Abstract
This study aimed to assess the in vitro biocompatibility of titanium (Ti) alloy orthodontic mini-implants by correlating human osteoblasts (HOb) response with chemical composition and surface morphology of mini-implants. HOb were cultivated with or without custom-made and commercial mini-implants, discs and filings. The surface morphology and chemical composition of the implants were assessed under the scanning electron microscopy (SEM) with energy-dispersive X-ray (EDX) microanalysis system. Cell viability, adhesion and proliferation were analyzed by optical microscopy and flow cytometry. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reduction and lactate dehydrogenase (LDH) release tests were used to assess the cytotoxicity of discs and filings-treated culture medium. Shape, adhesion, and multiplication of HOb were not significantly altered by the presence of mini-implants, discs or filings in culture, even though Ti alloy may exert in vitro a low cytotoxic effect on HOb adhered to discs. Morphology analysis by SEM demonstrated that custom-made mini-implants’ surface differs from that of commercial mini-screws in terms of surface finish and roughness, whilst EDX analysis showed largely similar percentages of Ti, aluminum and vanadium for the two types of implants. No major differences were noticed regarding the effect exerted in vitro on HOb by the investigated implants. The new mini-implants have a convenient in vitro cytotoxicity profile on HOb.
Keywords: orthodontic mini-implant, titanium alloy, biocompatibility, human osteoblast
⧉ Introduction
Orthodontic mini-implants (OMI) are skeletal anchorage devices temporarily inserted into oral bone structures either to supplement the orthodontic anchorage the teeth offer or to avoid using the latter as anchor units [1,2]. They are used for a limited period of time during the orthodontic treatment and then removed using a minimally invasive technique [3].
Many of the available mini-screws are manufactured from a grade 5 titanium alloy, also known as titanium 6-aluminum 4-vanadium (Ti-6Al-4V; Ti alloy). This material has replaced the commercially pure titanium (cp Ti) which, despite its higher degree of biocompatibility, is more prone to fracture [4,5]. Ti alloy benefits of higher tensile and yield strength than cp Ti [4]. Furthermore, its elastic modulus is superior compared to other non-Ti biomaterials, being closer to the bone’s, thus facilitating a more uniform force distribution along the bone–implant interface [6]. However, some concerns about Ti alloy releasing Al and V exist [4], which requires a thoroughly investigation of the potential cytotoxicity of the OMI to the host bone tissue.
Since ideal characteristics of orthodontic mini-screws are still open to debate [7], a custom-made design seems an interesting option for the variety of clinical needs. The main advantage is the possibility of customization in terms of material, length, diameter, thread features, head, body, and tip configuration. Primary stability is of major importance for the immediate loading of the orthodontic mini-screws and is a function of several characteristics: shape of the screw, length, diameter, and thread geometry (shape, pitch, depth, and width) [8,9,10,11,12,13]. Of the available shape forms, the square thread profile may provide the best primary stability for immediate loading [13], while reverse buttress thread makes the penetration easy without predrilling [14] and provides the greatest pull-out strength [15]. Thread pitch can be customized in case of experimental mini-implants, depending on the density of compact bone: low bone density requires a longer thread pitch distance and high bone density – a shorter one [13]. Moreover, a decrease screw pitch can be selected for increasing primary stability in cases where weaken conditions are present, such as poor bone quality or short implants [16]. This is because a shorter pitch distance makes surface area increase leading to a more favorable stress distribution [16]. Also, it is agreed that the greater the thread depth, the wider the surface area of the implant [16]. Depending on the clinical situation, deeper threads are advantageous in areas of softer bone, although shallow thread depths permit easier insertion into denser bone with no need for tapping [16]. Lastly, the microthread configuration, which includes implant material and surface treatment, and morphology, may improve bone formation and stress distribution for the implants inserted in the cancellous bone under immediate loading [13]. All these features could be selected by the orthodontist according to the specific clinical case. This kind of personalized approach would favor success of the orthodontic treatment with skeletal anchorage and would decrease the risk of potential complications.
Aim
The aim of this study was to evaluate in vitro the behavior of primary human osteoblasts (HOb) in contact with custom-made Ti alloy mini-implants, using regular commercial mini-screws as reference. Additionally, the mini-implants’ chemical structure and surface morphology were analyzed to identify whether these characteristics have an influence on HOb proliferation and adhesion.
⧉ Materials and Methods
Custom-made Ti alloy mini-implants and other samples for investigation
For the present study, custom-made orthodontic mini-implants (cmOMI) were manufactured using a particular experimental design, but the configuration can be modified according to particular clinical needs. Experimental cmOMI have a tapered core, reverse buttress thread and are single threaded. The latter characteristic seems to be the most favorable thread helix configuration in terms of implant stability [16]. The neck (the area that meets the soft tissue) and the crestal portion of the body are smooth, with no microthreads. The cmOMI are self-drilling due to its corkscrew tip and sharp threads. CmOMI were manufactured using Ti alloy (Ti-6Al-4V) and no special treatment was applied to their surface. CmOMI (which were labelled as Group A) were compared with samples belonging to the following groups:
(i) Group B: commercial mini-screws (Orlus, Ortholution, Korea), presenting a sandblasted acid-etched surface, previously reported to maximize the potential for osseointegration [17] and optimize biocompatibility [14].
(ii) Group C: alloy discs obtained from the same alloy used for cmOMI. They have a circular shape and a smooth surface.
(iii) Group D: alloy filings, composed from particles under 100 μm from the same alloy.
Samples from Groups A, C and D were provided by a manufacturing company (Tehnomed, Romania). Morphology of the four items investigated are presented in Figure 1A,1B,1C,1D and technical details for the samples are given in Table 1.
Figure 1.
