Abstract
Duplications of the alimentary tract are a diverse and complex spectrum of congenital malformations and can be found anywhere along the digestive tract. The management depends on multiple factors, such as age, location, size, macroscopic aspect, and the associated anomalies. This study reflects a 15-year single surgical team experience. We reviewed medical records of 35 consecutive patients presenting alimentary tract duplications, evaluated and managed between 2004 and 2019. The anatomical distribution included: oral structures (two cases), esophageal (three cases), gastric (three patients), jejunoileal (seven cases), ileocecal (12 cases), colonic (six cases), anorectal (one case), and one case of complex tubular duplication of the terminal ileum and entire colon with two anal openings at the perineum. Four patients had antenatal diagnosis, initially asymptomatic, were followed, after birth, with repeated ultrasound examinations for a medium period of 3.8 months. All cases were managed with open surgery. Excision of the lesion with preservation of the gut integrity could be performed in 28 of the cases, while in six cases, enterectomy followed by digestive anastomosis was required. In one complex caudal duplication syndrome, the duplicated tubular colon was left in place. The postoperative complications were gastroesophageal reflux disease (GERD) (two cases), Claude Bernard–Horner syndrome (one case), wound infection (one case), and in one case, massive tongue edema. Clinical findings may be misleading, imaging studies may be uncertain, therefore the surgeon remains to complete de picture with intraoperative findings. In complex duplication cases, a multidisciplinary approach is imperative for the best results.
Keywords: alimentary tract duplications, children, congenital anomalies, surgery
⧉ Introduction
Duplications of the alimentary tract are a diverse and complex spectrum of congenital malformations and can be found anywhere along the digestive tract from the oral cavity to the anorectal region. They can be cystic or tubular, single or multiple, have a muscular and mucosal layer with ectopic tissue, are usually benign in children (even though in adults have been reported malignant lesions [1]) and may communicate with the intestinal lumen [2].
Despite the close anatomic relation with the gastrointestinal tract, it is still difficult to summarize them in a comprehensive and clinically relevant classification [3,4,5]. Even more, the management depends on multiple factors, such as age, location, size, macroscopic aspect, and the associated anomalies, which might be the trigger for initiation of a workup and not the least, the degree of physician’s awareness of these types of lesions.
A review of the literature shows that individual centers have limited experience unless spanning over 10 years and from demographic areas with high number of pediatric populations [6,7]. For these reasons, we believe that sharing individual experience is particularly important in these heterogeneous cases.
Aim
The objective of this paper was to present one single surgical team’s experience and to emphasize the correlation between the variety of clinical presentations and the surgical approach, to evaluate the relationship with the importance of presurgical evaluation knowing that the definitive diagnosis can be made only intraoperatively or after the histopathological (HP) exam.
⧉ Patients, Materials and Methods
This study is a retrospective, single surgical team experience during a period of 15 years. We analyzed the medical records of 42 consecutive pediatric patients diagnosed and treated for alimentary tract duplications (ATDs) from 2004 and 2019, which were managed in the Clinic of Pediatric Surgery, Maria Skłodowska Curie Emergency Clinical Hospital for Children, Bucharest, Romania, by one single surgical team. The positive diagnosis was done by HP examination in 35 patients. The rest of the cases had a diagnosis of brachial cyst, mesenteric or omental cysts and were not considered prone to the aim of this research. The study was authorized by the Hospital Ethics Committee and the patients’ data were managed with personal information protection. The selected parameters were gender, age at diagnosis, age at surgery, antenatal diagnosis, duplication sites, associated malformations, clinical presentation, imaging studies, operative complications, surgical technique, HP examination results and follow-up.
⧉ Results
Most patients were diagnosed until the age of one year of life – 25 (71.5%) cases; among them, five were diagnosed at birth (two cases of tongue duplication, one complex caudal duplication syndrome (CDS), one jejuno-ileal duplication associating small bowel atresia and one incidental esophageal duplication cyst finding during surgery for esophageal atresia) and four patients had antenatal diagnosis of an intraabdominal mass. Four (11.5%) patients were diagnosed between one and three years old, and six (17%) cases after three years of life (Table 1).
