Abstract
Background: Lung is the third most frequent identified site of malignancy and lung cancer is the most lethal type of cancer in the world. Several benign lung diseases or proliferations may mimic lung carcinoma in its clinical, pathological, and radiological presentation, which makes the differential diagnosis life changing. This case series was designed to describe the main diagnosis encountered in a multidisciplinary emergency hospital during the last years in Romania. Results: The most challenging cases encountered during the recent years were those of lung hamartoma associated with eosinophilic pneumonia because of the multicentricity of the disease and the suspicion for metastasis in the clinical setting, pulmonary aspergillosis that presented as a cystic lesion with a 9 mm mural nodule, actinomycosis discovered as firm nodule showing aspects of false pleural invasion, cryptococcosis – a hilar mass for which a pneumectomy was prepared, pulmonary parasitosis that presented as a nodule with irregular borders, causing pleural retraction, one case of inflammatory myofibroblastic tumor of the lung, one case of tumorlet type neuroendocrine lesion in a patient with history of melanoma and renal oncocytoma, admitted under the suspicion of lung metastasis. Conclusions: These are some of the main mimickers of primary or secondary lung cancers and one must be aware of these similitudes to avoid higher cost procedures, psychological stress for the patient and higher mortality.
Keywords: lung neoplasm, respiratory tract infections, benign tumors
⧉ Introduction
Lung is the third most frequent identified site of malignancy and lung cancer is the most lethal type of cancer in the world. In Romania’s GLOBOCAN data from 2018, lung cancer was discovered in many patients, 13.6% of all new cases of cancer. In the top 5 most frequent cancers excluding non-melanoma skin cancer, lung cancer was first in men and forth in women. When talking about the overall mortality, lung cancer was by far the first, with 20.2%, followed by colonic cancer, with 7.6%. The 5-year relative survival rates for non-small-cell lung carcinoma (NSCLC), based on people diagnosed with NSCLC between 2009 and 2015 showed localized disease has a 61% chance, regional disease a 35% chance and distant disease a 6% chance of survival, according to Surveillance, Epidemiology, and End Results (SEER) Cancer Statistics Review (CSR) 1975–2016.
Being such an aggressive pathology, making a fast and correct diagnosis of lung cancer is crucial for the survival of that patient. However, there are several benign lung diseases or proliferations that may mimic lung malignancies, and caution must be taken when evaluating these cases.
⧉ Patients, Materials and Methods
The reported data encompasses seven patients with lung cancer mimicking lesions diagnosed between 2018 and 2020 in the Department of Pathology, Emergency University Hospital, Bucharest, Romania. Clinical, imagistic, and pathological investigations were done for each case. The tissue sent from the Department of Thoracic Surgery was fixed in 10% neutral buffered formalin, paraffin embedded and sectioned. The slides obtained were stained with Hematoxylin–Eosin (HE) and by immunohistochemistry techniques, when needed.
⧉ Case presentations
Case No. 1
This is the case of a 67-year-old man with history of recurrent gastric carcinoma. Three pulmonary nodules were received under the suspicion of lung metastasis from the ninth, eighth and sixth segment of the right lung. They were examined grossly, microscopically, and immunohistochemically.
On gross examination, the first one was a firm, gray, slightly lobulated, well circumscribed, 1.5 cm nodule and the other two fragments contained pale, firm 0.5 cm areas.
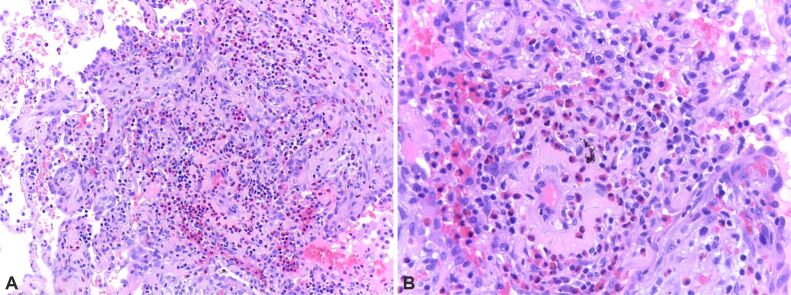
On microscopic examination, the first fragment showed pulmonary parenchyma, lobules of hyaline cartilage, areas of fibrosis, myxoid areas, rare adipocytes, and clefts of respiratory epithelial cells (Figure 1). All three fragments showed areas of focal nodular fibrosis with an inflammatory response composed of lymphocytes, plasma cells and eosinophils (Figure 2).
Figure 1.
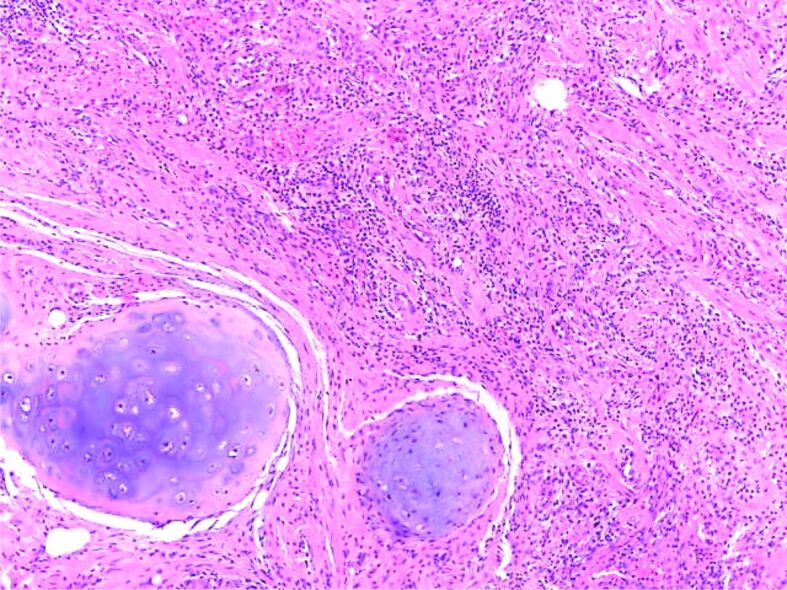
Proliferation of mixed mesenchymal tissue consisting of lobules of cartilaginous tissue some muscular fibers and fibrous tissue (HE staining ×40). HE: Hematoxylin–Eosin
Figure 2.
