Abstract
There have been several reports that the activity of echinocandin antifungal agents is not affected or decreased in the presence of human sera. It is known that these drugs are bound >80% in animal and human sera. The activity of the echinocandin caspofungin (MK-0991), a 1,3-β-d-glucan synthase inhibitor, against Aspergillus fumigatus with and without human sera was studied. Conidia of A. fumigatus in microtest plate wells formed germlings after overnight culture in RPMI 1640. Caspofungin was then added with or without serum, and the germlings were incubated at 37°C for 24 h. Human serum (5%) in RPMI 1640 alone did not significantly inhibit the growth of A. fumigatus in vitro. Caspofungin in RPMI 1640 exhibited dose-dependent inhibition, with concentrations of 0.1 and 0.05 μg/ml inhibiting 24.9% +/− 10.4% and 11.7% +/− 3.6%, respectively (n = 10; P < 0.01). The addition of 5% human serum to caspofungin at 0.1 or 0.05 μg/ml increased the inhibition to 78.6% +/− 5.8% or 58.3% +/− 19.2%, respectively (n = 10; P < 0.01 versus controls and versus the drug without serum). Lower concentrations of serum also potentiated drug activity. The effect of human sera was further seen when using caspofungin that had lost activity (e.g., by storage) against A. fumigatus at 0.1 μg/ml. Inactive caspofungin alone demonstrated no significant inhibition of hyphal growth, whereas the addition of 5% human serum to the inactive drug showed 83% +/− 16.5% inhibition (n = 5; P < 0.01). The restoration of activity of caspofungin was seen at concentrations as low as 0.05% human serum. In contrast to prior reports, this study suggests that human serum acts synergistically with caspofungin to enhance its inhibitory activity in vitro against A. fumigatus.
Caspofungin (MK-0991; Merck Research Laboratories, Rahway, N.J.) is a water-soluble, semisynthetic amine derivative of the natural product pneumocandin B0 and is a member of a new generation of echinocandins with enhanced activity against a variety of fungi (2). The agents act via the inhibition of the enzyme 1,3-β-d-glucan synthase, which synthesizes an essential component of the cell walls of many medically important fungi. The echinocandins do not give classic MICs, i.e., clear tube end points, for Aspergillus species when tested using in vitro broth dilution techniques. However, it has been shown that profound morphological effects on Aspergillus are produced with this class of drugs in vitro (3; D. A. Stevens, M. Martinez, and M. J. Devine, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F46, p. 107, 1996). Furthermore, in vivo studies with disseminated models of both mice and rats have shown potent effects of caspofungin against infection (1). Recently, it has been shown that, by using an XTT [(2,3)-Bis-(2-methoxy-4-nitro-5-sulphenyl-(2H)-tetrazolium-5-carboxanilide) sodium salt] dye assay, Aspergillus hyphal damage can be evaluated with several classes of drugs including echinocandins (Stevens et al., 36th ICAAC).
As this class of compounds is known to be highly protein bound (5), studies have looked for evidence that human sera adversely affect their activity. In this study we examined the effect of the interaction of human sera with caspofungin on the activity of caspofungin against Aspergillus fumigatus using an XTT assay. This interaction was studied under a variety of conditions.
MATERIALS AND METHODS
A. fumigatus.
A patient isolate of A. fumigatus, AF-10, was used for all experiments. The isolate was grown on Sabouraud's dextrose agar slants at 35°C for 24 h. Conidia were allowed to form at room temperature over 48 to 72 h. Conidia were harvested in distilled water, filtered through gauze, washed once, diluted in saline, and counted. Conidial suspensions consisted primarily of a single conidium (95%) or small groups of conidia with two or three per group (5%). Over 95% of the conidia germinated when incubated in RPMI 1640 medium (GIBCO, Grand Island, N.Y.) at 37°C for 2 h and then at room temperature (26°C) overnight. Each experiment was performed in 96-well flat-bottom plates, and all conditions were assayed in quadruplicate cultures.
Sera.
Human pooled AB sera were obtained from Scantibodies Lab Inc., Carlsbad, Calif. Human sera from donor whole blood were isolated by coagulation and centrifugation at room temperature. Fetal bovine sera were purchased from Sigma Chemical Co. (St. Louis, Mo.). Murine serum was obtained from CD-1 mice. Sera were either stored at −70°C and used later or used the same day as isolated. Fetal bovine sera were stored at −20°C. All sera were diluted in RPMI 1640, and 100 μl was used in experimental cocultures with 100 μl of A. fumigatus. Inactivation of complement was performed by heating undiluted sera for 1 h at 56°C. For experiments conducted with absorbed sera, the sera were first incubated at 37°C with and without A. fumigatus conidia at a concentration of 106/ml for 1 h, with and without agitation.