Table 1.
Age at diagnosis, clinical aspects, associated anomalies, intraoperative findings, and postoperative complications in our series
|
|
Oral structures |
Esophageal |
Gastric |
Jejunoileal |
Ileocecal |
Colonic |
Anorectal |
Complex |
|
Total No. of patients |
2 |
3 |
3 |
7 |
12 |
6 |
1 |
1 |
|
Gender (F/M) |
2 F |
2 F/1 M |
3 F |
3 F/3 M |
5 F/7 M |
2 F/4 M |
1 M |
1 F |
|
Prenatal diagnosis |
– |
– |
1 |
1 |
2 |
– |
– |
– |
|
Age at postnatal diagnosis |
At birth (2) |
At birth (1) |
At birth (–) |
At birth (1) |
At birth (–) |
At birth (–) |
5 years |
At birth |
|
<12 months (1) |
<12 months (1) |
<12 months (4) |
<12 months (7) |
<12 months (3) |
||||
|
12 years (1) |
1–3 years (–) |
1–3 years (1) |
1–3 years (2) |
1–3 years (1) |
||||
|
>3 years (1) |
>3 years (–) |
>3 years (1) |
>3 years (2) |
|||||
|
Symptoms and signs |
Suction difficulties, inefficient feeding (2) |
Pneumonia (1), palpable cervical mass (1), intraoperative incidental finding (1) |
Nausea, vomiting, abdominal pain (2), palpable mass (1) |
Incidental finding (3), abdominal pain (1), mobile palpable mass (3), nausea, vomiting (4), abdominal distension (3) |
Nausea vomiting (7), abdominal pain (3), palpable mass (2), intussusceptions (5) |
Abdominal pain (4), palpable mass (3), hematochezia (1) |
Draining fistula with recurrent infection |
Bowel obstruction, anorectal malformation |
|
Intraoperative findings |
1 cystic, 1 tongue-like structure |
All cystic |
All cystic |
6 cystic, 1 tubular |
All cystic |
3 tubular, 3 cystic |
Tubular |
Tubular |
|
Associated anomalies |
– |
Esophageal atresia (1) |
– |
Omental cyst (1) |
Right hydronephrosis (1), glandular hypospadias (1) |
– |
Bilateral renal cystic dysplasia, chronic renal insufficiency |
– |
|
Postoperative complication |
Tongue edema (1) |
Claude Bernard–Horner syndrome (1) |
GERD (2) |
– |
– |
– |
Wound infection (1) |
– |
F: Female; GERD: Gastroesophageal reflux disease; M: Male.
The clinical picture varied widely from asymptomatic to a large spectrum of non-specific symptoms, depending on the localization of the ATDs: incidental finding (four cases – 11.4%), suction difficulties (tongue duplication), pneumonia (thoracic mass), abdominal pain, palpable mass, nausea and vomiting, hematochezia, draining fistula (rectal duplication), intussusceptions (ileocecal, five cases) and bowel obstruction (four cases).
Anatomical distribution included oral structures – two (5.7%) cases, esophageal – three (8.6%) cases, gastric – three (8.6%) patients, jejunoileal – seven (20%) cases, ileocecal – 12 (34.3%) cases, colonic – six (17.1%) cases (Figure 1), anorectal – one (2.9%) case and one case of complex tubular duplication of the terminal ileum and entire colon and rectum, with two anal openings at the perineum.
Figure 1.

Cystic duplication of ileum (A), colon (B), and ileocecum (C)
Ultrasound (US) examination was used in 94% of the cases as the first imaging study performed after admission. The computed tomography (CT) scan completed US in 50%, magnetic resonance imaging (MRI) in two patients and abdominal X-ray in four cases who presented as intestinal obstruction. In four cases, the duplication cyst was an incidental finding (one case – during esophageal atresia surgery, and three cases – jejunoileal cystic duplication revealed during surgical intervention for bowel obstruction) and no specific workup was done. Concerning the associated anomalies, 86% of the cases did not have any other malformations while the rest presented: one case of esophageal atresia, one case of omental cyst, one case of right hydronephrosis, one case of jejunoileal duplication associating small bowel atresia, one case of glandular hypospadias and one case of bilateral renal cystic dysplasia.
