Abstract
Introduction:
The aim of this study was to identify factors associated with primary graft patency 1 year following open lower limb revascularisation (LLR) at a tertiary referral vascular service.
Methods:
A retrospective analysis of patients undergoing infra-inguinal bypass surgery between January 2016 and May 2017 at a tertiary vascular centre (St Mary’s Hospital, London) was performed. Data regarding patient demographics, comorbidities, type of operation and post-operative anti-thrombotic strategy were collected. Quality of run-off score was assessed from pre-operative imaging.
Results:
Seventy-seven cases were included in the analysis. Overall, the primary patency rate at 1-year was 63.6% (n = 49/77) and the secondary patency rate was 67.5% (n = 52/77). Independent variables with statistically significant inferior patency rates at 1-year were (1) bypasses with below knee targets (p = 0.0096), (2) chronic limb threatening ischaemia indication (p = 0.038), (3) previous ipsilateral revascularisation (p < 0.001) and (4) absence of hypertension history (p = 0.041). There was also a trend towards significance for American Society of Anesthesiologists (ASA) grade (p = 0.06). Independent variables with log-rank test p values of <0.1 were included in a Cox proportional hazards model. The only variable with a statistically significant impact on primary patency rates was previous open or endovascular ipsilateral revascularisation (HR 2.44 (1.04–5.7), p = 0.04).
Conclusion:
At 1-year follow-up, previous ipsilateral revascularisation was the most significant factor in affecting patency rates. Patients in this subgroup should therefore be deemed high-risk, which should be reflected in the informed consent and peri-operative management.
Keywords: lower limb, bypass revascularization, patency, anti-thrombotic
Introduction
Lower limb revascularisation is primarily reserved for patients with chronic limb threatening ischaemia (CLTI) and, on occasion, short distance claudication that has failed conservative management. The choice of lower limb revascularisation strategy has become contentious, with endovascular techniques increasingly prevalent. This choice relies on an assessment of a number of factors including the anatomical pattern of disease, patient specific risk and also patient preference. Despite this increase in endovascular procedures, open lower limb revascularisation (LLR) remains a key treatment modality, particularly in light of the BASIL trial, which highlights inferior long term patency following endovascular therapies. 1
In the United Kingdom, there have been over 17,000 LLR procedures conducted in the UK between 2014 and 2017. 2 Outcomes following LLR depend on a number of factors; these include patient factors (e.g. indication, co-morbidities), anatomical distribution of disease (e.g. quality and level of inflow and outflow) and technical (e.g. choice of conduit, technical errors). Retrospective studies which have previously helped to identify these factors are typically limited by short follow-up and missing co-variates, such as antithrombotic strategy and quantitative assessment of bypass run-off, which are crucial to determining graft patency in clinical practice.3,4
Antithrombotic strategies following LLR warrants further examination, in particular as there are large national and international discrepancies in practice; 5 with variations in the choice of agents, the length of treatment course(s) as well as the combination of agents. Better understanding of clinical practice and medium-term outcomes following surgery will serve to guide clinical practice as well as future research.
The aims of this study was to identify factors associated with primary graft patency 1 year following LLR at a tertiary referral vascular service and factors that may have influenced choice of post-operative antithrombotic treatment.
Methods
Inclusion and exclusion criteria
Institutional approval for this study was obtained (Imperial College NHS Healthcare NHS Trust Audit Department; ID VAS_06). A retrospective analysis of patients undergoing infra-inguinal bypass surgery between January 2016 and May 2017 at a tertiary vascular centre (St Mary’s Hospital, Imperial College Healthcare NHS Trust, London, UK) was performed. The timeframe was selected to enable accurate data collection from the hospital electronic health records system (PowerChart, Cerner, Missouri), which was fully implemented by January 2016. Patients were excluded if graft failure occurred in the first week following surgery or if they passed away during the admission. If a patient had multiple ipsilateral bypass procedures during the specified period, then only the index operation was included.