Caspofungin.
Caspofungin powder was supplied by Merck Research Laboratories. The powder was stored at −70°C. An appropriate amount was diluted in sterile distilled water to a concentration of 1 mg/ml. Aliquots of this solution were stored at −70°C and used only once per experiment. Final concentrations were attained by dilution on the day of the experiment in RPMI 1640 for incubation with A. fumigatus. When fresh drug was used, the powder was weighed and a solution of 1 mg/ml in sterile distilled water was then diluted to final concentrations in RPMI 1640. Caspofungin that became inactive, as determined by bioassay, by storage either at room temperature or for greater than 8 months at −70°C or by repeated freezing and thawing, was studied.
XTT assay.
Inhibition of hyphal growth was measured by the colorimetric XTT-coenzyme Q (2,3-dimethoxy-5-methyl-1,4-benzoquinone) method (6). XTT at 5 mg/ml plus coenzyme Q at 0.04 mg/ml in phosphate-buffered saline (pH 7.4; Sigma Chemical Co.) constituted the test solution.
A. fumigatus growth assay.
Ninety-six-well microtiter plates were inoculated with 103 conidia per well (100 μl), and conidia were allowed to germinate overnight as described above. Each treatment group was studied with quadruplicate wells; the appropriate amounts and combinations of caspofungin, sera, or manipulated sera were added in 100-μl volumes, and the cultures were incubated at 37°C for 24 h. The plates were centrifuged, supernatants were aspirated through a 27-gauge needle, distilled water was added, and the procedure was repeated. The XTT assay was performed by adding 0.2 ml of XTT test solution to wells of washed hyphae at 37°C. Supernatants (100 μl) from wells were transferred to wells of another microtest plate, and absorbance at 450 nm was recorded.
Quantitation of growth inhibition.
The absorbance at 450 nm of each well, measured with a 96-well microtest reader (Dynatech, McLean, Va.), was used to determine the difference in absorption (ΔA) between a tested well and control wells with XTT alone. The percentage of inhibition was calculated by the formula (ΔAcontrol − ΔAexperiment/ΔAcontrol) × 100. It has been previously shown (3, 8) that there is a linear relationship between the inoculum and the metabolism of XTT, as measured by the change in absorbance, and that drug-induced inhibition of hyphal growth, verified microscopically, correlates with diminished XTT metabolism. Thus, a decreased ΔA of XTT supernatants from caspofungin-treated cultures represents inhibition of growth.
Statistical analysis.
Student's t test was used for statistical analysis of the data, and significance was set at P < 0.05. The GB-STAT program (Microsoft, Richmond, Va.) was used for Bonferroni's adjustment to the t test where appropriate. All values for percentage of inhibition are presented as means from n experiments with their standard deviations.
RESULTS
Activity of caspofungin.
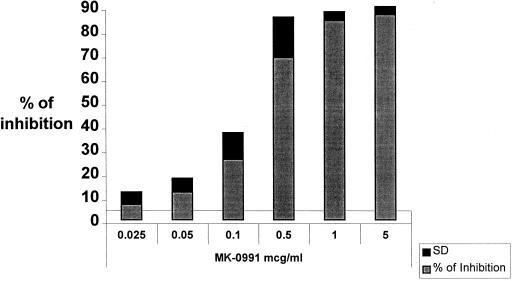
The percentage of hyphal growth inhibition of A. fumigatus with the XTT assay was determined using different concentrations of caspofungin as shown in Fig. 1. These results include data from 10 experiments, all done in quadruplicate wells. Inhibition of growth was found to be associated with club-like malformation of hyphal growth when cells were observed microscopically. A 70-mg dose of caspofungin produced a trough level in human serum of 2.66 ± 0.55 μg/ml (J. A. Stone, S. D. Holland, W. D. Ju, Z. Zhang, M. Schwartz, V. L. Hoagland, K. E. Mazina, T. L. Hunt, and S. Waldman, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A117, 1998). This is four to five times the 80% inhibitory level in our assay (Fig. 1).
FIG. 1.
Dose response of caspofungin against germlings of A. fumigatus. Vertical axis, percent inhibition of hyphal growth; horizontal axis, concentrations of drug in RPMI 1640. The standard deviation (SD) is shown at the top of each bar.