All our antenatally diagnosed patients, being initially asymptomatic, were followed after birth with repeated US examinations for a medium period of 3.8 months. The indication for surgery was gradual increase in size of the lesion or the debut of symptoms.
The rest of the patients were operated at the age of diagnosis. One patient with tubular rectal duplication was under long-term follow-up (five years) and conservative treatment because of the associated pathology – bilateral renal cystic dysplasia, advanced chronic kidney failure, renal transplant status.
Excision of the lesion with preservation of the gut integrity could be performed in 80% (28) of the cases, while in 17% (six) of the cases, enterectomy followed by digestive anastomosis was required (most commonly duplications in the ileocolic region). Enucleation of the duplication cyst was done in one case of esophageal location associated with esophageal atresia, one case of gastric duplication, two jejunoileal, six ileocecal and two colonic cases. Enucleation of the duplication cyst was implemented in the last five years whenever possible and allowed avoiding ileocecal valve sacrifice or other intestinal segment resection as for example the enucleation of the esophageal cyst with preservation of the muscular layer which allowed us to preserve the native esophagus and perform the primary anastomosis.
In the two cases of tongue duplications, both were diagnosed at birth, one was cystic, and one presented as a rudimentary tongue-like structure. The newborns presented difficulties in sucking with inefficient oral feeding which determined the indication for surgical removal. Both cases had an accessory sublingual gland which was removed. One case was associated with incomplete mandible duplication and was referred for further orthodontic treatment (Figure 2) [8].
Figure 2.
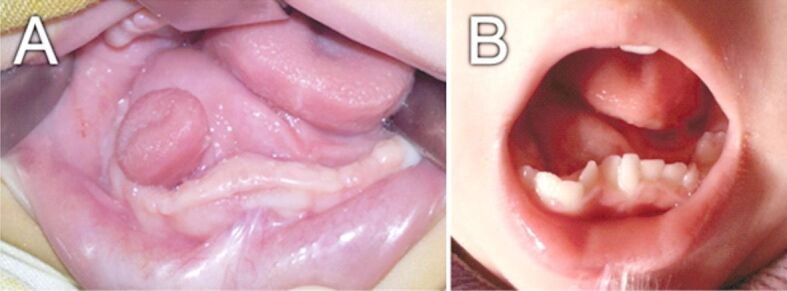
Tongue duplication associated with incomplete duplication of mandible: (A) Preoperative aspect at birth; (B) Postoperative follow-up at 2 years old
The esophageal duplications included one case of intraoperative cystic lesion located at the distal tracheal–esophageal fistula level, encountered during primary repair of an esophageal atresia in a newborn, one case of an infant with a history of pneumopathy and preoperative diagnosis of pulmonary cystic mass and one case of a painful and rapid growing cervical mass in 6-year-old children (Figure 3).
Figure 3.

(A) Thoracic cystic esophageal duplication, MRI aspect; (B) Intraoperative image of a cervical esophageal duplication. MRI: Magnetic resonance imaging
Regarding the esophageal atresia case up mentioned, it was considered as a type C after Gross’ system of classification (distal tracheo–esophageal fistula), which was suggested by gaseous distension of the abdomen. Preoperative barium swallowed defined the proximal esophagus ending position adjacent to the third thoracic vertebra. The distal esophagus caliber showed an illusory greater caliber than the proximal esophageal pouch. The positioning of the fistula disproportionate. The distance between the two esophageal ends was of 2 cm. Thorough dissection showed the muscular layer of the esophagus dissociated by a well-defined 2 cm clear fluid cystic mass, close to the tracheo–esophageal fistula. The submucosal and mucosal layer of the esophagus were integer. The well-defined cystic mass showing no communication with the esophageal lumen was excised. By this way, the muscular and mucosal layers of the surrounding esophagus could be secured, therefore the esophageal anastomosis between the two ends was done in safer circumstances.