(A and B) Thickening of the alveolar septa due to the increase in eosinophil-rich inflammatory infiltrate. HE staining: (A) ×100; (B) ×200
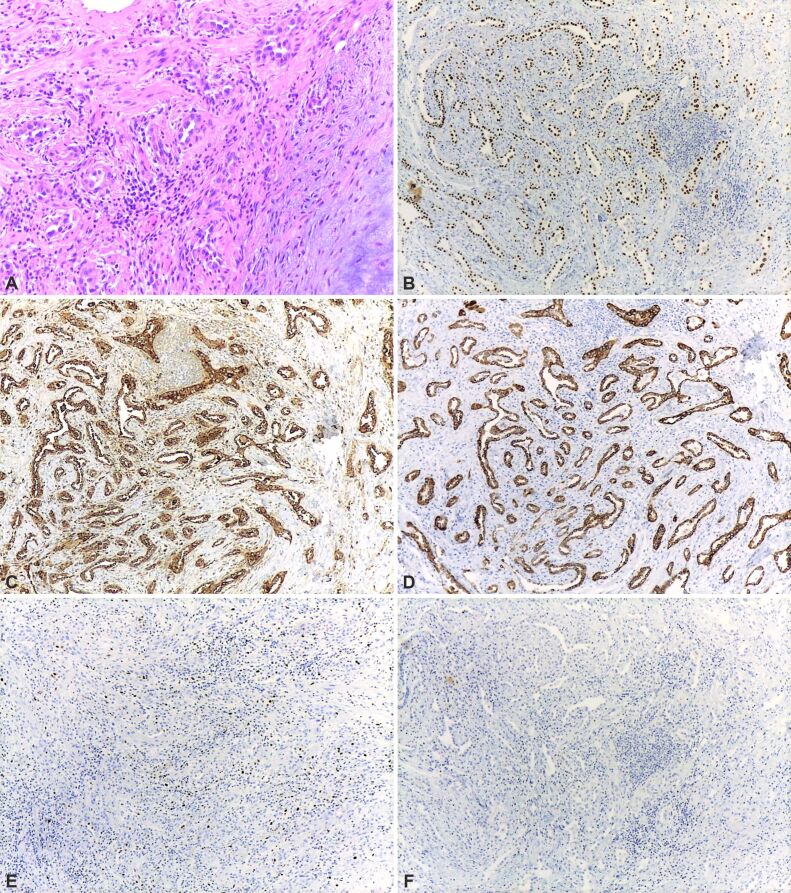
The immunohistochemistry showed: cytokeratin (CK)7 (+), thyroid transcription factor-1 (TTF-1) (+), Napsin A (+), CK20 (-), caudal-type homeobox 2 (CDX2) (-), Ki-67 labeling index <5% (Figure 3A,3B,3C,3D,3E,3F).
Figure 3.
(A) Lung parenchyma located around the hamartoma showing reactive pneumocyte hyperplasia; (B) Diffuse and strong nuclear TTF1 immunostaining in the normal pneumocytes; (C) Strong, diffuse cytoplasmic Napsin A immunostaining of the normal alveolar lining; (D) Diffuse and strong cytoplasmic CK7 immunostaining; (E) Ki-67 immunostaining shows nuclear positivity mostly in rare inflammatory cells; (F) CK20 and CDX2 immunostainings were completely negative (×100). HE staining: (A) ×100. Anti-TTF-1 antibody immunomarking: (B) ×100. Anti-Napsin A antibody immunomarking: (C) ×100. Anti-CK7 antibody immunomarking: (D) ×100. Anti-Ki-67 antibody immunomarking: (E) ×100. CDX2: Caudal-type homeobox 2; CK7: Cytokeratin 7; HE: Hematoxylin–Eosin; TTF-1: Thyroid transcription factor-1
The final diagnosis was lung hamartoma associated with chronic eosinophilic pneumonia.
This is an interesting case because of the unique association between a hamartoma and chronic eosinophilic pneumonia, both inducing changes suggestive for malignancy in a patient with history of gastric carcinoma.
Case No. 2
A 59-year-old man presented with dyspnea and thoracic pain. He had history of smoking, chronic ethanol consumption and occupational respiratory exposure to steel industry particles. He was admitted in our institution under the suspicion of mesothelioma after doing a computed tomography (CT) scan in another hospital. During surgery, the surgeons discovered in the eighth segment a firm nodule showing aspects of pleural invasion.
On gross examination, there was a pulmonary fragment measuring 8/7/2cm, with increased consistency and pale-grey appearance. On ice examination, there was marked acute non-specific inflammation and some reactive pneumocyte hyperplasia. The paraffin-embedded slide showed a lung abscess, chronic pleuritis and Actinomyces spp. colonies, so the final diagnosis was lung abscess due to Actinomyces spp. infection (Figure 4).
Figure 4.