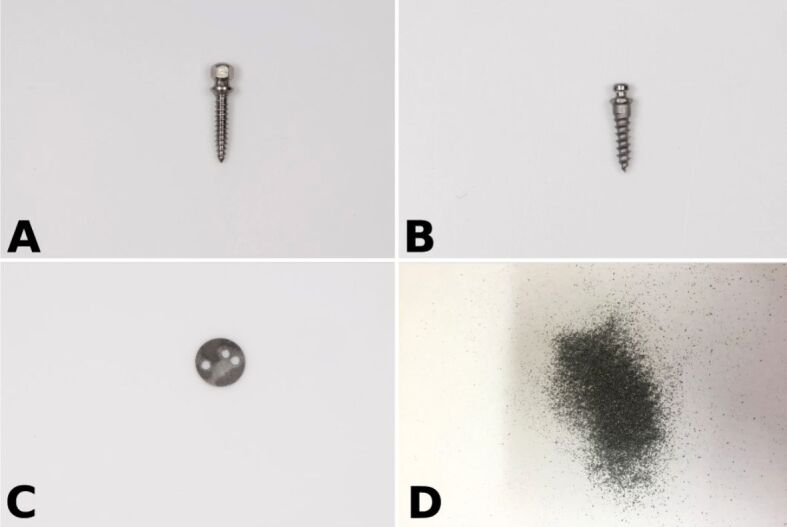
The analyzed Ti alloy sample groups: custom-made orthodontic mini-implant (A), Orlus reference mini-implant (B), alloy disc (C) and filings (D).
Table 1.
Description of the investigated Ti alloy mini-implants and discs
|
Device |
Size |
Material, surface characteristics |
|
Custom-made mini-implant |
1.8 × 1.1 × 10 mm (diameter × collar width × length) |
Ti-4Al-6V, smooth surface (untreated) |
|
Orlus mini-implant |
1.6 × 1 × 7 mm (diameter × collar width × length) |
Ti-4Al-6V sandblasted acid-etched surface |
|
Disc |
5 × 0.1 mm (diameter × thickness) |
Ti-4Al-6V |
|
Filings |
Particles <100 μm |
Ti-4Al-6V |
Al: Aluminum; Ti: Titanium; V: Vanadium
Study design
The study was approved by the Research Ethics Committee of Carol Davila University of Medicine and Pharmacy, Bucharest, Romania (Approval No. 102/2.12.2016). It was divided into two parts: (1) Surface characterization and chemical composition analysis of mini-implants; (2) In vitro investigations on HOb.
We used a total number of n=24 mini-implants (12 samples each, from Groups A and B), as well as n=6 discs (from Group C) and filings. All samples were sterilized by ultraviolet light for 24 hours.
Surface characterization and chemical composition analysis of mini-implants
We analyzed n=2 mini-implants from each of the Groups A and B, described above in Figure 1.
The surface morphology of mini-implants was analyzed under the environmental scanning electron microscope (SEM, Philips XL 30 ESEM TMP, The Netherlands). The operating conditions for SEM analysis were: 25 kV beam accelerating voltage, tilt angle of 0°, take-off angle of 35° and working distance between 10 μm and 1 mm. The mini-implants were evaluated at different levels (head, body, top) and power magnifications (25×, 100×, 200×, 500× and 2000×).
Quantitative evaluation of the concentration of each chemical element present in the alloy used for manufacturing the two analyzed groups of mini-implants (A/B) was carried out using energy-dispersive X-ray spectroscopy (EDX Sapphire UTW, 128 eV resolution). EDX uses the X-rays emitted by a sample to determine its elemental composition. For this analysis, mini-implants were fixed on the sample holder in order to facilitate the scan in a viewing angle of 120°.
In vitro investigations on human osteoblasts
Cell culture
Since bone cells are directly exposed to the cytotoxic effect of metallic ions released from the OMI, standardized human primary osteoblasts were considered. The biological impact of custom-made and commercial Ti alloy mini-implants on HOb was investigated relative to cell viability, morphology, adhesion and proliferation.
HOb (PromoCell, C-12720) were purchased from Biomedica Medizinprodukte, Bucharest, Romania. For cells propagation, HOb were plated in 25 cm2 flask at a density of 10 000–20 000 cells/cm2, and were cultivated in osteoblast growth medium (PromoCell, C-27001) at 37°C in 5% carbon dioxide (CO2) atmosphere. Culture medium was changed at 24–48 hours depending on cell morphology and confluence, the threshold being estimated at 70–90% confluence. HOb passage was performed by cell detachment with Trypsin/Ethylenediaminetetraacetic acid (EDTA) (0.25%/0.02%, w/v) in phosphate-buffered saline (PBS) w/o Ca2+ (Biochrom AG, Germany). The density of detached cells in suspension was measured by optical microscopy using a Bürker–Türk counting chamber. Cellular viability was evaluated by the Trypan Blue exclusion test (exclusion of Trypan Blue by living cells). For experiments, HOb from passages 3–14 were used. Cells were cultivated in osteoblast growth medium, in 24- or 96-well plates, in absence and presence of Ti alloy devices. Cells were analyzed at various time points after putting in contact HOb and Ti alloy devices (24, 48 and 72 hours) in terms of cell viability, adhesion, and proliferation, as described below. The shape of HOb and also cell adhesion to solid substrates (plastic or Ti alloy devices) were first investigated by optical microscopy (EVOS XL microscope, ThermoFisher Scientific). Cell proliferation was evaluated by flow cytometry using Carboxyfluorescein diacetate succinimidyl ester (CFDA–SE) as fluorescent dye. CFDA–SE is a non-toxic and long-term cell tracer that allows evaluation of the number of proliferating cells in successive daughter generations based on 1/2 dye dilution at each cell division. HOb were labeled with 10 μM CFDA–SE (Vybrant® CFDA–SE Cell Tracer Kit, ThermoFisher Scientific), according to the technical instructions provided by the kit manufacturer, and were then plated for experiments in 24-well plates, with or without Ti alloy samples. At the end of HOb cultivation, supernatants were discarded, cells were washed twice with PBS w/o Ca2+ and Mg2+ and were then detached with Trypsin/EDTA, as described above. Data on intracellular fluorescence of CFDA–SE-labeled HOb were acquired and processed using a FACSCalibur flow cytometer and the CellQuest software, respectively. The distribution of cells in daughter generations was obtained by processing flow cytometry data with the ModFit software. Specific tests were used for assessing the potential cytotoxic action of Ti alloy discs and of filings-treated culture medium (prepared as described below). Cell viability was evaluated by the 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reduction test that measures the number of metabolically active cells in culture. In parallel, membrane integrity was evaluated by the lactate dehydrogenase (LDH) release test that is a measure of cell death by necrosis. Briefly, HOb were cultivated in triplicates in 96-well plates (100 μL total culture volume), either on Ti alloy discs placed on the bottom of culture wells, or in regular culture plates where cells were cultivated in filings-treated culture medium. Control samples for assessing background contained only cell culture medium and no cells. At the end of cultivation, culture plates were centrifuged at 200 g at room temperature. 50 μL of cell-free culture supernatant were harvested from each well for the LDH release test. 50 μL of fresh culture medium were added instead in each well for restoring the 100 μL total volume of the cell culture.