From the three cases of gastric duplication, one had a thoracic cystic extension with no communication with the esophageal or gastric lumen causing compression on the distal esophagus, lesser curvature, and fornix (Figure 4). The cystic mass was removed through an abdominal approach with preservation of the native mucosal integrity of the stomach and reconstruction of the sero-muscular layer.
Figure 4.

MRI aspect of a gastric duplication extended into lower thorax. MRI: Magnetic resonance imaging
We encountered seven (20%) cases with jejunoileal duplication, 12 (34.3%) children with ileocecal duplication, and in six (17.1%) cases the duplicated segment was colonic (Figure 5). All cases were located on the mesenteric side on the bowel. The majority (21 cases) were cystic, with gastric, pancreatic, or respiratory ectopic tissue. Tubular duplications with luminal communication were between 4 cm and 15 cm long and occurred in jejunoileal region (one case) and colon region (three cases). In three cases was possible to remove the duplicated segment, while in one case (jejunoileal duplication) having a common wall resection included both duplicated and native segment.
Figure 5.

Duplication of the terminal ileum and appendix (A), and complete colon duplication (B)
In the case of the CDS, the last 10 cm of the ileum and the appendix, entire colon, rectum, and anus were duplicated completely (Figure 5). The baby also associated malrotation and duplication of the urinary bladder, urethra, vagina, and vulva.
The distal posterior wall of the left vagina was presenting the opening of the left rectum, which was hypoplastic. At six months of age, once diversification was started, voluntary stooling became inefficient with episodes of abdominal distention which determined the need for colorectal surgery at seven months. The right anal opening was stimulated, and its location was verified to be in the sphincter muscle. Consequently, we decided to resect the left anovestibular fistula and distal hypoplastic rectum, with a large latero-oblique colo–colic anastomosis. Because the duplicated hindgut included all the colon length and terminal ileum, we decided to preserve it to be able to produce formed stools.
In the case with anorectal duplication and chronic renal failure, the patient had recurrent infection episodes (with two cutaneous fistulae) and was managed conservatively. Because of the need for a second renal transplant, the patient was operated with complete removal of the tubular pararectal segment which did not communicate with the rectal lumen. Recovery was uneventful.
The immediate postoperative complications were gastro-esophageal reflux disease (two cases), Claude Bernard–Horner syndrome (one case), wound infection (one case), and in one case, massive tongue edema. The rest (85.7%) of the patients who underwent surgery did not present any complications.
⧉ Discussions
Our study shows that regardless of the anatomical location, all cases can be successfully and completely removed by an open approach with minimal complications and with the desired functional and cosmetic results. Given the low incidence of these types of lesions, lesser preoperative awareness from the physicians and the non-specific imaging characteristics, the preoperative diagnosis of ATDs is uncommon. The most common radiological modalities used to evaluate duplications are US (with evidence of inner hyperechoic and outer hypoechoic layers – double-wall sign) and contrast imaging. However, CT and MRI are more used in preoperative surgical approach evaluation than in preoperative diagnosis [9].
Duplications of the tongue are one of the rarest types of ATDs; in most of the cases, the lesion is evident at birth by clinical exam as a rudimentary tongue-like structure, a cystic mass [10] or part of a complex craniofacial malformation [11]. Patients can be asymptomatic or may present difficulty sucking and inefficient feeding [12]. This prompts early surgical treatment, but the diagnosis is missed preoperatively even in cases of MRI or CT scan evaluation due to the low awareness and non-specific aspect of the lesion.
In all our cases with esophageal location, the lesion had a cystic structure, symptoms being determined by volume and compression on the adjacent structures. Cervical lesions require drainage through endoscopic or US guidance in case of acute respiratory distress and posterior location followed by subsequent complete removal through a cervical incision approach. Intrathoracic and subdiaphragmatic cysts can be addressed by open or minimally invasive techniques being aware of the rare but possibly severe postoperative complications, such as chylothorax, esophageal injury, phrenic nerve injury, and tracheal injury [13] or Claude Bernard–Horner syndrome (in our case).