Data collection
Data regarding patient demographics, comorbidities, type of operation and post-operative anti-thrombotic strategy (single antiplatelet (SAP), dual antiplatelet therapy (DAPT), anticoagulant therapy only (anticoagulation), combined antiplatelet and anticoagulant therapy (combined)) were collected. The run-off score was calculated from the pre-operative angiogram using Rutherford criteria, 6 which are based on the appearance of the run-off vessels distal to the site of the anastomosis. The calculation is based on the severity of stenosis and the relative contribution of each vessel to the outflow in the limb. The best attainable score is 1, indicating a widely patent run-off. Higher scores indicate a greater degree of resistance to flow, with the worst attainable score of 10 indicating occluded run-off. Where a pre-operative angiogram was not available, run-off was estimated from the pre-operative duplex scan or computed tomography angiogram (CTA).
The primary end point for the study was the primary patency at 12-months follow-up. Primary patency was defined as patency attained without need for an adjunctive secondary surgical or endovascular procedure. Patency was assessed through the use of duplex ultrasonography undertaken by dedicated vascular sonographers. Secondary patency was defined as bypass patency after occlusion when treated successfully with endovascular or surgical therapy. Additionally, antithrombotic strategies were recorded.
Statistical analysis
The distribution of the continuous variables age and run-off score were visualised in histograms before being dichotomised to binary values (age <55 or ⩾55 years, run-off score <5 or ⩾5), maintaining roughly equal group sizes. The Kaplan-Meier method and log-rank test were used compare patency between groups in each variable. As the number of patients lost to follow-up was relatively small, censoring was not performed. Variables with logrank test p values ⩽0.1 were selected for inclusion in the Cox proportional hazards model. The Chi-squared test (X2) was used to test for differences in variables with log-rank test p values ⩽ 0.1 and the choice of antithrombotic strategy. All p-values ⩽0.05 were considered significant. All analyses were performed using R (R version 3.3.1; R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org).
Results
Patient demographics
After screening 101 bypasses for inclusion, 24 cases were excluded. Reasons for exclusion were re-intervention on already included cases (n = 11), mortality within admission (n = 5), loss to follow up (n = 8). The remaining 77 cases were included in the analysis. Demographic data are summarised in Table 1. Overall, the primary patency rate at 1-year was 63.6% (n = 49/77) and the secondary patency rate was 67.5% (n = 52/77). Median time to loss of primary patency was 3-months (IQR 2–9 months).