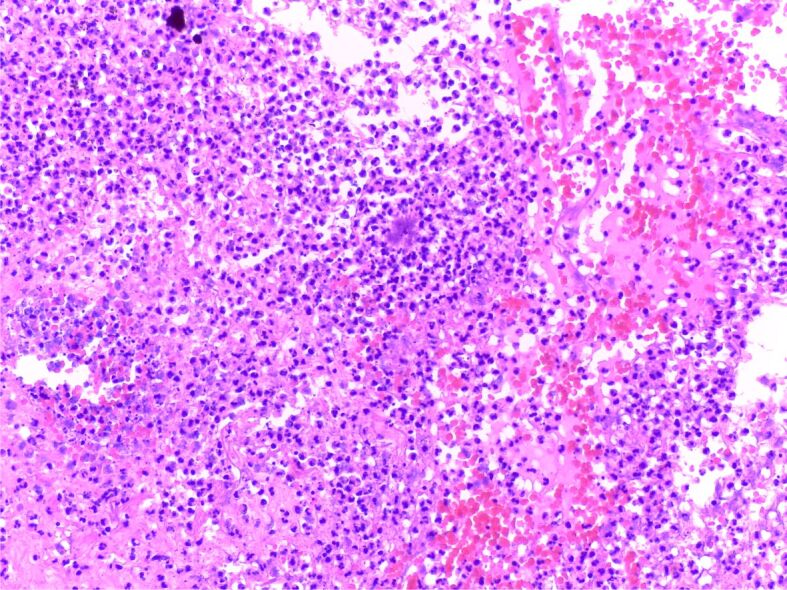
Small Actinomyces spp. colony composed of basophilic radiating filaments; the surrounding area consists of a suppurative inflammation with abundant lymphocytes, neutrophils, and fibroblasts and necrotic debris (HE staining, ×200)
Case No. 3
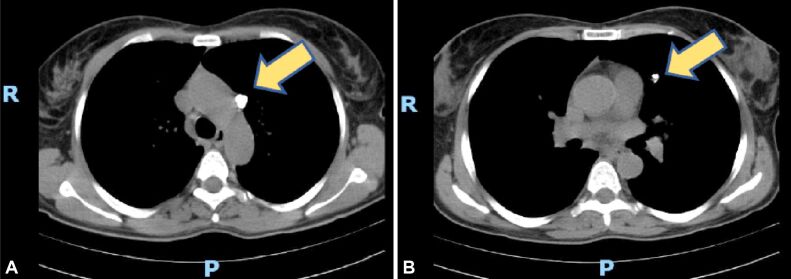
This is the case of a 52-year-old female with hemoptysis and a left lung tumor discovered on a CT scan. She presented two months later for a repeat CT scan and because there was a 14 mm cystic lesion with a 9 mm mural nodule and multiple calcifications located in the left superior lobe the excision is advised (Figure 5, A and B). The Department of Pathology received a fragment of the fifth segment of the left lung measuring 2.5/2/1.2 cm and showing a 10 mm nodular area with central calcification.
Figure 5.
(A and B) CT scan with left superior lobe centrally located cavity showing multiple calcifications, marked by yellow arrows. CT: Computed tomography
On microscopy, there was a fungal ball formed by dichotomous branching hyphae at an angle of 45°, with frequent septation, consistent with Aspergillus spp., surrounded by an acute, exudative inflammation and located in a cystically dilated bronchiole (Figure 6).
Figure 6.
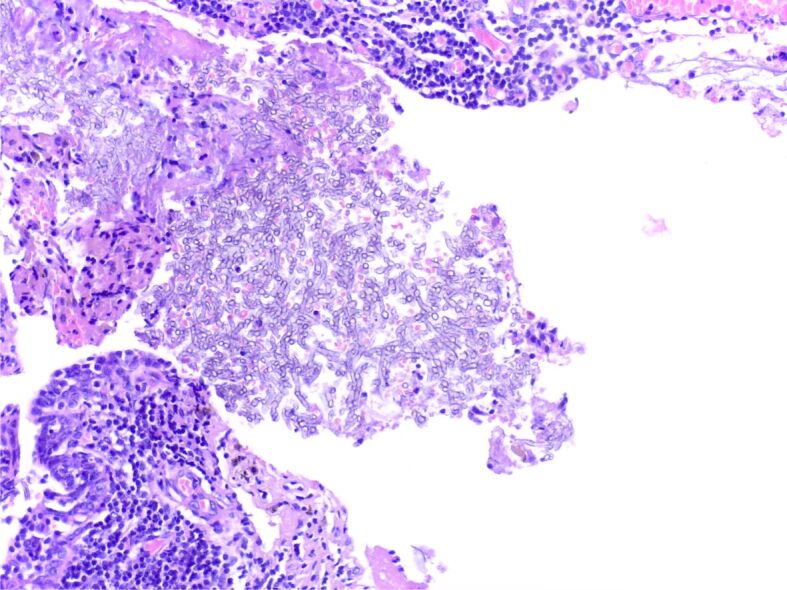
Fungus ball composed of Aspergillus spp. hyphae on a background of acute inflammation and cellular debris; a chronic inflammatory response can be noticed in the bronchial wall, as well as squamous metaplasia of the respiratory lining epithelium (HE staining, ×200)
Case No. 4
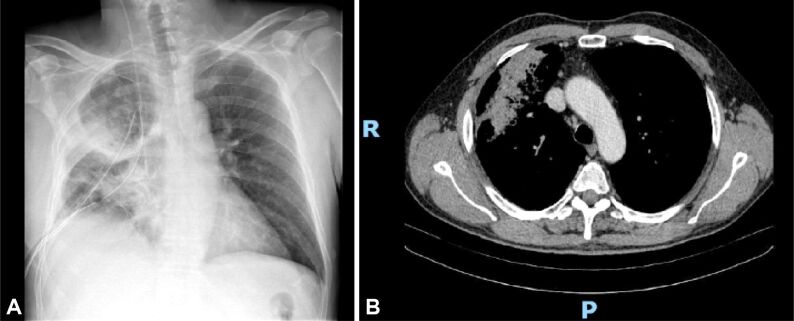
This is the case of a 64-year-old male who was admitted for the treatment of a right hilar mass (Figure 7, A and B). During the surgery, on ice examination showed non-specific granulomatous inflammation and some reactive pneumocyte changes and because there was no frank malignancy confirmed the procedure was limited.