In the case of foregut duplications associated with esophageal atresia, the atypical presentation, the reduced size of the lesion or its location outside of the surgical dissection site is circumstances for a delayed diagnosis. Ideally, the treatment consists in total excision because of the compression risk. If the cyst content is only evacuated, the left mucosa may lead to hemorrhage and anemia, recurrent pancreatitis, rupture, or malignant degeneration. Enucleation of the duplication of the cyst allows preservation of the esophageal wall length, which is essential in primary esophageal anastomosis [14]. Gastric duplications are misdiagnosed as cystic tumors of the pancreas [15] or adrenal gland but with normal tumor markers or in rare cases the ectopic tissue can be a cause of gastrointestinal bleeding. Although most cases are discovered in the pediatric population, some remain undetected until adult age when can be a cause of gastric malignancy [16] supporting the recommendation for complete surgical excision.
Midgut duplications are the most commonly encountered. Collection of secretions under high pressure inside the cyst’s cavity most often leads to recurrent abdominal pain, but ischemia and bowel necrosis have also been reported. Bowel intussusception (whereas the duplication cyst behaves as a head point) or bowel obstruction (intrinsic, or extrinsic to an adjacent bowel loop) are other ways of clinical presentation. The most threatening complications occur when gastric mucosa resemble de duplication cyst’s histological structure causing inflammation, hemorrhage, ulceration, and bowel perforation. Midgut duplications usually present as developing cysts on the mesenteric side infiltrating the muscular layer of the bowel. Very rarely they are identified on the opposite side or wholly separated from the intestinal wall [17].
Minimally invasive surgery is a safe and feasible approach for these cases of ATDs [18]. In the multicenter study of Guérin et al. [19] mentions about one out of three cases were converted to open surgery. The main causes of conversion were impossibility to dissect the cystic mass from the digestive tract most commonly secondary to the inability to separate the duplication cyst from the digestive tract, lack of visibility or indication for bowel resection. Conversion rate was greater in small children, weighing not more than 10 kg. Moreover, a 7% rate of complications in their cohort is reported.
Hindgut duplications can vary from cystic lesions in the colon wall to extensive duplications running along the entire colon up to the perineum, such as our case of CDS. Dominguez et al. (1993) [20] described CDS for the first time as an association of duplications of the hindgut, the lower urogenital tract, malformations of the spine (hemivertebrae, diplomyelia, myelomeningocele, sacral duplication) and anomalies of the abdominal wall (ventral herniation) at different grades of extent as an anomaly in different milestones of embryogenesis. Management of CDS is not standardized. Surgical treatment should target obtaining a normal function depending on the broad spectrum of associated anomalies, every patient needing a shaped approach for best results [21]. An essential checkbox in CDS assessment is evaluation of the spinal bone, particularly when spina bifida or anorectal malformations are present.
Crohn’s disease is not so common in children and its presentation is multifaceted, sometimes requiring experience and caution for an early diagnosis [22]. A noteworthy presentation of colonic duplications may be outlined when duplication cysts are identified in conjunction with a patient with known Crohn’s disease mimicking potential complications of the autoimmune disorder like colitis with bowel fistula [23].
Depending on the extent of colon duplication and its particularities in matter of sharing a common blood supply, different techniques may be considered. If there is no fusion between the two colons and each of them has its own blood source, removal of the duplicated segment is a good choice. Otherwise, mucosa stripping of the non-dominant duplication or assuring a functional emptying by stapling the two segments are procedures of choice. In this way, chronic constipation and its long-term complications may be avoided. Other reported complications of colon duplications are volvulus or neoplasms [24]. Latero-terminal anastomosis of the two lumens should be avoided because of the risk of stenosis at the anastomotic site in the long-term with fecal impaction and proximal colitis.
Ma et al. [1] did a review of 64 case reports with 67 patients with malignancies arising from ATDs. The most common location was the colorectum in patients aged between 40 and 60 years, the presenting symptoms were nonspecific for ATDs. Four patients (out of the 57 cases who underwent surgery) received biopsies or subtotal excisions were done for locally advanced extension of the malformation. There were 62 cases that described the duplication type and communication, including 50 cases of cystic duplication and 12 cases of tubular duplication. Adenocarcinoma, followed by squamous cell carcinoma and neuroendocrine tumors are the most common types of malignancies identified this series. Non-epithelial malignancies were noted in two cases.