LDH release was measured by the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, USA). Briefly, 50 μL of detection reagent were added on 50 μL culture supernatant. Reaction was allowed to develop for 30 minutes at room temperature, in the dark, and was finally stopped by adding 50 μL of the kit’s stop solution. Reaction intensity was measured as optical density (OD) at 470 nm using an enzyme-linked immunosorbent assay (ELISA) reader (Tecan Sunrise, Germany). From the OD of cell-containing wells, the background OD was subtracted.
MTS reduction was measured by the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, USA). We added 20 μL of the detection reagent in each cell culture well (up to 100 μL total volume) and samples were further incubated at 37°C in the dark for two hours 100 μL of culture supernatant were harvested from each well and were transferred in another 96-well plate. OD was read at 470 nm against the 620 nm reference wavelength, using an ELISA reader (Tecan Sunrise, Germany). From the OD of cell-containing wells, the background OD was subtracted.
Filings-treated medium
Ti alloy filings were incubated in cell culture medium for seven hours in a tube rotator, at a continuous rotational speed of 12 rpm (MACSmix Tube Rotator, Miltenyi Biotec GmbH, Germany). Filings-free culture medium was obtained by centrifugation at 4000 rpm for 20 minutes.
⧉ Results
Mini-implant morphology and chemical composition
Morphology analysis by SEM demonstrates that the surface of cmOMI mini-implants differs from that of commercial mini-screws. The cmOMI have a rough surface exhibiting a pattern of concentric groove morphology as result of surface mechanical machining. However, the reference group presents a smoother surface finish, especially in the area of head and neck of the implants (Figure 2A,2B,2C,2D,2E,2F). It also exhibited an irregular geometry with hairline cracks and narrow microspaces (Figure 3A,3B,3C,3D,3E,3F,3G,3H).
Figure 2.
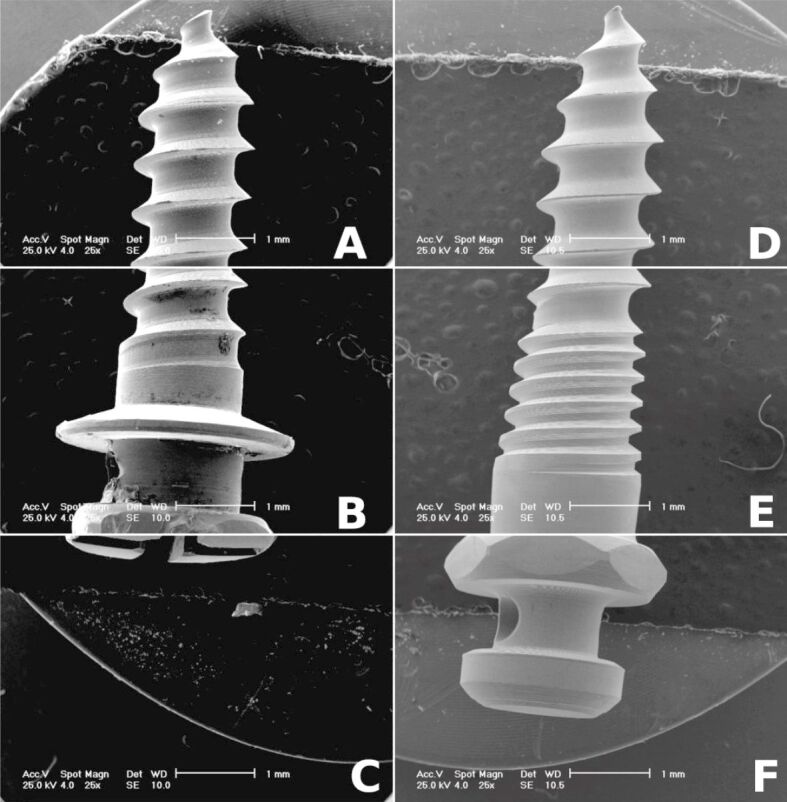
Scanning electron micrographs (25×) presenting active tip, body and head of custom-made mini-implant (A–C) and commercial mini-screw (D–F)
Figure 3.