Figure 7.
Centrally located right lung mass extending all the way to the visceral pleura, with irregular margins: (A) Radiograph (up); (B) CT scan (down)
On the permanent slide, there was a granulomatous inflammatory response with frequent giant cells containing round monomorphic intracellular microorganisms with a grey capsule and a clear halo on the HE slide (Figure 8).
Figure 8.
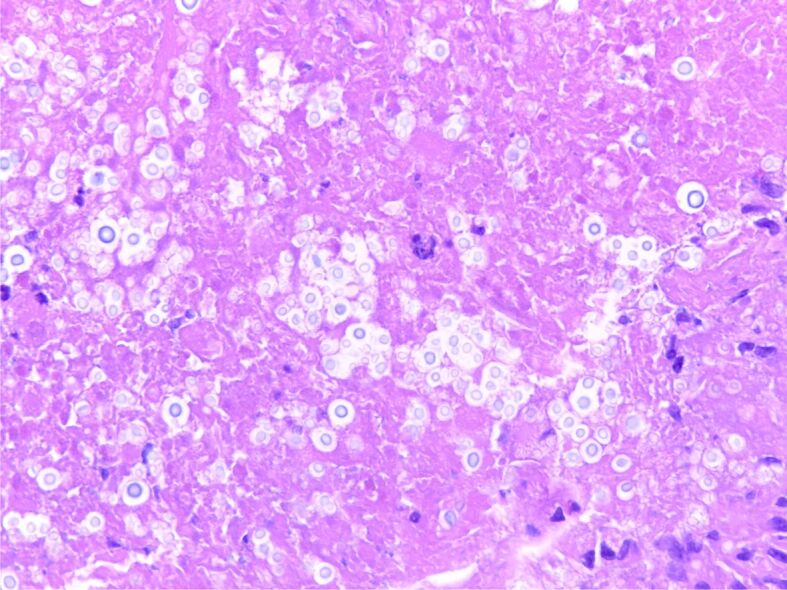
Image shows multiple, round, encapsulated microorganisms with clear halo, located in necrotic debris (Cryptococcus spp.) (HE staining, ×200)
The final diagnosis was pulmonary cryptococcosis (Figure 9).
Figure 9.
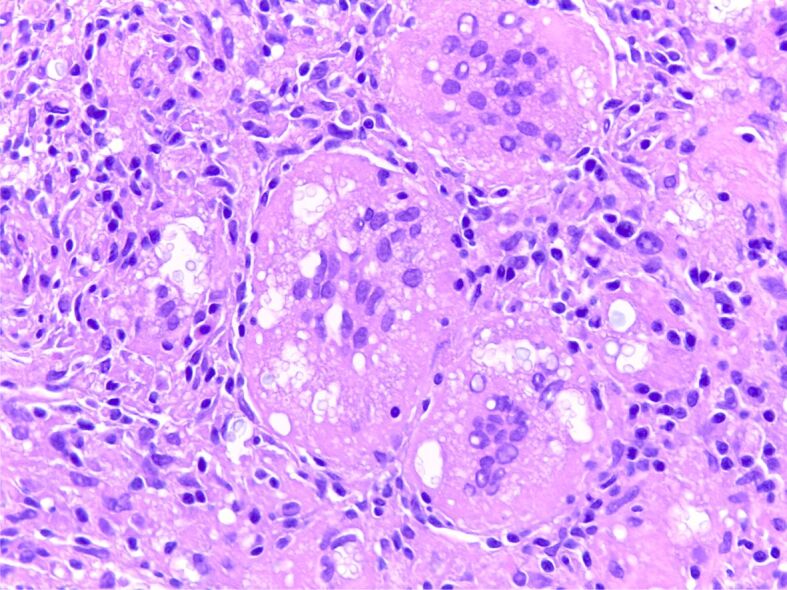
Cryptococcus spp. microorganisms in the cytoplasm of prominent multinucleated giant cells (HE staining, ×200)
Case No. 5
A 46-year-old man presented with the diagnosis of right lung tumor for surgical therapy. The CT scan showed a 17 mm nodule located in the medial lobe, with irregular borders, causing pleural retraction and a few small ones around the first nodule, couple of millimeters in diameter.
On gross examination, there was a 1.5 cm fragment with a white nodular area in the middle with low consistency, measuring 0.5 cm and a 1 cm fragment with a central white area of 0.5 cm.
On ice examination, there was a non-specific inflammatory response and on the permanent slide there was an admixed inflammation centered by a parasite (Figure 10).
Figure 10.