Human sera and caspofungin.
The addition of human sera to cultures containing caspofungin and hyphae resulted in a marked increase in the inhibition of fungal growth compared with caspofungin alone. This is shown in Table 1. Human sera did not significantly inhibit the growth of Aspergillus in media alone. The addition of human sera increased the inhibition of growth of Aspergillus by caspofungin up to fourfold (48.5%) compared to caspofungin alone (6.5%) at concentrations as low as 0.025 μg/ml. As is shown in Table 1, there is dose-dependent inhibition (48.5, 58.3, and 78.6%) with increasing concentrations of caspofungin and human sera. Similar results were obtained with sera from different donors as well as commercial pooled AB sera. This collaborative effect of serum and caspofungin was also seen where concentrations of serum as low as 0.05% were tested (Table 2). Increasing concentrations of sera increased the percent inhibition by caspofungin (Table 2). Human sera alone at concentrations higher than 20% significantly (P < 0.05) inhibited fungal growth. These higher concentrations of human sera did not enhance or decrease the activity of caspofungin compared to that of sera alone (data not shown). Experiments performed with freshly isolated sera, compared with those with sera repeatedly frozen and thawed and stored at −70°C, did not show any significant difference in the effect of the sera on caspofungin (data not shown).
TABLE 1.
Activity of caspofungin with and without human sera (5%) against A. fumigatus
| Treatmenta | Mean % inhibition ± SD (n = 10) |
P versus:
|
|
|---|---|---|---|
| RPMI 1640 | MK without HSb | ||
| RPMI 1640 | 0 ± 0 | ||
| HS | 5.1 ± 4.1 | NSc | |
| MK (0.025 μg/ml) | 6.5 ± 3.1 | NS | |
| MK (0.025 μg/ml) + HS | 48.5 ± 13.7 | <0.01 | <0.01 |
| MK (0.05 μg/ml) | 11.7 ± 3.6 | <0.01 | |
| MK (0.05 μg/ml) + HS | 58.3 ± 19.2 | <0.01 | <0.01 |
| MK (0.1 μg/ml) | 24.9 ± 10.2 | <0.01 | |
| MK (0.1 μg/ml) + HS | 78.6 ± 5.8 | <0.01 | <0.01 |
The treatment group represents 103 conidia of A. fumigatus per well. MK, caspofungin; HS, human sera.
Corresponding caspofungin concentration without human sera.
NS, not significant.
TABLE 2.
Activity of caspofungin at 0.1 μg/ml with decreasing concentrations of human sera against A. fumigatus
| Treatmenta | Mean % inhibition ± SD (n = 3) |
P versus:
|
|
|---|---|---|---|
| RPMI | RPMI + MK | ||
| RPMI | 0 | ||
| RPMI + MK | 16.4 ± 4.5 | <0.01 | |
| RPMI + MK + 5% HS | 69.0 ± 13.1 | <0.01 | <0.01 |
| RPMI + MK + 2.5% HS | 63.3 ± 10.4 | <0.01 | <0.01 |
| RPMI + MK + 1% HS | 62.3 ± 7.0 | <0.01 | <0.01 |
| RPMI + MK + 0.5% HS | 59.0 ± 10.1 | <0.01 | <0.01 |
| RPMI + MK + 0.1% HS | 33.0 ± 12.4 | <0.01 | <0.01 |
| RPMI + MK + 0.05% HS | 31.6 ± 12.2 | <0.01 | <0.01 |
The treatment group represents 103 conidia of A. fumigatus per well. RPMI, RPMI 1640; MK, caspofungin; HS, human sera.
Effect of temperature on potentiation.
Human sera were heated at temperatures of 56, 65, and 70°C for 1 h to determine if the ability to enhance the activity of caspofungin could be affected. As can be seen in Table 3, heat treatment did not change the ability of the human sera to enhance the activity of caspofungin. However, there was less activity noted in sera heated to 70°C.
TABLE 3.
Activity of caspofungin at 0.1 μg/ml plus heat-treated human sera or absorbed human sera against A. fumigatus
| Treatmenta | Mean % inhibition ± SD (n = 3) |
P versus:
|
|
|---|---|---|---|
| RPMI | HS + MK | ||
| RPMI | 0 | ||
| RPMI + MK | 18.0 ± 6 | <0.01 | <0.01 |
| HS | 5.1 ± 4.1 | NSb | <0.01 |
| HS + MK | 78.6 ± 5.8 | <0.01 | |
| HTHS at 56°C + MK | 75.2 ± 7.0 | <0.01 | NS |
| HTHS at 65°C + MK | 66.8 ± 10.1 | <0.01 | NS |
| HTHS at 70°C + MK | 34.8 ± 13.3 | <0.05 | <0.01 |
| AbHS + MK | 46.3 ± 16.5 | <0.01 | NS |
The treatment group represents 103 conidia of A. fumigatus per well. RPMI, RPMI 1640; MK, caspofungin; HS, human sera; HTHS, heat-treated HS; AbHS, absorbed HS.