Table 1.
Patient demographics.
| Variable | Maintained primary patency at 1-year (n = 49) | Loss of primary patency at 1-year (n = 28) | Log rank p value |
|---|---|---|---|
| Gender | 0.33 | ||
| M | 39 (79.6%) | 25 (89.3%) | |
| F | 10 (20.4%) | 3 (10.7%) | |
| Age | 0.92 | ||
| <55 | 11 (22.9%) | 7 (25%) | |
| >55 | 38 (79.2%) | 21(75%) | |
| Smoker | 0.97 | ||
| Non-smoker | 34 (69.4%) | 19 (67.9%) | |
| Current smoker | 15 (30.6%) | 9 (32.1%) | |
| ASA grade | 0.06 | ||
| 2 | 12 (24.5%) | 11 (39.3%) | |
| 3 | 36 (73.5%) | 15 (53.6%) | |
| 4 | 1 (2%) | 1 (3.6%) | |
| 5 | 0 (0%) | 1 (3.6%) | |
| Diabetes | 22 (44.9%) | 10 (35.7%) | 0.46 |
| HCL | 30 (61.2%) | 13 (46.4%) | 0.13 |
| HTN | 34 (69.4%) | 13 (46.4%) | 0.041* |
| IHD | 24 (49%) | 9 (32.1%) | 0.16 |
| Renal | 5 (10.2%) | 4 (14.3%) | 0.46 |
| Stroke | 4 (8.2%) | 4 (14.3%) | 0.43 |
| DVT | 2 (4.1%) | 2 (7.1%) | 0.65 |
| COPD | 7 (14.3%) | 3 (10.7%) | 0.57 |
| Statin therapy | 45 (91.8%) | 25 (89.3%) | 0.53 |
| Previous ipsilateral revascularisation | 11 (22.4%) | 17 (60.1%) | <0.001* |
| Indication | 0.038* | ||
| Claudication | 14 (28.6%) | 3 (10.7%) | |
| CLTI | 28 (57.1%) | 24 (85.7%) | |
| Aneurysm | 7 (14.3%) | 1 (3.6%) | |
| Runoff score | 0.38 | ||
| <5 | 34 (70.8%) | 22 (81.5%) | |
| >5 | 14 (29.2%) | 5 (18.5) | |
| n=48 | n=27 | ||
| Conduit | 0.47 | ||
| Vein | 40 (81.6%) | 21 (75%) | |
| Prosthetic | 9 (18.4%) | 7 (25%) | |
| Target level | 0.0096* | ||
| AK | 20 (40.8%) | 3 (10.7%) | |
| BK | 29 (59.2%) | 25 (89.3%) | |
| Urgency | 0.76 | ||
| Elective | 37 (75.5%) | 22 (78.6%) | |
| Emergency | 12 (24.5%) | 6 (21.4%) | |
| Antithrombotic strategy | 0.66 | ||
| SAP | 13 (26.5%) | 5 (17.9%) | |
| Anticoagulation | 4 (8.2%) | 2 (7.1%) | |
| Combined | 22 (44.9%) | 13 (46.4%) | |
| DAPT | 10 (20.4%) | 8 (28.6%) |
ASA: American Society of Anesthesiologists; HCL: hypercholesterolaemia; HTN: hypertension; IHD: ischaemic heart disease; DVT: deep vein thrombosis; COPD: chronic obstructive pulmonary disease; CLTI: chronic limb threatening ischaemia; AK: above knee; BK: below knee; SAP: single antiplatelet; Combined: combined antiplatelet and anticoagulation; DAPT: dual antiplatelet therapy.
p<0.05.
Factors associated with primary graft patency
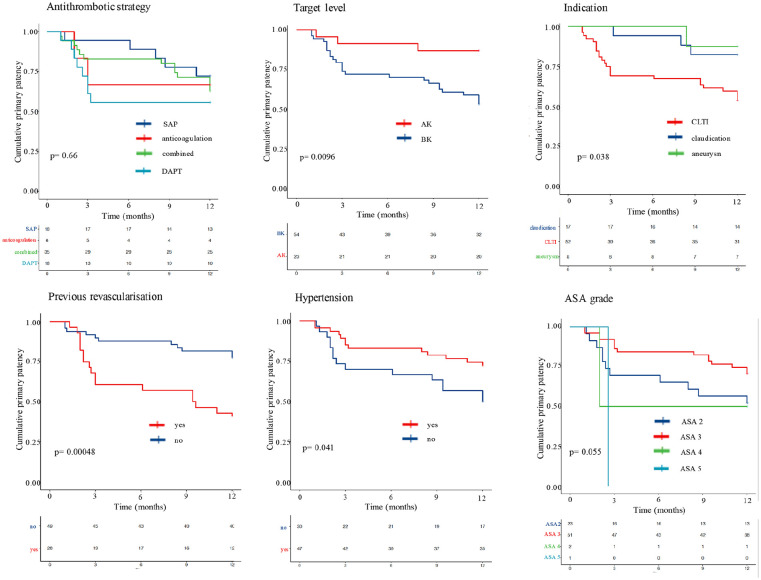
Independent variables with statistically significant inferior primary patency rates at 1-year were (1) bypasses with BK targets (p = 0.0096), (2) CLTI indication (p = 0.038), (3) previous ipsilateral revascularisation (p < 0.001) and (4) absence of hypertension history (p = 0.041). There was also a trend towards significance for American Society of Anesthesiologists (ASA) grade (p = 0.06).