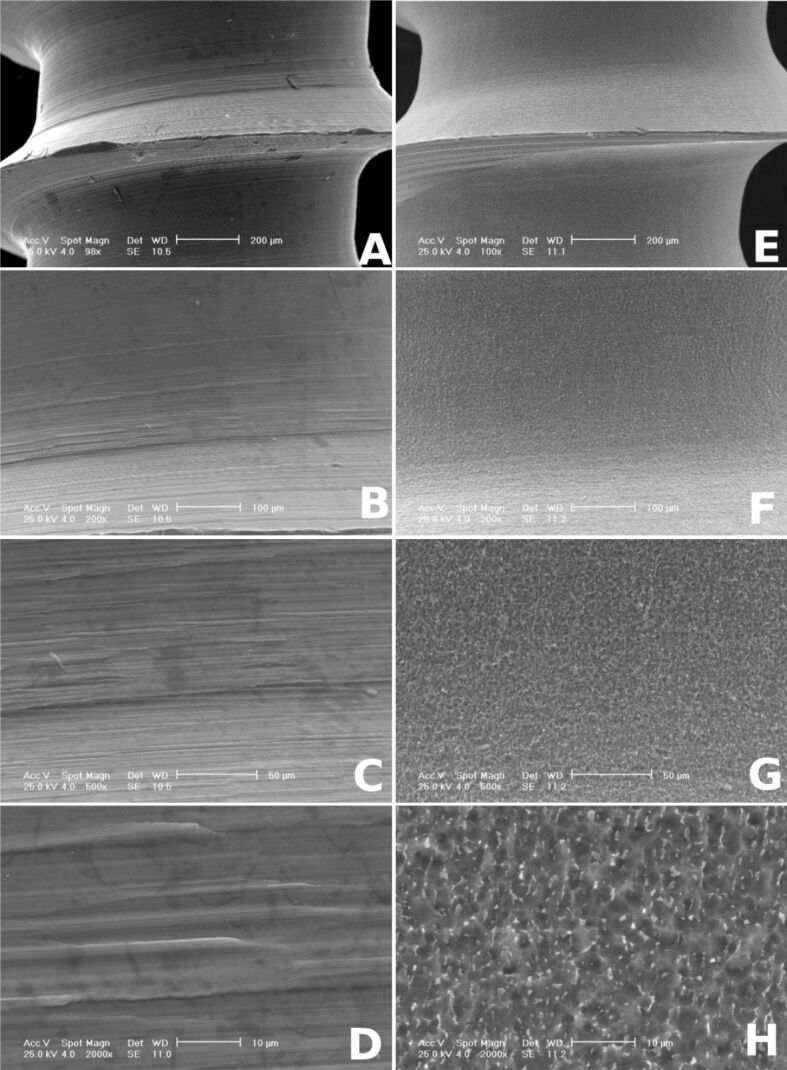
Scanning electron micrographs (100×, 200×, 500×, 2000×) showing the surface morphology of custom-made mini-implant (A–D) and commercial mini-screw (E–H)
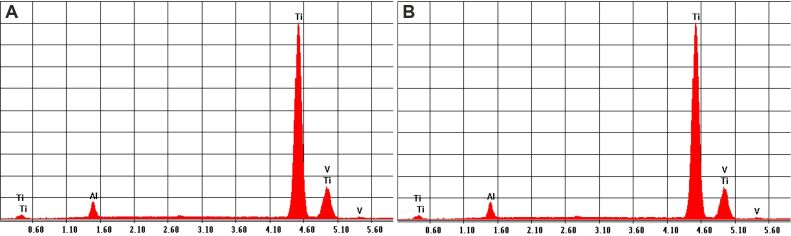
As featured from the EDX analysis charts, the alloys of the two types of investigated mini-implants showed largely similar percentages of Ti, Al and V (Figure 4, A and B; Table 2).
Figure 4.
EDX analyses for the chemical composition: (A) Graph for custom-made mini-implant; (B) Graph for commercial mini-implant. EDX: Energy-dispersive X-ray
Table 2.
Data for custom-made mini-implant and for commercial mini-implant
|
Custom-made mini-implant | ||||||
|
Element |
Wt % |
At % |
K-ratio |
Z |
A |
F |
|
Ti K |
89.69 |
84.85 |
0.8828 |
0.9935 |
0.9908 |
1 |
|
Al K |
7.57 |
12.71 |
0.031 |
1.0763 |
0.3794 |
1.0037 |
|
V K |
2.74 |
2.44 |
0.0268 |
0.9738 |
1.0021 |
1 |
|
Total |
100 |
100 |
|
|
|
|
|
Commercial mini-implant | ||||||
|
Element |
Wt % |
At % |
K-ratio |
Z |
A |
F |
|
Ti K |
90.39 |
86 |
0.8911 |
0.9942 |
0.9916 |
1 |
|
Al K |
6.8 |
11.49 |
0.0277 |
1.077 |
0.3771 |
1.0038 |
|
V K |
2.81 |
2.52 |
0.0275 |
0.9745 |
1.0028 |
1 |
|
Total |
100 |
100 |
|
|
|
|
Al: Aluminum; At: Atomic; Ti: Titanium; V: Vanadium; Wt: Weight
In vitro study
We investigated the putative cytotoxic effect that Ti alloy might exert in vitro on HOb. Ti alloy discs were used instead of mini-implants for enhancing the contact surface between HOb and material. Cytotoxicity was evaluated as number of metabolically active cells (MTS reduction test) and as membrane integrity (LDH release test). Experimental data showed that Ti alloy discs had decreased by 55% MTS reduction whilst increasing only by 12% LDH release by HOb, as shown in Table 3. These results indicate that HOb might have a lower adhesion on Ti alloy discs than on the usual surface of culture plates, either due to material or surface characteristics.
