of ethanol extract of Mentha spicata L. investigated on acute and chronic inflammation induced rats. Environmental Toxicology and Pharmacology . 2008;26(1):92–95. doi: 10.1016/j.etap.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 99.Jabbar A. A. S. A., Kathem S. H. The protective effect of mentha spicata ethanolic extract on irinotecan-induced mucositis in mice. Iraqi Journal of Pharmaceutical Sciences . 2019;28 [Google Scholar]
- 100.Al-Fartosi K. G., Radi H., Al-Rekabi E. A. Lipid profile of diabetic male rats treated with phenolic compounds of leaves extracts from mentha longifolia and mentha spicata. ISSN . 2014;3(2):p. 6. [Google Scholar]
- 101.Mushtaq A., Iqbal N., Jamil M., Khawaja N., Gohar U., Mehmood M. Anti-diabetic and anti-hyperlipidemic action of aqueous ethanolic extracts of mentha spicata (leaves), plumeria alba (leaves) and nymphaea alba (flowers and rhizomes) IJBPAS . 2017;6 [Google Scholar]
- 102.Nickavar B., Alinaghi A., Kamalinejad M. Evaluation of the antioxidant properties of five mentha species. Iranian Journal of Pharmaceutical Research . 2008;7(3):p. 7. [Google Scholar]
- 103.Abdel-Hady H., El-Wakil E. A., Abdel-Gawad M. GC-MS analysis, antioxidant and cytotoxic activities of mentha spicata. European Journal of Medicinal Plants . 2018;26(1):1–12. doi: 10.9734/ejmp/2018/45751. [DOI] [Google Scholar]
- 104.Dhifi W., Jelali N., Mnif W., Litaiem M., Hamdi N. Chemical composition of the essential oil ofmentha spicatal. From Tunisia and its biological activities. Journal of Food Biochemistry . 2013;37(3):362–368. doi: 10.1111/j.1745-4514.2012.00656.x. [DOI] [Google Scholar]
- 105.Fatiha B., Didier H., Naima G., et al. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae) Industrial Crops and Products . 2015;74:722–730. doi: 10.1016/j.indcrop.2015.04.038. [DOI] [Google Scholar]
- 106.Özer Z. Investigation of phenolic compounds and antioxidant activity of mentha spicata L. subsp. spicata and M. longifolia (L.) L. subsp. typhoides (briq.) harley decoction and infusion. Journal of the Turkish Chemical Society Section A: Chemistry . 2018;5:445–456. [Google Scholar]
- 107.Rita I., Pereira C., Barros L., Santos-Buelga C., Ferreira I. C. F. R. Mentha spicata L. infusions as sources of antioxidant phenolic compounds: emerging reserve lots with special harvest requirements. Food & Function . 2016;7(10):4188–4192. doi: 10.1039/c6fo00841k. [DOI] [PubMed] [Google Scholar]
- 108.Snoussi M., Noumi E., Trabelsi N., Flamini G., Papetti A., De Feo V. Mentha spicata essential oil: chemical composition, antioxidant and antibacterial activities against planktonic and biofilm cultures of Vibrio spp. strains. Molecules . 2015;20(8):14402–14424. doi: 10.3390/molecules200814402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prince Vijeya Singh J., Selvendiran K., Mumtaz Banu S., Padmavathi R., Sakthisekaran D. Protective role of Apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine . 2004;11(4):309–314. doi: 10.1078/0944711041495254. [DOI] [PubMed] [Google Scholar]
- 110.Aziz M., Saqib N., Akhtar N., et al. Phytochemical screening and evaluation of the diuretic activity of aqueous methanol extract from aerial parts of mentha viridis linn (labiatae) in albino rats. Tropical Journal of Pharmaceutical Research . 2014;13(7):1121–1125. doi: 10.4314/tjpr.v13i7.16. [DOI] [Google Scholar]
- 111.Saad A. B., Rjeibi I., Brahmi N., Elaloui E., Zouari N. Nicotine-induced oxidative stress, testis injury, AChE inhibition and brain damage alleviated by Mentha spicata. Inflammopharmacology . 2020;28(4):939–948. doi: 10.1007/s10787-019-00650-0. [DOI] [PubMed] [Google Scholar]
- 112.S Alqahtani A., Ullah R., Shahat A. A. Bioactive constituents and toxicological evaluation of selected antidiabetic medicinal plants of Saudi Arabia. Evidence-based Complementary and Alternative Medicine . 2022;2022:p. 23. doi: 10.1155/2022/7123521.7123521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Naidu J., Ismail R., Sasidharan S. Acute oral toxicity and brine shrimp lethality of methanol extract of mentha spicata L (Lamiaceae) Tropical Journal of Pharmaceutical Research . 2014;13(1):p. 101. doi: 10.4314/tjpr.v13i1.15. [DOI] [Google Scholar]
- 114.Mugisha M. K., Ndukui J. G., Namutembi A., Waako P., Karlson A.-K. B., Vudriko P. Acute and sub-acute toxicity of ethanolic leaf extracts of rumex abyssinica jacq. (Polygonaceae) and mentha spicata L. (Lamiaceae) Pharmacology & Pharmacy . 2014;05(03):309–318. doi: 10.4236/pp.2014.53038. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphical abstract of this study is attached in supplementary file.
Data Availability Statement
The data used to support this study are included within the article.