When considering primary patency rates at 1-year for different antithrombotic strategies, no statistically significant difference was found (p = 0.66). However, the DAPT and SAP group curves diverge early. The combined antithrombotic strategy group (combined anticoagulation and antiplatelet therapy) maintains a higher patency rate during early follow up as compared to the DAPT group, but catch up at 12-month follow up with a few late instances of loss of primary patency. The corresponding p values from the log-rank test for each independent variable are presented in Table 1. Kaplan Meier Survival curves for independent variables with p values <0.1 and for antithrombotic strategies are presented in Figure 1.
Figure 1.
Kaplan Meier Survival curves for independent variables.
SAP, single antiplatelet; Combined, combined antiplatelet and anticoagulation; DAPT, dual antiplatelet therapy; AK, above knee; BK, below knee; ASA, American Society of Anesthesiologists; HTN, hypertension.
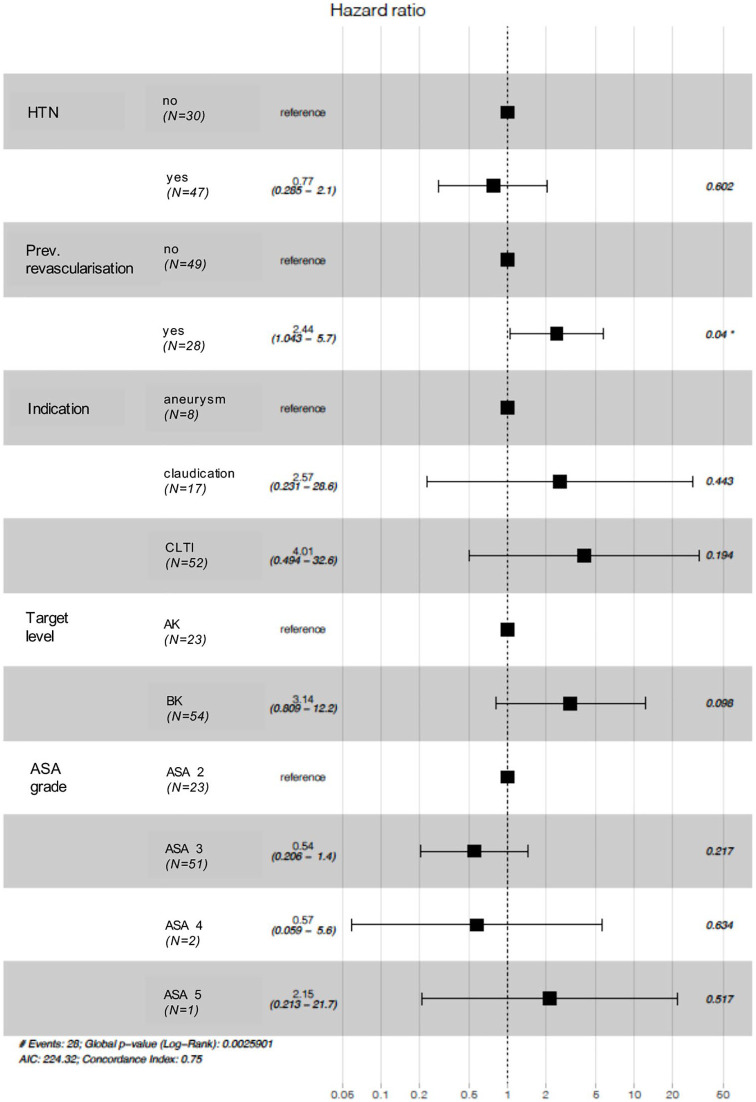
Independent variables with log-rank test p values of <0.1 were included in a Cox proportional hazards model (Figure 2). The only variable with a statistically significant impact on primary patency rates was previous open or endovascular ipsilateral revascularisation (HR 2.44 (1.04–5.7), p = 0.04).
Figure 2.
Forest plot for Cox proportional hazards model.