Table 3.
Cytotoxicity of Ti alloy discs evaluated as MTS reduction and LDH release by HOb in presence and absence of Ti alloy discs
|
LDH release [%] |
MTS reduction [%] |
|
|
Cell control |
0.556±0.014 |
0.289±0.033 |
|
Alloy discs |
0.623±0.017 |
0.128±0.017 |
HOb: Human osteoblasts; LDH: Lactate dehydrogenase; MTS: 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; Ti: Titanium. Data are presented as mean ± standard error of the mean for triplicate samples
We hypothesized that deterioration of mini-implants and release of free molecules may influence the behavior of HOb in culture. This process was mimicked in vitro by cultivation of HOb in culture medium that was pretreated for seven hours with Ti alloy filings. As demonstrated by the MTS reduction test (Figure 5), the filings-treated culture medium had no statistically significant effect on the number of metabolically active cells. Corroborated with the data presented in Table 3, results indicated that the investigated Ti alloy may reduce the number of metabolically active HOb adhered to discs, that might derive from surface effects and not from the release of free molecules.
Figure 5.
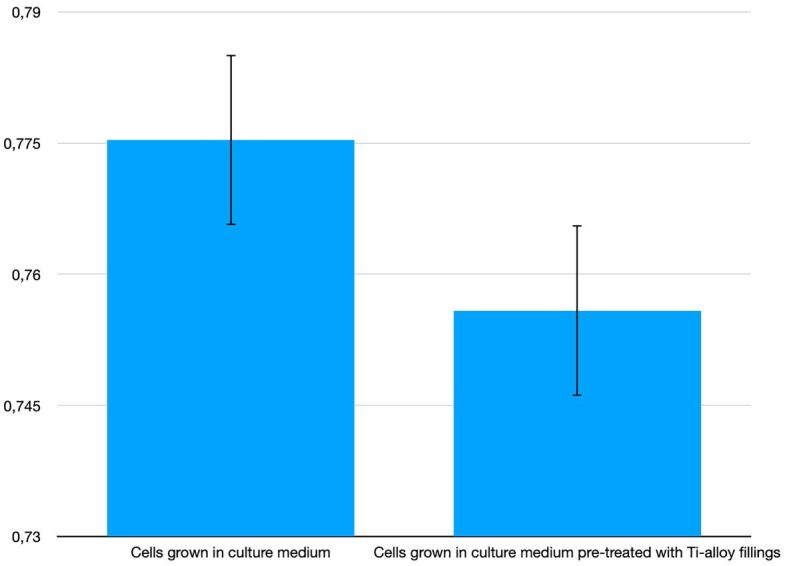
MTS reduction by HOb cultivated for 24 hours in culture medium pretreated with Ti alloy filings (in controlled rotation at 12 rpm). HOb: Human osteoblasts; MTS: 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium; Ti: Titanium
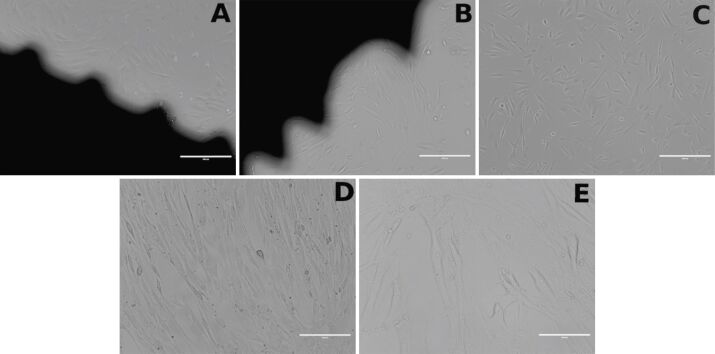
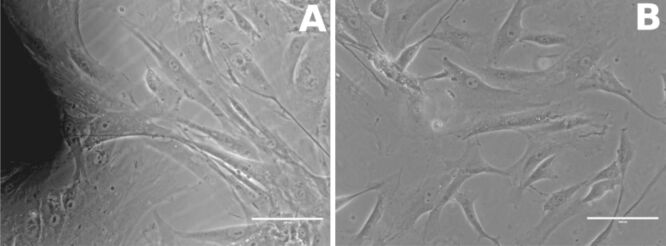
Regarding the interaction of HOb with mini-implants, the shape of HOb and their adhesion to the culture well bottom were not significantly influenced by the contact of cells with mini-implants (both custom-made and commercial), as shown by light microscopy images (Figure 6A,6B,6C,6D,6E; Figure 7, A and B). Moreover, HOb adhered well to the implant’s surface, as shown in Figure 7 (A and B).
Figure 6.
Human osteoblast cell culture: (A) With the custom-made orthodontic mini-implant (10×); (B) With the reference implant (10×); (C) Without any implant (control, 10×); (D) Without any implant (control, 20×); (E) Without any implant (control, 40×).
Figure 7.
Human osteoblast adhering to implant’s surface (40×): (A) In presence of the custom-made orthodontic mini-implant; (B) Without any implant (control)
We further investigated whether mini-implants could disturb the proliferation of HOb. Using flow cytometry with CFDA–SE, we assessed the distribution of HOb in daughter generations in absence and presence of mini-implants. Preliminary data indicated, as expected, that the growth of HOb was dependent on the seeding density of cells (20 000, 40 000 and 80 000 cells/cm2). Thus, the optimal seeding density of HOb was identified between 20 000 and 40 000 cells/cm2 (Figure 8) and, therefore, experiments were further done at a seeding density of 20 000 cells/cm2.
Figure 8.