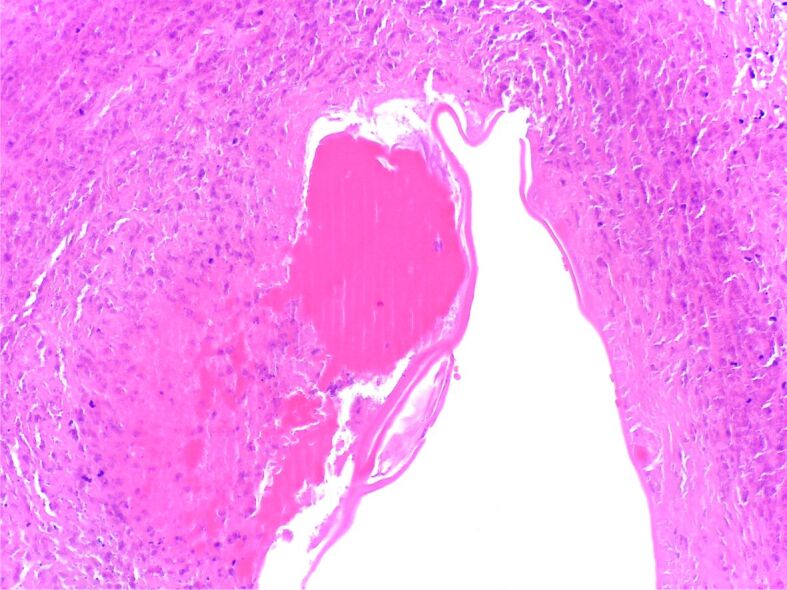
Granulomatous giant cell reaction with pseudo-palisading histiocytes at the periphery of a cavity containing necrotic debris and a proteinaceous structure suggestive of a parasitic wall (HE staining, ×40)
The final diagnosis was of lung parasitosis.
Case No. 6
A 65-year-old female with history of asthma, arterial hypertension, dyslipidemia, and depression presented with abdominal pain, dysphagia, and weight loss. On a CT scan, the radiologists discovered a 23/15/15 mm mass lesion in the inferior left lobe, relatively well-demarcated, showing iodophilia.
The clinicians recommended surgery and a lobectomy was performed.
Gross examination showed a 9/8/6 cm lobe with a 2 cm nodular white, well-demarcated lesion.
The microscopic examination showed fibroinflammatory response with abundant plasma cells, lymphocytes, histiocytes and myofibroblasts, consistent with inflammatory myofibroblastic tumor (IMT) of the lung (Figure 11).
Figure 11.
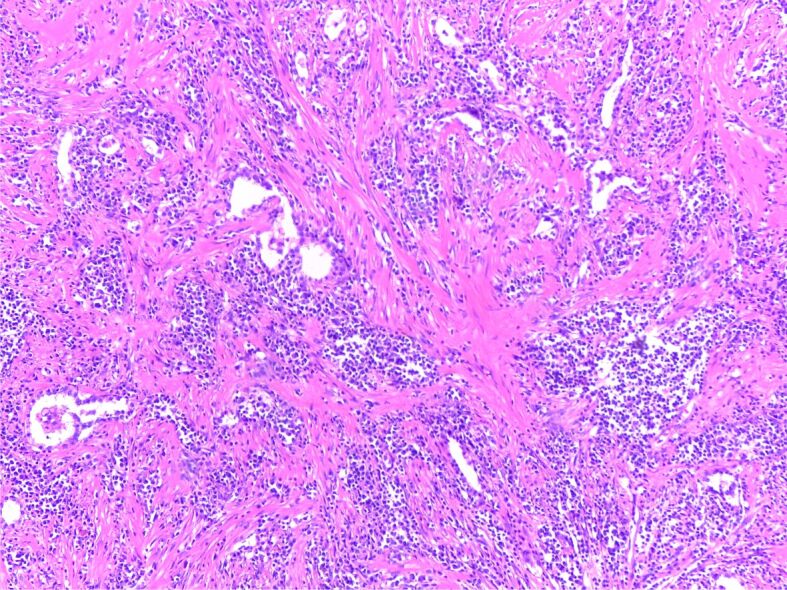
Inflammatory myofibroblastic tumor showing a spindle cell proliferation with short fascicular and storiform pattern, admixed with inflammatory cells and areas with hyalinized stroma (HE staining, ×40)
Case No. 7
A 70-year-old male with history of melanoma and renal oncocytoma was admitted under the suspicion of lung metastasis because of bilateral lung nodules.
The Department of Pathology received multiple samples consisting of lung fragments between 6 cm and 3 cm in the long axes, brown colored, with a 3 mm white-yellow nodule and a 4 mm brown nodule.
On microscopy, there were sclerotic nodules and a nodular proliferation of airway neuroendocrine cells (Kulchitsky cells) that extends beyond the epithelium into the adjacent wall or lung parenchyma consisting of nests of oval to spindle-shaped cells (Figure 12).
Figure 12.