HTN, hypertension; ASA, American Society of Anesthesiologists.
Factors associated with choice of antithrombotic strategy
Antithrombotic strategies utilised in the cohort are summarised in Table 1. In cases where therapeutic dose anticoagulation was used, either alone or in combination with an antiplatelet, warfarin was the anticoagulant in 41.5% of cases (n = 17/41), whilst direct acting oral anticoagulants (DOAC) were used in 58.5% of cases (n = 24/41). We did not observe any major haemorrhagic complications during the follow up period.
In order to explore whether there was an association between the antithrombotic strategy employed and the independent variables tested, further analyses were undertaken. A higher proportion of patients on formal anticoagulation alone or DAPT had a previous history of ipsilateral open or endovascular revascularisation (X2 = 10.6, p = 0.014). Additionally, although not statistically significant, a higher proportion of patients on anticoagulation alone, combined and DAPT antithrombotic strategies had BK targets (X2 = 4.7, p = 0.20) and were being treated for CLTI (X2 = 6.8, p = 0.34). Similarly, patients with ASA 4 or 5, who had poor primary patency outcomes, were treated with DAPT (X2 = 15.1, p = 0.087).
Discussion
Infra-inguinal bypasses remain a robust treatment option in those with lower limb ischaemia. Previous studies on this topic have mostly focused on retrospective analysis of large registry data sets.7,8 These have been invaluable in identifying factors associated with early graft failure. However, they are often limited by their focus upon early graft failure and absence of data regarding antithrombotic strategy or run-off quality; factors important to determining medium to long term graft patency. This study demonstrates not only the factors associated with primary patency but how antithrombotic strategies are employed in differing patient groups.
In our log-rank survival analysis, we identified four factors which were associated with early graft failure: previous ipsilateral revascularisation, no hypertension, indication and BK target. However, the Cox proportional hazards model only identified previous history of ipsilateral open or endovascular revascularisation as significantly associated with primary patency (HR 2.44 (1.04–5.7), p = 0.04). This is in keeping with the results of other studies, such as Lancaster et al. who found, from the UK National Surgical Quality Improvement Programme (NSQIP), that the odds of early graft failure were 1.39 times higher if the patient had undergone a prior operation for peripheral arterial disease. 7 However, unlike our study, their group did not specifically consider ipsilateral history or include previous endovascular intervention which has been shown in the BASIL trial to be associated with lower amputation-free survival. 9
The log-rank analysis and trends from the Cox proportional hazards model also suggest that the indication for LLR may be a useful predictor of primary patency, with the average patency being higher for those undergoing exclusion bypasses for popliteal artery aneurysms compared to LLR for claudication. Patency for those with CLTI was worst. This is consistent with findings from the literature, where early graft failure and ultimately limb salvage, have been shown to depend upon the initial indication for treatment.7,10 In the current study, however, whilst univariate analysis found indication to be statistically significant, it was not a predictive factor in the multivariate model. This may be due to the overlap between previous revascularisation attempts and indication.