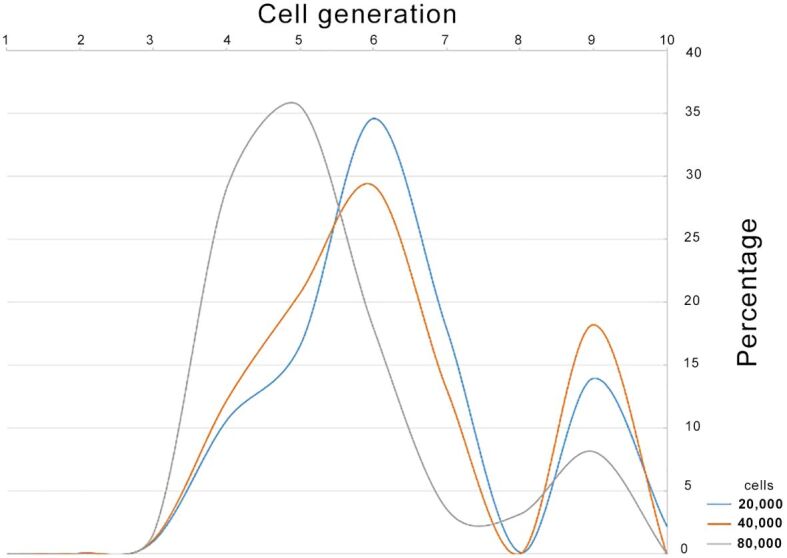
Identifying the optimal cell seeding density
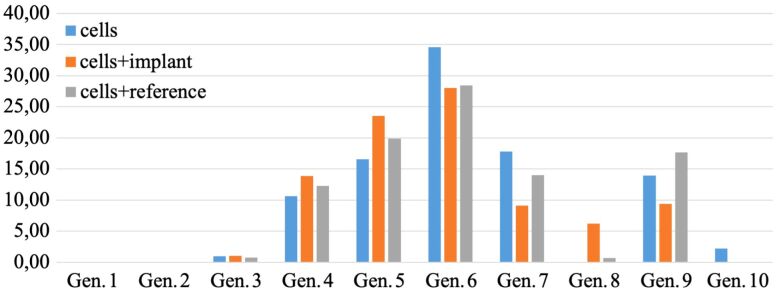
Multiplication of HOb was not significantly altered by the presence of mini-implants in the culture plates, as shown by almost identical distributions of cells in daughter cell generations in presence or absence of custom-made and commercial mini-implants (Figure 9).
Figure 9.
Multiplication pattern of human osteoblasts. Cells were cultivated for 24 hours (20 000 cells/cm2) with/without Ti alloy custom-made and commercial mini-implants (as reference). Distribution of cells in daughter cell generations (Gen.) was assessed by flow cytometry with Carboxyfluorescein diacetate succinimidyl ester (CFDA–SE)
⧉ Discussions
Orthodontic treatments involve usage of various metals and metal alloys that can release metal ions into the oral environment, hence triggering cytotoxicity, genotoxicity, carcinogenicity, and allergic reactions [18]. Most OMI are made from grade 5 Ti alloy, as defined by American Society for Testing and Materials (ASTM), an alloy consisting of Ti, Al and V [19]. Cytotoxic Al and V can be released and may accumulate in the tissues surrounding the mini-implant or diffuse in the circulatory system [18]. Hence, they might be either kept responsible for an implant failure due to osteolysis and allergic reactions or even for inducing various systemic effects, such as hypersensitivity, kidney disease or carcinogenesis [18].
Our study aimed to assess in vitro the biocompatibility of Ti alloy mini-implants, by using a model relevant for alveolar bone remodeling during the interaction with orthodontic mini-screws.
In a context dominated by a huge offer of serial manufactured mini-implants, a customized mini-screw aims to be an effective, convenient, and accessible alternative. It has the benefit of a possible customized selection in accordance with the specific clinical case in terms of dimension, shape, and thread design.
Experimental mini-implants were comparatively analyzed with a series of Ti alloy samples: commercial mini-screws, discs, and metal filings. Commercial mini-implants were used as controls to evaluate how mini-implant’s characteristics influence the adhesion, proliferation, and differentiation of bone cells. However, the main difference between the two types of mini-screws is the surface that comes in direct contact with the bone: the experimental mini-implant has a machined surface (no surface treatment), while the commercial one has a sandblasted acid-etched surface, known for favoring the screw’s biocompatibility.
Ti alloy discs were included in the analysis to mimic the flat surface between the mini-screw’s threads and evaluate the behavior of bone cells in relation to surface shape. The filings were used to further test the potential cytotoxicity of the alloy used for the experimental mini-implants.
SEM analysis was conducted to obtain a descriptive assay of the mini-implants’ design taking into consideration that the surface that encounters the bone influences the osteoblasts’ adhesion and activity. A rough surface favors better bone–implant contact and mechanical retention, which finally determines an enhanced stability [20], while a smooth surface stimulates fibrous connective tissue formation [21]. Our investigation revealed the differences between the surface roughness of the two types of investigated mini-screws, caused by their different processing technology. Correlating the influence of the two mini-screw types on HOb proliferation, differentiation, and adhesion with their surface characteristics, we might assume that surface treatment as well as the groove pattern influence the HOb behavior in close contact with the implant surface.
Furthermore, EDX analysis demonstrated that the two types of mini-implants have the same elements in the chemical composition, with little difference between the concentrations found within each alloy. Experimental mini-implants showed a lower amount of Ti (89.69%) compared with Orlus mini-screw (90.39%). Also, it has a larger amount of Al (7.57%) and a small amount of V (2.74%) compared to the values obtained for Orlus mini-implant (6.8% and 2.81%, respectively). Reducing the concentration of Ti and increasing the Al concentration for the experimental mini-implant might determine a greater mechanical resistance of the alloy.