⧉ Conclusions
ATDs are not only rare malformations but also have a broad anatomical spectrum and clinical particularities. The clinical presentation is usually imprecise, radiological evaluation has variable degrees of sensitivity and specificity in preoperative diagnosis, therefore the surgeon is left to complete the puzzle. Whenever possible, enucleation of the intramural cystic duplication is a good option, thus preserving the native digestive structures (in most cases – ileocecal valve or other digestive structures, such as the esophagus and gastric wall). In complex duplication, best results are obtained in interdisciplinary teams. ATDs management has a very good prognosis if a good management plan is done.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- 1.Ma H, Xiao W, Li J, Li Y. Clinical and pathological analysis of malignancies arising from alimentary tract duplications. Surg Oncol. 2012;21(4):324–330. doi: 10.1016/j.suronc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Holcomb GW, Gheissari A, O’Neill JA, Shorter NA, Bishop HC. Surgical management of alimentary tract duplications. Ann Surg. 1989;209(2):167–174. doi: 10.1097/00000658-198902000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladd WE, Gross RE. Duplications of the alimentary tract. South Med J. 1937;30(5):363–371. http://dx.org/10.1097/00007611-193704000-00002 https://sma.org/southern-medical-journal/article/duplications-of-the-alimentary-tract/ [Google Scholar]
- 4.Li L, Zhang JZ, Wang YX. Vascular classification for small intestinal duplications: experience with 80 cases. J Pediatr Surg. 1998;33(8):1243–1245. doi: 10.1016/s0022-3468(98)90159-2. [DOI] [PubMed] [Google Scholar]
- 5.Juillerat A, Rougemont AL, Wildhaber BE. Duplication de la vésicule biliaire avec hétérotopie : cas clinique et proposition de classification des duplications gastro-intestinales [Duplication of the gallbladder with heterotopic mucosa: a case report and proposal for a classification for gastrointestinal duplications] Arch Pediatr. 2016;23(6):607–611. doi: 10.1016/j.arcped.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Karnak I, Ocal T, Senocak ME, Tanyel FC, Büyükpamukçu N. Alimentary tract duplications in children: report of 26 years’ experience. Turk J Pediatr. 2000;42(2):118–125. [PubMed] [Google Scholar]
- 7.Xiang L, Lan J, Chen B, Li P, Guo C. Clinical characteristics of gastrointestinal tract duplications in children: a single-institution series review. Medicine (Baltimore) 2019;98(44):e17682–e17682. doi: 10.1097/MD.0000000000017682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spătaru RI, Nisipaşu C, Spătaru MD, Nisipaşu CI. Duplicaţia de limbă, entitate malformativă extrem de rară, prezentare de caz [Accessory tongue, a very rare anomaly - case presentation] Rom J Stomatol (Rev Rom Stomatol) 2013;59(4):282–283. https://rjs.com.ro/rjs-vol-lix-nr-4-an-2013/ [Google Scholar]
- 9.Hur J, Yoon CS, Kim MJ, Kim OH. Imaging features of gastrointestinal tract duplications in infants and children: from oesophagus to rectum. Pediatr Radiol. 2007;37(7):691–699. doi: 10.1007/s00247-007-0476-3. [DOI] [PubMed] [Google Scholar]
- 10.Binet A, El Ezzi O, Meagher-Villemure K, de Buys Roessingh AS. Intestinal duplication in the tongue: embryological and radiological point of view. Surg Radiol Anat. 2020;42(1):9–13. doi: 10.1007/s00276-019-02332-6. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi T, Sugiyama T, Sasaguri KI, Ono S, Maeda K, Nishino H, Jinbu Y, Mori Y. Surgical management of duplication of the pituitary gland-plus syndrome with epignathus, cleft palate, duplication of mandible, and lobulated tongue. J Craniofac Surg. 2017;28(2):e141–e144. doi: 10.1097/SCS.0000000000003324. [DOI] [PubMed] [Google Scholar]