Another variable identified in our log-rank analysis but not statistically significant in the Cox proportional hazards model was target level, with a trend towards those with BK targets (below knee popliteal and crural vessels) having a higher risk of graft failure at 1-year follow-up (HR 3.14 (0.81–12.2), p = 0.098). This is consistent with the findings of others who found that infrapopliteal bypass targets are twice as likely to occlude.7,11 However, we found no statistically significant relationship between run-off quality and primary patency at 1-year. Scoring run-off quality is challenging and no scoring system has been shown to correlate with graft failure. The utility of the scoring system used in this study is dependent on the accuracy of data collection and has been suggested to be most useful in comparison of similar bypass grafts with similar outflow sites. 6 An alternative approach may be to assess flow profile intraoperatively. A number of studies have observed that flow abnormalities, which may result from poor quality outflow or technical defects, detected on intraoperative completion duplex ultrasound are associated with high rates of reintervention.12,13
Interestingly, our log-rank survival analysis suggested a higher rate of graft failure for patients with no background history of hypertension (p = 0.041), but this did not reach statistical significance in the Cox proportional hazard model. Possible explanations for this observation may be that those patients with diagnosed hypertension are more likely to be prescribed anti-hypertensives. Another explanation for such a pattern, may be related to increasing clinician exposure on account of their hypertension. This could lead to improved general optimisation of comorbid patients compared to those with reduced health professional exposure. This trend has been seen in those undergoing other major operations, such as colectomies in which there is a 31% reduction in the odds of post-operative complications. 14
Other studies have identified a number of other potentially important risk factors for bypass failure including emergency procedure, 7 dialysis, 11 thrombocytosis, 7 current smoker,7,15 male gender, 7 female gender, 11 composite bypass grafts, 7 choice of conduit,11,16,17 younger age,8,15 ethnicity 7 and non-diabetic patient. 8
In addition to operative or patient factors, the importance of antithrombotic strategies is increasingly becoming evident. The COMPASS trial has demonstrated a reduction in adverse limb outcomes using low-dose rivaroxaban in peripheral artery disease. 18 These observations were extended in the recent VOYAGER PAD trial which evaluated the safety and efficacy of low dose rivaroxaban plus aspirin in reducing major thrombotic vascular events specifically in patients with symptomatic peripheral arterial disease undergoing revascularisation. 19 The results of the study suggest that low dose rivaroxaban plus aspirin is associated with a lower incidence of the composite outcome acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischaemic stroke or cardiovascular death as compared to aspirin alone. However, the study also showed a higher incidence of major bleeding, depending on the definition used, in the low dose rivaroxaban plus aspirin group.
Our study has demonstrated a possible trend towards improved primary patency in patients treated with SAP post-operatively. Additionally, early graft occlusions in the DAPT and anticoagulation groups were highest, whereas the combined antiplatelet and anticoagulation group appeared to have a higher number of late failures. These differences did not reach statistical significance and may be explained by the observation that a greater proportion of patients in the non-SAP group had higher ASA grades, BK targets, previous lower limb revascularisations and were being treated for limb salvage. All of these factors are associated with lower patency rates as suggested by our analysis. It is therefore possible, that in patients whom exhibit these high-risk indicators, the surgeon felt a greater antithrombotic effect was required to ensure patency. However, due to this confounding it was not possible to meaningfully describe what strategy was best in high-risk bypasses. There is some evidence to suggest that antithrombotic strategy should be tailored to individual circumstance,16,20 however, there remains little objective way of determining this choice.
A number of limitations exist within the current study. The use of structured electronic health records (EHR) allowed a detailed examination of patients’ medical history, radiology and surgical outcomes. This ensured we could get accurate follow-up data from patients 1 year post-operatively. The use of EHRs however, requires retrospective examination by its nature, which can impair the study validity with the introduction of selection bias. Also, the calculation of the run-off score was not based on a standardised modality which may limit the comparability and validity of run-off assessments. Furthermore, there is an assumption of compliance with medication, as this is not routinely tested in those on antiplatelet or DOAC therapy. In addition, data collection was limited to the time period since our centre had adopted an EHR, restricting data collection and thus potentially contributing to type II statistical error. Finally, our single-centre results may lack generalisability.
Future work should aim to address these limitations in order to achieve consensus on risk stratification. This would facilitate future studies evaluating optimal antithrombotic strategies, tailored to the individual’s needs. Future studies, including retrospective interrogation of large data sets and meta-analysis of existing literature, may help resolve this issue.
Conclusion
At 1-year follow-up, previous ipsilateral revascularisation was the most significant factor in affecting patency rates. Patients in this subgroup should therefore be deemed high-risk, which should be reflected in the informed consent process and peri-operative management.