Results highlighted that the investigated custom-made mini-implants were in the same biocompatibility range with the commercial reference relative to human normal osteoblasts.
Only few studies have reported the cytotoxic potential of OMI. Bueno & Basting evaluated the influence of Ti alloy mini-implants on osteoblasts and concluded that their proliferation has increased from 24 to 72 hours. Also, cell adhesion at 72 hours suggested the presence of bone remodeling, required for osseointegration initiation [22]. Malkoҫ et al. studied the cytotoxicity in five types of mini-implants made from two different alloys (stainless steel and Ti alloy) using a real-time cell analysis system. No adverse effects on gingival fibroblasts were observed in any material, but a significant decrease of osteoblasts viability was recorded for stainless steel mini-screws. Furthermore, same material proved to have different effects on osteoblasts, demonstrating that the cytotoxic effects depend on the composition, surface area and particle size [23].
Soft tissue surrounding the dental implant separates the dental implant from the oral cavity and provides a seal that prevents the development of peri-implant pathology [24]. Fibroblasts are encountered in large numbers in peri-implant mucosa [25]. The biocompatibility of five types of mini-implants was evaluated in relation with gingival fibroblasts using MTT and LDH cytotoxicity tests, showing no adverse effects of the elements solubilized from the mini-implants and no long-term cytotoxic effects on cells of the oral cavity [26]. Custom-made mini-implant from Ti-6Al-4V metal alloy did not induce impairment of the human gingival fibroblasts cell viable population [27]. However, in a three orthodontic implants test, the stainless-steel implant induced slight cytotoxic effects [28]. In another study, upon analyzing the cytotoxicity of another group of six mini-implants, the ones with highest amount of V and Al have shown the smallest cell viability at 24, 48, 72 and 168 hours. Moreover, their different effects on fibroblasts were reported to be caused by the presence of other elements in the composition, such as carbon (C), Ti, iron (Fe), copper (Cu), oxygen (O), and nitrogen (N) [18].
⧉ Conclusions
Ti alloy mini-implants investigated in the present study exerted no major effects on HOb proliferation, differentiation and adhesion. CmOMI had a low cytotoxicity profile on HOb, almost similar to the reference commercial mini-implants. Consequently, they are promising candidates for further development in animal models, that are mandatory for evaluating how these design features influence cmOMI’s stability. It should be pointed out that specific implant design features are of paramount for the success of skeletal anchorage and that every patient has a unique biological condition. Therefore, a customized selection of the mini-implant could increase treatment success with orthodontic mini-screws.
Conflict of interest
The authors declare that there are no financial or personal relationship with a third party, whose interests could be positively or negatively influenced by the article’s contents.
Author contribution
Cristian Funieru had similar contribution to the article as the first author.
Acknowledgments
This work has been supported by Projects Nucleus PN 19.29.02.02/2019 and PCCDI 58/2018 (Sanomat).
References
- 1.Candido C, Impellizzeri A, Galluccio G. Use of temporary anchorage devices in orthodontics: a review of the literature. WebmedCentral Orthod. 2013;4(12):WMC004458–WMC004458. http://www.webmedcentral.com/wmcpdf/Article_WMC004458.pdf [Google Scholar]
- 2.Freire JNO, Silva NRFA, Gil JN, Magini RS, Coelho PG. Histomorphologic and histomorphometric evaluation of immediately and early loaded mini-implants for orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2007;131(6):704e1–704e9. doi: 10.1016/j.ajodo.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Baumgaertel S, Razavi MR, Hans MG. Mini-implant anchorage for the orthodontic practitioner. Am J Orthod Dentofacial Orthop. 2008;133(4):621–627. doi: 10.1016/j.ajodo.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Anusavice KJ, Shen C, Rawls HR. In: Phillips’ science of dental materials. 12. Anusavice KJ, Shen C, Rawls HR, editors. Elsevier Health Sciences-Saunders; 2013. Dental casting alloys and metal joining; pp. 367–395.https://evolve.elsevier.com/cs/product/9781437724189?role=student [Google Scholar]
- 5.Elias CN, Lima JHC, Valiev R, Meyers MA. Biomedical applications of titanium and its alloys. JOM. 2008;60(3):46–49. https://link.springer.com/article/10.1007/s11837-008-0031-1 [Google Scholar]
- 6.Sana S, Majunath G. Mini-implant materials: an overview. IOSR J Dent Med Sci. 2013;7(2):15–20. https://www.iosrjournals.org/iosr-jdms/pages/v7i2.html https://www.iosrjournals.org/iosr-jdms/papers/Vol7-issue2/E0721520.pdf [Google Scholar]
- 7.Cope JB. Temporary anchorage devices in orthodontics: a paradigm shift. Semin Orthod. 2005;11(1):3–9. https://www.semortho.com/article/S1073-8746(04)00056-8/fulltext [Google Scholar]
- 8.Chang JZC, Chen YJ, Tung YY, Chiang YY, Lai EH, Chen WP, Lin CP. Effects of thread depth, taper shape, and taper length on the mechanical properties of mini-implants. Am J Orthod Dentofacial Orthop. 2012;141(3):279–288. doi: 10.1016/j.ajodo.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Cunha AC, Freitas AOA, Marquezan M, Nojima LI. Mechanical influence of thread pitch on orthodontic mini-implant stability. Braz Oral Res. 2015;29(1):1–6. doi: 10.1590/1807-3107BOR-2015.vol29.0042. [DOI] [PubMed] [Google Scholar]