- 12.Eaton D, Billings K, Timmons C, Booth T, Biavati JM. Congenital foregut duplication cysts of the anterior tongue. Arch Otolaryngol Head Neck Surg. 2001;127(12):1484–1487. doi: 10.1001/archotol.127.12.1484. [DOI] [PubMed] [Google Scholar]
- 13.Scarpa AA, Ram AD, Soccorso G, Singh M, Parikh D. Surgical experience and learning points in the management of foregut duplication cysts. Eur J Pediatr Surg. 2018;28(6):515–521. doi: 10.1055/s-0037-1607293. [DOI] [PubMed] [Google Scholar]
- 14.Spataru RI, Popoiu MC, Ivanov M. Foregut duplication cyst associated with esophageal atresia – one-stage neonatal surgical repair. Indian J Surg. 2015;77(Suppl 1):52–55. doi: 10.1007/s12262-014-1122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spătaru RI, Enculescu A, Popoiu MC. Gruber–Frantz tumor: a very rare pathological condition in children. Rom J Morphol Embryol. 2014;55(4):1497–1501. [PubMed] [Google Scholar]
- 16.Zhu Y, Lihong LV, Pan W, Ren P, Han T, Xu X. Gastric duplication complicated by malignant transformation in adults: report of three cases. J Gastrointest Dig Syst. 2015;5(6):374–374. https://www.omicsonline.org/open-access/gastric-duplication-complicated-by-malignant-transformation-in-adultsreport-of-three-cases-2161-069X-1000374.php?aid=65962 [Google Scholar]
- 17.Chiar CI, Elango T, Sivaneswaran L, Umasangar R, Mohan N. An unexpected gangrenous duplication of ileum. Med J Malaysia. 2017;72(1):83–84. [PubMed] [Google Scholar]
- 18.Sfoungaris D, Magdalini M, Patoulias I, Panteli C, Valioulis I. Antimesenteric gastrointestinal tract duplication undergoing non-ulcerative perforation. Rom J Morphol Embryol. 2018;59(4):1275–1278. [PubMed] [Google Scholar]
- 19.Guérin F, Podevin G, Petit T, Lopez M, de Lagausie P, Lardy H, Bonnard A, Becmeur F, Philippe P, Larroquet M, Sapin E, Kurzenne JY, le Mandat A, François-Fiquet C, Gaudin J, Valioulis I, Morisson-Lacombe G, Montupet P, Demarche M. Outcome of alimentary tract duplications operated on by minimally invasive surgery: a retrospective multicenter study by the GECI (Groupe d’Etude en Coeliochirurgie Infantile) Surg Endosc. 2012;26(10):2848–2855. doi: 10.1007/s00464-012-2259-7. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez R, Rott J, Castillo M, Pittaluga RR, Corriere JN. Caudal duplication syndrome. Am J Dis Child. 1993;147(10):1048–1052. doi: 10.1001/archpedi.1993.02160340034009. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Che X, Wang S, Chen G. Multiple-stage correction of caudal duplication syndrome: a case report. J Pediatr Surg. 2009;44(12):2410–2413. doi: 10.1016/j.jpedsurg.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Enculescu A, Lupusoru MD, Cirstoveanu C, Suceveanu AI, Andronache LF, Suceveanu AP, Voinea F, Puscasu M. Postoperative complicated appendectomy revealing Crohn’s disease in a pediatric patient. J Mind Med Sci. 2021;8(1):154–160. https://scholar.valpo.edu/jmms/vol8/iss1/21/ [Google Scholar]
- 23.Martin ST, Ko JS, Plesec TP, Ananthakrishnan L, Remzi FH. Colonic duplication mimicking fistulizing Crohn’s colitis. Inflamm Bowel Dis. 2011;17(8):E103–E105. doi: 10.1002/ibd.21768. [DOI] [PubMed] [Google Scholar]
- 24.Spătaru RI. The use of mechanical suture in the treatment of Hirschsprung’s disease: experience of 17 cases. Chirurgia (Bucharest) 2014;109(2):208–212. [PubMed] [Google Scholar]