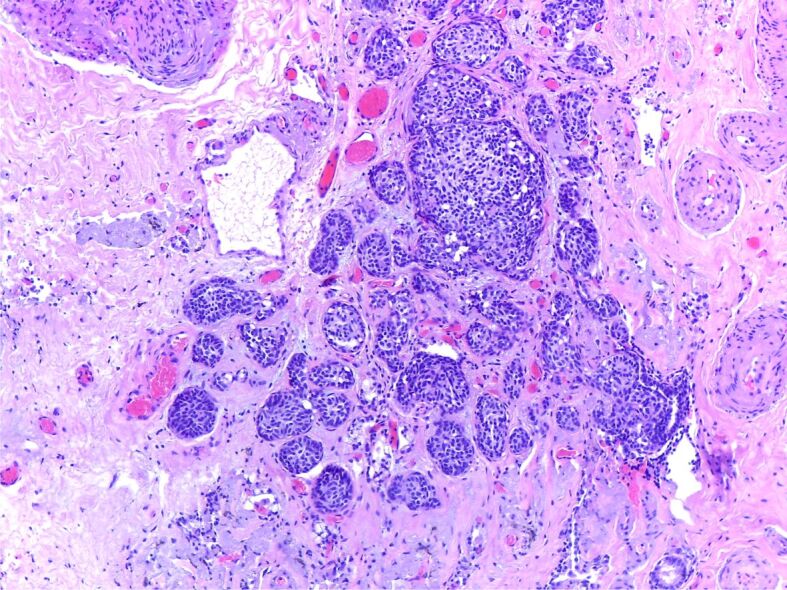
Small islands of bland neuroendocrine cells consistent with neuroendocrine tumorlet (HE staining, ×40)
The final diagnosis was pulmonary neuroendocrine lesion, tumorlet type.
⧉ Discussions
Hamartoma and chronic eosinophilic pneumonia
Pulmonary hamartomas are the most common benign tumors of the lung, composed of varying amounts of mesenchymal tissue: chondroid (most common), fat, connective tissue, smooth muscle and bone and entrapped respiratory epithelial cells. Typical CT findings consist of a round or lobulated nodule with smooth and well-defined borders [1]. If a hamartoma is made of little fat and has no calcification, it is difficult to distinguish it on a CT scan from primary lung cancer with a round or lobulated margin [2].
Chronic eosinophilic pneumonia is a rare disorder outlined by a marked accumulation of eosinophils in the interstitial septa and alveolar sacs. CT demonstrates multiple peripheral, non-segmental areas of airspace consolidation [3].
Actinomyces spp.
Actinomycosis is a chronic infection caused by Actinomyces spp., branched, Gram-positive bacilli, belonging to the normal flora of the oropharynx, intestinal and urogenital tract [4]. The pulmonary form of this infection accounts for 15% of all cases of actinomycosis.
The risk factors for pulmonary actinomycosis include poor oropharyngeal hygiene, chronic dental conditions, and alcoholism. In addition, chronic lung diseases, such as chronic obstructive pulmonary disease (COPD), bronchiectasis, chronic infection caused by mycobacteria or aspergilloma, are considered risk factors due to the anaerobic environment formed by the affected lung tissue [5].
Clinically, patients present with nonspecific symptoms, such as productive cough, hemoptysis, chest pain, weight loss, fever, and endobronchial dyspnea. The most common radiological changes include consolidations with mediastinal and hilar lymphadenopathy, atelectasis, cavitation, “ground glass” changes and pleurisy [6].
Actinomycosis is characterized by an admixture of suppurative and a granulomatous inflammation, proliferation of connective tissue and “sulfur granules”.
The granules measure 0.1–1 mm in diameter and macroscopically can be identified as yellow particles [7].
On histology, “sulfur granules” are bacterial colonies that appear as round basophilic masses, with radially arranged eosinophilic hyphae, surrounded by neutrophilic polymorphonuclear cells [8].
Because of the mass like shadow on thoracic radiography and the chest wall and bone invasion, this lesion can easily be mistaken for a lung malignancy and the physicians should be aware of this important differential diagnosis.
One retrospective study done in China showed that 50% of the cases were misdiagnosed as cancer (13 out of 26 patients) and almost 27% as tuberculosis [9].
Aspergillosis
The etiology of this disease is, more frequently, a saprophytic and soil related fungus called Aspergillus fumigatus. For developing pulmonary aspergillosis, the host requires some predisposing factors: asthma, bronchial dilatation, cystic fibrosis, lung cavities caused by various infections or immune deficiency.
Clinical manifestations, radiological and histological findings of pulmonary aspergillosis depend mainly on the immune status of the host and the pre-existing lung disease.
There are four main types of Aspergillus-related lung diseases: allergic bronchopulmonary aspergillosis, saprophytic aspergillosis infection (aspergilloma), chronic necrotizing aspergillosis (former semi-invasive form) and invasive aspergillosis: angio-invasive and airway-invasive forms [10].
Aspergilloma can mimic radiologically a malignancy by its abnormal, heterogeneous lung shadow.
One review of the literature evaluating a total of 28 cases of endobronchial aspergillosis showed that one quarter of patients had underlying malignancy and that all patients were diagnosed incidentally with aspergillosis [11].
Carcinoid tumorlet
This pathology is part of the diffuse idiopathic pulmonary neuroendocrine cell hyperplasia.
Carcinoid tumorlets are proliferations of neuroendocrine cells forming clusters that invade through lamina propria. They are poorly defined nests, with irregular margins and prominent fibrotic stroma. They are close related to an airway and are less than 5 mm in diameter.
On CT scan, there can be identified pulmonary nodules and on gross examination they can be identified as small, grey-white nodules [12].
Because of the multifocality of this disease, in the proper setting, the dissemination of a primary or secondary malignancy could be added in the differential diagnosis.
Cryptococcus spp.
Cryptococcosis is caused usually by Cryptococcus neoformans, and it represents a rare lung infection. This microorganism usually causes severe forms of pneumonia in immunocompromised patients, especially in those human immunodeficiency virus (HIV) positive and only mild symptoms in those immunocompetent.
Clinically, patients with an intact immune system have minimal cough and mild fever and those immunocompromised present with high fever, aggravated cough, dyspnea, and hemoptysis [13].