- 10.Hong C, Lee H, Webster R, Kwak J, Wu BM, Moon W. Stability comparison between commercially available mini-implants and a novel design: part 1. Angle Orthod. 2011;81(4):692–699. doi: 10.2319/092410-556.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marigo G, Elias CN, Marigo M. Surface analysis of 2 orthodontic mini-implants after clinical use. Am J Orthod Dentofacial Orthop. 2016;150(1):89–97. doi: 10.1016/j.ajodo.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Ciocan LT, Miculescu F, Miculescu M, Pătraşcu I. Retrieval analysis on dental implants biointegration phases. Rom J Morphol Embryol. 2010;51(1):117–122. [PubMed] [Google Scholar]
- 13.Ryu HS, Namgung C, Lee JH, Lim YJ. The influence of thread geometry on implant osseointegration under immediate loading: a literature review. J Adv Prosthodont. 2014;6(6):547–554. doi: 10.4047/jap.2014.6.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JS, Kim JK, Park YC, Vanarsdall RL. In: Applications of orthodontic mini-implants. Lee JS, Kim JK, Park YC, Vanarsdall RL, editors. Hanover Park IL USA: Quintessence Publishing Co Inc; 2007. Design and function of new, screw-type orthodontic mini-implants; pp. 29–50.https://lanakamal.com.ua/wp-content/uploads/lee-kim-park-vanarsdall-applications-of-orthodontic-mini-implants.pdf [Google Scholar]
- 15.Gracco A, Giagnorio C, Incerti Parenti S, Alessandri Bonetti G, Siciliani G. Effects of thread shape on the pullout strength of miniscrews. Am J Orthod Dentofacial Orthop. 2012;142(2):186–190. doi: 10.1016/j.ajodo.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Abuhussein H, Pagni G, Rebaudi A, Wang HL. The effect of thread pattern upon implant osseointegration. Clin Oral Implants Res. 2010;21(2):129–136. doi: 10.1111/j.1600-0501.2009.01800.x. [DOI] [PubMed] [Google Scholar]
- 17.Park YC, Lee KJ. In: Temporary anchorage devices in orthodontics. Nanda R, Uribe FA, editors. St Louis USA: Mosby; 2009. Biomechanical principles in miniscrew-driven orthodontics; pp. 93–144.https://www.worldcat.org/title/temporary-anchorage-devices-in-orthodontics/oclc/353997084 [Google Scholar]
- 18.Pithon MM, Santos RL, Martins FO, Medeiros PJ, Romanos MTV. Cytotoxicity of orthodontic mini-implants. Rev Clín Pesq Odontol Curitiba. 2010;6(2):141–146. http://www.matheuspithon.com.br/v2/wp-content/uploads/rcpo-0001-00003643-art_41.pdf [Google Scholar]
- 19.Sidambe AT. Biocompatibility of advanced manufactured titanium implants – a review. Materials (Basel) 2014;7(12):8168–8188. doi: 10.3390/ma7128168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulkarni M, Mazare A, Schmuki P, Iglič A. In: Nanomedicine. Seifalian A, de Mel A, Kalaskar DM, editors. One Central Press (OCP); 2014. Biomaterial surface modification of titanium and titanium alloys for medical applications; pp. 111–136.http://www.onecentralpress.com/biomaterial-surface-modification-of-titanium-and-titanium-alloys-for-medical-applications/ http://www.onecentralpress.com/nanomedicine/ [Google Scholar]
- 21.Schwartz Z, Raz P, Zhao G, Barak Y, Tauber M, Yao H, Boyan BD. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90(11):2485–2498. doi: 10.2106/JBJS.G.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bueno RC, Basting RT. In vitro study of human osteoblast proliferation and morphology on orthodontic mini-implants. Angle Orthod. 2015;85(6):920–926. doi: 10.2319/100714-717.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malkoç S, Öztürk F, Çörekçi B, Bozkurt BS, Hakki SS. Real-time cell analysis of the cytotoxicity of orthodontic mini-implants on human gingival fibroblasts and mouse osteoblasts. Am J Orthod Dentofacial Orthop. 2012;141(4):419–426. doi: 10.1016/j.ajodo.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Obădan F, Crăiţoiu Ş, Manolea HO, Hîncu MC, Iacov-Crăiţoiu MM. The evaluation of the morphological evolution of the tissue integration of dental implants through conventional histology and immunohistochemistry techniques. Rom J Morphol Embryol. 2018;59(3):851–859. [PubMed] [Google Scholar]
- 25.Iacov-Crăiţoiu MM, Crăiţoiu M. Clinical, histopathological and immunohistochemical behavior of peri-implant soft tissue. Rom J Morphol Embryol. 2020;61(1):121–128. doi: 10.47162/RJME.61.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birg J, Wheater M. Biocompatibility of temporary anchorage devices using an in vitro cell culture model. J Dent Orofac Surg. 2015;1(1):102–102. https://elynspublishing.com/index.php/journal/article/biocompatibility-of-temporary-anchorage-devices-using-an-in-vitro-cell-culture-model [Google Scholar]
- 27.Popa A, Dehelean C, Calniceanu H, Watz C, Brad S, Sinescu C, Marcu OA, Popa CS, Avram S, Nicolov M, Szuhanek CA. A custom-made orthodontic mini-implant-effect of insertion angle and cortical bone thickness on stress distribution with a complex in vitro and in vivo biosafety profile. Materials (Basel) 2020;13(21):4789–4789. doi: 10.3390/ma13214789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szuhanek CA, Watz CG, Avram Ş, Moacă EA, Mihali CV, Popa A, Campan AA, Nicolov M, Dehelean CA. Comparative toxicological in vitro and in ovo screening of different orthodontic implants currently used in dentistry. Materials. 2020;13(24):5690–5690. doi: 10.3390/ma13245690. [DOI] [PMC free article] [PubMed] [Google Scholar]