NS, not significant.
Effect of serum pretreatment with Aspergillus conidia, addition of serum components, and results with animal sera.
Human serum was incubated with Aspergillus conidia prior to use in an attempt to deplete serum antibody, complement, or other factors, which could be contributing to the collaborative effect seen with caspofungin. Table 3 shows a nonsignificant minimal change in the effect of the sera after absorption with conidia compared to similarly treated controls. In none of the individual experiments was there a significant difference between absorbed sera and normal sera.
Additional experiments not shown involving the addition of increasing concentrations of albumin (0 to 40 mg/ml) or apotransferrin (0 to 100 μg/ml) to RPMI 1640 alone or RPMI 1640 plus serum were performed to determine their effect on caspofungin activity. These two proteins did not significantly change the activity of caspofungin with or without serum present (data not shown).
Fetal bovine sera and sera from CD-1 mice at concentrations of 1 to 20% exhibited a trend to enhance the activity of caspofungin against Aspergillus (data not shown). These results were not found to be significant, indicating that the collaborative effect with caspofungin required human sera.
Human sera and inactive caspofungin.
The most dramatic effect of human sera combined with caspofungin was seen with minimally active or inactive drug. Caspofungin was considered inactive when the concentrations tested demonstrated no significant activity in our bioassay. Caspofungin was allowed to lose its activity after prolonged storage. The ability of inactive caspofungin to inhibit fungal growth at 0.025, 0.05, and 0.1 μg/ml was markedly decreased or absent as shown in Table 4 compared with Table 1. The addition of 5% human serum to the inactive drug restored its antifungal activity to levels much higher than that of the drug alone. The effect with sera and inactive drug is comparable to that of sera and active drug, as can be seen in Tables 1 and 4. This effect was again seen with concentrations of human sera as low as 0.05% in a dose-dependent manner.
TABLE 4.
Activity of degraded caspofungin with the addition of human sera against A. fumigatus
| Treatmenta | Mean % inhibition ± SD (n = 5) |
P versus:
|
|
|---|---|---|---|
| RPMI 1640 | MK without HSb | ||
| RPMI 1640 | 0 | ||
| HS | 5.1 ± 4.1 | NSc | |
| MK (0.025 μg/ml) | 0 ± 0 | NS | |
| MK (0.025 μg/ml) + HS | 38.4 ± 6.2 | <0.01 | <0.01 |
| MK (0.05 μg/ml) | 2.0 ± 3.1 | NS | |
| MK (0.05 μg/ml) + HS | 57.3 ± 17.4 | <0.01 | <0.01 |
| MK (0.1 μg/ml) | 2.1 ± 3.6 | NS | |
| MK (0.1 μg/ml) + HS | 83 ± 16.5 | <0.01 | <0.01 |
The treatment group represents 103 conidia of A. fumigatus per well. HS, human sera; MK, caspofungin.
Corresponding caspofungin concentration without human sera.
NS, not significant.
DISCUSSION
Previous studies have reported that echinocandins (caspofungin, LY 303366, and FK 463) have activity against A. fumigatus in vitro, even though this class of compounds does not exhibit classic MICs (2, 3; Stevens et al., 36th ICAAC). Our study demonstrates the inhibition of Aspergillus growth by caspofungin in vitro, both microscopically and in an XTT assay. This inhibition was seen whether ungerminated or germinated conidia were used in the system. The addition of human sera to the in vitro system resulted in marked enhancement of the antifungal activity of caspofungin against Aspergillus. This suggests that the activity of this drug in vivo might be increased by contact with sera.