Radiography shows an intrapulmonary mass located mainly in the lower lobes, with a diameter of up to 3 cm, occasionally with consolidation or reticulonodular appearance of the lung parenchyma [14]. Diffuse interstitial infiltrates and multiple small lung nodules can be found quite frequently [15].
Histologically, C. neoformans is a 5–10 μm fungus surrounded by a clear capsule, consisting of mucopolysaccharides, positive in special stainings for mucin [16,17,18]. It is characterized by granulomatous inflammation with multiple organisms in the form of extra- or intra-cellular yeasts.
In one retrospective study, 43.42% of cases (33 out of 76 patients) were initially misdiagnosed as cancer due to the clinical and radiological findings [19].
Parasitosis
Lung infections caused by parasites can have numerous pathogens from the helminthic and protozoal family with a non-specific clinical presentation and radiographic findings. The parasites affecting the respiratory system have been called “pneumatodes”.
The signs and symptoms of this infection include eosinophilic pneumonia, cough, wheezing, dyspnea, chest pain, right upper quadrant abdominal pain, fever, mild eosinophilia, hemoptysis, expectoration of cyst contents, acute respiratory distress syndrome (ARDS), epistaxis or nasal congestion [20].
Radiographic examination shows mediastinal or generalized lymphadenopathy, pleural effusion, interstitial pneumonia, and lung nodules.
Histologically, one may find diffuse alveolar damage, eosinophilic pneumonia, alveolar hemorrhage, foreign body-like lesion in bronchus, bronchial stenosis due to mucosal edema, abscesses, and the parasites [20].
Because of the rarity of these infections and the non-specific presentation, especially when it appears as a nodular lesion on thoracic radiography, the first thought is to exclude a lung malignancy, lung parasitosis being a good mimicker of this pathology.
Inflammatory myofibroblastic tumor (IMT)
IMT, also known as inflammatory pseudotumor or plasma cell granuloma, is a low-grade tumor, most commonly found in the lungs, pelvis, abdomen, head, neck and bone marrow [21,22].
The etiology of this process is still unclear. It occurs most frequently in the first two decades of life, with an average of 9–11 years, but can occur at any age and sex [21,22].
The inflammatory myofibroblastic lung tumors symptoms include cough, hemoptysis, dyspnea, chest pain, and constitutional symptoms [21].
On radiological examination of the thorax, the tumor presents as a nodular, peripheral lesion of variable size (diameter can be between 1.2 cm and 15 cm), more commonly located in the lower lobes. Rarely, patients may have multiple lesions [23,24].
On CT examination, these tumors are unique, well-defined, lobed, and frequently contain punctate calcifications that mimic a malignant tumor [25].
Histologically, this tumor is characterized by a varied spectrum of inflammatory cells (lymphocytes, plasma cells, histiocytes and occasional eosinophils) on a background of uniform spindle cells with limited cytological atypia and minimal mitotic activity. Therefore, it may be difficult to differentiate IMT from infections such as those caused by atypical mycobacteria, syphilis or other granulomatous pathologies [26].
One retrospective study of 16 cases of IMT showed that in five cases positron emission tomography (PET) was not able to distinguish between inflammation and malignancy, and thus excision remains the only certain method of diagnosis in this pathology [24].
⧉ Conclusions
A wide variety of pulmonary pathologies can have features similar with those of lung cancer. Keeping these conditions in mind and using them in the differential diagnosis, combined with careful examination of the clinical information, will help in making the correct diagnoses.
Conflict of interest
The authors declare that they have no conflict of interests.
Acknowledgments
Acknowledgments
The first author would like to thank to all the Departments of Emergency University Hospital, Bucharest, Romania, for their assistance with the collection of data.
References
- 1.Siegelman SS, Khouri NF, Scott WW, Leo FP, Hamper UM, Fishman EK, Zerhouni EA. Pulmonary hamartoma: CT findings. Radiology. 1986;160(2):313–317. doi: 10.1148/radiology.160.2.3726106. [DOI] [PubMed] [Google Scholar]
- 2.Furuya K, Yasumori K, Takeo S, Sakino I, Uesugi N, Momosaki S, Muranaka T. Lung CT: Part 1, Mimickers of lung cancer - spectrum of CT findings with pathologic correlation. AJR Am J Roentgenol. 2012;199(4):W454–W463. doi: 10.2214/AJR.10.7262. [DOI] [PubMed] [Google Scholar]
- 3.Cottin V, Cordier JF. Eosinophilic pneumonias. Allergy. 2005;60(7):841–857. doi: 10.1111/j.1398-9995.2005.00812.x. [DOI] [PubMed] [Google Scholar]
- 4.Hall V. Actinomyces – gathering evidence of human colonization and infection. Anaerobe. 2008;14(1):1–7. doi: 10.1016/j.anaerobe.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Kolditz M, End A. In: Complex pleuropulmonary infections. Rohde G, Subotic D, editors. Vol. 61. European Respiratory Society Monograph; 2013. Actinomycosis of the lung and pleura; pp. 66–80. [Google Scholar]