Footnotes
Author contributions: Conceptualisation: PN, UJ. Data collection and analysis: PN, IYA, AC. Drafting and editing manuscript: PN, UJ, IYA, AC, AA, VS, CM, JS, AHD, MJ, CR, RG, CB, DN, NJS.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Pasha Normahani  https://orcid.org/0000-0002-6362-7535
https://orcid.org/0000-0002-6362-7535
Chira Mustafa  https://orcid.org/0000-0002-0524-4384
https://orcid.org/0000-0002-0524-4384
References
- 1.Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010;51:5S–17S. [DOI] [PubMed] [Google Scholar]
- 2.Health Quality Improvement Partnership. National vascular registry 2017. annual report. 2017. https://www.hqip.org.uk/resource/national-vascular-registry-annual-report-2017/#.Xigu31P7RTY
- 3.Schanzer A, Hevelone N, Owens CD, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg 2007; 46: 1180–1190. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: analysis of amputation free and overall survival by treatment received. J Vasc Surg 2010; 51: 18S–31S. [DOI] [PubMed] [Google Scholar]
- 5.Lindblad B, Wakefield TW, Stanley TJ, et al. Pharmacological prophylaxis against postoperative graft occlusion after peripheral vascular surgery: a world-wide survey. Eur J Vasc Endovasc Surg 1995; 9: 267–271. [DOI] [PubMed] [Google Scholar]
- 6.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26: 517–538. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster RT, Conrad MF, Patel VI, et al. Predictors of early graft failure after infrainguinal bypass surgery: a risk-adjusted analysis from the NSQIP. Eur J Vasc Endovasc Surg 2012; 43: 549–555. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Sidawy AN, DeZee KJ, et al. Factors associated with early failure of infrainguinal lower extremity arterial bypass. J Vasc Surg 2008; 47: 556–561. [DOI] [PubMed] [Google Scholar]
- 9.Meecham L, Patel S, Bate GR, et al. Editor’s choice – a comparison of clinical outcomes between primary bypass and secondary bypass after failed plain balloon angioplasty in the bypass versus angioplasty for severe ischaemia of the limb (BASIL) trial. Eur J Vasc Endovasc Surg 2018; 55: 666–671. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin ZK, Pearce BJ, Curi MA, et al. Limb salvage after infrainguinal bypass graft failure. J Vasc Surg 2004; 39: 951–957. [DOI] [PubMed] [Google Scholar]
- 11.Nolan BW, De Martino RR, Stone DH, et al. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. J Vasc Surg 2011; 54: 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson BL, Bandyk DF, Back MR, et al. Intraoperative duplex monitoring of infrainguinal vein bypass procedures. J Vasc Surg 2000; 31: 678–690. [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie KS, Hill AB, Steinmetz OK. The predictive value of intraoperative duplex for early vein graft patency in lower extremity revascularization. Ann Vasc Surg 1999; 13: 275–283. [DOI] [PubMed] [Google Scholar]
- 14.Leeds IL, Canner JK, Gani F, et al. Increased healthcare utilization for medical comorbidities prior to surgery improves postoperative outcomes. Ann Surg 2020; 271: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galaria II, Surowiec SM, Tanski WJ, et al. Popliteal-to-distal bypass: identifying risk factors associated with limb loss and graft failure. Vasc Endovascular Surg 2005; 39: 393–400. [DOI] [PubMed] [Google Scholar]
- 16.Johnson WC, Williford WO. Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: a prospective randomized study. J Vasc Surg 2002; 35: 413–421. [DOI] [PubMed] [Google Scholar]
- 17.Suckow BD, Kraiss LW, Stone DH, et al. Comparison of graft patency, limb salvage, and antithrombotic therapy between prosthetic and autogenous below-knee bypass for critical limb ischemia. Ann Vasc Surg 2013; 27: 1134–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018; 391: 219–229. [DOI] [PubMed] [Google Scholar]
- 19.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med 2020; 382: 1994–2004. [DOI] [PubMed] [Google Scholar]
- 20.Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin Study): a randomised trial. Lancet 2000; 355: 346–351. [PubMed] [Google Scholar]