The finding that human sera enhance the activity of caspofungin is contrary to what other studies have reported for the treatment of other fungi with echinocandins. Hoban et al. (D. Hoban, T. Balko, D. Saunders, A. Kabani, J. Karlowsky, and G. Zhandel, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J9, p. 453, 1998) presented data in a recent abstract which demonstrated that the addition of human sera increased the MIC of LY 303366, an echinocandin, four- to eightfold against four different Candida species. In another study, Bartizal et al. (2) reported similar results, i.e., that both human serum and mouse serum increased the mean fungicidal concentration of caspofungin against Candida albicans. Our findings with Aspergillus are in sharp contrast to those two studies with C. albicans. There are no studies known to us that report on the effect of human sera and the activity of echinocandins against Aspergillus. The differences between our results and the results of others could be related to the antifungal target, i.e., Aspergillus versus Candida. On the other hand a similar enhancement of fluconazole and voriconazole activity with human sera against another fungus, Cryptococcus neoformans, was reported (4, 7).
In the present study, it is of particular interest that human sera were able to reactivate inactive drug that had no activity against Aspergillus in vitro. This phenomenon has not been described to our knowledge for any compounds, and there is no published report of this with other echinocandins or antifungal agents. This phenomenon warrants further investigation. One theory is that the “activation” of caspofungin by human sera represents enzymatic cleavage of an oxidative or phosphorylated product of drug storage.
Given that echinocandins are known to be highly protein bound, studies reporting increased MICs in the presence of sera might be expected. In this study, the addition of sera to the caspofungin itself, and furthermore sera with added high concentrations of albumin, did not decrease the activity of caspofungin but instead potentiated its activity. Attempts to eliminate the enhancement of caspofungin by human sera, e.g., by heating sera to less than 70°C or adsorption of sera with Aspergillus, resulted in no change in the enhancement of the caspofungin. This suggested that neither complement nor an Aspergillus antibody was contributing to the increased antifungal activity of the caspofungin with human sera. There was a consistent and stable enhancement in all experiments, and we were unable to change this by other manipulations of sera, e.g., by addition of other proteins or repeated freezing and thawing. All these studies indicate that the activity present in human sera that potentiates the antifungal caspofungin is very stable and is not associated with other potential immune mechanisms of drug enhancement, which might be removed by heat or conidium absorption.
In summary, the fact that human sera enhance the antifungal activity of caspofungin against Aspergillus is an encouraging property as it is a promising new and novel agent that will be used to treat Aspergillus infections.
ACKNOWLEDGMENT
This research was funded in part by a grant from Merck Research Laboratories.
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Pikounis V B, Balkovec J M, Bouffard A F, Dropinski J F, Rosen H, Kropp H, Bartizal K. Evaluation of the echinocandin antifungal caspofungin (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41:2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartizal K, Gill C J, Abruzzo G K, Flattery A M, Kong L, Scott P M, Smith J G, Leighton C E, Bouffard A F, Dropinski J F, Balkovec J. In vitro preclinical evaluation studies with the echinocandin antifungal caspofungin (L-743,872) Antimicrob Agents Chemother. 1997;41:2326–2332. doi: 10.1128/aac.41.11.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummer E, Chauhan S D, Stevens D A. Collaboration of human phagocytes with LY 303366 for antifungal activity against Aspergillus fumigatus. J Antimicrob Chemother. 1999;43:491–496. doi: 10.1093/jac/43.4.491. [DOI] [PubMed] [Google Scholar]
- 4.Brummer E, Kamei K, Miyaji M. Anticryptococcal activity of voriconazole against Cryptococcus var. gattii vs. var. neoformans; comparison with fluconazole and effect of human serum. Mycopathology. 1998;142:3–7. doi: 10.1023/a:1006999727607. [DOI] [PubMed] [Google Scholar]
- 5.Hadju R, Thompson R, Sundelof J G, Pelak B A, Bouffard F A, Dropinski J F, Kropp H. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743,872) Antimicrob Agents Chemother. 1997;41:2339–2344. doi: 10.1128/aac.41.11.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meshulam T, Levitz S M, Christin L, Diamond R D. A simplified new assay for the assessment of fungal cell damage with the tetrazolium dye (2,3)-Bis-(2-methoxy-4-nitro-5-sulphenyl-(2H)-tetrazolium-5-carboxanilide) sodium salt (XTT) J Infect Dis. 1995;172:1153–1156. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 7.Nassar F, Brummer E, Stevens D A. Different components in human serum inhibit multiplication of Cryptococcus neoformans and enhance fluconazole activity. Antimicrob Agents Chemother. 1995;39:2490–2493. doi: 10.1128/aac.39.11.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vora A, Chauhan S, Brummer E, Stevens D A. Activity of voriconazole combined with neutrophils or monocytes against Aspergillus fumigatus: effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Antimicrob Agents Chemother. 1998;42:2299–2303. doi: 10.1128/aac.42.9.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]