- 6.Han JY, Lee KN, Lee JK, Kim YH, Choi SJ, Jeong YJ, Roh MS, Choi PJ. An overview of thoracic actinomycosis: CT features. Insights Imaging. 2013;4(2):245–252. doi: 10.1007/s13244-012-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valour F, Sénéchal A, Dupieux C, Karsenty J, Lustig S, Breton P, Gleizal A, Boussel L, Laurent F, Braun E, Chidiac C, Ader F, Ferry T. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183–197. doi: 10.2147/IDR.S39601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099–d6099. doi: 10.1136/bmj.d6099. [DOI] [PubMed] [Google Scholar]
- 9.Sun XF, Wang P, Liu HR, Shi JH. A retrospective study of pulmonary actinomycosis in a single institution in China. Chin Med J (Engl) 2015;128(12):1607–1610. doi: 10.4103/0366-6999.158316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabi ML, Goracci A, Roche N, Paugam A, Lupo A, Revel MP. Pulmonary aspergillosis. Diagn Interv Imaging. 2015;96(5):435–442. doi: 10.1016/j.diii.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Ngu S, Narula N, Abureesh M, Li JJ, Chalhoub M. Endobronchial aspergilloma – a comprehensive literature review with focus on diagnosis and treatment modalities. Eur J Clin Microbiol Infect Dis. 2020;39(4):601–605. doi: 10.1007/s10096-019-03726-5. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I, WHO Panel The 2015 World Health Organization Classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 13.Lam CL, Lam WK, Wong Y, Ooi GC, Wong MP, Ho JC, Lam B, Tsang KW. Pulmonary cryptococcosis: a case report and review of the Asian–Pacific experience. Respirology. 2001;6(4):351–355. doi: 10.1046/j.1440-1843.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 14.Shirley RM, Baddley JW. Cryptococcal lung disease. Curr Opin Pulm Med. 2009;15(3):254–260. doi: 10.1097/MCP.0b013e328329268d. [DOI] [PubMed] [Google Scholar]
- 15.Wu B, Liu H, Huang J, Zhang W, Zhang T. Pulmonary cryptococcosis in non-AIDS patients. Clin Invest Med. 2009;32(1):E70–E77. doi: 10.25011/cim.v32i1.5090. [DOI] [PubMed] [Google Scholar]
- 16.Binford CH, Dooley JR. In: Pathology of tropical and extraordinary diseases. Binford CH, Connor DH, editors. Washington DC USA: Armed Forces Institute of Pathology (AFIP); 1976. Disease caused by fungi and actinomycetes; pp. 572–573.https://www.worldcat.org/title/pathology-of-tropical-and-extraordinary-diseases/oclc/3708131 [Google Scholar]
- 17.McAdam AJ, Sharpe AH. In: Robbins basic pathology. 9. Kumar V, Abbas AK, Aster JC, editors. Philadelphia PA USA: Elsevier-Saunders; 2015. General pathology of infectious disease; pp. 313–313.https://www.ncbi.nlm.nih.gov/nlmcatalog/101638769 [Google Scholar]
- 18.Husain AN. In: Robbins basic pathology. 9. Kumar V, Abbas AK, Aster JC, editors. Philadelphia PA USA: Elsevier-Saunders; 2015. Lung pathology; pp. 503–504.https://www.ncbi.nlm.nih.gov/nlmcatalog/101638769 [Google Scholar]
- 19.Zhang Y, Li N, Zhang Y, Li H, Chen X, Wang S, Zhang X, Zhang R, Xu J, Shi J, Yung RC. Clinical analysis of 76 patients pathologically diagnosed with pulmonary cryptococcosis. Eur Respir J, 2012, 40(5):1191-1200. Eur Respir J. 2012;2013;4041(5)(1):1191–1200. 252–252. doi: 10.1183/09031936.00168011. [DOI] [PubMed] [Google Scholar]
- 20.Khemasuwan D, Farver C, Mehta AC. In: Diseases of the central airways: a clinical guide Respiratory Medicine (RM) Book Series Humana Press. Mehta AC, Jain P, Gildea TR, editors. Switzerland: Springer International Publishing; 2016. Parasitic diseases of the lung; pp. 231–253.https://link.springer.com/book/10.1007/978-3-319-29830-6 [Google Scholar]
- 21.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. In: World Health Organization (WHO) Classification of tumours of soft tissue and bone. 4. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. Vol. 5. Lyon France: International Agency for Research on Cancer (IARC) Press; 2012. Inflammatory myofibroblastic tumour; pp. 83–85.https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Soft-Tissue-And-Bone-2013 [Google Scholar]
- 22.Coffin CM, Hornick JL, Fletcher CDM. Inflammatory myo-fibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31(4):509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 23.Choi BY, Kim WS, Cheon JE, Kim IO, Kim CJ, Yeon KM. Inflammatory myofibroblastic tumour of the liver in a child: CT and MR findings. Pediatr Radiol. 2003;33(1):30–33. doi: 10.1007/s00247-002-0786-4. [DOI] [PubMed] [Google Scholar]
- 24.Athanassiadi K, Laenger F, Dickgreber N, Haverich A. Multiple inflammatory myofibroblastic tumors involving lung and mediastinum: a rare clinical entity. Thorac Cardiovasc Surg. 2009;57(6):343–346. doi: 10.1055/s-0029-1185574. [DOI] [PubMed] [Google Scholar]
- 25.Takayama Y, Yabuuchi H, Matsuo Y, Soeda H, Okafuji T, Kamitani T, Kinoshita Y, Kubokura N, Sakai S, Oda Y, Hatakenaka M, Honda H. Computed tomographic and magnetic resonance features of inflammatory myofibroblastic tumor of the lung in children. Radiat Med. 2008;26(10):613–617. doi: 10.1007/s11604-008-0284-1. [DOI] [PubMed] [Google Scholar]
- 26.Sagar AES, Jimenez CA, Shannon VR. Clinical and histopathologic correlates and management strategies for inflammatory myofibroblastic tumor of the lung. A case series and review of the literature. Med Oncol. 2018;35(7):102–102. doi: 10.1007/s12032-018-1161-0. [DOI] [PubMed] [Google Scholar]